Abstract
Background:
We aimed to quantify the impact of PM2.5 and PM10 pollution on congenital hypothyroidism (CH) in Qingdao in the period 2014–2017.
Methods:
A generalized additive mixed model (GAMM) with time-series Poisson regression was conducted to quantify the association between PM2.5 and PM10 variables in the month when cases of CH were born or in the two preceding the months (lag0, lag1 and lag2) and monthly morbidity of people with CH across different populations.
Results:
A total of 480,633 newborns were screened for CH during 2014–2017 in Qingdao, and there were 268 cases of CH diagnosed. The count of days per month for which average concentrations of PM2.5 and PM10 exceed legal limits were positively associated with monthly CH morbidity at lag1 month among all the populations, and the adjusted relative risks (RRs) with exposure per 10 μg/m3 were close among different populations. However, the number of days per month of PM2.5 and PM10 concentrations exceeding limits were negatively associated with CH morbidity. Additionally, the RRs of CH increase with worsening air pollution.
Conclusions:
Concentrations of PM2.5 and PM10 exceeding the legal limits are significantly associated with CH in Qingdao. Moreover, it suggests that sudden and short-term particulate matter pollution events with high levels of particulates exceeding the legal limits may be related to risk of CH.
Keywords: congenital hypothyroidism, PM2.5, PM10, relative risk
Introduction
Air pollution is a complex environmental problem that can have adverse effects on the health of the exposed population. There is a perception that rapid economic development and urbanization in China over the last few decades led to an increase in the frequency and severity of PM2.5 and PM10 pollution episodes.1–5 Moreover, there is widespread concern that this situation creates risk on an unprecedented scale.6,7 In eastern China, higher particulate matter (PM) concentrations, as well as longer pollution event durations, were measured compared with other areas.8,9 Qingdao, as a coastal city of Shandong Province in eastern China, suffers from air pollution frequently and has been presented as a region with high PM2.5 and PM10 concentrations.10
There has been growing epidemiological and experimental evidence that short- or long-term exposure to air pollution is associated with a number of health risks, including morbidity and mortality resulting from respiratory problems, cardiovascular diseases and cancer, as well as causing reduced life expectancy more generally.7,11–13 It has been acknowledged that PM2.5 and PM10 may affect the health of pregnant women and their fetuses, and several studies have revealed that PM2.5 and PM10 could significantly increase the risks of preterm birth, fetal death, low birth weight, macrosomia fetus and congenital defects.14–16 However, more evidence is needed regarding PM2.5 and PM10 impacts on pregnancy and fetuses.
Congenital hypothyroidism (CH) is the most common endocrine system disorder in newborns, affecting an estimated 1 in 2000–4000 newborns.17 Previous studies have showed that the incidence of CH may be associated with race, low birth weight and preterm birth,18,19 but other risk factors have been considered to play a role in the occurrence of CH. Additionally, environmental factors may be associated with the incidence of CH;20 however, few relevant studies have been reported. In our study, we conducted a longitudinal data analysis with a time-series Poisson regression to quantify the association between PM pollution and CH in Qingdao city during 2014–2017.
Materials and methods
Study area
As shown in Figure 1, Qingdao, as a coastal city of Shandong Province, is situated in the eastern area of China. Due to the location, between longitude 119°30‘–121°00’ E and latitude 35°35‘–37°09’ N, Qingdao has a mid-temperate continental monsoon climate with an annual average temperature of 12.7°C and annual cumulative precipitation of 662.1 mm. Additionally, as a harbor city, Qingdao is the economic center of Shandong Province, with a population density of 801 persons per km2 (in 2014: population = 9,046,200; land size = 11,282 km2).
Figure 1.
Location of Qingdao in Shandong Province, China.
Data collection and management
Data on disease
CH is defined as the lack of thyroid hormones at birth, mainly as a consequence of thyroid dysgenesis/developmental abnormalities of the thyroid gland ranging from a lack of thyroid gland to a hypoplastic or ectopic gland.21 The study was approved by the Institutional Review Board of Qingdao Women and Children’s Hospital. After written informed consent was obtained from mothers, disease data on CH between January 2014 and December 2017 were obtained from the Qingdao Newborn Disease Screening Centre (QNDSC), which is responsible for CH screening of all local newborns and has a screening rate of 98.6%.22 The monthly morbidity of CH was calculated according to the date of birth rather than date of diagnosis. The preliminary screening test of CH was performed for each newborn by collecting plantar bloods within 72 h after delivery, to measure the thyroid-stimulating hormone (TSH) and thyroxine (T4). Newborns with positive results in the preliminary screening test (TSH ⩾20 mU/L) were then confirmed for CH by serum TSH, triiodothyronine (T3) and T4, followed by subsequent thyroid scanning, knee X-ray and complete physical examination.
Data on air pollution
Data on air pollution from 2014 to 2017 were collected from the China National Environmental Monitoring Centre, which publishes the daily air quality index and concentrations of major air pollutants, including PM2.5, PM10, sulfur dioxide (SO2), carbon monoxide (CO), nitrogen dioxide (NO2) and ozone (O3) for each city. According to the Ambient Air Quality Standards issued by the Ministry of Ecology and Environment of the People’s Republic of China in December 2012, the legal limits of PM2.5, PM10, SO2, CO and NO2 concentrations, equivalent to the 24 h means, are 75 μg/m3, 150 μg/m3, 150 μg/m3, 4 mg/m3 and 80 μg/m3, respectively, followed by the O3 concentration limit of 200 μg/m3 hourly average. As shown from the air pollution data, the principal pollutants during the study period were PM2.5 and PM10, and their daily concentrations exceeded the legal limits for most study period. However, the daily concentration of SO2, CO, NO2 and O3 were far below their legal limits for most of the study period. In order to detect the association between PM and CH, we quantified the relative risks of CH after controlling for other air pollutants, tendency and seasonality effects in our study.
Air pollution is defined as the phenomenon or event that the content of any substance in the atmosphere varies in a manner which is harmful for ecological stability and human survival, causing hazards for human, animals, vegetation or materials.23 The air quality index (AQI) is a number used by government agencies to communicate to the public how polluted the air currently is. The individual air quality index (IAQI) represents the state of individual contaminants. The IAQI was calculated as follows, according to the Technical Regulation on Ambient Air Quality Index (on trial):
IAQIP represents the IAQI of P contaminant. Cp represents the mass concentration of P contaminant. BPHi and BPLo represent the highest and lowest values of concentration limits like CP, respectively. IAQIHi and IAQILo represent the IAQI of BPHi and BPLo, respectively.
The AQI was calculated as follows:
IAQI represents the IAQI of contaminants, and n represents the specific contaminant.
AQI values are divided into ranges, and each range is assigned a descriptor. According to the Technical Regulation on Ambient Air Quality Index (on trial), air pollution is divided into four levels on the basis of AQI: mild pollution (AQI: 101–150); moderate pollution (AQI: 151–200); severe pollution (AQI: 201–300); and most severe pollution (AQI: >300).
Statistical analysis
First, a descriptive analysis was performed to describe the distribution of CH morbidity and air pollution variables during the study period. Then, longitudinal data analysis using a generalized additive mixed model (GAMM) was adopted to evaluate the association between monthly incidence of CH and monthly air pollution variables. Considering the potential lag effect of air pollution, the values of the air pollution variables at 0–2 months before birth (lag0, lag1, lag2) were analyzed, detecting the short-term effect of PM2.5 and PM10 on CH. The generalized additive model (GAM) method is a flexible and effective technique for conducting nonlinear regression analysis in time-series studies with a Poisson regression,24 which allows the Poisson regression to be fit as a sum of nonparametric smooth functions of predictor variables. Due to controlling the nonlinear confounding by nonparametric adjustment, GAM has been widely used in time-series studies of air pollution and morbidity.25–27
GAMM, allowing the parametric and nonparametric functions to be analyzed together in Poisson regression, was conducted in our study. In the model, the number of days for which average concentrations of PM2.5 and PM10 exceeded the legal limits were set as the parametric functions to quantify the association with CH. Other pollutants below the concentration limits for most of the study period were set as nonparametric functions to control their potential influence. Additionally, the tendency and seasonality effects were controlled in the models. The regression model was described as follows:
where Yt denotes the daily morbidity of scarlet fever at time t, which represented the specific day. Air pollution was a categorical variable including: no air pollution, mild air pollution, moderate air pollution, severe air pollution and most severe pollution, which were represented by 0, 1, 2, 3 and 4, respectively. The parameters were individually represented by β0 to β7 respectively, and the value and 95% confidence interval (95% CI) of relative risk (RR) of each parametric function was calculated by the corresponding natural logarithm of coefficient. Because the most severe days were few, the severe and most severe pollution days were combined as a variable in the model. Thus, there were three parametric functions set to estimate the RRs of CH related to air pollution levels, which included MPD1, MPD2 and BSMSD, representing the count of monthly mild pollution days, the count of monthly moderate pollution days, and the count of monthly both severe and most severe pollution days, respectively. In order to assess the RRs of CH related to PM2.5 and PM10, the monthly variables including the monthly average concentration of PM2.5 in the days exceeding the legal limit, the monthly average concentration of PM10 in the days exceeding the legal limit, the count of days of PM2.5 with concentration exceeding the legal limit and the count of days of PM10 with the concentration exceeding the legal limit were set as the parametric functions in the model, which were represented by EPM2.5, EPM10, CPM2.5 and CPM10, respectively. Moreover, the adjusted RRs with 95% CI were estimated for the risks associated with exposure per 10 μg/m3 increment for the monthly average concentration of PM2.5 in the days exceeding the legal limit and the monthly average concentration of PM10 in the days exceeding the legal limit. S1(SO2), S2(CO), S3(NO2) and S4(O3) were smooth nonparametric functions corresponding to the monthly average concentrations of SO2, CO, NO2, and O3, respectively. The tendency and seasonality term were S5(t) and S6(2πt/12), respectively. Considering the potential lag effect of the investigated exposure (lag0 to lag2), the monthly incidence of CH was analyzed as the dependent variable of interest in the model. The associations across different populations including overall population, male population and female population were quantified by corresponding monthly morbidity of CH. The statistical analysis was performed using SPSS 19.0 (SPSS Inc., Chicago) and software R version 2.3.1 (MathSoft Inc., Washington).
Results
Descriptive analysis for the disease
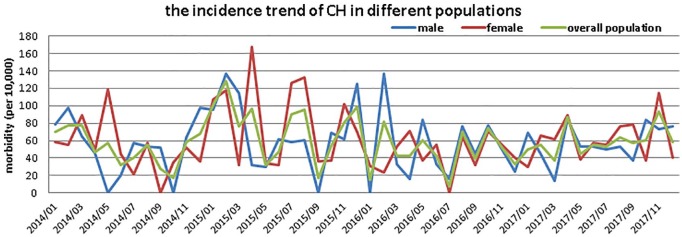
A total of 480,633 newborns were screened for CH during 2014–2017 in Qingdao, and there were 268 cases of CH diagnosed. Among all the cases, the CH caused by inherited defects account for a minor fraction, which was less than 3%. Table 1 shows the distribution of CH incidence in different populations. Figure 2 shows the monthly incidence trend of CH during the study period; there were 0, 2 and 4 months with zero incidence of CH among overall, male and female populations, respectively.
Table 1.
Description of congenital hypothyroidism (CH) cases and air pollution variables during 2014–2017.
| Variable | Mean ± SD | Min. | P25 | Median | P75 | Max. |
|---|---|---|---|---|---|---|
| Overall morbidity (1 × 106) | 59 ± 25 | 8 | 43 | 56 | 77 | 128 |
| Male morbidity (1 × 106) | 58 ± 34 | 0 | 33 | 55 | 77 | 137 |
| Female morbidity (1 × 106) | 60 ± 35 | 0 | 36 | 55 | 75 | 167 |
| DPM2.5 (μg/m3) | 47.0 ± 34.7 | 5.0 | 24.0 | 37.0 | 59.0 | 304.0 |
| MPM2.5 (μg/m3) | 47.0 ± 19.6 | 17.4 | 32.3 | 43.6 | 58.9 | 102.7 |
| EPM2.5 (μg/m3) | 67.7 ± 50.7 | 0 | 0 | 89.8 | 100.4 | 154.1 |
| DPM10 (μg/m3) | 92.9 ± 53.0 | 19.0 | 56.9 | 81.0 | 114.4 | 455.0 |
| MPM10 (μg/m3) | 92.8 ± 27.4 | 44.0 | 72.2 | 92.5 | 112.6 | 166.0 |
| EPM10 (days) | 125.1 ± 95.6 | 0 | 0 | 173.7 | 194.1 | 243.1 |
| SO2 (μg/m3) | 24.1 ± 13.3 | 7.8 | 15.6 | 21.2 | 27.2 | 65.4 |
| CO (mg/m3) | 0.8 ± 0.3 | 0.5 | 0.6 | 0.75 | 1.0 | 1.9 |
| NO2 (μg/m3) | 35.5 ± 10.0 | 19.5 | 26.7 | 34.7 | 40.4 | 58.0 |
| O3 (mg/m3) | 95.4 ± 30.3 | 32.0 | 68.8 | 99.3 | 120.5 | 156.2 |
Max., maximum; Min., minimum; P25, the 25th percentile; P75, the 75th percentile; SD, standard deviation.
Overall morbidity, monthly morbidity of CH in overall population; male morbidity, monthly morbidity of CH in male population; female morbidity, monthly morbidity of CH in female population; DPM2.5, daily average PM2.5 concentration; MPM2.5, monthly average PM2.5 concentration; EPM2.5, monthly average PM2.5 concentration in the days exceeding the legal limit; DPM10, daily average PM10 concentration; MPM10, monthly average PM10 concentration; EPM10, monthly average PM10 concentration in the days exceeding the legal limit; SO2, monthly average SO2 concentrations; CO, monthly average CO concentrations; NO2, monthly average NO2 concentrations; O3, hourly average O3 concentrations in a month.
Figure 2.
The monthly incidence trend of CH among different populations in Qingdao during 2014–2017.
Descriptive analysis for the air pollution variables
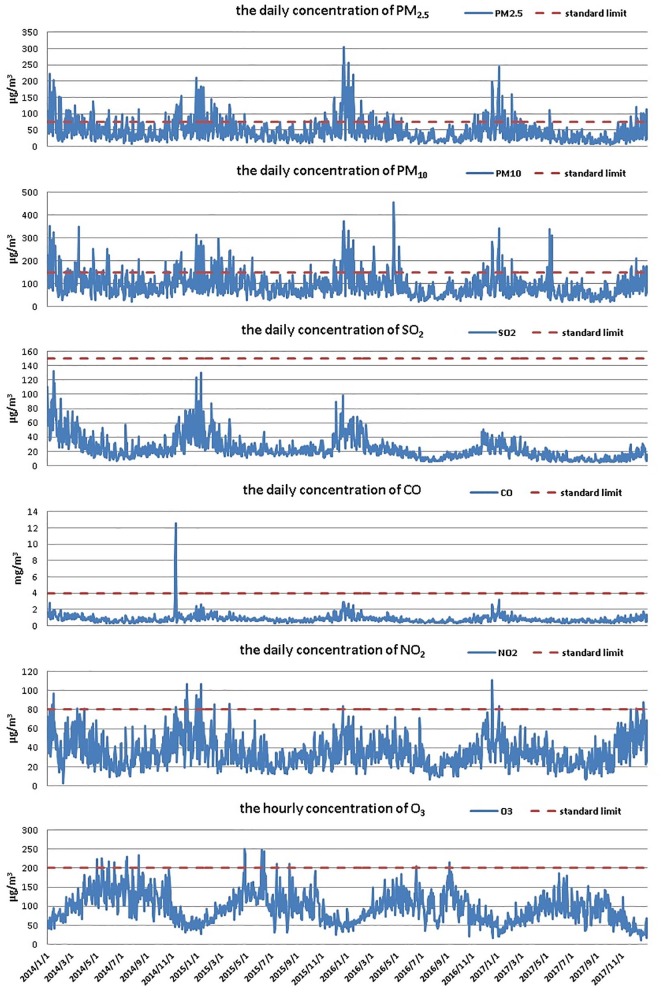
From 1 January 2014 to 31 December, 2017, there were 313 days of air pollution, including 233 days with mild pollution, 45 days with moderate pollution, 31 days with severe air pollution and 4 days with most severe air pollution. Figure 3 shows the distribution of air pollutants during the study period. It was observed that the principal pollutants in Qingdao during the study period were PM2.5 and PM10, whose concentrations exceed the legal limits for 222 days and 175 days, respectively. However, the concentrations of other pollutants such as SO2, CO, NO2 and O3 were far below the limits for most of the study period.
Figure 3.
The distribution of air pollutants in Qingdao during 2014–2017.
Regression analysis
Results showed that PM2.5 and PM10 variables were only significantly associated with monthly incidence of CH at lag1 month, after controlling for other air pollutants, tendency and seasonality effects. Moreover, the parameters and RR values were significant among the overall and different sexual populations, listed in Table 2.
Table 2.
Parameter coefficients, relative risks (RRs) and 95% confidence interval (95% CI) from the Poisson regression models for congenital hypothyroidism (CH) at 1 month lag.
| Populations | Variables | Coefficients (95% CI) | RR (95% CI) |
|---|---|---|---|
| Overall population | MPD1 | 0.128 (0.115–0.152) | 1.137 (1.122–1.164) |
| MPD2 | 0.428 (0.372–0.486) | 1.534 (1.451–1.626) | |
| BSMSD | 0.481 (0.464–0.532) | 1.618 (1.590–1.702) | |
| CPM2.5 | 0.018 (0.012–0.024) | 1.018 (1.012–1.024) | |
| NPM2.5 | −0.126 (–0.144 to –0.118) | 0.881 (0.866–0.889) | |
| EPM10 | 0.009 (0.008–0.010) | 1.009 (1.008–1.010) | |
| CPM10 | −0.086 (–0.112 to –0.071) | 0.917 (0.894–0.931) | |
| r 2 | 0.78 | ||
| Male population | MPD1 | 0.042 (0.032–0.058) | 1.043 (1.033–1.059) |
| MPD2 | 0.090 (0.064–0.102) | 1.094 (1.066–1.107) | |
| BSMSD | 0.484 (0.420–0.681) | 1.622 (1.522–1.976) | |
| EPM2.5 | 0.032 (0.022–0.048) | 1.033 (1.022–1.049) | |
| CPM2.5 | −0.162 (–0.176 to –0.143) | 0.850 (0.839–0.867) | |
| EPM10 | 0.006 (0.004–0.008) | 1.006 (1.004–1.008) | |
| CPM10 | −0.438 (–0.446 to –0.391) | 0.645 (0.640–0.676) | |
| r 2 | 0.87 | ||
| Female population | MPD1 | 0.162 (0.150–0.191) | 1.176 (1.162–1.210) |
| MPD2 | 0.691 (0.632–0.802) | 1.996 (1.881–2.230) | |
| BSMSD | 0.793 (0.722–0.856) | 2.210 (2.059–2.354) | |
| EPM2.5 | 0.041 (0.030–0.049) | 1.042 (1.030–1.050) | |
| CPM2.5 | −0.116 (–0.146 to –0.088) | 0.890 (0.864–0.916) | |
| EPM10 | 0.030 (0.022–0.039) | 1.030 (1.022–1.040) | |
| CPM10 | −0.035 (–0.052 to –0.016) | 0.966 (0.949–0.984) | |
| r 2 | 0.70 |
MPD1, the count of monthly mild pollution days; MPD2, the count of monthly moderate pollution days; BSMSD, the count of monthly both severe and most severe days; EPM2.5, the monthly average concentration of PM2.5 in the days exceeding the legal limit; CPM2.5, the count of monthly days of PM2.5 with concentration exceeding the legal limit; EPM10, the monthly average concentration of PM10 in the days exceeding the legal limit; CPM10, the count of monthly days of PM10 with concentration exceeding the legal limit; r2, R square of the model.
PM2.5 and PM10, as the principal pollutants during the study period, were significantly associated with CH incidence at lag1 month according to the model results. The number of days of the month for which average concentrations of PM2.5 and PM10 exceed the legal limits were positively related with monthly CH morbidity at lag1 month among all populations. Table 2 shows that the adjusted RRs with 95% CI were estimated for the risks associated with exposure at 10 μg/m3 increments. The adjusted RRs were close with different PMs among different populations. Moreover, the number of days of the month for which PM2.5 and PM10 concentrations exceed the legal limits were negatively related with CH morbidity among different populations with the same lag effect.
It was observed that the RRs of CH increases with worsening air pollution. The counts of monthly mild air pollution days, monthly moderate air pollution days and monthly both severe and most severe air pollution days were positively related with the CH morbidity at lag1 month.
Discussion
To our best knowledge, this is the first time it has been demonstrated that there is a quantitative relationship between monthly CH morbidity and PM (PM2.5 and PM10), based on longitudinal data used to evaluate the RRs with 95% CI. This has provided evidence that PM2.5 and PM10 are related to CH through a form of association. Many studies have applied monthly or daily mean of concentrations to research the association between air pollutants and birth defects, but results are inconsistent. The major reason may be that few studies have assessed this relationship based on the concentration exceeding the legal limit. The Chinese Ambient Air Quality Standards (CAAQS) issued by the Ministry of Ecology and Environment of the People’s Republic of China have played a positive role in promoting environmental enhancement, socioeconomic development and public health.28 In the CAAQS, the concentration limits of air pollutants are confirmed as national standards for the protection of the environment and human health. Compared with the recommendations of the World Health Organization (WHO) Air Quality Guidelines (AQGs), the CAAQS limit of CO concentration (1 h) is 20 mg/m3 lower. Moreover, the standard limit of NO2 (1 h) concentration in CAAQS is equal to the recommendation of the WHO AQGs, and the standard limits of PM2.5 (24 h), PM10 (24 h) and O3 (8 h) concentrations in CAAQS are equal to the interim target 1 (IT-1) in the WHO AQGs. However, the standard limit of SO2 (24 h) concentration in CAAQS is 25 ug/m3 higher than the IT-1 in the WHO AQGs. Compared with other studies, our study focused on the potential risks of PM2.5 and PM10 for human health based on extreme excessive PM events in which high concentrations exceed the limits according to CAAQS. PM2.5 and PM10 were the principal pollutants, with more days exceeding concentration limits than other pollutants during the study period in Qingdao. Using the concentrations that exceed the legal limits may be more accurate than using average values. Extreme PM pollution events with high concentrations may cause more serious impacts for health directly than pollution below the legal limits. Using the average concentrations of PMs may underestimate the effects on CH, and it may even be the case that the average values are significantly associated with CH because this averaging includes the impacts of extreme events with high concentrations. After statistical analysis, our study found that PM2.5 and PM10 with concentrations exceeding the legal limits were positively associated with morbidity of CH among different populations.
The monthly morbidity of CH was calculated based on the date of birth rather than the date of diagnosis. As a matter of fact, CH has already occurred at birth but is diagnosed in the subsequent stage after birth, which could be several months later. If the date of diagnosis is applied to the incidence of CH, it would cause bias due to adding the time of diagnosis. Thus, applying the date of birth to calculate monthly morbidity of CH, as the earliest time point of CH onset after delivery, is an appropriate way to precisely assess the relationship between CH and air pollutant variables. At present, effective techniques and methods for CH screening and diagnosis are applied widely in the neonatal period and during subsequent growth; however, there has been no progress in the fetal period.
We suppose that CH cases may be already affected during the fetal period, but are only screened and diagnosed in the subsequent stage starting from birth. Considering the characteristics of normal fetal thyroid gland development and thyroid hormone production, the months when newborns with CH are born or in the two preceding months (lag0, lag1, lag2) were investigated in our study. The fetal thyroid gland development is apparent early in gestation. Related substances, including thyroglobulin, iodine, T4, T3 and hypothalamic TRH, can be synthesized or trapped at 4–12 weeks.29 However, due to a lesser extent of T4 and T3, transplacental passage of maternal thyroid hormone plays an important role in fetal development in the first trimester. In the second trimester, fetal thyroid gland development begins to slowly accelerate. Fetal T4 production gradually rises from mid-gestation to term; however, fetal serum T3 levels are relatively lower owing to placental type 3 deiodinase activity, which coverts T4 to reverse T3.30 Maturation of the hypothalamic–pituitary–thyroid axis feedback relationships occurs by 30–35 weeks, which is in the third trimester. This is the primary stage for fetal thyroid gland rapid development, and fetal thyroid gland development and thyroid hormone production could be affected by the disorder of the hypothalamic–pituitary–thyroid axis feedback relationships caused by many factors during this period, which could be an important cause of CH. In addition, the primary period for fetal thyroid gland development and thyroid hormone production is involved in the lag0 to lag2 month period, which was applied to analyze the relationship between air pollution and CH. The monthly incidences of CH at lag0, lag1 and lag2 month were analyzed respectively by GAMM. The results show that PM2.5 and PM10 variables were only significantly associated with monthly incidence of CH at lag1 month, with no significant result found at lag0 or lag2 month. Moreover, the adjusted RRs with 95% CI were estimated for the risks associated with exposure per 10 μg/m3 increment. Lag1 month is in the primary period for fetal thyroid gland development and thyroid hormone production and maturation of the hypothalamic–pituitary–thyroid axis feedback relationships. For this period, PM pollution events with high concentrations were significantly related with CH.
From the results, a negative association at lag1 month was observed between the count of days per month of PM2.5 and PM10 at concentrations exceeding the legal limits and monthly morbidity of CH. However, these negative relationships could be explained by integrating with the monthly average concentration of PM2.5 and PM10 for the days exceeding the legal limit, which were positively associated with monthly incidence of CH. The two inverse associations indicate that the monthly CH incidence could increase after the period with both the increase of monthly average of PM concentration exceed limits and the decrease of exceed days. This could be reflected in PM pollution events where there is a rapid and short-term increase in PM pollution to high levels that exceed the legal limits, alongside the incidence of increases in cases of CH that may be related to this. Additionally, the relationship between the concentrations of PM2.5 and PM10 and CH morbidity at daily or weekly intervals was also analyzed, but no significant results were detected, which means that after several severe PM events have occurred, the accumulated impacts on population health would be detected at the monthly interval.
AQI is considered a summary assessment of ambient air pollutant, aiming at expressing the concentration of pollutants on a common scale of effects on human health. AQI is an indicator classifying air pollution into four levels, based on pollutants with high concentrations exceeding the legal limits. Rather than simply averaging concentrations, it may be more appropriate for situations in which there are high levels of air pollutants, which could accurately reflects the air quality during serious pollution events. AQI transforms the scientific concentration units of each pollutant into a nondimensional number, depending on the subindex of six criteria (IAQI) of air pollutants (PM2.5, PM10, SO2, NO2, CO and O3); the highest subindex value of the individual pollutants is taken as the AQI value for that particular period of time through the maximum function.31,32 The concentration of the air pollutant is just a reference for confirming the subindex of AQI, which is transformed into a nondimensional number by the relevant air pollutant concentration, which is completely different to the AQI value. In addition, the AQI value is not directly involved in the model, but only applied to assess the level of air pollution. In the model, there are three kinds of variables, without overlap. The first kind is the number of days of the month for each level of air pollution, aiming at detecting the relationship between degree of air pollution and CH. The second kind is the PM variables, used to quantify the relationship between PM and CH. The third kind is the adjusted variables used in the model to control other factors, including the monthly average concentrations of other air pollutants (SO2, CO, NO2, and O3), the specific month and trigonometric function. Thus, the proposed model is not overadjusted with the present variables. As shown in the results, mild air pollution, moderate air pollution and both severe and most severe air pollution were significantly related with CH morbidity at lag1 month. However, different levels of air pollution impacting CH to varying degrees, and the RRs of CH increase with worsening air pollution. Comparing to male infants, the RR values for females were higher, which indicates a stronger association between air pollution levels and monthly incidence of CH in female infants. Based on the variables in the model, it was not possible to represent the impacts of other air pollutants on CH; the results can only indicate that PMs were associated with an increase in the number of cases of CH, but nothing regarding the other pollutants.
Neonatal genetic metabolic disease screening programs are funded by local government, which built the network that covered all delivering facilities, which were dominated by the QNDSC. Due to it being free for public use, it can ensure a high coverage rate and compliance rate for newborns. According to government regulations, each local newborn should receive this screening service. Consequently, the incidence of CH was reliable in our study. It should be acknowledged that there are some limitations in our study. One of the limitations is that the potential effects of many factors, such as race, low birth weight, preterm birth, maternal factors and gestational weeks could not be analyzed because of lack of case data. Due to the limitation of air pollutant data, there were only 4 years of data for our study, which may be relatively short for a time-series analysis. Moreover, it needs to be emphasized that the quantitative association between PM variables and CH was only verified statistically in our study, but this cannot be equal to causation. Further studies, such as classic cohort studies and case–control studies, should be performed to detect the direct cause-and-effect correlation between air pollution and CH.
Conclusion
PM2.5 and PM10 at concentrations exceeding the legal limits are positively associated with CH in Qingdao. It is also suggested that rapid and short-term PM pollution events with high levels of PM exceeding the legal limit may be related to risk of CH. Our findings contribute to developing local strategies to prevent and reduce negative health impacts from CH in areas suffering from air pollution.
Acknowledgments
The authors thank the Qingdao Newborn Disease Screening Centre and the National Environmental Monitoring Centre of China for sharing the data needed for the study.
Footnotes
Author contributions: Quansheng Xing, Silin Pan and Wei Ni conducted the literature review and analyses, drafted the manuscript and approved the final manuscript as submitted. Wenjie Li and Guoju Li conducted the data collection and analysis, critically reviewed the manuscript and approved the final manuscript as submitted.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant no. 81770316).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Quansheng Xing  https://orcid.org/0000-0001-5670-0661
https://orcid.org/0000-0001-5670-0661
Contributor Information
Silin Pan, Qingdao Women and Children’s Hospital, Qingdao University, Qingdao City, Shandong Province, P.R. China.
Wei Ni, Qingdao Women and Children’s Hospital, Qingdao University, Qingdao City, Shandong Province, P.R. China.
Wenjie Li, Qingdao Women and Children’s Hospital, Qingdao University, Qingdao City, Shandong Province, P.R. China.
Guoju Li, Qingdao Women and Children’s Hospital, Qingdao University, Qingdao City, Shandong Province, P.R. China.
Quansheng Xing, Qingdao Women and Children’s Hospital, Qingdao University, Qingdao, Shandong Province, No. 6 Tongfu Road, Qingdao, 266000, P.R. China.
Reference
- 1. Xu P, Chen Y, Ye X. Haze, air pollution, and health in China. Lancet 2013; 382: 2067. [DOI] [PubMed] [Google Scholar]
- 2. Chen Z, Wang JN, Ma GX, et al. China tackles the health effects of air pollution. Lancet 2013; 382: 1959–1960. [DOI] [PubMed] [Google Scholar]
- 3. Chan CK, Yao X. Air pollution in megacities in China. Atmos Environ 2008; 42: 1–42. [Google Scholar]
- 4. Filonchyk M, Yan H. The characteristics of air pollutants during different seasons in the urban area of Lanzhou, Northwest China. Environ Earth Sci 2018; 77: 763. [Google Scholar]
- 5. Huang Y, Yan Q, Zhang C. Spatial–temporal distribution characteristics of PM 2.5 in China in 2016. J geovis spat anal 2018; 2: 12. [Google Scholar]
- 6. Wang S, Hao J. Air quality management in China: issues, challenges, and options. J Environ Sci 2012; 24: 2–13. [DOI] [PubMed] [Google Scholar]
- 7. Kan H, Chen R, Tong S. Ambient air pollution, climate change, and population health in China. Environ Int 2012; 42: 10–19. [DOI] [PubMed] [Google Scholar]
- 8. Rohde RA, Muller RA. Air pollution in China: mapping of concentrations and sources. PLoS One 2015; 10: e0135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang HJ, Chen HP, Liu J. Arctic sea ice decline intensified haze pollution in Eastern China. Atmos Oceanic Sci Lett 2015; 8: 1–9. [Google Scholar]
- 10. Zou JH, Xu FX, Zou B, et al. Spatial-temporal characteristics of haze in the key tourism cities of China (in Chinese). Trop Geo 2018; 38: 143–150. [Google Scholar]
- 11. Khoo KL. The haze and health: a blog about the fog. Ann Acad Med Singap 2006; 35: 909–910. [PubMed] [Google Scholar]
- 12. Lu K, Qin Y, He GX. The impact of haze weather on health: a view to future. Biomed Environ Sci 2013; 26: 945–946. [DOI] [PubMed] [Google Scholar]
- 13. Wu S, Ni Y, Li H, et al. Short-term exposure to high ambient air pollution increases airway inflammation and respiratory symptoms in chronic obstructive pulmonary disease patients in Beijing, China. Environ Int 2016; 94:76–82. [DOI] [PubMed] [Google Scholar]
- 14. Jiang LL, Zhang YH, Song GX, et al. A time series analysis of outdoor air pollution and preterm birth in Shanghai, China. Biomed Environ Sci 2007; 20: 426–431. [PubMed] [Google Scholar]
- 15. Zhang B, Liang S, Zhao J, et al. Maternal exposure to air pollutant PM2.5 and PM10 during pregnancy and risk of congenital heart defects. J Expo Sci Environ Epidemiol 2016; 26: 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang B, Zhao J, Yang R, et al. Ozone and other air pollutants and the risk of congenital heart defects. Sci Rep 2016; 6: 34852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rastogi MV, Lafranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis 2010; 5: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waller DK, Anderson JL, Lorey F, et al. Risk factors for congenital hypothyroidism: an investigation of infant’s birth weight, ethnicity, and gender in California, 1990–1998. Teratology 2000; 62: 36–41. [DOI] [PubMed] [Google Scholar]
- 19. Medda E, Olivieri A, Stazi MA, et al. Risk factors for congenital hypothyroidism: results of a population case–control study (1997–2003). Eur J Endocrinol 2005; 153: 765–773. [DOI] [PubMed] [Google Scholar]
- 20. Shapira SK, Lloyd PMC. Future research directions to identify causes of the increasing incidence rate of congenital hypothyroidism in the United States. Pediatrics 2010; 125: 64–68. [DOI] [PubMed] [Google Scholar]
- 21. LöF C, Patyra K, Kuulasmaa T, et al. Detection of novel gene variants associated with congenital hypothyroidism in a Finnish patient cohort. Thyroid 2016; 26: 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li WJ, Wang M, Wang Q, et al. The analysis of the congenital hypothyroidism screening among 1 million newborns in Qingdao (in Chinese). Lab Med Clin 2010; 7: 2732–2733. [Google Scholar]
- 23. Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut 2008; 151: 362–367. [DOI] [PubMed] [Google Scholar]
- 24. Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc 2004; 99: 673–686. [Google Scholar]
- 25. Schwartz J. Air pollution and hospital admissions for the elderly in Birmingham, Alabama. Am J Epidemiol 1994; 139: 589–598. [DOI] [PubMed] [Google Scholar]
- 26. Schwartz J. Assessing confounding, effect modification, and thresholds in the association between ambient particles and daily deaths. Environ Health Perspect 2000; 108: 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarnat JA, Schwartz J, Suh HH. Fine particulate air pollution and mortality in 20 U.S. cities. N Engl J Med 2001; 344: 1253–1254. [DOI] [PubMed] [Google Scholar]
- 28. Wang ZS, Wu T, Che F, et al. Comparison between domestic and international ambient air quality standards (in Chinese). Res Environ Sci 2010; 23: 253–260. [Google Scholar]
- 29. Brown RS, Huang SA, Fisher DA. The maturation of thyroid function in the perinatal period and during childhood. In: Braverman LE, Utiger RD. (eds) Werner’s and Ingbar’s the thyroid. Philadelphia, PA: Lippincott Williams and Wilkins, 2000, pp.1013–1028. [Google Scholar]
- 30. Thorpe-Beeston JG, Nicolaides KH, Felton CB, et al. Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. N Engl J Med 1991; 324: 532–536. [DOI] [PubMed] [Google Scholar]
- 31. Plaia A, Ruggieri M. Air quality indices: a review. Rev Environ Sci Bio 2011; 10: 165–179. [Google Scholar]
- 32. Shooter D, Brimblecombe P. Air quality indexing. Int J Environ Pollut 2009; 36: 305. [Google Scholar]