Abstract
Objective
To investigate whether illiteracy was associated with greater risk of prevalent and incident dementia and more rapid cognitive decline among older adults with low education.
Methods
Analyses included 983 adults (≥65 years old, ≤4 years of schooling) who participated in a longitudinal community aging study. Literacy was self-reported (“Did you ever learn to read or write?”). Neuropsychological measures of memory, language, and visuospatial abilities were administered at baseline and at follow-ups (median [range] 3.49 years [0–23]). At each visit, functional, cognitive, and medical data were reviewed and a dementia diagnosis was made using standard criteria. Logistic regression and Cox proportional hazards models evaluated the association of literacy with prevalent and incident dementia, respectively, while latent growth curve models evaluated the effect of literacy on cognitive trajectories, adjusting for relevant demographic and medical covariates.
Results
Illiterate participants were almost 3 times as likely to have dementia at baseline compared to literate participants. Among those who did not have dementia at baseline, illiterate participants were twice as likely to develop dementia. While illiterate participants showed worse memory, language, and visuospatial functioning at baseline than literate participants, literacy was not associated with rate of cognitive decline.
Conclusion
We found that illiteracy was independently associated with higher risk of prevalent and incident dementia, but not with a more rapid rate of cognitive decline. The independent effect of illiteracy on dementia risk may be through a lower range of cognitive function, which is closer to diagnostic thresholds for dementia than the range of literate participants.
Although rates of illiteracy are relatively low among developed countries, current estimates indicate that around 32 million adults in the United States are illiterate.1 This statistic is concerning given that literacy and formal education may be protective against risk of dementia.2–4
Prior epidemiologic studies have associated illiteracy or low to no formal education with increased prevalence and incidence of dementia.5–7 While low literacy is associated with greater cognitive decline,8–10 it is less clear whether illiterate people decline faster over time than literate people, thus increasing their risk of dementia. In addition, the relationship between literacy and dementia may be moderated by sex/gender, as several studies have found that the effect of education on risk for dementia is stronger among women.5,11–13
Given that prior studies were unable to distinguish between the effects of literacy and of low to no formal education, we evaluated literate and illiterate older adults with ≤4 years of education. To assess the unique contribution of literacy to dementia risk beyond that of years of schooling, we contrasted rates of dementia at baseline, risk for incident dementia, and rate of cognitive decline in illiterate vs literate participants. We hypothesized that illiteracy, independent of years of school and other confounders, would be associated with greater risk of prevalent and incident dementia, lower baseline cognitive abilities, and faster rate of cognitive decline when compared to being literate. Finally, we evaluated whether sex modified the effect of illiteracy on outcomes, such that associations would be stronger for women.
Methods
Participants and procedure
The current sample was selected from participants in the Washington Heights–Inwood Columbia Aging Project (WHICAP), a community-based, prospective cohort study of dementia in the ethnically diverse neighborhoods of Northern Manhattan, New York. The WHICAP study follows a sample of Medicare-eligible older adults residing in selected census tracts of Washington/Hamilton Heights and Inwood. Participants are followed up every 18–24 months to receive medical, neurologic, and neuropsychological evaluations.
Full study procedures and descriptions of the total sample have been reported previously.3 Briefly, participants were identified from Medicare records and recruited in 3 waves: 1992 (n = 2,338), 1999 (n = 2,183), and 2009 (n = 2,128). Baseline and follow-up evaluations include a battery of cognitive, functional, and health measures administered in the participant's preferred language (English or Spanish). The WHICAP battery was translated and validated in Spanish and has been reported previously.14 Race and ethnicity were determined via self-report using the format of the 2000 US Census. In addition, participants were asked to self-report as either male or female; however, this method does not allow us to know whether participants reported their sex or gender.15 As such, the term sex/gender is used to describe this variable.
The current study included participants who self-reported literacy status, had 4 or fewer years of schooling, and had baseline cognitive data. Participants who met criteria for dementia at their initial study visit were included only for the prevalent dementia analysis, and were excluded for the incident dementia and cognitive trajectory analyses. Figure 1 depicts how participants were selected from the full cohort for the current analyses. We included only participants with 4 or fewer years of schooling in order to (1) minimize the confounding effects of lifetime educational experiences with that of acquiring literacy, (2) avoid confounding lack of opportunity for schooling with learning disability, and (3) because 74% of illiterate adults in the sample had this range of education. Follow-up time was calculated as time in years between first and last visit. Average time in the study was 4.10 years, with a range from 0 (only baseline) up to 23 years. Characteristics of the sample are available in table 1.
Figure 1. Flow diagram for identifying participants from the 3 study cohorts.

NP = neuropsychological evaluation.
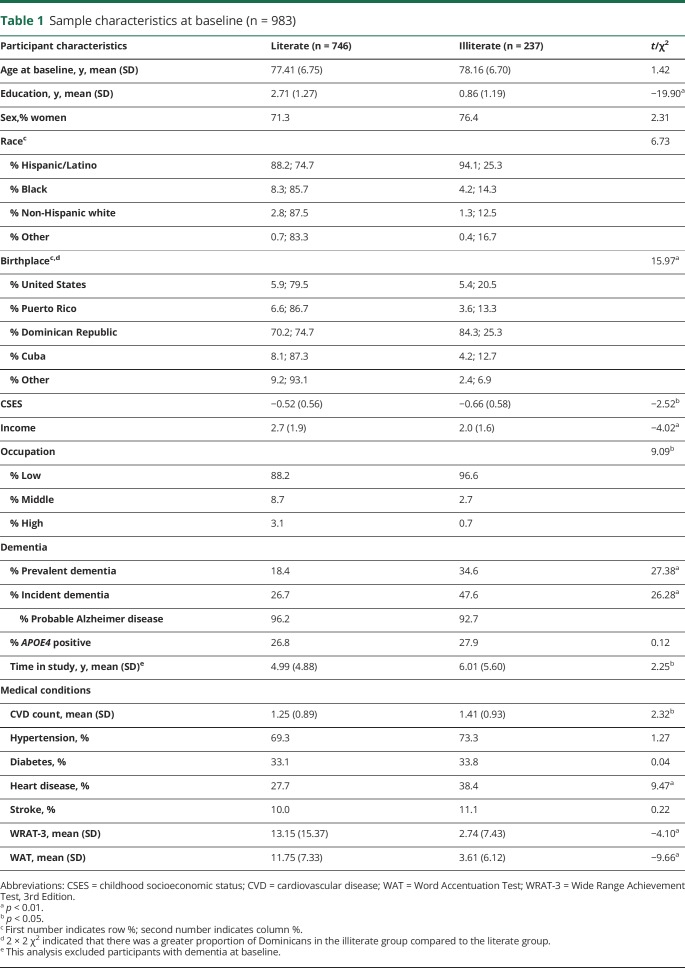
Table 1.
Sample characteristics at baseline (n = 983)
Standard protocol approvals, registrations, and patient consents
The study was approved by the Institutional Review Boards of Columbia University Medical Center and Columbia University Health Sciences and the New York State Psychiatric Institute and a voluntarily signed informed consent was obtained by all participants.
Neuropsychological evaluation
Participants completed a comprehensive neuropsychological battery that assessed the cognitive domains of memory, language, and visuospatial functioning. Composite cognitive domain scores were derived for each domain based on a previously published factor structure.14 Prior studies have established the validity of this battery to detect cognitive change among individuals from diverse educational and linguistic backgrounds (including illiterate individuals).14,16 The memory composite score comprised 3 scores from the Selective Reminding Test: total immediate recall, delayed recall, and delayed recognition.17 The language composite score comprised tests of naming, verbal fluency, verbal abstraction, repetition, and comprehension. The visuospatial functioning composite score comprised scores on the recognition and matching test of the Benton Visual Retention Test,18 the Rosen Drawing Test,19 and the Identities and Oddities subtest of the Mattis Dementia Rating Scale.20 All scores were standardized to z score metric using the larger WHICAP sample's means and SDs at the initial occasion.
Literacy
Literacy was self-reported by participants responding to the following question: “Did you ever learn to read or write?” Participants were classified as illiterate if they answered “no” or literate if they answered “yes.” Self-reported literacy was validated on a subsample of participants who were administered an objective reading test (Wide Range Achievement Test–Reading Subtest in English or the Word Accentuation Test in Spanish). As expected, illiterate participants performed worse on objective reading tests compared to literate participants (all ps < 0.001; table 1). While not formally measured, most participants with no formal schooling who attained literacy learned to read and write via informal lessons from family members during childhood.
Dementia risk factors
Demographics (i.e., age, sex/gender, years of school, race and ethnicity, country of origin, adult and childhood socioeconomic status) and medical risk factors (i.e., hypertension, diabetes, heart disease, APOE genotype) were all determined at baseline. Participant's occupation and income were used as proxies for adult socioeconomic status. Childhood socioeconomic status (CSES) was defined by the participant's parents' years of education and occupation. Each participant had a CSES factor score derived through factor analysis. Hypertension, diabetes, heart disease, and stroke were dichotomous variables based on self-report. Participants were classified as having a particular condition if they responded “yes” when asked about the condition or reported taking medication for the condition. A cardiovascular disease risk score was calculated by adding the number of conditions endorsed (0–4).21 APOE genotype was converted to a dichotomous variable reflecting the presence of at least 1 ε4 allele. APOE genotype is only provided for descriptive purposes and is not included as a covariate in our analyses.
Dementia diagnosis
Diagnosis of dementia was established by a review of all available clinical information (i.e., neuropsychological, medical, and neurologic, but not including radiologic data) and was based on standard research criteria. Greater detail of the development of this procedure can be found in prior studies.16,22 In brief, following each clinical evaluation, a consensus group of neurologists, psychiatrists, and neuropsychologists reviewed available data to assign a research diagnosis. A diagnosis of all-cause dementia was based on standard research criteria,23 and then dementia subtype was determined based on research criteria for probable or possible Alzheimer disease,23 Lewy body dementia,24 or vascular dementia.25 This approach has been validated in this population22 and has been used in numerous studies as an outcome to identify biological, genetic, and social risk factors for dementia.
Statistical analyses
Descriptive statistics, logistic regression, and Cox proportional hazard models were conducted in SPSS 23 (SPSS Inc., Chicago, IL). Covariates included age centered at the sample's mean (77 years) at baseline, years of education, sex/gender, CSES, study recruitment cohort, cardiovascular disease risk score, and Dominican nationality. At baseline, 12.6% of the sample was missing CSES data, but were not missing for any of the covariates. While there were no differences in race/ethnicity between literate and illiterate participants, there was a greater proportion of participants from the Dominican Republic in the illiterate group compared to the literate group. As such, Dominican nationality was included as a covariate. Furthermore, while cardiovascular disease may be considered a mediator between literacy and cognitive outcomes, we included it in our final models to determine if there was an effect of literacy even after accounting for cardiovascular disease. The analysis of mediators is the next step in this research, but beyond the scope of the current analyses.
Logistic regression was used to evaluate the relationship between literacy status (0 = literate vs 1 = illiterate) and prevalent dementia, first unadjusted and then adjusted by covariates. Cox proportional hazard models were used to test the relationship of literacy status to incident dementia; the time to event variable was in years from first assessment to the first assessment at which a diagnosis of dementia was assigned via consensus or years in study for participants who remained free from dementia over the course of follow-up. Both prevalent and incident dementia analyses included an interaction term of literacy by sex/gender. Incident and longitudinal analyses excluded participants with dementia at baseline (n = 217).
Longitudinal data were analyzed in Mplus version 8 with latent growth curve models to determine the relationship of literacy to intercept and change in cognitive function over time. The time score option in Mplus was used to accommodate differing intervals between study visits and time was parameterized as years from study entry. In total, 5 visits (follow-up up to 23 years) were analyzed to maximize covariance coverage. Average time between each follow-up was 3 years (SD 3.48). There was loss of follow-up with each visit, with 129 individuals who stopped after visit 1 (16.8% loss to follow-up), 229 after visit 2 (29.9% loss to follow-up), 153 after visit 3 (19.9% loss to follow-up), and 183 who returned for all 5 visits (23.8% of the baseline sample).
We evaluated model fit by the Bayesian Information Criterion (BIC).26 Missing data were managed with full information maximum likelihood using all available data at each occasion. All models included literacy as the principal predictor, in which the literate group was the reference category. Initially, 3 separate unadjusted latent growth curve models were estimated for each cognitive domain (memory, language, visuospatial). Allowing linear vs curvilinear change was compared in each of these models. To assess for evidence of practice effects, a spline modeling retest effects was included.27 The best fitting models were retained for subsequent analyses that included evaluating the effect of literacy status and covariates. Compared with models allowing only linear change, fit did not improve by allowing both linear and curvilinear change. Similarly, fit did not improve by modeling a spline for retest effects for each cognitive domain, so it was not included in subsequent models. Therefore, models estimating quadratic slopes and retest effects were not used. Subsequently, we built adjusted models in 2 steps: step 1 included all covariates except for cardiovascular disease, which was included in step 2. The covariate-adjusted models fit the data well as noted by the improved model fit for each cognitive trajectory as demonstrated by smaller BICs (memory BICs 3966.539, 3537.245; language BICs 2352.290, 2106.175; visuospatial BICs 3176.911, 2842.711). Multiple-group models were conducted in the fully adjusted models to examine sex/gender differences on the effect of literacy on initial levels and slope for each cognitive trajectory. Finally, sensitivity analyses were conducted to assess whether attrition due to death influenced our findings. To evaluate the effect of attrition due to death and dropout, we used joint modeling that included a discrete-time survival model with the latent growth curve model. The discrete-time survival model estimates a hazard function due to death and dropout, which is then regressed on growth trajectories to evaluate its influence on slope.
Data availability
Data are available upon reasonable request to the WHICAP Publications Committee. Data requests should be submitted at cumc.co1.qualtrics.com/jfe/form/SV_6x5rRy14B6vpoqN.
Results
Table 1 shows that illiterate participants had fewer years of school, lower CSES, and greater cardiovascular disease burden compared to literate participants. There was a greater proportion of illiterate individuals in the first recruitment cohort (50% in 1992 compared to 30% in 1999 and 20% in 2009; χ2 = 22.75, p < 0.001). Illiterate individuals had a higher proportion of dementia cases at baseline (35%) and over time (48%) compared to literate individuals (18% and 27%, respectively). A greater proportion of illiterate adults were born in the Dominican Republic (84%) compared to literate adults (70%). The vast majority (95%) of dementia cases were diagnosed in the clinical consensus conference as Alzheimer disease dementia. Diagnostically, there was a similar proportion of illiterate participants who received a diagnosis of Alzheimer disease dementia (93%) compared to literate participants (96%), around 1% of each group was diagnosed with vascular dementia, and 1 literate participant was diagnosed with Lewy body dementia.
Prevalent dementia
In the unadjusted model, being illiterate increased the odds of prevalent dementia by 2.35 (95% confidence interval [CI] 1.70, 3.26). After adjusting for covariates (step 1), illiterate participants were still at higher odds of prevalent dementia compared to literate participants (odds ratio 2.65, 95% CI 1.55, 4.50), and similarly after including cardiovascular disease (estimates in table 2). In this model, older age, early recruitment cohort (1992), and more cardiovascular disease were, besides illiteracy, additional variables to increase risk of prevalent dementia. Table 2 displays the results of the fully adjusted logistic regressions. There was no interaction between sex/gender and literacy status.
Table 2.
Prevalent and incident dementia analyses
Incident dementia
In the unadjusted Cox model, illiterate participants were 1.95 times (95% CI 1.46, 2.60) more likely to develop dementia compared to literate participants. The effect of illiteracy on dementia risk was slightly reduced after adjusting for covariates (step 1; hazard ratio 1.63, 95% CI 1.12, 2.36) and after including cardiovascular disease (table 2). In the fully adjusted model (table 2), older age and more cardiovascular disease predicted, in addition to illiteracy, higher risk of incident dementia. There was no interaction between sex/gender and literacy status.
Cognitive trajectories
Unadjusted growth models characterized trajectories of change within the 3 cognitive domains (table 3). For memory, language, and visuospatial functioning, illiterate participants had worse initial abilities compared to literate participants, but literacy status did not influence slope. Adjusting for covariates did not change the results (table 4). Multiple group models revealed that the association between literacy status and cognitive trajectories did not differ by sex/gender (all ps > 0.05).
Table 3.
Fixed effects of literacy group
Table 4.
Literacy group and covariate effects on cognitive trajectories
Results from the joint model indicated that survival and loss to follow-up was associated with lower initial level for memory (estimate −0.039, 95% CI −0.064, −0.014), but not language or visuospatial functioning. Rate of decline was not significantly affected by survival and loss to follow-up across any of the cognitive domains. Finally, the inclusion of a survival and loss to follow-up factor did not change the effect of literacy on initial levels or slope across domains (figures 2 and 3).
Figure 2. Estimated survival curves by literacy status in the adjusted Cox regression model.

Figure 3. Mean cognitive trajectories (95% confidence interval [CI]) for each cognitive domain by literacy group.

Discussion
Illiterate adults were almost 3 times as likely to have dementia at baseline and twice as likely to progress to dementia over time compared to literate participants. Illiterate adults demonstrated worse cognition at baseline compared to literate participants, but did not exhibit a faster rate of cognitive decline. These results suggest that illiterate adults are closer to the cognitive and functional thresholds for dementia than literate individuals. Furthermore, the effect of illiteracy on dementia risk and cognition did not differ between men and women.
Studies evaluating the association of illiteracy on prevalent and incident dementia within the United States and that provide reliable longitudinal assessment of cognition among illiterate participants are rare. A handful of studies on late-life cognitive decline from developing countries (i.e., China, India, and various countries in Latin America) included illiterate older adults, reporting mixed results. Inconsistent results of prior studies may have been due to the low sensitivity of cognitive screening instruments and difficulty with assessment of functional decline across cultures, particularly among illiterate adults.28,29 Our longitudinal study, in contrast, used measures of cognitive and daily functioning that were validated for use in this cohort.14,30 The effect of literacy on dementia risk remained robust even after including potential early and later life confounds of literacy (i.e., socioeconomic status, poorer overall health).29,31,32
Our unique cohort of illiterate and literate adults followed longitudinally allowed us to assess the influence of literacy on cognitive trajectories. While the effect of literacy on baseline cognition was robust, our hypothesis that illiteracy would increase rate of cognitive decline was not supported. There are at least 4 possible explanations for this. First, our hypothesis was based on prior studies that found more rapid decline among people with low literacy (not just illiterate participants) across the range of educational attainment (not limited to people with very few years of school).8–10 It is possible that literacy skills only affect cognitive change if the entire ranges of educational attainment and literacy are considered. This may be the most likely explanation given that prior studies from our laboratory observed that individuals with 8 or more years of education declined more slowly compared to those with fewer years of education.33 Future studies could evaluate if the effect of literacy on cognitive decline is modified by educational attainment. Second, it is possible that the effect of literacy on rate of decline occurs before age 65, perhaps in midlife. Thus, we would be unable to observe it in our Medicare-eligible community cohort. While a third possibility could be that death and dropout was higher in the illiterate adults whose cognitive scores, if they had been assessed, would have contributed to a more rapid decline, the results of our sensitivity analyses do not support this possibility as there was no effect of risk of death on cognitive trajectories for either illiterate participants or literate adults. Finally, a fourth possibility may be that literacy affects decline in other cognitive domains not formally assessed such as executive functioning.
We did not find that illiterate women were at greater risk for prevalent and incident dementia than illiterate men. Studies in Europe and China found an increase in prevalence5 and incidence11–13 among women with little to no formal education compared to men with similar educational backgrounds. The authors of these studies speculated that differences in quality of education may be driving the increased risk of dementia in women compared to men. However, these studies did not directly assess literacy and did not report the proportion of illiterate participants among participants with no formal education. We also speculate that sex/gender differences may only be apparent when evaluating a wider range of educational attainment. In other words, most prior work took place in well-educated cohorts. Sex/gender differences in the effects of education on health outcomes tend to be weaker in older birth cohorts.34 It may be that the interaction of sex/gender and literacy on dementia risk is more pronounced among samples with wider range of educational attainment and reading levels.
Results of this study shed light on potential mechanisms by which literacy reduces dementia risk. First, we found that literacy was associated with higher scores across multiple cognitive domains, not just those typically associated with reading or language skills. Throughout life, knowing how to read provides an important means of acquiring and structuring new knowledge that benefits not just language ability, but reinforces skills in working memory, visual memory, visuospatial processing, and visuomotor skills.35–37 The underlying mechanism for this generalized effect of literacy may be changes in cortical network organization and function.38,39 Literate adults have larger gray matter volume in the bilateral angular gyri, dorsal occipital lobes, and middle temporal gyri, and in the left supramarginal and posterior superior temporal gyri compared to illiterate adults.40 Literacy acquisition is also associated with white matter tract changes such as thickening of the splenium and the isthmus of the corpus callosum40,41 and increased integrity of the left arcuate fasciculus.42 Future studies with longitudinal cognitive and neuroimaging data may determine whether literacy lowers risk of dementia via brain reserve or brain maintenance (i.e., increasing the size or resistance of brain structures to neuropathology).
Literacy allows for engagement in socially and cognitively enriching activities (i.e., reading newspapers, obtaining a job that requires reading, helping children or grandchildren with homework) that have been associated to reduce the risk of dementia.43–45 Therefore, independent of brain reserve, literacy may increase cognitive reserve, or the ability to compensate for neuropathology once it is already present.
A limitation of the current study is that we were unable to directly determine how or when our literate participants learned to read and write while others remained illiterate. Lack of basic education during childhood may be a significant source of illiteracy and, in many parts of the world, economic constraints (i.e., needing to work) are a primary cause for dropping out of school, which increases rates of illiteracy.46 If access to education is not an obstacle, then issues with quality of education (i.e., shortage of teachers, lack of proper teaching materials and classroom equipment)47,48 may play a role. Another obstacle may be an individual's level of intellectual functioning. However, it is unlikely that the effect of illiteracy in this cohort can be explained by lower intellectual functioning in the illiterate group. The cohort included in this analysis are primarily people who were born and raised in rural areas (mostly in the Dominican Republic) where there were fewer opportunities to attend school. Anecdotal information obtained from prior discussions with participants reported that literacy was attained by opportunities presented to their younger siblings who attended school.49 That said, because we do not have information collected in childhood, we cannot entirely rule out if a subsample of illiterate participants never acquired literacy due to low intelligence. Understanding these factors may help clarify which literacy interventions should be employed to reduce dementia risk, and what the target age range for these interventions should be. Another limitation was that illiteracy was determined via self-report by asking “Did you ever learn to read or write?” It is unclear whether some participants knew how to read but not write. Acquiring one aspect of literacy over another may lead to differential dementia risks either through different direct effects on neuroanatomical structure or through engagement in different cognitive activities (i.e., differing work opportunities).
Our results suggest that acquisition of literacy skills may reduce the risk of dementia. While lower literacy is associated with other detrimental health outcomes (e.g., increased mortality),50 this study adds to this literature by associating illiteracy with increased risk of incident dementia and poorer cognitive abilities. It seems that illiteracy may increase dementia risk by conferring a lower range of cognitive function that is closer to diagnostic thresholds for dementia than the range of those who acquired literacy skills. Future studies may evaluate whether greater efforts placed on literacy programs to reduce illiteracy rates in both developed and developing countries may reduce risk of dementia and other health conditions.
Glossary
- BIC
Bayesian Information Criterion
- CI
confidence interval
- CSES
childhood socioeconomic status
- WHICAP
Washington Heights–Inwood Columbia Aging Project
Appendix. Authors

Study funding
Study funded by NIH/NIA grant RF1AG054023.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.US Department of Education, National Center for Education Statistics. The Condition of Education 2003. Washington, DC: NCES 2003–067: 2003. [Google Scholar]
- 2.Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia support for the cognitive reserve hypothesis. Neurology 2007;68:223–228. [DOI] [PubMed] [Google Scholar]
- 3.Manly JJ, Byrd D, Touradji P, Sanchez D, Stern Y. Literacy and cognitive change among ethnically diverse elders. Int J Psychol 2004;39:47–60. [Google Scholar]
- 4.Dotson VM, Kitner-Triolo MH, Evans MK, Zonderman AB. Effects of race and socioeconomic status on the relative influence of education and literacy on cognitive functioning. J Int Neuropsychol Soc 2009;15:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang M, Katzman R, Salmon D, et al. The prevalence of dementia and Alzheimer's disease in Shanghai, China: impact of age, gender, and education. Ann Neurol 1990;27:428–437. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M, Katzman R, Yu E, Liu W, Xiao S, Yan H. A preliminary analysis of incidence of dementia in Shanghai, China. Psychiatry Clin Neurosci 1998;52:291. [DOI] [PubMed] [Google Scholar]
- 7.He Y, Zhang X, Zhang M. Psychosocial risk factors for Alzheimer's disease. Hong Kong J Psychiatry 2000;10:2–7. [Google Scholar]
- 8.Manly JJ, Schupf N, Tang M-X, Stern Y. Cognitive decline and literacy among ethnically diverse elders. J Geriatr Psychiatry Neurol 2005;18:213–217. [DOI] [PubMed] [Google Scholar]
- 9.Manly JJ, Touradji P, Tang MX, Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol 2003;25:680–690. [DOI] [PubMed] [Google Scholar]
- 10.Sachs-Ericsson N, Blazer DG. Racial differences in cognitive decline in a sample of community-dwelling older adults: the mediating role of education and literacy. Am J Geriatr Psychiatry 2005;13:968–975. [DOI] [PubMed] [Google Scholar]
- 11.Launer L, Andersen K, Dewey M, et al. Rates and risk factors for dementia and Alzheimer's disease results from EURODEM pooled analyses. Neurology 1999;52:78–84. [DOI] [PubMed] [Google Scholar]
- 12.Ott A, Van Rossum C, Van Harskamp F, Van de Mheen H, Hofman A, Breteler M. Education and the incidence of dementia in a large population-based study: the Rotterdam Study. Neurology 1999;52:663–666. [DOI] [PubMed] [Google Scholar]
- 13.Letenneur L, Launer J, Andersen K, et al. Education and risk for Alzheimer's disease: sex makes a difference EURODEM pooled analyses. Am J Epidemiol 2000;151:1064–1071. [DOI] [PubMed] [Google Scholar]
- 14.Siedlecki KL, Manly JJ, Brickman AM, Schupf N, Tang M-X, Stern Y. Do neuropsychological tests have the same meaning in Spanish speakers as they do in English speakers? Neuropsychology 2010;24:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tannenbaum C, Greaves L, Graham ID. Why sex and gender matter in implementation research. BMC Med Res Methodol 2016;16:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manly JJ, Bell-McGinty S, Tang M-X, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol 2005;62:1739–1746. [DOI] [PubMed] [Google Scholar]
- 17.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 1974;24:1019–1025. [DOI] [PubMed] [Google Scholar]
- 18.Benton AL. Visual Retention Test Forms C, D, E. San Antonio, TX: Psychological Corporation; 1955. [Google Scholar]
- 19.Rosen W. The Rosen Drawing Test. Bronx, NY: Veterans Administration Medical Center; 1981. [Google Scholar]
- 20.Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellak L, Karasu TB, eds. Geriatric Psychiatry: A Handbook for Psychiatrists and Primary Care Physicians. New York: Harcourt Health Sciences Group: 1976. [Google Scholar]
- 21.Luchsinger J, Reitz C, Honig LS, Tang M-X, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005;65:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population: development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol 1992;49:453–460. [DOI] [PubMed] [Google Scholar]
- 23.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKeith I, Perry E, Perry R. Report of the second Dementia with Lewy Bodies International Workshop: diagnosis and treatment: Consortium on Dementia with Lewy Bodies. Neurology 1999;53:902–905. [DOI] [PubMed] [Google Scholar]
- 25.Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia diagnostic criteria for research studies: report of the NINDS‐AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz G. Estimating the dimension of a model. Ann Stat 1978;6:461–464. [Google Scholar]
- 27.McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Dev Psychol 2002;38:115. [PubMed] [Google Scholar]
- 28.Chandra V, Pandav R, Dodge H, et al. Incidence of Alzheimer's disease in a rural community in India: the Indo–US study. Neurology 2001;57:985–989. [DOI] [PubMed] [Google Scholar]
- 29.Nitrini R, Caramelli P, Herrera E Jr, et al. Incidence of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord 2004;18:241–246. [PubMed] [Google Scholar]
- 30.Tang M-X, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 2001;56:49–56. [DOI] [PubMed] [Google Scholar]
- 31.Scazufca M, Menezes PR, Vallada HP, et al. High prevalence of dementia among older adults from poor socioeconomic backgrounds in Sao Paulo, Brazil. Int Psychogeriatr 2008;20:394–405. [DOI] [PubMed] [Google Scholar]
- 32.Keskinoglu P, Giray H, Pıcakcıefe M, Bilgic N, Ucku R. The prevalence and risk factors of dementia in the elderly population in a low socio-economic region of Izmir, Turkey. Arch Gerontol Geriatr 2006;43:93–100. [DOI] [PubMed] [Google Scholar]
- 33.Zahodne LB, Stern Y, Manly JJ. Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology 2015;29:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zajacova A, Hummer RA. Gender differences in education effects on all-cause mortality for white and black adults in the United States. Social Sci Med 2009;69:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bramão I, Mendon A, Faísca L, Ingvar M, Petersson KM, Reis A. The impact of reading and writing skills on a visuo-motor integration task: a comparison between illiterate and literate subjects. J Int Neuropsychol Soc 2007;13:359–364. [DOI] [PubMed] [Google Scholar]
- 36.Petersson KM, Reis A, Ingvar M. Cognitive processing in literate and illiterate subjects: a review of some recent behavioral and functional neuroimaging data. Scand J Psychol 2001;42:251–267. [DOI] [PubMed] [Google Scholar]
- 37.Kosmidis MH, Zafiri M, Politimou N. Literacy versus formal schooling: influence on working memory. Arch Clin Neuropsychol 2011;26:575–582. [DOI] [PubMed] [Google Scholar]
- 38.Dehaene S. The massive impact of literacy on the brain and its consequences for education. Hum Neuroplast Educ 2011;117:19–32. [Google Scholar]
- 39.Dehaene S, Cohen L, Morais J, Kolinsky R. Illiterate to literate: behavioural and cerebral changes induced by reading acquisition. Nat Rev Neurosci 2015;16:234. [DOI] [PubMed] [Google Scholar]
- 40.Carreiras M, Seghier ML, Baquero S, et al. An anatomical signature for literacy. Nature 2009;461:983. [DOI] [PubMed] [Google Scholar]
- 41.Castro‐Caldas A, Nunes M, Maestú F, et al. Learning orthography in adulthood: a magnetoencephalographic study. J Neuropsychol 2009;3:17–30. [DOI] [PubMed] [Google Scholar]
- 42.Thiebaut de Schotten M, Cohen L, Amemiya E, Braga LW, Dehaene S. Learning to read improves the structure of the arcuate fasciculus. Cereb Cortex 2012;24:989–995. [DOI] [PubMed] [Google Scholar]
- 43.Scarmeas N, Levy G, Tang M-X, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology 2001;57:2236–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson RS, De Leon CFM, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002;287:742–748. [DOI] [PubMed] [Google Scholar]
- 45.Wilson R, Bennett D, Bienias J, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology 2002;59:1910–1914. [DOI] [PubMed] [Google Scholar]
- 46.Latin American Economic Outlook 2017. Santiago, Chile: CEPAL; 2016. [Google Scholar]
- 47.Card D, Krueger AB. School resources and student outcomes: an overview of the literature and new evidence from North and South Carolina. J Econ Perspect 1996;10:31–50. [Google Scholar]
- 48.Sisco S, Gross AL, Shih RA, et al. The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. J Gerontol B Psychol Sci Soc Sci 2014;70:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manly JJ, Jacobs DM, Sano M, et al. Effect of literacy on neuropsychological test performance in nondemented, education-matched elders. J Int Neuropsychol Soc 1999;5:191–202. [DOI] [PubMed] [Google Scholar]
- 50.Sudore RL, Yaffe K, Satterfield S, et al. Limited literacy and mortality in the elderly: the health, aging, and body composition study. J Gen Intern Med 2006;21:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request to the WHICAP Publications Committee. Data requests should be submitted at cumc.co1.qualtrics.com/jfe/form/SV_6x5rRy14B6vpoqN.