Abstract
Background:
First-line treatments for nonsmall cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutations have been evaluated in various clinical trials. However, it remains unclear which is the optimal treatment.
Methods:
A Bayesian network meta-analysis was used to assess the efficacy and safety profile of gefitinib, erlotinib, afatinib, dacomitinib, osimertinib, erlotinib plus bevacizumab and pemetrexed/carboplatin, or pemetrexed alone plus gefitinib. Literature was sourced from electronic databases. Data regarding objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), treatment-related adverse events (TRAEs), treatment-related adverse event grades 3–5 (TRAE 3–5), specific TRAEs [diarrhea, rash, and elevated aspartate aminotransferase/alanine aminotransferase (AST/ALT)] were extracted. The regimens were then ranked using the surface under the cumulative ranking curve (SUCRA).
Results:
A total of 19 studies involving 4607 EGFR-mutant NSCLC patients were analyzed. In regards to efficacy, pemetrexed/carboplatin (PC) plus gefitinib was superior in ORR and OS to chemotherapy and first-generation EGFR-tyrosine kinase inhibitors (EGFR-TKIs). All the TKI-based regimens had equivalent DCR and PFS. Patients with the L858R mutation treated with PC plus gefitinib achieved a better outcome than most EGFR TKI-related groups (except osimertinib) in the PFS subgroup. In regards to safety, no statistical significance for TRAEs was observed among the eight treatments. In regards to SUCRA, PC plus gefitinib ranked first in terms of PFS, OS, and TRAE grades 3–5.
Conclusions:
Pemetrexed/carboplatin plus gefitinib is a promising treatment option for EGFR-mutant NSCLC patients in the first-line setting.
Keywords: combination treatment, EGFR inhibitor, first line therapy, network meta-analysis, nonsmall cell lung cancer
Introduction
Lung cancer remains the leading cause of cancer incidence and cancer-related mortality worldwide.1 The most prevalent type is nonsmall cell lung cancer (NSCLC), accounting for 85% of all lung cancers.2
Epidermal growth factor receptor (EGFR) activating mutations have been identified as important drivers of NSCLC and as independent predictors of EGFR-tyrosine kinase inhibitor (EGFR-TKI) efficacy. These mutations refer mainly to deletion in exon 19 (19 del) or the Leu858Arg substitution in exon 21 (L858R).3–5 As a consequence, the therapeutic landscape of EGFR-mutant NSCLC has shifted profoundly in recent decades from traditional chemotherapy to multiple targeted agents and combination therapy.6
The current standard treatment for NSCLC EGFR mutations is a group of first-to-third generation EGFR-TKIs: gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib. Whilst these drugs have improved clinical benefits with a median progression-free survival (PFS) of 9.5–18.9 months when compared with platinum-based doublet therapy,7–22 overall survival (OS) using first-generation TKIs (gefitinib, and erlotinib) has not improved.
Subsequently, a head-to-head study compared afatinib with gefitinib as a first-line treatment. Afatinib demonstrated a significant improvement in PFS, but the difference was not clinically meaningful (median PFS of 11.0 and 10.9 months for afatinib and gefitinib, respectively).17 Strikingly, dacomitinib, an irreversible second-generation EGFR blocker, had a 5- to 5.5-month improvement in PFS and a 7-month improvement in OS when compared with gefitinib, though at the cost of increased toxicity.16,18 Although EGFR-TKI therapies have significantly prolonged PFS, they fail to demonstrate significant OS benefits. Disease progression occurs mostly within a year of first-line treatment of early generation EGFR-TKIs. This is coupled with the fact that 60% of patients acquire the T790M mutation during treatment and are recommended to undergo molecular testing.23
Based on the AURA 3 study, osimertinib, a newer third-generation EGFR-TKI, was confirmed as a superior second-line option for patients with T790M resistance to first- and second-generation EGFR-TKI therapy compared with standard platinum-pemetrexed chemotherapy with respect to PFS (10.1 months versus 4.4 months).24 Based on the positive PFS of the FLAURA study in 2018, osimertinib was recommended as the preferred first-line therapy, but the OS data was not published.21
In order to prevent or delay the emergence of acquired resistance to EGFR-TKIs, and to prolong OS, combination therapy with chemotherapy or antiangiogenic antibodies and EGFR-TKIs are an emerging trend, and have been evaluated in several clinical trials. Bevacizumab is one of the commonly used antiangiogenic monoclonal antibodies that targets the vascular endothelial growth factor (VEGF) signaling pathway. In the JO25567 and NEJ026 trials, bevacizumab plus erlotinib showed the potential to prolong PFS when compared with erlotinib monotherapy.25,26 Combination pemetrexed/carboplatin (PC), or pemetrexed alone with gefitinib, also improved PFS significantly in the NEJ009 and JMIT studies.27,28
Yet, data from head-to-head trials among these EGFR-TKI monotherapies and combination strategies are still lacking. It remains unclear which is the optimal first-line treatment for NSCLC patients with EGFR-mutation. So, we conducted a network meta-analysis of all the available evidence to compare the efficacy and toxicity among the regimens. Analyses included chemotherapy, EGFR-TKIs, chemotherapy plus EGFR-TKIs, and antiangiogenesis agents plus EGFR-TKIs.
Methods
Search strategy
We systematically searched PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials of the Cochrane Library databases using the following terms: nonsmall-cell lung cancer (NSCLC), untreated, first-line therapy, EGFR TKI, gefitinib, erlotinib, afatinib, dacomitinib, osimertinib, combination therapy, erlotinib and bevacizumab, chemotherapy, and gefitinib. Searches were filtered for clinical studies published between 1 January 2007 and 31 December 2018. We also searched the references of the primary research results, systematic reviews, abstracts from books, and conference proceedings. We also reexamined the reference lists of the related reviews for additional confirmation. Our last literature search was in February 2019. Details of the search strategy are displayed in Table S1. No protocol has been published for this study.
Selection criteria
Studies were included if they met the following inclusion criteria: patients with NSCLC who received no prior systemic therapy; intervention involving EGFR-TKI monotherapy or in combination; at least one available survival data regarding first-line treatment for advanced NSCLC patients; and prospective phase II or III randomized clinical trials. Studies that failed to meet the above criteria, or were not published in English, were excluded.
Data extraction
Two investigators (FL and ZZ) independently extracted the following data: authors of the study, publication year, patient types (chemotherapy-naïve or untreated), histopathological information, therapeutic regimens, sample size, EGFR mutation proportions, and efficacy outcomes [objective response rate (ORR), disease control rate (DCR), PFS, and OS] as well as safety outcomes [treatment related adverse events (TRAEs), treatment related adverse event grades 3–5 (TRAE 3–5) and specific concerned toxicities (diarrhea, rash, and elevated liver enzymes)].
Risk of bias assessment
The Cochrane Collaboration for Systematic Reviews’ guidelines were used to evaluate the quality of each study by two reviewers concerning the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. The overall methodologic quality of each study was assessed as low risk of bias, high risk of bias, or unclear risk of bias. Disagreements were discussed with a third author (KZ) to reach a consensus. For details concerning the risk of bias for the included studies, see Figure S1.
Outcome measures
In this study, ORR, DCR, PFS, and OS were used to assess clinical outcomes.29 The toxicity outcomes of TRAEs, TRAE grades 3–5 and the most common TRAEs [diarrhea, rash and elevated alanine transaminase (ALT)/aspartate transaminase (AST)] were also collected. This network meta-analysis followed the PRISMA for Network Meta-Analyses (PRISMA-NMA) guidelines.30 The Jadad score was used to evaluate the quality of each study by the two reviewers.31,32
Statistical analysis
We used a Bayesian framework to synthesize data from both direct and indirect comparisons of diverse regimens using R v3.5.1 (gemtc package).33 We chose a random-effects hierarchical model via the Markov chain Monte Carlo method using ADDIS 1.15 (Drugis.org) as we assumed there would be different comparisons for each outcome containing a common heterogeneity.34
First-generation EGFR-TKIs were grouped together because they had comparable clinical outcomes in previous studies.7–16 95% confidence intervals (CI) of the pooled HR excluding 1 or a two-sided p value of less than 0.05 was statistically significant. Results are presented as odds ratios (ORs) for binary outcomes (ORR, DCR, TRAEs including TRAE grades 3–5, and specific concerned TRAEs) and hazard ratios (HRs) for PFS and OS with the corresponding 95% CI. The ranking probabilities with respect to each clinical outcome were obtained using the surface under the cumulative ranking curve (SUCRA),35 which are displayed using a rank-heat plot. OR > 1 for ORR and DCR, or an HR < 1 for OS and PFS means better anti-tumor efficacy. For safety profile, OR > 1 means higher toxicity.
Inconsistencies within multiple treatment comparisons were assessed via Inconsistency Standard Deviation (ISD). A low risk of inconsistency was concluded if ISD included ‘1’ in the 95% CI. The loop-specific approach for inconsistency only occurs in closed loops in the evidence network and is exemplified by the inconsistency factor (IF) and node splitting models36–38 using R v3.5.1, RStudio (gemtc package) and ADDIS 1.15. A p value < 0.05 indicated significant inconsistency. Inconsistency evaluations are shown in Table S5–S14.
Results
Eligible studies and characteristics
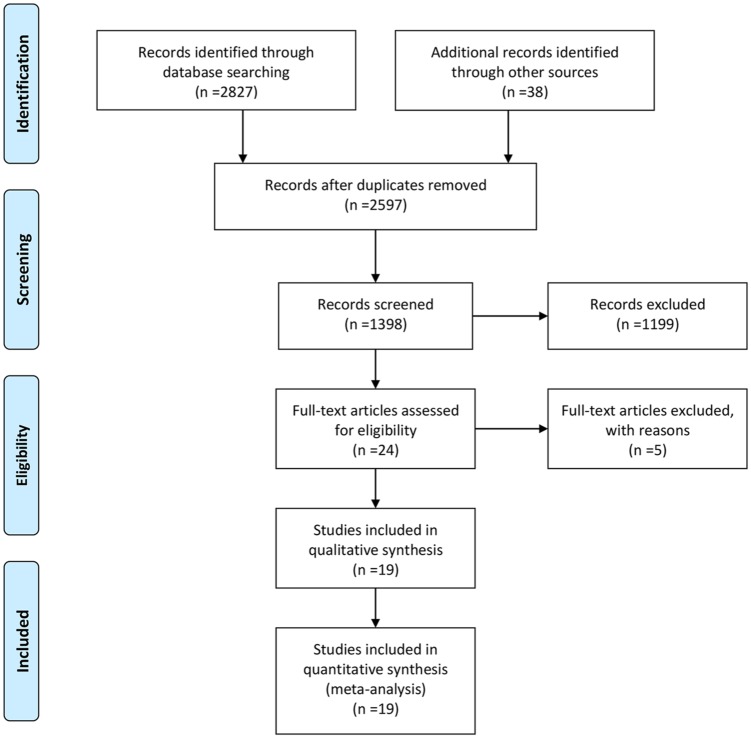
We identified 2597 original articles from our literature search. After removing duplicates and following title/abstract screening, 24 studies were considered for full-text assessment. 19 studies were eligible for this network meta-analyses, which involved 4607 untreated NSCLC patients with EGFR mutations. See Figure 1 for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Table 1 summarizes the characteristics of the 19 phase II/III randomized control trials.
Figure 1.
Flowchart of study selection and design.
Table 1.
Characteristics of included studies for network meta-analyses.
| Study | Year | Phase | Blindness | Patients | Treatment | EGFR mutation/ all population |
|---|---|---|---|---|---|---|
| IPASS | 2009 | III | open-label | Previously untreated | First-generation EGFR-TKIs (Gefitinib) | 132/609 |
| Chemotherapy (Paclitaxel/Carboplatin) | 129/608 | |||||
| WJTOG3405 | 2010 | III | open-label | Chemotherapy-naïve | First-generation EGFR-TKIs (Gefitinib) | 86/86 |
| Chemotherapy (Docetaxel/Cisplatin) | 86/86 | |||||
| NEJ002 | 2010 | III | open-label | Not previously received chemotherapy | First-generation EGFR-TKIs (Gefitinib) | 107/114 |
| Chemotherapy (Paclitaxel/Carboplatin) | 107/114 | |||||
| OPTIMAL | 2011 | III | open-label | Not previously received systemic anticancer therapy | First-generation EGFR-TKIs (Erlotinib) | 83/83 |
| Chemotherapy (Gemcitabine/Carboplatin) | 72/72 | |||||
| EURTAC | 2012 | III | open-label | No history of chemotherapy | First-generation EGFR-TKIs (Erlotinib) | 86/87 |
| Chemotherapy (Docetaxel/Cisplatin) | 86/87 | |||||
| First-SIGNAL | 2012 | III | open-label | Chemotherapy-naïve never-smokers | First-generation EGFR-TKIs (Gefitinib) | 26/159 |
| Chemotherapy (Gemcitabine/Cisplatin) | 16/150 | |||||
| LUX-Lung 3 | 2013 | III | open-label | Treatment-naïve | Afatinib | 204/230 |
| Chemotherapy (Pemetrexed/Cisplatin) | 104/115 | |||||
| LUX-Lung 6 | 2014 | III | open-label | Treatment-naïve | Afatinib | 216/242 |
| Chemotherapy (Gemcitabine/Cisplatin) | 108/122 | |||||
| JO25567 | 2014 | II | open-label | No previous chemotherapy | Erlotinib + Bevacizumab | 75/75 |
| First-generation EGFR-TKIs (Erlotinib) | 77/77 | |||||
| ENSURE | 2015 | III | open-label | Treatment-naïve | First-generation EGFR-TKIs (Erlotinib) | 110/110 |
| Chemotherapy (Gemcitabine/Cisplatin) | 107/107 | |||||
| JMIT | 2016 | II | open-label | Chemotherapy-naïve | Pemetrexed + Gefitinib | 126/126 |
| First-generation EGFR-TKIs (Gefitinib) | 65/65 | |||||
| ARCHER 1009 and A7471028 | 2016 | III + II | double-blind + open-label | Progression with one or two prior chemotherapy regimens | Dacomitinib | 53/53 |
| First-generation EGFR-TKIs (Erlotinib) | 48/48 | |||||
| CONVINCE | 2017 | III | open-label | No history of chemotherapy | First-generation EGFR-TKIs (Icotinib) | 148/148 |
| Chemotherapy (Pemetrexed/Cisplatin) | 137/137 | |||||
| NEJ026 | 2017 | III | open-label | No previous chemotherapy | Erlotinib + Bevacizumab | 112/112 |
| First-generation EGFR-TKIs (Erlotinib) | 112/112 | |||||
| NEJ009 | 2017 | III | open-label | No previous chemotherapy | Pemetrexed/Carboplatin + Gefitinib | 169/169 |
| First-generation EGFR-TKIs (Gefitinib) | 172/172 | |||||
| Han | 2017 | II | open-label | Untreated | Arm A: Pemetrexed/Carboplatin + Gefitinib | 40/40 |
| Arm B: 1st-generation EGFR-TKIs (Gefitinib) | 41/41 | |||||
| Arm C: Chemotherapy (Pemetrexed/Carboplatin) | 40/40 | |||||
| LUX-Lung 7 | 2017 | III | Double-blind | Treatment-naïve | Afatinib | 160/160 |
| First-generation EGFR-TKIs (Gefitinib) | 159/159 | |||||
| FLAURA | 2017 | III | Double-blind | No prior systemic anti-cancer/EGFR-TKI therapy | Osimertinib | 279/279 |
| First-generation EGFR-TKIs (Gefitinib/Erlotinib) | 277/277 | |||||
| ARCHER 1050 | 2017 | III | Double-blind | No prior systemic therapy | Dacomitinib | 227/227 |
| First-generation EGFR-TKIs (Gefitinib) | 225/225 |
EGFR-EGFR-TKIs, Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors; P, Pemetrexed; PC, Pemetrexed/Carboplatin.
Risk of bias in the included studies
All 19 studies were considered to have adequately performed random sequence generation and allocation concealment, as well as having a low risk of detection and reporting bias. Out of the 19 studies, 3 required the blinding of participants and personnel. The other 16 studies had an unclear risk of performance bias. Overall, 3 trials had incomplete outcome data, 5 trials had a low risk of attrition bias, and 11 had unclear risks of attrition bias. See Figure S1 for more detailed information.
Network geometry for multiple treatment comparisons
Network diagrams were established for multiple treatment comparisons (MTCs) based on the 19 random clinical trials (RCTs) (Figure 2). Solid lines between drugs represent direct comparisons. Outcome data for ORR, DCR, PFS, OS, TRAEs, TRAE grades 3–5, and specific TRAEs (rash, diarrhea and elevated ALT/AST) were extracted and analyzed. The eight recommended treatments (chemotherapy, first-generation EGFR-TKIs, afatinib, dacomitinib, osimertinib, pemetrexed plus gefitinib, pemetrexed/carboplatin plus gefitinib, and erlotinib plus bevacizumab) were compared directly and indirectly against each other. ISD and IF were low risk in this study (Table S5). The node-splitting analysis for direct and indirect evidence were consistent (Table S6–S14).
Figure 2.
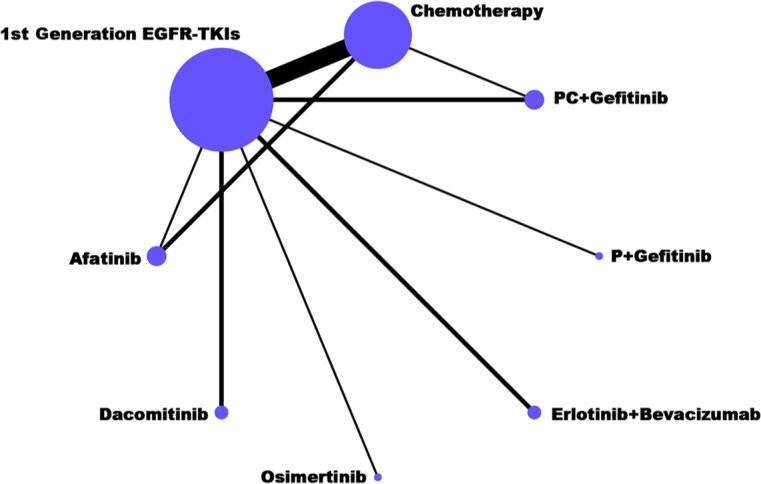
Network plot of first-line treatments for EGFR-mutant advanced NSCLC. The size of each dot represents the number of patients receiving the corresponding intervention. The width of each line represents the number of studies of corresponding comparison.
EGFR, epidermal growth factor receptor; NSCLC, nonsmall cell lung cancer.
Network meta-analyses for efficacy profile
PC plus gefitinib showed significantly longer OS than chemotherapy (HR = 1.71, 95% CI: 1.29–2.33), first-generation EGFR-TKIs (HR = 1.74, 95% CI: 1.34–2.33), and afatinib (HR = 1.54, 95% CI: 1.11–2.18) (Figure 2). PC plus gefitinib also showed a better ORR than chemotherapy, first-generation EGFR-TKIs, and dacomitinib. No significant differences in DCR and PFS among the regimens were observed except for chemotherapy. All other seven regimens based on EGFR-TKIs showed superior efficacy on ORR and PFS, when compared with chemotherapy (Table 2).
Table 2.
Multiple treatment comparison for efficacy based on network consistency model (OR > 1 for ORR and DCR, or an HR < 1 for PFS and OS means better anti-tumor efficacy).
| OR with 95% CI for ORR | |||||||
|---|---|---|---|---|---|---|---|
| Chemotherapy | 4.08 (3.20, 5.96) | 6.66 (3.91, 9.83) | 3.20 (1.82, 6.68) | 5.63 (2.65, 12.10) | 5.59 (2.47, 16.23) | 7.76 (4.60, 18.67) | 5.94 (3.02, 11.48) |
| 0.24 (0.17, 0.31) | 1st-generation EGFR-TKIs | 1.60 (0.85, 2.37) | 0.80 (0.44, 1.39) | 1.38 (0.61, 2.58) | 1.39 (0.58, 3.54) | 1.83 (1.11, 3.93) | 1.48 (0.75, 2.37) |
| 0.15 (0.10, 0.26) | 0.63 (0.42, 1.18) | Afatinib | 0.48 (0.26, 1.20) | 0.84 (0.38, 2.12) | 0.84 (0.36, 2.82) | 1.21 (0.67, 3.27) | 0.89 (0.43, 2.08) |
| 0.31 (0.15, 0.55) | 1.25 (0.72, 2.29) | 2.09 (0.83, 3.80) | Dacomitinib | 1.76 (0.65, 3.96) | 1.74 (0.63, 5.54) | 2.34 (1.12, 6.19) | 1.86 (0.74, 3.96) |
| 0.18 (0.08, 0.38) | 0.72 (0.39, 1.63) | 1.19 (0.47, 2.61) | 0.57 (0.25, 1.54) | Osimertinib | 1.01 (0.36, 3.48) | 1.36 (0.64, 4.21) | 1.06 (0.42, 2.64) |
| 0.18 (0.06, 0.41) | 0.72 (0.28, 1.72) | 1.20 (0.35, 2.80) | 0.57 (0.18, 1.58) | 0.99 (0.29, 2.79) | P + Gefitinib | 1.28 (0.49, 4.44) | 1.06 (0.32, 2.74) |
| 0.13 (0.05, 0.22) | 0.55 (0.25, 0.90) | 0.83 (0.31, 1.49) | 0.43 (0.16, 0.89) | 0.74 (0.24, 1.56) | 0.78 (0.23, 2.05) | PC + Gefitinib | 0.76 (0.27, 1.54) |
| 0.17 (0.09, 0.33) | 0.68 (0.42, 1.33) | 1.12 (0.48, 2.34) | 0.54 (0.25, 1.35) | 0.94 (0.38, 2.40) | 0.94 (0.37, 3.10) | 1.32 (0.65, 3.64) | Erlotinib + Bevacizumab |
| OR with 95% CI for DCR | |||||||
| Chemotherapy | 2.24 (1.31, 4.06) | 2.41 (1.08, 5.38) | 3.36 (0.87, 13.65) | 5.65 (1.37, 26.36) | 1.94 (0.32, 9.97) | 9.18 (1.40, 83.49) | 4.54 (1.23, 18.81) |
| 0.45 (0.25, 0.76) | 1st-generation EGFR-TKIs | 1.06 (0.43, 2.50) | 1.46 (0.44, 5.18) | 2.44 (0.66, 10.15) | 0.88 (0.15, 3.91) | 4.09 (0.66, 32.81) | 2.06 (0.61, 7.46) |
| 0.41 (0.19, 0.92) | 0.94 (0.40, 2.35) | Afatinib | 1.40 (0.31, 6.67) | 2.35 (0.50, 12.88) | 0.80 (0.12, 4.82) | 3.86 (0.50, 39.15) | 1.93 (0.44, 9.45) |
| 0.30 (0.07, 1.14) | 0.68 (0.19, 2.28) | 0.72 (0.15, 3.19) | Dacomitinib | 1.70 (0.27, 11.57) | 0.58 (0.07, 4.04) | 2.85 (0.29, 32.08) | 1.43 (0.24, 7.86) |
| 0.18 (0.04, 0.73) | 0.41 (0.10, 1.51) | 0.42 (0.08, 2.02) | 0.59 (0.09, 3.77) | Osimertinib | 0.35 (0.04, 2.60) | 1.64 (0.17, 19.40) | 0.84 (0.13, 5.56) |
| 0.52 (0.10, 3.10) | 1.14 (0.26, 6.62) | 1.24 (0.21, 8.26) | 1.73 (0.25, 14.84) | 2.87 (0.38, 28.00) | P + Gefitinib | 5.02 (0.44, 75.36) | 2.63 (0.35, 23.32) |
| 0.11 (0.01, 0.71) | 0.24 (0.03, 1.51) | 0.26 (0.03, 2.00) | 0.35 (0.03, 3.44) | 0.61 (0.05, 6.02) | 0.20 (0.01, 2.27) | PC + Gefitinib | 0.49 (0.04, 4.52) |
| 0.22 (0.05, 0.81) | 0.49 (0.13, 1.64) | 0.52 (0.11, 2.28) | 0.70 (0.13, 4.24) | 1.20 (0.18, 7.68) | 0.38 (0.04, 2.86) | 2.05 (0.22, 22.53) | Erlotinib + Bevacizumab |
| HR with 95% CI for PFS | |||||||
| Chemotherapy | 0.41 (0.32, 0.53) | 0.37 (0.23, 0.57) | 0.26 (0.15, 0.48) | 0.19 (0.09, 0.41) | 0.28 (0.12, 0.63) | 0.19 (0.11, 0.31) | 0.23 (0.13, 0.43) |
| 2.42 (1.87, 3.14) | 1st-generation EGFR-TKIs | 0.88 (0.56, 1.41) | 0.64 (0.37, 1.10) | 0.46 (0.22, 0.95) | 0.68 (0.31, 1.47) | 0.46 (0.29, 0.74) | 0.56 (0.32, 0.98) |
| 2.73 (1.76, 4.26) | 1.13 (0.71, 1.80) | Afatinib | 0.72 (0.36, 1.48) | 0.52 (0.22, 1.23) | 0.77 (0.31, 1.88) | 0.52 (0.27, 0.99) | 0.63 (0.31, 1.30) |
| 3.78 (2.06, 6.88) | 1.56 (0.91, 2.68) | 1.38 (0.67, 2.80) | Dacomitinib | 0.72 (0.29, 1.75) | 1.06 (0.41, 2.70) | 0.72 (0.35, 1.47) | 0.88 (0.40, 1.90) |
| 5.24 (2.46, 11.37) | 2.17 (1.06, 4.47) | 1.92 (0.81, 4.53) | 1.39 (0.57, 3.45) | Osimertinib | 1.47 (0.51, 4.22) | 1.00 (0.42, 2.36) | 1.22 (0.49, 3.02) |
| 3.56 (1.59, 8.07) | 1.47 (0.68, 3.19) | 1.30 (0.53, 3.21) | 0.94 (0.37, 2.45) | 0.68 (0.24, 1.95) | P + Gefitinib | 0.68 (0.28, 1.68) | 0.82 (0.32, 2.16) |
| 3.56 (1.59, 8.07) | 1.47 (0.68, 3.19) | 1.30 (0.53, 3.21) | 0.94 (0.37, 2.45) | 0.68 (0.24, 1.95) | 1.48 (0.59, 3.64) | PC + Gefitinib | 1.22 (0.59, 2.54) |
| 4.30 (2.35, 7.98) | 1.78 (1.02, 3.11) | 1.58 (0.77, 3.25) | 1.14 (0.53, 2.50) | 0.82 (0.33, 2.04) | 1.21 (0.46, 3.14) | 0.82 (0.39, 1.70) | Erlotinib + Bevacizumab |
| HR with 95% CI for OS | |||||||
| Chemotherapy | 1.02 (0.89, 1.16) | 0.90 (0.74, 1.09) | 0.77 (0.57, 1.04) | 0.64 (0.43, 0.96) | 0.58 (0.43, 0.78) | 0.82 (0.51, 1.33) | – |
| 0.98 (0.86, 1.12) | 1st-generation EGFR-TKIs | 0.88 (0.72, 1.08) | 0.75 (0.57, 0.99) | 0.63 (0.43, 0.92) | 0.57 (0.43, 0.75) | 0.81 (0.51, 1.28) | – |
| 1.12 (0.92, 1.35) | 1.14 (0.93, 1.39) | Afatinib | 0.86 (0.61, 1.21) | 0.71 (0.46, 1.10) | 0.65 (0.46, 0.90) | 0.92 (0.56, 1.52) | – |
| 1.30 (0.96, 1.77) | 1.33 (1.01, 1.75) | 1.17 (0.82, 1.64) | Dacomitinib | 0.83 (0.52, 1.33) | 0.76 (0.51, 1.11) | 1.07 (0.62, 1.84) | – |
| 1.56 (1.04, 2.33) | 1.59 (1.09, 2.33) | 1.40 (0.91, 2.16) | 1.20 (0.75, 1.92) | Osimertinib | 0.91 (0.56, 1.45) | 1.29 (0.71, 2.34) | – |
| 1.71 (1.29, 2.33) | 1.74 (1.34, 2.33) | 1.54 (1.11, 2.18) | 1.31 (0.90, 1.98) | 1.10 (0.69, 1.78) | PC + Gefitinib | 1.41 (0.84, 2.43) | – |
| 1.21 (0.75, 1.96) | 1.23 (0.78, 1.96) | 1.09 (0.66, 1.79) | 0.93 (0.54, 1.60) | 0.78 (0.43, 1.40) | 0.71 (0.41, 1.20) | Erlotinib + Bevacizumab | – |
CI, confidence interval; DCR, disease control rate; EGFR-TKIs, Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors; HR, hazard ratio; OR, odds ratio; ORR, objective response rate; OS, overall survival; P, Pemetrexed; PC, Pemetrexed/Carboplatin; PFS, progression-free survival.
As for the third-generation EGFR-TKI osimertinib, it had statistically longer OS and PFS outcomes than chemotherapy and first-generation EGFR-TKIs. Erlotinib plus bevacizumab showed a better PFS outcome than first-generation EGFR-TKIs (HR = 1.78, 95% CI: 1.02–3.11) and chemotherapy (HR = 4.30, 95% CI: 2.35–7.98). Dacomitinib had a better OS outcome compared with first-generation EGFR-TKIs (HR = 1.33, 95% CI: 1.01–1.75); however, there were no significant differences observed in ORR, DCR, or PFS between them.
Subgroup analyses for EGFR 19 del and L858R mutation
We extracted PFS and OS data for each subgroup based on EGFR mutation types: 19 del and L858R (Table S3–S4). Both subgroups had similar results in regards to longer PFS of patients treated with PC plus gefitinib than chemotherapy. In the L858R subgroup, PC plus gefitinib had a better PFS than first-generation EGFR-TKIs, afatinib, and dacomitinib. Unlike the L858R subgroup, the 19 del group showed that patients treated with erlotinib plus bevacizumab had a longer PFS than first-generation EGFR-TKIs (Figure S2 and Table S16).
As for OS, 11 studies on first-generation EGFR-TKIs, afatinib, dacomitinib, erlotinib plus bevacizumab and chemotherapy were available for subgroup analyses. Afatinib had a significantly more favorable OS in the EGFR 19 del subgroup compared with chemotherapy (HR = 0.64, 95% CI: 0.44–0.94). Other pairwise comparisons were insignificant (Figure S2 and Table S16).
Network meta-analyses for safety profile
For TRAEs and TRAE grades 3–5, there were no statistical significance differences for the eight treatment (Table 3). Rash and diarrhea were the most common treatment-related adverse events for EGFR-TKIs compared with chemotherapy (OR < 1, 95% CI < 1). Diarrhea typically occurred more often in patients treated with afatinib and dacomitinib. Elevated ALT/AST had a significantly higher incidence in the comparisons between pemetrexed plus gefitinib and osimertinib (OR = 0.07, 95% CI: 0.01–0.93). For all EGFR-TKIs, rash was a common occurrence, particularly among patients treated with afatinib, and dacomitinib.
Table 3.
Multiple treatment comparison for tolerability based on network consistency model (OR > 1 means higher toxicity).
| OR with 95% CI for TRAE | |||||||
|---|---|---|---|---|---|---|---|
| Chemotherapy | 0.65 (0.12, 3.98) | 2.30 (0.21, 34.62) | 1.76 (0.03, 126.31) | 0.55 (0.01, 27.75) | 0.81 (0.01, 47.69) | 0.25 (0.00, 17.55) | – |
| 1.53 (0.25, 8.02) | 1st-generation EGFR-TKIs | 3.44 (0.27, 53.89) | 2.60 (0.06, 123.26) | 0.83 (0.02, 29.44) | 1.22 (0.03, 53.71) | 0.39 (0.01, 17.21) | – |
| 0.44 (0.03, 4.74) | 0.29 (0.02, 3.65) | Afatinib | 0.75 (0.01, 70.02) | 0.24 (0.00, 16.79) | 0.36 (0.00, 26.66) | 0.11 (0.00, 9.70) | – |
| 0.57 (0.01, 30.78) | 0.38 (0.01, 15.78) | 1.33 (0.01, 123.00) | Dacomitinib | 0.31 (0.00, 54.00) | 0.44 (0.00, 84.11) | 0.14 (0.00, 31.06) | – |
| 1.82 (0.04, 92.99) | 1.20 (0.03, 46.01) | 4.20 (0.06, 373.35) | 3.21 (0.02, 766.68) | Osimertinib | 1.52 (0.01, 241.70) | 0.45 (0.00, 95.65) | – |
| 1.23 (0.02, 73.65) | 0.82 (0.02, 32.92) | 2.78 (0.04, 299.27) | 2.26 (0.01, 507.58) | 0.66 (0.00, 120.70) | P + Gefitinib | 0.31 (0.00, 66.39) | – |
| 3.95 (0.06, 259.87) | 2.59 (0.06, 123.07) | 9.06 (0.10, 1110.50) | 6.90 (0.03, 1660.94) | 2.20 (0.01, 449.84) | 3.27 (0.02, 699.85) | PC + Gefitinib | – |
| OR with 95% CI for TRAE 3–5 | |||||||
| Chemotherapy | 0.82 (0.15, 4.51) | 0.86 (0.12, 6.44) | 0.43 (0.01, 18.63) | 2.69 (0.07, 97.08) | 3.31 (0.08, 144.89) | 1.74 (0.04, 69.86) | – |
| 1.22 (0.22, 6.80) | 1st-generation EGFR-TKIs | 1.04 (0.11, 9.97) | 0.53 (0.02, 13.42) | 3.25 (0.12, 89.12) | 4.09 (0.16, 113.60) | 2.12 (0.08, 58.75) | – |
| 1.16 (0.16, 8.42) | 0.96 (0.10, 8.98) | Afatinib | 0.51 (0.01, 26.72) | 3.11 (0.06, 147.43) | 3.87 (0.07, 206.98) | 1.99 (0.04, 107.65) | – |
| 2.31 (0.05, 89.53) | 1.90 (0.07, 48.69) | 1.96 (0.04, 103.13) | Osimertinib | 6.04 (0.05, 682.93) | 7.68 (0.08, 826.09) | 3.97 (0.04, 427.83) | – |
| 0.37 (0.01, 14.74) | 0.31 (0.01, 8.02) | 0.32 (0.01, 16.36) | 0.17 (0.00, 18.93) | P + Gefitinib | 1.24 (0.01, 145.13) | 0.63 (0.01, 72.95) | – |
| 0.30 (0.01, 12.38) | 0.24 (0.01, 6.29) | 0.26 (0.00, 13.73) | 0.13 (0.00, 12.96) | 0.81 (0.01, 83.61) | PC + Gefitinib | 0.50 (0.00, 51.06) | – |
| 0.57 (0.01, 24.92) | 0.47 (0.02, 12.40) | 0.50 (0.01, 27.11) | 0.25 (0.00, 25.74) | 1.58 (0.01, 152.59) | 1.98 (0.02, 202.48) | Erlotinib + Bevacizumab | – |
| OR with 95% CI for Rash | |||||||
| Chemotherapy | 24.34 (11.21, 55.17) | 63.16 (19.35, 211.61) | 59.08 (7.87, 472.09) | 8.86 (1.24, 71.94) | 16.52 (2.03, 141.88) | 18.19 (3.79, 100.96) | 26.96 (4.09, 182.71) |
| 0.04 (0.02, 0.09) | 1st-generation EGFR-TKIs | 2.59 (0.71, 8.87) | 2.41 (0.38, 16.52) | 0.36 (0.05, 2.45) | 0.67 (0.09, 5.07) | 0.75 (0.18, 3.24) | 1.11 (0.20, 6.43) |
| 0.02 (0.00, 0.05) | 0.39 (0.11, 1.41) | Afatinib | 0.94 (0.10, 9.64) | 0.14 (0.01, 1.43) | 0.26 (0.03, 2.89) | 0.29 (0.05, 2.03) | 0.43 (0.05, 3.66) |
| 0.02 (0.00, 0.13) | 0.41 (0.06, 2.65) | 1.06 (0.10, 10.30) | Dacomitinib | 0.15 (0.01, 2.32) | 0.28 (0.02, 4.26) | 0.31 (0.03, 3.27) | 0.46 (0.03, 5.87) |
| 0.11 (0.01, 0.81) | 2.77 (0.41, 18.35) | 7.12 (0.70, 68.39) | 6.68 (0.43, 93.71) | Osimertinib | 1.88 (0.12, 29.86) | 2.08 (0.20, 22.76) | 3.09 (0.22, 40.78) |
| 0.06 (0.01, 0.49) | 1.48 (0.20, 10.61) | 3.79 (0.35, 38.36) | 3.53 (0.23, 55.17) | 0.53 (0.03, 8.33) | P + Gefitinib | 1.10 (0.10, 12.40) | 1.64 (0.13, 21.10) |
| 0.05 (0.01, 0.26) | 1.33 (0.31, 5.41) | 3.44 (0.49, 21.76) | 3.22 (0.31, 33.35) | 0.48 (0.04, 4.97) | 0.91 (0.08, 9.56) | PC + Gefitinib | 1.49 (0.15, 14.39) |
| 0.04 (0.01, 0.24) | 0.90 (0.16, 5.00) | 2.33 (0.27, 20.32) | 2.17 (0.17, 30.75) | 0.32 (0.02, 4.62) | 0.61 (0.05, 7.95) | 0.67 (0.07, 6.69) | Erlotinib + Bevacizumab |
| OR with 95% CI for Diarrhea | |||||||
| Chemotherapy | 5.17 (2.78, 9.68) | 59.57 (24.69, 152.55) | 28.18 (5.69, 134.90) | 5.21 (1.09, 24.07) | 4.57 (0.88, 22.84) | 5.50 (1.63, 19.52) | 6.63 (1.94, 22.59) |
| 0.19 (0.10, 0.36) | 1st-generation EGFR-TKIs | 11.55 (4.57, 31.11) | 5.41 (1.22, 22.83) | 1.01 (0.25, 4.09) | 0.88 (0.20, 3.97) | 1.07 (0.37, 3.27) | 1.28 (0.43, 3.69) |
| 0.02 (0.01, 0.04) | 0.09 (0.03, 0.22) | Afatinib | 0.47 (0.08, 2.58) | 0.09 (0.02, 0.47) | 0.08 (0.01, 0.45) | 0.09 (0.02, 0.39) | 0.11 (0.02, 0.47) |
| 0.04 (0.01, 0.18) | 0.18 (0.04, 0.82) | 2.11 (0.39, 12.76) | Dacomitinib | 0.19 (0.03, 1.47) | 0.16 (0.02, 1.37) | 0.20 (0.03, 1.23) | 0.23 (0.04, 1.49) |
| 0.19 (0.04, 0.91) | 0.99 (0.24, 4.05) | 11.39 (2.13, 62.89) | 5.38 (0.68, 39.83) | Osimertinib | 0.88 (0.11, 6.63) | 1.06 (0.18, 6.29) | 1.26 (0.21, 7.19) |
| 0.22 (0.04, 1.14) | 1.14 (0.25, 5.11) | 13.14 (2.22, 80.71) | 6.08 (0.73, 51.28) | 1.13 (0.15, 9.07) | P + Gefitinib | 1.20 (0.19, 7.92) | 1.45 (0.23, 9.44) |
| 0.18 (0.05, 0.61) | 0.94 (0.31, 2.67) | 10.82 (2.58, 47.57) | 5.06 (0.81, 29.41) | 0.94 (0.16, 5.58) | 0.83 (0.13, 5.18) | PC + Gefitinib | 1.19 (0.25, 5.21) |
| 0.15 (0.04, 0.51) | 0.78 (0.27, 2.31) | 9.07 (2.14, 40.02) | 4.28 (0.67, 25.54) | 0.79 (0.14, 4.75) | 0.69 (0.11, 4.40) | 0.84 (0.19, 3.93) | Erlotinib + Bevacizumab |
| OR with 95% CI for Elevated ALT/AST | |||||||
| Chemotherapy | 1.95 (0.92, 4.24) | 0.76 (0.20, 3.05) | 0.72 (0.10, 5.19) | 0.34 (0.05, 2.48) | 5.02 (0.64, 39.06) | 2.70 (0.61, 12.90) | 1.57 (0.35, 7.28) |
| 0.51 (0.24, 1.08) | 1st-generation EGFR-TKIs | 0.39 (0.10, 1.53) | 0.37 (0.06, 2.22) | 0.17 (0.03, 1.06) | 2.57 (0.38, 17.14) | 1.38 (0.38, 5.31) | 0.81 (0.22, 3.02) |
| 1.31 (0.33, 5.01) | 2.55 (0.65, 10.04) | Afatinib | 0.94 (0.10, 8.92) | 0.44 (0.05, 4.24) | 6.55 (0.62, 67.86) | 3.51 (0.55, 23.38) | 2.06 (0.31, 13.87) |
| 1.39 (0.19, 9.69) | 2.70 (0.45, 16.28) | 1.06 (0.11, 10.03) | Dacomitinib | 0.47 (0.04, 6.08) | 6.94 (0.52, 96.29) | 3.73 (0.41, 36.11) | 2.18 (0.23, 20.72) |
| 2.96 (0.40, 19.88) | 5.76 (0.94, 34.00) | 2.25 (0.24, 20.92) | 2.12 (0.16, 27.11) | Osimertinib | 14.75 (1.08, 196.61) | 7.99 (0.86, 74.33) | 4.68 (0.49, 43.19) |
| 0.20 (0.03, 1.55) | 0.39 (0.06, 2.62) | 0.15 (0.01, 1.62) | 0.14 (0.01, 1.92) | 0.07 (0.01, 0.93) | P + Gefitinib | 0.54 (0.05, 5.50) | 0.31 (0.03, 3.27) |
| 0.37 (0.08, 1.64) | 0.72 (0.19, 2.67) | 0.28 (0.04, 1.83) | 0.27 (0.03, 2.42) | 0.13 (0.01, 1.17) | 1.86 (0.18, 18.30) | PC + Gefitinib | 0.59 (0.09, 3.68) |
| 0.63 (0.14, 2.82) | 1.23 (0.33, 4.61) | 0.49 (0.07, 3.19) | 0.46 (0.05, 4.28) | 0.21 (0.02, 2.03) | 3.18 (0.31, 31.76) | 1.71 (0.27, 11.37) | Erlotinib + Bevacizumab |
CI, confidence interval; EGFR-TKIs, Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors; OR, Odds ratio; P, Pemetrexed; PC, Pemetrexed/Carboplatin; TRAE, Treatment-related Adverse Event; TRAE 3–5, Treatment-related Adverse Event Grade 3–5
Ranking probabilities
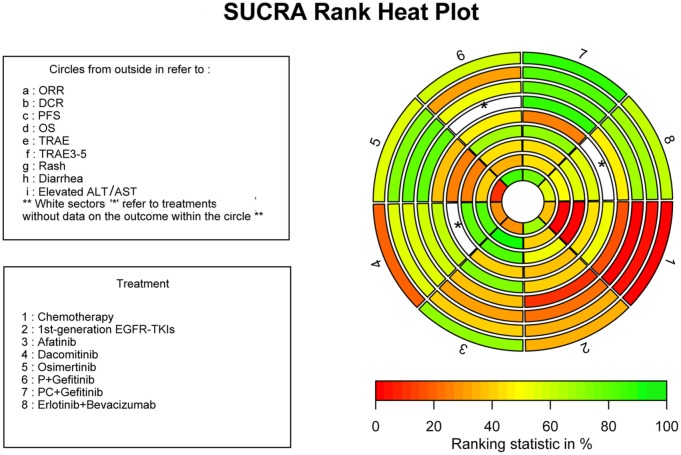
SUCRA was calculated for all of the available treatments. For SUCRA values, ‘1’ is the best and ‘0’ the worst. To present the data, we made a heat map plot integrating the efficacy and safety profile ranking of the treatments. Regimens with higher ranking probabilities for ORR, DCR, PFS, and OS and lower probabilities for TRAEs are more favorable in clinical practice (Figure 3, Table S15). The heat map plot is in Figure 3 and the SUCRA values for the eight regimens are displayed in Table S15. The scale consists of three colors: red (0%), yellow (50%), and green (100%). Green represents greater probabilities for better efficacy and red represents lower probabilities for incidence of TRAEs. Regimens colored green for efficacy outcomes and red for safety outcomes means high efficacy and low adverse effects.
Figure 3.
SUCRA rank heat plot of the first-line regimens for EGFR-mutant NSCLC patients with efficacy and toxicity profile. Each sector is colored according to the SUCRA value of the corresponding treatment and outcome. The scale consists of three colors: red (0%), yellow (50%), and green (100%). Each color is associated with a different pattern. Uncolored sectors show that the underlying treatment was not included in the network meta-analyses for that particular outcome.
DCR, disease control rate; EGFR, epidermal growth factor receptor; NSCLC, nonsmall cell lung cancer; ORR, objective response rate; OS, overall survival; P, pemetrexed; PC, pemetrexed and carboplatin; PFS, progression free survival; SUCRA, surface under the cumulative ranking; TKIs, tyrosine kinase inhibitors; TRAE, treatment related adverse event.
PC plus gefitinib ranked first for ORR (0.917), DCR (0.859), PFS (0.835), and OS (0.912), which indicated that it may have better efficacy compared with other treatments. Osimertinib (0.807) was second in the SUCRA OS rankings. As shown in the heat map plot (Figure 3), PC plus gefitinib, osimertinib, and erlotinib plus bevacizumab goes from green to light green in regards to efficacy outcomes, and light green to red in terms of safety profile. PC plus gefitinib also had the highest SUCRA of TRAE grades 3–5 (0.722). TRAEs, rash, and diarrhea outcomes occurred more often and had the highest ranked probabilities in patients treated with afatinib and dacomitinib. Pemetrexed plus gefitinib had the highest SUCRA value (0.893) for elevated ALT/AST.
Discussion
In this network meta-analysis of 19 head-to-head clinical trials, eight regimens were evaluated in regards to their therapeutic efficacy and toxicity as first-line treatments for patients with EGFR-mutant NSCLC. Since early studies on first-generation EGFR-TKIs showed no significant differences among gefitinib, erlotinib, or icotinib in terms of efficacy and toxicity, these three EGFR-TKIs were grouped together as a general class of first-generation EGFR-TKIs in our network meta-analysis.5–18
Our results showed that PC plus gefitinib had greater superiority in terms of ORR, DCR, PFS, and OS; however, this combination therapy had the highest incidence of TRAE grades 3–5. The incidence of TRAEs with PC plus gefitinib was comparable to EGFR-TKIs, and chemotherapy. The most common grade ⩾3 adverse events were hematological and gastrointestinal toxicities, including neutropenia (3.0–10.0% in the combinational group versus 0% in the gefitinib group), infection (4.7% versus 0%), vomiting (2.4% versus 0.6%), and pneumonitis (1.8% versus 1.2% as reported in the NEJ009 study), fatigue (7.5% versus 0%), liver dysfunction (10.0% versus 2.5%), and skin allergy (10.0% versus 0%) as reported in Han’s study.27,39 Whilst PC plus gefitinib caused a modest increase in toxicities compared with gefitinib, the toxicities appeared both tolerable and clinically manageable.
The results of our analysis indicated that gefitinib combined with carboplatin and pemetrexed as a first-line therapy provided the best survival benefits. The NEJ009 study and Han’s study showed that combination therapy improved PFS (20.9 months and 17.5 months, respectively) and improved median OS (52.2 months and 32.6 months, respectively) compared with monotherapy. Impressively, the PFS (20.9 months) of PC plus gefitinib surpassed osimertinib’s PFS (18.9 months), setting a record for first-line treatment in patients with EGFR-mutant NSCLC. It should be noted however, that Han’s research was a single center study with a small sample size.
Another study, JMIT, reported the clinical value of gefitinib plus pemetrexed with a median PFS reaching 15.8 months.28 The benefits of EGFR-TKI plus chemotherapy seemed to be based on platinum, but this needs further investigation. Such benefit may also be the case for PC plus gefitinib using platinum. Another possible merit of PC plus gefitinib is that subsequent treatment options will still be available if it is used as the first-line treatment.
As the disease inevitably progresses, patients are advised to check for molecular resistance, especially for the T790M mutation. Research has claimed the resistance mechanisms to first-generation EGFR-TKI monotherapy are on-target resistance (the emergence of the T790M mutation in exon 20 of the EGFR gene occurring in 50–70% of tumors), off-target resistance (like an amplification of the hepatocyte growth factor receptor-MET oncogene), pathologic transformation, or other unknown mechanisms.23 The resistance mechanism to PC plus gefitinib remains unclear and subsequent treatments in the NEJ009 and Han’s study have not been published, and the proportion of patients who develop the T790M mutation may affect the OS data of such studies. Still, testing for the T790M mutation is strongly recommended at the time of progression, which can make a difference to second-line therapies.
Osimertinib is the preferred option for patients with the T790M mutation when the disease progresses. Research shows patients who developed the T790M mutation during first-line standard of care clearly benefited from second-line osimertinib and had positive OS.24 Many other resistant mutations have no targeted agents. The combination of PC plus gefitinib might hinder patients’ second-line options of platinum-based chemotherapy as the disease progresses. The use of PC plus gefitinib as a first line treatment may allow osimertinib or anti-angiogenic regimens to be subsequent lines of treatment, which might further improve survival outcomes in such patients.
Another combination strategy recently investigated is EGFR-TKIs with antiangiogenic therapy. In two phase III trials (JO25567 and NEJ026), erlotinib combined with bevacizumab showed an improved median PFS (16.4–16.9 months) compared with erlotinib monotherapy.23,40 In the JO25567 study, the median OS for erlotinib plus bevacizumab was less favorable (47.0 months versus 47.4 months). However, in the NEJ026 study, treatment is ongoing for some patients receiving erlotinib plus bevacizumab, so the OS data remains immature. A subgroup analysis suggested that erlotinib plus bevacizumab improved PFS in patients with EGFR 19 del compared with those with the L858R mutation.41 Similarly, in our currently unpublished study: a multicenter, randomized, controlled phase III clinical trial comparing gefitinib plus apatinib (an orally multitargeted antiangiogenic TKI) with gefitinib plus placebo in patients with advanced NSCLC harboring EGFR mutations, the results are promising with a median PFS of up to 19 months.42 Indicating that EGFR-TKIs plus antiangiogenic agents might provide equivalent PFS benefits to osimertinib.
The EGFR 19 del and L858R mutations reportedly have different predictive and prognostic outcomes.43,44 As displayed in this NMA, PC plus gefitinib, when compared with first-generation EGFR-TKIs, afatinib and dacomitinib, had a greater PFS in patients with L858R than in patients with 19 del. An improvement in PFS with erlotinib plus bevacizumab compared with first-generation EGFR-TKIs was observed in the mutation subgroup of 19 del but not in the L858R subgroup. Similarly, in vivo, combining erlotinib with bevacizumab postponed the resistance to first-generation EGFR-TKIs of NSCLC cells harboring EGFR 19 del.25,26 A retrospective study revealed EGFR 19 del-positive subgroup had a higher risk of the secondary T790M mutation than the L858R-positive subgroup, which may affect PFS and OS.3
Nowadays, the increasing incidence of cancer coupled with the rise in the cost of new drugs and technologies needs to be weighed against the cost and value of various oncology treatments. Using the NCCN’s guidelines on efficacy, safety, quality, consistency of evidence, and affordability, first-generation EGFR-TKIs are high in efficacy and quality with moderate affordability, while osimertinib is quite expensive with low affordability.45,46 In China, pemetrexed, carboplatin and gefitinib are covered by medical insurance making them more affordable.47–49 From the perspective of cost-effectiveness, PC plus gefitinib showed better efficacy and costs less, therefore, is an excellent option for EGFR-mutant NSCLC as a first-line treatment. However, PC plus gefitinib requires chemotherapy hospitalization every 3 or 4 weeks, which, unlike oral EGFR-TKIs, might cause hypersensitivity or adverse reactions. Hopefully, PC plus osimertinib will become a potent combination strategy that might maximize the effects of currently available agents.20
This study has some limitations: risk of bias was reported in several studies with an unclear risk of performance and attrition bias. Our findings were also based on the available data and immature OS. Planned analyses for efficacy outcomes, including ORR, DCR, PFS, and OS, as well as safety outcomes, were not possible due to incomplete or immature data. The toxicity spectrum distinctly differed between EGFR-TKIs and chemotherapy. Some adverse events after chemotherapy, such as myelosuppression and nausea, were unable to be compared separately.50 Additionally, we performed subgroup analyses for EGFR 19 del and L858R mutations without other stratified clinical parameters due to incomplete and or immature data. In clinical practice, ethnicity, age, performance status (PS), the presence of brain metastasis, genomic characteristics, concurrent mutations and comorbidities, and patient wishes need to be considered in the decision-making process. Regardless of the above limitations, our study provides oncologists with strong evidence for the first-line treatment of NSCLC patients with activating EGFR mutations, as well as, a general view of different regimens’ efficacy and toxicity profiles; helping clinicians to make more informed decisions.
Conclusion
Pemetrexed/carboplatin plus gefitinib is a promising treatment option for EGFR-mutant NSCLC patients in the first-line setting.
Clinical practice points
Eight regimens, including five EGFR-TKIs (gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib), pemetrexed plus gefitinib, PC plus gefitinib, and erlotinib plus bevacizumab were evaluated in first-line setting of advanced NSCLC patients harboring EGFR mutations in this network meta-analysis.
Pemetrexed/carboplatin plus gefitinib had a synergistic antitumor effect and is potentially superior to osimertinib in term of efficacy with a similar toxicity, indicating that chemotherapy plus EGFR-TKIs could be a first-line treatment option for NSCLC patients with an activated EGFR mutation.
Supplemental Material
Supplemental material, Supplemental_Data_2_Supplemental_Table_and_Figure_changes_marked_yellow for Pemetrexed/carboplatin plus gefitinib as a first-line treatment for EGFR-mutant advanced nonsmall cell lung cancer: a Bayesian network meta-analysis by Zhonghan Zhang, Kangmei Zeng, Shen Zhao, Yuanyuan Zhao, Xue Hou, Fan Luo, Feiteng Lu, Yaxiong Zhang, Ting Zhou, Yuxiang Ma, Yunpeng Yang, Wenfeng Fang, Yan Huang, Li Zhang and Hongyun Zhao in Therapeutic Advances in Medical Oncology
Acknowledgments
Authors Zhonghan Zhang, Kangmei Zeng, Shen Zhao contributed equally. We would like to thank all the patients, their families, study investigators, and personnel from the RCT trials.
Footnotes
Contributors: Conception design and Protocol Writing: Zhonghan Zhang, Hongyun Zhao, Li Zhang
Literature retrieval and review: Feiteng Lu, Zhonghan Zhang
Data extraction and quality assessment: Xue Hou, Kangmei Zeng, Ting Zhou, Yunpeng Yang, Wenfeng Fang, Yan Huang
Statistical guidance: Yaxiong Zhang, Shen Zhao
Data analysis and interpretation: Zhonghan Zhang, Yuanyuan Zhao, Fan Luo
Writing of the manuscript: Zhonghan Zhang, Kangmei Zeng
Primary revision before submitting: Hongyun Zhao, Li Zhang
Final approval of manuscript: All authors
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:This work was supported by National Key R&D Program of China (Grant number: 2016YFC0905500, 2016YFC0905503), The Science and Technology Planning Project of Guangdong Province of China (Grant number: 2017B020227001), Natural Science Foundation of Guangdong Province of China (Grant number: 2018A0303130243) and The 5010 Clinical Research Foundation of Sun Yat-sen University (Grant number: 2016001) and Jiangsu Hengrui Medicine Co. Ltd. (Jiangsu, China).
Li Zhang has received research support from the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA12020101 to J.D.). Yan Huang has received research support from Science and Technology Program of Guangzhou (201704020072). Yunpeng Yang was supported by Outstanding Young Talents Program of Sun Yat-sen University Cancer Center (16zxyc03) and Central Basic Scientific Research Fund for Colleges-Young Teacher Training Program of Sun Yat-sen University (17ykpy85).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Kangmei Zeng  https://orcid.org/0000-0003-4361-0805
https://orcid.org/0000-0003-4361-0805
Data sharing statement: No additional data are available.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Zhonghan Zhang, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Kangmei Zeng, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Shen Zhao, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Yuanyuan Zhao, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Xue Hou, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Fan Luo, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Feiteng Lu, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Yaxiong Zhang, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Ting Zhou, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Yuxiang Ma, Department of Clinical Research, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Yunpeng Yang, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Wenfeng Fang, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Yan Huang, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, Guangdong, P.R. China.
Li Zhang, Department of Medical Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, 651 Dongfeng Road East, Guangzhou, Guangdong 510060, P.R. China.
Hongyun Zhao, Department of Clinical Research, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, 651 Dongfeng Road East, Guangzhou, Guangdong, 510060, P.R. China.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidemiol Biomarkers Prev 2017; 26: 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ke EE, Zhou Q, Zhang QY, et al. A higher proportion of the EGFR T790M mutation may contribute to the better survival of patients with exon 19 deletions compared with those with L858R. J Thorac Oncol 2017; 12: 1368–1375. [DOI] [PubMed] [Google Scholar]
- 4. Rossi S, D’Argento E, Basso M, et al. Different EGFR gene mutations in exon 18, 19 and 21 as prognostic and predictive markers in NSCLC: a single institution analysis. Mol Diagn Ther 2016; 20: 55–63. [DOI] [PubMed] [Google Scholar]
- 5. Zheng Z, Xie D, Su H, et al. Treatment outcome comparisons between exons 19 and 21 EGFR mutations for non-small-cell lung cancer patients with malignant pleural effusion after first-line and second-line tyrosine kinase inhibitors. Tumour Biol 2017; 39: 1010428317706211. [DOI] [PubMed] [Google Scholar]
- 6. Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017; 15: 504–535. [DOI] [PubMed] [Google Scholar]
- 7. Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012; 30: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 8. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–128. [DOI] [PubMed] [Google Scholar]
- 9. Miyauchi E, Inoue A, Kobayashi K, et al. Efficacy of chemotherapy after first-line gefitinib therapy in EGFR mutation-positive advanced non-small cell lung cancer-data from a randomized phase III study comparing gefitinib with carboplatin plus paclitaxel (NEJ002). Jpn J Clin Oncol 2015; 45: 670–676. [DOI] [PubMed] [Google Scholar]
- 10. Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013; 24: 54–59. [DOI] [PubMed] [Google Scholar]
- 11. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–246. [DOI] [PubMed] [Google Scholar]
- 12. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol 2017; 28: 2443–2450. [DOI] [PubMed] [Google Scholar]
- 13. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol 2015; 16: 990–998. [DOI] [PubMed] [Google Scholar]
- 14. Wu YL, Chu DT, Han B, et al. Phase III, randomized, open-label, first-line study in Asia of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer: evaluation of patients recruited from mainland China. Asia Pac J Clin Oncol 2012; 8: 232–243. [DOI] [PubMed] [Google Scholar]
- 15. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015; 26: 1883–1889. [DOI] [PubMed] [Google Scholar]
- 16. Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015; 26: 1877–1883. [DOI] [PubMed] [Google Scholar]
- 17. Ramalingam SS, O’Byrne K, Boyer M, et al. Dacomitinib versus erlotinib in patients with EGFR-mutated advanced nonsmall-cell lung cancer (NSCLC): pooled subset analyses from two randomized trials. Ann Oncol 2016; 27: 423–429. [DOI] [PubMed] [Google Scholar]
- 18. Schuler M, Tan EH, O’Byrne K, et al. First-line afatinib vs gefitinib for patients with EGFR mutation-positive NSCLC (LUX-Lung 7): impact of afatinib dose adjustment and analysis of mode of initial progression for patients who continued treatment beyond progression. J Cancer Res Clin Oncol 2019; 145: 1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu Y, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017; 18: 1454–1466. [DOI] [PubMed] [Google Scholar]
- 20. Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141–151. [DOI] [PubMed] [Google Scholar]
- 21. Planchard D, Boyer M, Lee JS, et al. Osimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with untreated EGFRm advanced NSCLC: FLAURA post-progression outcomes. Ann Oncol 2018; 29(Suppl. 9): abstract 4890. [Google Scholar]
- 22. Popat S. Osimertinib as first-line treatment in EGFR-mutated non-small-cell lung cancer. New Engl J Med 2018; 378: 192–193. [DOI] [PubMed] [Google Scholar]
- 23. Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell 2010; 17: 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015; 372: 1689–1699. [DOI] [PubMed] [Google Scholar]
- 25. Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014; 15: 1236–1244. [DOI] [PubMed] [Google Scholar]
- 26. Furuya N, Fukuhara T, Saito H, et al. Phase III study comparing bevacizumab plus erlotinib to erlotinib in patients with untreated NSCLC harboring activating EGFR mutations: NEJ026. J Clin Oncol 2018; 36: 9006. [Google Scholar]
- 27. Nakamura A, Inoue A, Morita S, et al. Phase III study comparing gefitinib monotherapy (G) to combination therapy with gefitinib, carboplatin, and pemetrexed (GCP) for untreated patients (pts) with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). J Clin Oncol 2018; 36(Suppl. 15): 9005. [Google Scholar]
- 28. Cheng Y, Murakami H, Yang PC, et al. Randomized phase II trial of gefitinib with and without pemetrexed as first-line therapy in patients with advanced nonsquamous non-small-cell lung cancer with activating epidermal growth factor receptor mutations. J Clin Oncol 2016; 34: 3258–3266. [DOI] [PubMed] [Google Scholar]
- 29. Imai H, Kaira K, Minato K. Clinical significance of post-progression survival in lung cancer. Thorac Cancer 2017; 8: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 31. Moher D, Jadad AR, Tugwell P. Assessing the quality of randomized controlled trials. Current issues and future directions. Int J Technol Assess Health Care 1996; 12: 195–208. [DOI] [PubMed] [Google Scholar]
- 32. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods 2012; 3: 161–176. [DOI] [PubMed] [Google Scholar]
- 34. Valkenhoef GV, Tervonen T, Zwinkels T, et al. ADDIS: a decision support system for evidence-based medicine. Decis Support Syst 2013; 55: 459–475. [Google Scholar]
- 35. Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010; 29: 932–944. [DOI] [PubMed] [Google Scholar]
- 37. Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 2012; 3: 80–97. [DOI] [PubMed] [Google Scholar]
- 38. Veroniki AA, Vasiliadis HS, Higgins JP, et al. Evaluation of inconsistency in networks of interventions. Int J Epidemiol 2013; 42: 332–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han B, Jin B, Chu T, et al. Combination of chemotherapy and gefitinib as first-line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: a randomized controlled trial. Int J Cancer 2017; 141: 1249–1256. [DOI] [PubMed] [Google Scholar]
- 40. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019; 20: 625–635. [DOI] [PubMed] [Google Scholar]
- 41. Sheng M, Wang F, Zhao Y, et al. Comparison of clinical outcomes of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations after tyrosine kinase inhibitors treatment: a meta-analysis. Eur J Clin Pharmacol 2016; 72: 1–11. [DOI] [PubMed] [Google Scholar]
- 42. Li Z, Hongyun Z. Gefitinib plus Apatinib compared with Gefitinib plus placebo in patients with stage IIIB or IV non-squamous non-small-cell lung cancer harbouring EGFR mutations, a multicenter, randomized, controlled clinical trial Paper presented at the IASLC 19th World Conference on Lung Cancer, 2018, Toronto, Canada. [Google Scholar]
- 43. Yu JY, Yu SF, Wang SH, et al. Clinical outcomes of EGFR-TKI treatment and genetic heterogeneity in lung adenocarcinoma patients with EGFR mutations on exons 19 and 21. Chin J Cancer 2016; 35: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Y, He D, Fang W, et al. The difference of clinical characteristics between patients with exon 19 deletion and those with L858R mutation in nonsmall cell lung cancer. Medicine 2015; 94: e1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eaton KD, Jagels B, Martins RG. Value-based care in lung cancer. Oncologist 2016; 21: 903–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schnipper LE, Bastian A. New frameworks to assess value of cancer care: strengths and limitations. Oncologist 2016; 21: 654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aguiar PN, Jr, Haaland B, Park W, et al. Cost-effectiveness of osimertinib in the first-line treatment of patients with EGFR-mutated advanced non-small cell lung cancer. JAMA Oncol 2018; 4: 1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Skinner KE, Fernandes AW, Walker MS, et al. Healthcare costs in patients with advanced non-small cell lung cancer and disease progression during targeted therapy: a real-world observational study. J Med Econ 2018; 21: 192–200. [DOI] [PubMed] [Google Scholar]
- 49. Wu B, Gu X, Zhang Q. Cost-effectiveness of osimertinib for EGFR mutation-positive non-small cell lung cancer after progression following first-line EGFR TKI therapy. J Thorac Oncol 2018; 13: 184–193. [DOI] [PubMed] [Google Scholar]
- 50. Okamoto I, Schuette WH, Stinchcombe TE, et al. Meta-analysis of pemetrexed plus carboplatin doublet safety profile in first-line non-squamous non-small cell lung cancer studies. Curr Med Res Opin 2017; 33: 937–941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Data_2_Supplemental_Table_and_Figure_changes_marked_yellow for Pemetrexed/carboplatin plus gefitinib as a first-line treatment for EGFR-mutant advanced nonsmall cell lung cancer: a Bayesian network meta-analysis by Zhonghan Zhang, Kangmei Zeng, Shen Zhao, Yuanyuan Zhao, Xue Hou, Fan Luo, Feiteng Lu, Yaxiong Zhang, Ting Zhou, Yuxiang Ma, Yunpeng Yang, Wenfeng Fang, Yan Huang, Li Zhang and Hongyun Zhao in Therapeutic Advances in Medical Oncology