Abstract
Cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) are two structurally distinct messengers that mobilize the endoplasmic and endolysosomal Ca2+ stores, respectively. Both are synthesized by the CD38 molecule (CD38), which has long been thought to be a type II membrane protein whose catalytic domain, intriguingly, faces to the outside of the cell. Accordingly, for more than 20 years, it has remained unresolved how CD38 can use cytosolic substrates such as NAD and NADP to produce messengers that target intracellular Ca2+ stores. The discovery of type III CD38, whose catalytic domain faces the cytosol, has now begun to clarify this topological conundrum. This article reviews the ideas and clues leading to the discovery of the type III CD38; highlights an innovative approach for uncovering its natural existence; and discusses the regulators of its activity, folding, and degradation. We also review the compartmentalization of cADPR and NAADP biogenesis. We further discuss the possible mechanisms that promote type III CD38 expression and appraise a proposal of a Ca2+-signaling mechanism based on substrate limitation and product translocation. The surprising finding of another enzyme that produces cADPR and NAADP, sterile α and TIR motif–containing 1 (SARM1), is described. SARM1 regulates axonal degeneration and has no sequence similarity with CD38 but can catalyze the same set of multireactions and has the same cytosolic orientation as the type III CD38. The intriguing finding that SARM1 is activated by nicotinamide mononucleotide to produce cADPR and NAADP suggests that it may function as a regulated Ca2+-signaling enzyme like CD38.
Keywords: cyclic ADP ribose (cADPR), CD38, nicotinic acid adenine dinucleotide phosphate (NAADP), signal transduction, calcium intracellular release, protein topology, SARM1, calcium signaling
Introduction
Mobilization of intracellular Ca2+ stores is a universal signaling mechanism that cells employ for responding to a wide range of external stimuli. Ligands, such as hormones, bind to surface receptors and activate production of second messengers in the cytosol, which in turn activate the Ca2+-release mechanisms of the stores and result in cytosolic Ca2+ changes. The first such messenger identified was inositol trisphosphate (IP3),2 which targets the cytosolic portion of its receptor channel in the endoplasmic reticulum (ER) (1, 2). Cells, however, possess multiple Ca2+ stores. Soon after, two other Ca2+ messengers, cyclic ADP-ribose (cADPR) (3, 4) and nicotinic acid adenine dinucleotide phosphate (NAADP) (5, 6), were discovered, with cADPR acting on the ryanodine channel (7, 8) present also in the ER, whereas NAADP targets the Ca2+-releasing mechanisms in the endolysosomes (9, 10). Like IP3, both nucleotide messengers are known to regulate a wide range of physiological functions and in a variety of cell types spanning three biological kingdoms (reviewed in Refs. 11–16), including mediating the adrenergic receptor activation in the salivary gland (17) and the hippocampal mGluR1-dependent long-term potentiation (18), shown recently.
The synthesis enzymes for both nucleotide messengers were soon identified, even though both messengers were unknown molecules that had never been described before. The first enzyme shown to be able to cyclize NAD to cADPR was named ADP-ribosyl cyclase (cyclase) (19, 20). It is a small soluble protein of about 30 kDa found only in Aplysia (mollusks). Soon afterward, sequence comparison revealed that the carboxyl domain (C-domain) of human CD38 was homologous (19). CD38 is a single-pass membrane protein consisting of a short amino tail, a transmembrane segment, and a C-domain (21).
Surprisingly, despite their distinct differences in structure and function, both messengers are produced by the same enzymes, CD38 and the cyclase (22, 23). Both are multifunctional enzymes that not only can cyclize NAD to produce cADPR but also can catalyze a base-exchange reaction to produce NAADP from NADP and nicotinic acid (24–27). Crystallography in combination with mutagenesis identified the catalytic site in the C-domain where NAD binds (Fig. 1) and delineated the mechanism of this multifunctionality (22, 23, 28). CD38 has since remained the only fully characterized enzyme for producing cADPR and NAADP. Although CD157, a glycosylphosphatidylinositol-anchored homolog of CD38, also can produce cADPR from NAD, its activity is much lower than CD38's (29, 30). Both its catalysis and function remain undercharacterized.
Figure 1.
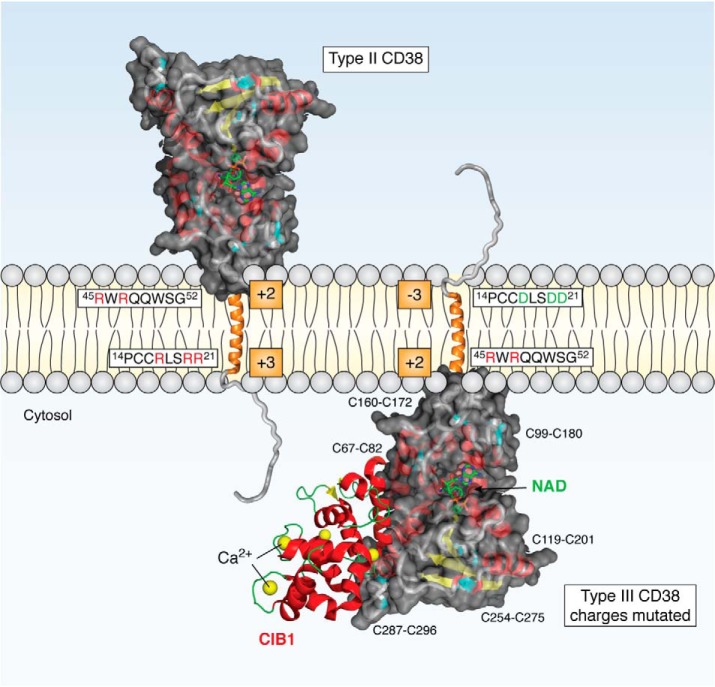
Type II and III CD38. The structure of the catalytic C-domain is based on crystallography (PDB entry 1YH3) and is shown with NAD at the active site. Its six disulfides are indicated and colored cyan. The N-terminal side of the transmembrane segment (orange helix) of type II CD38 has three positive arginines (red R), one more than the C-terminal side. Changing these positive residues to aspartates (green D) converts type II to type III with the catalytic domain facing the cytosol. CIB1 (PDB entry 1XO5) has four Ca2+-binding sites, and it interacts with the cytosolic C-domain of the type III CD38.
Ablation of the CD38 gene in mice produces multiple physiological defects (reviewed in Refs. 11–14), including altering the response of skeletal muscle contractile force to β-adrenergic stimulation (31) and impaired development of astrocytes and oligodendrocytes (32), confirming its biological relevance. Gene ablation also provides strong evidence that CD38 is responsible for producing cADPR and NAADP. For example, in lymphokine-activated killer (LAK) cells, interleukin-8 induced NAADP formation after cADPR production, resulting in Ca2+ changes and activation of cell migration in these cells. Formation of both nucleotide messengers was abrogated if the CD38 gene was deleted, indicating that CD38 is responsible for their synthesis (33).
CD38 has long been thought to be a type II membrane protein expressing mostly on the cell surface and with its C-domain facing outside (Fig. 1) (21). In fact, it was first identified as an antigen on the surface of lymphocytes, the expression of which varies with the developmental stages (34) but, strangely, having no known biological function. Because the C-domain is also the catalytic domain of CD38, this raises a topological puzzle: how a type II protein with its catalytic domain facing outside can use cytosolic substrates, NAD and NADP, to produce messengers that target the cytosolic portions of the Ca2+ release machineries.
Cells are organized and separated into two main compartments, cytosol and extracytosol, by lipid bilayer that also defines the topology of all transmembrane proteins. The lumen of organelles, such as ER and endolysosomes, is topologically contiguous with the outside and constitutes part of the extracytosolic compartment. In Ca2+ signaling, surface receptors with their ligand-binding portions facing outside serve to transduce external signals, such as interleukin-8 described above, into the production of messenger molecules, such as NAADP and cADPR, in the cytosolic compartment.
The topological enigma raised by CD38 being a type II protein has defied resolution for more than 20 years. This has recently begun to be clarified by studies focusing on the membrane orientation of CD38, which have led to the proposal of the existence of type III CD38 with an opposite membrane orientation. With its catalytic domain facing the cytosol, type III CD38 is fully consistent with the regular topology of a Ca2+-signaling enzyme.
Type III CD38
Clues to type III CD38
Based on sequence analyses, the possible existence of type III CD38 with its catalytic domain facing the cytosol was first proposed (11, 35). If correct, it should readily resolve the topological issue. An accepted rule that governs the orientation of single-pass transmembrane proteins is the “positive inside rule” (36), which states that the more positively charged side of the two sides of the transmembrane segment is the one more likely to be in the cytosol. In the case of CD38, the N-terminal side has, overall, about three net positive charges (red R, Fig. 1) and two on the carboxyl side of the transmembrane segment, consistent with CD38 being preferentially expressed as a type II protein. The difference in the charges is minimal, however, less than one charge, suggesting a good possibility of CD38 being expressed also in the opposite orientation, as a type III protein.
Proteins expressing in two opposite membrane orientations are not common; nonetheless, there are many documented cases (15), including prion (37), epoxide hydrolase (38), and ductin (39). Likewise, bacterial EmrE (40) and melanocortin-2 receptor accessory protein (41) both are expressed as a homodimer with the two monomers in opposite orientation. In the case of cytochrome P450 2E1, it is localized to the ER but has opposite transmembrane orientation when transported to the plasma membrane (42).
Cytosolic CD38 is enzymatically active
Whether type III CD38 exists or not is physiologically relevant only if it is enzymatically active. The issue is far from straightforward because of the two critical characteristics of CD38 that must be considered, namely glycosylation and disulfides (Fig. 1). Glycosylation is immaterial for activity because recombinant CD38 with the glycosylation sites mutated and produced in yeast is fully active (43, 44). On the other hand, disulfides are known to be important for the enzymatic activities of CD38. It is not a certainty that the critical disulfides of the C-domain can be formed when it is facing the cytosol in the type III orientation.
The issue of disulfide was directly tested by expressing the catalytic domain in the cytosol. An inducible construct was made by splicing the catalytic domain of CD38 to enhanced green fluorescent protein, which directed the expression of the construct in the cytosol (35). Induction by tetracycline resulted in progressive elevation of cellular cADPR levels, indicating that the catalytic C-domain expressed in the cytosol is active and can cyclize cytosolic NAD to cADPR. A combination of mutational and immunoanalyses showed that the disulfides of the catalytic domain are formed and remain intact in the cytosol (35). Cytosolic proteins with disulfides are not common, but an analysis of disulfide bonds in the Protein Data Bank shows that 298 cytosolic or nuclear proteins contain 509 structurally defined disulfide bonds (45). Indeed, many cytosolic proteins, such as chaperones, glycolytic enzymes, and kinases, are found to contain disulfides (46).
Construction of type III CD38
The next step in verifying the idea of type III CD38 was to actually construct it. If the charges indeed govern the orientation of CD38, mutating the positive residues in its N-terminal tail should result in expression of the CD38 in the type III orientation. This is the case (47, 48). Mutation of even one of the positive residues to aspartate resulted in expression of a significant amount of type III CD38. With all mutated, more than 95% of CD38 was expressed as type III (Fig. 1). It was expressed in the ER instead of the plasma membrane, where the type II CD38 was localized. The membrane orientation of type III CD38 was verified by independent assays (47). As expected, type III CD38 was shown to be nonglycosylated, as the C-domain was facing the cytosol instead of the lumen of the ER, where glycosylation normally occurs (47).
Most importantly, the constructed type III CD38 was enzymatically active with specific activity similar to the WT (47, 48), indicating that the expressed type III CD38 was folded correctly even with its catalytic domain facing the cytosol. In live cells, cellular cADPR contents progressively increased concomitant with the induced expression of the constructed type III CD38 (47).
Endogenous type III CD38
The construction of the active type III CD38 represents a definite proof of principle for the proposal of the type III CD38. Whether it exists naturally needs to be established. The endogenous type III CD38 has so far defied detection because most of the CD38 is expressed preferentially as type II, and it is identical to type III CD38 except in membrane orientation. The assay must thus be topologically specific and highly sensitive. The first and simpler assay is to detect the N-terminal tail of the type III CD38 exposed to the outside of live cells using specific antibodies. The integrity of the plasma membrane serves to ensure that the antibodies can only target the tails exposed to the outside. This approach assumes that the type III CD38 is expressed not only in the ER but also in the plasma membrane (Figs. 1 and 2).
Figure 2.
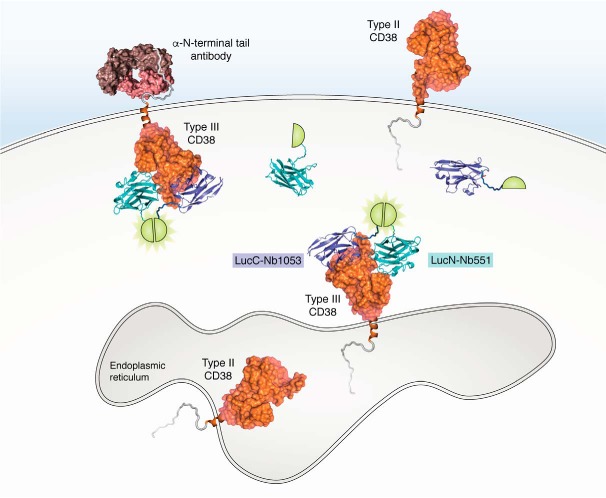
Immunoassay and DepID for detecting type III CD38 inside cells. The immunoassay uses externally applied antibody against the N-terminal tail to detect the tail of the type III CD38 exposed to the outside. DepID probes are constructed with Nbs (blue and cyan) against two different epitopes on the catalytic domain of type III CD38, and each is fused with a luciferase fragment (LucN and LucC, semicircle). Binding of the Nbs to the epitopes on the type III CD38 (PDB entry 3RAJ) brings the luciferase fragment together, reconstituting the luciferase and producing luminescence (light green).
Anti-tail immunofluorescence
Highly specific monoclonal and polyclonal antibodies (Fig. 2) have been raised against the N-terminal tail of CD38, the sequence of which is quite unique (47). Immunofluorescence staining of live human HL-60 cells following retinoic acid induction showed increased signals of the tail on the surface, suggesting an increased expression of the endogenous type III CD38 concomitant with the granulocytic differentiation of the cells. Similar results were seen in U937 cells, a human monocytic cell line, after activation by interferon γ. A population of type III CD38-positive cells in human peripheral blood mononuclear cells was also detected by immunostaining with anti-tail (47).
Likewise, endogenous type III CD38 was detected in mouse spleen cells and the LAK cells using mouse anti-tail (49). It was found to be nonglycosylated, consistent with the results obtained from the constructed human type III CD38 described above (47, 48). Together, these results show that type III CD38 is naturally present in both human and mouse cells and that its expression is physiologically regulated.
The DepID assay
The main limitation of the anti-tail approach is that it can only detect type III CD38 expressed in the plasma membrane. As described above, most of the constructed type III CD38 is expressed in the ER instead (47, 48). A novel method called DepID (dual-epitope protein identification) has been devised to detect the type III CD38 from inside live cells (50). It is based on the use of nanobodies (Nbs), single-domain antibodies that can be functionally expressed inside cells. Nbs have been widely used to modulate the functions of intracellular proteins (51–53) and, when tagged with a fluorescent protein (chromobody), to visualize their dynamic changes in live cells (54, 55).
A series of Nbs targeting three separate epitopes on the catalytic domain of CD38 has been generated (56). For DepID, two of them, Nb1053 and Nb551 (Fig. 2), were each fused with a segment of a luciferase, LucN and LucC (Fig. 2). Separately, the segments are inactive. But when the constructs bind to the two epitopes on the same type III CD38, the segments are brought together to reconstitute the active luciferase and produce luminescence. The assay has low background, because the unbound probes are inactive, and high sensitivity, because of the activity of the reconstituted luciferase. The specificity of DepID is also much higher than standard immunoassay, as it requires the target protein to have both epitopes on the same molecule.
The DepID assay was verified using HEK293 cells expressing the constructed type III CD38 described above as control (50). To detect endogenous type III CD38, the DepID assay was applied to two human multiple myeloma cell lines, LP-1 and OPM2. Strong luminescence signal was seen in LP-1 cells but not when CD38 was ablated by using the CRISPR/Cas9 technique. Even stronger signals were seen in OPM2 cells, but only when both DepID probes were expressed in them (50). Thus, two totally distinct assays, anti-tail and DepID, both document the natural existence of type III CD38 in the plasma membrane and internally in human and mouse cells.
Regulation of type III CD38
That the catalytic domain of the endogenous type III CD38 is facing the cytosol raises the possibility that it is interacting with a variety of regulators present in the cytosol, allowing elucidation of its regulation. In turn, the identification of these cytosolic factors can further substantiate the membrane topology of the natural type III CD38.
CIB1 regulates cellular cADPR levels
Using the yeast two-hybrid technique with the catalytic domain of CD38 as bait, a number of interacting regulators have been identified, including a cytosolic Ca2+-binding protein, CIB1 (50). It has four Ca2+-binding sites, and two of them are of high affinity with the EF-hand motif (57) (Fig. 1). CIB1 is a multifunctional regulator involved in a wide range of cellular activities, including modulation of the IP3 receptor Ca2+ release channel (58).
That CIB1 indeed binds to the type III CD38 was verified in vitro using recombinant proteins, in cell lysates using immunoprecipitation and in live cells using the bimolecular fluorescence complementation technique (50). Mutational analyses further identified the N terminus of CIB1 as being involved in interacting with the catalytic domain of type III CD38 (50). The functional consequence of the interaction was assessed using shRNA to knock down and Cas9/guide RNA to knock out CIB1. A direct correlation between the cellular cADPR and CIB1 levels was demonstrated. These results indicate that the type III CD38 is functionally active in producing cellular cADPR and that the activity is specifically modulated through interaction with cytosolic CIB1 (50).
Chaperones regulate folding
As described above, the catalytic domain of the type III CD38 is highly unusual in that, when expressed in the cytosol, it can readily form the six disulfides needed for its enzymatic activities (35, 47) (Fig. 1). It is possible that the information for the disulfide formation is encoded in its primary sequence, such that during its translation and folding, the microenvironment and proximity of appropriate cysteines facilitate the disulfide formation. Indeed, it is documented that the local microenvironment of protein can greatly influence the ionization and reactivity of the thiolate groups of cysteines at physiological pH (59). Specific binding of cytosolic chaperones can further help the disulfide formation. This may well be the general process that allows not only type III CD38 but also the hundreds of cytosolic proteins to form and retain disulfide bonds (45, 46).
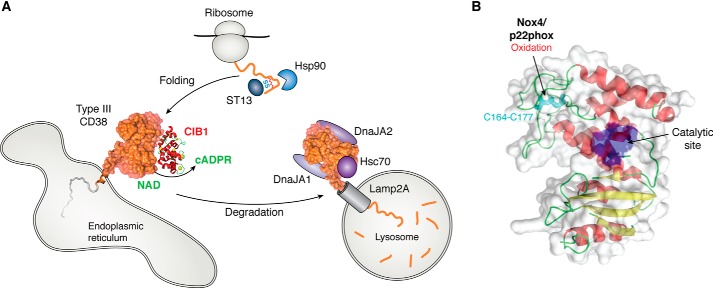
Consistently, it was shown using the yeast two-hybrid technique that ST13, a co-chaperone also called Hip, is another cytosolic protein associated with the catalytic domain of CD38 (60). In addition, a complex of chaperones, including also Hsp90, Hsc70, DnaJA1, and DnaJA2, was identified by MS in the immunoprecipitates of the C-domain of CD38 (60). Knockdown of ST13 or Hsp90 resulted in reduction of the type III CD38, suggesting that both chaperones are involved in the correct folding of type III CD38, including its disulfide formation. Their knockdown may thus increase misfolding and degradation (Fig. 3A).
Figure 3.
Regulators of the type III CD38. A, CIB1 interacts with type III CD38 and modulates its cADPR-producing activity. The correct folding and disulfide formation of type III CD38 are assisted by chaperones Hsp90 and ST13. On the other hand, chaperones Hsc70, DnaJA1, and DnaJA2 mediate degradation of type III CD38 via the lysosomal receptor Lamp2A. B, Nox4 and its associated component p22phox activate mouse type III CD38 (PDB entry 2EG9) by oxidizing cysteine 164 to form disulfide with cysteine 177.
Chaperones regulate degradation
In contrast, knockdown of Hsc70, DnaJA1, or DnaJA2 caused a large increase in type III CD38 levels by as much as 2–3-fold. Likewise, an inhibitor of Hsp70 and Hsc70, VER-155008, dramatically increased the levels of type III CD38 and concomitantly raised the cellular cADPR levels (60), further documenting that type III CD38 is responsible for cellular cADPR. The results suggest that these chaperones are facilitating the degradation of type III CD38 and their knockdown reduces degradation, allowing the proteins to accumulate. This was verified by chase experiments using cycloheximide to block translation, and subsequent degradation of type III CD38 was expectedly slowed by the knockdown of Hsc70 (60).
It is known that Hsc70 facilitates protein degradation via chaperone-mediated autophagy (CMA) (61). Consistently, blocking the lysosomal degradation with bafilomycin increased the levels of type III CD38, whereas MG-132, an inhibitor of the proteasome degradation pathway for soluble proteins, had no effect. The import of the type III CD38 into lysosomes for degradation was via the lysosomal receptor, Lamp2A (61), and its knockdown increased the level of type III CD38 by 2–3-fold (60) (Fig. 3A). Immunostaining showed significant co-localization of type III CD38 and Lamp2A, which could be further increased by geldanamycin, an inducer of CMA degradation of the ryanodine receptor (62). The binding region on the type III CD38 for Lamp2A was identified in the last 19 residues from the C terminus, which was also the region where Hsc70 and DnaJA2 bound to the type III CD38 (Fig. 3A) (60).
CMA of soluble proteins has been most commonly studied. But type III CD38 is not the only membrane protein degraded through CMA (63). Others include the ryanodine receptor (62), a calcium channel in the ER targeted by cADPR, and the epidermal growth factor receptor (64). Exactly how membrane proteins are transported to the lysosomes has not been elucidated. It can involve p97/Cdc48-mediated protein dislocation or intramembrane proteolysis extracting transmembrane segments and transporting to lysosomes, as has been proposed (65).
NADPH oxidase 4 (Nox4) regulates disulfide formation
Type III CD38 in mice has been characterized and is shown to be a nonglycosylated protein of about 36 kDa (49), similar to the constructed human type III CD38 (47, 48). Its disulfide within the last 19 carboxyl residues is not formed, in contrast to type II CD38. A mAb, M19, raised against this part of the molecule can thus specifically recognize the type III CD38 (49).
As described in the Introduction, mouse CD38 is shown to mediate interleukin-8 signaling in LAK cells (33, 49). A recent study further identifies type III CD38 as responsible for mediating the Ca2+ changes induced by reactive oxygen species in these cells (49). Inhibiting Nox4 in the cells using either specific inhibitor, shRNA or gene knockout, inhibited the interleukin-8–activated cADPR production and Ca2+ changes (49). Nox4 makes reactive oxygen species/superoxide, which can oxidize cysteines, leading to disulfide bond formation. In the cell lysates, Nox4 was shown to activate the cADPR-synthesizing activity, which also required the presence of the phosphorylated p22phox, an accessory component of Nox4 (49). H2O2, a product of Nox4, could directly stimulate the activity. The results suggest that Nox4 and the phosphorylated p22phox bind to and activate type III CD38 to produce cADPR through oxidization of its cysteines. That disulfides are important for cADPR-synthesizing activity of type III CD38 is consistent with what was seen in human type III CD38 (47). Immunoprecipitation of type III CD38 using M19 shows that Nox4 and p22phox were indeed associated with the type III CD38. Mutating individual cysteines of CD38 identified cysteine 164 as the Nox4 target, which was oxidized to form a disulfide with cysteine 177 (Fig. 3B) (49). Enzymes such as Nox4 and/or disulfide isomerases (66), in association with chaperones, may well be responsible for the disulfide formation of type III CD38 during its translation and folding. Taken together, these results further establish that the type III CD38 is a regulated Ca2+-signaling enzyme.
Potential mechanisms for regulating the expression of type III CD38
The existence of type III CD38 with its catalytic domain facing the cytosol readily resolves the topological enigma of Ca2+ signaling mediated by cADPR. It is thus of clear interest to survey and explore what known mechanisms may be consistent with and may be involved in regulating the expression of type III CD38 in particular and single-pass membrane proteins in general. This is not a trivial issue because, theoretically, the existence and co-expression of an alternate membrane orientation for any membrane protein can potentially endow it with entirely different functions.
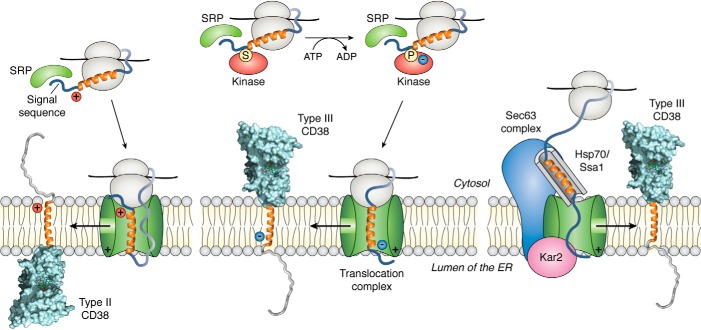
That type III CD38 can be constructed by altering the charges in the N-terminal tail of CD38 indicates that the “positive inside” rule governs its expression. The molecular basis of the rule is depicted in Fig. 4 (left). Protein synthesis generally begins in the cytosolic ribosomes. Binding of the signal recognition particle (SRP; Fig. 4) to the nascent polypeptide then directs the continuation of the translation process of the membrane protein to the ER. The electrostatic repulsion of the positive charge of the nascent polypeptide with the translocation complex leads to its folding and exiting though the side channel of the complex and inserting into the membrane in the type II orientation (67).
Figure 4.
Potential mechanisms for expression of type III CD38. Left, translation begins in the cytosolic ribosomes. Binding of the SRPs to the signal sequence of the nascent polypeptide directs it to the translocation complex in the ER. The positive charges of the nascent polypeptide interact with the complex and result in folding of the polypeptide. Subsequent exit from the side channel of the complex results in type II insertion into the ER membrane (left). Middle, kinases are known to associate with the ribosomes, and the phosphorylation of the nascent polypeptide can reduce its charge interaction with the translocation complex and thus the folding of the polypeptide, resulting in the type III orientation. The lipid content of the ER can also modulate the membrane orientation of CD38, as is observed in bacteria. Right, chaperones such as Hsp70 or Ssa1 can bind to the hydrophobic segment of the nascent polypeptide and, through the Sec63 complex, direct its insertion into the ER membrane in the type III orientation via the translocation complex.
Phosphorylation of the tail
In the case of CD38, the charge difference between the two sides spanning the transmembrane segment is small, and the charge repulsion of its two sides with the translocation complex is more similar. The folding of the nascent polypeptide may thus become stochastic, but still with a preference for the type II orientation. Consistent with this notion, cells transfected with the WT CD38 expressed a significant portion in the type III orientation as detected by DepID (50), even though the major portion was type II.
Increasing the negative charges at the N-terminal side of the nascent polypeptide should increase the charge attraction with the translocation complex and reduce its folding inside the complex (Fig. 4, middle), which can result in a graded increase in the portion of the expressed proteins being in the type III orientation. This is the case. With the positive residues changed to negative, essentially all of the expressed CD38 was found to be in the type III orientation (47, 48). Similar results were seen in mouse CD38 (49), attesting to the species generality of the rule.
A possible and natural means to increase the negative charge in the tail is phosphorylation. There are three serines in the N-terminal tail of CD38. Changing them to aspartates to simulate phosphorylation indeed increased the expression of type III CD38 (48). Naturally, co-translational phosphorylation of the nascent polypeptide has been observed (68–70) (Fig. 4, middle). In particular, mTORC2 kinase has been shown to be associated with a large ribosomal subunit protein at the tunnel exit and can phosphorylate the nascent polypeptide on either serine (69) or threonine (70). Whether a similar phosphorylation of the serines in the N-terminal tail during its translation can lead to an increase in the expression of type III CD38 would be worthy of investigation.
Lipid modulation
Another factor that can affect the orientation of the membrane proteins is the lipid content of the membrane. In bacteria, lactose permease exhibited a mixture of topological conformations from one orientation to a complete inversion of the N-terminal helical bundle, depending on the phosphatidylethanolamine content. The topological orientations were even interconvertible by post-assembly synthesis or dilution of the lipid in vivo (71). Using fluorescence energy transfer, the rate of the protein flipping in the lipid bilayer in both directions triggered by a change in the proteoliposome lipid composition occurred rapidly in the range of seconds (72). The possibility that lipid binding to type II CD38 might reduce the energy barrier sufficiently to allow orientation flipping is intriguing.
SRP-independent ER insertion
Although the SRP-dependent insertion of protein into the ER membrane (Fig. 4, left and middle) is generally thought to be the dominant process, the SRP-independent insertion has also been described (73, 74). In yeast, nascent polypeptides with signal sequences not recognized efficiently by SRP are bound by soluble chaperone Ssa1, targeting the nascent chains to the Sec63 complex (Fig. 4, right), which in turn interacts and transfers the nascent chains to the translocation complex. The movement and folding of the nascent chains through the channel of the complex is facilitated by Kar2, a luminal chaperone (74).
In mammalian cells, it is believed that Hsp70s serves a function similar to that of Ssa1. Hsc70, a member of the Hsp70 chaperone family, is associated with the type III CD38 (60). Its sequence, however, only has minimal homology with Ssa1. It remains a possibility that binding of an appropriate chaperone to the hydrophobic segment of CD38 may prevent aggregation and direct the nascent polypeptide to the translocation complex (Fig. 4, right) or allow the entire protein to be translated in the cytosol. Subsequent insertion into the ER membrane in the type III orientation can be mediated by this SRP-independent pathway.
Type II CD38
The discovery of type III CD38, with its cytosolic orientation and its efficient production of intracellular cADPR, solves one long-standing CD38 mystery. But another mystery remains: Although CD38 is capable of making both cADPR and NAADP, the optimal pH values for the two reactions are substantially different, meaning that type III CD38 cannot make NAADP in the cytosol. So how then does NAADP get made? Solving this mystery requires returning to earlier proposals of CD38 trafficking.
Type II CD38 on the cell surface is the dominant form of CD38 found in many cells, especially in blood cells, and is enzymatically active. Its catalytic domain facing the extracytosolic compartment is topologically inappropriate to contribute to production of cellular cADPR. However, the topological barrier of the bilayer membrane, in principle, can be specifically and selectively relaxed by membrane transporters. Among them, connexin 43 is permeant to NAD (75), whereas nucleoside transporters can mediate both concentrative and equilibrative movement of cADPR (76). It has thus been proposed that the surface type II CD38 is internalized through endocytosis together with surface transporters into the endolysosomal compartment (16, 77–79). The cytosolic NAD can enter via connexin 43 and get converted to cADPR inside the compartment, which is then transported out by the nucleoside transporter back to the cytosol to target the ER Ca2+ stores.
Biogenesis of NAADP
The endolysosomal compartment is highly acidic and not conducive for the cyclization reaction of CD38 to produce cADPR, even if NAD is made available by the transporters. It is, however, well-suited for the base-exchange reaction to produce NAADP by CD38, which has an optimum at pH 4 (22, 80). This is because the acidic pH neutralizes the charge repulsion between the negative Glu-146 at the catalytic site and the base-exchange substrate, nicotinic acid (80). Ever since the first documentation of this peculiar acidic requirement of the NAADP synthesis (22), the endolysosomes have been suggested to be a special cellular compartment for the biogenesis of NAADP (77–79). The idea has been made more substantive with the findings that the endolysosomes are functional Ca2+ stores (81, 82) and are targeted by NAADP, which specifically activates the endolysosomal two-pore channels (9, 10) to effect Ca2+ release from the stores in response to various physiological stimuli. It is a novel notion that the two forms of CD38 are topologically segregated into two compartments so as to produce two structurally and functionally distinct Ca2+ messengers.
The first test for this proposal is to identify a stimulus for endocytosis and to determine whether the surface type II CD38 can indeed be internalized into the endolysosomes via endocytosis. Nb against the catalytic domain of CD38 is such a stimulus. Phenomenologically, it is very effective in inducing the endocytosis of the endogenous type II CD38 expressed on the surface of human myeloma LP-1 cells, as well as that expressed in HeLa and HEK293 cells transfected with CD38 (83). The Nb can be fluorescently labeled, and imaging showed that the endocytosed complex reached the endolysosomes. Concomitant with the Nb-activated endocytosis, the cellular NAADP level in LP-1 cells progressively increased in the presence of exogenous nicotinic acid (NA) (83). These results show that endocytosis can indeed deliver surface type II CD38 to the endolysosomes and that the internalized CD38 remains enzymatically active for at least a period of time, even though it was eventually degraded (84). This was further substantiated by constructing a lysosomal CD38 specifically directed to express in the lysosomes by splicing the catalytic domain of CD38 to the C-terminal segment of LAMP1 for lysosomal retention. The construct, likewise, efficiently elevated the cellular NAADP levels in the presence of NA (83).
The requirement for NA, however, indicates that the access of substrate to the extracytosolic compartment of the endolysosomes is still limited. This tight compartmentation suggests the possibility of a novel mechanism for signaling. Type II CD38 is constitutively active, and the regulation is not on CD38 itself but on the access of substrates through activation of specific membrane transporters. For example, the permeability of connexin 43 was shown to be regulated by phosphorylation by protein kinase C (85). Whether connexin 43 or other transporters are mediating the movement of NADP and NA, substrates for the base-exchange reaction of CD38, remains to be investigated.
Biogenesis of cADPR
Evidence described above suggests that the type II CD38 may contribute to the production of NAADP, but intriguing results indicate that production of cellular cADPR is likely to be tightly compartmentalized in the cytosol and that type II CD38 may not contribute substantially to the cellular cADPR synthesis. This was first seen in cells transfected with WT human CD38 or with charge mutants with increasing numbers of the N-terminal positive residues changed. Normalizing the cellular cADPR contents to the amounts of CD38 expressed, it was seen that cells expressing CD38 with all N-terminal positive charges mutated, and thus in the type III orientation, had the highest normalized cADPR contents, higher than the cells expressing WT CD38 (47, 48). As most of the WT was expressed as type II on the surface and only a small amount as type III inside the cells (50), the result is the first indication that type II CD38 may not contribute much to the production of cytosolic cADPR.
Consistently, in mouse LAK cells, the type II CD38 is constitutively active, but the activity cannot be stimulated by interleukin-8. Type III CD38, in contrast, has low basal activity but can be stimulated by the cytokine (49). The elevation of the cellular cADPR levels activated by the cytokine is thus mediated by the type III and not the type II CD38.
To directly address whether the surface type II CD38 contributes to the production of cellular cADPR, a cross-linking technique was developed to identify possible regulators of surface CD38. The results show that the transferrin receptor, CD71, specifically interacts with surface CD38 and that its knockdown reduces type II CD38 expression (84). To simulate this regulatory effect of CD71, a construct was made by splicing the amino tail of CD71 to a nanobody against CD38. As described above, binding of the nanobody to surface CD38 induced endocytosis. Its anchoring to the surface by the amino tail of CD71 greatly increased its effectiveness and resulted in more than 80% reduction of type II CD38 via endocytosis, which was then degraded in the lysosomes (84). Remarkably, the cellular cADPR levels did not decrease correspondingly. Bafilomycin-mediated blockade of lysosomal degradation greatly elevated active type II CD38 by trapping it in the lysosomes, but also did not increase cADPR levels. Retention of type II CD38 in the ER by expressing an ER construct that prevented its transport to the cell surface likewise did not change cADPR levels (84). These results provide the first and direct evidence that cADPR biogenesis occurs in the cytosol and is catalyzed mainly by type III CD38 and that type II CD38, compartmentalized in the ER, lysosomes, or cell surface, contributes only minimally to cADPR biogenesis.
A new enzyme emerges
As described in the Introduction, CD38 has been the only fully characterized enzyme for synthesizing cADPR and NAADP. It has, however, been observed that ablation of the CD38 gene in mice resulted in large decreases of cADPR contents in many tissues except in the brain (86), suggesting the possibility of another enzyme for synthesizing cADPR in the brain. However, searches in the mouse genome database have produced no promising candidates.
It is thus surprising that sterile α and Toll/interleukin-1 receptor motif-containing 1 (SARM1), a protein unrelated to and having no sequence similarity with CD38, has recently been shown to be able to catalyze the synthesis of cADPR (87, 88). This unexpected discovery is a convergence of two different and independent lines of investigation.
SARM1 is known to play an important role in axonal degeneration underlying several neurological disorders. Its activation triggered axon degeneration locally and induced NAD depletion (89), suggesting that it may have NAD-hydrolyzing activity. Consistently, the Toll/interleukin-1 receptor (TIR) domain of SARM1, recombinantly produced and purified, was shown to be an effective NADase (87, 88). Surprisingly, analysis of its hydrolysis products by HPLC showed, in addition to ADP-ribose, a small peak corresponding to cADPR (87, 88).
That SARM1 is indeed an endogenous and regulated enzyme capable of producing cADPR in live cells was demonstrated through an entirely separate effort, which was aimed at developing specific inhibitors of CD38. One of the most potent found was sulfo-araF-NMN (CZ48), a catalysis-based inhibitor and a cell-permeant mimetic of nicotinamide mononucleotide (NMN) (90). It blocked CD38 by forming a covalent bond with its catalytic residue, Glu-226, as revealed by crystallography (90). It is thus expected that CZ48 should inhibit CD38 and cause reduction in cellular cADPR.
Most surprisingly, when CZ48 was applied to HEK293 cells, it effectively raised the cellular level of cADPR instead (88). CRISPR/Cas9 deletion of the CD38 gene did not prevent the induced cADPR increase, indicating that the production was not mediated by CD38, the hitherto only known enzyme that could increase cellular cADPR. Deletion of SARM1, however, eliminated the cADPR increase, whereas overexpressing SARM1 enhanced it, indicating that the target of CZ48 is SARM1 (88).
That CZ48 is a mimetic of NMN suggests that SARM1 may be stimulated by NMN as well. This is the case. Both NMN and CZ48 were equally effective in activating SARM1 to produce cADPR from NAD with essentially identical half-maximal effective concentrations (88). Consistently, knockout of NMN adenylyltransferase 1, the main NMN-utilizing enzyme in cells, elevated cellular NMN and activated cADPR production, indicating that NMN is an endogenous activator of SARM1.
In contrast to CZ48, NMN cannot activate cADPR increases when applied to live cells. Structure-function studies indicate that the sulfur attached to the phosphate group of CZ48 is important for its cell permeability. A wide range of human and mouse cells are responsive to CZ48, including primary mouse dorsal root ganglions (88). The extents of cADPR increases in response to CZ48 correlated well with the cellular SARM1 levels. CZ48 is clearly a cell-permeant, versatile, useful, and specific activator of cellular cADPR production via SARM1.
Unlike CD38, SARM1 possesses multiple domains, including an N-terminal domain with multiple armadillo repeat motifs (ARMs), two tandem sterile α motif (SAM) domains, and a catalytic C-terminal TIR domain. The N-terminal segment is believed to be associated with the mitochondria, whereas the catalytic TIR domain is facing the cytosol (91, 92). The ARM domain is autoregulatory because its deletion removed the regulatory response of SARM1 to CZ48 and NMN. The remaining SAM-TIR portion became constitutive active in producing cADPR both in vitro and in vivo (88). The results suggest that CZ48 activates SARM1 by inducing a conformational change (Fig. 5) to release the catalytic TIR domain from the sequestration of the ARM domain. In addition, CZ48 also induced dimerization of the TIR domain as part of the process of activating the enzymatic activity of SARM1 (Fig. 5) (88). A similar conclusion on the conformational changes of the SARM1 domains was reached separately from the functional studies on axonal degeneration (92).
Figure 5.
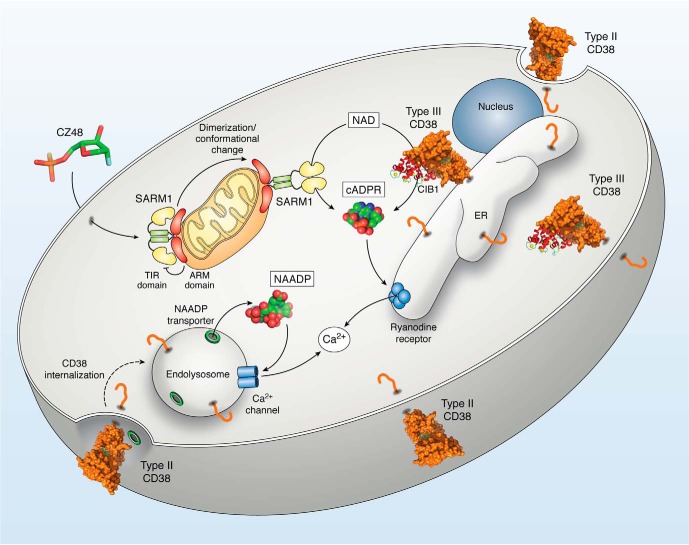
The CD38/cADPR/NAADP-signaling pathway. Type III CD38, present in the ER and plasma membrane, cyclizes cytosolic NAD to produce cellular cADPR (PDB entry 2O3Q), which targets the ryanodine receptor in the ER and releases Ca2+ from the store. The activity of the type III CD38 is modulated by CIB1, a Ca2+-binding regulator. Type II CD38 is expressed on the cell surface and can be internalized through endocytosis to the endolysosomes, where the acidic lumen is conducive for NAADP production. Transporters internalized together with the type II CD38 facilitate the movement of NAADP (PDB entry 4F45) into the cytosol, where it targets Ca2+ channels, such as the two-pore channel, in the acidic Ca2+ stores. CZ48 (PDB entry 3ROM) is a mimetic of NMN and is cell-permeant. It induces dimerization and conformational changes in SARM1, releasing the catalytic TIR domain from the autoinhibition of the ARM domain. The amino segment of SARM1 is associated with the mitochondria, whereas the TIR is facing the cytosol. The activated SARM1 cyclizes NAD to produce cADPR in a manner similar to CD38.
SARM1 and CD38 are clearly two totally different proteins with no sequence and structural similarity. It is thus surprising that they are virtually identical catalytically (88). Both enzymes catalyze NAD hydrolysis and cyclization of NAD to cADPR as well as a base-exchange reaction using NADP and nicotinic acid as substrates to produce NAADP. Similar catalysis of the two enzymes would suggest similarity in their active sites. This seems not to be the case, as CZ48 formed a covalent bond with the catalytic residue of CD38 and irreversibly inhibited its enzymatic activity, whereas it activated SARM1 reversibly without forming a covalent linkage (88). Another notable difference was that SARM1 had much less cADPR-hydrolyzing activity than CD38, making SARM1 more efficient for cADPR synthesis. It is thus catalytically more similar to Aplysia ADP-ribosyl cyclase (20) than to human CD38.
Studies on SARM1 have hitherto mostly focused on its role in axonal degeneration. This is mainly because it is highly expressed in neuronal cells. In nonneuronal HEK293 cells overexpressing SARM1, CZ48 did induce nonapoptotic cell death with elevation of cADPR and depletion of NAD, similar to that observed during axonal degeneration (88). However, SARM1 is a ubiquitous enzyme endogenously present in a wide range of nonneuronal cells as well. In these cells, it was also fully efficient in elevating cADPR in response to CZ48 activation, but without inducing cell death (88). It is thus certain that SARM1 is not just a mediator of cell death. The question of what physiological roles SARM1 plays in these cells is undoubtedly of wide interest. As cADPR is documented as a second messenger for mediating a variety of physiological functions, SARM1 may well be a regulable enzyme involved in some or all of these signaling processes.
Summary
Evidence amassed in the past 30 years firmly establishes the wide range of signaling functions of cADPR and NAADP in cells spanning three biological kingdoms. Much is now known about their Ca2+-signaling mechanisms and the stores that the two nucleotide messengers target. Despite the fact that both messengers were unknown molecules that had never been described before, their synthesis enzymes, the cyclase and CD38, were soon identified, and their catalysis was elucidated. In contrast to these advances is the conspicuous weakness in the understanding of the regulation of CD38. The conceptual bottleneck is centered around the topological issues described in this review. It is puzzling how a type II enzyme with its catalytic domain facing outside can be regulated by the cell.
The discovery of the type III CD38 expressed both in the ER and the plasma membrane as summarized in Fig. 5 has now begun to clarify the issue. With the catalytic domain facing the cytosol, type III CD38 can readily use the cytosolic NAD as substrate and produce cADPR (Fig. 5), which targets the ryanodine receptor in the ER. Regulators of type III CD38 have since been identified, including CIB1, chaperones and Nox4. The type II CD38 is expressed on the cell surface and can be internalized via endocytosis, together with specific transporters. The acidic lumen of the endolysosomes is conducive for the type II CD38 to catalyze the base-exchange reaction to produce NAADP, which is then transported out via the transporters to target the two-pore channel.
The ground-breaking finding that SARM1 can catalyze the production of cADPR and NAADP, the same as CD38, should usher in a new frontier. It is an autoregulated enzyme. NMN or its mimetic CZ48 activates dimerization of SARM1, releasing the catalytic TIR domain from the inhibition of the ARM domain (Fig. 5). SARM1 is known to regulate axonal degeneration. That it may be involved in regulating other functions and function as a Ca2+-signaling enzyme is intriguing and should warrant further investigation.
Another advance derived from the study of type III CD38 that may well have widespread application is the novel approach of the DepID assay specifically designed to detect the topology of cellular proteins. Although it was developed for CD38, it is, in fact, quite versatile and can readily be applied to any intracellular protein. A variation in the format of ELISA has also been used as a diagnostic test to precisely quantify the levels of soluble CD38 in the plasma of multiple myeloma patients. Myeloma cells are known to overexpress surface CD38 and show increased shedding as soluble CD38. Using the DepID as an ultrasensitive and specific assay, it was shown that the plasma levels of soluble CD38 were significantly higher than those from healthy donors and were correlated with the progress of the disease (93). DepID is thus a topologically selective, highly sensitive and specific approach for in vivo and in vitro monitoring of proteins.
This work was supported by National Science Foundation of China Grants 31671463, 31571438, 31871401, and 31871403.
- IP3
- inositol trisphosphate
- ER
- endoplasmic reticulum
- cADPR
- cyclic ADP-ribose
- NAADP
- nicotinic acid adenine dinucleotide phosphate
- cyclase
- ADP-ribosyl cyclase
- C-domain
- carboxyl domain
- LAK
- lymphokine-activated killer
- DepID
- dual-epitope protein identification
- Nb
- nanobody
- CMA
- chaperone-mediated autophagy
- Nox4
- NADPH oxidase 4
- SRP
- signal recognition particle
- NA
- nicotinic acid
- TIR
- Toll/interleukin-1 receptor
- SARM1
- sterile α and TIR motif–containing 1
- NMN
- nicotinamide mononucleotide
- ARM
- armadillo repeat motif
- SAM
- sterile α motif
- PDB
- Protein Data Bank.
References
- 1. Streb H., Irvine R. F., Berridge M. J., and Schulz I. (1983) Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306, 67–69 10.1038/306067a0 [DOI] [PubMed] [Google Scholar]
- 2. Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., and Mikoshiba K. (1989) Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature 342, 32–38 10.1038/342032a0 [DOI] [PubMed] [Google Scholar]
- 3. Lee H. C., Walseth T. F., Bratt G. T., Hayes R. N., and Clapper D. L. (1989) Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J. Biol. Chem. 264, 1608–1615 [PubMed] [Google Scholar]
- 4. Lee H. C., Aarhus R., and Levitt D. (1994) The crystal structure of cyclic ADP-ribose. Nat. Struct. Biol. 1, 143–144 10.1038/nsb0394-143 [DOI] [PubMed] [Google Scholar]
- 5. Lee H. C., and Aarhus R. (1997) Structural determinants of nicotinic acid adenine dinucleotide phosphate important for its calcium-mobilizing activity. J. Biol. Chem. 272, 20378–20383 10.1074/jbc.272.33.20378 [DOI] [PubMed] [Google Scholar]
- 6. Lee H. C., and Aarhus R. (1995) A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 270, 2152–2157 10.1074/jbc.270.5.2152 [DOI] [PubMed] [Google Scholar]
- 7. Lee H. C., Aarhus R., Graeff R., Gurnack M. E., and Walseth T. F. (1994) Cyclic ADP ribose activation of the ryanodine receptor is mediated by calmodulin. Nature 370, 307–309 10.1038/370307a0 [DOI] [PubMed] [Google Scholar]
- 8. Galione A., Lee H. C., and Busa W. B. (1991) Ca2+-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science 253, 1143–1146 10.1126/science.1909457 [DOI] [PubMed] [Google Scholar]
- 9. Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang X. J., Rietdorf K., Teboul L., Chuang K.-T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., et al. (2009) NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600 10.1038/nature08030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brailoiu E., Churamani D., Cai X., Schrlau M. G., Brailoiu G. C., Gao X., Hooper R., Boulware M. J., Dun N. J., Marchant J. S., and Patel S. (2009) Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 186, 201–209 10.1083/jcb.200904073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee H. C. (2012) Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J. Biol. Chem. 287, 31633–31640 10.1074/jbc.R112.349464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guse A. H., and Lee H. C. (2008) NAADP: a universal Ca2+ trigger. Sci. Signal. 1, re10 10.1126/scisignal.144re10 [DOI] [PubMed] [Google Scholar]
- 13. Galione A., Morgan A. J., Arredouani A., Davis L. C., Rietdorf K., Ruas M., and Parrington J. (2010) NAADP as an intracellular messenger regulating lysosomal calcium-release channels. Biochem. Soc. Trans. 38, 1424–1431 10.1042/BST0381424 [DOI] [PubMed] [Google Scholar]
- 14. Lee H. C. (2001) Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu. Rev. Pharmacol. Toxicol. 41, 317–345 10.1146/annurev.pharmtox.41.1.317 [DOI] [PubMed] [Google Scholar]
- 15. Lee H. C. (2012) The cyclic ADP-ribose/NAADP/CD38-signaling pathway: past and present. Messenger 1, 16–33 10.1166/msr.2012.1005 [DOI] [Google Scholar]
- 16. De Flora A., Zocchi E., Guida L., Franco L., and Bruzzone S. (2004) Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann. N.Y. Acad. Sci. 1028, 176–191 10.1196/annals.1322.021 [DOI] [PubMed] [Google Scholar]
- 17. Imbery J. F., Iqbal A. K., Desai T., and Giovannucci D. R. (2019) Role of NAADP for calcium signaling in the salivary gland. Cell Calcium 80, 29–37 10.1016/j.ceca.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 18. Foster W. J., Taylor H. B. C., Padamsey Z., Jeans A. F., Galione A., and Emptage N. J. (2018) Hippocampal mGluR1-dependent long-term potentiation requires NAADP-mediated acidic store Ca2+ signaling. Sci. Signal. 11, eaat9093 10.1126/scisignal.aat9093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. States D. J., Walseth T. F., and Lee H. C. (1992) Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human lymphocyte antigen CD38. Trends Biochem. Sci. 17, 495 10.1016/0968-0004(92)90337-9 [DOI] [PubMed] [Google Scholar]
- 20. Lee H. C., and Aarhus R. (1991) ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 2, 203–209 10.1091/mbc.2.3.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackson D. G., and Bell J. I. (1990) Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J. Immunol. 144, 2811–2815 [PubMed] [Google Scholar]
- 22. Aarhus R., Graeff R. M., Dickey D. M., Walseth T. F., and Lee H. C. (1995) ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J. Biol. Chem. 270, 30327–30333 10.1074/jbc.270.51.30327 [DOI] [PubMed] [Google Scholar]
- 23. Howard M., Grimaldi J. C., Bazan J. F., Lund F. E., Santos-Argumedo L., Parkhouse R. M., Walseth T. F., and Lee H. C. (1993) Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 262, 1056–1059 10.1126/science.8235624 [DOI] [PubMed] [Google Scholar]
- 24. Liu Q., Kriksunov I. A., Graeff R., Munshi C., Lee H. C., and Hao Q. (2005) Crystal structure of human CD38 extracellular domain. Structure 13, 1331–1339 10.1016/j.str.2005.05.012 [DOI] [PubMed] [Google Scholar]
- 25. Graeff R., Liu Q., Kriksunov I. A., Kotaka M., Oppenheimer N., Hao Q., and Lee H. C. (2009) Mechanism of cyclizing NAD to cyclic ADP-ribose by ADP-ribosyl cyclase and CD38. J. Biol. Chem. 284, 27629–27636 10.1074/jbc.M109.030965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Graeff R., Munshi C., Aarhus R., Johns M., and Lee H. C. (2001) A single residue at the active site of CD38 determines its NAD cyclizing and hydrolyzing activities. J. Biol. Chem. 276, 12169–12173 10.1074/jbc.M011299200 [DOI] [PubMed] [Google Scholar]
- 27. Zhang H., Graeff R., Chen Z., Zhang L., Zhang L., Lee H., and Hao Q. (2011) Dynamic conformations of the CD38-mediated NAD cyclization captured in a single crystal. J. Mol. Biol. 405, 1070–1078 10.1016/j.jmb.2010.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee H. C. (2006) Structure and enzymatic functions of human CD38. Mol. Med. 12, 317–323 10.2119/2006-00086.Lee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Itoh M., Ishihara K., Tomizawa H., Tanaka H., Kobune Y., Ishikawa J., Kaisho T., and Hirano T. (1994) Molecular cloning of murine BST-1 having homology with CD38 and Aplysia ADP-ribosyl cyclase. Biochem. Biophys. Res. Commun. 203, 1309–1317 10.1006/bbrc.1994.2325 [DOI] [PubMed] [Google Scholar]
- 30. Hirata Y., Kimura N., Sato K., Ohsugi Y., Takasawa S., Okamoto H., Ishikawa J., Kaisho T., Ishihara K., and Hirano T. (1994) ADP ribosyl cyclase activity of a novel bone marrow stromal cell surface molecule, BST-1. FEBS Lett. 356, 244–248 10.1016/0014-5793(94)01279-2 [DOI] [PubMed] [Google Scholar]
- 31. Park D. R., Nam T. S., Kim Y. W., Lee S. H., and Kim U. H. (2018) CD38-cADPR-SERCA signaling axis determines skeletal muscle contractile force in response to β-adrenergic stimulation. Cell Physiol. Biochem. 46, 2017–2030 10.1159/000489441 [DOI] [PubMed] [Google Scholar]
- 32. Hattori T., Kaji M., Ishii H., Jureepon R., Takarada-Iemata M., Minh Ta H., Manh Le T., Konno A., Hirai H., Shiraishi Y., Ozaki N., Yamamoto Y., Okamoto H., Yokoyama S., Higashida H., Kitao Y., and Hori O. (2017) CD38 positively regulates postnatal development of astrocytes cell-autonomously and oligodendrocytes non-cell-autonomously. Glia. 65, 974–989 10.1002/glia.23139 [DOI] [PubMed] [Google Scholar]
- 33. Rah S. Y., Mushtaq M., Nam T. S., Kim S. H., and Kim U. H. (2010) Generation of cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate by CD38 for Ca2+ signaling in interleukin-8-treated lymphokine-activated killer cells. J. Biol. Chem. 285, 21877–21887 10.1074/jbc.M109.066290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., and Schlossman S. F. (1980) Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc. Natl. Acad. Sci. U.S.A. 77, 1588–1592 10.1073/pnas.77.3.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao Y. J., Zhang H. M., Lam C. M. C., Hao Q., and Lee H. C. (2011) Cytosolic CD38 protein forms intact disulfides and is active in elevating intracellular cyclic ADP-ribose. J. Biol. Chem. 286, 22170–22177 10.1074/jbc.M111.228379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. von Heijne G. (1989) Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature 341, 456–458 10.1038/341456a0 [DOI] [PubMed] [Google Scholar]
- 37. Stewart R. S., and Harris D. A. (2003) Mutational analysis of topological determinants in prion protein (PrP) and measurement of transmembrane and cytosolic PrP during prion infection. J. Biol. Chem. 278, 45960–45968 10.1074/jbc.M307833200 [DOI] [PubMed] [Google Scholar]
- 38. Zhu Q., von Dippe P., Xing W., and Levy D. (1999) Membrane topology and cell surface targeting of microsomal epoxide hydrolase: evidence for multiple topological orientations. J. Biol. Chem. 274, 27898–27904 10.1074/jbc.274.39.27898 [DOI] [PubMed] [Google Scholar]
- 39. Dunlop J., Jones P. C., and Finbow M. E. (1995) Membrane insertion and assembly of ductin: a polytopic channel with dual orientations. EMBO J. 14, 3609–3616 10.1002/j.1460-2075.1995.tb00030.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seppälä S., Slusky J. S., Lloris-Garcerá P., Rapp M., and von Heijne G. (2010) Control of membrane protein topology by a single C-terminal residue. Science 328, 1698–1700 10.1126/science.1188950 [DOI] [PubMed] [Google Scholar]
- 41. Sebag J. A., and Hinkle P. M. (2007) Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc. Natl. Acad. Sci. U.S.A. 104, 20244–20249 10.1073/pnas.0708916105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neve E. P., and Ingelman-Sundberg M. (2000) Molecular basis for the transport of cytochrome P450 2E1 to the plasma membrane. J. Biol. Chem. 275, 17130–17135 10.1074/jbc.M000957200 [DOI] [PubMed] [Google Scholar]
- 43. Munshi C. B., Fryxell K. B., Lee H. C., and Branton W. D. (1997) Large scale production of human CD38 in yeast by fermentation. Methods Enzymol. 280, 318–330 10.1016/S0076-6879(97)80123-1 [DOI] [PubMed] [Google Scholar]
- 44. Fryxell K. B., O'Donoghue K., Graeff R. M., Lee H. C., and Branton W. D. (1995) Functional expression of soluble forms of human CD38 in Escherichia coli and Pichia pastoris. Protein Expr. Purif. 6, 329–336 10.1006/prep.1995.1043 [DOI] [PubMed] [Google Scholar]
- 45. Chiu J., and Hogg P. J. (2019) Allosteric disulfides: sophisticated molecular structures enabling flexible protein regulation. J. Biol. Chem. 294, 2949–2960 10.1074/jbc.REV118.005604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cumming R. C., Andon N. L., Haynes P. A., Park M., Fischer W. H., and Schubert D. (2004) Protein disulfide bond formation in the cytoplasm during oxidative stress. J. Biol. Chem. 279, 21749–21758 10.1074/jbc.M312267200 [DOI] [PubMed] [Google Scholar]
- 47. Zhao Y. J., Lam C. M. C., and Lee H. C. (2012) The membrane-bound enzyme CD38 exists in two opposing orientations. Sci. Signal. 5, ra67 10.1126/scisignal.2002700 [DOI] [PubMed] [Google Scholar]
- 48. Zhao Y. J., Zhu W. J., Wang X. W., Zhang L.-H., and Lee H. C. (2015) Determinants of the membrane orientation of a calcium signaling enzyme CD38. Biochim. Biophys. Acta 1853, 2095–2103 [DOI] [PubMed] [Google Scholar]
- 49. Park D. R., Nam T. S., Kim Y. W., Bae Y. S., and Kim U. H. (2019) Oxidative activation of type III CD38 by NADPH oxidase-derived hydrogen peroxide in Ca2+ signaling. FASEB J. 33, 3404–3419 10.1096/fj.201800235R [DOI] [PubMed] [Google Scholar]
- 50. Liu J., Zhao Y. J., Li W. H., Hou Y. N., Li T., Zhao Z. Y., Fang C., Li S. L., and Lee H. C. (2017) Cytosolic interaction of type III human CD38 with CIB1 modulates cellular cyclic ADP-ribose levels. Proc. Natl. Acad. Sci. U.S.A. 114, 8283–8288 10.1073/pnas.1703718114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van den Abbeele A., De Clercq S., De Ganck A., De Corte V., Van Loo B., Soror S. H., Srinivasan V., Steyaert J., Vandekerckhove J., and Gettemans J. (2010) A llama-derived gelsolin single-domain antibody blocks gelsolin-G-actin interaction. Cell Mol. Life Sci. 67, 1519–1535 10.1007/s00018-010-0266-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kirchhofer A., Helma J., Schmidthals K., Frauer C., Cui S., Karcher A., Pellis M., Muyldermans S., Casas-Delucchi C. S., Cardoso M. C., Leonhardt H., Hopfner K. P., and Rothbauer U. (2010) Modulation of protein properties in living cells using nanobodies. Nat. Struct. Mol. Biol. 17, 133–138 10.1038/nsmb.1727 [DOI] [PubMed] [Google Scholar]
- 53. Caussinus E., Kanca O., and Affolter M. (2011) Fluorescent fusion protein knockout mediated by anti-GFP nanobody. Nat. Struct. Mol. Biol. 19, 117–121 10.1038/nsmb.2180 [DOI] [PubMed] [Google Scholar]
- 54. Rothbauer U., Zolghadr K., Tillib S., Nowak D., Schermelleh L., Gahl A., Backmann N., Conrath K., Muyldermans S., Cardoso M. C., and Leonhardt H. (2006) Targeting and tracing antigens in live cells with fluorescent nanobodies. Nat. Methods 3, 887–889 10.1038/nmeth953 [DOI] [PubMed] [Google Scholar]
- 55. Moutel S., Bery N., Bernard V., Keller L., Lemesre E., de Marco A., Ligat L., Rain J. C., Favre G., Olichon A., and Perez F. (2016) NaLi-H1: a universal synthetic library of humanized nanobodies providing highly functional antibodies and intrabodies. eLife 5, e16228 10.7554/eLife.16228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li T., Qi S., Unger M., Hou Y. N., Deng Q. W., Liu J., Lam C. M. C., Wang X. W., Xin D., Zhang P., Koch-Nolte F., Hao Q., Zhang H., Lee H. C., and Zhao Y. J. (2016) Immuno-targeting the multifunctional CD38 using nanobody. Sci. Rep. 6, 27055 10.1038/srep27055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gentry H. R., Singer A. U., Betts L., Yang C., Ferrara J. D., Sondek J., and Parise L. V. (2005) Structural and biochemical characterization of CIB1 delineates a new family of EF-hand-containing proteins. J. Biol. Chem. 280, 8407–8415 10.1074/jbc.M411515200 [DOI] [PubMed] [Google Scholar]
- 58. White C., Yang J., Monteiro M. J., and Foskett J. K. (2006) CIB1, a ubiquitously expressed Ca2+-binding protein ligand of the InsP3 receptor Ca2+ release channel. J. Biol. Chem. 281, 20825–20833 10.1074/jbc.M602175200 [DOI] [PubMed] [Google Scholar]
- 59. Bulaj G., Kortemme T., and Goldenberg D. P. (1998) Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry 37, 8965–8972 10.1021/bi973101r [DOI] [PubMed] [Google Scholar]
- 60. Wu Y., Zhang J., Fang L., Lee H. C., and Zhao Y. J. (2019) A cytosolic chaperone complex controls folding and degradation of type III CD38. J. Biol. Chem. 294, 4247–4258 10.1074/jbc.RA118.005844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kaushik S., and Cuervo A. M. (2018) The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 19, 365–381 10.1038/s41580-018-0001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pedrozo Z., Torrealba N., Fernández C., Gatica D., Toro B., Quiroga C., Rodriguez A. E., Sanchez G., Gillette T. G., Hill J. A., Donoso P., and Lavandero S. (2013) Cardiomyocyte ryanodine receptor degradation by chaperone-mediated autophagy. Cardiovasc. Res. 98, 277–285 10.1093/cvr/cvt029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang D. W., Peng Z. J., Ren G. F., and Wang G. X. (2015) The different roles of selective autophagic protein degradation in mammalian cells. Oncotarget 6, 37098–37116 10.18632/oncotarget.5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shen S., Zhang P., Lovchik M. A., Li Y., Tang L., Chen Z., Zeng R., Ma D., Yuan J., and Yu Q. (2009) Cyclodepsipeptide toxin promotes the degradation of Hsp90 client proteins through chaperone-mediated autophagy. J. Cell Biol. 185, 629–639 10.1083/jcb.200810183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Avci D., and Lemberg M. K. (2015) Clipping or extracting: two ways to membrane protein degradation. Trends Cell Biol. 25, 611–622 10.1016/j.tcb.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 66. Stockton J. D., Merkert M. C., and Kellaris K. V. (2003) A complex of chaperones and disulfide isomerases occludes the cytosolic face of the translocation protein Sec61p and affects translocation of the prion protein. Biochemistry 42, 12821–12834 10.1021/bi035087q [DOI] [PubMed] [Google Scholar]
- 67. Higy M., Junne T., and Spiess M. (2004) Topogenesis of membrane proteins at the endoplasmic reticulum. Biochemistry 43, 12716–12722 10.1021/bi048368m [DOI] [PubMed] [Google Scholar]
- 68. Dasari V. R., Anandatheerthavarada H. K., Robin M. A., Boopathi E., Biswas G., Fang J. K., Nebert D. W., and Avadhani N. G. (2006) Role of protein kinase C-mediated protein phosphorylation in mitochondrial translocation of mouse CYP1A1, which contains a non-canonical targeting signal. J. Biol. Chem. 281, 30834–30847 10.1074/jbc.M510725200 [DOI] [PubMed] [Google Scholar]
- 69. Dai N., Christiansen J., Nielsen F. C., and Avruch J. (2013) mTOR complex 2 phosphorylates IMP1 cotranslationally to promote IGF2 production and the proliferation of mouse embryonic fibroblasts. Genes Dev. 27, 301–312 10.1101/gad.209130.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Oh W. J., Wu C. C., Kim S. J., Facchinetti V., Julien L. A., Finlan M., Roux P. P., Su B., and Jacinto E. (2010) mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. EMBO J. 29, 3939–3951 10.1038/emboj.2010.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vitrac H., Bogdanov M., and Dowhan W. (2013) In vitro reconstitution of lipid-dependent dual topology and postassembly topological switching of a membrane protein. Proc. Natl. Acad. Sci. U.S.A. 110, 9338–9343 10.1073/pnas.1304375110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vitrac H., MacLean D. M., Jayaraman V., Bogdanov M., and Dowhan W. (2015) Dynamic membrane protein topological switching upon changes in phospholipid environment. Proc. Natl. Acad. Sci. U.S.A. 112, 13874–13879 10.1073/pnas.1512994112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Craig E. A. (2018) Hsp70 at the membrane: driving protein translocation. BMC Biol. 16, 11 10.1186/s12915-017-0474-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Barlowe C. K., and Miller E. A. (2013) Secretory protein biogenesis and traffic in the early secretory pathway. Genetics 193, 383–410 10.1534/genetics.112.142810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bruzzone S., Guida L., Zocchi E., Franco L., and De Flora A. (2001) Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 15, 10–12 10.1096/fj.00-0566fje [DOI] [PubMed] [Google Scholar]
- 76. Guida L., Bruzzone S., Sturla L., Franco L., Zocchi E., and De Flora A. (2002) Equilibrative and concentrative nucleoside transporters mediate influx of extracellular cyclic ADP-ribose into 3T3 murine fibroblasts. J. Biol. Chem. 277, 47097–47105 10.1074/jbc.M207793200 [DOI] [PubMed] [Google Scholar]
- 77. Lee H. C. (2000) Enzymatic functions and structures of CD38 and homologs. Chem. Immunol. 75, 39–59 10.1159/000058774 [DOI] [PubMed] [Google Scholar]
- 78. Lee H. C. (2003) Calcium signaling: NAADP ascends as a new messenger. Curr. Biol. 13, R186–R188 10.1016/S0960-9822(03)00120-9 [DOI] [PubMed] [Google Scholar]
- 79. Lee H. C. (1997) Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol. Rev. 77, 1133–1164 10.1152/physrev.1997.77.4.1133 [DOI] [PubMed] [Google Scholar]
- 80. Graeff R., Liu Q., Kriksunov I. A., Hao Q., and Lee H. C. (2006) Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine NAADP synthesis and hydrolysis activities. J. Biol. Chem. 281, 28951–28957 10.1074/jbc.M604370200 [DOI] [PubMed] [Google Scholar]
- 81. Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S., and Galione A. (2002) NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111, 703–708 10.1016/S0092-8674(02)01082-6 [DOI] [PubMed] [Google Scholar]
- 82. Galione A. (2006) NAADP, a new intracellular messenger that mobilizes Ca2+ from acidic stores. Biochem. Soc. Trans. 34, 922–926 10.1042/BST0340922 [DOI] [PubMed] [Google Scholar]
- 83. Fang C., Li T., Li Y., Xu G. J., Deng Q. W., Chen Y. J., Hou Y. N., Lee H. C., and Zhao Y. J. (2018) CD38 produces nicotinic acid adenosine dinucleotide phosphate in the lysosome. J. Biol. Chem. 293, 8151–8160 10.1074/jbc.RA118.002113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Deng Q. W., Zhang J., Li T., He W. M., Fang L., Lee H. C., and Zhao Y. J. (2019) The transferrin receptor CD71 regulates type II CD38, revealing tight topological compartmentalization of intracellular cyclic ADP-ribose production. J. Biol. Chem. 294, 15293–15303 10.1074/jbc.RA119.010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bruzzone S., Franco L., Guida L., Zocchi E., Contini P., Bisso A., Usai C., and De Flora A. (2001) A self-restricted CD38-connexin 43 cross-talk affects NAD+ and cyclic ADP-ribose metabolism and regulates intracellular calcium in 3T3 fibroblasts. J. Biol. Chem. 276, 48300–48308 10.1074/jbc.M107308200 [DOI] [PubMed] [Google Scholar]
- 86. Partida-Sánchez S., Cockayne D. A., Monard S., Jacobson E. L., Oppenheimer N., Garvy B., Kusser K., Goodrich S., Howard M., Harmsen A., Randall T. D., and Lund F. E. (2001) Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat. Med. 7, 1209–1216 10.1038/nm1101-1209 [DOI] [PubMed] [Google Scholar]
- 87. Essuman K., Summers D. W., Sasaki Y., Mao X., DiAntonio A., and Milbrandt J. (2017) The SARM1 Toll/interleukin-1 receptor domain possesses intrinsic NAD+ cleavage activity that promotes pathological axonal degeneration. Neuron 93, 1334–1343.e5 10.1016/j.neuron.2017.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhao Z. Y., Xie X. J., Li W. H., Liu J., Chen Z., Zhang B., Li T., Li S. L., Lu J. G., Zhang L., Zhang L. H., Xu Z., Lee H. C., and Zhao Y. J. (2019) A cell-permeant mimetic of NMN activates SARM1 to produce cyclic ADP-ribose and induce non-apoptotic cell death. iScience 15, 452–466 10.1016/j.isci.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gerdts J., Brace E. J., Sasaki Y., DiAntonio A., and Milbrandt J. (2015) SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 348, 453–457 10.1126/science.1258366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kwong A. K., Chen Z., Zhang H., Leung F. P., Lam C. M., Ting K. Y., Zhang L., Hao Q., Zhang L. H., and Lee H. C. (2012) Catalysis-based inhibitors of the calcium signaling function of CD38. Biochemistry 51, 555–564 10.1021/bi201509f [DOI] [PubMed] [Google Scholar]
- 91. Panneerselvam P., Singh L. P., Ho B., Chen J., and Ding J. L. (2012) Targeting of pro-apoptotic TLR adaptor SARM to mitochondria: definition of the critical region and residues in the signal sequence. Biochem. J. 442, 263–271 10.1042/BJ20111653 [DOI] [PubMed] [Google Scholar]
- 92. Gerdts J., Summers D. W., Sasaki Y., DiAntonio A., and Milbrandt J. (2013) Sarm1-mediated axon degeneration requires both SAM and TIR interactions. J. Neurosci. 33, 13569–13580 10.1523/JNEUROSCI.1197-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li T., Li S. L., Fang C., Hou Y. N., Zhang Q., Du X., Lee H. C., and Zhao Y. J. (2018) Nanobody-based dual epitopes protein identification (DepID) assay for measuring soluble CD38 in plasma of multiple myeloma patients. Anal. Chim. Acta 1029, 65–71 10.1016/j.aca.2018.04.061 [DOI] [PubMed] [Google Scholar]