Abstract
OBJECTIVES:
Human anti-chimeric antibodies (HACAs) and subtherapeutic infliximab concentrations are associated with decreased duration of response. We evaluated the clinical utility of measuring HACA and infliximab concentrations.
METHODS:
The medical records of patients with inflammatory bowel disease (IBD) who had HACA and infliximab concentrations measured were reviewed to determine whether the result affected clinical management.
RESULTS:
One hundred fifty-five patients had HACA and infliximab concentrations measured. The main indications for testing were loss of response to infliximab (49%), partial response after initiation of infliximab (22%), and possible autoimmune/delayed hypersensitivity reaction (10%). HACAs were identified in 35 patients (23%) and therapeutic infliximab concentrations in 51 patients (33%). Of 177 tests assessed, the results impacted treatment decisions in 73%. In HACA-positive patients, change to another anti-tumor necrosis factor (TNF) agent was associated with a complete or partial response in 92% of patients, whereas dose escalation had a response of 17%. In patients with subtherapeutic infliximab concentrations, dose escalation was associated with complete or partial clinical response in 86% of patients, whereas changing to another anti-TNF agent had a response of 33%. Patients with clinical symptoms and therapeutic infliximab concentrations were continued at the same dose 76% of the time and had no evidence of active inflammation by endoscopic/radiographic assessment 62% of the time.
CONCLUSIONS:
Measurement of HACA and infliximab concentration impacts management and is clinically useful. Increasing the infliximab dose in patients who have HACAs is ineffective, whereas in patients with subtherapeutic infliximab concentrations, this strategy may be a good alternative to changing to another anti-TNF agent.
INTRODUCTION
Infliximab (Remicade, Centocor, Horsham, PA) is a chimeric monoclonal IgG1 antibody against tumor necrosis factor (TNF) that is effective for the treatment of Crohn’s disease and ulcerative colitis (1–3). Treatment with infliximab can result in immunogenicity and the formation of human anti-chimeric antibodies (HACAs), also known as antibodies to infliximab (4). The incidence of HACAs has been shown to be as high as 37–61% in patients receiving episodic infliximab (4). Scheduled infliximab therapy decreases the incidence of HACAs to 6–16 % (2,5). Concomitant immunosuppressive therapy also decreases the formation of HACAs, but this may only be important in those receiving episodic therapy (2,4–9). Immunogenicity to infliximab is not a unique phenomenon related to its chimeric structure, as treatment with any exogenous protein can lead to the development of antibodies (10,11). In fact, similar rates of antibodies have been reported in patients treated with adalimumab and certolizumab pegol (12–15).
Some have questioned whether the presence of antibodies to anti-TNF agents directly correlates with decreased efficacy (16). Comparisons can be drawn from the rheumatoid arthritis literature. Several groups have shown that the development of antibodies to infliximab and adalimumab correlates with not only decreased drug concentrations but also decreased clinical response (17–21). In inflammatory bowel disease (IBD), studies have shown that there is a shorter duration of clinical response in patients with detectable HACA concentrations (4,22,23). A subgroup analysis of a larger randomized controlled trial showed a trend toward decreased remission in patients who underwent episodic therapy and had detectable antibodies (6).
The clinical efficacy of infliximab may be dependent not only on the absence of HACA but also on infliximab concentrations. In a study of Crohn’s disease patients on scheduled maintenance infliximab therapy, patients with detectable trough concentrations had a higher rate of clinical remission, a lower serum C-reactive protein (CRP) concentration, and a higher rate of endoscopic improvement (5). HACAs have also been associated with an increased risk of infusion reactions, which in turn can also lead to decreased infliximab concentrations (4–6,23,24).
Although the associations between clinical efficacy and infusion reactions with infliximab concentrations and HACA status have been described, the clinical utility of these tests in routine practice remains unclear. The clinical indications for measuring HACA and infliximab concentrations in patients with IBD have not been previously assessed. Furthermore, the optimal patient management based on the results of testing has not been clearly elucidated. We retrospectively studied the utility of measuring HACA and infliximab concentrations and compared subsequent clinical management and response. We propose a treatment algorithm based on the results of testing.
METHODS
Overview
We conducted a retrospective review of the medical records of all patients at our institution who underwent HACA and infliximab concentration testing. No systematic strategy was used to test all patients who were failing or who were intolerant to infliximab. Physicians working in the Inflammatory Bowel Disease Clinic at Mayo Clinic, Rochester can, at their discretion, order HACA and infliximab concentrations as a send-out test from Mayo Medical Laboratories to Prometheus Laboratories (San Diego, CA). Medical records were electronically searched to identify patients who had received infliximab and who underwent testing for HACA and infliximab concentrations between 1 January 2003 and 1 August 2008. All patients included in the analysis had provided authorization for medical record review for research purposes, and the study was approved by the Mayo Clinic Institutional Review Board.
Inclusion and exclusion criteria
All patients with a diagnosis of Crohn’s disease, ulcerative colitis, or indeterminate colitis who were treated with infliximab and underwent HACA and infliximab concentration testing were included in the study. Exclusion criteria were limited to: the absence of follow-up after being tested, and the infusion of infliximab as a part of a clinical trial.
Measures and analyses
Demographic and clinical characteristics were abstracted from the electronic medical record in those patients that met entry criteria. Characteristics included age, gender, smoking status, type of IBD, anatomic distribution, duration of disease, previous surgery, prior and concurrent treatment for IBD, date of infliximab initiation, dose, duration of treatment, clinical response, change in dose or frequency, acute or delayed hypersensitivity reactions, autoimmune reactions, and change to another anti-TNF agent. Acute infusion reactions were defined as an adverse event that occurred within 1 h after infusion. Delayed hypersensitivity reactions were defined as the occurrence of myalgias, arthralgias, fever, or rash occurring 1–14 days after infusion. Clinical response was retrospectively determined as defined earlier (25). In patients with Crohn’s disease, complete response was defined as cessation of diarrhea and abdominal cramping, or, in the cases of patients with fistulas, cessation of fistula drainage and complete closure of all draining fistulas. Partial response was defined as a reduction in the amount of diarrhea and abdominal cramping, or, in the case of fistula patients, a decrease in the drainage, size, or number of fistulas. Outcomes not meeting one of the above definitions were classified as non-response (25). In patients with ulcerative colitis, complete response was defined as cessation of diarrhea, hematochezia, and abdominal cramping whereas partial response was defined as a reduction in the amount of diarrhea, hematochezia, and abdominal cramping. The results of radiological and/or endoscopic imaging were documented when available.
The testing date, the reason for testing, and the rationale for changing treatment post-testing were obtained from the medical record. Results of HACA and infliximab concentration testing from Prometheus Laboratories were categorized in the following manner. Infliximab concentrations ≥ 12 mcg/ml at 4 weeks after infusion were considered therapeutic (4). Patients with a detectable infliximab concentration (> 1.4 mcg/ml) at dosing trough were considered to have therapeutic concentrations (5). Patients with any detectable HACA concentration were considered to have a positive antibody status and by definition, had an undetectable infliximab concentration (the presence of infliximab in the sample interferes with the HACA assay).
Subtherapeutic infliximab concentrations were defined as an undetectable trough concentration or an infliximab concentration < 12 mcg/ml at 4 weeks after infusion. Testing results that were non-interpretable because testing was performed at an inappropriate time were not included in the analysis (e.g., infliximab concentration > 12 mcg/ml before 4 weeks or < 12 mcg/ml after 4 weeks, but before trough dosing). Clinical response (as defined above) to any change in therapeutic treatment was also assessed. C-reactive protein and erythrocyte sedimentation rate at initiation of infliximab, before change in treatment, and post-treatment were abstracted when data were available.
The clinical utility of testing was assessed retrospectively. A priori, we determined that when the results of testing changed treatment or helped to avoid inappropriate clinical management, the test was considered useful. Testing was considered to have no impact on clinical decision making when a counterintuitive treatment plan was instituted, for example, when the treating clinicians: dose-increased infliximab when HACAs were detected, maintained the same therapy when subtherapeutic infliximab concentrations were found and either dose-increased infliximab or changed anti-TNF agents when therapeutic infliximab concentrations were detected.
Statistical analysis
Descriptive statistics were used to analyze baseline characteristics. Fisher’s exact test, χ2 test, and log-rank test for discontinuation were used for statistical analysis between groups. A P value of 0.05 was considered significant. Statistical analyses were conducted using Statistical Analysis Software (SAS).
RESULTS
Patient characteristics
One hundred fifty-five patients underwent HACA and infliximab concentration testing between 1 January 2003 and 1 August 2008. One hundred twelve patients (71.8%) of the initial tests were ordered by a single physician (W.J.S.). The baseline demographic and clinical characteristics are shown in Table 1. One hundred twenty-seven patients (82%) received induction followed by scheduled dosing. Among the 28 patients who did not receive induction dosing, 18 (64%) subsequently received scheduled dosing. Forty-seven percent of patients were on concurrent immunosuppressant medication consisting of azathioprine, 6-mercaptopurine, or methotrexate. The median time to initial testing after infliximab initiation was 50 weeks (interquartile ratio [IQR]: 22.7–120), and the median number of infusions (per patient) before testing was 8 (IQR: 4–15). Initial complete clinical response to infliximab therapy was seen in 100 patients (65%), partial response was observed in 45 patients (29%), and no response was seen in 10 patients (6%). Forty-three patients (28%) had the dose or frequency of infliximab increased before testing. The results of testing stratified by the presence or absence of concomitant immunosuppressive therapy are summarized in Table 2. Concurrent immunosuppressive therapy was significantly associated with negative HACA status (14% in those on concomitant immunosuppressive therapy vs. 29% in those not, P < 0.032), as well as therapeutic infliximab concentrations (48% vs. 21%, P < 0.001). HACA status did not significantly differ in patients receiving scheduled dosing compared with episodic treatment, but only 10 patients (6%) were receiving episodic treatment.
Table 1.
Baseline characteristics of inflammatory bowel disease patients who underwent testing for human anti-chimeric antibodies and infliximab concentrations
| Characteristic | N =155 |
|---|---|
| Females, n (%) | 86 (55%) |
| Median age at diagnosis of inflammatory bowel disease (IQR) | 25 (18–36) |
| Median age at time of initial test, years (IQR) | 39 (26–50) |
| Smoking status, n (%) | |
| Current | 82 (21%) |
| Former (> 1 month with no smoking) | 24 (15%) |
| Crohn’s disease | 121 (78%) |
| Ileal | 19 (16%) |
| Colonic only | 35 (29%) |
| Ileocolonic | 67 (55%) |
| Perianal disease | 29 (24%) |
| Surgical management before anti-TNF | 65 (54%) |
| Ulcerative colitis | 31 (20%) |
| Left-sided disease | 7 (23%) |
| Pan-colonic disease | 24 (77%) |
| Indeterminate colitis | 3 (2%) |
| Concomitant medication (at the time of initial test) | |
| Mesalamine | 11 (7%) |
| Corticosteroids (> 20 mg/day) | 16 (10%) |
| Azathioprine/6-merca ptopuri ne | 57 (37%) |
| Methotrexate | 12 (10%) |
| Induction dosing (0, 2, 6 weeks) | 127 (82%) |
| History of steroid pretreatment | 16 (10%) |
| Initial response to infliximab | |
| Complete response | 100 (65%) |
| Partial response | 45 (29%) |
| No response | 10 (6%) |
| Median time to initial testing after infliximab initiation, weeks (IQR) | 50 (22.7–120) |
| Median number of infusions (per patient) before test (IQR) | 8 (4–15) |
IQR, interquartile range; TNF, tumor necrosis factor.
Table 2.
Testing results stratified by concomitant immunosuppressive therapy
| HACAs | Therapeutic IFX | Non-therapeutic IFX | Non-interpretable | |
|---|---|---|---|---|
| Concurrent IS (n=69) | 10 (14) | 33 (48) | 22 (32) | 4 (6) |
| No concurrent IS (n=86) | 25 (29) | 18 (21) | 41 (48) | 2 (2) |
HACAs, human anti-chimeric antibodies; IFX, infliximab; IS, immunosuppressive therapy.
Indications for testing
The indications for testing are listed in Table 3. The main indications for initial testing were: loss of response to infliximab (49%), partial response after initiation of infliximab (22%), and possible autoimmune/delayed hypersensitivity reaction (10%). HACAs were identified in 35 patients (23%), therapeutic infliximab concentrations were found in 51 patients (33%), and subtherapeutic concentrations were found in 69 patients (44%). Out of the initial 155 tests, only 6 (4%) could not be assessed because they were completed at an inappropriate time leading to non-interpretable results. The results of testing stratified by indication are summarized in Table 4. Out of 110 patients tested for loss of response or partial response, 19 (17%) had detectable HACAs and 50 (45%) had non-therapeutic concentrations. Out of 16 patients tested for autoimmune/delayed hypersensitivity reactions, 6 (38%) had detectable HACAs and in 5 patients tested for an allergic reaction to infliximab, only 1 patient (20%) had detectable HACAs.
Table 3.
Primary indication for testing for human anti-chimeric antibodies and infliximab concentrations
| Indication, n (%) | N =155 |
|---|---|
| Loss of response | 76 (49) |
| Partial response on initiation | 34 (22) |
| Autoimmune/delayed hypersensitivity reaction | 16 (10) |
| Primary non-response | 8 (5) |
| Reintroduction after drug holiday | 7 (5) |
| Endoscopic/CTE recurrence | 6 (4) |
| Acute infusion reaction | 5 (3) |
| Unclear reason | 3 (2) |
CTE, computed tomography enterography.
Table 4.
Testing results stratified by indication
| HACAs | Therapeutic IFX | Non-therapeutic IFX | Non-interpretable | |
|---|---|---|---|---|
| Loss of response/partial response (n=110) | 19 (17) | 36 (33) | 50 (45) | 5 (5) |
| Autoimmune/delayed hypersensitivity reactions (n=16) | 6 (38) | 5 (31) | 4 (25) | 1 (6) |
| Acute infusion reaction (n=5) | 1 (20) | 3 (60) | 1 (20) | |
| Reintroduction after drug holiday (n=7) | 6 (86) | 1 (14) |
HACAs, human anti-chimeric antibodies; IFX, infliximab.
Clinical management based on HACA and infliximab concentrations
Of 177 total tests assessed (including 22 subsequent HACA status and infliximab concentration testing), the results impacted treatment decisions as defined above in 130 clinical situations (73%; 95% CI: 66–79%). The clinical scenarios where testing had no impact on clinical management are shown in Table 5.
Table 5.
Scenarios in which testing did not impact clinical management
| Clinical management | n = 47 | |
|---|---|---|
| Detectable HACA | Increase infliximab | 7 |
| Continue infliximab | 4 | |
| Therapeutic concentrations | Change to another anti-TNF | 10 |
| Increase infliximab | 2 | |
| Continue infliximab despite endoscopic/CTE recurrence | 3 | |
| Subtherapeutic concentrations | Continue infliximab (same dose) | 15 |
| Change treatment secondary to adverse eventsa | 6 |
CTE, computed tomography enterography; HACA, human anti-chimeric antibody; TNF, tumor necrosis factor.
These included delayed hypersensitivity reactions (n = 4), tuberculosis (n = 1), and lymphoma (n = 1).
Thirty-five patients were positive for HACAs. Among the 12 HACA-positive patients who changed to another anti-TNF agent, a complete or partial response was noted in 11 (92%) (Table 6). On the other hand, increasing the dose of infliximab in 6 HACA-positive patients was associated with response in 1 patient (17%, P < 0.004). During subsequent testing, none of these patients achieved therapeutic infliximab concentrations with dose escalation. Of the remaining patients, six discontinued infliximab, three continued on the same dose, three proceeded to surgery, and five patients could not be assessed as adequate follow-up information was not available.
Table 6.
Clinical outcomes of patients with detectable human anti-chimeric antibodies or subtherapeutic infliximab concentrations
| Response to test | Complete/partial response (%) | P value | |
|---|---|---|---|
| Detectable HACA | Increase infliximab | 1/6 (17) | P < 0.004 |
| Change anti-TNF | 11/12 (92) | ||
| Subtherapeutic concentration | Increase infliximab | 25/29 (86) | P < 0.016 |
| Change anti-TNF | 2/6 (33) |
HACA, human anti-chimeric antibody; TNF, tumor necrosis factor.
Sixty-three patients had subtherapeutic infliximab concentrations. Among 29 patients with subtherapeutic infliximab concentrations, increasing the infliximab dose was associated with complete or partial clinical response in 25 (86%). Six patients with subtherapeutic infliximab concentrations were changed to another anti-TNF, and this was associated with a response in two patients (33%, P < 0.016). Of the remaining patients, 10 continued on the same dose, 9 discontinued infliximab, 8 proceeded to surgery, and 7 patients could not be assessed as adequate follow-up information was not available.
Fifty-one patients had therapeutic infliximab concentrations. In 21 situations where patients had clinical symptoms and therapeutic infliximab concentrations, and radiological and/or endoscopic imaging was available, patients had no evidence of active inflammation 62% of the time (95% CI: 38–82%), and continued at the same dose 76% (95% CI: 54–90%). In the eight patients (38%) that had active inflammation despite therapeutic infliximab concentrations, three patients continued on the same dose, two patients had surgery, two patients were changed to another anti-TNF (no follow-up information available), and one was treated with an additional immunosuppressive agent.
In 5 patients evaluated for acute infusion reactions, 1 patient (20%; 95% CI: 2–64%) had detectable HACAs, whereas among 16 patients assessed for delayed hypersensitivity or autoimmune reactions, 6 had detectable antibody concentrations (38%; 95% CI: 18–61 %). In the seven patients assessed before possible reintroduction of infliximab after a drug holiday, six patients (86%) had detectable HACAs.
Infliximab discontinuation
By the end of the study period (August 2008), among 140 patients with available information, 51 were still on infliximab (36%; 95% CI: 29%–45). The main indications for infliximab discontinuation were loss of response in 38 patients (27%; 95% CI: 20–35%) and continued partial response in 20 (14%; 95% CI: 9–21%). Other indications for infliximab discontinuation (all < 10%) were primary non-response, surgical intervention, autoimmune or delayed hypersensitivity reaction, infusion reactions, or other side-effects. In patients with therapeutic infliximab concentrations who continued the same dose and those with subtherapeutic concentrations who were dose escalated, the median time to discontinuation of infliximab after the test date was similar at 75 weeks (IQR: 46–116 and 45–92, respectively, P > 0.61).
DISCUSSION
Infliximab has become a common treatment for both Crohn’s disease and ulcerative colitis. Among patients who initially respond to infliximab, up to 40% will subsequently lose response (2). The clinical management of patients who respond to infliximab and then lose response remains largely empiric. Management strategies include escalation of the dose or shortening of the infusion interval, switching to another anti-TNF agent, or switching to another therapeutic class (26–29). A decision analysis suggested that dose escalation was more likely to be effective than switching drugs within the class, but this empiric strategy likely leads to dose escalation in some patients who are HACA positive (30). It is logical that the incorporation of routine measurement of HACA and infliximab concentrations could lead to a more nuanced approach, but the use of these tests in clinical practice has not been reported.
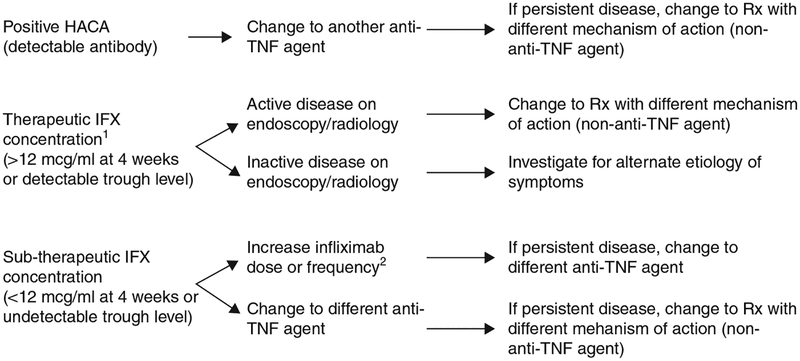
Our study shows that measurement of HACA and infliximab concentrations is useful in clinical practice. The patients in our study were tested, on average, approximately 1 year after the initiation of infliximab. The majority of tests were performed for loss of response or partial clinical response (71%) and in these patients 62% had detectable HACAs or non-therapeutic infliximab concentrations. It is in this cohort of patients that the test results are most important in determining appropriate treatment. When ordering HACA and infliximab concentrations, there are three possible permutations (Figure 1).
Figure 1.
Treatment algorithm in patients with clinical symptoms (infliximab and HACA concentrations). 1Patients should save endoscopic or radiologic imaging. 2This strategy may be preferable. HACA, human anti-chimeric antibody; TNF, tumor necrosis factor.
The presence of HACAs provides clear evidence that immunogenicity to infliximab has developed and that further treatment would result in a decreased clinical response or possible infusion reactions (4–6,16,22,23). Similar to the study by Maser et al. (5), patients with any detectable HACA concentration were considered to have a positive antibody status because they likely have developed some degree of immunogenicity to infliximab. In our study, a change to another anti-TNF agent in HACA-positive patients was associated with a complete or partial response in 92%, whereas increasing the dose of infliximab resulted in a 17% response (P < 0.004). This would suggest that increasing the infliximab dose in the face of positive HACA is unlikely to be a successful strategy, and the results of HACA testing are indeed useful in this cohort of patients.
In patients with subtherapeutic concentrations, infliximab dose escalation was associated with a significantly increased clinical response compared with changing to another anti-TNF (86% vs. 33%, P < 0.016). In addition, patients with therapeutic infliximab concentrations who continued the same dose and those with subtherapeutic concentrations who were dose escalated had a similar median time to infliximab discontinuation (75 weeks). These results suggest that increasing the infliximab dose may be a successful strategy in treating patients with subtherapeutic concentrations. At present, there are no comparative effectiveness studies assessing dose escalation vs. changing to another anti-TNF agent, in patients that lose response to infliximab. Previous exposure to an anti-TNF agent is associated with a reduced clinical response to a second anti-TNF agent compared with anti-TNF naive patients and this could perhaps explain the increased clinical response rate seen with dose intensification in our study (31). Both strategies are likely effective and further studies need to be performed to determine whether one treatment strategy is superior to the other.
A 4-week post-infusion infliximab concentration of > 12 mcg/ml and a detectable trough concentration have both been found to be significantly associated with decreased infusion reactions, increased clinical remission, lower C-reactive protein, and endoscopic healing (4,5). In the presence of therapeutic infliximab concentrations and clinical symptoms, confirmatory testing with ileocolonoscopy and/or computed tomography or magnetic resonance imaging enterography should be performed. To underscore this recommendation, in patients with clinical symptoms and a therapeutic infliximab concentration, patients continued at the same dose 76% of the time and had no evidence of active inflammation by endoscopic/radiographic assessment 62% of the time. Increasing the dose or changing to another anti-TNF agent in these patients would have led to inappropriate management. If therapeutic infliximab concentrations are present and there is persistent disease, then increasing the dose of infliximab or changing to another anti-TNF with the same mechanism of action would likely be of little benefit, and consideration should be given to switching to a medication with a different mechanism of action. In this cohort of patients, infliximab concentration testing would be clinically useful and would help to avoid inappropriate management.
Although 10 % of HACA and infliximab concentration testing was completed to assess for delayed hypersensitivity reactions (HACAs detected in only 38% of patients), there is little data to support a link between antibody presence and these reactions. In the study by Baert et al. (4), there was no relationship between delayed hypersensitivity reactions and HACA concentrations. On the other hand, testing before retreatment with infliximab may be more useful as HACAs were detectable in 86% of these patients in our study. Similarly, in a study published in abstract form, all patients with Crohn’s disease who were retreated with infliximab after a drug holiday and developed a delayed hypersensitivity reaction had detectable HACAs after infusion (32).
Patients receiving concurrent immunosuppressive therapy were significantly more likely to have therapeutic infliximab concentrations and less likely to have detectable antibodies, as compared with those not receiving concomitant immunosuppressive therapy. These results should be interpreted with caution given that this is a retrospective study. There are also several other potential limitations to this study. Patient selection for testing was at the discretion of the treating physician, and the resultant cohort of patients represent only a small subset of the total population of patients on infliximab at Mayo Clinic. Clinical response was abstracted through review of patient charts using pre-defined clinical criteria. Validated instruments such as the Crohn’s Disease Activity Index, Harvey-Bradshaw Index, and endoscopic improvement could not be obtained retrospectively. In addition, there is no specific comparator/control group in which no testing was performed and so absolute conclusions regarding the superiority of testing over clinical judgment alone cannot be made. However, the results of this study do suggest that testing in specific circumstances could potentially help to avoid inappropriate management.
In conclusion, our data suggest that HACA and infliximab concentration testing impact treatment decisions in 73% of patients and that these tests are a useful adjunct to clinical and endoscopic/radiological assessment. Use of these tests can potentially avoid inappropriate management and optimize patient treatment algorithms (Figure 1). A prospective randomized trial should be conducted to confirm these findings.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Therapeutic infliximab concentrations likely correlate with increased clinical response, whereas detectable human anti-chimeric antibody (HACA) concentrations may result in decreased clinical efficacy.
The clinical utility of measuring infliximab and HACA concentrations in routine practice remains unclear, and the optimal patient management based on the results of testing has not been clearly elucidated.
WHAT IS NEW HERE
In our study, patients with detectable human anti-chimeric antibodies (HACAs) who changed to another anti-tumor necrosis factor (TNF) agent had a significantly increased response compared with patients who were dose escalated.
Patients with subtherapeutic infliximab concentrations had a significantly increased response when they were dose escalated compared with changing to another anti-TNF agent.
In the presence of therapeutic infliximab concentrations and clinical symptoms, confirmatory testing with ileocolonoscopy and/or computed tomography or magnetic resonance imaging enterography should be performed before changes in treatment.
Incorporation of routine measurement of HACA andinfliximab concentrations is clinically useful and may help to optimize patient treatment algorithms.
ACKNOWLEDGMENTS
The project described was supported by grant number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Waqqas Afif, MD.
Potential competing interests: William Sandborn is a consultant to and has received research support from Centocor, Schering-Plough, Abbott Laboratories, and UCB Pharma. Edward Loft us has received research support from Schering-Plough and has participated in continuing medical education activities sponsored in part by Centocor. William Faubion is a consultant to and has received research support from Centocor and Abbot Laboratories. Dr Kane is a consultant to Abbott Laboratories.
REFERENCES
- 1.Targan SR, Hanauer SB, van Deventer SJ et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med 1997;337:1029–35. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB, Feagan BG, Lichtenstein GR et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 3.Rutgeerts P, Sandborn WJ, Feagan BG et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- 4.Baert F, Noman M, Vermeire S et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003;348:601–8. [DOI] [PubMed] [Google Scholar]
- 5.Maser EA, Villela R, Silverberg MS et al. Association of trough serum infliximab to clinical outcome aft er scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol 2006;4:1248–54. [DOI] [PubMed] [Google Scholar]
- 6.Hanauer SB, Wagner CL, Bala M et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol 2004;2:542–53. [DOI] [PubMed] [Google Scholar]
- 7.Van Assche G, Magdelaine-Beuzelin C, D’ Haens G et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology 2008;134:1861–8. [DOI] [PubMed] [Google Scholar]
- 8.Vermeire S, Noman M, Van Assche G et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007;56:1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Diamond RH, Wagner CL et al. Benefits and risks of immunomodulators and maintenance infliximab for IBD: subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009;30:210–26. [DOI] [PubMed] [Google Scholar]
- 10.Bray GL, Gomperts ED, Courter S et al. A multicenter study of recombinant factor VIII (recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. The Recombinate Study Group. Blood 1994;83:2428–35. [PubMed] [Google Scholar]
- 11.Fineberg SE, Galloway JA, Fineberg NS et al. Immunogenicity of recombinant DNA human insulin. Diabetologia 1983;25:465–9. [DOI] [PubMed] [Google Scholar]
- 12.Sandborn WJ, Feagan BG, Stoinov S et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 2007;357:228–38. [DOI] [PubMed] [Google Scholar]
- 13.Sandborn WJ, Hanauer SB, Rutgeerts P et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 2007;56:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiber S, Khaliq-Kareemi M, Lawrance IC et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med 2007;357:239–50. [DOI] [PubMed] [Google Scholar]
- 15.West RL, Zelinkova Z, Wolbink GJ et al. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn’s disease. Aliment Pharmacol Ther 2008;28:1122–6. [DOI] [PubMed] [Google Scholar]
- 16.Cassinotti A, Travis S. Incidence and clinical significance of immunogenicity to infliximab in Crohn’s disease: a critical systematic review. Inflamm Bowel Dis 2009;15:1264–75. [DOI] [PubMed] [Google Scholar]
- 17.Bartelds GM, Wijbrandts CA, Nurmohamed MT et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis 2007;66:921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendtzen K, Geborek P, Svenson M et al. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum 2006;54:3782–9. [DOI] [PubMed] [Google Scholar]
- 19.Radstake TR, Svenson M, Eijsbouts AM et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis 2009;68:1739–45. [DOI] [PubMed] [Google Scholar]
- 20.Svenson M, Geborek P, Saxne T et al. Monitoring patients treated with anti-TNF-alpha biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodies. Rheumatology (Oxford) 2007;46:1828–34. [DOI] [PubMed] [Google Scholar]
- 21.Wolbink GJ, Voskuyl AE, Lems WF et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2005;64:704–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ainsworth MA, Bendtzen K, Brynskov J. Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn’s disease. Am J Gastroenterol 2008;103:944–8. [DOI] [PubMed] [Google Scholar]
- 23.Farrell RJ, Alsahli M, Jeen YT et al. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: a randomized controlled trial. Gastroenterology 2003;124:917–24. [DOI] [PubMed] [Google Scholar]
- 24.Miele E, Markowitz JE, Mamula P et al. Human antichimeric antibody in children and young adults with inflammatory bowel disease receiving infliximab. J Pediatr Gastroenterol Nutr 2004;38:502–8. [DOI] [PubMed] [Google Scholar]
- 25.Swoger JM, Loft us EV, Pardi DS et al. Adalimumab for Crohn’s disease in clinical practice at an academic medical center; the first 118 patients. Gastroenterology 2008;134:A662. [Google Scholar]
- 26.Rutgeerts P, Van Assche G, Vermeire S. Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology 2004;126:1593–610. [DOI] [PubMed] [Google Scholar]
- 27.Sandborn WJ, Colombel JF, Enns R et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med 2005;353:1912–25. [DOI] [PubMed] [Google Scholar]
- 28.Sandborn WJ, Rutgeerts P, Enns R et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med 2007;146:829–38. [DOI] [PubMed] [Google Scholar]
- 29.Targan SR, Feagan BG, Fedorak RN et al. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology 2007;132:1672–83. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan GG, Hur C, Korzenik J et al. Infliximab dose escalation vs. initiation of adalimumab for loss of response in Crohn’s disease: a cost-effectiveness analysis. Aliment Pharmacol Ther 2007;26:1509–20. [DOI] [PubMed] [Google Scholar]
- 31.Colombel JF, Sandborn WJ, Rutgeerts P et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 32.Hanauer SB, Rutgeerts PJ, D’ Haens G et al. Delayed hypersensitivity to infliximab (Remicade) re-infusion after 2–4 year interval without treatment. Gastroenterology 1999;116. [Google Scholar]