Abstract
We describe an optical assay for glucose based on the luminescence decay time of a long lifetime metal–ligand complex. Concanavalin A was covalently labeled with Ruthenium metal–ligand complex (RuCon A) which served as the donor. The acceptor was malachite green which was covalently linked to insulin. The malachite green insulin was also covalently labeled with maltose (MIMG) to provide binding affnity to RuCon A. Binding of RuCon A to MIMG resulted in a decreased intensity and decay time of RuCon A. Glucose was detected by competitive displacement of MIMG from RuCon A, resulting in increased intensity and decay time. This glucose assay has several favorable features. The long life time of RuCon A allows phase-modulation decay time measurements using an amplitude-modulated blue-light-emitting diode as the light source. Reversibility of the assay can be controlled by the extent of sugar labeling of the insulin. Finally, the glucose-sensitive range can be adjusted by selection of the sugar structure and extent of labeling of the insulin.
Noninvasive optical sensing of glucose is the “Holy Grail” of clinical diagnostics research. The availability of such a glucose assay would allow improved control of blood glucose in diabetics, for whom fluctuations in glucose concentrations result in long-term health consequences of blindness and/or cardiovascular disease. Insulin pumps are currently available, but a means is lacking to control insulin delivery based on blood glucose.
Numerous laboratories and companies have attempted to develop noninvasive glucose assays. These include the use of near infrared spectroscopy (1–4) and optical activity (5–7). To date, none of these methods have been successfully used as clinically useful assays, which is the result of the weak optical signals obtain able from glucose over the strong background of the scattering and absorption properties of skin.
In this laboratory we are attempting to develop glucose-sensing methods which will allow transdermal measurements of glucose using sensing patches implanted below the skin. This quasi-noninvasive approach was chosen because of the difficulties in developing completely noninvasive glucose measurements. The basic idea is to develop a glucose-sensitive patch in which the fluorescence decay time changes in response to the glucose concentrations. During the past several years we have demonstrated the feasibility of lifetime-based sensing of a variety of clinically relevant analytes (8), including glucose (9). Lifetime measurements can be advantageous in clinical sensing because the measured decay times are mostly independent of the probe concentrations and optical properties of the sample. Lifetime measurements can be performed in highly scattering media (10, 11), through skin (12), and more recently in scattering media with two-photon excitation (13).
Additionally, it is known that tissue glucose closely follows blood glucose with only a 15-min time delay (14). Based on these independent observations we believe that a glucose-sensitive patch, with optical transdermal measurements, can provide a clinically useful method for continuous monitoring of blood glucose.
In a previous report we demonstrated lifetime-based sensing of glucose using fluorescence resonance energy transfer (FRET).3 This assay (9) was based on the reversible association of concanavalin A (Con A) with dextran. This approach (9) is similar to that described by Schultz and co-workers (15–17), except that we use lifetimes instead of fluorescence intensities. The Con A was labeled with a fluorescent donor (D) and the dextran with an acceptor (A). Association of D-Con A with A-dextran resulted in FRET, decreasing the intensity and lifetime of the donor. Glucose was measured by its competitive displacement of A-dextran from D-Con A, resulting in an increased lifetime. While this assay performed acceptably well using some donor–acceptor pairs, the glucose reversibility was often inadequate. Additionally, the donor fluorophores displayed nanosecond decay times, requiring high-frequency light modulation for phase-modulation lifetime measurements.
In the present report we describe another competitive assay based on Con A (Scheme 1). During the past 2 years we have developed several long-lifetime probes based on the luminescent complex ruthenium tris-(2,2′-bipyridyl), [Ru(bpy)3]2+. These complexes dis play lifetimes near 400 ns and have been covalently attached to proteins to study microsecond hydrodynamics of proteins (18), fluorescence polarization immunoassays (19–20), and FRET immunoassays (21). The long decay times are advantageous because the light source for phase-modulation lifetime measurements can be a simple LED (22, 23), and MLCs are known which absorb at long wavelengths beyond the absorption of skin (12, 20). Additionally, the long decay times of the luminescent MLCs allow elimination of the nanosecond autofluorescence by off-gating the detector.
SCHEME 1.
Donor-labeled Con A and sugar-acceptor-labeled insulin.
Finally, we chose to use an acceptor-labeled reagent other than dextran. The multiple Con A binding sites for dextran may have contributed to problems of Con A–dextran aggregation and lack of reversibility with glucose. We chose to use a saccharide-labeled protein, maltose-labeled insulin, to allow control of the number of saccharides and the probability for aggregation with Con A. Additionally, by changing the saccharide and/or extent of insulin labeling with saccharide, it should be possible to adjust the glucose-sensitive range of the sensor.
MATERIALS AND METHODS
Con A, bovine insulin, and maltose were purchased from Sigma and malachite green isothiocyanate was purchased from Molecular Probes. Bis(2,2′-bipyridyl)-(4 - succinyl - 2,2′- bipyridyl) - ruthenium(II)hexafluoro-phosphate [Ru(bpy)2msubpy] was synthesized as described in Ref. (24). Preparation of the maltose–insulin conjugate has been previously reported by Jeong et al. (25). The degree of glycosylation was determined by the phenol–sulfuric acid method (26) and was found to be 1 maltose per insulin molecule.
Ru-labeled Con A was prepared by adding 5 mg (4.5 μmol) Ru(bpy)2msubpy dissolved in 100 μl DMF to 5 mg (0.19 μmol) Con A and 7.8 mg α-methyl mannoside that are dissolved in 2 ml of a 0.2 m borate buffer, pH8.0. The reaction was allowed to proceed overnight at 4°C and the resulting conjugate was separated on a Biogel P6 column. The extent of labeling was 5–6 Ru per Con A.
To prepare the maltose–insulin–malachite green (MIMG), 5.9 mg (12 μmol) of malachite green isothiocyanate dissolved in 100 μl DMF was added to 1.9 μmol insulin–maltose that was dissolved in 1.0 ml of 0.2 m bicarbonate buffer, pH 8.5. The reaction was stirred for an hour at room temperature and then quenched by the addition of 100 μl 1.5 m hydroxylamine with incubation for an additional hour at room temperature. The conjugate was purified by gel filtration on a Biogel P6 column equilibrated with 0.1 m phosphate buffer, pH8.0. Approximately 38% of the protein was recovered with 1.5 malachite green dyes per insulin molecule. All measurements were done in pH 6.8 buffer containing 0.25 m NaCl, 0.005 m NaH2PO4, 0.2 mm CaCl2, 0.1 mm MnCl2, and 0.02% sodium azide.
Emission spectra were recorded on an Aminco SLM AB2 spectrofluorometer using an excitation wavelength of 460 nm. Polarizers were used to eliminate the effect of Brownian rotation.
Frequency-domain lifetime measurements were per formed using the FD data acquisition card and Koala optical module from ISS, Inc. (Urbana, IL). The light source was an air-cooled argon ion laser (543-AP Omnichrome) at 488 nm. The excitation wavelength was amplitude modulated by an electrooptical low-frequency modulator (K2.LF from ISS, Inc.). The emission was isolated using a bandpass filter which transmits light from 610 to 660 nm (from Intor, Inc.).
The frequency-domain intensity data were fit to a multiexponential decay law
| [1] |
where αi and τi are the preexponential factors and de cay times, respectively. The parameters values (αi, τi) were determined from frequency-domain data as described previously (27, 28) and used to calculate the mean lifetime, :
| [2] |
For sensing applications a full multiexponential resolution of the intensity decay is not necessary. In these cases we used phase angle (ϕ) and modulation (m) measurements at a single light modulation frequency (ω). The measured values can be related to apparent phase (τp) and modulation (τm) lifetimes using
| [3] |
| [4] |
The apparent values represent a weighted average of the decay times (τi) displayed by the sample.
RESULTS
The absorption (A) and emission (C) spectra of RuCon A are shown in Fig. 1. These spectra show several favorable properties of the Ru MLCs as luminescent probes. These complexes display strong absorption near 460 nm (ϵ = 11,500 M−1 cm−1). This absorption is ideally matched to the optical output of blue LED light sources. The Ru complexes display a large Stokes shift, from 460 to 650 nm, so that it is relatively easy to filter the excitation light from the emission. Finally, this MLC, when bound to protein, displays an average lifetime near 500 ns (18, 24), making it a promising fluorophore for low-cost lifetime-based sensing.
FIG. 1.
Absorption (-·-) and emission (---) spectra of the donor RuCon A and absorption spectrum (—) of the acceptor maltose-insulin-malachite green (MIMG).
The acceptor for energy transfer was designed to have three key features: a sugar moiety, the acceptor dye, and a small protein spacer (Scheme 1). Bovine insulin was chosen as the protein spacer because it is a relatively small protein (Mr 5700) whose structure is very well established. It has three free amino groups at positions A-1, B-1, and B-29 that are available for labeling with the acceptor dye and the sugar moiety. With careful reaction conditions, it is possible to limit conjugation to one dye and one sugar per insulin, thus reducing aggregation to a minimum.
Brownlee and Cerami (29) have shown that the distribution of the sugar moiety in the 1:1 maltose–insulin conjugate is as follows: 49% on B-1; 29% on B-29, and 22% on A-1. The distribution of the sugar to these three sites depends on the relative reactivities of the amine functionalities, which are constant, and the re action conditions such as pH, temperature, etc., which are controllable. Thus, one can say with confidence that individual preparations will yield essentially the same products as long as the same conditions are followed. The same rationale can be applied to the reaction of the malachite green to glycosylated insulin and the Ru complex to Con A.
The sugar moiety serves as the recognition site for Con A binding. It docks onto the sugar binding site of Con A, allowing the acceptor dye to get in close proximity with the donor, thereby allowing energy transfer to take place. In addition, it acts as the competitor for glucose, thus providing the basis for measuring glucose concentrations. We chose maltose for this purpose because when conjugated to insulin, it was reported to have comparable binding affinity for Con A as for glucose (30). The absorption spectrum of the acceptor, MIMG, is shown in Fig. 1. It has an extinction coefficient of 61,000 m−1 cm−1 at 630 nm where it overlaps very strongly with the emission spectrum of RuCon A. Based on the spectral properties of RuCon A and malachite green, the estimated Förster distance is about 42 Å.
Emission spectra of RuCon A in the presence of increasing concentrations of MIMG are shown in Fig. 2. Decreased intensity of RuCon A with increased concentration of MIMG is an effect of energy transfer and inner filter effects. The latter is evident from the shift of the emission maxima toward longer wavelengths at higher MIMG concentrations. To confirm that significant quenching is due to energy transfer, intensity de cay measurements were performed. Time-resolved data are unaffected by inner filter effects and can be used to estimate energy transfer parameters for a D– A system using appropriate models.
FIG. 2.
Emission spectra of RuCon A in the presence of increasing concentrations of MIMG. The RuCon A concentration was 5 μm.
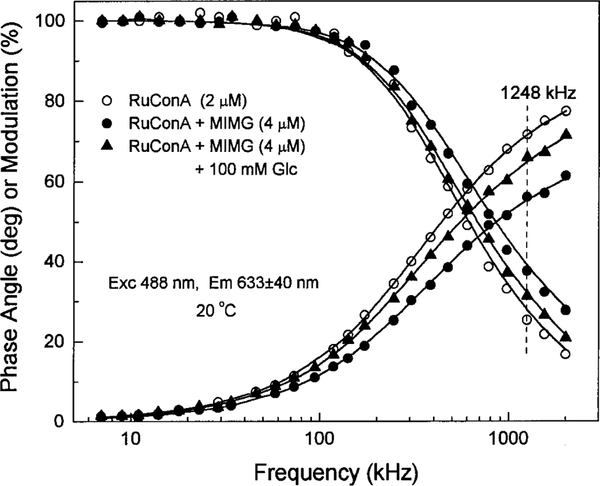
Multiexponential analysis was used to calculate the changes in mean lifetime of RuCon A in the presence of MIMG (Eqs. [1] and [2]). The representative frequency-domain data (RuCon A alone and in the presence of 4 μm MIMG) are shown in Fig. 3. The FD data show that MIMG results in a decrease in the mean lifetime of RuCon A, as seen by the shift of the data to higher frequencies. Analysis of the decay data showed that the mean lifetime of RuCon A decreases from 431 ns alone to 337 ns in the presence of 4 μm MIMG.
FIG. 3.
Frequency-domain intensity decays of 2 μm RuCon A, 2 μm RuCon A with 4 μm MIM, and the latter with 100 mm glucose.
To determine whether this observed decrease in mean lifetime of RuCon A is indeed due to energy transfer and binding of MIMG to RuCon A and not to other quenching processes, insulin labeled with malachite green (IMG), but not with maltose, was added to RuCon A in a similar manner. In contrast to MIMG, IMG was not expected to bind to Con A because it lacks the sugar moiety. The mean lifetime of RuCon A decreased only from 431 to 426 ns in the presence of 4 μm IMG, resulting in a small decrease in the phase angle (Fig. 4). Quenching becomes more significant at higher concentrations, but still only a fraction of that observed with MIMG. This small change may, therefore, be attributed to diffusion-dependent energy transfer that occurs because of the long lifetime of RuCon A. It may also be due to very weak nonspecific binding of IMG with RuCon A.
FIG. 4.
Phase angle of RuCon A at 1248 kHz in the presence of MIMG and the control IMG without maltose.
To understand our D–A system in terms of energy transfer parameters we analyzed the frequency-do main data using a method described in detail elsewhere(31). This method (31) determines the fraction of free donor (RuCon A), fraction of D–A pairs (RuCon A–MIMG), average energy transfer efficiency for D–A pairs, average distance between D and A in D–A pairs, and the distance distribution.
We measured intensity decays of RuCon A (2 μm) in the presence of 0, 1, 2, 4, and 8 μm MIMG. The following parameters were obtained: average energy transfer efficiency and average distance between Ru and MG in RuCon A–MIMG pairs are 0.81 and 31 A, respectively. The fraction of RuCon A–MIMG increased with increased MIMG concentration from 0.42 (1 μm MIMG) to 0.60 (2 μm) to 0.74 (4 μm) to 0.83 (8 μm). The distance distribution parameter hw (full width at half-maximum height of distribution) was held fixed during the fitting procedure and was estimated at about 18 Å (the best fit). The average distance of 31 Å and distance distribution of 18 Å are obtained for Förster distance, R0 of 42 Å calculated based on spectral properties of RuCon A and MIMG (32). The recovered parameters cannot be regarded as precise values since multi-MIMG binding to RuCon A cannot be excluded. Nonetheless, obtained values reasonably describe our D–A system and are valuable for future design of such D–A-sensing probes. For example, helpful information can be estimated on affinity binding between the macromolecules from fractions of free and D–A pairs. Also, energy transfer efficiency from D to A depends on structure and size of labeled macromolecules and density of D and A.
Steady-state fluorescence spectra of 2 μm RuCon A in the presence of 4 μm MIMG and varying amounts of glucose are shown in Fig. 5. This figure shows that the emission intensity of RuCon A increased by the addition of glucose. Importantly, the FD data shift back to lower frequencies in the presence of 100 mm glucose, with a mean lifetime of 413 ns (Fig. 3). The increased RuCon A lifetime indicates competitive displacement of MIMG by glucose.
FIG. 5.
Emission spectra of RuCon A (2 μm) with MIM (4 μm), in the presence of increasing concentrations of glucose.
For glucose sensing it is not necessary to resolve the multiexponential decays. Phase or modulation measurements at a single modulation frequency are adequate to determine the analyte concentration. Such data are shown for the phase angle data in Fig. 6. Addition of glucose results in increases in phase angle consistent with increased decay times of RuCon A. However, increase in decay time depends on the order by which MIMG and glucose are added to RuCon A. In designing a glucose sensor, one would intuitively start with a mixture of the donor and acceptor which is then made to come in contact with a solution of glucose. This is represented in Fig. 6 by the (D + A) + Glc data. A maximum change in the phase angle of ~7° occurs within the range of 0–50 mm glucose. Better glucose sensitivity can be obtained when MIMG is added to a solution of RuCon A and glucose (~9°). This is shown in Fig. 6 by (D + Glc) + A. In both cases, the full reversibility of 15° is not observed. These observations may be attributed to an additional irreversible pathway in the RuCon A–MIMG binding.
FIG. 6.
Glucose measurements from phase or modulation at 1248 kHz. The assay mixture contained 1 μm RuCon A and 2 μm MIM.
To clarify whether the irreversibility is related to the efficiency of binding of glucose to MIMG, we titrated D + A with α-d-mannoside. This sugar is known to dis place polysaccharides bound to Con A 30 times more efficiently than glucose (33). The mannoside-dependent phase angle for D + A is shown in Fig. 6. To produce the same degree of change in the phase angle as mannoside, about a 25-fold increase in concentration of glucose is required; thus, the assay is about 25 times more sensitive to mannose. This means that mannose competes with MIMG better than glucose, consistent with what is known about native Con A. Thus, these data tell us that labeling of Con A with Ru(bpy)2msubpy probably creates only minor perturbations in the native Con A structure and the binding site is essentially un altered. Despite this, the full reversibility of 15° is not achieved. This may be partially attributed to the same processes observed for IMG (see Fig. 4), as well as non-reversible RuCon A–MIMG aggregates.
Examination of Fig. 6 reveals that the glucose-sensitive range of our assay is somewhat above the desired physiological range. In particular, our own assay displays a KD near 40 mm, whereas blood glucose levels are typically in the range of 6–11 mm. Hence, the clinical uses of our assay would require that the glucose-sensitive range be varied. We believe this would be possible by changing the structure of the sugar moiety which is linked to the insulin donor. This work is currently in progress.
DISCUSSION
The experiments described in this report demon strate that lifetime-based sensing of glucose is possible using long-lifetime metal–ligand probes. What additional developments are needed to result in a useful glucose sensor? One obvious need is for longer wave length excitation. The currently used 450-nm excitation is not likely to penetrate skin and tissues which absorb light up to about 600 nm. We have already developed a red-absorbing osmium MLC which can be excited at wavelengths as long as 690 nm (20). However, this probe has a short lifetime near 20 ns and a low quantum yield. Fortunately, we have already shown that the use of tridentate ligands on osmium can result in lifetimes near 200 ns (34) with absorption to 650 nm. These results (34) are in general agreement with the report of Brewer and co-workers (35) or the long decay times of tridentate–osmium complexes, although our measured decay times are about twofold shorter than the 400-ns decay times they report (35). Nonetheless, the existence of these long lifetime complexes indicates it will be possible to develop long-life time probes for transdermal excitation.
A significant potential difficulty is whether the implanted patch will remain in equilibrium or follow the blood glucose concentrations. It is already known that tissue glucose closely follows blood glucose (14). However, it is possible that the body will react to the implanted patch by capsule formation. Work is currently in progress in our laboratories to evaluate this possibility. Preliminary results have shown that a period of capsule formation in the area surrounding the patch becomes revascularized, suggesting access to blood glucose (36).
Finally, there are alternative methods to use for fluorescence sensing of glucose. During the past 2 years several reports have appeared on fluorophores which are sensitive to glucose (40). These fluorophores have two or more boron groups for binding to the hydroxyl group of sugars. While the effects of glucose on the lifetime of these probes have not been reported, it seems probable that these fluorophores will display glucose-sensitive lifetimes.
In summary, quasi-noninvasive sensing of glucose seems feasible with available technology. In addition, immediate applications can be foreseen in the bio-process arena. A dialysis membrane bag containing MIMG and RuCon A can be used to fabricate a fiber-optic glucose sensor with a modulated blue LED and a lock-in amplifier. Such an approach has been demonstrated previously for oxygen sensing in bioreactors (41).
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (RR-10955) to Dr. Govind Rao and from the National Center for Resource Resources (RR-08119) to Joseph R. Lakowicz.
Footnotes
Abbreviations used: A, acceptor; bpy, 2,2′-bipyridine; D, donor; DMF, dimethylformamide; FD, frequency domain; FRET, fluorescence resonance energy transfer; IMG, insulin labeled with the malachite green; LED, light-emitting diode; mcbpy, 4-methyl-4′-carboxyl-2,2′-bipyridine; MG, malachite green isothiocyanate; MIMG, maltose–insulin labeled with the malachite green; MLC, metal–ligand complex; msubpy, 4-methyl-4′-succinyl-2,2′-bipyridine; RuCon A, concanavalin A labeled with the NHS ester of Ru(bpy)2(mcbpy).
REFERENCES
- 1.Heise HM, Marbach R, Koschinsky, Th., and Gries, F. A. (1994) Artif. Organs 18(6), 439–447. [DOI] [PubMed] [Google Scholar]
- 2.Bauer B, and Floyd TA (1987) Anal. Chim. Acta 197, 295–301. [Google Scholar]
- 3.Kaiser N (1977) J. Horm. Metab. Res Suppl. 8, 30–33. [PubMed] [Google Scholar]
- 4.Robinson MR, Eaton RP, Haaland DM, Koepp GW, Thomas EV, Stallard BR, and Robinson PL (1992) Clin. Chem 38(9), 1618–1622. [PubMed] [Google Scholar]
- 5.March WF, Rabinovitch B, Adams R, Wise JR, and Mel ton M (1982) Trans. Am. Soc. Artif. Intern. Organ 28, 232–235. [PubMed] [Google Scholar]
- 6.Rabinovitch B, March WF, and Adams RL (1982) Diabetes Care 5(3), 254–258. [DOI] [PubMed] [Google Scholar]
- 7.Gunby P (1980) JAMA 243(4), 317. [PubMed] [Google Scholar]
- 8.Szmacinski H, and Lakowicz JR (1994) in Topics in Fluores cence Spectroscopy (Lakowicz JR Ed.), Vol. 4, pp. 295–334, Plenum Press, New York. [Google Scholar]
- 9.Lakowicz JR, and Maliwal B (1993) Anal. Chim. Acta 271, 155–164. [Google Scholar]
- 10.Szmacinski H, and Lakowicz JR (1995) Sensors Actuators B 29, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burch CL, Lakowicz JR, and Sevick EM (1995) Biophys.J 68, 1574–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bambot SB, Rao G, Romauld M, Carter GM, Sipior J, Terpetschnig E, and Lakowicz JR (1995) Biosensors Bioelec tronics 10(6/7), 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szmacinski H, Gryczynski I, and Lakowicz JR (1997) Sub mitted for publication. [Google Scholar]
- 14.Velho G, Froguel Ph, Thevenot DR, and Reach G (1988) Diabetes Nutr. Metab 3, 227–233. [Google Scholar]
- 15.Meadows D, and Schultz JS (1988) Talanta 35(2), 145–150. [DOI] [PubMed] [Google Scholar]
- 16.Schultz JS, Mansouri S, and Goldstein IJ (1982) Diabetes Care 5(3), 245–253. [DOI] [PubMed] [Google Scholar]
- 17.Schultz JS, and Sims G (1979) Biotechnol. Bioeng. Symp 9, 65–71. [PubMed] [Google Scholar]
- 18.Terpetschnig E, Szmacinski H, Malak H, and Lakowicz JR (1995) Biophys. J 68, 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terpetschnig E, Szmacinski H, and Lakowicz JR (1995) Anal. Biochem 227, 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terpetschnig E, Szmacinski H, and Lakowicz JR (1996) Anal. Biochem 240, 54–59. [DOI] [PubMed] [Google Scholar]
- 21.Youn HJ, Terpetschnig E, Szmacinski H, and Lakowicz JR (1995) Anal. Biochem 232, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fantini S, Franceschini MA, Fishkin JB, Barbieri B, and Gratton E (1994) Appl. Optics 33(22), 5204–5213. [DOI] [PubMed] [Google Scholar]
- 23.Sipior J, Carter GM, Lakowicz JR, and Rao G (1996) Rev. Sci. Instrum 67(11), 3795–3798. [Google Scholar]
- 24.Szmacinski H, Terpetschnig E, and Lakowicz JR (1996) Bio phys. Chem 62(1–3), 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong SY, Kim SW, Eenink MJD, and Feijen J (1984)J. Controlled Release 1, 57–66. [Google Scholar]
- 26.Dubois M, Gilles KA, Hamilton JK, Rebers PA, and Smith F (1956) Anal. Chem 28, 350–356. [Google Scholar]
- 27.Lakowicz JR, Gratton E, Laczko G, Cherek H, and Limke man M (1984) Biophys. J 46, 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gratton E, Lakowicz JR, Maliwal B, Cherek H, Laczko G, and Limkeman M (1984) Biophys. J 46, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brownlee M, and Cerami A (1983) Diabetes 32, 499–504. [DOI] [PubMed] [Google Scholar]
- 30.Sato S, Jeong SY, McRea JC, and Kim SW (1984) Pure Appl. Chem 56(10), 1323–1328. [Google Scholar]
- 31.Lakowicz JR, Gryczynski I, Wiczk W, Kus¬ba J, and Johnson ML (1991) Anal. Biochem 195, 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Förster Th. (1948) Ann. Phys 2, 55–75. [Translated by R. S. Knox, 1974]. [Google Scholar]
- 33.Goldstein IJ (1976) in Concanavalin A as a Tool (Bittiger H, and Schnebli HP, Eds.), Wiley, London. [Google Scholar]
- 34.Murtaza Z, and Lakowicz JR (1996) Unpublished observations. [Google Scholar]
- 35.Brewer RG, Jensen GE, and Brewer KJ (1994) Inorg. Chem 33, 124–129. [Google Scholar]
- 36.Rao G, et al. , preliminary results. [Google Scholar]
- 37.Kataoka K, Hisamitsu I, Sayama N, Okano T, and Sakurai Y (1995) J. Biochem 117, 1145–1147. [DOI] [PubMed] [Google Scholar]
- 38.Deng G, James TD, and Shinkai S (1994) J. Am. Chem. Soc 116, 4567–4572. [Google Scholar]
- 39.Yoon J, and Czarnik AW (1992) J. Am. Chem. Soc 114, 5874–5875. [Google Scholar]
- 40.Takeshita M, Uchida K, and Irie M (1996) Chem. Commun 1807–1808. [Google Scholar]
- 41.Bambot SB, Holavanahali R, Lakowicz JR, Carter GM, and Rao G (1994) Biotechnol. Bioeng 43, 1139–1145. [DOI] [PubMed] [Google Scholar]