Abstract
Due to contrasting results from clinical trials, remote monitoring devices have so far rarely been approved for heart failure (HF) management in European countries. Implementation of telemedicine into clinical practice of heart failure outpatient care is still limited. As part of an expert meeting on physiological monitoring in the complex mutimorbid HF patient, the needs to establish evidence supporting the use of devices in heart failure outpatient care was discussed according to a trialist’s perspective. This document reflects the key points debated by a multidisciplinary panel of leading international experts on this topic.
Keywords: Heart failure, Remote monitoring, Telemonitoring
Introduction
Hospital admissions for worsening heart failure (HF) represent an enormous burden worldwide on a societal, economic and healthcare level, and days lost due to HF-related hospital stays or to death constitute a key clinical outcome measure for the individual patient suffering from HF. Healthcare providers and payers are therefore attaching increasing importance to preventive strategies to avoid worsening HF requiring hospital admission in outpatients.
Technologically advanced implantable haemodynamic monitoring systems in HF have been developed and marketed, and to an extent taken up in routine practice.1 More developments and novel approaches to using information from these remote monitoring devices are in progress. In the future these developments will hopefully allow for optimized monitoring and better self-management of advanced HF patients. Informed and self-responsible patients would then collaborate better and be able to make more targeted use of healthcare resources thus reducing adverse clinical event rates and eventually health care costs. Nevertheless, today implementation of telemedicine into clinical practice in European countries is still limited.
Telemonitoring devices in heart failure: state-of-the-art
Implantable devices with inbuilt remote monitoring capability have been developed with the premise is that close regular surveillance of selected physiological variables, including for example pulmonary artery pressure (PAP) as a surrogate of cardiac filling pressure, intrathoracic impedance, or autonomic function parameters, which reflect early (preclinical) stages of worsening HF would facilitate early detection of cardiac deterioration.2 Irrespective of whether patients have HF with reduced or preserved left ventricular ejection fraction, changes in these physiological variables are, in most cases, induced by excess intravascular volume that typically begins to accumulate days or weeks before the onset of clinical congestion, characterized by worsening clinical symptoms or weight changes,3,4 Therapeutic interventions in the asymptomatic patient may prevent further clinical deterioration and avert the need for hospital admission. For example, the results of the CHAMPION trial,5,6 suggested a direct causal relationship between elevated filling pressures and adverse clinical outcomes in patients with New York Heart Association (NYHA) Class III HF, while lower cardiac filling pressures were shown to be associated with less clinical decompensation and reduced mortality risk.7
Despite encouraging results from randomized and observational trials demonstrating the safety, feasibility, and efficacy of this novel HF disease management paradigm,4,5,8,9 others have failed to demonstrate any clinical benefit,10,11 As a result, telemedicine has remained under-utilized, and devices have rarely been approved for clinical HF management. This raises the important question of why some telemedicine trials have failed. One possibility is that investigators may not always have realized that telemonitoring needs to always be complemented by clearly pre-specified management algorithms, and relies on patients’ adherence to suggested medication changes. Therefore, evaluation of device-based monitoring in clinical trials requires a complex environment, in which dedicated healthcare professionals (e.g. interventional cardiologists, HF specialists, and nurse practitioners) join forces with motivated and knowledgeable patients to ensure study success, a valid but necessarily complex process to ensure safety12 and one appreciated by patients,13 but sadly one that is increasingly expensive and the main cause for fewer trials being conducted when there is no major commercial sponsor. As a result, major effort is going into devising ways we can obtain and depend on evidence obtained by ways other than the conventional large scale RCT, including what is now called ‘Real-World Evidence’.14–16
Updated indications for device-based heart failure management
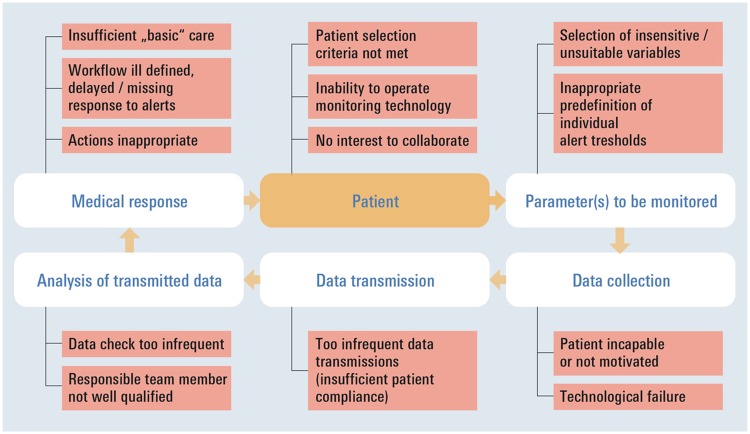
Figure 1 illustrates key elements implicated in a device-based HF management cycle and shows factors that may challenge success at each step. Patient selection is crucial. If patients at low risk of adverse clinical events are enrolled in trials evaluating device-based monitoring, the number of events detectable over time by any given intervention may be too small to yield significant differences between study arms. Monitoring may also fail to improve outcomes if patients are incapable or unwilling to comply with HF management requirements. The choice of actionable monitoring tools, parameters accurately reflecting HF pathophysiology and individualized alert thresholds must enable recognition of cardiac deterioration events at an early stage, when clinical decompensation requiring hospital admission may still be prevented. Data capture needs to be easy and appealing for patients, as well as technically robust enough to ensure patient compliance and regular, reliable data transmission. Information must be regularly received and interpreted by personnel qualified to initiate timely therapeutic actions and/or interventions. It is important to recognize that benefits are best achieved within the context of high-quality HF care and in a setting where well-established workflows facilitate prompt notice of monitoring alerts and implementation of adequate actions in response. As also noted by Desai and Stevenson,17 the final step in the cycle is ensuring that patient reassessment takes place routinely, allowing the supervising clinician to clarify in time whether the perturbation has resolved or requires further intervention. Overall, monitoring-devices are one essential element of the HF care cycle, but their effects on clinical outcomes must be evaluated in the entire context.
Figure 1.
Key elements required for seamless disease management in outpatients with advanced heart failure based on telemonitoring as an important novel adjunct. The care cycle may be interrupted at each step, and multiple factors could challenge success.
The CHAMPION trial,4,5 which evaluated the CardioMEMS™ system, was performed at study sites in the USA with locally-developed HF disease management programmes. As outlined by Jermyn et al.,18 such facilities typically employ advanced HF specialists and nurses dedicated to implement guideline-recommended HF therapies in educated outpatients. Non-randomized ‘real-life’ application of the device has also yielded positive results.9 Importantly, a recent large propensity matched analysis demonstrated also that HF patients, who were monitored with the CardioMEMS™ system, had significant and clinically highly relevant reductions in days lost due to death or hospitalization, with associated improvement of survival in the monitored group.19 However, whether approval and successful implementation of PAP-guided HF management with the CardioMEMS™ system will be reproducible elsewhere, e.g. in European countries, remains to be determined, and will most likely depend on the availability of healthcare settings that facilitate incorporation of this important novel diagnostic adjunct into existing HF disease management strategies.
Barriers in the implementation of remote monitoring devices and future perspective
In countries like Germany, the sector-based organization of the healthcare system may represent a barrier to seamless HF outpatient management, and to date there is no reimbursement for the workload associated with close follow-up and monitoring of high-risk HF patients. The prospective, observational MEMS-HF study is currently underway in Germany, The Netherlands and Ireland20 to evaluate the safety and feasibility of PAP-guided HF management with the CardioMEMS™ system outside the USA. The study results will provide additional data to help demonstrate the role of haemodynamic-guided HF management in improving clinical outcomes. To ensure effective HF outpatient care within the trial framework, the protocol provides detailed recommendations for standardized, structured post-implant HF care, and for the management of ambulatory PAP trends, including the request for individualized PAP thresholds to trigger medication adjustments during follow-up by specifically trained nurses. In the future, one of the most important requirements to enable routine application of device-based monitoring tools would be the training and reimbursement of sufficient numbers of nurses and physician assistants to offer multidisciplinary HF management for all patients with advanced HF,21 a feature we know our present systems do not reliably provide,22,23 The still-considerable HF-related hospitalization and mortality rates highlight an urgent need for appropriate HF management strategies as for example recently demonstrated for Germany.24 Provided significant reductions in HF-related hospitalizations are achievable by novel invasive or non-invasive device-based telemonitoring strategies25–28 this might be feasible at little extra cost.
Conflict of interest: none declared.
References
- 1. Gensini GF, Alderighi C, Rasoini R, Mazzanti M, Casolo G.. Value of telemonitoring and telemedicine in heart failure management. Card Fail Rev 2017;3:1–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nagueh SF. Non-invasive assessment of left ventricular filling pressure. Eur J Heart Fail 2018;20:38–48. [DOI] [PubMed] [Google Scholar]
- 3. Klein L. Discovering the neurohormonal and hemodynamic duality of heart failure. J Am Coll Cardiol 2017;70:1887–1889. [DOI] [PubMed] [Google Scholar]
- 4. Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep 2009;6:287–292. [DOI] [PubMed] [Google Scholar]
- 5. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS.. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
- 6. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB; CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet 2016;387:453–461. [DOI] [PubMed] [Google Scholar]
- 7. Zile MR, Bennett TD, El Hajj S, Kueffer FJ, Baicu CF, Abraham WT, Bourge RC, Warner Stevenson L.. Intracardiac pressures measured using an implantable hemodynamic monitor: relationship to mortality in patients with chronic heart. Failure. Circ Heart Fail 2017;10:e003594. [DOI] [PubMed] [Google Scholar]
- 8. Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, Kautzner J, Sack S, Husser D, Piorkowski C, Sogaard P; IN-TIME study group. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 2014;384:583–590. [DOI] [PubMed] [Google Scholar]
- 9. Heywood JT, Jermyn R, Shavelle D, Abraham WT, Bhimaraj A, Bhatt K, Sheikh F, Eichorn E, Lamba S, Bharmi R, Agarwal R, Kumar C, Stevenson LW.. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation 2017;135:1509–1517. [DOI] [PubMed] [Google Scholar]
- 10. Boehm M, Drexler H, Oswald H, Rybak K, Bosch R, Butter C, Klein G, Gerritse B, Monteiro J, Israel C, Bimmel D, Kaab S, Huegl B, Brachmann J; OptiLink HF Study Investigators . Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J 2016;37:3154–3163. [DOI] [PubMed] [Google Scholar]
- 11. Morgan JM, Kitt S, Gill J, McComb JM, Ng GA, Raftery J, Roderick P, Seed A, Williams SG, Witte KK, Wright DJ, Harris S, Cowie MR.. Remote management of heart failure using implantable electronic devices. Eur Heart J 2017;38:2352–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swedberg K. Lessons for the monitoring of safety in clinical trials. Eur J Heart Fail 2018;20:148.. [DOI] [PubMed] [Google Scholar]
- 13. Lucas R, Wang SF, Riley J, Pepper J, Cowie M.. Patient experience in clinical trials: results of a survey. Eur J Heart Fail 2018;20:612–614. [DOI] [PubMed] [Google Scholar]
- 14. James S. Importance of post-approval real-word evidence. Eur Heart J Cardiovasc Pharmacother 2018;4:10–11. [DOI] [PubMed] [Google Scholar]
- 15. Agewall S. Cardiovascular pharmacotherapy and real-world data. Eur Heart J Cardiovasc Pharmacother 2018;4:65–66. [DOI] [PubMed] [Google Scholar]
- 16. Agewall S. Adherence to guidelines and registry data. Eur Heart J Cardiovasc Pharmacother 2017;3:183–184. [DOI] [PubMed] [Google Scholar]
- 17. Desai AS, Stevenson LW.. Connecting the circle from home to heart-failure disease management. N Engl J Med 2010;363:2364–2367. [DOI] [PubMed] [Google Scholar]
- 18. Jermyn R, Alam A, Kvasic J, Saeed O, Jorde U.. Hemodynamic-guided heart-failure management using a wireless implantable sensor: infrastructure, methods, and results in a community heart failure disease-management program. Clin Cardiol 2017;40:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abraham J, Bharmi R, Jonsson O, Oliveira GH, Artis A, Valika A, Capodilupo R, Adamson PB, Roberts G, Dalal N, Desai A, Benza RL. Association of ambulatory hemodynamic monitoring with clinical outcomes: a concurrent matched control analysis. JAMA Cardiol 2019;4:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Angermann CE, Adamson PB, Assmus B, Anker SD, Brachmann J, Ertl G, Köhler F, Rosenkranz S, Tschöpe C, Böhm M.. CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF): rationale for and design of a prospective cohort study to demonstrate the safety and to report the clinical performance of the CardioMEMS™ HF System. Clin Res Cardiol 2018;107:991–1002. [DOI] [PubMed] [Google Scholar]
- 21. Metra M. October 2017 at a glance: phenotyping heart failure, co-morbidities, use of evidence-based therapy and new treatments. Eur J Heart Fail 2017;19:1216–1217. [DOI] [PubMed] [Google Scholar]
- 22. Komajda M, Cowie MR, Tavazzi L, Ponikowski P, Anker SD, Filippatos GS; QUALIFY Investigators. Physicians' guideline adherence is associated with better prognosis in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail 2017;19:1414–1423. [DOI] [PubMed] [Google Scholar]
- 23. Dovancescu S, Pellicori P, Mabote T, Torabi A, Clark AL, Cleland J.. The effects of short-term omission of daily medication on the pathophysiology of heart failure. Eur J Heart Fail 2017;19:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stork S, Handrock R, Jacob J, Walker J, Calado F, Lahoz R, Hupfer S, Klebs S.. Epidemiology of heart failure in Germany: a retrospective database study. Clin Res Cardiol 2017;106:913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brahmbhatt DH, Cowie MR.. Remote management of heart failure: an overview of telemonitoring technologies. Card Fail Rev 2019;5:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sponga S, Bagur R, Livi U.. Teleconsultation for left ventricular assist device patients: a new standard of care. Eur J Heart Fail 2018;20:818–821. [DOI] [PubMed] [Google Scholar]
- 27. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Winkler S, Vettorazzi E, Polze A, Stangl K, Hartmann O, Marx A, Neuhaus P, Scherf M, Kirwan BA, Anker SD.. Telemedical Interventional Management in Heart Failure II (TIM-HF2), a randomised, controlled trial investigating the impact of telemedicine on unplanned cardiovascular hospitalisations and mortality in heart failure patients: study design and description of the intervention. Eur J Heart Fail 2018;20:1485–1493. [DOI] [PubMed] [Google Scholar]
- 28. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Störk S, Butter CSechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, ESchulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047–1057. [DOI] [PubMed] [Google Scholar]