Abstract
Background.
Racial/ethnic minorities have lower rates of deceased kidney transplantation (DDKT) and living donor kidney transplantation (LDKT) in the United States. We examined whether social determinants of health (eg, demographics, cultural, psychosocial, knowledge factors) could account for differences in the Veterans Affairs (VA) Kidney Transplantation (KT) Program.
Methods.
We conducted a multicenter longitudinal cohort study of 611 Veterans undergoing evaluation for KT at all National VA KT Centers (2010–2012) using an interview after KT evaluation and tracking participants via medical records through 2017.
Results.
Hispanics were more likely to get any KT (subdistribution hazard ratios [SHR] [95% confidence interval (CI)]: 1.8 [1.2–2.8]) or DDKT (SHR [95% CI]: 2.0 [1.3–3.2]) than non-Hispanic white in univariable analysis. Social determinants of health, including marital status (SHR [95% CI]: 0.6 [0.4–0.9]), religious objection to LDKT (SHR [95% CI]: 0.6 [0.4–1.0]), and donor preference (SHR [95% CI]: 2.5 [1.2–5.1]), accounted for some racial differences, and changes to Kidney Allocation System policy (SHR [95% CI]: 0.3 [0.2–0.5]) mitigated race differences in DDKT in multivariable analysis. For LDKT, non-Hispanic African American Veterans were less likely to receive an LDKT than non-Hispanic white (SHR [95% CI]: 0.2 [0.0–0.7]), but accounting for age (SHR [95% CI]: 1.0 [0.9–1.0]), insurance (SHR [95% CI]: 5.9 [1.1–33.7]), presenting with a living donor (SHR [95% CI]: 4.1 [1.4–12.3]), dialysis duration (SHR [95% CI]: 0.3 [0.2–0.6]), network of potential donors (SHR [95% CI]: 1.0 [1.0–1.1]), self-esteem (SHR [95% CI]: 0.4 [0.2–0.8]), transplant knowledge (SHR [95% CI]: 1.3 [1.0–1.7]), and changes to Kidney Allocation System policy (SHR [95% CI]: 10.3 [2.5–42.1]) in multivariable analysis eliminated those disparities.
Conclusions.
The VA KT Program does not exhibit the same pattern of disparities in KT receipt as non-VA centers. Transplant centers can use identified risk factors to target patients who may need more support to ensure they receive a transplant.
INTRODUCTION
End-Stage Kidney Disease (ESKD) results in significant morbidity and mortality in the United States. It disproportionately affects racial/ethnic minority populations (eg, non-Hispanic blacks, Hispanics, and other minorities), with incidence rates ranging from 1.5 to 3.4 times greater compared with non-Hispanic whites.1 However, minorities are substantially less likely to undergo kidney transplantation (KT), the optimal treatment for ESKD.1–5 Prior research in Veterans and non-Veterans demonstrated that minorities experience longer time to complete the medical evaluation for KT,6,7 to be waitlisted for KT,8–10 or to receive a KT. They are also less likely to receive living donor KT (LDKT) versus deceased donor KT (DDKT).2,3,5,11–14 Disparities persist despite controlling for medical factors, such as the cause of ESKD, comorbidities, and dialysis type/duration.7,15–17 These findings suggest that social determinants of health, such as demographics,18,19 culturally related factors,6,20,21 transplant knowledge,22–24 and psychosocial characteristics25,26 may contribute to race/ethnicity disparities in KT.
It is important to distinguish between transplantation in the Veterans Affairs (VA) National Transplant Program (versus non-VA) settings because the VA process is quite different for referral, evaluation, and transplantation.27–29 Patients are referred to one of a limited number of VA transplant centers VA throughout the country, but VA coverage includes travel to the center and necessary lodging for the patient and caregiver. Private insurers and Medicare do not provide this coverage for transplant recipients at non-VA centers.30 Thus, an important innovation of our work has been to highlight the unique aspects of VA care that may be responsible for mitigating disparities observed in the non-VA public and private insurer settings. Our most recent work demonstrated that the VA did not exhibit the significant racial disparities in time from evaluation to waitlisting for KT as have been found in non-VA transplant centers.27 We also found that younger age, being married, having both private and public insurance, fewer comorbidities, and moderate or greater levels of depression predicted a shorter time to waitlisting. Before these findings, research regarding the impact of social determinants of health on disparities in KT experienced by patients in the VA was limited to just 2 studies.3,12 But those studies relied on secondary data analysis and had limited information regarding social determinants of health. They also assessed disparities across multiple stages of the KT process simultaneously (ie, referral, evaluation, waitlisting), which limited their ability to identify the phases of the process which contribute most to the observed disparities.
Because national data and previous research demonstrate that (a) disparities occur at every stage of the transplant process, including referral, evaluation, transplantation, and posttransplant outcomes1,31–34 but (b) different factors may contribute to disparities in KT waitlisting versus receipt of KT,5,11 it is important to determine whether different variables predict disparities in receipt of KT (the goal of the current article) independent of waitlisting in the VA transplant program as well (findings from our previous article). Thus, the current work is a major advance from our previous article because of the distinct and important role that social determinants of health may play in disparities of receiving a KT and the type of KT received. Although we cannot argue that these variables caused the outcome, we hope to better predict KT outcome with the variables we measured.
In addition, 2 critical and unique advantages of our article make this research a significant contribution to disparities in KT research. First, we recruited the whole Veteran population that was evaluated for KT within the National VA Kidney Transplant program between 2010 and 2012 (rather than just a sample of Veterans). During this time period, only 4 KT centers existed within the VA, and we recruited almost every patient within this system. Second, ours was a longitudinal study wherein we interviewed those Veterans prospectively and determined how social determinants of health, assessed at the time of initial KT clinic evaluation, predict disparities in receiving a KT, and the type of transplant received. We wanted to determine whether social determinants of health predict transplant outcomes, as has been found for health behaviors and outcomes in other diseases.35–44 With a few exceptions,45–50 little work has focused on transplantation, and what has been done was limited by small sample sizes, cross-sectional design, or a limited number of predictors.8,51 We designed our study to address those limitations. Our study is prognostic rather than etiological research because we aimed to predict the probability of a given outcome at a given time for an individual patient rather than a causal relationship between risk factors and a given outcome.52 In light of a recent editorial calling for more transparency in the VA KT program, we feel the results of our study are especially timely.53
MATERIALS AND METHODS
Study Design
This multicenter longitudinal study was approved by the Institutional Review Board at all 4 VA KT Centers. A detailed description of the VA Transplant evaluation process is included in our previous work27 and further explained by the VA National Director of Surgery.29 Participants completed a semistructured telephone interview 1 to 3 weeks after they initiated evaluation at one of the 4 VA transplant centers. We used a ≈70-minute interview comprised of existing valid measures6 to assess our hypothesized predictor variables. We conducted medical record review to assess KT outcomes at the conclusion of our study period.
Study Sample
We recruited participants from all 4 National VA KT Centers during their initial transplant evaluation appointment between February 2010 and December 2012. Inclusion criteria were (1) referral for KT; (2) no previous KT; and (3) age 18 or older. Of all the Veterans being evaluated for KT within the VA National KT Program, 648 patients met these criteria, and we enrolled 617 of them, yielding a 95% recruitment rate of the whole population (rather than a sample). Of the 617 patients enrolled, 6 formally withdrew their participation (final n=611). We continued to collect transplant status data through February 2017.
Interview Procedures and Measures
Interviewers at the University of Pittsburgh Survey Research Program, independent from any transplant service, conducted the telephone interviews. We offered a Spanish-language interview for participants who preferred it so as not to exclude any Spanish-speaking participants. The interviewers entered data directly into the interview software using a computer-aided telephone interview system.
Predictor Variables
We provide extended descriptions, ranges, and psychometric properties of all predictor variables in Table 1.
TABLE 1.
Potential predictorsa of transplant outcomes
| Predictor categories | Variables | Description and scoring |
|---|---|---|
| Demographic characteristics | ||
| Race/ethnicity | During the first interview, respondents could select as many of the following race categories as appropriate: “American Indian or Alaska Native,” “Asian,” “Black or African American,” “Native Hawaiian or Other Pacific Islander,” “White,” or “Other.” If the “Other” category was specified, respondents had the opportunity to provide a verbatim response that was used to recategorize any “Other” responses into a known category, if possible. For the purposes of this article, “Non-Hispanic African American” refers to respondents who self-identified with only one race group (“Black or African American”) and were also non-Hispanic. “Non-Hispanic white” refers to respondents who self-identified with only one race group (“white”) and were also non-Hispanic. “Hispanic” refers to respondents who were Hispanic (regardless of race). “Other Minorities” refers to non-Hispanic respondents who could not be directly classified as either “Non-Hispanic African American” or “Non-Hispanic white.” | |
| Sex | Categories listed in Table 2 | |
| Age | This variable was defined as the difference (in y) between the completion date of the first interview and the date of birth. | |
| Marital status | Married vs not | |
| Education | Categories listed in Table 2 | |
| Income | Categories listed in Table 2 | |
| Insurance status | During the first interview, respondents could select as many of the following current healthcare coverage categories as appropriate: “VA,” “Medicare,” “Medicaid,” “Private Health Insurance,” “Self-pay,” “None,” and “Other.” If the “Other” category was specified, respondents had the opportunity to provide a verbatim response that was used to recategorize any “Other” responses into a known category, if possible. For purposes of this article, “Public” coverage included any mention of “VA,” “Medicare,” or “Medicaid” without any mention of “Private Health Insurance.” “Private Only” coverage included the reporting of “Private Health Insurance” without the mention of any public coverage (“VA,” “Medicare,” “Medicaid”). “Private and Public” coverage included the mention of “Private Health Insurance” along with at least one mention of “VA,” “Medicare,” or “Medicaid.” | |
| Rural residence | We obtained every participant’s zip code from their medical record. We then coded the zip codes into rural vs urban based on the following website from the HRSA: https://www.hrsa.gov/rural-health/about-us/definition/datafiles.html. | |
| Occupation | During the first interview, respondents were asked if they currently had paid employment. If the answer was “yes,” the respondent was asked to provide a verbatim response describing the kind of the work they currently perform. If the answer was “no,” the respondent was asked to provide a verbatim response describing the kind of work they performed when they last worked. These verbatim responses were used to classify the occupation verbatim responses into a categorization based on the Hollingshead Occupational Scale (1) as follows: Score 9—higher executives, proprietors of large businesses, and major professionals Score 8—administrators, lesser professionals, proprietors of medium-sized businesses Score 7—smaller business owners, farm owners, managers, minor professionals Score 6—technicians, semiprofessionals, small business owners Score 5—clerical and sales workers, small farm and business owners Score 4—smaller business owners, skilled manual workers, craftsmen, and tenant farmers Score 3—machine operators and semiskilled workers Score 2—unskilled workers Score 1—farm laborers/menial service workers We dichotomized this variable at a score of 4. Those with 4 or less were coded as “lower-status occupations” and those with a score of 5 or greater were coded as “higher status occupations.” |
|
| KAS changes | The introduction of changes to KAS in 2014 resulted in a bolus effect of reduction in waitlist disparities for DDKT.10 We added KAS as a time-varying covariate to control for its potential effect in our model. | |
| Medical/health factors | ||
| Dialysis type | Center-based hemodialysis or peritoneal dialysis (at time of evaluation) | |
| Dialysis duration | Time on dialysis (at time of evaluation). Because dialysis duration was skewed, we used established literature54,55 to determine the following categories for dialysis duration: 1. 0 y on dialysis 2. <1 y on dialysis 3. 1− <5 y on dialysis 4. 5− <10 y on dialysis 5. >10 y on dialysis |
|
| BMI | Calculated with patient height and weight using NHLBIʼs calculator available at https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmicalc.htm | |
| Perceived burden of kidney disease23,24 | We assessed this factor with 3 items adapted from the Kidney Transplant Questionnaire23,24 and used in our previous pilot work.6 Participants rated the extent to which they felt burdened by their kidney disease on a scale of 1 (definitely true) to 5 (definitely false) for each item (eg, “My kidney disease interferes with my life”). We calculated an overall mean score for this variable. Cronbach alpha for the current sample = 0.743. | |
| Charlson Comorbidity Index56,57 | For all study participants, VA inpatient and outpatient medical utilization records were examined for the purposes of calculating the Charlson Comorbidity Index.56 Any applicable ICD-9-CM code occurring no >12 mo before presentation for evaluation at the VA kidney transplant center was utilized. | |
| Number of potential living donors in the patientʼs social network58,59 | The network of potential living donors available for evaluation was determined by asking participants to indicate how many living relatives and friends they had aged 18–70 y, the age range of living kidney donors. Actual living donors were individuals who were undergoing, had already undergone, or were planning to undergo evaluation for living donation to a specific patient. For our analyses, we summed across these 3 groups for an overall number of living donors. | |
| Actual donors evaluated | Yes/no | |
| Culturally related factors | ||
| Experience of discrimination35,37,60 | Assesses the extent to which participants have experienced a set of discriminatory practices (eg, “When getting health care, I was treated with less respect than other people because of my race or color”; range = 1 [never] to 5 [always]). We summed across these items for an overall experience of discrimination score. Cronbach alpha for the current sample = 0.922. | |
| Perceived racism43,61 | Assesses the extent to which patients believe that racism is common in health care (eg, “Doctors treat African American and white people the same”; range = 1 [strongly disagree] to 5 [strongly agree]). Item responses range from 1 (strongly disagree) to 5 (strongly agree). An overall mean score was calculated for this variable. Cronbach alpha for the current sample = 0.769. | |
| Medical mistrust43,61,62 | Assesses the degree to which participants believe their hospital to be trustworthy, competent, and acting in their best interests (eg, “I trust hospitals”; 1 = strongly disagree to 5 = strongly agree). An overall mean score was calculated for this variable. Cronbach alpha for the current sample = 0.868. | |
| Trust in physicians63 | Assesses the degree of patient trust in their physician (eg, “I doubt that my doctor really cares about me as a person”; range = 1 [totally disagree] to 5 [totally agree]). An overall mean score was calculated for this variable. Cronbach alpha for the current sample = 0.815 | |
| Family loyalty64 | Assesses feelings of loyalty and mutual support regarding the family (eg, “The family should consult close relatives [uncles, aunts, first cousins] concerning its important decisions”; range = 1 [strongly disagree] to 5 [strongly agree]). An overall mean score was calculated for this variable. Cronbach alpha for the current sample = 0.786 | |
| Religious preference | Assessed religious affiliation and level of importance/influence of religious beliefs (eg, “Regardless of whether you attend religious services, please indicate on a scale from 1 [not at all] to 9 [extremely] how important your religious beliefs are to you.”) An overall mean score was calculated for importance/influence of religious beliefs. Cronbach alpha for the current sample = 0.912. | |
| Religious objections to LDKT6,65,66 | Assessed with a revised subscale of the ODAS.65 The ODAS was created by experts in the psychological evaluation of religious beliefs as a measure of individualsʼ attitudes towards organ donation. We revised this 8-item scale to assess religious beliefs as they relate to living donor KT (eg, “I believe that living donor KT is against my religion”; 1 [strongly disagree] to 5 [strongly agree]). | |
| Psychosocial characteristics | ||
| Emotional distress67 | Measured with the anxiety and depression subscales of the BSI.67 Each subscale comprises 6 items related to either anxiety or depression (eg, “Please indicate how bothered or distressed you have been by that feeling during the past 2 wk: nervousness or shakiness inside”; 1 [not at all] to 5 [extremely]). An overall mean score was calculated for this variable. Cronbach alpha for the current sample = 0.747 for anxiety, 0.823 for depression. | |
| Social support68,69 | Measured with a 12-item version of the ISEL-12.68,69 The ISEL assesses patients’ perceived availability of 3 separate functions of social support. The “tangible” subscale is intended to measure perceived availability of material aid; the “appraisal” subscale, the perceived availability of someone to talk to about one’s problems; and the “belonging” subscale, the perceived availability of people with whom one can do things (eg, “I feel that there is no one I can share my most private worries and fears with”; 1 [definitely false] to 4 [definitely true]). An overall mean score was calculated for this variable. Cronbach alpha for the current sample = 0.940. | |
| Self-esteem70 | Measured using the Rosenberg Self-Esteem Scale.70 The self-esteem scale assesses patients’ feelings of self-worth and self-respect (eg, “I feel that I am a person of worth, at least on an equal plane with others”). Individual responses range from 1(strongly agree) to 4 (strongly disagree). An overall mean score was calculated for this variable. Cronbach alpha for the current sample = 0.901. | |
| Sense of mastery71 | Assessed using the Sense of Mastery Scale.71 The Sense of Mastery Scale assesses the degree to which participants feel they have personal control over the things that happen to them (eg, “I have little control over the things that happen to me.”) Individual responses range from 1(strongly agree) to 4 (strongly disagree). An overall mean score was calculated for this variable. Cronbach alpha for the current sample = 0.803. | |
| Locus of control72 | Assessed with the 18-item MHLC scales, Form C.72 The scale includes separate subscales to assess the extent to which recipients view their health condition is due to their own behavior (Internal Locus of Control) or the behavior of doctors, other people not including doctors, chance, luck, or fate (External Locus of Control). Responses to items range from 1 (strongly disagree) to 6 (strongly agree). An overall mean score was calculated for this variable. Cronbach alpha for the current sample = 0.755 for Internal Locus of Control and 0.810 for External Locus of Control. | |
| Transplant knowledge, concerns, and preference | ||
| Transplant knowledge | Assessed with items adapted from the KT Knowledge Survey73 and the KT Questionnaire.23,24 This measure includes 27 multiple choice and true-false items. A summative score is created for the total number of items that patients answered correctly. | |
| Transplant learning activities | The type, number, and time spent in each educational activity were assessed by self-report. Patients were asked to indicate whether they had engaged in any of a list of activities to learn or think about transplantation (eg, “Read brochures about kidney transplant from living donors”). Then, patients were asked to indicate how much time was spent on each of the activities that they checked. A summative score was calculated for the total number of items checked and total time spent on all learning activities. | |
| Transplant concerns | Assessed using 30 items adapted from the KT Questionnaire.23,24 This measure asks patients to indicate whether any of a list of concerns affected their decisions about getting a transplant, including concerns about transplant for themselves and concerns about the potential donor’s future health status. The items can be summed to indicate overall level of concern about transplantation or examined individually to determine particular concern items that vary by race. | |
| Transplant preference | Assessed via self-report by asking participants whether they preferred a living or deceased donor kidney transplant and if they had someone being worked up as a potential LD.6 | |
We included these measures because they (a) are widely used in organ donation or transplantation studies, other medical populations, or both; (b) have known psychometric properties, including (for scaled measures) Cronbach α of ≈0.80–0.92 (see references cited with each instrument for psychometric data); and (c) used in our previous research.
BMI, body mass index; BSI, Brief Symptom Inventory; DDKT, deceased donor kidney transplantation; HRSA, Health Resources and Services Administration; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ISEL, Interpersonal Support Evaluation List; KAS, Kidney Allocation System; KT, kidney transplantation; LD, living donor; LDKT, living donor kidney transplantation; MHLC, Multidimensional Health Locus of Control; NHLBI, National Heart, Lung, and Blood Institute; ODAS, Organ Donation Attitude Survey; VA, Veterans Affairs.
Outcome Variables
At the conclusion of our study period, we abstracted participants’ medical record to determine their final KT outcome and the date the outcome occurred. We identified 11 potential categories:
Received LDKT: Patient received LDKT (endpoint = date of transplant).
Received DDKT: Patient received DDKT (endpoint = date of transplant).
Still on waiting list: Patient was accepted for transplant and added to the United Network for Organ Sharing (UNOS) waiting list but was still on the waitlist at the time of the final medical record review (endpoint = date of final medical record review).
Died before waitlist: Patient passed away before completing the evaluation (endpoint = date of death).
Died after waitlist: Patient passed away while on the UNOS waitlist, but never received transplant (endpoint = date of death).
Discontinued evaluation: Patient ended the evaluation process before being listed for KT (endpoint = date evaluation was closed).
Withdrew from waitlist: Patient removed him/herself from the waitlist, no longer wished to pursue transplant (endpoint = date of UNOS waitlist removal).
Ineligible for transplant: Patient completed the evaluation but was determined ineligible by the transplant team (endpoint = date of ineligibility).
Transplant program removed the patient from the waitlist: The transplant program removed the patient from the UNOS waitlist (eg, too sick for transplant, did not keep up with testing; endpoint = date of UNOS removal).
Received DDKT at another transplant center: (Endpoint = date of transplant, if noted, or date transplant is first noted in medical record).
Received LDKT at another transplant center: (Endpoint = date of transplant, if noted, or date transplant is first noted in medical record).
Statistical Analysis
We divided participants’ race/ethnicity into 4 groups—non-Hispanic white, non-Hispanic black, Hispanic, and other minorities (ie, American Indian/Alaskan Native, Asian, Native Hawaiian or Other Pacific Islander, other–unspecified). We dichotomized several predictor variables due to small sample size: occupation, experience of discrimination, anxiety, depression. We summarized all binary and categorical variables in frequencies and proportions. We examined continuous variables by mean and SD for normally distributed variables and median and interquartile range for nonnormally distributed variables. To handle missing data, we used an available-case approach and utilized all cases where the variables of interest are nonmissing. Specific missing data are detailed in the notes of each table.
We performed time-to-event analysis, in the presence of competing risks, to examine the association of baseline risk factors with time from evaluation to time of transplant. It is of major importance to be aware of the presence of any competing risks when performing survival analyses. The Kaplan-Meier method for unadjusted survival analysis can handle only one outcome and yields unreliable results for the estimation of survival probability in the presence of competing risks. We fit 3 nested models using an SAS macro produced by Kohl et al,74 as recommended by Noordzij et al52 using the cumulative incidence competing risk method instead.
When the main event of interest was time to receive any KT (categories 1 and 2 above, received LDKT or DDKT, respectively), we treated categories 4, 5, 10, 11 (death or receiving a KT at a non-VA center) as competing events because they hinder the occurrence of the event of interest (receiving a KT within the VA transplant system).52 We treated categories 3 and 6 through 9 (still on waitlist or removed from waitlist) as noninformative censoring because those participants neither experienced the outcome of interest during the observation period nor did they experience a competing event. We also fit separate regression models for DDKT and LDKT to assess factors associated with the different types of transplant because the literature has shown that different factors are associated with, and predict, whether a patient receives a deceased or living donor KT.2,3,5,11–14,31–34 For DDKT, we treated category 2 as the main event, categories 1, 4, 5, 10, 11 as competing events, and categories 3 and 6 through 9 as noninformative censoring. For LDKT, we treated category 1 as the main event, categories 2, 4, 5, 10, 11 as competing events, and categories 3 and 6 through 9 as noninformative censoring.
We used cumulative incidence plots to visualize the estimated probability of events. We calculated an unadjusted cumulative incidence for each outcome event (any transplant, DDKT, or LDKT, competing event, or other removal) as a function of years from evaluation. To conduct the regression analyses, we fit Fine-Gray75 models, which directly estimate the relationship between predictor variables and the cumulative incidence function of the main event, and computed subdistribution hazard ratios with 95% confidence intervals. We incorporated the revision of the Kidney Allocation System (KAS) in the regression model as a time-varying covariate, which equaled 0 before the implementation of KAS (December 4, 2014) and 1 after its implementation. We considered all other predictor variables fixed at baseline. We fit 3 models for time to receiving any KT, a DDKT, and an LDKT, separately. Model 1 was a univariable Fine-Gray model with race/ethnicity. Model 2 was a multivariable Fine-Gray model with race/ethnicity, demographics, and medical/health factors. Model 3 was a multivariable Fine-Gray model in which we added KAS, culturally related factors, psychosocial characteristics, and transplant knowledge variables to model 2. In models 2 and 3, we constructed multivariable models by including potential predictors either that were significant at the P < 0.10 level or that had an effect size (subdistribution hazard ratios) > 2.0 in the univariable model (table not shown), to reduce the likelihood of type 2 error.
RESULTS
Descriptive Statistics by Race/Ethnicity
Of the 611 veterans being evaluated for transplant in our analysis, 270 were non-Hispanic white, 198 were non-Hispanic black, 77 were Hispanic, and 66 were other minorities. One hundred sixty-eight participants received a transplant (142 DDKT and 26 LDKT), 192 died before receiving a transplant, 144 were removed from evaluation or waitlist for various reasons, 29 were transplanted at another non-VA center, and 78 were still on the waitlist at the end of the study (see Table 2 for details of the complete sample by race/ethnicity). Of the 168 veterans who received KT, 18.5% (31) was transplanted at site A, 24.4% (41) at site B, 23.2% (39) at site C, and 33.9% (57) at site D. Table 2 also lists all the variables of our complete sample by race/ethnicity. The proportions in the VA differ from national non-VA data.76 Figure 1 depicts the unadjusted cumulative incidence function of each outcome category and its 95% confidence interval. Based on the plot, for those receiving an LDKT, the waiting time is generally <1.5 years but longer for those receiving a DDKT.
TABLE 2.
List of transplant status and other variables by race/ethnicity
| Variablesa | Total (N = 611) | Non-Hispanic white (n = 270) | Non-Hispanic African American (n = 198) | Hispanic (n = 77) | Other minorities (n = 66) |
|---|---|---|---|---|---|
| Transplant statusx | |||||
| Y from enrollment to transplant or last follow-up (median [IQR]) | 2.7 (1.4–4.6) | 2.7 (1.4–4.3) | 3.1 (1.5–5.0) | 2.3 (1.4–4.6) | 1.9 (0.9–4.0) |
| Received a transplant at a VA center (n [%]) | 168 (27.5) | 68 (25.2) | 50 (25.3) | 30 (39.0) | 20 (30.3) |
| LDKT | 26 (4.3) | 17 (6.3) | 2 (1.0) | 5 (6.5) | 2 (3.0) |
| DDKT | 142 (23.2) | 51 (18.9) | 48 (24.2) | 25 (32.5) | 18 (27.3) |
| Transplant at a non-VA center | 29 (4.7) | 11 (4.1) | 8 (4.0) | 5 (6.5) | 5 (7.6) |
| Died before receiving a transplantb | 192 (31.4) | 106 (39.3) | 49 (24.7) | 18 (23.4) | 19 (28.8) |
| Still on waiting list | 78 (12.8) | 22 (8.1) | 43 (21.7) | 8 (10.4) | 5 (7.6) |
| Other removalc | 144 (23.6) | 63 (23.3) | 48 (24.2) | 16 (20.8) | 17 (25.8) |
| Demographic characteristics | |||||
| Sex (female) (n [%]) | 19 (3.1) | 5 (1.9) | 11 (5.6) | 2 (2.6) | 1 (1.5) |
| Age (in y) (mean [SD]) | 59.6 (9.1) | 61.4 (8.5) | 57.4 (9.6) | 59.7 (9.2) | 58.3 (9.1) |
| Education (n [%]) | |||||
| Less than high school | 28 (4.6) | 17 (6.3) | 6 (3.0) | 3 (3.9) | 2 (3.0) |
| High school graduate | 164 (26.8) | 80 (29.6) | 53 (26.8) | 13 (16.9) | 18 (27.3) |
| Some college | 270 (44.2) | 108 (40.0) | 103 (52.0) | 40 (51.9) | 19 (28.8) |
| College degree | 89 (14.6) | 41 (15.2) | 25 (12.6) | 14 (18.2) | 9 (13.6) |
| Graduate degree | 49 (8.0) | 24 (8.9) | 11 (5.6) | 7 (9.1) | 7 (10.6) |
| Household income (n [%]) | |||||
| < US $15 000 | 100 (16.4) | 42 (15.6) | 43 (21.7) | 10 (13.0) | 5 (7.6) |
| $15 000–$24 999 | 121 (19.8) | 51 (18.9) | 41 (20.7) | 18 (23.4) | 11 (16.7) |
| $25 000–$49 999 | 212 (34.7) | 99 (36.7) | 57 (28.8) | 28 (36.4) | 28 (42.4) |
| $50 000–74 999 | 101 (16.5) | 41 (15.2) | 43 (21.7) | 10 (13.0) | 7 (10.6) |
| > $75 000 | 57 (9.3) | 33 (12.2) | 12 (6.1) | 8 (10.4) | 4 (6.1) |
| Insurance (public only) (n [%]) | 440 (72.0) | 198 (73.3) | 145 (73.2) | 58 (75.3) | 39.0 (59.1) |
| Rural residence, % yes (n [%]) | 101 (16.5) | 64 (23.7) | 19 (9.6) | 4 (5.2) | 14 (21.2) |
| Occupation (>skilled manual worker) (n [%]) | 279 (45.7) | 125 (46.3) | 90 (45.5) | 41 (53.2) | 23.0 (34.8) |
| Marital status (not married) (n [%]) | 243 (39.8) | 95 (35.2) | 89 (44.9) | 35 (45.5) | 24.0 (36.4) |
| Study location (n [%]) | |||||
| Site A | 124 (20.3) | 44 (16.3) | 53 (26.8) | 15 (19.5) | 12.0 (18.2) |
| Site B | 116 (19.0) | 60 (22.2) | 36 (18.2) | 12 (15.6) | 8.0 (12.1) |
| Site C | 218 (35.7) | 95 (35.2) | 92 (46.5) | 13 (16.9) | 18.0 (27.3) |
| Site D | 153 (25.0) | 71 (26.3) | 17 (8.6) | 37 (48.1) | 28.0 (42.4) |
| Event after KAS (yes) (n [%]) | 40 (6.5) | 16 (5.9) | 17 (8.6) | 5 (6.5) | 2 (3.0) |
| Medical factors | |||||
| BMI (mean [SD]) | 29.6 (4.6) | 29.9 (4.6) | 29.2 (4.8) | 29.2 (3.9) | 30.4 (4.5) |
| Charlson Comorbidity index (range: 1–13) (median [IQR]) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 5.0 (4.0–5.0) | 4.0 (3.0–5.0) |
| Dialysis duration (median [IQR]) Type of dialysis (n [%]) | 1.6 (0.9–2.8) | 0.7 (0.0–1.7) | 1.3 (0.3–3.0) | 1.4(0.5–1.9) | 1.5 (0.7–2.8) |
| None | 165 (27.0) | 99 (36.7) | 41 (20.7) | 15 (19.5) | 10 (15.2) |
| Hemodialysis | 372 (60.9) | 146 (54.1) | 134 (67.7) | 57 (74.0) | 35 (53.0) |
| Peritoneal dialysis | 63 (10.3) | 25 (9.3) | 23 (11.6) | 5 (6.5) | 10 (15.2) |
| Burden of kidney disease (range: 1–5) (median [IQR]) | 3.7 (3.0–4.3) | 3.7 (3.0–4.3) | 3.7 (3.0–4.3) | 3.7 (3.0–4.3) | 4.0 (2.7–4.7) |
| No. of potential donors in social network (median [IQR]) | 17.0 (11–29) | 14.0 (10–24) | 20.0 (13.0–35.0) | 18.0 (12.0–43.0) | 17.0 (10.0–25.0) |
| Have a living donor at T1 (yes) (n [%]) | 240 (39.3) | 110 (40.7) | 78 (39.4) | 34 (44.2) | 18.0 (27.3) |
| Cultural factors | |||||
| Experience of discrimination (any) (n [%]) | 221 (36.2) | 54 (20.0) | 117 (59.1) | 30 (39.0) | 20.0 (30.3) |
| Perceived racism (range: 1–5) (median [IQR]) | 2.3 (2.0–2.8) | 2.0 (1.8–2.5) | 2.5 (2.0–3.0) | 2.0 (1.8–2.8) | 2.0 (2.0–2.8) |
| Medical mistrust (range: 1–5) (mean [SD]) | 2.5 (0.5) | 2.4 (0.5) | 2.6 (0.5) | 2.5 (0.5) | 2.5 (0.5) |
| Trust in physician (range: 1–5) (mean [SD]) | 2.3 (0.5) | 2.2 (0.4) | 2.3 (0.5) | 2.3 (0.6) | 2.4 (0.5) |
| Family loyalty (range: 8–80) (mean [SD]) | 50.9 (9.1) | 49.4 (7.9) | 51.6 (9.7) | 53.8 (9.8) | 51.7 (10.1) |
| Religious objection (range: 1–5) (median [IQR]) | 2.0 (1.8–2.3) | 2.0 (1.6–2.3) | 2.0 (1.9–2.3) | 2.0 (1.8–2.5) | 2.0 (1.5–2.3) |
| Psychosocial characteristics | |||||
| Social support (range: 11–48) (median [IQR]) | 44.0 (38.0–47.0) | 44.0 (38.0–47.0) | 45.0 (38.0–47.0) | 44.0 (37.0–48.0) | 45.0 (35.0–48.0) |
| Self-esteem (range: 1–4) (median [IQR]) | 3.1 (2.9–3.5) | 3.0 (2.8–3.5) | 3.1 (2.9–3.5) | 3.2 (2.9–3.7) | 3.0 (2.7–3.5) |
| Mastery (range: 1–4) (median [IQR]) | 3.0 (2.7–3.2) | 3.0 (2.6–3.1) | 3.0 (2.8–3.3) | 3.0 (2.7–3.4) | 3.0 (2.6–3.3) |
| Locus of control | |||||
| Internal (range: 1–6) (mean [SD]) | 4.2 (1.1) | 4.1 (1.0) | 4.2 (1.1) | 4.4 (1.1) | 4.2 (1.1) |
| External (range: 1–6) (mean [SD]) | 3.5 (0.8) | 3.4 (0.8) | 3.6 (0.8) | 3.5 (0.8) | 3.4 (0.8) |
| Anxiety (≥ moderate) (n [%]) | 9 (1.5) | 4 (1.5) | 2 (1.0) | 2 (2.6) | 1.0 (1.5) |
| Depression (≥ moderate) (n [%]) | 17 (2.8) | 11 (4.1) | 3 (1.5) | 2 (2.6) | 1.0 (1.5) |
| Transplant knowledge and education | |||||
| Transplant knowledge (range: 0–27) (median [IQR]) | 22.0 (20.0–23.0) | 22.0 (21.0–24.0) | 22.0 (20.0–23.0) | 21.0 (20.0–23.0) | 21.0 (19.0–23.0) |
| No. learning activities (range: 0–8) (median [IQR]) | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 5.0 (3.0–6.0) | 5.0 (3.0–6.0) | 5.0 (4.0–6.0) |
| Total h of learning activities (median [IQR]) | 14.0 (6.5–28.5) | 14.0 (7.2–25.5) | 12.0 (5.5–29.5) | 15.3(7.0–32.5) | 12.5 (7.0–31.0) |
| Transplant concerns (range: 0–30) (mean [SD]) | 10.6 (4.7) | 10.3 (4.5) | 10.3 (4.5) | 11.8 (5.0) | 10.8 (5.6) |
| Donor preference (n [%]) | |||||
| No preference | 82 (13.4) | 37 (13.7) | 26 (13.1) | 12 (15.6) | 7 (10.6) |
| Deceased donor | 70 (11.5) | 38 (14.1) | 16 (8.1) | 8 (10.4) | 8 (12.1) |
| Living donor | 448 (73.3) | 195 (72.2) | 156 (78.8) | 57 (74.0) | 40 (60.6) |
| Living donor recruitment | |||||
| Willing to accept volunteer (n [%]) | 526 (86.1) | 242 (89.6) | 176 (88.9) | 64 (83.1) | 44 (66.7) |
| Willing to ask for donation (n [%]) | 340 (55.6) | 139 (51.5) | 132 (66.7) | 42 (54.5) | 27 (40.9) |
Higher value = greater amount (or higher score on) a particular variable.
n = 20 missing for household income; n = 15 missing for occupation, n = 7 missing for Charlson score, dialysis duration, n = 4 missing for BMI; n = 12 missing for age, insurance type, external beliefs; n = 26 missing for living donor at T1; n = 13 missing for discrimination, internal beliefs; n = 17 missing for total h of learning activities; n = 20 missing for willing to accept volunteer; n = 23 missing for willing to ask for donation; n = 11 sex, education, marriage status, anxiety, depression, medical mistrust, trust in physician, family loyalty, transplant concerns, burden of kidney disease, number of potential donors, racism, social support, esteem, mastery, transplant knowledge, no. of learning activities, dialysis type, donor preference; n = 13 missing for religious objection to LDKT
To explore the potentially higher mortality of non-Hispanic whites in our sample, we examined the group of participants who died before being transplanted. We examined factors including age, Charlson comorbidity, and insurance coverage to explore whether any of these factors were collinear with race/ethnicity among those who died, and only found a significant difference for age. Specifically, among those who died before receiving a transplant the non-Hispanic white patients (mean [SD] = 63.9 [6.6]) were older than all other groups: Hispanic patients (mean [SD]
Other removal includes discontinued evaluation (n = 9), withdrew from waitlist (n = 10), ineligible for transplant (n = 67), and transplant program removed the patient from the waitlist (n = 58).
BMI, body mass index; DDKT, deceased donor kidney transplantation; IQR, interquartile range; KAS, Kidney Allocation System; LDKT, living donor kidney transplantation; SD, standard deviation; VA, Veterans Affairs.
FIGURE 1.
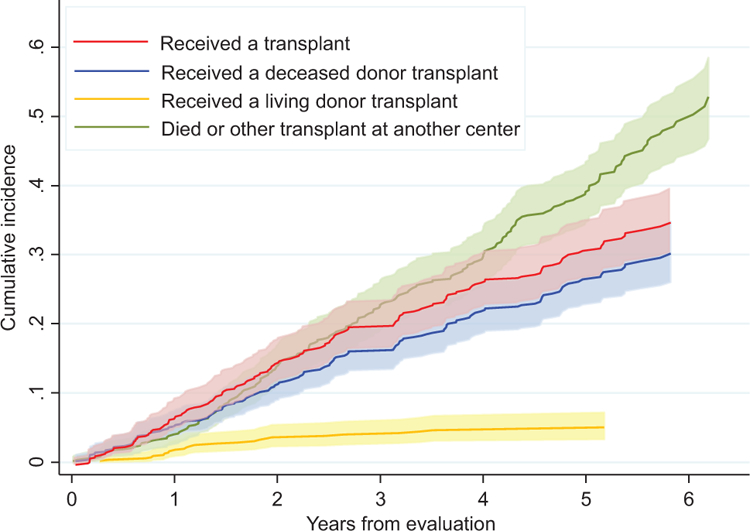
Unadjusted cumulative incidence function by event status.
Predictors of Time to Transplant
We present the results of our Fine-Gray competing regression analyses for time to KT in Table 3. In our unadjusted model, only Hispanics were more likely to get a KT than non-Hispanic whites (after adjusting for multiple comparisons using Bonferroni correction). The other race/ethnicity groups did not differ from non-Hispanic whites. After adjusting for demographics and medical/health factors (model 2), the racial differences disappeared. Veterans who were younger, married, or from study site B (rather than site A) were more likely to get a KT, but those from site C (rather than site A) or who had more comorbid conditions were less likely to get a KT. The addition of KAS in model 3 mitigated the effects of study site; however, the effect Hispanics were once again more likely to be transplanted than non-Hispanic whites.
TABLE 3.
Unadjusted and adjusted Fine-Gray proportional subdistribution hazards model for time from evaluation to receiving a transplanta
| Variables | Model 1 |
Model 2 |
Model 3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SHR | 95% CI | P value | SHR | 95% CI | P value | SHR | 95% CI | P value | |||
| Model 1 | |||||||||||
| Race/ethnicity | 0.036b | 0.343c | 0.179d | ||||||||
| Non-Hispanic white | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||
| Non-Hispanic African American | 1.015 | 0.708–1.454 | 0.936 | 1.021 | 0.687–1.519 | 0.917 | 1.227 | 0.813–1.851 | 0.331 | ||
| Hispanic | 1.799 | 1.166–2.777 | 0.008 | 1.485 | 0.944–2.335 | 0.087 | 1.692 | 1.049–2.730 | 0.031 | ||
| Other minorities | 1.373 | 0.825–2.284 | 0.223 | 1.249 | 0.707–2.207 | 0.443 | 1.064 | 0.574–1.970 | 0.844 | ||
| Model 2 | |||||||||||
| Other demographic characteristics | |||||||||||
| Age (in y) | 0.963 | 0.948–0.979 | <0.001 | 0.962 | 0.946–0.979 | <0.001 | |||||
| Marital status (not married) | 0.865 | 0.421–0.816 | 0.586 | 0.613 | 0.436–0.862 | 0.002 | |||||
| Study location | 0.001f | ||||||||||
| Site A | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| Site B | 1.697 | 1.051–2.739 | 0.030 | 1.623 | 0.985–2.675 | 0.057 | |||||
| Site C | 0.545 | 0.343–0.867 | 0.010 | 0.698 | 0.426–1.145 | 0.154 | |||||
| Site D | 1.458 | 0.923–2.301 | 0.106 | 1.552 | 0.974–2.473 | 0.064 | |||||
| Medical factors | |||||||||||
| Charlson Comorbidity Index | 0.897 | 0.804–1.000 | 0.050 | 0.886 | 0.791–0.993 | 0.037 | |||||
| Model 3 | |||||||||||
| KAS | 0.247 | 0.176–3.29 | <0.001 | ||||||||
Main event = received a transplant, competing event = died or transplant at a non-VA center, censoring = still on waitlist or other removal.
Other pairwise comparisons: black to Hispanic P = 0.013, black to other P = 0.260, Hispanic to other P = 0.361.
Other pairwise comparisons: black to Hispanic P = 0.147, black to other P = 0.502, Hispanic to other P = 0.595.
Other pairwise comparisons: black to Hispanic P = 0.227, black to other P = 0.656, Hispanic to other P = 0.183.
Other pairwise comparisons: site B to site C P < 0.001, site B to site D P = 0.486, site C to D P < 0.001.
Other pairwise comparisons: site B to site C P < 0.001, site B to site D P = 0.844, site C to D P < 0.001.
CI, confidence interval; KAS, Kidney Allocation System; SHR, subdistribution hazard ratio; VA, Veterans Affairs.
We present the results of our regression analysis for receiving a DDKT in Table 4. After adjusting for multiple comparisons, Hispanics had a higher probability of receiving DDKT than non-Hispanic whites. In model 2, the probability of DDKT was higher among participants who were younger, married, from study site B (rather than site A), or had a longer dialysis duration but lower among those with higher comorbidity. Our inclusion of KAS and other variables in model 3 showed that religious objection to LDKT and outcome event occurring after KAS was associated with a lower likelihood of DDKT, but a preference for deceased donation predicted a higher likelihood of DDKT. We evaluated the interaction effect of race/ethnicity by site and race/ethnicity by KAS, but neither of them was significant.
TABLE 4.
Unadjusted and adjusted Fine-Gray proportional subdistribution hazards model for time from evaluation to receiving a DDKTa
| Variables | Model 1 |
Model 2 |
Model 3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SHR | 95% CI | P value | SHR | 95% CI | P value | SHR | 95% CI | P value | |||
| Model 1 | |||||||||||
| Race/ethnicity | 0.028b | 0.023c | 0.005d | ||||||||
| Non-Hispanic white | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | ||
| Non-Hispanic African American | 1.356 | 0.920–2.000 | 0.124 | 1.557 | 1.029–2.356 | 0.036 | 1.924 | 1.245–2.975 | 0.003 | ||
| Hispanic | 1.988 | 1.226–3.223 | 0.005 | 1.971 | 1.198–3.243 | 0.008 | 2.253 | 1.313–3.869 | 0.003 | ||
| Other minorities | 1.692 | 0.977–2.930 | 0.061 | 1.757 | 0.958–3.224 | 0.069 | 1.558 | 0.824–2.944 | 0.172 | ||
| Model 2 | |||||||||||
| Other demographic characteristics | |||||||||||
| Marital status (not married) | 0.614 | 0.427–0.884 | 0.009 | 0.612 | 0.412–0.908 | 0.015 | |||||
| Occupation (>skilled manual worker) | NS | 0.706 | 0.493–1.010 | 0.057 | |||||||
| Study location | 0.002e | 0.021f | |||||||||
| Site A | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| Site B | 1.926 | 1.125–3.300 | 0.017 | 1.720 | 0.983–3.009 | 0.057 | |||||
| Site C | 0.677 | 0.402–1.138 | 0.141 | 0.840 | 0.480–1.471 | 0.543 | |||||
| Site D | 1.494 | 0.885–2.522 | 0.133 | 1.502 | 0.864–2.612 | 0.149 | |||||
| Medical factors | |||||||||||
| Charlson Comorbidity Index | 0.825 | 0.736–0.924 | 0.001 | 0.806 | 0.715–0.907 | <0.001 | |||||
| Dialysis duration | 1.090 | 1.034–1.149 | 0.001 | 1.107 | 1.040–1.178 | 0.001 | |||||
| Model 3 | |||||||||||
| Event after KAS | 0.324 | 0.224–0.468 | <0.001 | ||||||||
| Cultural factors | |||||||||||
| Religious objection to LDKT | 0.642 | 0.431–0.956 | 0.029 | ||||||||
| Donor preference | 0.039 | ||||||||||
| No preference | 1 (reference) | 1 (reference) | 1 (reference) | ||||||||
| Deceased donor | 2.484 | 1.217–5.071 | 0.012 | ||||||||
| Living donor | 1.382 | 0.817–2.339 | 0.228 | ||||||||
Higher value = greater amount (or higher score on) a particular variable.
Main event = received a deceased donor transplant, competing event = received a living donor transplant, died or transplant at a non-VA center, censoring = still on waitlist or other removal.
Other pairwise comparisons: black to Hispanic P = 0.123, black to other P = 0.432, Hispanic to other P = 0.612.
Other pairwise comparisons: black to Hispanic P = 0.383, black to other P = 0.702, Hispanic to other P = 0.741.
Other pairwise comparisons: black to Hispanic P = 0.578, black to other P = 0.532, Hispanic to other P = 0.328.
Other pairwise comparisons: site B to site C P < 0.001, site B to site D P = 0.283, site C to D P = 0.001.
Other pairwise comparisons: site B to site C P = 0.007, site B to site D P = 0.594, site C to D P = 0.019.
CI, confidence interval; DDKT, deceased donor kidney transplantation; KAS, Kidney Allocation System; LDKT, living donor kidney transplantation; NS, nonsignificant; SHR, subdistribution hazard ratio; VA, Veterans Affairs.
We present the results of our regression analysis of LDKT in Table 5. Non-Hispanic African American patients were less likely to receive an LDKT than non-Hispanic whites. With the addition of demographic and medical factors in model 2, younger age, presenting with a living donor at evaluation, and fewer years on dialysis were significant predictors of LDKT. In model 3, the outcome event occurring after KAS and lower self-esteem were significant predictors of LDKT. Higher transplant knowledge was approaching significance as another predictor of LDKT. In the multivariable analyses, the maximum number of missing values for any single variable was 12 (2.0%) for KT, 15 (2.5%) for DDKT, and 26 (4.3%) for LDKT. Among all candidate variables, the highest proportion of missing was 4.2% (n = 26 missing for the asking for a living donor item); the maximum proportion of missing was smaller than 5%.
TABLE 5.
Unadjusted and adjusted Fine-Gray proportional subdistribution hazards model for time from evaluation to receiving an LDKTa
| Variables | Model 1 |
Model 2 |
Model 3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SHR | 95% CI | P value | SHR | 95% CI | P value | SHR | 95% CI | P value | |||
| Model 1 | |||||||||||
| Race/ethnicity | 0.078b | 0.095c | 0.115d | ||||||||
| Non-Hispanic white | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1(reference) | 1 (reference) | 1 (reference) | ||
| Non-Hispanic African American | 0.159 | 0.037–0.691 | 0.014 | 0.166 | 0.034–0.823 | 0.028 | 0.349 | 0.060–2.020 | 0.240 | ||
| Hispanic | 1.043 | 0.386–2.823 | 0.933 | 1.504 | 0.520–4.353 | 0.451 | 2.972 | 0.960–9.204 | 0.059 | ||
| Other minorities | 0.493 | 0.115–2.110 | 0.341 | 1.132 | 0.360–3.558 | 0.832 | 1.633 | 0.448–5.954 | 0.458 | ||
| Model 2 | |||||||||||
| Other demographic characteristics | |||||||||||
| Age (in y) | 0.942 | 0.909–0.977 | 0.001 | 0.959 | 0.927–0.992 | 0.015 | |||||
| Insurance, public only | 4.329 | 0.953–19.664 | 0.058 | 5.924 | 1.074–32.667 | 0.041 | |||||
| Medical factors | |||||||||||
| Have a living donor at T1 (yes) | 4.744 | 1.710–13.165 | 0.001 | 4.071 | 1.347–12.309 | 0.013 | |||||
| Dialysis duration | 0.375 | 0.223–0.632 | <0.001 | 0.328 | 0.181–0.594 | <0.001 | |||||
| Number of potential donors in network | NS | 1.019 | 1.009–1.029 | 0.0001 | |||||||
| Model 3 | |||||||||||
| Event after KAS | 10.260 | 2.498–42.140 | 0.001 | ||||||||
| Psychosocial factors | |||||||||||
| Self-esteem | 0.430 | 0.231–0.802 | 0.008 | ||||||||
| Transplant knowledge and education | |||||||||||
| Transplant knowledge | 1.301 | 0.987–1.714 | 0.062 | ||||||||
Higher value = greater amount (or higher score on) a particular variable.
Main event = received a living donor transplant, competing event = received a deceased donor transplant, died or transplant at a non-VA center, censoring = still on waitlist or other removal.
Other pairwise comparisons: black to Hispanic P = 0.025, black to other P = 0.256, Hispanic to other P = 0.368.
Other pairwise comparisons: black to Hispanic P = 0.0.014, black to other P = 0.033, Hispanic to other P = 0.680.
Other pairwise comparisons: black to Hispanic P = 0.028, black to other P = 0.156, Hispanic to other P = 0.403.
CI, confidence interval; KAS, Kidney Allocation System; LDKT, living donor kidney transplantation; SHR, subdistribution hazard ratio; VA, Veterans Affairs.
DISCUSSION
Our study is the largest prospective study of veterans who were evaluated for KT in the National VA transplant program. Unlike most studies that collect a sample of a target population,8,30,51 our study recruited virtually all Veterans who received a KT within every VA National Transplant Program Center during our study period. It also represents the first of its kind to focus on pretransplant social determinants of health as potential predictors of racial disparities in transplant status outcomes in Veterans transplanted within the National VA Transplant Program. It is a major advance from our previous study27 that focused on disparities in waitlisting for KT in the VA because the current work tracked Veterans through 2017 to study disparities in receiving a KT and the type of KT received, distinct steps in the transplantation process that have been shown to have different rates of disparities with different factors influencing outcomes at each of those steps.1,5,11,31,32,34
In addition to variables that are known to predict transplant outcomes (eg, age, comorbid conditions, or dialysis) we identified several social determinants of health that predicted type of transplant received, including marital status, self-esteem, and transplant knowledge. As we expected, the revision of the KAS policy in 2014 affected rates of DDKT but not LDKT. We found that Hispanic veterans were significantly more likely to receive a DDKT than non-Hispanic white veterans. This result indicates that the VA National Transplant Program does not exhibit the same pattern of racial disparities in receipt of DDKT as has been found in other non-VA centers, where minorities repeatedly have been shown to receive transplants at lower rates than non-Hispanic whites.1–5,11–14,31–34 Of particular interest in our sample of veterans was that non-Hispanic black, Hispanic, and other minority veterans received transplants at similar rates to non-Hispanic white veterans, which is in stark contrast to national non-VA outcomes.1–3,5,11–14,31–34,76 These analyses, along with the results demonstrating no race/ethnicity disparities in time from evaluation to listing in our previous article,27 indicate that the VA’s approach to KT evaluation surmounts the disparities that are consistently found in non-VA settings. This reduction in race/ethnicity disparities was strengthened for Hispanic veterans but not non-Hispanic black veterans after the introduction of KAS in 2014.
Marital status consistently has been found to be a psychosocial asset for positive outcomes in transplant, other clinical populations, and community-dwelling adults, as it is often a proxy of social support.77–85 Marriage is particularly advantageous in transplant because the spouse most often serves as the patient’s primary support person. In the past, patients have been turned down for transplant if they lacked a support person or if that person was not reliable. Thus, it is not surprising that being married was a significant predictor of transplant receipt across both transplant types in our study. Similarly, our findings on transplant knowledge and engagement in kidney transplant-related learning activities support our own6,27,36 and other’s work86–89 showing knowledge as a predictor of successful transplant outcomes.
We also found a transplant center effect for receiving a transplant, which was most likely influenced by the local difference in time to receive a deceased donor KT. These findings support other research that demonstrated geographic variation in transplant rates to be an important variable influencing racial disparities in rates of DDKT.90 Geographic variation may be due to local economic differences, local practice patterns, differences in referral rates by regional VAs that the transplant centers have no control over, local organ recovery rates, and local and regional organ wait times (also not under the control of any center). Although a direct comparison of transplant center rates between VA and non-VA centers is not equivalent because VA transplant centers serve patients from a larger geographic distribution than non-VA centers (who typically only serve patients within close proximity), we used Organ Procurement and Transplantation Network data76 across the same time period and combined across UNOS regions to make a rough comparison of transplant rates between each VA transplant center and the respective UNOS regions. We found that VA transplant center rates were equivalent for Hispanics and other minorities to similar UNOS regions but VA centers had higher rates of KT for AA patients than similar UNOS regions.
Overall, these results point to the important role that addressing social determinants of health may play in reducing disparities in transplantation. Although transplant centers cannot select patients based on marital status, nor can they force people to learn about transplantation, they can identify patients at risk for not obtaining a transplant due to a possible lack of social support or transplant knowledge and target those patients for additional support throughout the evaluation and transplant process. Furthermore, our results demonstrate that variation between centers can help identify key areas to target for process improvements so that the VA can provide high-quality care across all sites. The National Director of Surgery recently addressed how the VA continues to make process improvements in transplant care.29
Limitations
One important limitation of our work, because we limited our study to the VA National Transplant Program, is that we cannot definitively determine that the differences between our VA-specific findings and other published reports of national data are not an inherent result of the population served by the VA rather than the VA system itself. It is also important to note that some of our patients had to be censored because they were still on the waitlist at the end of our study period. Also, because there were relatively few Veterans who received LDKT, only very large differences between the groups were detected for this outcome. Furthermore, although our study investigated many potential predictors of KT outcomes, there are other predictors, such as time from ESKD diagnosis to referral, or county/state of residence, which we did not measure. Finally, it is important to note that race/ethnicity persisted in being a significant predictor of outcome but in ways that favored Hispanic veterans for DDKT. This finding indicates that other unique factors that we did not measure may influence KT outcomes in veterans who are transplanted within the VA National Transplant Program. Future research should examine other potential factors that may contribute to differences in transplant outcomes in this group.
CONCLUSIONS
Our results indicate that the VA National Transplant Program does not exhibit the same pattern of racial disparities in receipt of KT as has been found in non-VA transplant centers in that non-Hispanic black and Hispanic Veterans were transplanted at equal or greater rates than non-Hispanic white Veterans.2,3,5,11–14,31–34 Even though race/ethnicity and several medical factors persisted in predicting KT, we found that social determinants of health contributed as well (ie, marital status, self-esteem, and transplant knowledge). We also identified important risk factors for receipt of transplant among Veterans. VA transplant centers may be able to use these risk factors to target patients who may be in need of more social support or transplant education to increase their likelihood of receiving a transplant.
ACKNOWLEDGMENTS
The authors wish to thank the transplant teams and support staff at each study site and the site research coordinators who helped recruit participants and run the study: Malia Reed, Natalie Suiter, and Jackie Walczyk. We are especially grateful to our Veteran participants and their family members who made this project possible.
This material is based upon work supported (or supported in part) by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Health Services Research and Development. Work on this project was funded in part by a grant from the VA Health Services Research and Development Department (IIR 06-220), a grant from the National Institute of Diabetes Digestive and Kidney Diseases (R01DK081325), and a grant from Dialysis Clinic, Inc, a nonprofit corporation.
The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
The data that support the findings of this study are available on request from the first author. The data are not publicly available due to privacy or ethical restrictions.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.United States Renal Data System. 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: United Stated Renal Data System, 2018. [Google Scholar]
- 2.Fan PY, Ashby VB, Fuller DS, et al. Access and outcomes among minority transplant patients, 1999–2008, with a focus on determinants of kidney graft survival. Am J Transplant 2010;10(4 Pt 2):1090–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill JS, Hussain S, Rose C, et al. Access to kidney transplantation among patients insured by the United States Department Of Veterans Affairs. J Am Soc Nephrol 2007;18(9):2592–2599. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigue J, Kazley AS, Mandelbrot DA, et al. Living donor kidney transplantation: overcoming disparities in live kidney donation in the US - recommendations from a consensus conference. Clin J Am Soc Nephrol 2015;10(9):1687–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taber DJ, Gebregziabher M, Hunt KJ, et al. Twenty years of evolving trends in racial disparities for adult kidney transplant recipients. Kidney Int 2016;90(4):878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myaskovsky L, Almario Doebler D, Posluszny DM, et al. Perceived discrimination predicts longer time to be accepted for kidney transplant. Transplantation 2012;93(4):423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weng FL, Joffe MM, Feldman HI, et al. Rates of completion of the medical evaluation for renal transplantation. Am J Kidney Dis 2005;46(4):734–745. [DOI] [PubMed] [Google Scholar]
- 8.Joshi S, Gaynor JJ, Bayers S, et al. Disparities among blacks, Hispanics, and whites in time from starting dialysis to kidney transplant waitlisting. Transplantation 2013;95(2):309–318. [DOI] [PubMed] [Google Scholar]
- 9.Johansen KL, Zhang R, Huang Y, et al. Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clin J Am Soc Nephrol 2012;7(9):1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Melanson TA, Plantinga LC, et al. Racial/ethnic disparities in waitlisting for deceased donor kidney transplantation 1 year after implementation of the new national kidney allocation system. Am J Transplant 2018;18(8):1936–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purnell TS, Xu P, Leca N, et al. Racial differences in determinants of live donor kidney transplantation in the United States. Am J Transplant 2013;13(6):1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakkera HA, O’Hare AM, Johansen KL, et al. Influence of race on kidney transplant outcomes within and outside the Department of Veterans Affairs. J Am Soc Nephrol 2005;16(1):269–277. [DOI] [PubMed] [Google Scholar]
- 13.Young CJ, Kew C. Health disparities in transplantation: focus on the complexity and challenge of renal transplantation in African Americans. Med Clin North Am 2005;89(5):1003–1031, ix. [DOI] [PubMed] [Google Scholar]
- 14.Gander JC, Zhang X, Plantinga L, et al. Racial disparities in preemptive referral for kidney transplantation in Georgia. Clin Transplant 2018;32(9):e13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keith D, Ashby VB, Port FK, et al. Insurance type and minority status associated with large disparities in prelisting dialysis among candidates for kidney transplantation. Clin J Am Soc Nephrol 2008;3(2):463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayanian JZ, Cleary PD, Keogh JH, et al. Physicians’ beliefs about racial differences in referral for renal transplantation. Am J Kidney Dis 2004;43(2):350–357. [DOI] [PubMed] [Google Scholar]
- 17.Gordon EJ, Ladner DP, Caicedo JC, et al. Disparities in kidney transplant outcomes: a review. Semin Nephrol 2010;30(1):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schold JD, Gregg JA, Harman JS, et al. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol 2011;6(7):1760–1767. [DOI] [PubMed] [Google Scholar]
- 19.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol 2010;5(12):2276–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prieto LR, Miller DS, Gayowski T, et al. Multicultural issues in organ transplantation: the influence of patients’ cultural perspectives on compliance with treatment. Clin Transplant 1997;11(6):529–535. [PubMed] [Google Scholar]
- 21.Warsame F, Haugen CE, Ying H, et al. Limited health literacy and adverse outcomes among kidney transplant candidates. Am J Transplant 2019;19(2):457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigue JR, Cornell DL, Kaplan B, et al. A randomized trial of a home-based educational approach to increase live donor kidney transplantation: effects in blacks and whites. Am J Kidney Dis 2008;51(4):663–670. [DOI] [PubMed] [Google Scholar]
- 23.Waterman AD, Barrett AC, Stanley SL. Optimal transplant education for recipients to increase pursuit of living donation. Prog Transplant 2008;18(1):55–62. [DOI] [PubMed] [Google Scholar]
- 24.Waterman AD, Stanley SL, Covelli T, et al. Living donation decision making: recipients’ concerns and educational needs. Prog Transplant 2006;16(1):17–23. [DOI] [PubMed] [Google Scholar]
- 25.Chilcot J, Spencer BW, Maple H, et al. Depression and kidney transplantation. Transplantation 2014;97(7):717–721. [DOI] [PubMed] [Google Scholar]
- 26.Noohi S, Khaghani-Zadeh M, Javadipour M, et al. Anxiety and depression are correlated with higher morbidity after kidney transplantation. Transplant Proc 2007;39(4):1074–1078. [DOI] [PubMed] [Google Scholar]
- 27.Freeman MA, Pleis JR, Bornemann KR, et al. Has the Department of Veterans Affairs found a way to avoid racial disparities in the evaluation process for kidney transplantation? Transplantation 2017;101(6):1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunnar W The VA transplant program: a rebuttal to criticism and a look to the future. Am J Transplant 2019;19(5):1288–1295. [DOI] [PubMed] [Google Scholar]
- 29.Gunnar W, Bronson DA, Cupples SA. Access to transplant care and services within the Veterans Health Administration. Fed Pract 2018;35(8):12–21. [PMC free article] [PubMed] [Google Scholar]
- 30.Melancon JK, Kucirka LM, Boulware LE, et al. Impact of Medicare coverage on disparities in access to simultaneous pancreas and kidney transplantation. Am J Transplant 2009;9(12):2785–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harding K, Mersha TB, Pham PT, et al. Health disparities in kidney transplantation for African Americans. Am J Nephrol 2017;46(2):165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malek SK, Keys BJ, Kumar S, et al. Racial and ethnic disparities in kidney transplantation. Transpl Int 2011;24(5):419–424. [DOI] [PubMed] [Google Scholar]
- 33.Melanson TA, Hockenberry JM, Plantinga L, et al. New kidney allocation system associated with increased rates of transplants among black and Hispanic patients. Health Aff (Millwood) 2017;36(6):1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waterman AD, Peipert JD, Hyland SS, et al. Modifiable patient characteristics and racial disparities in evaluation completion and living donor transplant. Clin J Am Soc Nephrol 2013;8(6):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bird ST, Bogart LM. Perceived race-based and socioeconomic status(SES)-based discrimination in interactions with health care providers. Ethn Dis 2001;11(3):554–563. [PubMed] [Google Scholar]
- 36.Myaskovsky L, Switzer GE, Crowley-Matoka M, et al. Psychosocial factors associated with ethnic differences in transplantation. Curr Opin Organ Transplant 2007;12(2):182–187. [Google Scholar]
- 37.Thorburn Bird S, Bogart LM. Birth control conspiracy beliefs, perceived discrimination, and contraception among African Americans: an exploratory study. J Health Psychol 2003;8(2):263–276. [DOI] [PubMed] [Google Scholar]
- 38.Bird ST, Bogart LM, Delahanty DL. Health-related correlates of perceived discrimination in HIV care. AIDS Patient Care STDS 2004;18(1):19–26. [DOI] [PubMed] [Google Scholar]
- 39.Krieger N. Discrimination and Health Inequities In: Berkman L, Kawachi I, eds. Social Epidemiology Oxford, UK: Oxford University Press; 2000:36–75. [Google Scholar]
- 40.Landrine H, Klonoff EA. The schedule of racist events: a measure of racial discrimination and a study of its negative physical and mental health consequences. J Black Psychology 1996;22(2):114–168. [Google Scholar]
- 41.Paradies Y A systematic review of empirical research on self-reported racism and health. Int J Epidemiol 2006;35(4):888–901. [DOI] [PubMed] [Google Scholar]
- 42.Finnegan JR Jr, Meischke H, Zapka JG, et al. Patient delay in seeking care for heart attack symptoms: findings from focus groups conducted in five U.S. regions. Prev Med 2000;31(3):205–213. [DOI] [PubMed] [Google Scholar]
- 43.LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev 2000;57 Suppl 1:146–161. [DOI] [PubMed] [Google Scholar]
- 44.Krieger N Does racism harm health? Did child abuse exist before 1962? On explicit questions, critical science, and current controversies: an ecosocial perspective. Am J Public Health 2003;93(2):194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holley JL, McCauley C, Doherty B, et al. Patients’ views in the choice of renal transplant. Kidney Int 1996;49(2):494–498. [DOI] [PubMed] [Google Scholar]
- 46.Louis ON, Sankar P, Ubel PA. Kidney transplant candidates’ views of the transplant allocation system. J Gen Intern Med 1997;12(8):478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klassen AC, Hall AG, Saksvig B, et al. Relationship between patients’ perceptions of disadvantage and discrimination and listing for kidney transplantation. Am J Public Health 2002;92(5):811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boulware LE, Ephraim PL, Ameling J, et al. Effectiveness of informational decision aids and a live donor financial assistance program on pursuit of live kidney transplants in African American hemodialysis patients. BMC Nephrol 2018;19(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipford KJ, McPherson L, Hamoda R, et al. Dialysis facility staff perceptions of racial, gender, and age disparities in access to renal transplantation. BMC Nephrol 2018;19(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozminkowski RJ, White AJ, Hassol A, et al. Minimizing racial disparity regarding receipt of a cadaver kidney transplant. Am J Kidney Dis 1997;30(6):749–759. [DOI] [PubMed] [Google Scholar]
- 51.Siegel JT, O’Brien EK, Alvaro EM, et al. Barriers to living donation among low-resource Hispanics. Qual Health Res 2014;24(10):1360–1367. [DOI] [PubMed] [Google Scholar]
- 52.Noordzij M, Leffondré K, van Stralen KJ, et al. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 2013;28(11):2670–2677. [DOI] [PubMed] [Google Scholar]
- 53.Bennett WM. VA solid organ transplant programs: maximizing the resources for current and future veterans. Am J Transplant 2019;19(5):1259–1261. [DOI] [PubMed] [Google Scholar]
- 54.Stewart DE, Klassen DK. Early experience with the new kidney allocation system: a perspective from UNOS. Clin J Am Soc Nephrol 2017;12(12):2063–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart DE, Kucheryavaya AY, Klassen DK, et al. Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant 2016;16(6):1834–1847. [DOI] [PubMed] [Google Scholar]
- 56.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 57.Jassal SV, Schaubel DE, Fenton SS. Baseline comorbidity in kidney transplant recipients: a comparison of comorbidity indices. Am J Kidney Dis 2005;46(1):136–142. [DOI] [PubMed] [Google Scholar]
- 58.Delmonico F; Council of the Transplantation Society. A report of the Amsterdam forum on the care of the live kidney donor: data and medical guidelines. Transplantation 2005;79(6 Suppl):S53–S66. [PubMed] [Google Scholar]
- 59.Arthur T The role of social networks: a novel hypothesis to explain the phenomenon of racial disparity in kidney transplantation. Am J Kidney Dis 2002;40(4):678–681. [DOI] [PubMed] [Google Scholar]
- 60.Williams DR, Yan Yu, Jackson JS, et al. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol 1997;2(3):335–351. [DOI] [PubMed] [Google Scholar]
- 61.Boulware LE, Ratner LE, Ness PM, et al. The contribution of sociode-mographic, medical, and attitudinal factors to blood donation among the general public. Transfusion 2002;42(6):669–678. [DOI] [PubMed] [Google Scholar]
- 62.LaVeist TA, Isaac LA, Williams KP. Mistrust of health care organizations is associated with underutilization of health services. Health Serv Res 2009;44(6):2093–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson LA, Dedrick RF. Development of the trust in physician scale: a measure to assess interpersonal trust in patient-physician relationships. Psychol Rep 1990;67(3 Pt 2):1091–1100. [DOI] [PubMed] [Google Scholar]
- 64.Bardis PD. A familism scale. J Marriage Fam 1959;21:340–341. [Google Scholar]
- 65.Rumsey S, Hurford DP, Cole AK. Influence of knowledge and religiousness on attitudes toward organ donation. Transplant Proc 2003;35(8):2845–2850. [DOI] [PubMed] [Google Scholar]
- 66.Myaskovsky L, Burkitt KH, Lichy AM, et al. The association of race, cultural factors, and health-related quality of life in persons with spinal cord injury. Arch Phys Med Rehabil 2011;92(3):441–448. [DOI] [PubMed] [Google Scholar]
- 67.Derogatis L, Spencer P. The Brisef Symptom Inventory (BSI): Administration, Scoring, and Procedures Manual In: Clinical Psychometric Research Baltimore, MD; 1975. [Google Scholar]
- 68.Cohen S, Underwood LG, Gottlieb BH. Social Support Measurement and Intervention: A Guide for Health and Social Scientists New York, NY: Oxford University Press; 2000. [Google Scholar]
- 69.Cohen S, Zdaniuk B. ISEL 12 Psychometric Properties 2006. Available at http://www.psy.cmu.edu/~scohen/. Accessed October 26, 2011.
- 70.Rosenberg M. Society and the adolescent self-image Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- 71.Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav 1978;19(1):2–21. [PubMed] [Google Scholar]
- 72.Wallston KA, Stein MJ, Smith CA. Form C of the MHLC scales: a condition-specific measure of locus of control. J Pers Assess 1994;63(3):534–553. [DOI] [PubMed] [Google Scholar]
- 73.Murray LR, Conrad NE, Bayley EW. Perceptions of kidney transplant by persons with end stage renal disease. Anna J 1999;26(5):479–83, 500; discussion 484. [PubMed] [Google Scholar]
- 74.Kohl M, Plischke M, Leffondré K, et al. PSHREG: a SAS macro for proportional and nonproportional subdistribution hazards regression. Comput Methods Programs Biomed 2015;118(2):218–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94(446):496–509. [Google Scholar]
- 76.Organ Procurement and Transplantation Network. National Data Available at optn.transplant.hrsa.gov. Accessed November 30, 2018.
- 77.Müller HH, Englbrecht M, Wiesener MS, et al. Depression, anxiety, resilience and coping pre and post kidney transplantation - initial findings from the psychiatric impairments in kidney transplantation (PI-KT)-study. PLOS One 2015;10(11):e0140706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nevins TE, Nickerson PW, Dew MA. Understanding medication nonadherence after kidney transplant. J Am Soc Nephrol 2017;28(8):2290–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the caregiver health effects study. JAMA 1999;282(23):2215–2219. [DOI] [PubMed] [Google Scholar]
- 80.Cronkite RC, Moos RH. The role of predisposing and moderating factors in the stress-illness relationship. J Health Soc Behav 1984;25(4):372–393. [PubMed] [Google Scholar]
- 81.Feldman LB. Depression and marital interaction. Fam Process 1976;15(4):389–395. [DOI] [PubMed] [Google Scholar]
- 82.Giunta CT, Compas BE. Coping in marital dyads: patterns of associations with psychological symptoms. J Marriage Fam 1993;55(4):1011–1017. [Google Scholar]
- 83.Gruen RJ, Folkman S, Lazarus RS. Dyadic response patterns in married couples, depressive symptoms, and somatic dysfunction. J Fam Psychol 1987;1(2):168–186. [Google Scholar]
- 84.Kahn J, Coyne JC, Margolin G. Depression and marital disagreement: the social construction of despair. J Soc Pers Relatsh 1985;2(4):447–461. [Google Scholar]
- 85.Myaskovsky L, Dew MA, Switzer GE, et al. Quality of life and coping strategies among lung transplant candidates and their family caregivers. Soc Sci Med 2005;60(10):2321–2332. [DOI] [PubMed] [Google Scholar]
- 86.Browne T, Amamoo A, Patzer RE, et al. Everybody needs a cheer-leader to get a kidney transplant: a qualitative study of the patient barriers and facilitators to kidney transplantation in the Southeastern United States. BMC Nephrol 2016;17(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wachterman MW, McCarthy EP, Marcantonio ER, et al. Mistrust, misperceptions, and miscommunication: a qualitative study of preferences about kidney transplantation among African Americans. Transplant Proc 2015;47(2):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodrigue J, Paek M, Schold J, et al. Predictors and moderators of educational interventions to increase the likelihood of potential living donors for Black patients awaiting kidney transplantation. J Racial Ethn Health Disparities 2017;4(5):837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Axelrod DA, Kynard-Amerson CS, Wojciechowski D, et al. Cultural competency of a mobile, customized patient education tool for improving potential kidney transplant recipients’ knowledge and decision-making. Clin Transplant 2017;31(5):e12944. [DOI] [PubMed] [Google Scholar]
- 90.Saunders MR, Lee H, Alexander GC, et al. Racial disparities in reaching the renal transplant waitlist: is geography as important as race? Clin Transplant 2015;29(6):531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]


