Abstract
Endothelial cells have key functions in endothelial barrier integrity and in responses to angiogenic signals that promote cell proliferation, cell migration, cytoskeletal reorganization, and formation of new blood vessels. These functions highly depend on protein–protein interactions in cell–cell junction and cell attachment complexes and on interactions with cytoskeletal proteins. Protein phosphatase 2A (PP2A) dephosphorylates several target proteins involved in cytoskeletal dynamics and cell adhesion. Our goal was to find new interacting and substrate proteins of the PP2A-B55α holoenzyme in bovine pulmonary endothelial cells. Using LC-MS/MS analysis, we identified flotillin-1 as a protein that binds recombinant GSH S-transferase–tagged PP2A-B55α. Immunoprecipitation experiments, proximity ligation assays, and immunofluorescent staining confirmed the interaction between these two endogenous proteins in endothelial cells. Originally, flotillins were described as regulatory proteins for axon regeneration, but they appear to function in many cellular processes, such as membrane receptor signaling, endocytosis, and cell adhesion. Ser315 is a known PKC-targeted site in flotillin-1. Utilizing phosphomutants of flotillin-1 and the NanoBiT luciferase assay, we show here that phosphorylation/dephosphorylation of Ser315 in flotillin-1 significantly affects its interaction with PP2A-B55α and that PP2A-B55α dephosphorylates phospho-Ser315. Spreading, attachment, migration, and in vitro tube formation rates of S315A variant–overexpressing cells were faster than those of nontransfected or S315D-transfected cells. These results indicate that the PP2A–flotillin-1 interaction identified here affects major physiological activities of pulmonary endothelial cells.
Keywords: protein phosphatase 2 (PP2A), endothelial cell, angiogenesis, cell migration, phosphorylation
Introduction
Protein phosphatase 2A (PP2A) is one of the main serine/threonine phosphatases in the cell involved in the regulation of numerous signaling pathways, and it is also known as a potent tumor suppressor (1, 2). PP2A is a heterotrimeric holoenzyme composed of a core dimer structure containing a scaffolding A subunit (PP2A A) and a catalytic C subunit (PP2A C) (3). Specificity of PP2A is achieved through association of the core dimer with a third, variable B regulatory subunit. At least four structurally different families of the regulatory subunits have been identified: B (B55), B' (B56), B” (PR72), and B”' (PR93) (4). The type of the B subunit is the key to determining the substrate specificity and subcellular localization of the holoenzyme. Our recent data point out that the ABαC holoenzyme form of PP2A is essential in endothelial cell (EC)2 barrier integrity in micro- and macrovascular endothelial cells. Furthermore, we detected association of Bα (also known as B55) and adherent junction proteins, suggesting that the activity of the ABαC holoenzyme form of PP2A is necessary for functional adherent junctions in ECs (5–7).
The flotillin protein family consists of two ubiquitously expressed proteins, flotillin-1 and flotillin-2, also known as reggie-2 and reggie-1, respectively. They are highly conserved, and their protein sequences are about 50% identical (8). Flotillins contain an N-terminal stomatin/prohibitin/flotillin/HflK/C or prohibitin homology domain and a C-terminal “flotillin domain” containing alanine and glutamic acid repeats (8). Flotillin-1 is expressed most abundantly in hematopoietic cells and the brain, heart, and lungs (9, 10). Within cells, flotillin-1 has been shown to be present in membranes, endosomes, the Golgi, and the nucleus (11–13). Flotillins are present in cells as monomers and homo- or heteromers (14). Although flotillin-1 is recognized as a lipid raft protein (15), earlier findings also indicate its wide subcellular distribution depending on the cell type (16). In some cell lines, flotillin-1 localizes in the plasma membrane (HepG2 and HeLa), in contrast with its intracellular localization found in Madin-Darby canine kidney cells (17).
Several posttranslational sites have been identified on flotillins. Flotillins attach to the cytosolic side of the plasma membrane via fatty acid modification (18, 19). Palmitoylation has been shown at Cys34 of flotillin-1 (19) and Cys4, Cys19, and Cys20 of flotillin-2 (18, 20). This modification of Cys34 is essential in membrane localization of flotillin-1 in kidney cells (19) but not in adipocytes (17). Fyn kinase phosphorylates flotillin-1 at Tyr160 (21) and flotillin-2 at Tyr163. The Tyr163 residue of flotillin-2 and the flotillin domain are important in hetero- and homo-oligomer formation of flotillin-1 and flotillin-2 (18, 22).
Recent results indicate that activated PKC triggers endocytosis by phosphorylating flotillin-1 on Ser315 (23). Beyond endocytosis, flotillin-1 is considered to function in signaling and interactions with the cytoskeleton (24). Dephosphorylation of phospho-Ser315 of flotillin-1 has not yet been studied, and the phosphatase responsible for dephosphorylation of this site is still unknown.
In this work, flotillin-1 was identified as a new interacting partner of the Bα subunit–containing PP2A holoenzyme. We show evidence that dephosphorylation of phospho-Ser315 of flotillin-1 promotes angiogenesis and cell migration of pulmonary artery endothelial cells.
Results
The PP2A Bα holoenzyme interacts with flotillin-1 in endothelial cells
Regulatory B subunits of PP2A strongly influence substrate specificity and protein interactions of PP2A. Therefore, to reveal new interacting partners or substrates of the Bα regulatory subunit–containing PP2A holoenzyme in endothelial cells, a recombinant construct of PP2A Bα suitable for bacterial protein expression was made. GST-PP2A Bα was successfully expressed and purified on GSH-Sepharose 4B beads. First, a pulldown assay was carried out to test the ability of the recombinant GST-PP2A Bα to bind endogenous PP2A A and C subunits from endothelial cell lysate (Fig. S1A). Then pulldown samples resolved by SDS-PAGE were stained with Coomassie Blue dye solution, and the patterns of protein bands were compared (Fig. S1B). A band with the approximate size of 46–48 kDa was present in the GST-PP2A Bα sample incubated with bovine pulmonary artery endothelial cell (BPAEC) lysate, but it was missing from all control samples. Flotillin-1 (FLOT-1, UniProt Q08DN8) was identified by MS from that piece of the gel. The interaction between PP2A Bα and flotillin-1 was confirmed by Western blot analysis of the GST-PP2A Bα pulldown samples (Fig. 1A).
Figure 1.
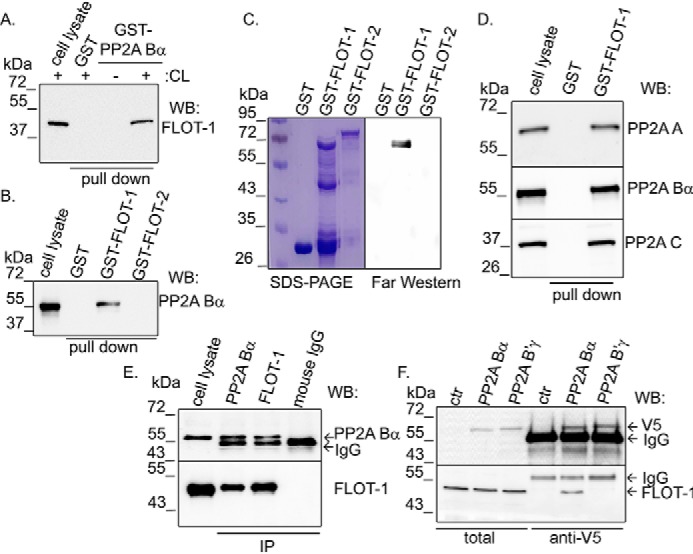
Interaction between PP2A and flotillin-1. A, bacterially expressed GST and GST-tagged PP2A Bα were loaded onto GSH-Sepharose. Samples were incubated with lysis buffer or BPAEC lysate (CL). Western blotting (WB) of the endothelial cell lysate and the eluted fractions after pulldown were probed with a flotillin-1 (FLOT-1)–specific antibody. B, GST, GST–flotillin-1, or GST–flotillin-2 immobilized on GSH-Sepharose was incubated with BPAEC lysate (pulldown). Interaction with PP2A Bα protein was checked in a Western blot experiment. C, interaction of GST, GST–flotillin-1, and GST–flotillin-2 with PP2A Bα was analyzed by far-Western blotting. Efficiency of protein purification was visualized by Coomassie Blue staining. D, GST and GST–flotillin-1 recombinant proteins were immobilized on GSH–Sepharose 4B and incubated with BPAEC lysate. Eluted proteins were analyzed with antibodies specific for PP2A subunits by Western blotting. E, PP2A Bα or flotillin-1 was immunoprecipitated from BPAEC lysate. Total cell lysate and IP complexes were probed for PP2A Bα and flotillin-1 by Western blotting. Control IP was made with isotype-matched mouse IgG. F, lysates of pcDNA3.1 V5-His PP2A Bα– and pcDNA3.1 V5-His PP2A B'γ–transfected BPAECs were incubated with anti-V5-agarose affinity gel. Total cell lysates and samples eluted from the affinity gel were tested with flotillin-1– and V5 tag–specific antibodies by Western blotting. ctr, control.
Flotillin-1 and flotillin-2 are highly similar and abundant proteins in the cell; therefore, the specificity of the flotillin-PP2A interaction was tested. Both flotillin-1 and flotillin-2 were cloned from endothelial cell cDNA, and pGEX-4T-2 constructs were created to produce GST–flotillin-1 and GST–flotillin-2 proteins. A GST pulldown assay (Fig. 1B and Fig. S1C) and far-Western blotting (Fig. 1C) were performed with GST, GST–flotillin-1, and GST–flotillin-2 as bait. Although GST–flotillin-1 is more sensitive to protein degradation compared with GST–flotillin-2, the results showed that only GST–flotillin-1 was able to interact with PP2A Bα. To prove that GST–flotillin-1 binds to the holoenzyme form of PP2A, pulldown samples were also tested with PP2A A subunit– and PP2A C subunit–specific antibodies (Fig. 1D and Fig. S1D). Further, immunoprecipitation using flotillin-1– and PP2A Bα–specific antibodies confirmed that there is an interaction between endogenous flotillin-1 and PP2A Bα in endothelial cells (Fig. 1E). Flotillin-1 was coimmunoprecipitated with PP2A Bα and vice versa.
The specificity of flotillin-1 interaction with the PP2A B subunit was studied further. Endothelial cells were transfected with the pcDNA V5-His PP2A Bα and pcDNA V5-His PP2A B'γ constructs, and the V5-tagged PP2A Bα and PP2A B'γ proteins were purified with anti-V5 affinity gel. The efficiency of overexpression and purification of Bα and B'γ was sufficient (Fig. 1F, top). Western blot analysis demonstrated binding of flotillin-1 to Bα, but no interaction was detected with B'γ (Fig. 1F, bottom). Taken together, all of the above results prove that flotillin-1 specifically interacts with the Bα subunit–containing PP2A holoenzyme in endothelial cells.
Localization of flotillin-1 is affected by PP2A activity
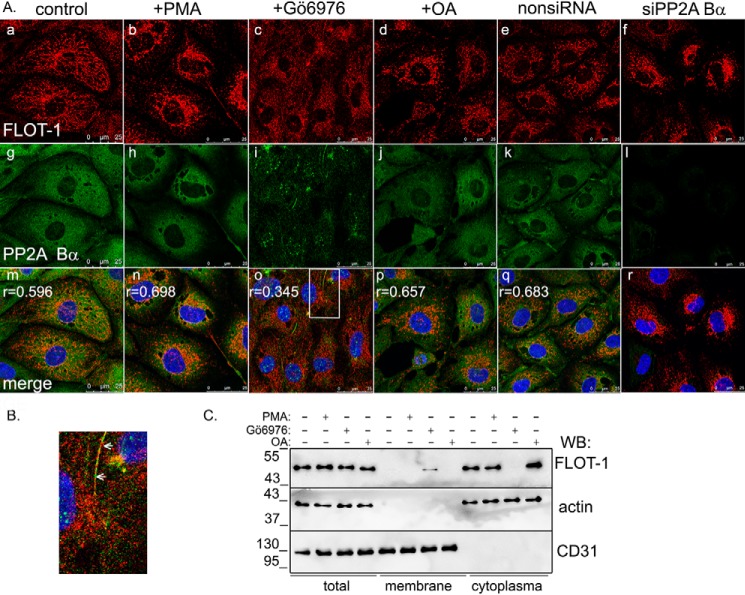
Next we intended to examine the physiological relevance of the interaction. Endogenous PP2A Bα showed homogenous localization in control cells, as expected (Fig. 2A, g) (5). Flotillin-1 was present in the cytoplasm of endothelial cells (Fig. 2A, a), as shown by immunofluorescent staining and merging of the confocal images, and the high value of the Pearson's coefficient demonstrates colocalization of the two proteins (Fig. 2A, m). As flotillin-1 is able to interact with membranes, possible colocalization with cell organelles was checked (Fig. S2B), but no specific interaction was found. Flotillin-1 is subjected to phosphorylation by Fyn and PKC kinases on Tyr160 and Ser315, respectively (21, 23). PP2A is a phospho-Ser/Thr–specific phosphatase; therefore, we focused on the latter as a potential phosphosite for PP2A-driven dephosphorylation. To test the probable participation of PP2A in dephosphorylation of flotillin-1, treatments affecting PP2A or PKC activities were utilized before immunofluorescent staining of ECs (Fig. 2A and Fig. S2A). PKC was stimulated by addition of PMA or inhibited by Gö6976. PP2A was inhibited by okadaic acid. siRNA-mediated depletion of the Bα regulatory subunit was also employed to affect PP2A activity, as PP2A was expected to fail to bind its substrates because of loss of the Bα-targeting subunit. Silencing of Bα had no effect on the expression of PP2A A, PP2A C, or PP2A Bγ subunits (Fig. S3A). Experiments were repeated with two individual siRNA, as indicated under “Experimental procedures.” When PP2A was inhibited by okadaic acid treatment of the cells (Fig. 2A, j) or via depletion of PP2A Bα (Fig. 2A, l), apparent accumulation of flotillin-1 was observed in the perinuclear region of the cells (Fig. 2A, d and f). Upon activation of PKC by PMA, the same localization change of flotillin-1 was detected (Fig. 2A, b), and the Pearson's coefficient value indicated more pronounced colocalization. No significant change occurred, however, in localization of PP2A Bα in cells challenged with okadaic acid or PMA (Fig. 2A, h and j). Interestingly, inhibition of PKC activity by Gö6976 resulted in translocation of flotillin-1 into the cell membrane (Fig. 2B and Fig. S2A, j). Subcellular fractionation of endothelial cells also proved the more pronounced cytoplasmic localization of flotillin-1 in control and okadaic acid– or PMA-treated cells, in contrast to its appearance in the membrane fraction of Gö6976-treated cells (Fig. 2C). We also observed a shift in the electrophoretic mobility of flotillin-1 in PMA-treated cells, suggesting that the protein was subjected to PKC phosphorylation (Fig. S3B). We hypothesized that the detected localization change of flotillin-1 is related to the reversible phosphorylation of flotillin-1.
Figure 2.
Subcellular localization of PP2A Bα and flotillin-1. A, immunofluorescence staining of control BPAECs and BPAECs treated with PMA (1 μm, 30 min), Gö6976 (1 μm, 30 min), OA (5 nm, 30 min), nonspecific siRNA (nonsiRNA), or PP2A Bα–specific siRNA (50 nm) using anti-flotillin-1 (red) and anti-PP2A Bα (green) primary antibodies. Nuclei of cells were stained with DAPI (blue). Scale bars = 25 μm; r values (m–q) are Pearson's cross-correlation coefficients indicating colocalization of flotillin-1 and PP2A Bα. B, magnified region (marked by the white rectangle in A, o) of Gö6976-treated BPAECs. Arrows indicates flotillin-1 in the membrane region. C, cytoplasmic and membrane fractions were isolated from control BPAECs and BPAECs treated with PMA (1 μm, 30 min), Gö6976 (1 μm, 30 min), or OA (5 nm, 30 min). Total lysate and cell fractions were analyzed with anti-flotillin-1, anti-actin (cytoplasmic marker), and anti-CD31 (membrane marker) antibodies. WB, Western blot.
Phosphorylation state of Ser315 in flotillin-1 affects the interaction with PP2A Bα
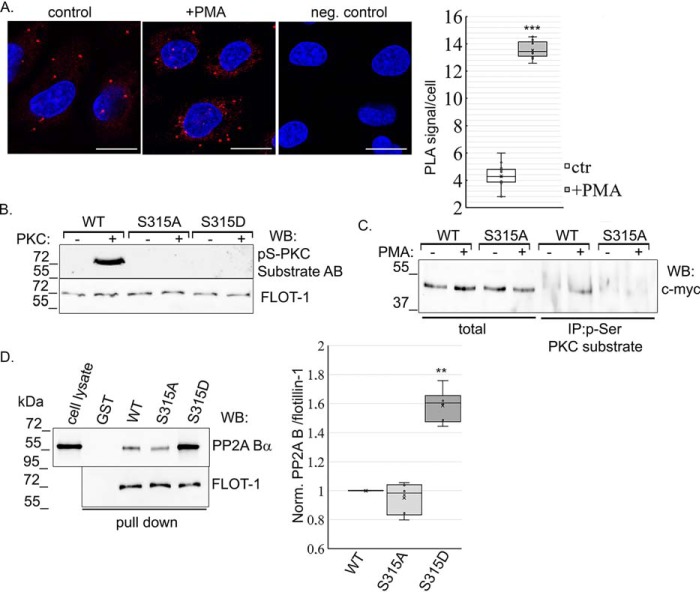
To further analyze the interaction upon PKC activation, a proximity ligation assay (PLA) was carried out on control and PMA-treated cells (Fig. 3A). PLA is an efficient method to test endogenous protein–protein interaction in cells, where signals are visualized as individual fluorescent dots (25). PLA signals were counted and expressed as signal per cell. In untreated control cells, interaction was detected between flotillin-1 and PP2A Bα. However, significantly more spots were present after PMA treatment, indicating enhanced association between the phosphorylated form of flotillin-1 and PP2A Bα.
Figure 3.
PKC enhances the PP2A Bα–flotillin-1 interaction. A, direct interaction between PP2A Bα and flotillin-1 was confirmed by Duolink in situ PLA using anti-PP2A Bα and anti-flotillin-1 primary antibodies on control (ctrl) and PMA-treated (1 μm, 30 min) endothelial cells. As a negative control (neg. control), cells were stained only with PP2A Bα antibody. Nuclei were stained with DAPI (blue). Scale bar = 50 μm. PLA signals were counted and expressed as signal per cell. Statistical analysis of PLA was done with a t test. B, recombinant GST–flotillin-1 WT, GST–flotillin-1 S315A, and GST–flotillin-1 S315D proteins were purified, and in vitro PKC assays were performed. Proteins were incubated with or without active PKC for 30 min at 30 °C. Phosphorylation of flotillin-1 was analyzed in a Western blot experiment using anti-phospho-Ser PKC substrate and flotillin-1 antibodies (AB). C, BPAECs were transfected with the pcDNA 3.1 myc-His flotillin-1 WT and flotillin-1 S315A recombinant plasmids. Immunoprecipitation was performed using untreated and PMA-treated (1 μm, 30 min) cell lysates using phospho-Ser PKC substrate antibody. Total cell lysates and IP complexes were tested with anti-c-myc antibody by Western blotting (WB). D, bacterially expressed GST and GST-tagged flotillin-1 WT, phospho-null (S315A) and phosphomimic (S315D) recombinant proteins immobilized on GSH-Sepharose were incubated with BPAEC lysate. Endothelial cell lysate and the eluted proteins were tested with PP2A Bα– and flotillin-1–specific antibodies by Western blotting. Evaluation of data is reported as mean ± S.D. Statistical analysis was done using Student's t test. **, p < 0.01; and ***, p < 0.001.
PKC site (Ser315) mutants of flotillin-1 were then created. Ser-to-Ala–encoding (phospho-null) and Ser-to-Asp–encoding (phosphomimic) plasmids were made by site-directed mutagenesis, and GST-tagged proteins were produced. In vitro PKC assays were performed using the purified GST–flotillin-1 WT, GST–flotillin-1 S315A, and GST–flotillin-1 S315D proteins. Phosphorylation of the recombinants was detected by Western blotting using a phospho-Ser PKC substrate–specific antibody (Fig. 3B). This antibody recognizes proteins phosphorylated at a serine residue at the PKC consensus sequence. An equal amount of loaded proteins was verified using a flotillin-1–specific antibody. PKC phosphorylated WT GST–flotillin-1, but no phosphorylation was detected in the phospho-null or phosphomimic flotillin-1 samples, indicating the specificity of the antibody and that S315 is the only PKC phosphorylation site in flotillin-1. Also, endothelial cells were transfected with pcDNA 3.1 myc-His A flotillin-1 (WT) and pcDNA 3.1 myc-His A–flotillin-1 S315A (phospho-null) constructs to perform immunoprecipitation from control and PMA-treated cells using the phospho-Ser PKC substrate–specific antibody. Total lysates and IP complexes were analyzed with c-myc–specific antibody by Western blotting (Fig. 3C). The overexpression level of proteins was about the same in the total lysates. Similar to the in vitro kinase assay results, only phosphorylated WT flotillin-1 was detectable in the IP samples, implying that Ser315 is indeed the sole PKC site in flotillin-1.
In agreement with the above result of PLA studies, more PP2A Bα binds to the phosphomimic (S315D) mutant of flotillin-1 than to the WT or phospho-null (S315A) forms of flotillin-1, as shown by a pulldown assay (Fig. 3D). This result suggests that phosphorylation of flotillin-1 evokes a conformational change of the protein that augments the protein–protein interaction.
PP2A dephosphorylates flotillin-1 in endothelial cells
NanoBiT is a luminescence-based, two-subunit system that can be used to detect protein–protein interaction, and it can be followed in real time in living cells (26). The LgBiT (17.6 kDa) and SmBiT (11 amino acids) subunits are fused to two specific proteins. When interaction occurs between the expressed proteins of interest, LgBiT and SmBiT are linked and generate a luminescent signal. Constructs suitable for the NanoBiT system were created, and BPAECs were cotransfected with pBiT1.1-C TK/LgBiT-PP2A Bα in pairs with pBiT2.1-C-TK/SmBiT flotillin-1 WT, pBiT2.1-C-TK/SmBiT flotillin-1 S315A, and pBiT2.1-C-TK/SmBiT flotillin-1 S315D constructs. The stable, highly luminescent signals verified the interaction between all three forms of flotillin-1 and PP2A Bα. Similar to the pulldown results (Fig. 3C), the strongest luminescent signal (beside the positive control of the NanoBit system (Fig. S4)) was produced when PP2A Bα interacted with the phosphomimic S315D form of flotillin-1 (Fig. 4A, blue line). In parallel experiments, PKC was activated by addition of 1 μm PMA at 5 min of measurement. No apparent change was observed with the phosphomutant forms of flotillin-1 in which Ser315, the PKC site, was replaced with Ala or Asp. However, the luminescent signal reflecting the interaction between WT flotillin-1 and PP2A Bα started to become stronger upon addition of PMA, and after about 10 min, the signal reached its maximum (Fig. 4A, dark green line). After that, the signal decreased with time, indicating a weakening in the interaction of the proteins. In contrast, the WT flotillin-1 and PP2A Bα association signal decreased when the samples were treated with okadaic acid (Fig. 4A, yellow line). The observed reversible change in the interaction can be associated with the consecutive phosphorylation and dephosphorylation of flotillin-1.
Figure 4.
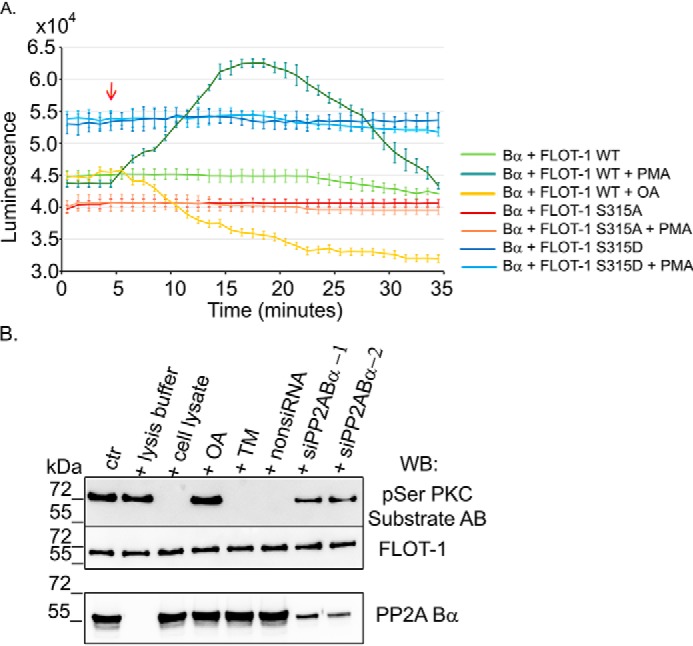
Phosphorylation of Ser315 in flotillin-1 augments its interaction with PP2A. A, a NanoBiT luciferase complementation assay was used to follow the interaction between WT and phosphomutant forms of flotillin-1 and PP2A Bα with or without treatment in real time. BPAECs were cotransfected with LgBiT-PP2A Bα in pair with SmBiT-flotillin-1 WT, -S315A, or -S315D constructs. 24 h post-transfection, luminescence was recorded for 35 min. 1 μm PMA, 5 nm OA, or vehicle was added at 5 min (red arrow) to follow the effect of PKC activation or PP2A inhibition on the interactions. B, dephosphorylation of phospho-flotillin-1 (ctr) was tested with addition of lysis buffer, nontransfected BPAEC cell lysates with or without pretreatment for 10 min with 5 nm OA or 1 μm tautomycetin (TM), and nonspecific siRNA (nonsiRNA) or PP2A Bα–specific siRNA–transfected BPAEC cell lysates. Lysates were tested for PP2A Bα levels by Western blotting (WB). Samples were analyzed by Western blotting using an antibody specific for phospho-Ser PKC substrate proteins and a flotillin-1 antibody.
In vitro dephosphorylation of phospho-flotillin-1 was also tested. GST–flotillin-1 was phosphorylated in vitro by active PKC and then incubated with lysis buffer, cell lysate, or cell lysate pretreated with okadaic acid, tautomycetin, nonspecific siRNA, or siPP2A Bα (Fig. 4B). The cell lysate dephosphorylated the recombinant. Employment of okadaic acid and depletion of PP2A Bα blocked dephosphorylation of flotillin-1, but no effect of tautomycetin, a specific inhibitor of protein phosphatase 1 (PP1), was found. These results together indicate that phospho-flotillin-1 can be a substrate of PP2A Bα.
The phospho-null form of flotillin-1 shows enhanced membrane localization and plays a role in cell migration and angiogenesis
Next, subcellular localization of recombinant flotillin-1 proteins was examined. BPAECs were transfected with flotillin-1 WT–, flotillin-1 S315A–, and flotillin-1 S315D–encoding plasmids. Immunofluorescent staining of the overexpressed proteins revealed that the WT form of flotillin-1 showed a distribution similar to the endogenous protein and that the phosphomimic S315D form was enriched around the nucleus of cells, resembling the localization of endogenous flotillin-1 after activation of PKC or inhibition of PP2A (Figs. 5A and 2A). The phospho-null mutant spread in the entire cell, except for the nucleus, and VE-cadherin costaining indicated its membrane localization as well (Fig. 5A). In line with this, subcellular fractionation of transfected cells showed that only S315A flotillin-1 was present in the membrane fraction, but the WT or S315D recombinants were not detectable (Fig. 5B). In agreement with the observed localizations, WT flotillin-1 appeared in the membrane region when cells were challenged with a PKC inhibitor (Fig. 5, A and C).
Figure 5.

Subcellular localization of flotillin-1 phosphomutants. A, immunofluorescent staining of pcDNA3.1/myc-His A-flotillin-1 WT, Gö6976-treated (1 μm, 30 min) WT, and phospho-null (S315A) and phosphomimic (S315D) flotillin-1–transfected BPAECs was performed using a tag-specific c-myc antibody (green). A VE-cadherin (red) antibody was used to detect cell membranes, and nuclei were stained with DAPI (blue). Magnified sections (marked by white rectangles) on merged images show membrane regions of the cells. Scale bars = 25 μm. B, the membrane fraction was isolated from control, flotillin-1 WT and flotillin-1 S315A– and flotillin-1 S315D–transfected BPAECs. Total lysates and membrane fractions were analyzed with anti-c-myc, anti-actin (cytoplasmic marker), and anti-CD31 (membrane marker) antibodies. CTR, control; WB, Western blot. C, membrane and cytoplasmic fractions were made from untreated and Gö6976-treated cells transfected with pcDNA3.1/myc-His A-flotillin-1 WT. Fractions were analyzed by Western blotting.
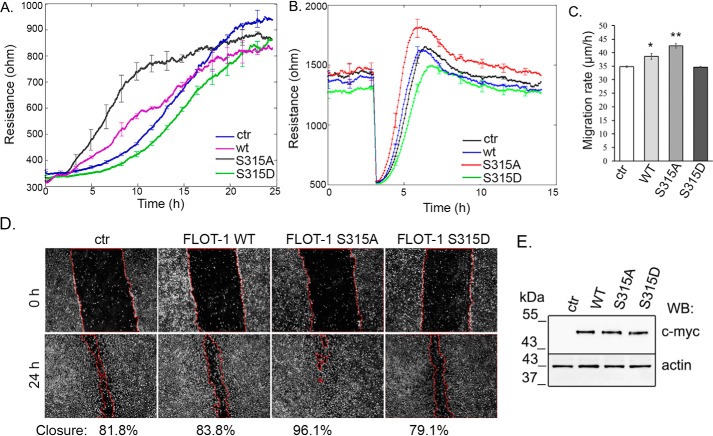
To explore the function of dephosphorylated flotillin-1 at the cell membrane of endothelial cells, electric cell substrate impedance sensing (ECIS) measurements were made on BPAECs overexpressing different forms of flotillin-1. First, cell spreading and attachment of control and flotillin-1 WT and flotillin-1 S315A– and flotillin-1 S315D–overexpressing cells were investigated (Fig. 6A). At high frequency, ECIS measures only the cell–matrix interaction. The resistance value is directly proportional to the number of cells attaching to the surface. WT and flotillin-1 S315A–overexpressing cells showed faster attachment and spreading compared with control or flotillin-1 S315D–overexpressing ones. Next, the migration rate of cells was compared using an in vitro wound healing assay, also measured by ECIS (Fig. 6B). At lower frequency, ECIS provides information about cell–cell interactions. After the cells achieved monolayer density (about 1200–1400 ohm), an alternate current was applied for 30 s to establish wounds in the cell layer. Neighboring healthy cells immediately migrated inward to replace the dead cells. The impedance in each wounded well increased gradually until it reached a maximum plateau value. The migration rates of flotillin-1 WT and flotillin-1 S315A–expressing cells were significantly higher than those of the control or flotillin-1 S315D–expressing ones (Fig. 6C). A scratch assay performed on control cells and cells overexpressing different flotillin-1 forms showed the same results (Fig. 6D). Western blot analysis of cells after the scratch assay showed the same expression levels of different forms of flotillin-1 (Fig. 6E). The angiogenic properties of cells in endothelial tube formation were also compared. Cells were seeded on Matrigel, and light microscopy and confocal microscopy pictures were taken (Fig. 7A). Capillary network formation was evaluated 5 h after seeding, and the tube length and branching points of flotillin-1–expressing cells were compared (Fig. 7B). Both the total length of tubes and the number of branching points were significantly higher for flotillin-1 WT and flotillin-1 S315A–expressing cells. These results demonstrate the regulatory role of the dephosphorylated form of flotillin-1 in important properties of endothelial cells related to their movement and attachment.
Figure 6.
The regulatory role of flotillin-1 is phosphorylation-dependent. A, spreading and attachment of nontransfected (ctr), WT, and mutant flotillin-1–transfected cells were followed in time using ECIS measurements. Results are presented as means ± S.D. of four chambers for each sample. B, an in vitro wound healing assay was performed with ECIS to measure the rate of cell migration as described under “Experimental procedures.” Results are presented as means ± S.D. of four chambers for each sample. C, statistical analysis of the in vitro wound healing assay was done with Student's t test. Data are reported as mean ± S.D. D, representative images from the wound healing scratch assay of control, WT, and S315A flotillin-1– and S315D flotillin-1–transfected cells. The pictures were taken 0 and 24 h after scratching. E, overexpression of different forms of flotillin-1 from the scratch assay was checked by Western blotting (WB) using anti-c-myc– and anti-actin–specific antibodies. *, p < 0.05; **, p < 0.01.
Figure 7.

Role of flotillin-1 in angiogenesis. A, nontransfected cells (ctr) and cells transfected with different forms of flotillin-1 were plated on Matrigel-coated μ-Slide angiogenesis plates. Tube formation of endothelial cells is shown by pictures taken with a Leica MC 120 HD microscope (a–d) and a Leica TCS SP8 confocal microscope (e–h) 5 h after seeding. Actin microfilaments were stained with Texas Red phalloidin (red). Scale bars = 250 μm (a–d) and 100 μm (e–h). B, evaluation of in vitro angiogenesis of control, flotillin-1 WT, and flotillin-1 S315A– and flotillin-1 S315D–transfected BPAEC samples. Data are reported as mean ± S.D. Statistical analysis was done with Student's t test.
Discussion
PP2A is a highly ubiquitous phospho-Ser/phospho-Thr–specific protein phosphatase. Two isoforms of the catalytic C subunit and the structural A subunit are known. The C isoforms are almost identical, and the β isoform of A exclusively binds the members of the B72 family. On the other hand, the primary sequences of the more than 20 members of the B subunit families are not even similar, except for a few conserved amino acids that are responsible for the interaction with the A subunit. The high variability in the multisubunit structure of the enzyme allows wide substrate specificity. Consequently, it was proven that PP2A is an active component in many signaling pathways of the cell. Our previous work showed a role of PP2A in barrier regulation of pulmonary artery endothelial cells by influencing the phosphorylation level of cytoskeletal and cell junction proteins (5–7). Overexpression of PP2Ac reduced the effects of thrombin and nocodazole on the actin cytoskeleton and the microtubule structure. Simultaneously, overexpression attenuated the weakening of the endothelial barrier because of administration of these agents (6). Specific inhibition of PP2A activity or silencing of the Bα subunit of PP2A, however, eliminated the reductions in the agonist-induced effects (5, 6). To acquire more definitive data regarding the role of PP2A in this cell type, we searched for protein partners of the most abundant regulatory subunit of PP2A, the Bα subunit. Flotillin-1 (also known as reggie-2), a 48-kDa protein, was identified by MS after selecting a specific band containing the protein(s) binding to the Bα subunit (bait) from an EC lysate in GST pulldown. The interaction has been proven by several further experimental methods, such as direct pulldown of recombinant proteins, immunoprecipitation, proximity ligation, and a NanoBit assay of native proteins.
Flotillin-1 and flotillin-2 are well-conserved proteins. Among flotillin proteins in vertebrates, there is a similarity of about 90% (8). The exact function of flotillin-1 is not yet known; nevertheless, several results suggest its involvement in numerous processes, including cell adhesion (27, 28), cellular trafficking (24, 29, 30), and signal transduction (31).
Our earlier findings regarding the essential role of the Bα containing PP2A in functional adherent junctions and barrier integrity of ECs (5) fit well the fact that, in several reports, flotillins are connected to cadherin-mediated intercellular adhesion (for a review, see Ref. 27). Further, because flotillin-1 bears a PKC phosphorylation site at Ser315 but there is no homologous site present in flotillin-2, our working hypothesis was that the role of flotillin-1 related to endothelial function is probably regulated via reversible phosphorylation of Ser315.
Although flotillins are mainly referred to as membrane-associated proteins (24, 32), their cellular distribution may change, and it is highly dependent on the cell type and conditions (21, 33–35). Fork et al. (31) reported flotillin-1 being colocalized with caveloin-1 predominantly within human umbilical vein endothelial cells. In the case of pulmonary artery ECs, we detected flotillin-1 in the cytoplasm with no specific pattern. Interestingly, when the cells were challenged to inhibit PP2A or activate/inhibit PKC, the localization of flotillin-1 changed. When PKC was activated, confocal images demonstrated a higher degree of colocalization of flotillin-1 and Bα. Also, augmented interaction of flotillin-1 and the phosphatase subunit was detected by proximity ligation assay after PMA treatment. Pulldown experiments revealed increased amounts of Bα binding to the phosphomutant S315D form of flotillin-1. Furthermore, comparison of the subcellular localization of the WT and phosphomutants of flotillin-1 demonstrated that only the phospho-null mutant was detectable in the membrane fraction, and inhibition of PKC evoked membrane localization of the WT. These findings suggest that the subcellular localization of flotillin-1 is phosphorylation-dependent. Similar phosphorylation-dependent localization changes are known for other proteins, including several PP2A substrates such as histone deacetylase 5 (36) and the kinase suppressor of Ras or Raf-1 (37).
A further conclusion of the colocalization studies is that flotillin-1 can be the substrate of PKC in endothelial cells. To prove this, as shown earlier (38), phosphosite-specific mutants of flotillin-1 and a phospho-Ser PKC-specific antibody were employed. An in vitro PKC kinase assay and PMA challenge of ECs overexpressing flotillin-1 resulted in phosphorylation of WT flotillin-1 only; however, the S315A and S315D forms of flotillin-1 could not be phosphorylated, strongly suggesting that PKC phosphorylates exclusively Ser315 in flotillin-1. Our results are in line with earlier findings of Cremona et al. (23) regarding stably transfected HEK293 cells. They reported PKC-triggered dopamine transporter endocytosis in connection with phosphorylation of flotillin-1 on Ser315.
Subcellular localization of flotillin-1 and Bα with and without PMA challenge and reversible modification of their interaction upon PKC activation detected by NanoBiT assay both imply that phospho-Ser315 flotillin-1 is a substrate for PP2A. Most importantly, we confirmed that PKC-phosphorylated flotillin-1 is dephosphorylated by a type 2A phosphatase.
Angiogenesis, endothelial barrier formation, and maintenance depends on migration, adhesion, and intercellular junctions of endothelial cells. Overexpression of the WT and phospho-null form of flotillin-1 significantly facilitated cell spreading, attachment, and migration of endothelial cells and increased tube length and number of branches during in vitro tube formation. PKC activation has been shown by others to cause gap formation and reduced adhesion of endothelial cells (39). Taken together, flotillin-1 assists with endothelial barrier formation and angiogenesis, and the phosphorylation state of Ser315 in flotillin-1, governed by PKC and PP2A activities, is crucial for the function of flotillin-1 in endothelial cells.
Another likely signaling consequence of the flotillin–PP2A interaction may be related to the important lipid signaling substance sphingosine-1-phosphate (S1P). The PP2A catalytic subunit has been described to deactivate sphingosine kinase 1, which converts sphingosine into S1P, but the relevant PP2A holoenzyme form was not identified (40). A more recent paper claims an essential role of flotillins in recruitment of sphingosine to the membrane to sustain the cellular S1P level (41). One may hypothesize that various holoenzyme forms of PP2A may cooperate to control the phosphorylation level of flotillin and sphingosine kinase and, consequently, cellular S1P concentration, which is thought to regulate the physiological properties of endothelial cells, such as vascular permeability, inflammation, and angiogenesis (42).
Conclusion
We have shown protein–protein interaction between flotillin-1 and the Bα subunit of protein phosphatase 2A. Ser315 in flotillin-1 is phosphorylated by PKC, and phospho-Ser315 is dephosphorylated by PP2A. The phosphatase–flotillin interaction is important for the physiological activities of endothelial cells. When flotillin-1 is dephosphorylated at Ser315, it facilitates endothelial barrier formation and angiogenesis.
Experimental procedures
Reagents
Materials were obtained from the following vendors: the pGEX-4T-2 vector from Clontech Laboratories Inc. (Mountain View, CA); the pcDNATM 3.1/myc-His (−) A vector from Life Technologies; anti-flotillin-1, anti-PP2A A, anti-PP2A Bα (rabbit polyclonal antibody), anti-PP2A Bα (2G9) mouse mAb, anti-PP2A B', anti-PP2A C, anti-phospho-(Ser) PKC substrate, anti-β-catenin, anti-VE-cadherin, anti-CD31 primary antibodies, and anti-rabbit IgG HRP-linked and anti-mouse IgG HRP-linked secondary antibodies from Cell Signaling Technology (Beverly, MA); Gö6976 (CAS 136194-77-9), PP2A-B55-a siRNA, and anti-lamin A/C (H-110) antibody from Santa Cruz Biotechnology (Santa Cruz, CA); anti-V5-agarose affinity gel from Sigma; restriction enzymes, T4 DNA ligase, and Phire Hot Start II DNA polymerase from Thermo Scientific (Vantaa, Finland); Alexa 488- and Alexa 594-conjugated secondary antibodies and ProLong Gold Antifade reagent with DAPI, Texas Red–phalloidin, LysoTrackerTM Deep Red, and MitoTrackerTM Red CMXRos special packaging from Molecular Probes/Invitrogen; and albumin bovine serum from VWR (Radnor, PA). All other chemicals were obtained from Sigma.
Cell culture and treatments
BPAECs (ATCC, culture line CCL 209) were maintained as described earlier (43). Phorbol 12-myristate 13-acetate (PMA), tautomycetin, Gö6976, and okadaic acid (OA) were dissolved in DMSO and utilized in serum-free medium.
Generation of constructs
The coding region of PP2A Bα was amplified for subcloning from the previously prepared pcDNA V5-His PP2A Bα template (5) using the following primers containing EcoRI and XhoI restriction sites: 5′-TTG AAT TCC CAT GGC AGG AGC TGG-3′ (forward) and 5′-CCA CTC GAG CTA ATT CAC TTT GTC TTG-3′ (reverse). The coding sequences of WT flotillin-1 and flotillin-2 were amplified from BPAEC cDNA using the following primer pairs containing EcoRI-XhoI and BamHI-XhoI restriction cloning sites, respectively: 5′-TGG AAT TCC TAT GTT TTT CAC TTG TGG CCC A-3′ (forward) and 5′-ATG CTC GAG TCA GGC TGT TCT CAA AGG C-3′ (reverse); 5′-TAT GGA TCC ATG GGC AAT TGC CAC ACG GT-3′ (forward) and 5′-AAA CTC GAG TCA CAC CTG GAC ACC AGT G-3′ (reverse). Each of these coding DNA fragments were inserted into pGEX-4T-2 vector suitable for bacterial protein expression. Ser315 mutants of flotillin-1 were derived from the pGEX-4T-2 flotillin-1 construct using back-to-back primer pairs with the same reverse primer (5′-/5Phos/TGC CTC CGC CTG CAT AAT TAG TTG GG-3′) and the following forward primers: S315A, 5′-/5Phos/GAA GCC GCG GCT GTG CGG ATG C-3′; S315D, 5′-/5Phos/GAA GCC GCG GAT GTG CGG ATG C-3′. Flotillin-1 WT and phosphomutant inserts were also subcloned into the pcDNA3.1/myc-His A (−) mammalian expression vector using the 5′-TGG AAT TCC TAT GTT TTT CAC TTG TGG CCC A-3′ (forward) and 5′-ATA TAA GCT TGG CTG TCC TCA AAG GCT TGT G-3′ (reverse) primers.
For the NanoBiT Protein:Protein Interaction System, the coding regions of PP2A Bα and flotillin-1 were amplified by PCR using the following primers: PP2A Bα, 5′-TAG AAT TCC ATG GCA GGA GCT GGA G-3′ (forward) and 5′-ATA CTC GAG GCA TTC ACT TTG TCT TGA AAT ATA TA-3′ (reverse); flotillin-1, 5′-GCG AAT TCC ATG TTT TTC ACT TGT GGC CCA-3′ (forward) and 5′-TAT CTC GAG ATG GCT GTT CTC AAA GGC TTG-3′ (reverse). PP2A Bα and flotillin-1 coding sequences were subcloned into the pBiT1.1-C TK/LgBiT and pBiT2.1-C-TK/SmBiT vectors, respectively. The pcDNA V5-His PP2A B'γ construct was prepared previously (5).
Bacterial expression, GST pulldown assay, and in vitro kinase assay
BL21 (DE3) Escherichia coli cells were transformed with pGEX-4T-2 containing GST and the pGEX-4T-2 PP2A Bα, pGEX-4T-2 flotillin-1 WT, pGEX-4T-2 flotillin-1 S315A, pGEX-4T-2 flotillin-1 S315D, and pGEX-4T-2 flotillin-2 constructs. Cells were grown to A600 = 0.3, recombinant protein expression was induced with 0.1 mm isopropyl 1-thio-β-d-galactopyranoside. 3 h post-induction, culturing at room temperature, a GST pulldown assay was carried out as described earlier (43). In each experiment, equal amounts of recombinant proteins were loaded onto SDS-PAGE and checked by Coomassie Blue staining. The in vitro PKC assay was done as described previously (38).
SDS-PAGE and LC-MS/MS analysis
Proteins were resolved by SDS-PAGE and stained with Coomassie Blue. Liquid chromatography with tandem mass spectrometry detection was performed by Dr. Tamás Janáky and Zoltán Szabó (University of Szeged, Faculty of Medicine, Department of Medical Chemistry (43)).
Western blotting and far-Western blotting
Immunoblotting and far-Western blotting were done using nitrocellulose membranes as described earlier (44). Antibodies were diluted according to the manufacturer's recommendations in Tris-buffered saline (TBS) with 0.1% Tween (TBST) containing 1% BSA. For far-Western blotting, blotted proteins were incubated with BPAEC lysate overnight at 4 °C, washed with 1× TBS, and then incubated with PP2A Bα–specific primary antibody for 4 h at 4 °C and anti-rabbit IgG HRP-conjugated secondary antibody for 1 h at room temperature.
IP, immunofluorescence, and microscopy
Immunoprecipitation and immunostaining of desirable proteins using appropriate antibodies were done as described before (43). For LysoTracker or MitoTracker staining, cells were incubated with 100 nm LysoTracker-containing medium for 30 min or 50 nm MitoTracker-containing serum-free medium for 20 min at 37 °C and then fixed with 3.7% paraformaldehyde for 10 min. Confocal images were acquired by a Leica TCS SP8 confocal microscope using an HC PL APO CS2 63 × 1.40 numeric aperture oil immersion objective on a DMI6000 CS microscope at 25 °C. Nonspecific binding of the secondary antibodies was checked in a control experiment. Pearson's coefficient was evaluated using the JACoP plugin in ImageJ (45).
Transfection and siRNA silencing
BPAECs were transfected with the pcDNA3.1/myc-His A (−) flotillin-1 WT, pcDNA3.1/myc-His A (−) flotillin-1 S315A, and pcDNA3.1/myc-His A (−) flotillin-1 S315D plasmids using Lipofectamine 3000 transfection reagent (Invitrogen) according to the manufacturer's instructions.
PP2A Bα was silenced using 50 nm PP2A Bα-specific SMARTpool siRNA (GE Healthcare Dharmacon, Inc., Lafayette, CO; marked as siPP2A Bα-1) or 50 nm PP2A Bα–specific RNA (marked as siPP2A Bα-2) (Santa Cruz Biotechnology) in complex with Lipofectamine RNAiMAX transfection reagent (Invitrogen) in serum-free medium. The ON-TARGETplus siCONTROL nontargeting pool was used as a negative control. Cells were utilized after 48 h of further incubation.
Cell fractionation
Membrane and cytoplasmic fractions were isolated using the ProteoJET Membrane Protein Extraction Kit (Thermo Scientific, Inc.). Cells were collected by centrifugation at 300 × g for 5 min. The cell pellet was washed with cell wash solution and centrifuged at 300 × g for 5 min. Permeabilization buffer was added to the cell pellet and vortexed briefly. After 10 min of incubation at 4 °C with constant mixing, the cytosolic fraction was separated by centrifugation at 16,000 × g for 15 min. Solubilization buffer was added to the pellet and resuspended. The membrane fraction was made by incubation at 4 °C for 30 min with constant mixing, followed by centrifugation at 16,000 × g for 15 min at 4 °C. The efficiency of fractionation was analyzed by immunoblotting using CD31 antibody as a membrane marker and actin antibody as a cytoplasmic marker.
Anti-V5-agarose affinity gel
Interaction between flotillin-1 and PP2A Bα or PP2A B'γ was examined by anti-V5-agarose affinity gel as described previously (46).
PLA
BPAECs grown on coverslips were fixed with 3.7% paraformaldehyde for 10 min, permeabilized with 0.5% Triton X-100 in TBS, and blocked with 2% BSA in TBS. Samples were incubated with anti-flotillin-1 and anti-PP2A Bα primary antibodies for 1 h. PLA was performed with the Duolink In Situ kit (Sigma-Aldrich) according to the manufacturer's protocol.
NanoBiT Protein:Protein Interaction System
The NanoBiT Protein:Protein Interaction System was purchased from Promega (Madison, WI). BPAECs were cotransfected with pBiT1.1-C TK/LgBiT-PP2A Bα (later referred as LgBiT-PP2A Bα) and pBiT2.1-C-TK/SmBiT flotillin-1 (later referred as SmBiT flotillin-1) WT, -S315A, and -S315D constructs. Positive control vectors provided by the manufacturer were SmBit-PRKACA and LgBit-PRKAR2A, coding the catalytic and regulatory subunits of PKA. As a negative control, the NanoBiT negative control vector, which encodes HaloTag-SmBiT, was cotransfected with LgBiT-PP2A Bα. Luminescent signals were detected with a Tecan Spark multimode microplate reader (Tecan Group).
ECIS measurements
ECIS model Zθ (Applied BioPhysics Inc., Troy, NY) was used to monitor transendothelial electric resistance. Control and transfected cells were seeded on type 8W10E arrays, and impedance was followed in time. For seeding and attachment experiments, resistance values were measured at 64 kHz, and for the wound healing assay, 4 kHz was used. The in vitro wound healing assay was performed as described previously (46). BPAECs transfected with various forms of flotillin-1 were plated onto two 8W10E arrays 24 h post-transfection. When cells achieved monolayer density (about 1000–1300 ohm impedance), an alternate current of 5 mA at 60 kHz was applied for 30 s to establish wounds in the cell layer, and the impedance was measured for 10 h. The migration rate of cells was evaluated by measuring the time needed for recovery of the normal confluent monolayer impedance, and velocity was calculated by v = r/time, where r is the radius of the electrode (125 μm).
In vitro angiogenesis
Control, pcDNA3.1/myc-His A flotillin-1 WT and pcDNA3.1/myc-His A flotillin-1 S315A– and pcDNA3.1/myc-His A flotillin-1 S315D–transfected BPAECs were seeded on Matrigel-coated μ-Slide plates (Ibidi). Bright-field images were taken by a Leica MC 120 HD microscope. F-actin staining was done 5 h after seeding, and images were captured using a Leica TCS SP8 confocal microscope using an HC PLA APO CS2 63 × 1.40 NA oil immersion objective on a DMI6000 CS microscope. Quantification of capillary formation was determined using ImageJ. Data are reported as mean ± S.E. Statistical analysis was done with Student's t test (paired). Asterisks mark significance compared with control samples.
Scratch assay
Endothelial cells were transfected with pcDNA3.1/myc-His A (−) plasmids encoding flotillin-1 WT, -S315A, or -S315D. 24 h post-transfection, cells were scratched with a 1-ml pipette tip, and pictures were taken at from 0–24 h with a Leica MC 120 HD microscopy. Wound closure of cells was evaluated using ImageJ.
Statistical analysis
Statistical evaluations were performed as indicated. Significant differences were as follows: *, p < 0.05; **, p < 0.01; and ***, p < 0.001. Densitometry of immunoblots was done using ImageJ.
Author contributions
Z. T. formal analysis; Z. T. and A. B. investigation; Z. T., N. K., M. F., and A. B. methodology; C. C. supervision; C. C. funding acquisition; A. B. writing-original draft; A. B. project administration.
Supplementary Material
Acknowledgment
We thank Kitti Barta for technical assistance.
This work was supported National Research, Development and Innovation Fund (NKFI) Grant PD116262 (to A. B.), Grants ÚNKP-18-4, ÚNKP-19-4 (to A. B.), and ÚNKP-19-3 (to Z. T.), New National Excellence Program of the Ministry of Human Capacities, and a Bolyai Fellowship from the Hungarian Academy of Sciences (to A. B.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S4.
- EC
- endothelial cell
- BPAEC
- bovine pulmonary artery endothelial cell
- cDNA
- complementary DNA
- PMA
- phorbol 12-myristate 13-acetate
- PLA
- proximity ligation assay
- IP
- immunoprecipitation
- ECIS
- electric cell substrate impedance sensing
- OA
- okadaic acid
- TBS
- Tris-buffered saline
- VE
- vascular endothelial.
References
- 1. Janssens V., and Goris J. (2001) Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353, 417–439 10.1042/0264-6021:3530417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Janssens V., Goris J., and Van Hoof C. (2005) PP2A: the expected tumor suppressor. Curr. Opin. Genet. Dev. 15, 34–41 10.1016/j.gde.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 3. Xu Y., Xing Y., Chen Y., Chao Y., Lin Z., Fan E., Yu J. W., Strack S., Jeffrey P. D., and Shi Y. (2006) Structure of the protein phosphatase 2A holoenzyme. Cell 127, 1239–1251 10.1016/j.cell.2006.11.033 [DOI] [PubMed] [Google Scholar]
- 4. Seshacharyulu P., Pandey P., Datta K., and Batra S. K. (2013) Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett. 335, 9–18 10.1016/j.canlet.2013.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kása A., Czikora I., Verin A. D., Gergely P., and Csortos C. (2013) Protein phosphatase 2A activity is required for functional adherent junctions in endothelial cells. Microvasc. Res. 89, 86–94 10.1016/j.mvr.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tar K., Birukova A. A., Csortos C., Bakó E., Garcia J. G., and Verin A. D. (2004) Phosphatase 2A is involved in endothelial cell microtubule remodeling and barrier regulation. J. Cell Biochem. 92, 534–546 10.1002/jcb.20036 [DOI] [PubMed] [Google Scholar]
- 7. Tar K., Csortos C., Czikora I., Olah G., Ma S. F., Wadgaonkar R., Gergely P., Garcia J. G., and Verin A. D. (2006) Role of protein phosphatase 2A in the regulation of endothelial cell cytoskeleton structure. J. Cell Biochem. 98, 931–953 10.1002/jcb.20829 [DOI] [PubMed] [Google Scholar]
- 8. Rivera-Milla E., Stuermer C. A., and Málaga-Trillo E. (2006) Ancient origin of reggie (flotillin), reggie-like, and other lipid-raft proteins: convergent evolution of the SPFH domain. Cell Mol. Life Sci. 63, 343–357 10.1007/s00018-005-5434-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edgar A. J., and Polak J. M. (2001) Flotillin-1: gene structure: cDNA cloning from human lung and the identification of alternative polyadenylation signals. Int. J. Biochem. Cell Biol. 33, 53–64 10.1016/S1357-2725(00)00069-8 [DOI] [PubMed] [Google Scholar]
- 10. Rajendran L., Masilamani M., Solomon S., Tikkanen R., Stuermer C. A., Plattner H., and Illges H. (2003) Asymmetric localization of flotillins/reggies in preassembled platforms confers inherent polarity to hematopoietic cells. Proc. Natl. Acad. Sci. U.S.A. 100, 8241–8246 10.1073/pnas.1331629100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santamaría A., Fernández P. L., Farré X., Benedit P., Reventós J., Morote J., Paciucci R., and Thomson T. M. (2003) PTOV-1, a novel protein overexpressed in prostate cancer, shuttles between the cytoplasm and the nucleus and promotes entry into the S phase of the cell division cycle. Am. J. Pathol. 162, 897–905 10.1016/S0002-9440(10)63885-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gkantiragas I., Brügger B., Stüven E., Kaloyanova D., Li X. Y., Löhr K., Lottspeich F., Wieland F. T., and Helms J. B. (2001) Sphingomyelin-enriched microdomains at the Golgi complex. Mol. Biol. Cell 12, 1819–1833 10.1091/mbc.12.6.1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Gassart A., Geminard C., Fevrier B., Raposo G., and Vidal M. (2003) Lipid raft-associated protein sorting in exosomes. Blood 102, 4336–4344 10.1182/blood-2003-03-0871 [DOI] [PubMed] [Google Scholar]
- 14. Solis G. P., Hoegg M., Munderloh C., Schrock Y., Malaga-Trillo E., Rivera-Milla E., and Stuermer C. A. (2007) Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem. J. 403, 313–322 10.1042/BJ20061686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salzer U., and Prohaska R. (2001) Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood 97, 1141–1143 10.1182/blood.V97.4.1141 [DOI] [PubMed] [Google Scholar]
- 16. Babuke T., and Tikkanen R. (2007) Dissecting the molecular function of reggie/flotillin proteins. Eur. J. Cell Biol. 86, 525–532 10.1016/j.ejcb.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 17. Liu J., Deyoung S. M., Zhang M., Dold L. H., and Saltiel A. R. (2005) The stomatin/prohibitin/flotillin/HflK/C domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J. Biol. Chem. 280, 16125–16134 10.1074/jbc.M500940200 [DOI] [PubMed] [Google Scholar]
- 18. Neumann-Giesen C., Falkenbach B., Beicht P., Claasen S., Lüers G., Stuermer C. A., Herzog V., and Tikkanen R. (2004) Membrane and raft association of reggie-1/flotillin-2: role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem. J. 378, 509–518 10.1042/bj20031100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morrow I. C., Rea S., Martin S., Prior I. A., Prohaska R., Hancock J. F., James D. E., and Parton R. G. (2002) Flotillin-1/reggie-2 traffics to surface raft domains via a novel Golgi-independent pathway: identification of a novel membrane targeting domain and a role for palmitoylation. J. Biol. Chem. 277, 48834–48841 10.1074/jbc.M209082200 [DOI] [PubMed] [Google Scholar]
- 20. Li Y., Martin B. R., Cravatt B. F., and Hofmann S. L. (2012) DHHC5 protein palmitoylates flotillin-2 and is rapidly degraded on induction of neuronal differentiation in cultured cells. J. Biol. Chem. 287, 523–530 10.1074/jbc.M111.306183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riento K., Frick M., Schafer I., and Nichols B. J. (2009) Endocytosis of flotillin-1 and flotillin-2 is regulated by Fyn kinase. J. Cell Sci. 122, 912–918 10.1242/jcs.039024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Babuke T., Ruonala M., Meister M., Amaddii M., Genzler C., Esposito A., and Tikkanen R. (2009) Hetero-oligomerization of reggie-1/flotillin-2 and reggie-2/flotillin-1 is required for their endocytosis. Cell Signal. 21, 1287–1297 10.1016/j.cellsig.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 23. Cremona M. L., Matthies H. J., Pau K., Bowton E., Speed N., Lute B. J., Anderson M., Sen N., Robertson S. D., Vaughan R. A., Rothman J. E., Galli A., Javitch J. A., and Yamamoto A. (2011) Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat. Neurosci. 14, 469–477 10.1038/nn.2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otto G. P., and Nichols B. J. (2011) The roles of flotillin microdomains: endocytosis and beyond. J. Cell Sci. 124, 3933–3940 10.1242/jcs.092015 [DOI] [PubMed] [Google Scholar]
- 25. Bagchi S., Fredriksson R., and Wallén-Mackenzie A. (2015) In situ proximity ligation assay (PLA). Methods Mol. Biol. 1318, 149–159 10.1007/978-1-4939-2742-5_15 [DOI] [PubMed] [Google Scholar]
- 26. Dixon A. S., Schwinn M. K., Hall M. P., Zimmerman K., Otto P., Lubben T. H., Butler B. L., Binkowski B. F., Machleidt T., Kirkland T. A., Wood M. G., Eggers C. T., Encell L. P., and Wood K. V. (2016) NanoLuc complementation reporter optimized for accurate measurement of protein interactions in cells. ACS Chem. Biol. 11, 400–408 10.1021/acschembio.5b00753 [DOI] [PubMed] [Google Scholar]
- 27. Bodin S., Planchon D., Rios Morris E., Comunale F., and Gauthier-Rouvière C. (2014) Flotillins in intercellular adhesion: from cellular physiology to human diseases. J. Cell Sci. 127, 5139–5147 10.1242/jcs.159764 [DOI] [PubMed] [Google Scholar]
- 28. Banning A., Babuke T., Kurrle N., Meister M., Ruonala M. O., and Tikkanen R. (2018) Flotillins regulate focal adhesions by interacting with α-actinin and by influencing the activation of focal adhesion kinase. Cells 7, E28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meister M., and Tikkanen R. (2014) Endocytic trafficking of membrane-bound cargo: a flotillin point of view. Membranes 4, 356–371 10.3390/membranes4030356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langhorst M. F., Solis G. P., Hannbeck S., Plattner H., and Stuermer C. A. (2007) Linking membrane microdomains to the cytoskeleton: regulation of the lateral mobility of reggie-1/flotillin-2 by interaction with actin. FEBS Lett. 581, 4697–4703 10.1016/j.febslet.2007.08.074 [DOI] [PubMed] [Google Scholar]
- 31. Fork C., Hitzel J., Nichols B. J., Tikkanen R., and Brandes R. P. (2014) Flotillin-1 facilitates toll-like receptor 3 signaling in human endothelial cells. Basic Res. Cardiol. 109, 439 10.1007/s00395-014-0439-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solis G. P., Schrock Y., Hülsbusch N., Wiechers M., Plattner H., and Stuermer C. A. (2012) Reggies/flotillins regulate E-cadherin-mediated cell contact formation by affecting EGFR trafficking. Mol. Biol. Cell 23, 1812–1825 10.1091/mbc.e11-12-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Browman D. T., Hoegg M. B., and Robbins S. M. (2007) The SPFH domain-containing proteins: more than lipid raft markers. Trends Cell Biol. 17, 394–402 10.1016/j.tcb.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 34. Langhorst M. F., Reuter A., Jaeger F. A., Wippich F. M., Luxenhofer G., Plattner H., and Stuermer C. A. (2008) Trafficking of the microdomain scaffolding protein reggie-1/flotillin-2. Eur. J. Cell Biol. 87, 211–226 10.1016/j.ejcb.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 35. Neumann-Giesen C., Fernow I., Amaddii M., and Tikkanen R. (2007) Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J. Cell Sci. 120, 395–406 10.1242/jcs.03336 [DOI] [PubMed] [Google Scholar]
- 36. Weeks K. L., Ranieri A., Karaś A., Bernardo B. C., Ashcroft A. S., Molenaar C., McMullen J. R., and Avkiran M. (2017) β-Adrenergic stimulation induces histone deacetylase 5 (HDAC5) nuclear accumulation in cardiomyocytes by B55α-PP2A-mediated dephosphorylation. J. Am. Heart Assoc. 6, e004861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ory S., Zhou M., Conrads T. P., Veenstra T. D., and Morrison D. K. (2003) Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites. Curr. Biol. 13, 1356–1364 10.1016/S0960-9822(03)00535-9 [DOI] [PubMed] [Google Scholar]
- 38. Boratkó A., and Csortos C. (2017) PKC mediated phosphorylation of TIMAP regulates PP1c activity and endothelial barrier function. Biochim. Biophys. Acta Mol. Cell Res. 1864, 431–439 10.1016/j.bbamcr.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 39. Waschke J., Golenhofen N., Kurzchalia T. V., and Drenckhahn D. (2006) Protein kinase C-mediated endothelial barrier regulation is caveolin-1-dependent. Histochem. Cell Biol. 126, 17–26 10.1007/s00418-005-0140-7 [DOI] [PubMed] [Google Scholar]
- 40. Barr R. K., Lynn H. E., Moretti P. A., Khew-Goodall Y., and Pitson S. M. (2008) Deactivation of sphingosine kinase 1 by protein phosphatase 2A. J. Biol. Chem. 283, 34994–35002 10.1074/jbc.M804658200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riento K., Zhang Q., Clark J., Begum F., Stephens E., Wakelam M. J., and Nichols B. J. (2018) Flotillin proteins recruit sphingosine to membranes and maintain cellular sphingosine-1-phosphate levels. PLoS ONE 13, e0197401 10.1371/journal.pone.0197401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hannun Y. A., and Obeid L. M. (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 10.1038/nrm2329 [DOI] [PubMed] [Google Scholar]
- 43. Boratkó A., Gergely P., and Csortos C. (2013) RACK1 is involved in endothelial barrier regulation via its two novel interacting partners. Cell Commun. Signal. 11, 2 10.1186/1478-811X-11-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boratkó A., Péter M., Thalwieser Z., Kovács E., and Csortos C. (2015) Elongation factor-1A1 is a novel substrate of the protein phosphatase 1-TIMAP complex. Int. J. Biochem. Cell Biol. 69, 105–113 10.1016/j.biocel.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 45. Bolte S., and Cordelières F. P. (2006) A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 10.1111/j.1365-2818.2006.01706.x [DOI] [PubMed] [Google Scholar]
- 46. Boratkó A., Gergely P., and Csortos C. (2012) Cell cycle dependent association of EBP50 with protein phosphatase 2A in endothelial cells. PLoS ONE 7, e35595 10.1371/journal.pone.0035595 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.