Abstract
We characterized the pH-dependent intensity decays of three fluorophores, Oregon green 514 carboxylic acid, Cl-NERF, and DM-NERF, using frequency-domain fluorometry, with the objective of identifying lifetime-based sensors for low pH values. These three probes were originally designed as dual excitation wavelength–ratiometric probes, with high photostability and high quantum yields in aqueous solutions. We found that their fluorescence intensity decays were strongly dependent on pH. Moreover, global intensity decays analysis reveals that these probes have double exponential intensity decays at intermediate pH values and that the decay time amplitudes are greatly dependent on pH. The longer lifetime components originated from the unprotonated forms and the shorter components from the protonated forms. Both forms can emit fluorescence at intermediate pH values. The apparent pKa values were also determined from the titration curves of phase angles and modulations versus pH for the purpose of pH sensing. The apparent pKa values range from pH 3 to 5, a range where lifetime-based sensors are not presently reported. Since these probes show low pKa values and display substantial phase and modulation changes with pH, they are suitable as lifetime-based pH sensors to monitor the pH changes in acidic environments. One potential application of these probes is to trace the pH in different cellular compartments.
Fluorescence sensing techniques are advancing very rapidly and have been widely used for quantitative measurements in clinical chemistry and cell biology. Fluorescence detection can provide high sensitivity, and suitably designed fluorophores provide the ability to monitor living cells with high analyte selectivity. For example, fluorescence wavelength ratiometric sensing has been widely used to follow the biochemical and ionic changes in cells (1). Furthermore, with the help of confocal microscopy, fluorescence lifetime imaging (2, 3), and two-photon excitation techniques (4), imaging of pH, calcium, or proteins (5) in organelles and cellular compartments can be accomplished. In recent years a number of new pH probes have been synthesized and utilized for pH sensing based on fluorescence emission ratios (6–8), excitation ratios (6, 7, 9, 10), and fluorescence lifetimes (11–13).
Cellular pH measurements based on fluorescence have also been widely utilized. As examples, fluorescence has been used to measure the cytosolic pH gradient in developing zygotes (14, 15), to trace the endosomal pH evolution along the endocytosis pathway (16, 17), and to determine the phagosomal pH of microphages (18). In recent years, we have seen the introduction of fluorescence lifetime-based techniques for cellular fluorescence imaging. Fluorescence lifetime-based sensing can be accomplished by several approaches, such as time-resolved measurements (19, 20), time-gated techniques (21, 22), and frequency domain measurements (23–26). These methods allow localized quantities and imaging of chemical species in cells.
In the present report, we describe the spectrometric characteristics of three lifetime-based pH sensors, Oregon green 514 carboxylic acid, Cl-NERF, and DM-NERF. These probes were first introduced by Molecular Probes (Eugene, OR) and used as excitation ratio probes for the pH determinations in acidic environments (27, 28). Both their protonated and unprotonated forms have high extinction coefficients between 470 and 520 nm and emit bright yellowish light. The absorption spectra of the protonated species are blue-shifted relative to the unprotonated forms. They are considered better pH sensors than fluorescein because of their greater photostability and the possibility of wavelength-ratiometric measurements. We found that both forms (protonated and unprotonated) display emission with distinct intensity decays that result in significant pH-dependent phase angles and modulations. Using their appropriate conjugates, these probes could be introduced into the cells and trace the pH distribution in acidic cellular compartments.
MATERIALS AND METHODS
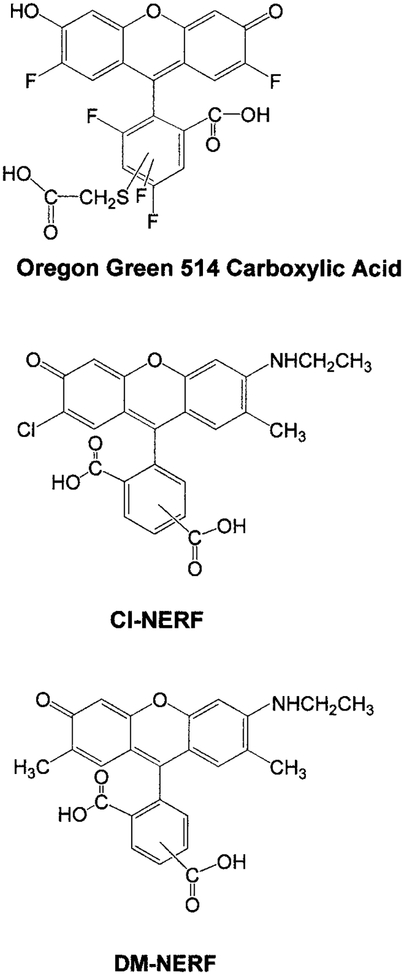
The pH probes, Oregon green carboxylic acid 514, DM-NERF, and Cl-NERF, were purchased from Molecular Probes (Eugene, OR) and used without further purification. Their chemical structures are shown in Fig. 1. The other chemicals used for preparing different pH buffer solutions were obtained from Sigma (St. Louis, MO) and Aldrich (Milwaukee, WI). The buffer series included 50 mM of citrate buffer, phosphate buffer, Tris buffer, and carbonate buffer, covering the pH range from 2 to 9. All these buffers contained 100 mM of KCl to maintain a constant ionic strength. Aqueous stock solutions of each probe were added to the buffer solutions to prepare the samples of final concentration of 1.5 μM for all the spectrophotometric measurements.
FIG. 1.
Chemical structures of the three pH probes: Oregon green 514 carboxylic acid, Cl-NERF, and DM-NERF.
Absorption spectra were performed with a Hewlett–Packard 8453 Spectrophotometer. Fluorescence emission spectra were measured using an Aminco Bowman Series AB2 Luminescence Spectrometer with excitation wavelengths of 488 and 514 nm. The time-resolved fluorescence intensity decays were measured using frequency-domain phase/modulation instrumentation (ISS, Inc.). The excitation source for the frequency-domain measurements was an air-cooled argon ion laser (543-AP Omnichrome), with its 488-nm output amplitude modulated by an electrooptic modulator (ISS, Inc.). A mode-locked argon ion laser with output at 514 nm was also used for the phase and modulation acquisition at a single frequency of 75.47 MHz. An aqueous solution of erythrosin B (BDH, UK) with a fluorescence lifetime of 80 ps was used as the lifetime reference. In order to cut off the scattered excitation light, long pass filters from Corning (above 510 and 540 nm) were placed on the emission side of the sample.
The fluorescence intensity decays were recovered from the multifrequency data and analyzed with single or double exponential decay models,
| (1) |
where Ik(t) is the fluorescence intensity decay at each pH (k) value, and αik is the amplitude for individual lifetime τi(Σαik = 1). The fractional intensity fik of each decay component (fik = αikτi/Σαikτi) was also determined. Detailed descriptions of the theories and instruments have been published previously (29, 30). For global intensity decays analysis, the individual lifetime τi was set as the global parameter, but the amplitudes αik were dependent on the pH environments.
RESULTS
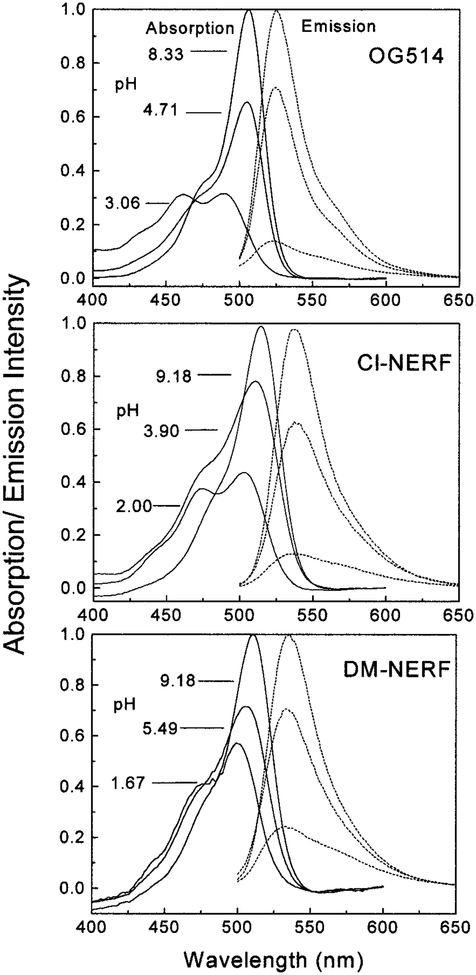
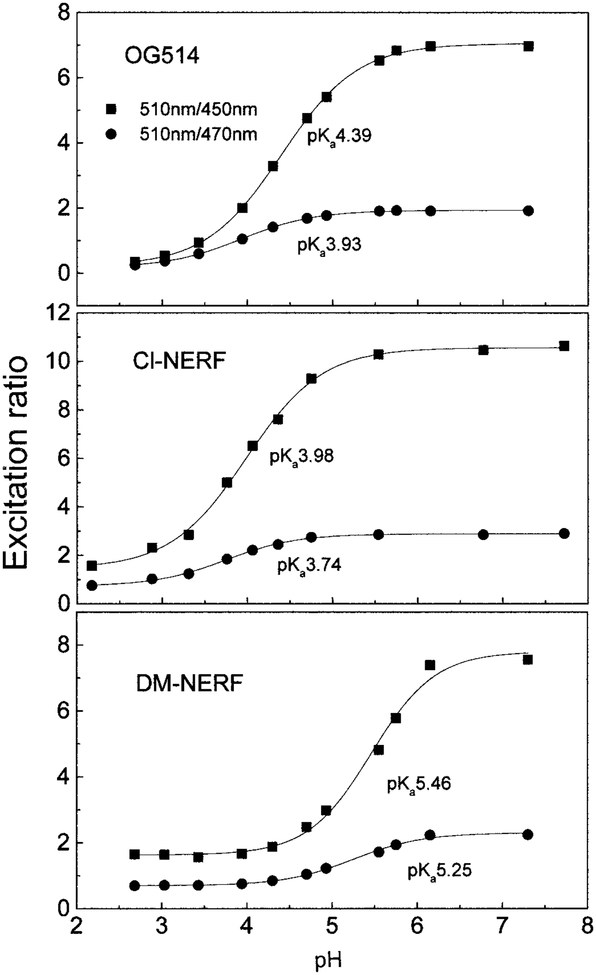
Oregon green 514 carboxylic acid (OG514)2 is a pentaflouorofluorescein derivative with its absorption maximum red-shifted about 25 nm relative to that of fluorescein. DM-NERF and Cl-NERF are derived from dimethyl and chloro substitution on Rhodol Green carboxylic acid and can be considered as structural hybrids of fluorescein and rhodamine (Fig. 1). All these probes are claimed to have greater photostability than fluorescein (Molecular Probes’ Handbook of Fluorescence Probes and Research Chemicals, 6th ed., p. 563). Absorption and emission spectra of the three probes at different pH values are shown in Fig. 2. Protonation of these probes resulted in decreased extinction coefficients and quantum yields, as well as the blue shift of their absorption spectra. The pH-dependent shifts of the absorption spectra indicate these probes are suitable for excitation wavelength ratiometric measurements. The emission maxima of these probes are shown to be pH-insensitive by comparing the normalized spectra of these probes at high and low pH values. However, the emission intensities increase dramatically with increasing of pH due to the higher quantum yields of the protonated forms than those of the unprotonated forms (Table 1). In Fig. 3, we show the excitation intensity ratios of the three probes at various pH values. The pKa values observed by fluorescence measurements are quiet close to the ground state pKa values listed in Table 1, which suggests that pKa changes and excited state reactions do not substantially affect the spectrum of these probes. Similar results for these probes at different excitation wavelengths have been reported (1, 16, 27). The intensity ratios are dependent on the excitation wavelengths and instrumentation we used.
FIG. 2.
The pH-dependent absorption and emission spectra of OG514, Cl-NERF, and DM-NERF. The excitation wavelength was 488 nm.
TABLE 1.
Spectral Changes and pKa Values for the Three pH Probes
| pH probe | Quantum yield | pKaa | λexc (nm) | Phase angle | Modulation | |||
|---|---|---|---|---|---|---|---|---|
| Protonated | Unprotonated | pKa | Δθ(deg) | pKa | Δm | |||
| OG514 | 0.22 | 0.65 | 4.7 | 488 | 4.10 | 15.3 | 3.77 | 0.24 |
| 514 | 3.21 | 16.3 | 3.28 | 0.25 | ||||
| Cl-NERF | 0.19 | 0.78 | 3.8 | 488 | 3.40 | 27.2 | 3.08 | 0.36 |
| 514 | 3.02 | 29.1 | 2.79 | 0.37 | ||||
| DM-NERF | 0.37 | 0.88 | 5.4 | 488 | 5.35 | 13.8 | 5.00 | 0.19 |
| 514 | 4.70 | 13.3 | 4.30 | 0.19 | ||||
The actual pKa is shown in the first column; apparent phase and modulation pKa values are also listed.
FIG. 3.
The pH dependency of the emission intensity of OG514, Cl-NERF, and DM-NERF based on wavelength-ratiometric measurement. The excitation wavelength pairs used here were 510 nm/450 nm and 510 nm/470 nm, and the emission intensity was monitored at 570 nm.
The shift of the absorption spectra and the changes of emission intensity indicate that more than one fluorescent species exist in aqueous solution. These species are the probes at different protonation levels, showing different extinction coefficients and quantum yields. If all these forms can fluoresce, changes of the quantum yields could be attributed to the different intensity decays of these forms. If multiple species exist with different decay times, then these probes can be used for lifetime-based measurements, as well as for wavelength-ratiometric measurements. It has been reported earlier that the pH sensitive range could be significantly expanded by using wavelength-ratiometric probes with lifetime-based measurements (11).
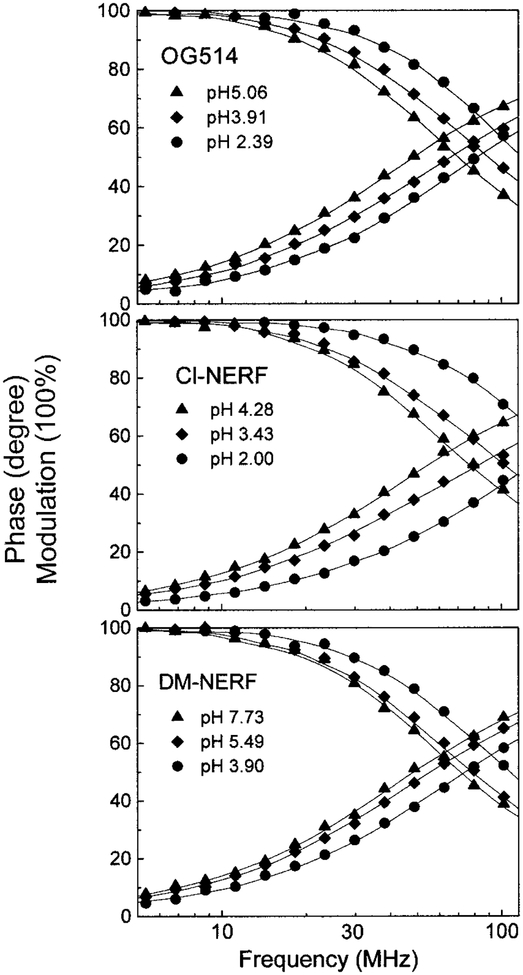
To characterize these probes as lifetime-based sensors, we measure their frequency domain intensity decays (Fig. 4). The frequency responses of all these probes display a significant shift to high-modulation frequencies in low pH environments, indicating a shorted mean lifetime at low pH. The intensity decays of each probe were satisfactorily fitted to the two-exponential model for a wide range of pH values, except for very low values. Global analysis was performed by assuming that two fluorescent species exist in aqueous solution and that each form displays the single exponential decays τ1 (acid form) and τ2 (basic form). The two lifetimes were taken as global parameters, which are independent of pH. In the fitting process, the values of the preexponential factors (α1 and α2) were variable, reflecting the relative amount of each form at each pH value. The results of intensity decay analysis are summarized in Table 2, including the fractional intensity fi of each decay component (fi = αiτi/Σ αiτi). Intensity decays at extremely low pH values of 0.9 were also measured. The analysis results revealed another decay time of 1.55 ns for OG514, 1.22 ns for Cl-NERF, and 2.18 ns for DM-NERF. Nevertheless, there was only a minor change in the absorption and emission spectrum below pH 2.0. This additional component at low pH may be due to a second protonated form of these probes, which can fluoresce and is present only at very low pH. All three probes displayed significant changes in lifetime as the pH varied from 3 to 6. The largest change in lifetime was observed for Cl-NERF τ2/τ1 = 2.74). Significant changes were also found for OG514 (τ2/τ1 = 1.78) and DM-NERF (τ2/τ1 = 1.63).
FIG. 4.
Frequency-domain intensity decays of the three probes at different pH values. The excitation wavelength was 488 nm.
TABLE 2.
Global Intensity Decay Analysis of the pH Probes for a Range of pH Values
| pH of the solution | α1 | f1 | (ns)a |
|---|---|---|---|
| Oregon green 514 carboxylic acid | |||
| 3.06 | 0.916 | 0.859 | 2.56 |
| 3.43 | 0.865 | 0.782 | 2.70 |
| 3.91 | 0.657 | 0.518 | 3.18 |
| 4.28 | 0.431 | 0.298 | 3.58 |
| 4.71 | 0.176 | 0.107 | 3.93 |
| 5.06 | 0.122 | 0.072 | 3.99 |
| 5.49 | 0.000 | 0.000 | 4.12 |
| Global decay time: τ1 = 2.31 ns, τ2 = 4.12 ns, square root of variance = 3.37. | |||
| Cl-NERF | |||
| 2.00 | 0.936 | 0.842 | 1.67 |
| 2.39 | 0.881 | 0.730 | 1.93 |
| 2.88 | 0.787 | 0.576 | 2.28 |
| 3.43 | 0.550 | 0.309 | 2.89 |
| 3.91 | 0.278 | 0.123 | 3.31 |
| 4.28 | 0.079 | 0.030 | 3.52 |
| Global decay time: τ1 = 1.31 ns, τ2 = 3.59 ns, square root of variance = 2.86. | |||
| DM-NERF | |||
| 3.43 | 0.978 | 0.964 | 2.47 |
| 3.91 | 0.905 | 0.854 | 2.64 |
| 4.28 | 0.861 | 0.792 | 2.73 |
| 4.71 | 0.739 | 0.635 | 2.97 |
| 5.06 | 0.613 | 0.494 | 3.18 |
| 5.49 | 0.349 | 0.248 | 3.55 |
| 5.93 | 0.184 | 0.122 | 3.74 |
| 6.30 | 0.025 | 0.016 | 3.90 |
| 7.73 | 0.019 | 0.012 | 3.91 |
| Global decay time: τ1 = 2.42 ns, τ2 = 3.93 ns, square root of variance = 3.10. | |||
stands for the mean lifetime and equals to Σαiτi2/Σαiτi.
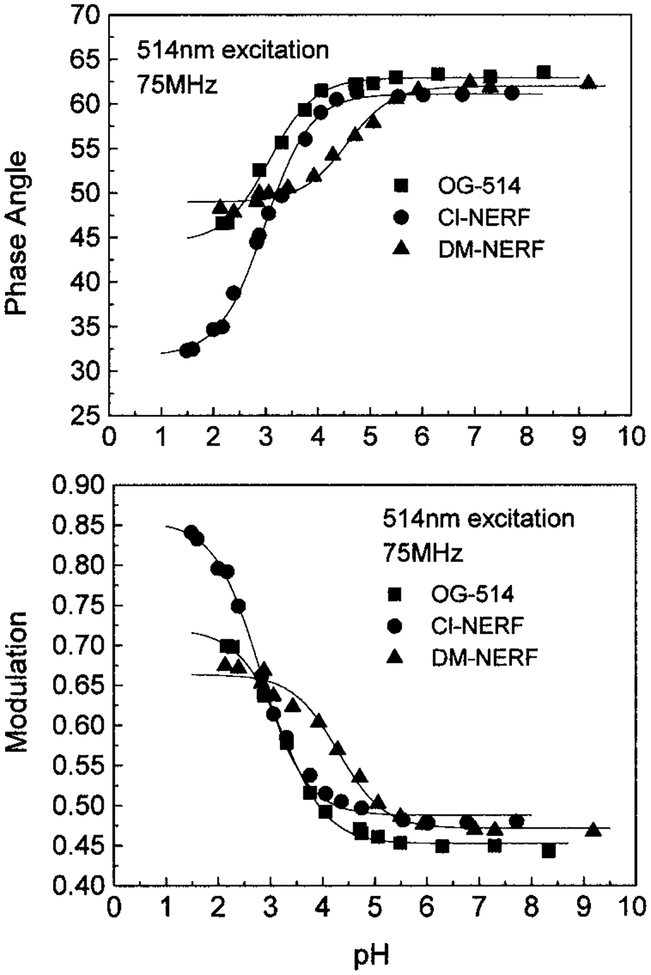
The high pH sensitivity of the intensity decays of these probes results in significant changes of the phase angle and modulation, especially at modulation frequencies ranging from 40 to 100 MHz (Fig. 4). The modulation frequency of 75.47 MHz was chosen to describe the pH-dependent phase angle and modulation changes (Fig. 5). This frequency of 75.47 MHz is the fundamental frequency of the mode-locked Ar-ion laser and close to that of many mode-locked lasers. In Table 1, we summarized the apparent pKa values and the magnitude of the changes in phase angle and modulation at the excitation wavelengths of 488 and 514 nm (Ar-ion laser). Since OG514 is a polyfluorinated fluorescein-based dye, its pKa values should be much lower than those determined for fluorescein because of the presence of electron-withdrawing fluorine. Similarly, the amine substituents of DM-NERF and Cl-NERF reduce their pKa values, the presence of methyl groups on DM-NERF increases the values to around five, and the presence of chloro groups on Cl-NERF reduces the values to around three. While it is clear that the probes display pH-dependent ionization, we can not assign with certainty the pKa values with ionizable groups on the probes. Consistent with the lifetime changes, Cl-NERF displayed the largest changes in phase angle (27°) and modulation (0.36) from its protonated form to unprotonated form, and DM-NERF displayed the smallest changes (Table 1). These pH-dependent phase and modulation data were fitted to the Henderson–Hasellboch equation to determine the apparent pKa values. Cl-NERF displays the lowest apparent pKa value, and DM-NERF displays the highest value. Since the useful pH sensing range will be pKa ± 1, the pH probes discussed here provide a working range from pH 2 to 6. Moreover, the apparent pKa values from phase angle and modulation are dependent on the excitation wavelength. The main reason for the increase of pKa at 488 nm excitation is due to the greater contribution of the protonated form to the mean fluorescence lifetime (presenting as the lower phase angle and higher modulation) at the shorter excitation wavelength. Higher apparent pKa values are expected if the excitation wavelength will be shorter than 488 nm.
FIG. 5.
The pH dependency of the phase angles and modulations for OG514, Cl-NERF, and DM-NERF.
DISCUSSION
The increasing availability of lifetime sensing instruments and lifetime imaging microscopes requires a selection of lifetime sensors. New fluorophores with desirable selectivity to different cellular components have been introduced into living systems as indicators to study different intracellular species. It has already been reported that some ratiometric probes display not only changes of their intensity ratios, but also useful changes in their fluorescence intensity decays (11). Several pH-sensitive probes like carboxy-SNAFL-1, BCECF, and DM-NERF have been used in time-resolved fluorescence microscopy to determine the cytosolic pH (21, 22).
All three probes discussed here display significant phase angle and modulation sensitivity to pH, especially in acidic pH regions close to their pKa values. Table 1 shows that apparent pKa can be shifted to lower values by changing the excitation wavelength from 488 to 514 nm, which provides a possibility to extend the pH working range of these probes. While not studied here, it is likely that the apparent pKa values can also be modified by selection of the emission wavelength. The other fluorescence characteristics, such as high quantum yields, good photostability, and being excitable by the major emitting lines of Ar-ion laser, support these probes as promising pH indicators for fluorescence lifetime microscopy. Because their responses are more sensitive to the low pH environment than to the near-neutral cytosol, these probes may be used to trace the pH evolution in acidic cellular compartments. These compartments can be vacuoles of yeast (31), endosomes (4), and phagosomes (18) of macrophages and endocytic systems in different cell lines (16, 32, 33) or in microorganisms (17). Similar to the strategies used for pH sensing with fluorescein (16, 34), these lifetime sensors can be linked to different macromolecules such as dextran, zymosans, and soluble exogenous antigens. Then these conjugates can be introduced into different cellular compartments through endocytosis, fluid-phase pinocytosis, or phagocytosis. After that they will be processed and transported to different destinations via diverse pathways. If these conjugates do not degrade and keep all their spectrometric properties, they can reflect the pH differences within the cellular compartments.
Footnotes
Abbreviation used: OG514, Oregon Green 514 carboxylic acid.
REFERENCES
- 1.Dunn KW, Mayor S, Myers JN, and Maxfield FR (1994) FASEB 8, 573–582. [DOI] [PubMed] [Google Scholar]
- 2.Gadella TWJ Jr., Jovin TM, and Clegg RM (1993) Biophys. Chem 48, 221–239. [Google Scholar]
- 3.Dong CY, So PTC, French T, and Gratton E (1995) Biophys. J 69, 2234–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.French T, So PTC, Weaver DJJ, Coelho-Sampaio T, Gratton E, Voss EWJ, and Carrero J (1997) J. Microsc 185, 339–353. [DOI] [PubMed] [Google Scholar]
- 5.Harootunian AT, Adams SR, Wen W, Meinkoth JL, Tayor SS, and Tsien RY (1993) Mol. Biol 4, 993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Y, Marcus EM, Haugland RP, and Opas M (1995) J. Cell. Physiol 164, 9–16. [DOI] [PubMed] [Google Scholar]
- 7.Whitetaker JE, Haugland RP, and Prendergast FG (1991) Anal. Biochem 194, 330–344. [DOI] [PubMed] [Google Scholar]
- 8.Kurtz I, and Balaban RS (1985) Biophys. J 48, 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nedergarrd M, Desai S, and Pulsinelli W (1990) Anal. Biochem 187, 109–114. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Zaguilan R, Tompkins LS, and Lynch RM (1994) SPIE 2137, 17–28. [Google Scholar]
- 11.Szmacinski H, and Lakowicz JR (1993) Anal. Chem 65, 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson RB, and Lakowicz JR (1993) Anal. Chem 65, 853–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draxler S, and Lippitsch M (1993) Sens. Actuators B 11, 421–424. [Google Scholar]
- 14.Gibbon BC, and Kropf DL (1994) Science 263, 1419–1421. [DOI] [PubMed] [Google Scholar]
- 15.Kropf DL, Henry CA, and Gibbon BC (1995) Eur. J. Cell Biol 68, 297–305. [PubMed] [Google Scholar]
- 16.Lee RJ, Wang S, and Low PS (1996) Biochim. Biophys. Acta 1312, 237–242. [DOI] [PubMed] [Google Scholar]
- 17.Aubry L, Klein G, Martiel J-L, and Satre M (1993) J. Cell Sci 105, 861–866. [DOI] [PubMed] [Google Scholar]
- 18.Vergne I, Constant P, and Lanéelle G (1998) Anal. Biochem 255, 127–132. [DOI] [PubMed] [Google Scholar]
- 19.Herman B, Wodnicki P, Kwon S, Periasamy A, Gordon GW, Mahajan N, and Wang XF (1997) J. Fluoresc 7, 85–91. [Google Scholar]
- 20.Srivastava A, and Kirshnamoorthy G (1997) Anal. Biochem 249, 140–146. [DOI] [PubMed] [Google Scholar]
- 21.Sanders R, Gerritsen HC, Draaijer A, Houpt PM, van Veen SJF, and Levine YK (1994) SPIE 2137, 56–62. [Google Scholar]
- 22.Sanders R, Draaijer A, Gerritsen HC, Houpt PM, and Levine YK (1995) Anal. Biochem 227, 302–308. [DOI] [PubMed] [Google Scholar]
- 23.Lakowicz JR, Szmacinski H, and Johnson ML (1992) J. Fluoresc 2, 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakowicz JR, Szmacinski H, Nowaczyk K, and Johnson M (1992) Proc. Natl. Acad. Sci. USA 89, 1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakowicz JR, and Szmacinski H (1993) Sens. Actuators B 11, 133–143. [Google Scholar]
- 26.Lakowicz JR, Szmacinski H, Nowaczyk K, Lederer WJ, Kirby MS, and Johnson M (1994) Cell Calcium 15, 7–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitaker JE, Haugland RP, Ryan D, Dunn K, Maxfield FR, and Haugland RP (1991) Biophys. J 59, 358a. [Google Scholar]
- 28.Dunn K, Maxfield FR, Whitaker JE, Haugland RP, and Haugland RP (1991) Biophys. J 59, 345a. [Google Scholar]
- 29.Lakowicz JR, and Maliwal BP (1985) Biophys. Chem 21, 61–78. [DOI] [PubMed] [Google Scholar]
- 30.Lakowicz JR (1991) in Topics in Fluorescence Spectroscopy, Vol. 1, Techniques (Lakowicz JR, Ed.), pp. 293–355, Plenum, New York. [Google Scholar]
- 31.Pena A, Ramírez J, Rosas G, and Calahorra M (1995) J. Bacteriol 177, 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zen K, Biwersi J, Periasamy N, and Verkman AS (1992)J. Cell Biol 119, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Weert AWM, Dunn KW, Geuze HJ, Maxfield FR, and Stoorvogel W (1995) J. Cell Biol 130, 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Deurs B, Ropke C, and Thorball N (1984) Eur. J. Cell Biol 34, 96–102. [PubMed] [Google Scholar]