Abstract
Children may be more vulnerable to the combined interactions of chemical and non-chemical stressors from their built, natural, and social environments when compared to adults. Attention deficit/hyperactivity disorder (ADHD) is the most commonly diagnosed childhood neurodevelopmental disorder and is considered a major public health issue, as 75% of childhood cases persist into adulthood. ADHD is characterized by developmentally inappropriate levels of hyperactivity, impulsivity, and inattention, with the neurotransmitter serotonin regulating these symptoms. Monoamine oxidase A (MAOA) aids in serotonin uptake and is often implicated in behavioral and emotional disorders, including ADHD. When children are exposed to cigarette smoke, bisphenol A (BPA), or organophosphate pesticides, MAOA activity is inhibited. Non-chemical stressors, such as traumatic childhood experiences, and lifestyle factors, complicate the relationship between genotype and exposures to chemical stressors. But the co-occurrence among outcomes between exposures to chemical stressors, non-chemical stressors, and the low activity MAOA genotype suggest that mental illness in children may be influenced by multiple interacting factors.
In this systematic review, we examine the existing literature that combines exposures to chemical and non-chemical stressors (specifically childhood trauma), MAOA characteristics, and ADHD diagnosis to investigate the interrelationships present. We observe that chemical (lead [Pb], phthalates/plasticizers, persistent organic pollutants, and cigarette smoke) exposure is significantly related to ADHD in children. We also observed that existing literature examining the interaction between MAOA, exposures to chemical stressors, and traumatic experiences and their effect on ADHD outcomes is sparse. We recommend that future studies investigating childhood ADHD include chemical and non-chemical stressors and inherent characteristics to gain a holistic understanding of childhood mental health outcomes.
Keywords: mental health, one health, childhood development, psychosocial, child, stressors
Graphical Abstract
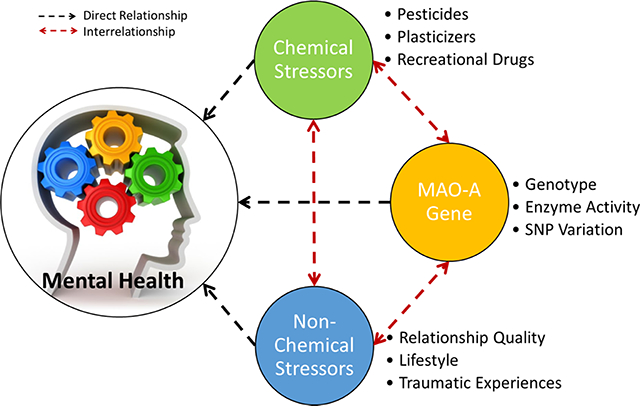
A summary figure depicting the interrelationships between chemical and non-chemical stressors, genetic characteristics, and the collective influence on children’s mental health outcomes, as discussed in this review.
1. Introduction
Attention deficit/hyperactivity disorder (ADHD) is characterized by developmentally inappropriate levels of inattention, impulsivity and/or hyperactivity (Danielson et al., 2018; Mahone and Denckla, 2017; Perou et al., 2013). ADHD is the most commonly diagnosed childhood neurodevelopmental disorder and is considered a major public health issue, as 75% of childhood cases persist into adulthood. From 2005 to 2011, U.S. children (3–17 years old) were more frequently diagnosed with ADHD (6.8%) than conduct disorders (3.5%), anxiety (3.0%), depression (2.1%), autism spectrum disorders (1.1%), or Tourette syndrome (0.2%) (Perou et al., 2013). Between 1999 and 2014 the annual percentage of U.S. children diagnosed with ADHD rose from 7.0% to 10.2% (Pastor et al., 2015). However, the percentage of diagnosed cases fluctuates based on the method of evaluation and the selected edition of the Diagnostic and Statistical Manual of Mental Disorders referenced (DSM; published by the American Psychiatric Association) (Thomas et al., 2015). The American Psychological Association (APA) highlights mental health as a critical component of children’s overall health and well-being and underscores the complex interrelationships between mental and physical health as well as the ability to succeed in society, since both components affect how children think and feel.
ADHD has been linked to exposure to chemicals (e.g., plasticizers, pesticides, metals) and children may be more susceptible to the risks of exposure than adults because of their smaller body size, continuing development, and increased respiratory rate (Bouchard et al., 2010b; Braun et al., 2006; Daston et al., 2004; Heilbrun et al., 2015; Hoffman et al., 2010; Polańska et al., 2012). Children can also experience greater exposures to chemicals than adults, with children reported to have approximately double the phthalate burden than similarly exposed adults, further increasing their risk of exposure related effects (Heudorf et al., 2007; Koch et al., 2004). While children are similarly exposed to other classes of chemicals, such as pesticides and toxic metals (lead, arsenic, cadmium, mercury), their increased metabolism compared to adults may make children more susceptible to the effects of these exposures as well (Llobet et al., 2003; Radomski et al., 1971).
Non-chemical stressors arising from the built, natural, and social environments (e.g., noise pollution, physiological stress, and family dynamics), including psychosocial adversities (e.g., deficiencies in environmental quality, resources, and negative social factors), have also been shown to be risk factors for ADHD (Biederman et al., 2002; Gordon, 2003; Rider et al., 2012). The combination of these factors is likely responsible for the biological component of ADHD, which has been shown to be related to a genetic predisposition, and/or a family history of mental health diagnoses and/or the learned behaviors of the family dynamic (Kessler et al., 2009, 2010; Mahone and Denckla, 2017). A gene involved in serotonin and neurotransmitter metabolism, monoamine oxidase A (MAOA), is influenced by exposures to chemical stressors, traumatic experiences, and is associated with ADHD outcomes (Kalgutkar et al., 2001; Oades et al., 2008; Oreland and Shaskan, 1983; Shih et al., 1999).
The varied risk factors for ADHD and the sensitivity of children to chemical stressors make it necessary to examine this issue holistically. By doing this, we can consider a child’s total environment (where they live, learn, and play), including the built, natural and social influences they experience (Tulve et al., 2016). The interrelationships between stressors is important to consider, as they can modulate, mediate, and overlap, potentially resulting in a synergistic effect (Kraemer et al., 2001). Environmental stressors are recognized as one of the largest challenges in mental health science today and increased interdisciplinary research is needed to improve care (Collins et al., 2011). The children’s health framework developed by Tulve et al. (2016) outlines the interrelationships between both chemical and non-chemical stressors in the total (built, natural, and social) environment, as well as activities and behaviors and inherent characteristics that affect children’s health and well-being and can be used to guide interdisciplinary studies (Tulve et al., 2016).
The objective of this review was to systematically survey the literature for the combination of ADHD diagnoses, childhood exposures to chemical and non-chemical stressors, and their genetic predisposition for susceptibility to the diagnosis through the MAOA gene using the Tulve et al. (2016) framework as the organizing tool. Previous systematic reviews concerning chemical exposures and neurodevelopmental outcomes have focused on one class of chemicals, an individual chemical, and/or a wide range of outcomes including memory, IQ, and behavioral disorders (Goodlad et al., 2013; Lam et al., 2017; Rodríguez-Barranco et al., 2013). To the best of our knowledge, our review is the first to include multiple classes of chemical exposures, childhood traumatic experiences, and genetic MAOA characteristics for the analysis of a specific developmental outcome (ADHD).
2. Methods
This review followed the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines (Liberati et al., 2009; Moher et al., 2015). Included references were peer-reviewed, written in English, published between 1980 and 2017, classified as research on children (consisting of preschool aged children (2–5 years) through adolescence (13–18 years) as defined by the PubMed Medical Subject Headings) and used either the DSM-IV mental health classification system or a comparable system. In 1980, the APA changed the name and definition of hyperkinetic impulse disorder to attention deficit disorder (ADD) with subtypes relating to hyperactivity and later to attention deficit hyperactivity disorder (ADHD) with no subtypes. 1980 marks the beginning of the diagnosis of ADD/ADHD as we understand it today, so this year was used as the starting point for this review (Connor and Doerfler, 2008; Ghosh and Sinha, 2012).
2.1. Search Details
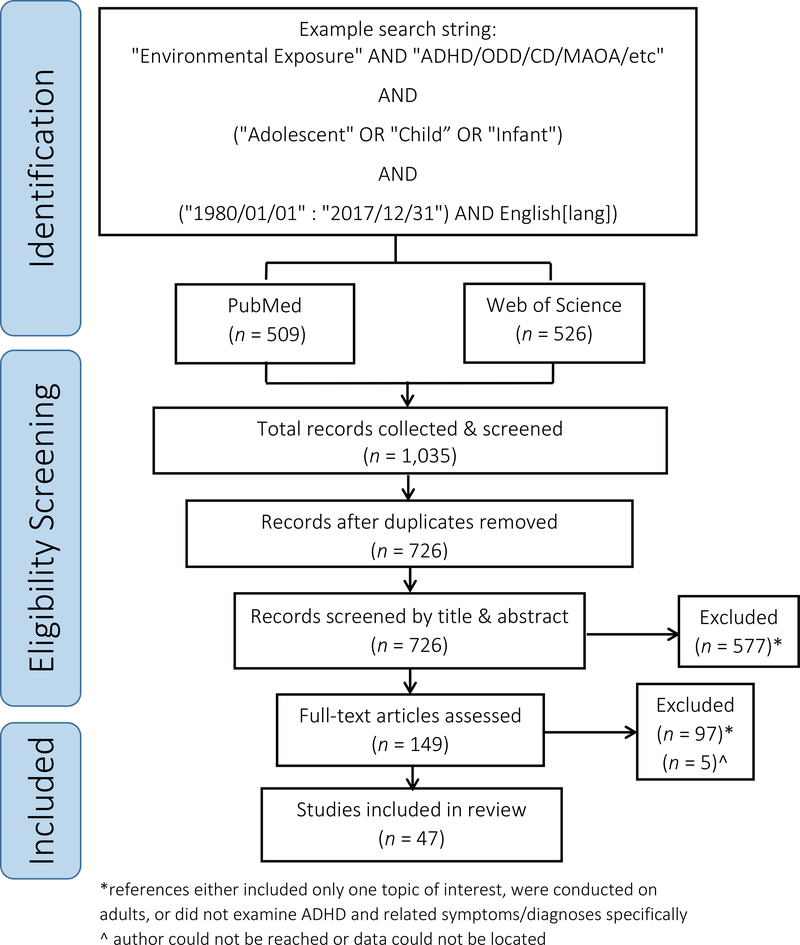
Web of Science (Core Collection) and PubMed were the two databases used for identification of relevant primary references using search strings related to “environmental exposure” AND “Attention Deficit and Disruptive Behavior Disorders” AND “monoamine oxidase”. An example of the search and subsequent screening process can be found in Fig. 1 and is detailed in the Supplement.
Figure 1.
PRISMA diagram of the process to select studies for inclusion in this review.
2.2. Reference Screening Process
An initial screening included a review of the title and abstract. Inclusion criteria for the title and abstract screening was determined based on responses to the following three questions.
Does the study include MAOA, ADHD, environmental/chemical exposure, or trauma?
Does the paper report a relationship between the topics?
Are children the focus of this study?
References meeting all criteria questions were included in the full text screening. The full text screening excluded references that focused on a single topic and not an interactive relationship, as well as any references that did not explicitly use ADHD or the associated symptoms based on the DSM-IV/V criteria as a mental health diagnosis (i.e., references grouping “externalizing behaviors” into one category were omitted) (Ghosh and Sinha, 2012; Jensen et al., 2001). Studies focused on other mental health outcomes, terrorist events, physical illnesses, or indirect relationships were excluded. Studies focused on prenatal exposures were excluded from this review, as few examined ADHD outcomes, and classified behaviors as ‘externalizing’. The prenatal exposure studies presented different data for monitoring and quantification, as well as included different chemical stressors for consideration. Thus, the prenatal exposure data was separated from the childhood exposure data presented herein and is analyzed in a forthcoming publication. Included references that did not provide enough information related to the original study design and statistical analysis were marked, and authors were contacted to obtain the necessary information for inclusion in our meta-analysis. If the authors could not be reached, the study was removed from the review (Fig. 1).
2.3. Data extraction & analysis
Chemical exposure data (including any details regarding blood/urine measurements or survey data), the effect size, confidence interval, and any accompanying statistical data related to ADHD as a mental health outcome in the examined population was extracted from relevant tables, graphs and text within each reference. The extracted data were put into Microsoft Excel and sorted according to exposure and outcome details. All effect size data were converted to odds ratios for meta-analysis and visualization using R Studio and the meta, metafor, metaviz, and ggplot 2 packages.
2.4. Quality assessment
The screening process described in section 2.2 was conducted on the 1035 references initially identified on two separate occasions. Any discrepancies between the two separate screenings were reconciled based on the relevance of the reference’s content to this review. The extracted data mentioned in section 2.3 were checked for accuracy by two individual reviewers on separate occasions.
2.4.1. Quality of evidence
The quality of the individual references included in this review was determined using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach, which is used worldwide and provides a systematic, transparent framework for summarizing evidence and making a recommendation (Guyatt et al., 2011). The GRADE method determines a quality ranking of a data set based on the imprecision (statistical significance), sample size (n </> 1000), indirectness (specificity of variables used for the relationship examined) and risk of bias (likelihood of population, publication, or other biases) of the data (Guyatt et al., 2011; Schünemann et al., 2013). The summary of findings table was created using the freely available Guideline Development Tool (GDT) software (GRADEpro_GDT, 2015). The individual quality rankings were used to determine the study’s quality in the meta-analysis, which was a contributing factor in the certainty of evidence presented in the summary of findings table. The individual GRADE quality rankings we generated referred to this review and the question we sought to answer. The rankings were not an evaluation of the scientific merit of the included peer-reviewed references, so we do not present our individual reference rankings. The summary of findings table presents the anticipated absolute effects of the risk of ADHD onset based on the participants with a DSM ADHD diagnosis, and those without. The estimate is pooled from the data provided in the included studies. The risk of ADHD with chemical exposure is based on the pooled OR from the included studies.
2.4.2. Publication & overall bias
The risk of publication bias in the meta-analyses was determined by assessing the symmetry of the data by using the inverse number of participants in each original study (1/n) and the standard error (SE) for comparison to the log odds ratio (OR). The statistical presence of asymmetry denotes that there is publication bias present in the data, as non-biased data would have a symmetric shape around the line of no effect (1.0) (Macaskill et al., 2001; Peters et al., 2006; Sutton et al., 2000). Each statistical comparison has different strengths for various datasets; the SE comparison (Egger’s test) is common practice in meta-analytics and uses the within study precision to estimate the symmetry of the data. However, when the OR is the summary estimate using the SE comparison can be problematic. There is a correlation between the SE and the magnitude of the OR, which can lead to inflated type 1 error especially when there are large ORs in the dataset (Peters et al., 2006). The 1/n method (modified Macaskill’s test) removes the likelihood of type 1 error being an artifact of the correlation between SE and OR magnitude, as well as lowers the likelihood that asymmetry would be observed when there is between study heterogeneity and/or when there are small studies that show greater effects than larger studies (Peters et al., 2006). We present both metrics for the meta-analyses herein but use the 1/n method to determine if there is publication bias present in the included data based on the strengths of that method and the included dataset.
Two independent reviewers assessed the risk of bias across this entire review with the Risk of Bias in Systematic Reviews (ROBIS) tool for etiology-based systematic reviews (Whiting et al., 2016). Their combined assessment indicated that there was a low risk of bias across this review.
2.5. Statistical analysis
2.5.1. Data grouping
Chemical exposures were separated into four different groups, based on similar chemical characteristics and/or mechanism of action, in order to be systematically compared to childhood ADHD symptoms and diagnoses. The four chemical classes were metals (Pb, Hg, Mn), phthalates/plasticizers (PhPl), organic contaminants (OCs), and cigarette smoke. Even though the PhPl and OCs act through the endocrine system, the PhPl studies were separated because they included large amounts of data and described sex-specific effects, which the OCs did not (Diamanti-Kandarakis et al., 2009). The statistical data extracted from each included study were the OR(s) for the onset of ADHD comparing children with higher and lesser exposures to chemicals. The OR data extracted from each reference were used in the relevant meta-analysis, and the general estimate of ADHD onset in relation to childhood exposures to chemicals is reported in the meta-analysis OR statistics in this review.
2.5.2. Meta-Analysis
Meta-analyses were conducted individually for each class of chemicals using the Random Effects Model (RMA), which is used for the analysis of studies that have different parameters and accounts for variation in the effects observed between studies included in the meta-analysis. The RMA model was calculated using the restricted (residual) maximum likelihood (REML) estimator, which provides a greater estimate of variance compared to the maximum likelihood (ML) estimator, which we deemed appropriate for this dataset (Vasdekis and Vlachonikolis, 2005). The heterogeneity measures used in this review are T2, which is a measure of the residual variance between the studies included in each meta-analysis not captured by the meta-analytic model and is expressed in squared units of the effect estimate (ln (OR)2). The I2 value is a measure of true heterogeneity to total variation in the observed effects of the meta-analysis (i.e., a signal to noise ratio) (Borenstein et al., 2011). More specifically, I2 is an estimate of how much of the unaccounted variability (residual heterogeneity + sampling error) is attributable to residual heterogeneity (vs. sampling error) and is expressed as a percentage (Columbia University, 2019). T2 and I2 provide a metric to assess the certainty of the data within the meta-analysis: T2 allows an understanding of the variance of studies within the meta-analysis; I2 is a metric that can be used to compare the current meta-analyses to other relevant meta-analyses (Borenstein et al., 2011). Columbia University (2019) provides that the I2 heterogeneity percentage values can be interpreted as:
0%–40%: might not be important.
30%–60%: may represent moderate heterogeneity.
50%–90%: may represent substantial heterogeneity.
75%–100%: considerable heterogeneity.
The results from the meta-analyses are visually presented with Forest plots. In a Forest plot, the OR is provided on the X-axis and the included references are listed on the Y-axis. The blue boxes corresponding to each reference’s OR are sized based on their weight in the meta-analysis, which is based on the study’s statistical significance, variance, and number of participants (Borenstein et al., 2011). The error bars on each box represent the upper and lower 90% confidence intervals (CI90%) that were calculated from the original studies to reflect the variance in the dataset (as most studies presented the 95% CI). The meta-analysis summary OR is provided by a red diamond and a vertical dashed red line. The ‘line of no effect’ (the line at which the odds of an outcome occurring are equal to the odds of the outcome not occurring) is represented by a dotted vertical line at an OR of 1.
2.5.3. Meta-regression
When the I2 value was above 75% in the meta-analyses, a meta-regression was performed to account for the considerable residual heterogeneity in the data. To better understand the sources of residual heterogeneity in the meta-analysis, all variables that were represented across all included data were analyzed in the meta-regression. The resulting meta-regression I2 values were compared to the I2 values from the meta-analysis to determine if any of the heterogeneity could be attributed to the examined variables. Meta-regression bubble plots are presented in the Supplement when appropriate.
2.5.4. Sensitivity analysis
To determine the robustness of the data included in the meta-analysis, 7 meta-analytic estimation methods were compared post-hoc to the REML meta-analyses we conducted (Thabane et al., 2013). The 7 estimation methods were the REML, the ML, the fixed-effects (FE), the Hedges (HE), the DerSimonian-Laird (DL), the empirical Bayesian (EB), and the Paul-Mandel (PM) estimators. The robustness of the data is presented as a percentage that was calculated by the number of estimators that yielded ORs within 10% of each other. All results presented were robust in the sensitivity analysis, with at least 70% agreement between estimation models (Table S1).
3. Results
Forty-seven references examining ADHD in children were included in the systematic review and meta-analyses. Seventeen references related to metal exposures (13 = Pb, 3 = Hg, 3 = Mn, 2 = As; analyzed separately), 8 related to PhPl exposures, 9 related to OCs (6 = pesticides/ polychlorinated biphenyls, 2 = perfluorinated compounds (PFCs), 1= nonylphenol; analyzed separately), and 12 related to exposure to cigarette smoke. There were 9 references related to ADHD and MAOA characteristics (e.g., genotype, activity), and 2 references discussed the relationship between ADHD, MAOA characteristics, and traumatic experiences. Individual categories totaled more than 47 as some references included chemical analyses of more than one class of chemical. All classes of chemical data were statistically analyzed except As, nonylphenol, and PFCs because there were less than 3 references in each of those groups. Due to the wide range of different methodologies and outcomes examined in the traumatic experiences and MAOA data, a statistical analysis was not conducted, and this data were qualitatively assessed instead.
3.1. Summary of Exposures to Chemical Stressors and ADHD Findings
When the chemical data were separated by chemical class (metals, Ph/Pl, OCs and cigarette smoke) and compared to the cumulative odds ratio of the random effects based meta-analysis, exposures to chemical stressors were consistently associated with greater incidences of ADHD in children compared to lesser exposed children (Table 1).
Table 1.
Summary of findings from the systematic review of exposures to chemical stressors related to childhood ADHD. Table was made using GRADEpro software.
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with DSM criteria | Risk with chemical exposure | |||||
| Meta-analysis details for DSM ADHD (total), hyperactivity, impulsiveness and inattention diagnoses. | ||||||
| ADHD assessed with: Pb | 369 per 1,000 | 643 per 1,000 (463 to 733) | OR 3.08 (1.47 to 4.69) | 25253 (26 observational studies) | ⨁⨁⨁◯ MODERATE a,b | Lead (Pb) exposure likely results in an increase in DSM criteria-based ADHD diagnosis. |
| ADHD assessed with: PhPl | 62 per 1,000^ | 182 per 1,000 (133 to 226) | OR 3.36 (2.32 to 4.40) | 21594 (29 observational studies) | ⨁⨁⨁◯ MODERATE a,b | Phthalate exposure likely results in an increase in DSM criteria-based ADHD diagnosis. |
| ADHD (females) assessed with: PhPl | 35 per 1,000^ | 102 per 1,000 (80 to 122) | OR 3.12 (2.39 to 3.82) | 6036 (21 observational studies) | ⨁⨁⨁◯ MODERATE a,b | Phthalate exposure likely results in an increase in DSM criteria-based ADHD diagnoses in girls. |
| ADHD (males) assessed with: PhPl | 95 per 1,000^ | 267 per 1,000 (155 to 352) | OR 3.48 (1.76 to 5.20) | 6516 (21 observational studies) | ⨁⨁⨁◯ MODERATE a,b | Phthalate exposure likely results in an increase in DSM criteria-based ADHD diagnoses in boys. |
| ADHD assessed with: OCs | 144 per 1,000 | 148 per 1,000 (132 to 163) | OR 1.03 (0.90 to 1.15) | 15898 (29 observational studies) | ⨁⨁◯◯ LOW a,b | POP exposure may result in an increase in ADHD. |
| ADHD assessed with: Cigarette Smoke | 142 per 1,000 | 309 per 1,000 (133 to 425) | OR 2.70 (0.93 to 4.47) | 23602 (19 observational studies) | ⨁⨁⨁⨁ HIGH a,b | Cigarette smoke exposure results in large increase in DSM criteria-based ADHD diagnosis. |
| Meta-analysis details for DSM ADHD (total) diagnoses. | ||||||
| ADHD assessed with: Pb | 313 per 1,000 | 635 per 1,000 (425 to 733) | OR 3.82 (1.62 to 6.02) | 17158 (56 observational studies) | ⨁⨁⨁◯ MODERATE a,b | Lead (Pb) exposure likely results in an increase in DSM criteria-based ADHD diagnosis. |
The risk in chemical exposure group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the exposure OR (and its 95% CI).
CI: Confidence interval; OR: Odds ratio
Non-significant confidence intervals in original studies
Number of participants < 1000 in original studies.
Estimate is based on included studies that provided DSM ADHD and Control information for participants, actual risk may differ.
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
The publication bias in each meta-analysis was assessed using the 1/n method described in Section 2.4. Using this method, the publication bias was not statistically significant for any of the included meta-analyses, except for the OC meta-analysis (p = 0.01, Table S1). Under the SE method, all meta-analyses were subject to publication bias, except the OC, and Cigarette Smoke meta-analyses (Table S1). Despite the greater likelihood of type 1 error, and small study effects in the SE method, the contrast between the two methods indicate that there is likely some publication bias in all meta-analyses. Unfortunately, publication bias is common in meta-analytics since negative data is often difficult to publish/not published, and the ‘grey’ literature (e.g., conference abstracts, non-peer reviewed sources) seldom presents comprehensive results that can be used for additional analyses.
3.2. Metal Exposures
3.2.1. Lead (Pb)
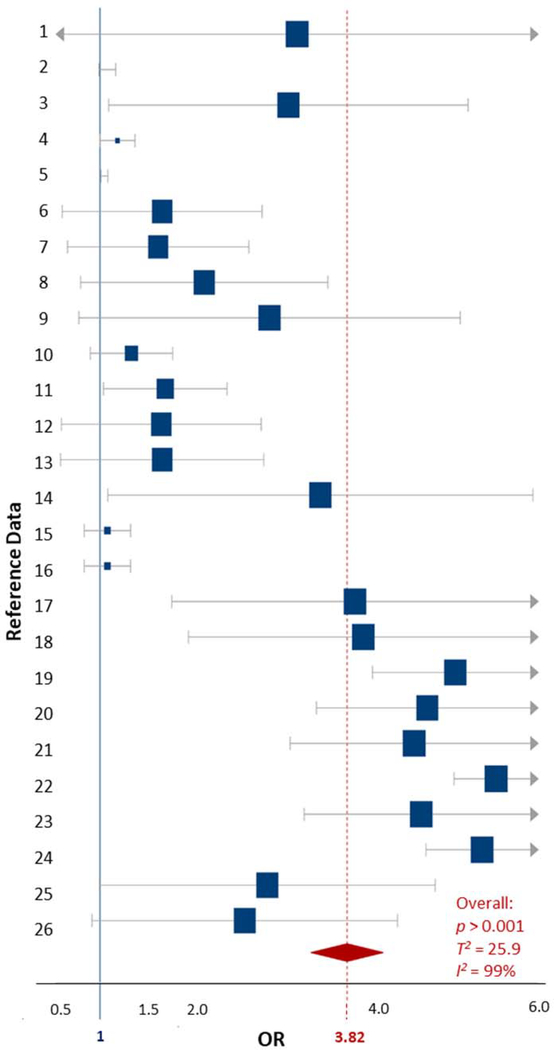
The Pb exposure data were composed of 14 studies that included a total of 56 data comparisons, with participant ages ranging from 3 to 12 years (Boucher et al., 2012; Ha et al., 2009; Huang et al., 2016; Joo et al., 2017; Kim et al., 2010; Liu et al., 2014; Nicolescu et al., 2010; Nigg et al., 2010; Park et al., 2016; Roy et al., 2009; Wang et al., 2008; Yousef et al., 2011; Zhang et al., 2015). All studies examined blood Pb concentrations (Table S2). The mental health assessments separated the diagnostic criteria by a complete ADHD diagnosis, and the Inattention and Hyperactive/Impulsive ADHD subtypes. Several studies separated Impulsive and Hyperactive into distinct categories, but all extracted subtype data were included in the overall and subtype specific meta-analyses. The heterogeneity for the overall ADHD outcomes related to Pb exposures meta-analysis was ‘considerable’ (T2 = 6.3, I2 = 96%). The overall OR for Pb exposure being associated with an ADHD (all subtypes and diagnoses) diagnosis was 3.39 (90%CI 2.66–4.12, p < 0.001) (Table 1, Fig. S1). Since there was much data available for comparison, a meta-analysis was also conducted on ADHD diagnosis separately. The total variance between the studies increased, and the heterogeneity remained ‘considerable’ (T2 = 9.3, I2 = 96%). The OR for specific ADHD diagnoses was 4.06 (2.89–5.23, p < 0.001) which was greater than when all ADHD diagnoses were combined (Table 1, Fig. 2).
Figure 2.
Forest plot showing the ADHD-specific random effects meta-analysis for childhood exposures to Pb based on concentration data. The blue squares represent the mean OR from each reference datum (Table S1), the size variation represents the weight each datum had in the meta-analysis with larger sized indicating greater weights. The horizontal bars are the upper and lower CI95%. The red diamond is the summary OR based on the meta-analysis, with the mean indicated by a red dashed line. The ‘line of no effect’ at an OR of 1 is indicated with a solid blue line. Plot was made using the DistillerSR software (Evidence Partners).
Since the heterogeneity of the meta-analysis was considerable, a meta-regression was conducted to explain the variability in the data. The variables that were consistent across all extracted data were the original OR, sample size, ADHD diagnosis, the number of participants that were diagnosed with ADHD, and those that were considered control participants (Table S1, Fig. S2). When all variables were put into a meta-regression, the significant variables were observed to be the specific ADHD diagnosis (not a symptom-specific diagnosis) and the number of participants that were diagnosed with ADHD (p values = 0.02). All other variables in the model were not statistically significant (Fig. S3). The meta-regression model accounted for 29.7% of the residual heterogeneity and some of the total variability (T2 = 4.4). However, the I2% decreased slightly in the meta-regression, but remained ‘considerable’ at 92% overall. The residual heterogeneity in the data that was not able to be accounted for (62.2%) by either of the analyses could stem from the route of exposure (i.e., dietary, drinking water, paint), as well as the concentration of Pb that the children had in their blood. Not all studies provided concentration data, so it was not possible to analyze the ADHD outcome according to Pb concentration measurements, which may have provided greater insight than the variables we were able to examine (Table S1).
The certainty of the evidence in the ADHD specific and all-symptom meta-analysis was determined to be ‘moderate’ based on the imprecision of the original studies, specifically the confidence intervals not always being significant, as well as the number of participants being >1000 in the included studies (Table S2). However, based on the included data, the large effect size observed in the original studies, and the ORs of our meta-analyses, our findings suggest that Pb exposure likely results in an increase in ADHD and its subtypes in children (Table 1).
3.2.2. Mercury (Hg) & Manganese (Mn)
The Hg and Mn data included 3 studies for each metal, comprised of 10 and 6 data comparisons, respectively. The age ranges for the participants was 5–15 years (Bouchard et al., 2007; Boucher et al., 2012; Ha et al., 2009; Oulhote et al., 2014; Yousef et al., 2011). All Hg studies use measurements observed in blood samples to determine the Hg exposure, while the Mn studies used measurements observed in either hair or blood samples (Table S2). To provide the consensus of the existing data regarding childhood Hg and Mn exposures and the relationship to ADHD outcomes, our meta-analysis combined the studies for each metal.
For Hg exposure, the heterogeneity between studies was negligible (T2 = 0, I2 = 0%). The OR for Hg exposure and all ADHD outcomes was 2.68 (2.16–3.19, p < 0.0001). The heterogeneity of the included Mn exposure studies was ‘moderate’ (T2 = 0, I2 = 49%). The OR for Mn exposure and all ADHD outcomes was 2.63 (1.27–4.00, p < 0.002). Despite the significant results of these meta-analyses, the small number of studies cannot be overlooked. Meta-analyses with few data are often inaccurate and the data are presented here to fill an existing data gap to drive future research questions but not to conclude a relationship as having been proven (Higgins et al., 2003).
3.3. Phthalate/Plasticizer Exposures (PhPl)
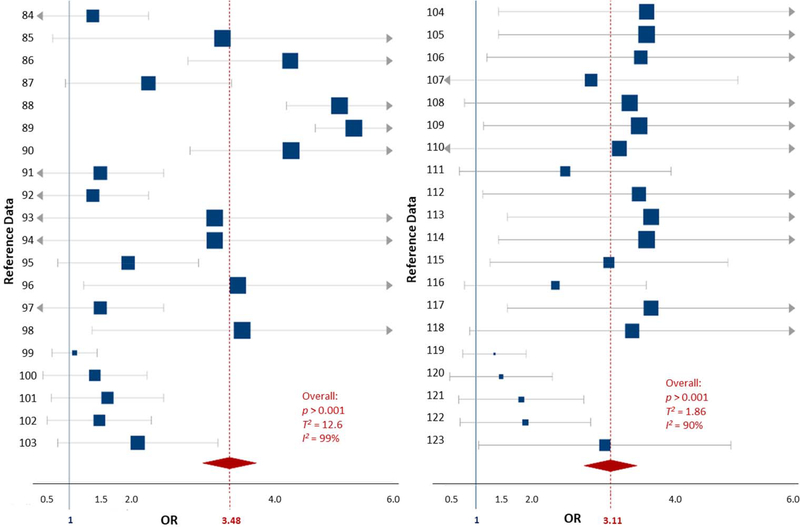
The PhPl exposure data included 5 studies comprised of 71 data comparisons collected from participants ranging in age from 6 to 18 years (Harley et al., 2013; Hu et al., 2017; Li et al., 2018; Tewar et al., 2016; Won et al., 2016). These included studies examined 10 different PhPl compounds in urine samples (Table S3). Due to the known sex-based effect differences that occur with PhPl exposures, 4 of the included studies examined differences in ADHD outcomes specific to sex, equaling 21 data comparisons for each sex, and 29 combined-sex data comparisons. The OR for an ADHD diagnosis (all subtypes) being associated with PhPl exposure in both sexes was 3.31 (2.59–4.02, p < 0.0001) (Table 1, Fig. S2). When males and females were evaluated separately, the odds ratio was 3.54 (2.23–4.86, p < 0.0001) for males and 3.12 (2.54–3.70, p < 0.0001) for females (Table 1, Fig. 3) (see Fig. 4).
Figure 3.
Forest plots showing the random effects meta-analysis of exposures to phthalate/plasticizers and ADHD outcomes for both male and female children. Plots are separated by sex: males (left) and females (right). The blue squares represent the mean OR from each reference datum (Table S2), the size variation represents the weight each datum had in the meta-analysis with larger sized indicating greater weights. The horizontal bars are the upper and lower CI95%. The red diamond is the summary OR based on the meta-analysis, with the mean indicated by a red dashed line. The ‘line of no effect’ at an OR of 1 is indicated with a solid blue line. Plot was made using the DistillerSR software (Evidence Partners).
Figure 4.
Forest plot showing the ADHD-specific fixed effects meta-analysis for childhood exposures based on OCs concentration data. The blue squares represent the mean OR from each reference datum (Table S3), the size variation represents the weight each datum had in the meta-analysis with larger sized indicating greater weights. The horizontal bars are the upper and lower CI95%. The red diamond is the summary OR based on the meta-analysis, with the mean indicated by a red dashed line. The ‘line of no effect’ at an OR of 1 is indicated with a solid blue line. Plot was made using the DistillerSR software (Evidence Partners).
The certainty of the evidence included in the PhPl meta-analysis when both sexes were combined and when the sexes were separated was ‘moderate’ (Table 1). Despite the large OR observed in the meta-analysis for the PhPl exposures of both sexes, there was variance (T2 = 6.0) and ‘considerable’ heterogeneity (I2 = 98%) in the data. The male-specific meta-analysis had increased overall variance and the same amount of heterogeneity as the combined sex meta-analysis (T2 = 10.5; I2 = 98%), while the female-specific meta-analysis had decreased overall variance and heterogeneity (T2 = 1.7, I2 = 91%).
The considerable amount of heterogeneity in the sex-specific meta-analyses provided rationale to conduct a meta-regression for each data set. For each sex-specific dataset, the available variables (i.e., specific PhPl exposure, original OR, sample size, and ADHD diagnosis) were analyzed in the meta-regressions (Table S3, Fig. S4 [males], and S5 [females]). The female-specific meta-regression yielded one significant variable, BPA exposure (p < 0.0001, T2 = 1.8, I2 = 92.8/%). The male-specific meta-regression did not have any significantly influential variables (T2 = 12.6, I2 = 98.5%). Despite the additional relationships examined for each dataset, the amount of variability and heterogeneity did not decrease, and no residual heterogeneity could be accounted for in either model.
The amount of variance and heterogeneity in these analyses is likely due to the 10 different PhPl compounds compared, as well as the inclusion of all ADHD diagnoses and subtypes. Despite these differences, the data evaluated here are valuable as there are few cohorts of PhPl data for children available. To account for these differences, the imprecision of the meta-analyses was rated ‘very serious’, and the plausible confounding factors were included to reduce the demonstrated effect in the GRADE rating. Despite the amount of heterogeneity observed, the ORs indicate a large effect of PhPl exposures on ADHD outcomes, yielding a quality score of ‘moderate’ certainty (Table 1). These findings suggest that PhPl exposures will likely result in an increase in all ADHD outcomes across both sexes, whether analyzed together or separately (Table 1).
3.4. Organic Contaminants (OCs) Exposures
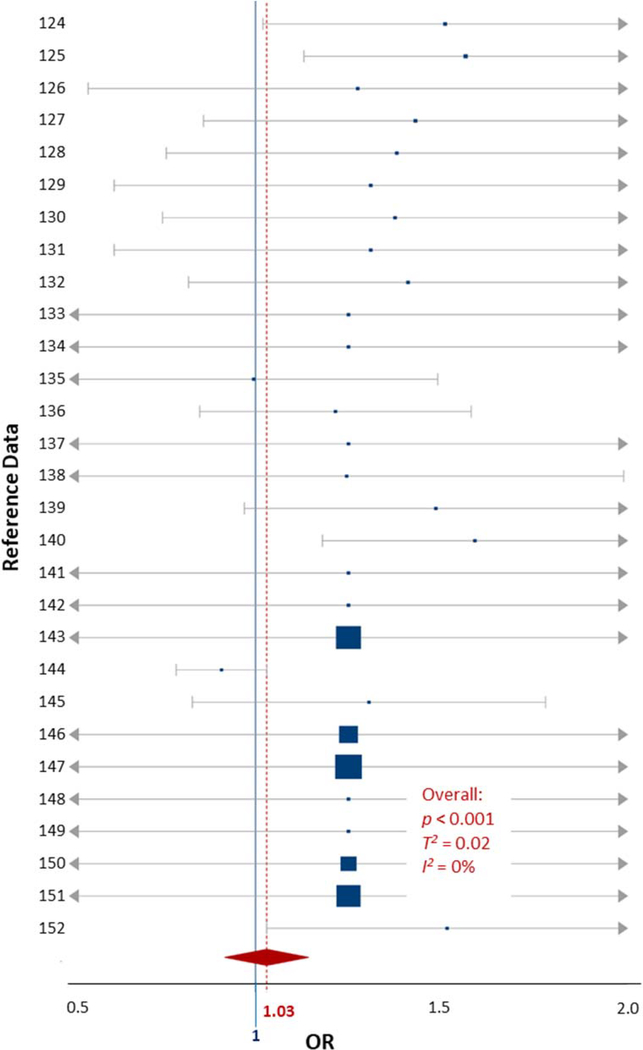
The OC exposure data included 6 studies comprised of 29 data comparisons which examined four classes of OCs (organophosphate pesticides, polychlorinated biphenyls [PCBs], pyrethroid pesticides, and trichlorophenol [TCP]) in blood and urine measurements (Table S4) (Bouchard et al., 2010a; Newman et al., 2014; Quiros-Alcala et al., 2014; Wagner-Schuman et al., 2015; Xu et al., 2011; Yu et al., 2016a). While these four classes of OCs have different toxicity profiles, and likely have various biochemical mechanisms of action, they are grouped here because the number of individual OC class studies were too few for meta-analysis, and to provide a general risk estimate for OCs as a chemical group. Participants in the included studies ranged in age from 4 to 17 years. The variance and heterogeneity between included studies was negligible (T2 = 0, I2 = 0%). The OR for OCs exposures coinciding with an ADHD outcome was 0.99 (0.96–1.02), which is approximately the line of ‘no effect’ (1.0) and does not allow a specific conclusion to be drawn. The certainty of the evidence used in this meta-analysis was considered ‘very low’ by GRADE standards (Table 1). The low sample sizes of the included studies, non-significant results, and diversity of pollutants included in the meta-analysis likely resulted in the evidence being weak compared to other analyses included in this review. However, since the variability and heterogeneity of the OC data were observed to be negligible, a meta-regression was not conducted, and we conclude that the quality of the data was not enough to warrant a comprehensive analysis. Future studies could narrow the scope of their work to a single class of OCs to draw a more precise conclusion.
3.5. Chemical Mixture Exposures-Cigarette Smoke
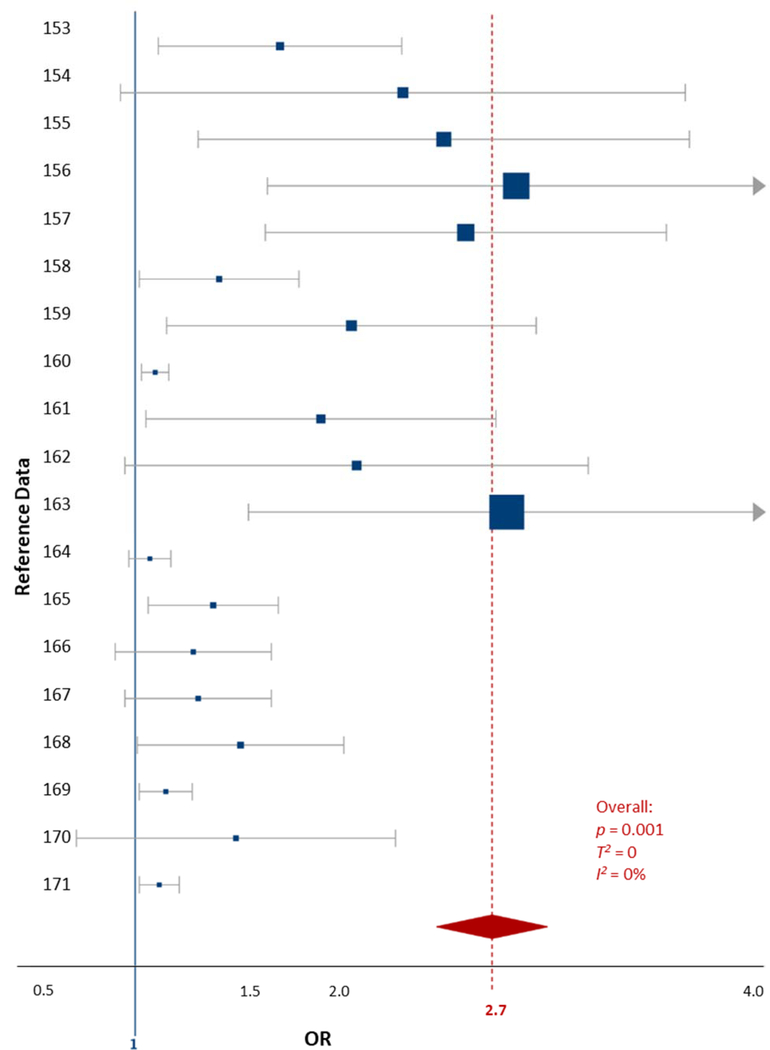
The childhood cigarette smoke exposure data were extracted from 6 studies comprised of 19 data comparisons (Cho et al., 2013; Desrosiers et al., 2013; Joo et al., 2017; Padron et al., 2016; Tiesler et al., 2011; Twardella et al., 2010). The data were collected from participants aged 4–12 years, by urine metabolite measurement (cotinine) and parental survey (Table S5). The heterogeneity between the included studies was negligible (T2 = 0, I2 = 0%). The OR for cigarette smoke exposures and all ADHD outcomes in children was 2.7 (1.22–4.18, p = 0.01, Fig. 5).
Figure 5.
Forest plot showing the ADHD-specific fixed effects meta-analysis for childhood exposures to cigarette smoke data. The blue squares represent the mean OR from each reference datum (Table S4), the size variation represents the weight each datum had in the meta-analysis with larger sized indicating greater weights. The horizontal bars are the upper and lower CI95%. The red diamond is the summary OR based on the meta-analysis, with the mean indicated by a red dashed line. The ‘line of no effect’ at an OR of 1 is indicated with a solid blue line. Plot was made using the DistillerSR software (Evidence Partners).
The certainty of the evidence used in this meta-analysis was considered ‘high’ by GRADE standards (Table 1). The statistical significance of the included data is likely the rationale for the impressive GRADE ranking. The confidence intervals crossing the line of no effect (1.0) highlights the amount of variation present even in analyses considered high quality (Fig. 5). Our analysis suggests that exposure to cigarette smoke will result in an increase in ADHD symptoms and diagnoses in children (Table 1).
3.6. Qualitative Assessment of Arsenic (As), Nonylphenol and Perfluorinated Compounds (PFCs)
The included references detailing the relationships between As, nonylphenol, and the PFCs with ADHD outcomes provided enough data for qualitative assessment. The data are valuable in understanding the relationship between ADHD outcomes in children and exposures to chemical stressors, in addition to highlighting a knowledge gap for PFCs, As, and nonylphenol exposures. The two PFC references had sample sizes > 1000 participants and measured PFCs in children’s serum samples but were not in agreement with their results (Hoffman et al., 2010; Stein et al., 2014). Stein et al. (2014) reported that there is not a significant relationship between increasing PFC exposures and ADHD outcomes in children aged 6–12 years but observed a sex-based relationship. Hoffman et al. (2010) observed a significant relationship between increasing PFC exposures and ADHD outcomes in children aged 12–15 years, but no sex-based differences were discussed. Both studies indicated that more research is needed to draw a conclusion.
The single As study included in this review observed a significant relationship between As exposure and ADHD in a cohort of 261 children aged 6–9 years (Rodriguez-Barranco et al., 2016). The relationship was observed at urinary As concentrations considered safe (Rodriguez-Barranco et al., 2016). The single included nonylphenol study examined the relationship between nonylphenol exposures and ADHD but observed no significant relationships in a group of 207 highly exposed children aged 4–15 years using blood and urine samples (Yu et al., 2016b). Both studies highlight the importance of age at exposure/effect and sample size in various cohorts.
3.7. MAOA and ADHD
Nine references investigated the relationship between MAOA characteristics and ADHD outcomes in children (Brookes et al., 2006; Das et al., 2006; Karmakar et al., 2014; Kiive et al., 2005; Kwon et al., 2014; Li et al., 2007; Liu et al., 2011; Rommelse et al., 2008; Stoff et al., 1989). However, these studies did not examine the same MAOA characteristics which made them difficult to compare. Seven studies compared functional single nucleotide polymorphisms (SNPs) along the MAOA gene sequence, but only 2 of those 7 compared the same SNPs (Brookes et al., 2006; Karmakar et al., 2014; Kwon et al., 2014; Li et al., 2007; Liu et al., 2011; Qian et al., 2009; Rommelse et al., 2008). Another study examined the variable number of tandem repeated segments (VNTRs) in the promoter region of the MAOA genomic sequence (Das et al., 2006). Two other studies compared MAOA activity in platelet samples to ADHD outcomes (Kiive et al., 2005; Stoff et al., 1989).
Despite the variety of genetic markers examined by the studies included in this review, all studies observed a significant relationship between MAOA characteristics and childhood ADHD outcomes (Brookes et al., 2006; Das et al., 2006; Karmakar et al., 2014; Kwon et al., 2014; Li et al., 2007; Liu et al., 2011; Rommelse et al., 2008). The two studies that examined MAOA activity rather than genetic sequence had conflicting results, with Stoff et al. (1989) highlighting that MAOA activity alone may not be enough to determine a child’s ADHD outcome (Kiive et al., 2005; Stoff et al., 1989). Li et al. (2007) and Liu et al. (2011) compared the same MAOA SNPs, and both observed a relationship between sex, MAOA genotype, and ADHD outcome, but concede that more research is needed before a conclusion can be made regarding those findings.
3.8. ADHD, MAOA, and Trauma
Three studies investigated the complex interrelationships between MAOA genotype, traumatic childhood experiences, and ADHD outcomes in children (Enoch et al., 2010; Li and Lee, 2012; Zohsel et al., 2015). Each study examined a different age group ranging from 6 months to 15 years of age, but all studies examined the VNTR in the promoter region of MAOA. Enoch et al. (2010) examined the relationship between MAOA VNTRs and stressful life events from 6 months–3.5 years of age and observed that the combination of a low-activity VNTR and early life stress can result in increased hyperactive behavior at 7 years of age compared to the high-activity VNTR participants. There were also gender specific differences observed, with boys having a shorter window (1.5–2.5 years) for stressful life events to influence their behavior at 7 years old, compared to the window observed in girls (6 months–3.5 year) (Enoch et al., 2010). Li and Lee (2012) examined the relationship between negative parenting, MAOA VNTR, and ADHD outcomes in boys aged 6–9 years. A relationship was observed between negative parenting and increased inattention in boys with a high-activity VNTR (Li and Lee, 2012). Zohsel et al. (2015) examined VNTRs and severe life events in adolescents aged 11–15 years. They observed low-activity VNTR carriers that experienced a severe life event during adolescence were more likely to be categorized as having attention problems and that the problems were more likely to persist through adolescence into adulthood than in their high-activity counterparts (Zohsel et al., 2015). The conflicting results of these studies demonstrate the complexity of the MAOA VNTR relationship with trauma and behavior. Traumatic life events appear to influence the low-activity VNTR, while continual negative experiences (i.e., negative parenting) appear to influence the high-activity VNTR. However, more research that examines the interaction between trauma, behavior, and MAOA VNTR is needed to draw a conclusion.
4. Discussion
The data evaluated in this review highlight the relationships between chemical and non-chemical stressors, genetics, and children’s ADHD diagnoses (Fig. 6). The results from the chemical stressor meta-analyses support the idea that exposures to chemical stressors are related to mental health outcomes in children (Fig. 6, solid blue arrow). The qualitative analysis of the MAOA characteristic data highlights the body of work regarding the relationship between MAOA and chemical stressors, as well as the relationship between MAOA and childhood ADHD outcomes (Fig. 6, dashed black arrows). The qualitative examination of the three studies describing the interrelationships between MAOA characteristics, childhood traumatic experiences, and ADHD outcomes elucidates the relationship between non-chemical stressors and MAOA, as well as the relationship between exposure to non-chemical stressors and childhood ADHD outcomes (Fig. 6, dashed black arrows). The data evaluated in this review do not describe the relationship between chemical and non-chemical stressors, but highlight the complex interactions surrounding this relationship that are discussed in the wider body of literature on this topic (Fig. 6, dotted red line). The specific relationships elucidated by each type of data are discussed below.
Figure 6.
Visual representation depicting the interrelationships between chemical and non-chemical stressors, genetic characteristics, and the collective influence on children’s ADHD outcomes.
4.1. Chemical Stressor Data
The chemical stressors data examined in this review support a relationship between exposure to chemical stressors and ADHD outcomes in children (Table 1, Fig. 6). Despite the variations in the data included in each meta-analysis, the number of primary studies and significance of the primary relationships resulted in statistically significant meta-analyses (Figs. 2–5). The significant relationships observed in this review are likely due to a distinct biochemical mechanism linking environmental chemicals to the MAOA pathway (Aziz and Knowles, 1973; Fowler et al., 1996; Kalgutkar et al., 2001; Nakamura et al., 2010; Neal and Guilarte, 2010). Exposure to environmental chemicals, including naphthylamine, nicotine (found in cigarette smoke), bisphenol A (a plasticizer), organophosphate pesticides (an OC class), and Pb (a metal), has been linked to the diagnosis of ADHD and related symptoms, MAOA genotype, and MAOA enzyme activity (Fig. 6, black dashed arrows) (Aziz and Knowles, 1973; Evans et al., 2014; Hauptmann and Shih, 2001; Roen et al., 2015; Verity, 1990).
The Pb exposure data evaluated, in tandem with the significant relationship we observed in the Hg and Mn exposure data, suggest that there may be a common mechanism of action related to ADHD for metals. Several metals, including Pb, Hg, Mn, and As, have been shown to interfere with the calcium channels involved with neurotransmitter regulation and the N-methyl-D-aspartate receptor (NMDAR), which is an upstream regulator of MAOA in the serotonin pathway (Bortolato et al., 2012; Büsselberg, 1995; Neal and Guilarte, 2010). Transgenic mouse models that have MAOA silenced are hypersensitive to NMDAR agonists (Bortolato et al., 2012). While Pb is not an agonist for NMDAR, Pb does inhibit and interfere with the normal function of NMDAR by disrupting the upstream calcium channels required for function (Büsselberg, 1995; Neal and Guilarte, 2010). Interestingly, the NMDAR that is inhibited by Pb’s interaction with the calcium channels is involved in the biochemical pathway implicated in Post-Traumatic Stress Disorder (PTSD) (Steckler and Risbrough, 2012). The interconnection of the two diagnoses biochemically may account for the relationship observed between trauma and ADHD outcomes, as well as the similarity of some symptoms of ADHD and PTSD (Biederman et al., 2013; Büsselberg, 1995; Neal and Guilarte, 2010; Steckler and Risbrough, 2012). Future studies could investigate the effects of the MAOA low and high activity VNTRs under metal exposure to determine how the calcium channels and NMDAR are related to the onset of ADHD in children.
The data gap observed for childhood As, PFCs, and nonylphenol exposures in relation to ADHD could be due to an indirect biochemical mechanism relating exposure to the symptoms of ADHD, such that primary studies are not focused on those relationships, or that children’s exposures to these chemicals are low. For PFCs, we posit that many studies have not yet been published since PFCs are considered ‘emerging’, ‘of concern’, and ubiquitous in the environment. Whereas, the lack of data for As and nonylphenol may be due to limited exposure during childhood and/or limited knowledge of the exposures that children may experience.
The chemical exposure data evaluated and the examined biochemical modes of action in relation to ADHD outcomes reflect relationships between chemical stressors in the total (built, natural, and social) environment and children’s health, as well as policy and decision-making services outlined in the conceptual framework depicting children’s health and well-being (Tulve et al., 2016). All chemicals examined in this review are known to have toxic effects at high concentrations and have exposure limits to prevent toxicity set by the U.S. Center for Disease Control and Prevention’s Agency for Toxic Substances and Disease Registry (U.S. CDC). The specific toxicity details are listed in the U.S. Environmental Protection Agency’s Integrated Risk Information System (U.S. EPA). The link between the toxicity limits and children’s mental health outcomes were made clear through the conceptual children’s health framework (Tulve et al., 2016). When future toxicity limits are enacted considering epidemiological data such as those presented here could provide an additional perspective to protect children’s health.
4.2. Inherent Characteristics Data
The MAOA data examined suggest that when the MAOA gene is exposed to environmental chemicals, the activity of the MAOA enzyme can be altered, and ADHD outcomes are more likely (Fig. 6, black dashed arrows). The studies described in section 3.7 highlight the variety of loci along the MAOA gene that have been shown to be related to ADHD and may be influenced by exposure to chemical stressors, while additional loci may be identified in the future. The significant relationship observed in the chemical exposure meta-analyses suggests that there may be many genetic loci that influence ADHD outcomes and are sensitive to environmental chemicals. Section 3.8 describes the interaction of non-chemical stressors with MAOA genotypes, and their combined relationship with ADHD outcomes in children. Future work could examine the cohorts from the included studies for MAOA gene characteristics and enzyme activity to determine if there is a relationship in the existing data.
There were sex-based differences identified in two of the MAOA studies (Li et al., 2007; Liu et al., 2011). The MAOA enzyme is encoded from the X-linked MAOA gene, which makes genotyping females complicated because females have two X chromosomes and have the potential to be heterozygous with two different copies of MAOA. Determining the genotype of heterozygous females is complicated because only one of the two copies of MAOA is active due to X-linked inactivation of one copy by DNA methylation (Plath et al., 2002; Wong et al., 2010). If the DNA methylation pattern is not accounted for when genotyping females, the active copy may not be identified which may explain the sex-specific differences observed (Chen et al., 2013; Kuepper et al., 2013). Future studies examining MAOA in females could include DNA methylation measures to ensure that the active copy of MAOA is analyzed. Several studies linked DNA methylation changes along the MAOA enzyme to chemical exposures, and a few studies have demonstrated that trauma affects MAOA epigenetically and that epigenetic changes can be passed to children trans-generationally (Bottiglieri et al., 2000; Domschke et al., 2012; Gillett and Tamatea, 2012; Kellermann, 2013; Melas et al., 2013; Shumay et al., 2012; Thayer and Kuzawa, 2011; Youssef et al., 2018; Ziegler et al., 2016). The epigenetic changes that result from chemical stressor and traumatic experience exposures add an additional layer to what is already a set of complex interrelationships.
The genetic data examined in this review are related to childhood ADHD outcomes, as well as bidirectionally influenced by exposure to chemical stressors and traumatic experiences. The conceptual framework put forth by Tulve et al. (2016) aided in linking the exposures and genetic data through the childhood biological timepoints (toddler through adolescence) to prenatal and preconception timepoints. The exposure data evaluated in this review represents a snapshot in time, but the exposures to chemical and non-chemical stressors children experience are not singular. The genetic data persists throughout the entire life course and can be modified epigenetically but cannot ultimately be changed (Shumay et al., 2012). Future studies examining the epigenetics of MAOA could investigate various biological timepoints to determine epigenetic plasticity throughout the life course, since epigenetic effects are not always permanent, to better understand children’s susceptibility to exposures, as well as the persistence of the epigenetic effects related to their exposures to chemical and non-chemical stressors.
4.3. Non-chemical Stressor Data
4.3.1. Early Childhood Traumatic Experiences
The non-chemical stressor data reported in section 3.8 indicate that under negative and stressful experiences certain MAOA VNTRs are more prone to ADHD outcomes than others. Stressful and severe experiences during childhood affected the low-activity VNTRs leading to ADHD hyperactive symptom prevalence, while persistent negative experiences appeared to influence the high-activity VNTRs and the inattentive symptoms of ADHD (Enoch et al., 2010; Li and Lee, 2012; Zohsel et al., 2015). These studies illustrate that the relationship between the MAOA VNTR and traumatic experiences is complicated in children, and that both chronic and acute trauma can result in ADHD symptoms and behavioral outcomes (Fig. 6, black dashed arrows). There is not a consensus in the literature between the low and high activity MAOA VNTRs and the behavioral effects of childhood trauma, but these data support the conclusion drawn by Nikolas and Burt (2010), that future studies should disambiguate the inattentive and hyperactive ADHD diagnoses when examining the genetic influences of ADHD to achieve a more precise conclusion that can fuel future research (Guo et al., 2008; Hinds et al., 1992; Kuepper et al., 2013; Voltas et al., 2015).
The Adverse Childhood Experiences (ACE) study retrospectively examined adult illness to adverse experiences during childhood. The ACE study observed that a variety of illnesses, including mental health outcomes, could be attributed to adverse childhood experiences (Anda et al., 2002; Brown et al., 2007; Chapman et al., 2004; Edwards et al., 2003; Felitti et al., 1998). Greater amounts of adverse experiences have been linked to greater incidences of ADHD in children, especially when related to parental involvement and/or family adversities (Biederman et al., 2002; Rogers et al., 2009). When childhood traumatic experiences are combined with the low activity MAOA variant, the mental health outcomes reported in the literature are more extreme (Brunner et al., 1993; Caspi et al., 2002; Ducci et al., 2008; Ehlers and Gizer, 2013; Falk, 2014; McDermott et al., 2009, 2013; Stetler et al., 2014; Weder et al., 2009; Widom and Brzustowicz, 2006). Future studies examining both MAOA and traumatic childhood experiences could incorporate the ACE study framework to ascertain an approximate likelihood of a future mental health diagnosis related to traumatic experiences as well as a current ADHD evaluation.
4.4. Multiple stressors influence mental health
This review supports the idea that multiple stressors as well as the interaction between multiple stressors can influence ADHD in children. Going forward, the interconnections between stressors found in children’s total environment should be considered in mental health evaluations. The principles for addressing the grand challenges facing mental health professionals today specifically include using life-course and system-wide approaches, evidence-based interventions, and understanding environmental influences (Collins et al., 2011). The conceptual framework developed by Tulve et al. (2016) highlights the overlapping factors children experience daily and provides a tool to aid in the understanding of multiple stressor research. In our review, the conceptual framework provided a visual representation of the interrelationships between the chemical and non-chemical stressors we evaluated in relation to children’s ADHD outcomes. Future studies can use the framework as a guide for greater understanding of the interrelationships of multiple stressors on a children’s health outcomes.
4.4. Limitations
The limitations of this work are like those that plague most meta-analyses – the low number of comparable studies, the heterogeneity between the included studies, and publication bias. Based on the inclusion criteria and our goal of examining existing data that described the interrelationships between exposures, genetics, and ADHD outcomes, there were few studies that qualified. The heterogeneity between studies stemmed mostly from the varied cohorts examined and the different goals of the original researchers. Despite these limitations, we believe our results are a valuable contribution to the study of childhood ADHD outcomes and should be used to drive future research in this area. As the importance of negative data becomes a more prolific ideology, publication bias may decrease as more negative results are published.
5. Conclusions
Our review highlights the interrelationships between exposures to chemicals in childhood, genetic predisposition via MAOA, and the influence of non-chemical stressors on childhood ADHD outcomes (Fig. 6). The chemical exposure meta-analyses suggest that exposure is a significant influence on ADHD outcomes in children with increased exposures to chemical stressors (metals, Ph/Pl, cigarette smoke; Table 1; Fig. 6, solid blue arrow). The data also suggests that there is a relationship between MAOA, exposures to chemical stressors, and ADHD outcomes, but the gene loci examined were too diverse to statistically interpret (Fig. 6, dashed black arrows). The effect of traumatic childhood experiences cannot be overlooked as the studies included in this review as well as many others indicate a significant relationship between childhood stress and adverse mental health outcomes throughout the life course (Fig. 6, dashed black arrows).
The combination of these 3 risk factors (MAOA VNTR, trauma experienced, and exposures to chemical stressors) appears to increase the probability of an ADHD diagnosis in children. However, the severity and duration of traumatic experiences and exposures to chemical stressors will likely influence neurological processes as well as the subsequent ADHD outcome and should be accounted for in understanding mental health. Individual variations in MAOA genotype, childhood traumatic experiences, and exposures to various concentrations of chemicals suggest that a mental health outcome related to these risk factors is a challenge of resilience for children exposed to these factors. The understanding of childhood trauma is continually increasing, and the effects of trauma can be affected by MAOA VNTR, exposures to chemical stressors, and the quality of the parental relationships each child experiences. Caregiver support in reaction to trauma is an incredibly effective mediator in a child’s long-term response to a traumatic situation as well as the type, frequency, and duration of the traumatic experience (Cook et al., 2017; Van der Kolk et al., 2005; Walsh, 2002; Whitson et al., 2015).
This review collected and evaluated the existing literature regarding the interrelationships between exposures to chemical stressors, MAOA characteristics and activity, and traumatic experiences on ADHD outcomes and highlighted areas where more specific research is needed to further elucidate the complex interrelationships observed. We suggest:
Examining the relationship between chemical and non-chemical stressors in all capacities
Including the ACE framework in studies that examine both MAOA and traumatic childhood experiences to ascertain the likelihood of a future mental health diagnosis related to traumatic experiences as well as a current ADHD evaluation.
Future studies examining the epigenetics of MAOA could investigate various biological timepoints to determine epigenetic plasticity throughout the life course, since epigenetic effects are not always permanent, to better understand children’s susceptibility to exposures.
Investigate the effects of the MAOA low and high activity VNTRs under metal exposure to determine how the calcium channels and NMDAR are related to the onset of ADHD in children.
Supplementary Material
Acknowledgments
We acknowledge and thank Drs. William Boyes and Kim Rogers for their insightful reviews of this manuscript; as well as the scientists that provided additional unpublished data from their studies for inclusion in this review. This project was supported by an appointment to the Internship/Research Participation Program at the U.S. Environmental Protection Agency (EPA), Office of Research and Development, National Exposure Research Laboratory, administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy and EPA. The views expressed in this manuscript are those of the author(s) and do not necessarily represent the views or policies of the EPA. It has been subjected to Agency administrative review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Funding
This project was supported by an appointment to the Internship/Research Participation Program at the U.S. Environmental Protection Agency (EPA), Office of Research and Development, National Exposure Research Laboratory, administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy and EPA.
Footnotes
Declaration of competing interest
The authors declare no conflict of interest.
Human subject research
The data provided in this manuscript were extracted from existing peer-reviewed literature and were de-identified prior to their original publication. We did not have access to the Personally Identifying Information (PII), and the primary authors of the included studies gained all required approvals prior to original publication.
Appendix A. Supplementary data
References
- Anda RF, et al. , 2002. Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatr Serv. 53, 1001–9. [DOI] [PubMed] [Google Scholar]
- Aziz SA, Knowles CO, 1973. Inhibition of monoamine oxidase by the pesticide chlordimeform and related compounds. Nature. 242, 417–418. [DOI] [PubMed] [Google Scholar]
- Biederman J, et al. , 2002. Differential effect of environmental adversity by gender: Rutter’s index of adversity in a group of boys and girls with and without ADHD. American journal of psychiatry. 159, 1556–1562. [DOI] [PubMed] [Google Scholar]
- Biederman J, et al. , 2013. Examining the nature of the comorbidity between pediatric attention deficit/hyperactivity disorder and post-traumatic stress disorder. Acta psychiatrica Scandinavica. 128, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, et al. , 2007. Meta-analysis: fixed effect vs random effects. www.meta-analysis.com. Access date. 16, 2012.
- Borenstein M, et al. , 2011. Introduction to meta-analysis. John Wiley & Sons. [Google Scholar]
- Bortolato M, et al. , 2012. NMDARs mediate the role of monoamine oxidase A in pathological aggression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 32, 8574–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M, et al. , 2007. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 115, 122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, et al. , 2010a. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 125, e1270–e1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, et al. , 2010b. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 125, e1270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, et al. , 2012. Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Quebec. Environ Health Perspect. 120, 1456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. , 2006. Exposures to environmental toxicants and attention deficit hyperactivity disorder in US children. Environmental health perspectives. 114, 1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes K, et al. , 2006. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 11, 934–53. [DOI] [PubMed] [Google Scholar]
- Brown DW, et al. , 2007. Adverse childhood experiences and childhood autobiographical memory disturbance. Child Abuse Negl. 31, 961–9. [DOI] [PubMed] [Google Scholar]
- Brunner HG, et al. , 1993. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 262, 578–580. [DOI] [PubMed] [Google Scholar]
- Büsselberg D, 1995. Calcium channels as target sites of heavy metals. Toxicology letters. 82, 255–261. [DOI] [PubMed] [Google Scholar]
- Caspi A, et al. , 2002. Role of genotype in the cycle of violence in maltreated children. Science. 297, 851–854. [DOI] [PubMed] [Google Scholar]
- Chapman DP, et al. , 2004. Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of affective disorders. 82, 217–225. [DOI] [PubMed] [Google Scholar]
- Chen H, et al. , 2013. The MAOA gene predicts happiness in women. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 40, 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SC, et al. , 2013. Association between urine cotinine levels, continuous performance test variables, and attention deficit hyperactivity disorder and learning disability symptoms in school-aged children. Psychological Medicine. 43, 209–219. [DOI] [PubMed] [Google Scholar]
- Collins PY, et al. , 2011. Grand challenges in global mental health. Nature. 475, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor DF, Doerfler LA, 2008. ADHD with comorbid oppositional defiant disorder or conduct disorder: discrete or nondistinct disruptive behavior disorders? Journal of Attention Disorders. 12, 126–134. [DOI] [PubMed] [Google Scholar]
- Cook A, et al. , 2017. Complex trauma in children and adolescents. Psychiatric annals. 35, 390–398. [Google Scholar]
- Danielson ML, et al. , 2018. Prevalence of parent-reported ADHD diagnosis and associated treatment among US children and adolescents, 2016. Journal of Clinical Child & Adolescent Psychology. 47, 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, et al. , 2006. MAOA promoter polymorphism and attention deficit hyperactivity disorder (ADHD) in indian children. Am J Med Genet B Neuropsychiatr Genet. 141b, 637–42. [DOI] [PubMed] [Google Scholar]
- Daston G, et al. , 2004. A framework for assessing risks to children from exposure to environmental agents. Environmental health perspectives. 112, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers C, et al. , 2013. Associations between prenatal cigarette smoke exposure and externalized behaviors at school age among Inuit children exposed to environmental contaminants. Neurotoxicology and Teratology. 39, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, et al. , 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine reviews. 30, 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, et al. , 2008. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Molecular psychiatry. 13, 334–347. [DOI] [PubMed] [Google Scholar]
- Edwards VJ, et al. , 2003. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. American Journal of Psychiatry. 160, 1453–1460. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, 2013. Evidence for a genetic component for substance dependence in Native Americans. American Journal of Psychiatry. 170, 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, et al. , 2010. Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Genes Brain and Behavior. 9, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SF, et al. , 2014. Prenatal bisphenol A exposure and maternally reported behavior in boys and girls. Neurotoxicology. 45, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk AE, 2014. Gene-Environment Interplay for Childhood and Adolescent Antisocial Behavior. University of California, Los Angeles. [Google Scholar]
- Felitti VJ, et al. , 1998. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American journal of preventive medicine. 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Fowler JS, et al. , 1996. Brain monoamine oxidase A inhibition in cigarette smokers. Proceedings of the National Academy of Sciences. 93, 14065–14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Sinha M, 2012. ADHD, ODD, and CD: Do They Belong to a Common Psychopathological Spectrum? A Case Series. Case reports in psychiatry. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlad JK, et al. , 2013. Lead and attention-deficit/hyperactivity disorder (ADHD) symptoms: A meta-analysis. Clinical Psychology Review. 33, 417–425. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, 2003. Role of environmental stress in the physiological response to chemical toxicants. Environmental research. 92, 1–7. [DOI] [PubMed] [Google Scholar]
- Guo G, et al. , 2008. The VNTR 2 repeat in MAOA and delinquent behavior in adolescence and young adulthood: associations and MAOA promoter activity. European Journal of Human Genetics. 16, 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M, et al. , 2009. Low blood levels of lead and mercury and symptoms of attention deficit hyperactivity in children: a report of the children’s health and environment research (CHEER). Neurotoxicology. 30, 31–6. [DOI] [PubMed] [Google Scholar]
- Harley KG, et al. , 2013. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res. 126, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann N, Shih JC, 2001. 2-Naphthylamine, a compound found in cigarette smoke, decreases both monoamine oxidase A and B catalytic activity. Life sciences. 68, 1231–1241. [DOI] [PubMed] [Google Scholar]
- Heilbrun LP, et al. , 2015. Maternal chemical and drug intolerances: potential risk factors for autism and attention deficit hyperactivity disorder (ADHD). The Journal of the American Board of Family Medicine. 28, 461–470. [DOI] [PubMed] [Google Scholar]
- Heudorf U, et al. , 2007. Phthalates: toxicology and exposure. International journal of hygiene and environmental health. 210, 623–634. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, et al. , 2003. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.). 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds HL, et al. , 1992. Characterization of a highly polymorphic region near the first exon of the human MAOA gene containing a GT dinucleotide and a novel VNTR motif. Genomics. 13, 896–897. [DOI] [PubMed] [Google Scholar]
- Hoffman K, et al. , 2010a. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12–15 years of age. Environ Health Perspect. 118, 1762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, et al. , 2010b. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in US children 12–15 years of age. Environmental health perspectives. 118, 1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, et al. , 2017. Associations of phthalates exposure with attention deficits hyperactivity disorder: A case-control study among Chinese children. Environ Pollut. 229, 375–385. [DOI] [PubMed] [Google Scholar]
- Huang S, et al. , 2016. Childhood Blood Lead Levels and Symptoms of Attention Deficit Hyperactivity Disorder (ADHD): A Cross-Sectional Study of Mexican Children. Environ Health Perspect. 124, 868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PS, et al. , 2001. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. Journal of the American Academy of Child & Adolescent Psychiatry. 40, 147–158. [DOI] [PubMed] [Google Scholar]
- Joo H, et al. , 2017. Secondhand Smoke Exposure and Low Blood Lead Levels in Association With Attention-Deficit Hyperactivity Disorder and Its Symptom Domain in Children: A Community-Based Case-Control Study. Nicotine & Tobacco Research. 19, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalgutkar AS, et al. , 2001. Interactions of nitrogen-containing xenobiotics with monoamine oxidase (MAO) isozymes A and B: SAR studies on MAO substrates and inhibitors. Chemical research in toxicology. 14, 1139–1162. [DOI] [PubMed] [Google Scholar]
- Karmakar A, et al. , 2014. Potential Contribution of Monoamine Oxidase A Gene Variants in ADHD and Behavioral Co-Morbidities: Scenario in Eastern Indian Probands. Neurochemical Research. 39, 843–852. [DOI] [PubMed] [Google Scholar]
- Kessler RC, et al. , 2009. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiologia e psichiatria sociale. 18, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, et al. , 2010. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British Journal of Psychiatry. 197, 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiive E, et al. , 2005. Changes in platelet monoamine oxidase activity, cholesterol levels and hyperactive behaviour in adolescents over a period of three years. Neurosci Lett. 384, 310–5. [DOI] [PubMed] [Google Scholar]
- Kim Y, et al. , 2010. Association between blood lead levels (<5 mug/dL) and inattention-hyperactivity and neurocognitive profiles in school-aged Korean children. Sci Total Environ. 408, 5737–43. [DOI] [PubMed] [Google Scholar]
- Koch HM, et al. , 2004. Internal exposure of nursery-school children and their parents and teachers to di (2-ethylhexyl) phthalate (DEHP). International Journal of Hygiene and Environmental Health. 207, 15–22. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, et al. , 2001. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American journal of psychiatry. 158, 848–856. [DOI] [PubMed] [Google Scholar]
- Kuepper Y, et al. , 2013. MAOA-uVNTR genotype predicts interindividual differences in experimental aggressiveness as a function of the degree of provocation. Behavioural brain research. 247, 73–78. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, et al. , 2014. Association between monoamine oxidase gene polymorphisms and attention deficit hyperactivity disorder in Korean children. Genet Test Mol Biomarkers. 18, 505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, et al. , 2017. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environmental health perspectives. 125, 086001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. , 2007. Monoamine oxidase A gene polymorphism predicts adolescent outcome of attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 144b, 430–3. [DOI] [PubMed] [Google Scholar]
- Li JJ, Lee SS, 2012. Association of positive and negative parenting behavior with childhood ADHD: interactions with offspring monoamine oxidase A (MAO-A) genotype. J Abnorm Child Psychol. 40, 165–75. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. , 2018. Relationship between bisphenol A exposure and attention-deficit/ hyperactivity disorder: A case-control study for primary school children in Guangzhou, China. Environ Pollut. 235, 141–149. [DOI] [PubMed] [Google Scholar]
- Liu JH, et al. , 2014. Blood Lead Concentrations and Children’s Behavioral and Emotional Problems A Cohort Study. Jama Pediatrics. 168, 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, et al. , 2011. Association analyses of MAOA in Chinese Han subjects with attention-deficit/hyperactivity disorder: family-based association test, case-control study, and quantitative traits of impulsivity. Am J Med Genet B Neuropsychiatr Genet. 156b, 737–48. [DOI] [PubMed] [Google Scholar]
- Llobet J, et al. , 2003. Concentrations of arsenic, cadmium, mercury, and lead in common foods and estimated daily intake by children, adolescents, adults, and seniors of Catalonia, Spain. Journal of Agricultural and Food Chemistry. 51, 838–842. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Denckla MB, 2017. Attention-Deficit/Hyperactivity Disorder: A Historical Neuropsychological Perspective. Journal of the International Neuropsychological Society : JINS. 23, 916–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott R, et al. , 2013. MAOA and aggression: A gene–environment interaction in two populations. Journal of Conflict Resolution. 57, 1043–1064. [Google Scholar]
- McDermott R, et al. , 2009. Monoamine oxidase A gene (MAOA) predicts behavioral aggression following provocation. Proceedings of the National Academy of Sciences. 106, 2118–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, et al. , 2010. Prenatal and lactational exposure to low-doses of bisphenol A alters brain monoamine concentration in adult mice. Neuroscience letters. 484, 66–70. [DOI] [PubMed] [Google Scholar]
- Neal AP, Guilarte TR, 2010. Molecular neurobiology of lead (Pb 2+): effects on synaptic function. Molecular neurobiology. 42, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J, et al. , 2014. PCBs and ADHD in Mohawk adolescents. Neurotoxicol Teratol. 42, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolescu R, et al. , 2010. Environmental exposure to lead, but not other neurotoxic metals, relates to core elements of ADHD in Romanian children: performance and questionnaire data. Environ Res. 110, 476–83. [DOI] [PubMed] [Google Scholar]
- Nigg JT, et al. , 2010. Confirmation and extension of association of blood lead with attention-deficit/hyperactivity disorder (ADHD) and ADHD symptom domains at population-typical exposure levels. J Child Psychol Psychiatry. 51, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolas MA, Burt SA, 2010. Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: a meta-analysis. Journal of abnormal psychology. 119, 1. [DOI] [PubMed] [Google Scholar]
- Oades RD, et al. , 2008. The influence of serotonin-and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): Findings from a family-based association test (FBAT) analysis. Behavioral and Brain Functions. 4, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreland L, Shaskan EG, 1983. Monoamine oxidase activity as a biological marker. Trends in Pharmacological Sciences. 4, 339–341. [Google Scholar]
- Oulhote Y, et al. , 2014. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ Health Perspect. 122, 1343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padron A, et al. , 2016. Exposure to secondhand smoke in the home and mental health in children: a population-based study. Tob Control. 25, 307–12. [DOI] [PubMed] [Google Scholar]
- Park JH, et al. , 2016. Blood lead concentrations and attention deficit hyperactivity disorder in Korean children: a hospital-based case control study. Bmc Pediatrics. 16, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor PN, et al. , 2015. Quickstats: Percentage of children and adolescents aged 5–17 years with diagnosed attention-deficit/hyperactivity disorder (ADHD), by race and hispanic ethnicity—National health interview survey, united states, 1997–2014. MMWR Morb. Mortal. Wkly. Rep. 64, 925. [Google Scholar]
- Perou R, et al. , 2013. Mental health surveillance among children—United States, 2005–2011. MMWR Surveill Summ. 62, 1–35. [PubMed] [Google Scholar]
- Plath K, et al. , 2002. Xist RNA and the mechanism of X chromosome inactivation. Annual review of genetics. 36, 233–278. [DOI] [PubMed] [Google Scholar]
- Polańska K, et al. , 2012. Exposure to environmental and lifestyle factors and attention-deficit / hyperactivity disorder in children — A review of epidemiological studies. International Journal of Occupational Medicine and Environmental Health. 25, 330–355. [DOI] [PubMed] [Google Scholar]
- Qian QJ, et al. , 2009. Attention Deficit Hyperactivity Disorder comorbid oppositional defiant disorder and its predominately inattentive type: evidence for an association with COMT but not MAOA in a Chinese sample. Behavioral and Brain Functions. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros-Alcala L, et al. , 2014. Pyrethroid pesticide exposure and parental report of learning disability and attention deficit/hyperactivity disorder in U.S. children: NHANES 1999–2002. Environ Health Perspect. 122, 1336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski JL, et al. , 1971. Blood levels of organochlorine pesticides in Argentina: occupationally and nonoccupationally exposed adults, children and newborn infants. Toxicology and applied pharmacology. 20, 186–193. [DOI] [PubMed] [Google Scholar]
- Rider CV, et al. , 2012. Incorporating nonchemical stressors into cumulative risk assessments. Toxicological Sciences. 127, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Barranco M, et al. , 2016. Postnatal arsenic exposure and attention impairment in school children. Cortex. 74, 370–82. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Barranco M, et al. , 2013. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Science of the total environment. 454, 562–577. [DOI] [PubMed] [Google Scholar]
- Roen EL, et al. , 2015. Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age. Environmental Research. 142, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, et al. , 2009. Parental involvement in children’s learning: Comparing parents of children with and without Attention-Deficit/Hyperactivity Disorder (ADHD). Journal of school psychology. 47, 167–185. [DOI] [PubMed] [Google Scholar]
- Rommelse NN, et al. , 2008. Differential association between MAOA, ADHD and neuropsychological functioning in boys and girls. Am J Med Genet B Neuropsychiatr Genet. 147b, 1524–30. [DOI] [PubMed] [Google Scholar]
- Roy A, et al. , 2009. Lead exposure and behavior among young children in Chennai, India. Environ Health Perspect. 117, 1607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JC, et al. , 1999. Role of MAO A and B in neurotransmitter metabolism and behavior. Polish journal of pharmacology. 51, 25–29. [PubMed] [Google Scholar]
- Shumay E, et al. , 2012. Evidence that the methylation state of the monoamine oxidase A (MAOA) gene predicts brain activity of MAO A enzyme in healthy men. Epigenetics. 7, 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Risbrough V, 2012. Pharmacological treatment of PTSD–established and new approaches. Neuropharmacology. 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, et al. , 2014. Perfluorooctanoate exposure in a highly exposed community and parent and teacher reports of behaviour in 6–12-year-old children. Paediatr Perinat Epidemiol. 28, 146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler DA, et al. , 2014. Association of low-activity MAOA allelic variants with violent crime in incarcerated offenders. Journal of psychiatric research. 58, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoff DM, et al. , 1989. Elevated platelet MAO is related to impulsivity in disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 28, 754–60. [DOI] [PubMed] [Google Scholar]
- Tewar S, et al. , 2016. Association of Bisphenol A exposure and Attention-Deficit/Hyperactivity Disorder in a national sample of U.S. children. Environ Res. 150, 112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, et al. , 2015. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 135, e994–e1001. [DOI] [PubMed] [Google Scholar]
- Tiesler CMT, et al. , 2011. Passive smoking and behavioural problems in children: Results from the LISAplus prospective birth cohort study. Environmental Research. 111, 1173–1179. [DOI] [PubMed] [Google Scholar]
- Tulve N, et al. , 2016. Development of a Conceptual Framework Depicting a Childs Total (Built, Natural, Social) Environment in Order to Optimize Health and Well-Being. Ommega Internationals. 2, 1–8. [Google Scholar]
- Twardella D, et al. , 2010. Exposure to secondhand tobacco smoke and child behaviour - results from a cross-sectional study among preschool children in Bavaria. Acta Paediatrica. 99, 106–111. [DOI] [PubMed] [Google Scholar]
- Van der Kolk BA, et al. , 2005. Disorders of extreme stress: The empirical foundation of a complex adaptation to trauma. Journal of Traumatic Stress: Official Publication of The International Society for Traumatic Stress Studies. 18, 389–399. [DOI] [PubMed] [Google Scholar]
- Verity MA, 1990. COMPARATIVE OBSERVATIONS ON INORGANIC AND ORGANIC LEAD NEUROTOXICITY. Environmental Health Perspectives. 89, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voltas N, et al. , 2015. Association study of monoamine oxidase-A gene promoter polymorphism (MAOA-uVNTR) with self-reported anxiety and other psychopathological symptoms in a community sample of early adolescents. Journal of anxiety disorders. 31, 65–72. [DOI] [PubMed] [Google Scholar]
- Wagner-Schuman M, et al. , 2015. Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environ Health. 14, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F, 2002. A family resilience framework: Innovative practice applications. Family relations. 51, 130–137. [Google Scholar]
- Wang HL, et al. , 2008. Case-control study of blood lead levels and attention deficit hyperactivity disorder in Chinese children. Environmental Health Perspectives. 116, 1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weder N, et al. , 2009. MAOA genotype, maltreatment, and aggressive behavior: the changing impact of genotype at varying levels of trauma. Biological psychiatry. 65, 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson ML, et al. , 2015. The mediating role of parenting stress for children exposed to trauma: Results from a school-based system of care. Journal of child and family studies. 24, 1141–1151. [Google Scholar]
- Widom CS, Brzustowicz LM, 2006. MAOA and the “Cycle of Violence:” Childhood Abuse and Neglect, MAOA Genotype, and Risk for Violent and Antisocial Behavior. Biological Psychiatry. 60, 684–689. [DOI] [PubMed] [Google Scholar]
- Won EK, et al. , 2016. Association of current phthalate exposure with neurobehavioral development in a national sample. Int J Hyg Environ Health. 219, 364–71. [DOI] [PubMed] [Google Scholar]
- Wong CCY, et al. , 2010. A longitudinal study of epigenetic variation in twins. Epigenetics. 5, 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, et al. , 2011. Urinary trichlorophenol levels and increased risk of attention deficit hyperactivity disorder among US school-aged children. Occup Environ Med. 68, 557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef S, et al. , 2011. Attention deficit hyperactivity disorder and environmental toxic metal exposure in the United Arab Emirates. J Trop Pediatr. 57, 457–60. [DOI] [PubMed] [Google Scholar]
- Yu CJ, et al. , 2016a. Increased risk of attention-deficit/hyperactivity disorder associated with exposure to organophosphate pesticide in Taiwanese children. Andrology. 4, 695–705. [DOI] [PubMed] [Google Scholar]
- Yu CJ, et al. , 2016b. Attention Deficit/Hyperactivity Disorder and Urinary Nonylphenol Levels: A Case-Control Study in Taiwanese Children. PLoS One. 11, e0149558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, et al. , 2015. Attention-deficit/hyperactivity symptoms in preschool children from an e-waste recycling town: assessment by the parent report derived from DSM-IV. BMC Pediatr. 15, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohsel K, et al. , 2015. Monoamine oxidase A polymorphism moderates stability of attention problems and susceptibility to life stress during adolescence. Genes Brain Behav. 14, 565–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.