Abstract
Apoptosis has emerged as a primary cause of tendinopathy. CD44 signaling pathways exert anti-apoptotic and -inflammatory effects on tumor cells, chondrocytes, and fibroblast-like synoviocytes. The aim of this study was to examine the association among CD44, apoptosis, and inflammation in tendinopathy. Expression of CD44 and apoptotic cell numbers in tendon tissue from patients with long head of biceps (LHB) tendinopathy were determined according to the histological grades of tendinopathy. Primary tenocytes from Achilles tendon of Sprague–Dawley rats 1 week after collagenase injection were cultured with an antagonizing antibody against CD44. Treatment responses were determined by evaluating cell viability and expression of tendon-related proliferation markers, inflammatory mediators, and apoptosis. The expression of CD44 and apoptosis were positively correlated with the severity of tendinopathy in the human LHB tendinopathy. Furthermore, CD44 expression and apoptotic cells were co-stained in tendinopathic tendon. Blocking the CD44 signaling pathways in rat primary tenocytes by OX-50 induced cell apoptosis and the elevated levels of cleaved caspase-3. Furthermore, they had decreased cell viability and expression of collagen type I, type III, tenomodulin, and phosphorylated AKT. In contrast, there were elevated levels of inflammatory mediators, including interleukin (IL)-1β, IL-6, tumor necrosis factor-α, cyclooxygenase-2, and phosphorylated NF-κB, as well as matrix metalloproteinase (MMP) family members including MMP-1, -3, -9, and -13 in tenocytes upon OX-50 treatment. This study is the first to demonstrate the association of CD44 and apoptosis in tendinopathy. Our data imply that CD44 may play a role in tendinopathy via regulating apoptosis, inflammation, and extracellular matrix homeostasis.
Keywords: apoptosis, tendon, cytokine induction, inflammation, matrix metalloproteinase (MMP), CD44, tendinopathy
Introduction
Tendinopathy is a chronic painful tendon disorder and accounts for 30–50% of musculoskeletal and sports-related problems (1, 2). The pathological features of tendinopathy comprise proliferation of tenocytes, intracellular abnormalities in tenocytes, disruption of collagen fibers, and a subsequent increase in noncollagenous matrix (3). The pathophysiology of tendinopathy remains largely unclear thus far. Nevertheless, the mechanisms that have been proposed include mechanical overload, inflammation, imbalance between matrix metalloproteinases (MMPs)3 and tissue inhibitors of metalloproteinases, and dysregulated apoptosis (1, 4–6).
Excessive apoptosis has long been linked to a spectrum of degenerative disorders, including osteoarthritis (OA) and tendinopathy (6–9). Hypoxia, mechanical loading, inflammation, and genetic predisposition are the risk factors that may lead to enhanced apoptotic tenocyte death (10–12). Tenocytes play a critical role in maintaining homeostasis of extracellular matrix (ECM). A potential association between changes in ECM composition and increased tenocyte apoptosis has been established (13, 14). Therefore, identification of the molecular mechanisms driving dysregulated tenocyte apoptosis is important in understanding the pathophysiology of tendinopathy.
CD44 is a principal cell-surface receptor for hyaluronan (hyaluronic acid (HA)), which is a constituent of the ECM. We have previously demonstrated that CD44 is clearly located at the plasma membranes and cell-cell junctions of the cultured tenocytes and mediates the HA-induced down-regulation of MMP-1 and MMP-3 expression in interleukin (IL)-1β–stimulated tenocytes (15). Growing evidence indicates that signaling through CD44 can induce the anti-apoptotic effects on OA chondrocytes (16). Furthermore, RNAi or mAb targeting CD44 induces apoptosis in various kinds of cancer cells (17–19). However, the association between CD44 and apoptosis in tendinopathy has yet to be clarified. As such, this study was undertaken to address the role of CD44-mediated apoptotic process in tendinopathy. Our hypotheses are as follows: (i) that the expression of CD44 and apoptotic cell number are positively correlated with disease severity in human long head of biceps (LHB) tendinopathy and (ii) that blocking CD44 induces cell apoptosis and the expressions of MMPs, inflammatory mediators, and phosphorylated NF-κB in primary tenocytes from rats following collagenase injection, as well as the down-regulation of tendon-related proliferation marker expression. Our results implicate that CD44-mediated signaling pathways may not only inhibit apoptosis, but also mediate anti-inflammation and maintain ECM homeostasis during tendinopathy.
Results
Increased CD44 expression and enhanced apoptosis in tendon tissue from patients with LHB tendinopathy
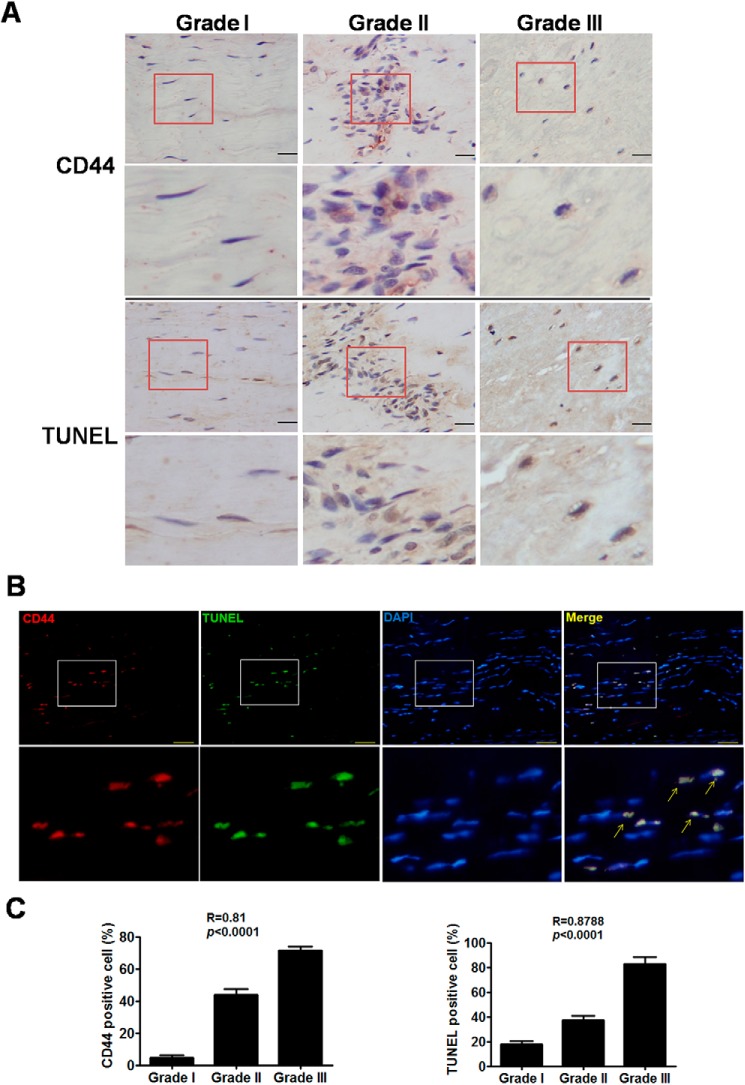
The expression of CD44 was first correlated with apoptosis in the tendon of patients with LHB tendinopathy. Immunohistochemical and TUNEL stainings showed that CD44 and apoptotic cells were detected with increased expression levels and positively correlated with the histological grades of patients during progression of tendinopathy (Fig. 1, A and C). Immunofluorescence and TUNEL stainings demonstrated co-staining of CD44 expression and apoptotic cells in tendon from patients with LHB tendinopathy (Fig. 1B).
Figure 1.
Expression of CD44 and apoptosis in tendon from patients with LHB tendinopathy. A, immunohistochemical staining of CD44 and TUNEL stain in tendon from patients with different grades of LHB tendinopathy. Scale bars, 50 μm in ×400 magnifications. Boxed areas are shown at higher magnification in the panels below them. B, immunofluorescence staining of CD44 (Alexa Fluor 594–stained (red)), TUNEL stain (green), and nucleus (4′,6-diamidino-2-phenylindole–stained (blue)) in tendon tissue from patients with LHB tendinopathy. Scale bars, 50 μm in ×400 magnifications. Boxed areas are shown at higher magnification in the panels below them. Arrows, positivity for both CD44 and apoptotic cells. C, calculated CD44- and TUNEL-positive cells in tendon from patients with different grades of LHB tendinopathy. Values are the mean ± S.E. (error bars). R, Spearman correlation coefficients. Results are representative of at least two independent experiments.
Tendinopathic and myofibroblastic characteristics and increased CD44 expression in primary tenocytes from rats' Achilles tendon treated with collagenase
Tenocytes from the collagenase-treated rat tendon at week 1 expressed higher levels of IL-1β, IL-6, cyclooxygenase-2 (COX-2), MMP-1, MMP-3, α-smooth muscle actin expression (α-SMA), and CD44 but lower levels of collagen type I (COL1A1) and type III COL3A1 than those from normal rat tendon (Fig. 2), indicating that these cells were under both tendinopathic and myofibroblastic conditions (p < 0.05 for all results; Fig. 2).
Figure 2.
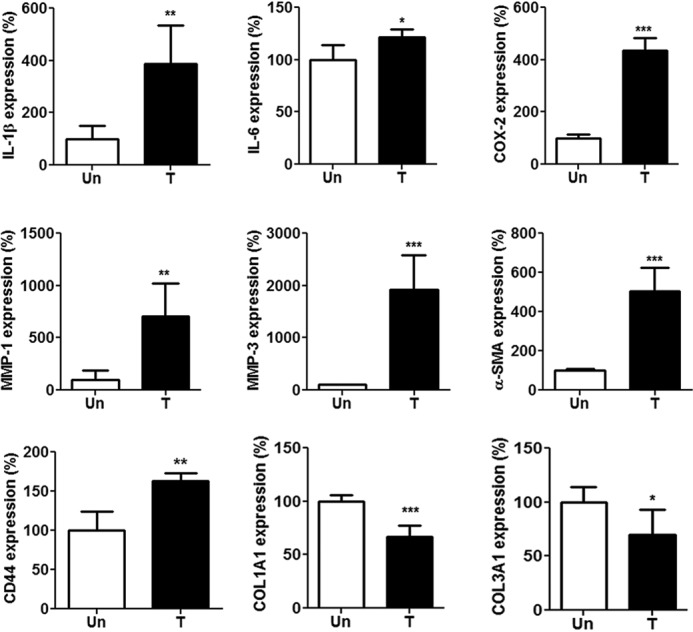
Expression of inflammation-related mediators, MMPs, α-SMA, CD44, and tendon-related markers in rat primary tenocytes. Tenocytes were isolated from Achilles tendons of Sprague–Dawley rats treated (T) or untreated (Un) with intratendinous injection of collagenase I (10 lambda/rat, 1.5 mg/lambda) for 1 week. Shown is expression of IL-1β, IL-6, COX-2, MMP-1, MMP-3, α-SMA, CD44, COL1A1, and COL3A1 in primary tenocytes, as determined by qRT-PCR. Values are the mean ± S.E. (error bars) (n = 5). Results are representative of at least two independent experiments.
Blocking CD44 induces cell apoptosis of primary tenocytes from rats following collagenase injection
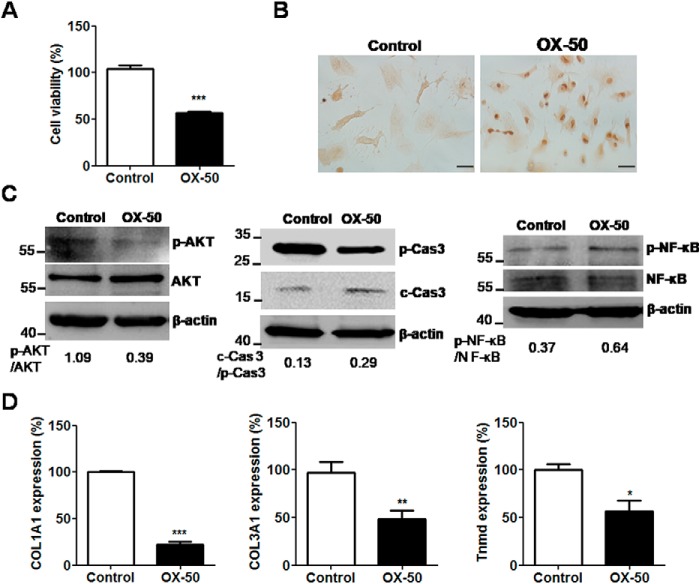
To determine and clarify the role of CD44-associated apoptosis in tendinopathy, OX-50, a CD44-antagonizing antibody, was added to the cell culture of primary tenocytes from rats following collagenase injection. After treatment with OX-50 (10 μg/ml) for 24 h, cell viability was significantly reduced, as determined by WST-8 analysis (p < 0.001; Fig. 3A). Furthermore, TUNEL-positive cells were observed in the OX-50–treated tenocytes for 24 h (Fig. 3B). Furthermore, a reduction in phosphorylated AKT levels and, in contrast, induction in the ratios of cleaved caspase-3 to pro-caspase-3 and phosphorylated NF-κB to NF-κB in the OX-50–treated tenocytes were simultaneously detected, as determined by immunoblotting (Fig. 3C).
Figure 3.
Cell viability, apoptosis, and expression of tendon-related markers in rat primary tenocytes via CD44 blockage. Tenocytes were isolated from Achilles tendons of Sprague–Dawley rats treated with ultrasound-guided intratendinous injection of collagenase I (20 lambda/rat, 1.5 mg/lambda) for 1 week. A, cell viability was determined by WST-8 assay after OX-50 treatment (10 μg/ml) for 24 h and expressed as the percentages of surviving cells relative to those in control cells. Each value shown represents the mean ± S.E. (error bars) (n = 4). B, TUNEL staining in tenocytes after OX-50 treatment (10 μg/ml) for 24 h. Scale bars, 100 μm in ×200 magnifications. C, expression of phospho-AKT, AKT, cleaved caspase-3 (c-Cas 3), pro-caspase-3 (p-Cas 3), phospho-NF-κB, NF-κB, and β-actin in tenocytes after OX-50 treatment (10 μg/ml) for 30 min (p-AKT and AKT), 8 h (cleaved caspase-3) and 60 min (p-NF-κB and NF-κB), as determined by immunoblotting. D, expression of COL1A1, COL3A1, and tnmd in tenocytes after OX-50 treatment (10 μg/ml) for 24 h, as determined by qRT-PCR. Values are the mean ± S.E. (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001. Results are representative of at least three independent experiments.
Blocking CD44 regulates expression of tendon-related markers, MMPs, and inflammatory mediators
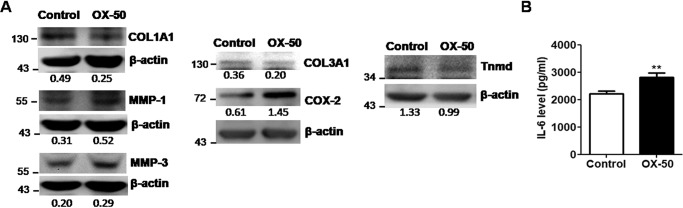
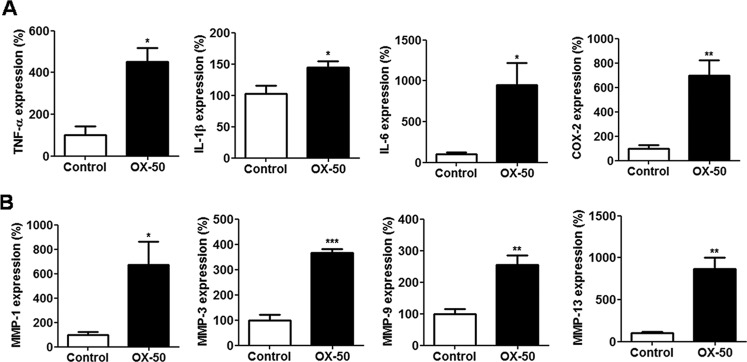
We further investigated the expression of COL1A1, COL3A1, and tenomodulin (tnmd), which are related molecules produced by proliferative tenocytes in OX-50–treated cells. Their mRNA and protein expression levels were significantly down-regulated, as determined by quantitative RT-PCR (qRT-PCR) (p < 0.05 for all results; Fig. 3D) and immunoblotting (see Fig. 5A). In contrast, the mRNA expression of MMP family members, including MMP-1, -3, -9, and -13, and inflammation-related genes, including IL-1beta, IL-6, TNFalpha, and COX-2, was concomitantly up-regulated in OX-50–treated tenocytes, as measured by qRT-PCR (p < 0.05 for all results; Fig. 4). Furthermore, the protein expression levels of MMP-1 and -3, COX-2, and IL-6 were increased in OX-50–treated tenocytes compared with those in the control cells, as determined by immunoblotting and ELISA analyses (Fig. 5B). However, TNFα and IL-1β levels were undetectable between the control and OX-50–treated cells by ELISA tests.
Figure 5.
Protein expression of inflammation-related mediators, MMPs, and tendon-related markers in rat primary tenocytes via CD44 blockage. A, expression of COL1A1, COL3A1, tnmd, COX-2, MMP-1, and MMP-3 in OX-50–treated rat primary tenocytes for 72 h, as determined by immunoblotting. B, expression of IL-6 in the supernatant of OX-50–treated rat primary tenocytes for 72 h, as determined by ELISA. Results are representative of at least two independent experiments. See the legend to Fig. 3 for other definitions. Error bars, S.E.
Figure 4.
mRNA expression of inflammation-related mediators and MMPs in rat primary tenocytes via CD44 blockage. A, expression of TNFα, IL-1β, IL-6, and COX-2 in OX-50–treated rat primary tenocytes for 6 h (TNFα and IL-1β) and 24 h (IL-6 and COX-2), as determined by qRT-PCR. Values are the mean ± S.E. (error bars) (n = 3). B, expression of MMP-1, -3, -9, and -13 in OX-50–treated rat primary tenocytes for 24 h, as determined by qRT-PCR. Values are the mean ± S.E. (n = 3). Results are representative of at least three independent experiments. See the legend to Fig. 3 for other definitions.
Discussion
This is the first study to demonstrate the association of CD44 and apoptosis in tendinopathy. As the severity of tendinopathy progressed, the expression of CD44 and apoptosis correlatively increased in patients with LHB tendinopathy. CD44 expression and TUNEL-positive cells were co-stained in tendinopathic tendon. Blocking the CD44 signaling pathways using OX-50 significantly induced cell apoptosis and the levels of cleaved caspase-3 in tenocytes from rat Achilles tendons following collagenase injection. Moreover, OX-50 decreased tenocyte viability and the expression of COL1A1, COL3A1, tnmd, and phosphorylated AKT. On the other hand, there were elevated levels of inflammatory mediator (including IL-1β, IL-6, TNFα, COX-2, and phosphorylated NF-κB) as well as MMP family member (including MMP-1, -3, -9, and -13) expression in tenocytes upon OX-50 treatment.
HA is known to regulate various cellular mechanisms through interacting with CD44, including cell adhesion, growth, survival, migration, and differentiation. It has been documented that enrichment of HA to the chondrocyte surface by binding to the CD44 receptor induces cell proliferation and matrix synthesis. Similarly, it has been found that HA reduces IL-1β–stimulated MMP-13 expression and attenuates oxidative stress-induced apoptosis in human OA chondrocytes through engaging with CD44 (20). The anti-apoptotic effects of HA on anti-FAS–induced apoptosis of chondrocytes from OA patients are abrogated by anti-CD44 antibodies (16). Short-hairpin RNA targeting CD44 inhibits cell proliferation and promotes apoptosis of colon carcinoma cells, whereas anti-CD44 small interference RNA impairs chronic lymphocytic leukemia (CLL) cell viability, whereas engagement of CD44 by HA protects CLL cells from apoptosis (17, 18). Moreover, anti-CD44 antibody could induce apoptosis in chondrosarcoma cell line SW1353 (19). Various cancer-related studies also demonstrated that CD44 promoted cell survival and suppressed apoptosis through AKT signaling and HA synthesis (21–23). These observations correlate with our findings that increased expression of CD44 is related to enhanced apoptosis during progression of LHB tendinopathy (Fig. 1). Furthermore, the OX-50–treated rat tenocytes from collagenase-injected Achilles tendons showed higher cleaved caspase-3 level and apoptotic cell number than control counterparts. In addition, they had down-regulated phosphorylated AKT expression and reduced cell viability in response to OX-50 treatment (Fig. 3). To our knowledge, this is the first study to reveal the anti-apoptotic role of CD44 in tendinopathy.
Proinflammatory cytokines such as IL-1β have been recognized as the main initiators of tendinopathy and can be released by tenocytes in response to mechanical overload, resulting in the production of proinflammatory mediators such as COX-2, MMP-1, and MMP-3 through an NF-κB–mediated mechanism (24). IL-1beta not only induces IL-6, IL-1beta, COX-2, MMP-1, MMP-3, and MMP-13 gene expression in human tendon cells, but also reduces the expression of tnmd and type I and III collagen in injured tendon–derived cells (25, 26). Interestingly, administration of a monoclonal anti-CD44 antibody IRAWB14 exacerbates the inflammatory signs in mice with proteoglycan-induced arthritis (27). In our results, blocking CD44 using OX-50 down-regulates the expression of COL1A1, COL3A1, and tnmd (Fig. 3D), but up-regulates the expression of inflammatory mediators, such as IL-1β, IL-6, TNFα, and COX-2, in tenocytes from rats with Achilles tendinopathy (Fig. 4). In particular, the phosphorylated expression of NF-κB was up-regulated in OX-50–treated tenocytes (Fig. 3C). Furthermore, IL-1β had been reported to stimulate apoptosis in tendons (28). In our results, increased expression of inflammatory cytokines, especially IL-1beta, was shown together with increased apoptosis in tenocytes from rats with tendinopathy after OX-50 treatment. Accordingly, our findings suggest that a positive feedback loop may exist as an IL-1–mediated mechanism through NF-κB activation in tendon diseases, and this loop can be broken down by disturbing the CD44 signaling pathways.
MMPs, a large family of endopeptidases, have been reported be involved in tendinopathy, pain generation, and even tendon rupture due to their cleavage of ECM proteins (29, 30). MMPs has been proposed to be activated by inflammatory cytokines, especially IL-1β (28). The MMP-1, MMP-9, and MMP-13 expression levels are significantly higher in the human torn rotator cuffs than in the intact rotator cuffs (31). MMP-3 is involved in the failure of normal matrix remodeling and in Achilles tendinopathy (32, 33). Moreover, activation of CD44 using HA can down-regulate IL-6–induced MMP-13 expression in human OA osteoblasts (34). In our results, blocking CD44 using OX-50 down-regulated MMP-1, -3, -9, and -13 expression in tenocytes from rat Achilles tendons following collagenase injection (Fig. 4B). Our findings implied that CD44 might be involved in the ECM homeostasis via regulating MMP expression.
This study has some limitations. First, an in vivo gene regulatory system should be applied to validate the CD44-induced anti-apoptotic effect in a tendinopathy animal model. Further studies using viral vectors to overexpress the CD44 gene in the tendinopathic animal model will be performed to confirm the role of CD44 in tendinopathy. Second, tenocytes suitable for a CD44 inhibition study should be primarily cultured from the tendinopathic tendons. However, tenocytes isolated from the tendinopathic tendons in a mature rat model are too old (usually more than 14 weeks old) to be primarily cultured according to our pilot study.4 An alternative way was to use the primary tenocytes isolated from the rat Achilles tendon, which were injected with collagenase for 1 week. These tenocytes have higher expression levels of inflammatory mediators, MMP-1, MMP-3, α-SMA, and CD44 but lower levels of COL1A1 and COL1A3, demonstrating both tendinopathic and myofibroblastic characteristics (Fig. 2). Therefore, we suggested that this is an appropriate model to study the association of CD44 and tendinopathy.
In conclusion, by using tendon specimens from patients with LHB tendinopathy and tenocytes from rats following collagenase injection, we demonstrated that blocking the CD44 signal pathway induces tenocyte apoptosis, decreases tenocyte viability and proliferation, and increases inflammatory cytokines and MMP expression. CD44 might play a role in tendinopathy via regulating apoptosis, inflammation, and ECM homeostasis. These findings provide a new approach to treating patients with tendinopathy.
Materials and methods
Ethics statement
All of the experimental rats were purchased from the Animal Center at National Cheng Kung University, and the following animal experiments were done strictly in accordance with protocols approved by the Institutional Animal Care and Use Committee of National Cheng Kung University (approval 105-156). The human study was approved by the Institutional Review Board of National Cheng Kung University Hospital (approval A-ER-106-063) and was done strictly in accordance with the approved guidelines. Informed consent was obtained from all patients. The human study abides by the Declaration of Helsinki principles.
LHB specimens and histopathological grades
Fourteen consecutive patients (6 men, 8 women; mean age, 62.1 years; age range, 52–76 years) undergoing arthroscopic treatment for a rotator cuff tear and LHB tendinopathy at our university hospital were recruited. The tenodesis or tenotomy of LHB was done, and the pathological tendon area was harvested for the following histological examinations. The specimens were fixed in fresh 4% paraformaldehyde for 16–24 h at 4 °C and then subsequently dehydrated, paraffin-embedded, and longitudinally sectioned. Sequential 4-μm sections were stained with hematoxylin and eosin and examined under a light microscope for changes in tenocyte morphology and collagen bundle characteristics. Based on our previous study (35), a modified semiquantitative method scored each factor (tenocyte morphology and collagen bundle characteristics) on a four-point scale. According to the sum of scores, the tendinopathy was graded as 0–3 (0, ≤2, 3–4, or ≥5 points). The histological grading was assessed by two observers unaware of the clinical and arthroscopic conditions of the patients. If an inconsistency existed, the field was reassessed, and a final score was decided upon.
Immunohistochemical, immunoblotting, and immunofluorescence assessments
The sections were deparaffinized in xylene, dehydrated in alcohol, epitope-unmasked by heating, washed with H2O2 in PBS, and stained with antibodies against CD44 (1:100; #37259, Cell Signaling Technology), in combination with the chromogen 3-amino-9-ethylcarbazole (Zymed Laboratories Inc.) or subjected to the DeadEndTM colorimetric TUNEL system (#G7360, Promega). Cell lysates of OX-50 (10 μg/ml; #GTX76383, GeneTex)–treated tenocytes were subjected to immunoblot analyses with antibodies against phosphorylated AKT (1:1000; #9271, Cell Signaling), total AKT (1:1000; #9272, Cell Signaling), phosphorylated NF-κB (1:1000; #3033, Cell Signaling), total NF-κB (1:200; #SC-109, Santa Cruz Biotechnology, Inc.), cleaved caspase-3 (1:500; #9664, Cell Signaling), caspase-3 (1:1000; #9662, Cell Signaling), MMP-1 (1:1000; #GTX00674, GeneTex), MMP-3 (1:1000; #14351, Cell Signaling), COL1A1 (1:1000; #GTX112731, GeneTex), COL3A1 (1:1000, #GTX26310, GeneTex), tenomodulin (1:1000; #bs-7525R, Bioss), and COX-2 (1:1000; #sc-1747, Santa Cruz Biotechnology) in combination with a horseradish peroxidase–conjugated secondary antibody (1:10,000; Jackson ImmunoResearch) and quantitative control anti-β-actin antibodies (1:10,000; #A3854, Sigma-Aldrich). The signal intensity in immunoblot analyses was further quantitated using ImageJ software (National Institutes of Health). Cells with immunohistochemical stainings of TUNEL and CD44 were identified and counted in five high-power fields (×400) to determine the average percentages of TUNEL and CD44-positive cells corresponding to hematoxylin-stained total cells. For CD44 and TUNEL dual-immunofluorescence staining, paraffin-embedded sections receiving similar pretreatment were subjected to the DeadEndTM fluorometric TUNEL system (#G3250, Promega) and incubated with antibodies against CD44 (1:100; #37259, Cell Signaling), followed by Alexa Fluor 594–conjugated secondary antibodies (1:200; #A-11012, Thermo Fisher Scientific).
Primary culture of tenocytes from collagenase-injected rat Achilles tendon
Male Sprague–Dawley rats (4–6 weeks old) were purchased from LASCO (Taipei, Taiwan) and housed in the specific pathogen-free condition with a light/dark cycle of 12 h in the Animal Center of National Cheng Kung University (Tainan, Taiwan). Twenty-four rats were treated with ultrasound-guided intratendinous injection of collagenase I (1.5 mg/lambda, 10 lambda injection/rat, Sigma-Aldrich). One week after the index procedure, Achilles tendons were harvested after the rats had been killed with an overdose of isoflurane. The preparation of tendon samples and tenocyte culture methods are the same as in our previous study (15). Well-characterized second-to-fourth passage cells were used in this experiment; they showed no phenotypic drift of major tenocyte markers, such as cell shape (elongated and spindle-shaped with apposition), and tenomodulin expression identified using anti-tenomodulin antibody (Santa Cruz Biotechnology) as described previously (15). Because low-density plating and subsequent colony formation were the standard methods to isolate the tendon progenitor cells (36) and not used in our primary culture, the cell culture system we utilized contained mostly the differentiated tenocytes but not the tendon progenitor cells. Furthermore, tenocytes were identified by their tendinopathic and myofibroblastic natures identified by detecting proliferative markers (COL1A1 and COL1A3), inflammatory mediators (IL-1β, IL-6, and COX-2), MMP-1, MMP-3, CD44, and α-SMA with qRT-PCR (Fig. 2).
Cell viability and apoptosis analyses
After treatment with OX-50 for 24 h, tenocytes were analyzed using WST-8 assay with measurement of absorbance at 450 nm. Cell viability was represented as the percentages normalized with the average WST-8 values of the control group. The DeadEndTM colorimetric TUNEL system (#G7360, Promega) was used for detecting apoptotic cells in OX-50–treated tenocytes according to the standard protocol.
qRT-PCR and ELISA
Total RNA from tenocytes was isolated with TRIzol reagents (#15596018, Invitrogen), and cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (#4368813, Applied Biosystems) for qRT-PCR by SYBR® Green PCR kit (#208054, Qiagen) with primer pairs specific to COL1A1 (forward, 5′-ATCAGCCCAAACCCCAAGGAGA-3′; reverse, 5′-CGCAGGAAGGTCAGCTGGATAG-3′), COL3A1 (forward, 5′-TGATGGGATCCAATGAGGGAGA-3′; reverse, 5′-GAGTCTCATGGCCTTGCGTGTTT-3′), tnmd (forward, 5′-CGCCACACCAGACAAGCA-3′; reverse, 5′-CCAGCATTGGGTCAAATTCA-3′), MMP-1 (forward, 5′-CATACTGTACTGAGAGGATTCCCCACAGA-3′; reverse, 5′-ACATCATCAACTTTATCGTCAATTCCAGG-3′), MMP-3 (forward, 5′-TCCCAGGAAAATAGCTGAGAACTT-3′; reverse, 5′-GAAACCCAAATGCTTCAAAGACA-3′), MMP-9 (forward, 5′-TACTTTGGAAACGCAAATGG-3′; reverse, 5′-GTGTAGAGATTCTCACTGGG-3′), MMP-13 (forward, 5′-CGTGTGGAGTTATGATGATG-3′; reverse, 5′-ATCTACTTTGTCGCCAATTC-3′), IL-1β (forward, 5′-CACCTCTCAAGCAGAGCACAG-3′; reverse, 5′-GGGTTCCATGGTGAAGTCAAC-3′), IL-6 (forward, 5′-CGAAAGTCAACTCCATCTGCC-3′; reverse, 5′-GGCAACTGGCTGGAAGTCTCT-3′), TNFα (forward, 5′-TGCCTCAGCCTCTTCTCATTCCT-3′; reverse, 5′-GAGCCCATTTGGGAACTTCTCC-3′), COX-2 (forward, 5′-CACGGACTTGCTCACTTTGTT-3′; reverse, 5′-AAGCGTTTGCGGTACTCATT-3′), α-SMA (forward, 5′-ATCCGATAGAACACGGCATCAT-3′; reverse, 5′-CATGGCAGGGACATTGAAGG-3′), CD44 (forward, 5′-AAGACATCGATGCCTCAAAC-3′; reverse, 5′-CTCCAGTAGGCTGTGAAGTG-3′), and GAPDH (forward, 5′-CCATCTTCCAGGAGCGAGATC-3′; reverse, 5′-GCCTTCTCCATGGTGGTGAA-3′). The comparative Ct method was used to calculate the relative abundance of each gene compared with GAPDH expression.
Supernatants of tenocytes were subjected to ELISA for detecting the IL-6, TNFα, and IL-1β levels (R&D Systems).
Statistical analysis
Data are expressed as mean ± S.E. Statistical significance between two groups was analyzed using Student's t test because of the normal distribution of our data that was assessed using the SigmaPlot software). The correlation among CD44 expression, TUNEL-positive cell number, and the different histological grades of tendinopathy were analyzed using the Spearman correlation rank test. p < 0.05 was considered significant.
Author contributions
P.-T. W., I.-M. J., and S.-Y. C. conceptualization; P.-T. W., W.-R. S., C.-L. L., C.-H. M., and I.-M. J. resources; P.-T. W., W.-R. S., C.-L. L., J.-L. H., and S.-Y. C. data curation; P.-T. W., C.-L. L., J.-L. H., C.-L. W., and S.-Y. C. formal analysis; P.-T. W., W.-R. S., I.-M. J., and S.-Y. C. supervision; P.-T. W., I.-M. J., and S.-Y. C. funding acquisition; P.-T. W., W.-R. S., C.-L. L., J.-L. H., C.-H. M., C.-L. W., L.-C. K., I.-M. J., and S.-Y. C. validation; P.-T. W., W.-R. S., J.-L. H., C.-H. M., C.-L. W., I.-M. J., and S.-Y. C. investigation; P.-T. W., C.-L. L., and S.-Y. C. methodology; P.-T. W., I.-M. J., and S.-Y. C. writing-original draft; P.-T. W., I.-M. J., and S.-Y. C. project administration; P.-T. W., I.-M.J., and S.-Y. C. writing-review and editing; L.-C. K. software.
Acknowledgments
We are grateful to Skeleton Materials and Bio-compatibility Core Lab, Research Center of Clinical Medicine, National Cheng Kung University Hospital, for the assistance of this study. We are also grateful to I-Ting Lee and Yu-Ying Chen for their excellent assistance.
This work was supported by Ministry of Science and Technology, Taiwan, Grants MOST 106–2622-E-006–029-CC2, MOST 108–2314-B-006–037-MY3, MOST 107–2314-B-006–065-MY3, and MOST 105–2622-E-650–001-CC2 and National Cheng Kung University Hospital (Tainan, Taiwan) Grants NCKUH-10802005, and NCKUEDA 10605. The authors declare that they have no conflicts of interest with the contents of this article.
P.-T. Wu, W.-R. Su, C.-L. Li, J.-L. Hsieh, C.-H. Ma, C.-L. Wu, L.-C. Kuo, I-M. Jou, and S.-Y. Chen, unpublished data.
- MMP
- matrix metalloproteinase
- OA
- osteoarthritis
- ECM
- extracellular matrix
- HA
- hyaluronic acid
- IL
- interleukin
- LHB
- long head of biceps
- TUNEL
- terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
- α-SMA
- α-smooth muscle actin
- tnmd
- tenomodulin
- qRT-PCR
- quantitative RT-PCR
- TNF
- tumor necrosis factor
- CLL
- chronic lymphocytic leukemia
- COX-2
- cyclooxygenase-2
- COL1A1 and COL3A1
- collagen type I and III, respectively.
References
- 1. Riley G. (2005) Chronic tendon pathology: molecular basis and therapeutic implications. Expert Rev. Mol. Med. 7, 1–25 10.1017/S1462399405008963 [DOI] [PubMed] [Google Scholar]
- 2. Lui P. P., Maffulli N., Rolf C., and Smith R. K. (2011) What are the validated animal models for tendinopathy? Scand. J. Med. Sci. Sports 21, 3–17 10.1111/j.1600-0838.2010.01164.x [DOI] [PubMed] [Google Scholar]
- 3. Longo U. G., Ronga M., and Maffulli N. (2009) Achilles tendinopathy. Sports Med. Arthrosc. 17, 112–126 10.1097/JSA.0b013e3181a3d625 [DOI] [PubMed] [Google Scholar]
- 4. Arnoczky S. P., Tian T., Lavagnino M., and Gardner K. (2004) Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J. Orthop. Res. 22, 328–333 10.1016/S0736-0266(03)00185-2 [DOI] [PubMed] [Google Scholar]
- 5. Del Buono A., Battery L., Denaro V., Maccauro G., and Maffulli N. (2011) Tendinopathy and inflammation: some truths. Int. J. Immunopathol. Pharmacol. 24, 45–50 10.1177/03946320110241S209 [DOI] [PubMed] [Google Scholar]
- 6. Yuan J., Murrell G. A., Wei A. Q., and Wang M. X. (2002) Apoptosis in rotator cuff tendonopathy. J. Orthop. Res. 20, 1372–1379 10.1016/S0736-0266(02)00075-X [DOI] [PubMed] [Google Scholar]
- 7. Lotz M., Hashimoto S., and Kühn K. (1999) Mechanisms of chondrocyte apoptosis. Osteoarthritis Cartilage 7, 389–391 10.1053/joca.1998.0220 [DOI] [PubMed] [Google Scholar]
- 8. Pearce C. J., Ismail M., and Calder J. D. (2009) Is apoptosis the cause of noninsertional Achilles tendinopathy? Am. J. Sports Med. 37, 2440–2444 10.1177/0363546509340264 [DOI] [PubMed] [Google Scholar]
- 9. Lian Ø., Scott A., Engebretsen L., Bahr R., Duronio V., Khan K. (2007) Excessive apoptosis in patellar tendinopathy in athletes. Am. J. Sports Med. 35, 605–611 10.1177/0363546506295702 [DOI] [PubMed] [Google Scholar]
- 10. Benson R. T., McDonnell S. M., Knowles H. J., Rees J. L., Carr A. J., and Hulley P. A. (2010) Tendinopathy and tears of the rotator cuff are associated with hypoxia and apoptosis. J. Bone Joint Surg. Br. 92, 448–453 10.1302/0301-620X.92B3.23074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsieh J. L., Jou I. M., Wu C. L., Wu P. T., Shiau A. L., Chong H. E., Lo Y. T., Shen P. C., and Chen S. Y. (2018) Estrogen and mechanical loading-related regulation of estrogen receptor-β and apoptosis in tendinopathy. PLoS One 13, e0204603 10.1371/journal.pone.0204603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nell E. M., van der Merwe L., Cook J., Handley C. J., Collins M., and September A. V. (2012) The apoptosis pathway and the genetic predisposition to Achilles tendinopathy. J. Orthop. Res. 30, 1719–1724 10.1002/jor.22144 [DOI] [PubMed] [Google Scholar]
- 13. Egerbacher M., Arnoczky S. P., Caballero O., Lavagnino M., and Gardner K. L. (2008) Loss of homeostatic tension induces apoptosis in tendon cells: an in vitro study. Clin. Orthop. Relat. Res. 466, 1562–1568 10.1007/s11999-008-0274-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chuen F. S., Chuk C. Y., Ping W. Y., Nar W. W., Kim H. L., and Ming C. K. (2004) Immunohistochemical characterization of cells in adult human patellar tendons. J. Histochem. Cytochem. 52, 1151–1157 10.1369/jhc.3A6232.2004 [DOI] [PubMed] [Google Scholar]
- 15. Wu P. T., Kuo L. C., Su F. C., Chen S. Y., Hsu T. I., Li C. Y., Tsai K. J., and Jou I. M. (2017) High-molecular-weight hyaluronic acid attenuated matrix metalloproteinase-1 and -3 expression via CD44 in tendinopathy. Sci. Rep. 7, 40840 10.1038/srep40840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lisignoli G., Grassi F., Zini N., Toneguzzi S., Piacentini A., Guidolin D., Bevilacqua C., and Facchini A. (2001) Anti-Fas-induced apoptosis in chondrocytes reduced by hyaluronan: evidence for CD44 and CD54 (intercellular adhesion molecule 1) involvement. Arthritis Rheum. 44, 1800–1807 [DOI] [PubMed] [Google Scholar]
- 17. Park Y. S., Huh J. W., Lee J. H., and Kim H. R. (2012) shRNA against CD44 inhibits cell proliferation, invasion and migration, and promotes apoptosis of colon carcinoma cells. Oncol. Rep. 27, 339–346 10.3892/or.2011.1532 [DOI] [PubMed] [Google Scholar]
- 18. Fedorchenko O., Stiefelhagen M., Peer-Zada A. A., Barthel R., Mayer P., Eckei L., Breuer A., Crispatzu G., Rosen N., Landwehr T., Lilienthal N., Möllmann M., Montesinos-Rongen M., Heukamp L., Dürig J., et al. (2013) CD44 regulates the apoptotic response and promotes disease development in chronic lymphocytic leukemia. Blood 121, 4126–4136 10.1182/blood-2012-11-466250 [DOI] [PubMed] [Google Scholar]
- 19. Yoshida M., Yasuda T., Hiramitsu T., Ito H., and Nakamura T. (2008) Induction of apoptosis by anti-CD44 antibody in human chondrosarcoma cell line SW1353. Biomed. Res. 29, 47–52 10.2220/biomedres.29.47 [DOI] [PubMed] [Google Scholar]
- 20. Mongkhon J. M., Thach M., Shi Q., Fernandes J. C., Fahmi H., and Benderdour M. (2014) Sorbitol-modified hyaluronic acid reduces oxidative stress, apoptosis and mediators of inflammation and catabolism in human osteoarthritic chondrocytes. Inflamm. Res. 63, 691–701 10.1007/s00011-014-0742-4 [DOI] [PubMed] [Google Scholar]
- 21. Liu S., and Cheng C. (2017) Akt signaling is sustained by a CD44 splice isoform-mediated positive feedback loop. Cancer Res. 77, 3791–3801 10.1158/0008-5472.CAN-16-2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anand V., Khandelwal M., Appunni S., Gupta N., Seth A., Singh P., Mathur S., and Sharma A. (2019) CD44 splice variant (CD44v3) promotes progression of urothelial carcinoma of bladder through Akt/ERK/STAT3 pathways: novel therapeutic approach. J. Cancer Res. Clin Oncol. 145, 2649–2661 10.1007/s00432-019-03024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song J. M., Im J., Nho R. S., Han Y. H., Upadhyaya P., Kassie F.. Hyaluronan-CD44/RHAMM interaction-dependent cell proliferation and survival in lung cancer cells. Mol. Carcinog. 58, 321–333 10.1002/mc.22930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shakibaei M., Buhrmann C., and Mobasheri A. (2011) Anti-inflammatory and anti-catabolic effects of TENDOACTIVE(R) on human tenocytes in vitro. Histol. Histopathol. 26, 1173–1185 10.14670/HH-26.1173 [DOI] [PubMed] [Google Scholar]
- 25. Tsuzaki M., Guyton G., Garrett W., Archambault J. M., Herzog W., Almekinders L., Bynum D., Yang X., and Banes A. J. (2003) IL-1β induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1β and IL-6 in human tendon cells. J. Orthop. Res. 21, 256–264 10.1016/S0736-0266(02)00141-9 [DOI] [PubMed] [Google Scholar]
- 26. Zhang K., Asai S., Yu B., and Enomoto-Iwamoto M. (2015) IL-1β irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem. Biophys. Res. Commun. 463, 667–672 10.1016/j.bbrc.2015.05.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mikecz K., Dennis K., Shi M., and Kim J. H. (1999) Modulation of hyaluronan receptor (CD44) function in vivo in a murine model of rheumatoid arthritis. Arthritis Rheum. 42, 659–668 [DOI] [PubMed] [Google Scholar]
- 28. Mobasheri A., and Shakibaei M. (2013) Is tendinitis an inflammatory disease initiated and driven by pro-inflammatory cytokines such as interleukin 1β? Histol. Histopathol. 28, 955–964 10.14670/HH-28.955 [DOI] [PubMed] [Google Scholar]
- 29. Del Buono A., Oliva F., Longo U. G., Rodeo S. A., Orchard J., Denaro V., and Maffulli N. (2012) Metalloproteases and rotator cuff disease. J. Shoulder Elbow Surg. 21, 200–208 10.1016/j.jse.2011.10.020 [DOI] [PubMed] [Google Scholar]
- 30. Ji R. R., Xu Z. Z., Wang X., and Lo E. H. (2009) Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol. Sci. 30, 336–340 10.1016/j.tips.2009.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lakemeier S., Braun J., Efe T., Foelsch C., Archontidou-Aprin E., Fuchs-Winkelmann S., Paletta J. R., and Schofer M. D. (2011) Expression of matrix metalloproteinases 1, 3, and 9 in differing extents of tendon retraction in the torn rotator cuff. Knee Surg. Sports Traumatol. Arthrosc. 19, 1760–1765 10.1007/s00167-010-1367-y [DOI] [PubMed] [Google Scholar]
- 32. Lo I. K., Marchuk L. L., Hollinshead R., Hart D. A., and Frank C. B. (2004) Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am. J. Sports Med. 32, 1223–1229 10.1177/0363546503262200 [DOI] [PubMed] [Google Scholar]
- 33. Raleigh S. M., van der Merwe L., Ribbans W. J., Smith R. K., Schwellnus M. P., and Collins M. (2009) Variants within the MMP3 gene are associated with Achilles tendinopathy: possible interaction with the COL5A1 gene. Br. J. Sports Med. 43, 514–520 10.1136/bjsm.2008.053892 [DOI] [PubMed] [Google Scholar]
- 34. Hiraoka N., Takahashi K. A., Arai Y., Sakao K., Mazda O., Kishida T., Honjo K., Tanaka S., and Kubo T. (2011) Intra-articular injection of hyaluronan restores the aberrant expression of matrix metalloproteinase-13 in osteoarthritic subchondral bone. J. Orthop. Res. 29, 354–360 10.1002/jor.21240 [DOI] [PubMed] [Google Scholar]
- 35. Wu P. T., Jou I. M., Yang C. C., Lin C. J., Yang C. Y., Su F. C., and Su W. R. (2014) The severity of the long head biceps tendinopathy in patients with chronic rotator cuff tears: macroscopic versus microscopic results. J. Shoulder Elbow Surg. 23, 1099–1106 10.1016/j.jse.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 36. Durgam S., Schuster B., Cymerman A., Stewart A., and Stewart M. (2016) Differential adhesion selection for enrichment of tendon-derived progenitor cells during in vitro culture. Tissue Eng. Part C Methods 22, 801–808 10.1089/ten.tec.2016.0152 [DOI] [PubMed] [Google Scholar]