Abstract
Introduction:
Salivary duct carcinoma (SDC) is a high-grade salivary gland carcinoma that is associated with frequent metastasis and poor outcome. Androgen receptor (AR) immunoexpression in SDC is reported in 69% to 100% SDC. Androgen deprivation therapy (ADT) has shown a response rate of 18% to 42% in SDC. Therefore, AR immunoexpression may serve as a diagnostic and predictive marker for ADT response in SDC.
Methods:
We investigated AR immunopositivity and staining pattern in a large retrospective cohort of 188 SDCs from 163 patients, including 22 paired primary and metastatic SDCs from the same patients, focusing specifically on staining heterogeneity and concordance. A control cohort of 61 non-SDC salivary gland carcinomas was also included.
Results:
AR immunopositivity defined as ≥1% of tumor cell nuclear staining was found in 94% (177/188) of SDCs, including 95% of primary tumors, 100% of regional metastases, and 90% of distant metastases. Most of the cases (75%, 86/114) showed homogeneous and diffuse AR positivity. However, a subset (25%) exhibited focal or heterogeneous AR staining pattern. Although most metastases (21/22, 95%) had concordant AR expression with the primary tumors, one treatment-naïve tumor (5%) had complete loss of AR immunoexpression in the metastasis without detectable molecular alterations in AR or AR co-regulators. AR positive staining in non-SDC salivary carcinomas was infrequent (15%, 9/61), and mostly heterogeneous or focal.
Conclusion:
AR immunoexpression is highly prevalent in SDC, in both primary (94%) and metastatic tumors (93%). The cumulative AR immunopositivity rate in SDC is 90% based on data from the current study and previous literature. A small subset may show intratumoral AR heterogeneity and discordant AR expression in metastasis. AR immunoexpression may be seen in non-SDC salivary gland carcinomas but it is uncommon and usually focal.
Keywords: salivary duct carcinoma, androgen receptor, immunohistochemistry, salivary carcinoma
Introduction
Salivary duct carcinoma (SDC) is a high-grade salivary gland carcinoma, accounting for approximately 10% of all salivary gland malignancies (1). Histologically, SDC typically demonstrates apocrine cytomorphology with enlarged central nuclei with prominent nucleoli, abundant eosinophilic cytoplasm, and apical snouts (2). SDC may arise from pre-existing pleomorphic adenoma (SDC ex-PA) or de novo. Overall, SDC is associated with an adverse clinical outcome, due to a high frequency of local, regional, and distant metastasis (1, 3).
In 2000, Fan et al. was the first to report nuclear androgen receptor (AR) immunopositivity in SDC (4, 5). Subsequently, overactive AR signaling was detected as strong and diffuse AR immunoexpression. AR immunoexpression has been reported in 69 to 100% of SDC (6-9). Other than SDC, AR immunoexpression is also reported in prostatic carcinoma, carcinoma of skin adnexa, and a subset of breast carcinoma (10-12). Androgen-deprivation therapy (ADT) has been considered as a well-established treatment modality in advanced prostatic carcinoma for the past seven decades (10), and has been used in selected cases of AR-positive salivary gland carcinoma showing a response rate of 55% (13, 14). In SDC, the reported overall response rate to ADT, partial or complete, is 18% to 42% (5, 13, 14).
Given the reported high frequency of AR immunopositivity and the potential therapeutic application of ADT in SDC, AR immunohistochemistry (IHC) becomes a widely-used diagnostic marker and a possible predictive biomarker for these patients. However, most of the prior studies focused solely on primary tumors with AR expression being reported simply as positive or negative. In the current study, we aimed to investigate intensity and distribution pattern of AR immunoexpression in a large retrospective cohort of 188 SDCs from 163 patients, including 22 cases with matched primary tumors and metastases, focusing specially on staining heterogeneity and concordance.
Materials and methods
Study cohort
After obtaining Institutional Review Board approval, the pathology database was searched for SDC diagnosed at Memorial Sloan-Kettering Cancer Center (MSKCC, New York, NY, USA) and Sunnybrook Health Sciences Centre (SHSC, Toronto, ON, Canada) between 2000 and 2018. A total of 188 SDCs from 163 patients were included in the present study (MSKCC: 173 tumors from 154 patients; SHSC: 15 tumors from 9 patients). Among the 188 SDCs studied, 148 (79%) were primary tumors, 11 were lymph node metastases (6%), and 29 were distant metastases (15%). Twenty-two patients in our cohort had paired pathologic specimens from the primary tumors and metastases, including 21 patients with distant metastases and 1 with regional lymph node recurrence. The primary tumors were further subtyped as SDC ex-pleomorphic adenoma (PA) when there was histologic evidence of a co-existent or prior PA, or SDC de novo.
Moreover, a control group of 61 salivary gland carcinomas composed of histologic types other than SDC were included in the study and tested for AR expression. These non-SDC cases included 17 adenoid cystic carcinomas, 9 myoepithelial carcinomas, 7 acinic cell carcinomas, 6 mucoepidermoid carcinoma, 2 epithelial-myoepithelial carcinomas, 2 secretory carcinomas, and 17 carcinomas of other histologic types (12 carcinoma not otherwise classified, 2 adenocarcinoma NOS, 1 adenocarcinoma NOS ex pleomorphic adenoma, 1 adenosquamous carcinoma ex pleomorphic adenoma, and 1 undifferentiated carcinoma). All cases were reviewed by at least one head and neck pathologist (BX or NK) to confirm the diagnosis. AR IHC was performed on representative tumor section in all cases.
AR immunohistochemistry (IHC) interpretation
IHC staining for AR was performed with AR m/onoclonal antibody (clone: SP107, dilution: 1:250, Spring Bioscience Corporation, Pleasanton, CA, US) and Ventana system (Ventana Medical Systems Inc., Tucson, AZ, US) according to the manufacturer’s recommendations. Based on previous literature (2, 7, 15-19), AR was considered positive if ≥1% of tumor cell nuclei were immunoreactive. Additionally, the following three parameters were recorded: 1) the percentage of tumor cell nuclei positive for AR; 2) staining intensity: 1+ (weak), 2+ (moderate), and 3+ (strong); and 3) staining pattern, being focal, heterogeneous/patchy, and homogeneous.
Statistics
All statistical analyses were performed using the SPSS software 24.0 (IBM Corporation, New York, NY, U.S.). The differences of AR immunopositivity and its staining details (percentage of positive tumor cell nuclei, staining intensity, and staining pattern) was assessed in primary tumors, nodal metastases, and distant metastases using appropriate statistical tests, i.e. Fisher’s exact test for nonparametric variables and one-way analysis of variance (ANOVA) for continuous variables. P values less than 0.05 were considered to be statistically significant.
Results
AR immunoexpression in SDC
The frequency of AR immunopositivity in the SDC cohort (188 samples from 163 patients) was 94% (177/188), including 140/148 (94%) primary tumors, 11 of 11 (100%) regional lymph node metastases, and 26 of 29 (90%) distant metastases (Table 1). The site of primary tumors was available in 110 cases, including 90 (82%) in the parotid gland, 12 (11%) in the submandibular gland, 3 (3%) in the lacrimal gland, and 5 (5%) in the minor salivary glands. Among the 118 primary tumors with available H&E slides from the resection specimens, 33 were classified as SDCs ex-PA, and 85 were classified as SDCs de novo. The rate of AR immunopositivity did not differ between de novo and CA ex-PA cases, being 94% and 95% respectively. In SDC ex-PA cases, AR staining was identified to be strong and homogenous in the SDC component but focal and weak in the PA component.
Table 1.
Androgen receptor (AR) immunoexpression in salivary duct carcinoma (SDC).
| AR immunopositivity (n=190) | |
| All | 177/188 (94%) |
| Primary tumor | 140/148 (95%) |
| Ex-Pleomorphic adenoma | 31/33 (94%) |
| De novo | 81/85 (95%) |
| Regional lymph node metastasis | 11/11 (100%) |
| Distant metastasis | 26/29 (90%) |
| Percentage of AR positivity in positive cases (n=114) | |
| 1-10% of tumor cell nuclei | 10 (9%) |
| 11-33% of tumor cell nuclei | 10 (9%) |
| 34-66% of tumor cell nuclei | 7 (6%) |
| 67-100% of tumor cell nuclei | 87 (76%) |
Values are expressed as number of cases (column %).
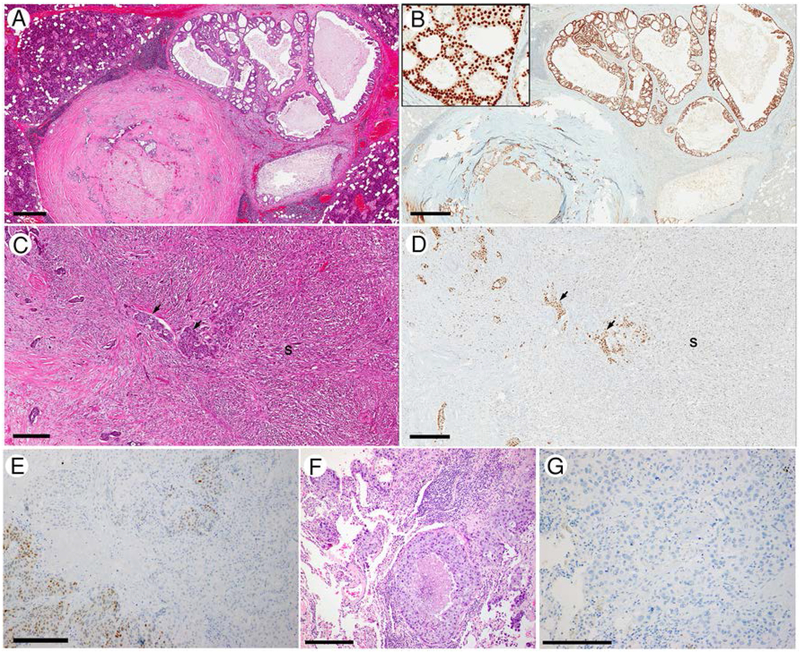
AR immunostaining details were assessed in 114 cases, 70 (61%) of which showed homogeneous diffuse and strong (3+) staining in at least 67% of tumor cell nuclei. The mean percentage of positive tumor cell nuclei was 74% and the median was 90%. Eighty-seven (76%) cases showed AR immunopositivity in at least 67% of tumor cell nuclei. The staining intensity was weak (1+) in 11 (10%), moderate (2+) in 29 (25%), and strong (3+) in 74 (65%). Eighty-six SDCs (75%) had homogeneous AR staining within the tumor, 19 (17%) showed staining heterogeneity, whereas the remaining 9 (8%) cases had only focal AR immunopositivity. One of the tumors with heterogeneous AR staining showed morphologic heterogeneity with a classical SDC histology and a sarcomatoid tumor component. Interestingly, AR was strong (3+) and diffuse in the classical SDC areas, whereas weak (1+) and focal in the sarcomatoid tumor component (Figure 1). The rest of the tumors with heterogeneous AR staining exhibited a classical SDC morphology. Overall, when comparing primaries to metastases (regional and distant), the staining frequency, the percentage of positive tumor cell nuclei, the staining intensity, and the staining patterns did not differ significantly between the primary and metastatic tumors (Table 2, p=0.240 for rate of positivity; p=0.135 for percentage of positive tumor cell nuclei; p=0.310 for intensity; and p=0.178 for staining pattern).
Figure 1. AR expression in salivary duct carcinoma (SDC).
(A/B) a SDC ex-PA shows homogeneous strong (3+) AR immunoexpression in 100% of tumor cell nuclei. (C/D) a SDC with sarcomatoid component shows heterogeneous AR expression. AR immunoreactivity is strong and diffuse in the conventional SDC component (black arrows), while is weak and patchy (staining about 40% of tumor cell nuclei) in the sarcomatoid component (S) (E-G) A SDC with discordant AR immunoexpression: AR is heterogeneously positivity in the primary tumor (E) The distant metastasis to lung has typical histologic appearance of SDC with comedo-type necrosis and apocrine cytologic features (F) but is completely negative for AR (G). Scale bar: 600 microns in A and B; 300 microns in C, D and E; 200 microns in F and G.
Table 2.
AR immunoexpression in AR-positive primary and metastatic SDC.
| Percentage of positive TC | Staining intensity | Staining pattern | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean±SEM | Median (range) | 1+ | 2+ | 3+ | Focal | Heter. | Homog. | |
| All cases (n=114) | 74%±3% | 90% (1-100%) | 11 (10%) | 29 (25%) | 74 (65%) | 9 (8%) | 19 (17%) | 86 (75%) |
| Primary tumor (n=85) | 70%±3% | 80% (1-100%) | 10 (12%) | 23 (21%) | 52 (61%) | 6 (7.1%) | 18 (21%) | 61 (72%) |
| Lymph node metastasis (n=8) | 82%±10% | 92% (20-100%) | 0 (0%) | 3 (37.5%) | 5 (62.5%) | 0 (0%) | 1 (12.5%) | 7 (87.5%) |
| Distant metastasis (n=21) | 85%±7% | 100% (1-100%) | 1 (5%) | 3 (14%) | 17 (81%) | 3 (14%) | 0 (0%) | 18 (86%) |
TC: tumor cell nuclei; heter.: heterogeneous; homog.: homogeneous, SEM: standard error of mean.
In 22 patients, AR IHC was performed in both primary and recurrent tumors, including 21 distant metastases and 1 regional lymph node recurrence. The AR immunoexpression in the primary and metastatic tumors are provided in Supplementary Table 1. Although most of the tumors (21/22, 95%) showed concordant AR IHC results, there was one discordant case (patient #22) where the AR expression was lost completely in the distant metastasis. The primary tumor was a 3.0 cm SDC de novo of submandibular gland that showed strong (3+) and heterogeneous AR immunopositivity in 40% of tumor cell nuclei, whereas the 1.2-cm lung metastasis (that was treatment naïve and resected at the same time) was negative for TTF1 and completely negative for AR. The data of the subsequent targeted exome next generation sequencing using MSK-IMPACT platform (20) was available on the lung metastasis and showed TP53 c.267delC frameshift mutation and ERBB2 amplification without genetic alteration affecting AR or AR coregulators, e.g. FOXA1, GATA2, SPOP, and NCOR1.
AR in non-SDC salivary gland carcinoma group
Among the 61 salivary gland carcinomas of non-SDC type tested, AR immunoreactivity was detected in 9 (16%, Table 3). The histopathologic diagnoses for these 9 cases were: 2 of 17 adenoid cystic carcinoma; 2 of 10 myoepithelial carcinoma, 2 of 2 secretory carcinoma, 1 of 6 mucoepidermoid carcinoma, 1 of 2 epithelial-myoepithelial carcinoma, and 1 adenocarcinoma NOS. The AR positive mucoepidermoid carcinoma was intermediate grade. In one case of high-grade secretory carcinoma (ETV6-NTRK3 fusion positive), the AR staining was homogeneous and moderate to strong (2-3+) in 95% of tumor cell nuclei, whereas the remaining positive cases exhibited either focal (1 case) or heterogeneous (5 cases) staining. The details of AR immunostaining were not available in the other two positive cases because the immunostained slides were not obtainable for re-review.
Table 3.
AR expression in other types of salivary gland carcinoma.
| AR positivity | AR staining patterns in positive cases | |
|---|---|---|
| All | 9/61 (15%) | |
| Adenoid cystic carcinoma | 2/17 | Both cases: 20%, heterogeneous |
| Myoepithelial carcinoma | 2/10 | Case 1: 40%, 2+, heterogeneous; Case 2: 40%, 1+, heterogeneous |
| Acinic cell carcinoma | 0/7 | |
| Mucoepidermoid carcinoma | 1/6 | 50%, 1+-2+, heterogeneous |
| Epithelial-myoepithelial carcinoma | 1/2 | NA |
| Secretory carcinoma | 2/2 | Case 1: 95%, 2+-3+, homogeneous; Case 2: Focal |
| Others | 1/17 | NA |
Sensitivity and specificity
In our cohort, the overall sensitivity and specificity of AR in diagnosing SDC using a definition of ≥1% as positive threshold were 94% and 85% respectively. If using a diffuse ≥50% staining as a cutoff for AR positivity, the sensitivity and specificity were 74% and 97%, respectively.
Discussion
In this study, we found a high frequency of AR immunopositivity in SDC, being 93%. A literature review on AR immunoexpression in SDC was also conducted, and the findings are summarized in Table 4. In brief, AR immunopositivity has been reported in 90% of SDCs, ranging from 69% to 100%. Variable criteria were utilized to define positive AR expression in these studies ranging from any nuclear staining to diffuse nuclear staining. However, the published frequency of AR immunopositivity in SDC was not drastically affected by such variability, since the majority of SDCs show diffuse and strong AR staining. Several studies, including ours, have used a cutoff of any positivity to ≥1% positive tumor cell nuclei, and have reported a frequency of AR positivity of 92% (ranging from 75% to 100%) (2, 7, 15-19). On the other hand, some other studies did require strong and/or diffuse nuclear staining to consider AR positive, and reported a AR positivity rate of 88% (ranging from 69% to 100%) (3, 5, 6, 9, 21). In concordance with these previous studies (2, 3, 5-7, 9, 15-19, 21), our study cohort had a high prevalence of AR immunopositivity in SDC with most of the cases showing diffuse and strong staining. However, a subset of cases, being 25% in our study, may show focal and/or heterogeneous AR staining, and this should be interpreted in the context of the morphology and should not by itself exclude the diagnosis of SDC if it is otherwise typical.
Table 4.
Literature review: AR immunoexpression in salivary duct carcinoma.
| Reference | Threshold for AR positivity | AR overall |
|---|---|---|
| (4, 26, 27) | NA | 12/13 (92%) |
| (28) | NA | 20/28 (71%) |
| (22) | NA | 6/6 (100%) |
| (29) | NA | 3/4 (75%) |
| (30) | NA | 11/15 (73%) |
| (8) | NA | 7/7 (100%) |
| (25) | NA | 12/16 (75%) |
| (31) | NA | 124/133 (93%) |
| (21) | Strong and homogeneous nuclear, and/or cytoplasmic staining in >70% tumor cells. | 27/35 (77%) |
| (9) | Diffuse nuclear staining | 16/16 (100%) |
| (3) | Diffuse nuclear staining | 161/168 (96%) |
| (5) | Diffuse nuclear staining | 70/78 (90%) |
| (6) | Allred score≥6 | 43/62 (69%) |
| (20) | Any nuclear staining | 54/61 (89%) |
| (19) | >0% tumor cells (IRS score ≥ 2) | 56/67 (84%) |
| (18) | ≥1% tumor cells (Allred score>2) | 36/42 (86%) |
| (7) | ≥1% tumor cells (Allred score≥3) | 30/30 (100%) |
| (17) | ≥1% tumor cells | 24/32 (75%) |
| (2) | ≥1% tumor cells (Allred score≥3) | 179/183 (98%) |
| (16) | ≥1% tumor cells (Allred score≥3) | 21/23 (91%) |
| Current study | ≥1% tumor cells | 177/188 (94%) |
| All | 1089/1207 (90%) |
IRS: immunoreactive score (IRS) of Remmele and Stegner,
In contrast to SDC, AR immunoexpression in other types of salivary carcinoma seems relatively infrequent, being detected in 15% of our control cohort, which is consistent with what has been previously reported (13, 22-24). Moreover, similarly to prior studies, we found that AR-immunopositivity in non-SDC carcinomas is typically focal or heterogeneous (13, 22-24). The only exception in our control cohort was a secretory carcinoma with high grade transformation which showed a homogeneous moderate to strong nuclear AR staining in 95% of tumor cell nuclei. Given the high frequency of diffuse and strong AR immunopositivity in SDC and the low rate of AR immunopositivity in other carcinoma types (where AR is often focal or heterogeneous when it is positive), AR IHC can be a helpful diagnostic marker in classifying salivary gland carcinoma in combination with other available ancillary testing techniques, including molecular testing for characteristic fusions in salivary gland tumors.
One potential weakness of our study is that all cases included were surgical cases, being either resections or biopsies. Therefore, we cannot address the utility of AR IHC in fine needle aspiration cytology samples to differentiate SDC from other salivary gland tumors.
At the molecular level, AR splice variants (including the ligand-binding independent form AR-V7), mutations, and copy number gain, as well as alteration of AR-coregulators (e.g. FASN, FOXA1, and NCOR1) have been reported in ADT treatment-naïve SDC (21, 25). AR-knockdown in vitro results in growth inhibition in SDC-cell line (21), suggesting a role of AR in tumorigenesis and tumor progression of SDC. In addition, several recent studies, including a prospective phase II trial, have shown that ADT may be used in AR-positive salivary gland carcinomas, including SDC, with a response rate of 18 to 55% (5, 13, 14). Therefore, AR IHC may be utilized as a predictive biomarker to select patients with salivary gland carcinoma for ADT in the appropriate clinical setting.
All previous studies (summarized in Table 4) have focused solely on AR expression in primary SDC except for one study by Jalal et al. However, that study included only 5 distant metastases in a small cohort of 23 SDCs and did not report the frequency of AR positivity in distant metastases alone (15). The current study cohort is the largest to explore AR expression in primary, recurrent and metastatic SDC. In our laboratory, the rate of AR expression appears similar in primary and metastatic sites, being 95% in primary tumors, 100% in lymph node metastases, and 90% in distant metastases. Given the high frequency of AR immunopositivity in metastatic setting, AR IHC can be used clinically to establish the diagnosis of SDC in metastasis, especially in small material in which the classical histologic features of SDC may not be appreciated. In addition, we were the first to investigate in a detailed fashion AR concordance in primary and metastatic SDC. Twenty-one of the 22 SDCs (95%) with both primary and metastasis tested displayed concordant AR immunopositivity, whereas one treatment naïve case (5%) showed discordant results, being heterogeneously positive in the primary tumor and completely negative in the lung metastasis. As the primary SDC showed heterogeneous AR staining in this case, it is postulated that the metastatic tumor originated from the AR-negative subclone. However, the exact mechanism of lost AR immunoexpression in the distant metastasis remains to be investigated.
Conclusions
AR immunoexpression in SDC is frequent, being detected in more than 90% of cases. The staining pattern of AR in SDC is typically diffuse, homogeneous, and strong. However, a subset (25%) may show focal or heterogeneous staining, and this should not exclude the diagnosis of SDC. Metastatic SDC usually retains AR expression; although, on occasion, AR immunoexpression can be completely lost in the metastasis. In non-SDC salivary gland carcinoma, AR immunoexpression is infrequent and the staining are typically focal or homogeneous.
Supplementary Material
Highlights:
AR immunoexpression is highly prevalent in primary and metastatic SDC.
The cumulative AR immunopositivity rate in SDC is 90%.
A small subset of SDC may show intratumoral AR heterogeneity and discordant AR expression in metastasis.
AR immunoexpression is uncommon and usually focal in non-SDC salivary gland carcinoma.
Acknowledgments
Conflicts of Interest and Source of Funding:
The authors have disclosed that they have no significant relationships with, or financial interest in any commercial companies pertaining to this article. Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. World Health Organization Classification of Tumours: pathology and genetics of head and neck tumours (4th edition). Lyon: International Agency for Research on Cancer (IARC), 2017. [Google Scholar]
- 2.Williams L, Thompson LD, Seethala RR, Weinreb I, Assaad AM, Tuluc M, Ud Din N, Purgina B, Lai C, Griffith CC, Chiosea SI. Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol 2015; 39, 705–713. [DOI] [PubMed] [Google Scholar]
- 3.Boon E, Bel M, van Boxtel W, van der Graaf WTA, van Es RJJ, Eerenstein SEJ, Baatenburg de Jong RJ, van den Brekel MWM, van der Velden LA, Witjes MJH, Hoeben A, Willems SM, Bloemena E, Smit LA, Oosting SF, Jonker MA, Flucke UE, van Herpen CML. A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from The Netherlands. Int J Cancer 2018; 143, 758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan CY, Wang J, Barnes EL. Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: an immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol 2000; 24, 579–586. [DOI] [PubMed] [Google Scholar]
- 5.Boon E, van Boxtel W, Buter J, Baatenburg de Jong RJ, van Es RJJ, Bel M, Fiets E, Oosting SF, Slingerland M, Hoeben A, Tesselaar MET, Jonker MA, Flucke UE, van der Graaf WTA, van Herpen CML. Androgen deprivation therapy for androgen receptor-positive advanced salivary duct carcinoma: A nationwide case series of 35 patients in The Netherlands. Head Neck 2018; 40, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang L, Williams MD, Bell D. Expression of PTEN, Androgen Receptor, HER2/neu, Cytokeratin 5/6, Estrogen Receptor-Beta, HMGA2, and PLAG1 in Salivary Duct Carcinoma. Head Neck Pathol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiosea SI, Williams L, Griffith CC, Thompson LD, Weinreb I, Bauman JE, Luvison A, Roy S, Seethala RR, Nikiforova MN. Molecular characterization of apocrine salivary duct carcinoma. Am J Surg Pathol 2015; 39, 744–752. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto N, Minami S, Fujii M. Clinicopathologic study of salivary duct carcinoma and the efficacy of androgen deprivation therapy. Am J Otolaryngol 2014; 35, 731–735. [DOI] [PubMed] [Google Scholar]
- 9.Beck ACC, Lohuis P, Al-Mamgani A, Smit LA, Klop WMC. Salivary duct carcinoma: evaluation of treatment and outcome in a tertiary referral institute. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies 2018; 275, 1885–1892. [DOI] [PubMed] [Google Scholar]
- 10.Perlmutter MA, Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Reviews in urology 2007; 9 Suppl 1, S3–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Vasiliou SK, Diamandis EP. Androgen receptor: A promising therapeutic target in breast cancer. Critical reviews in clinical laboratory sciences 2019, 1–24. [DOI] [PubMed] [Google Scholar]
- 12.Rollins-Raval M, Chivukula M, Tseng GC, Jukic D, Dabbs DJ. An immunohistochemical panel to differentiate metastatic breast carcinoma to skin from primary sweat gland carcinomas with a review of the literature. Arch Pathol Lab Med 2011; 135, 975–983. [DOI] [PubMed] [Google Scholar]
- 13.Dalin MG, Watson PA, Ho AL, Morris LG. Androgen Receptor Signaling in Salivary Gland Cancer. Cancers 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fushimi C, Tada Y, Takahashi H, Nagao T, Ojiri H, Masubuchi T, Matsuki T, Miura K, Kawakita D, Hirai H, Hoshino E, Kamata S, Saotome T. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol 2018; 29, 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jalaly JB, Sanati S, Chernock RD, Dibe DG, El-Mofty SK. Salivary Duct Carcinoma and Invasive Ductal Carcinoma of the Breast: A Comparative Immunohistochemical Study. Head Neck Pathol 2018; 12, 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masubuchi T, Tada Y, Maruya S, Osamura Y, Kamata SE, Miura K, Fushimi C, Takahashi H, Kawakita D, Kishimoto S, Nagao T. Clinicopathological significance of androgen receptor, HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. International journal of clinical oncology 2015; 20, 35–44. [DOI] [PubMed] [Google Scholar]
- 17.Di Palma S, Simpson RH, Marchio C, Skalova A, Ungari M, Sandison A, Whitaker S, Parry S, Reis-Filho JS. Salivary duct carcinomas can be classified into luminal androgen receptor-positive, HER2 and basal-like phenotypes. Histopathology 2012; 61, 629–643. [DOI] [PubMed] [Google Scholar]
- 18.Haderlein M, Scherl C, Semrau S, Lettmaier S, Hecht M, Erber R, Iro H, Fietkau R, Agaimy A. Impact of postoperative radiotherapy and HER2/new overexpression in salivary duct carcinoma : A monocentric clinicopathologic analysis. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al] 2017; 193, 961–970. [DOI] [PubMed] [Google Scholar]
- 19.Villepelet A, Lefevre M, Verillaud B, Janot F, Garrel R, Vergez S, Bertolus C, Malard O, de Gabory L, Mauvais O, Baujat B. Salivary duct carcinoma: Prospective multicenter study of 61 cases of the Reseau d'Expertise Francais des Cancers ORL Rares. Head Neck 2019; 41, 584–591. [DOI] [PubMed] [Google Scholar]
- 20.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, Brannon AR, O'Reilly C, Sadowska J, Casanova J, Yannes A, Hechtman JF, Yao J, Song W, Ross DS, Oultache A, Dogan S, Borsu L, Hameed M, Nafa K, Arcila ME, Ladanyi M, Berger MF. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics : JMD 2015; 17, 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitani Y, Rao PH, Maity SN, Lee YC, Ferrarotto R, Post JC, Licitra L, Lippman SM, Kies MS, Weber RS, Caulin C, Lin SH, El-Naggar AK. Alterations associated with androgen receptor gene activation in salivary duct carcinoma of both sexes: potential therapeutic ramifications. Clin Cancer Res 2014; 20, 6570–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasser SM, Faquin WC, Dayal Y. Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors. Frequent expression of androgen receptor in a subset of malignant salivary gland tumors. American journal of clinical pathology 2003; 119, 801–806. [DOI] [PubMed] [Google Scholar]
- 23.Aquino G, Collina F, Sabatino R, Cerrone M, Longo F, Ionna F, Losito NS, De Cecio R, Cantile M, Pannone G, Botti G. Sex Hormone Receptors in Benign and Malignant Salivary Gland Tumors: Prognostic and Predictive Role. Int J Mol Sci 2018; 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Can NT, Lingen MW, Mashek H, McElherne J, Briese R, Fitzpatrick C, van Zante A, Cipriani NA. Expression of Hormone Receptors and HER-2 in Benign and Malignant Salivary Gland Tumors. Head Neck Pathol 2018; 12, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalin MG, Desrichard A, Katabi N, Makarov V, Walsh LA, Lee KW, Wang Q, Armenia J, West L, Dogan S, Wang L, Ramaswami D, Ho AL, Ganly I, Solit DB, Berger MF, Schultz ND, Reis-Filho JS, Chan TA, Morris LG. Comprehensive Molecular Characterization of Salivary Duct Carcinoma Reveals Actionable Targets and Similarity to Apocrine Breast Cancer. Clin Cancer Res 2016; 22, 4623–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.