Abstract
Several key attributes of zebrafish make them an ideal model system for the discovery and development of regeneration promoting therapeutics; most notably their robust capacity for self-repair which extends to the central nervous system. Further, by enabling large-scale drug discovery directly in living vertebrate disease models, zebrafish circumvent critical bottlenecks which have driven drug development costs up. This review summarizes currently available zebrafish phenotypic screening platforms, HTS-ready neurodegenerative disease modeling strategies, zebrafish small molecule screens which have succeeded in identifying regeneration promoting compounds and explores how intravital imaging in zebrafish can facilitate comprehensive analysis of nanocarrier biodistribution and pharmacokinetics. Finally, we discuss the benefits and challenges attending the combination of zebrafish and nanoparticle-based drug optimization, highlighting inspiring proof-of-concept studies and looking toward implementation across the drug development community.
Keywords: Neurodegeneration, Neuroregeneration, Nanocarrier, Drug optimization
1. Introduction
In this review, we cover three topics spanning the use of the zebrafish model system for the discovery and optimization of transformative neurodegenerative disease therapeutics; nanotechnology-enhanced drugs designed to stimulate dormant neuroregenerative capacities in patients. First, we discuss how zebrafish facilitate large-scale phenotypic screening paradigms that place living disease models at the start, rather than the end, of the drug discovery process. Next, we explore how inducible cell-specific neurodegenerative disease models facilitate the identification of regeneration-modulating compounds by bridging classic regenerative biology studies with the therapeutic ends of regenerative medicine. Lastly, we speculate on how combining nanomedicine strategies for drug optimization with zebrafish assays for evaluating improvements in drug targeting and/or efficacy provide a powerful platform for advancing promising regeneration-enhancing lead drug candidates.
2. Zebrafish and drug discovery
The zebrafish system allows large-scale genetic [1] and chemical [2] screens to be performed in a vertebrate model system, the latter providing a whole-organism perspective to drug discovery and development [3]. Notably, a high degree of pharmacological conservation has been observed between fish and humans; similar effects have been observed for a broad range of compounds including anticoagulants, narcotics, anti-angiogenic, chemotherapeutics, as well as modulators of hematopoiesis, lipid metabolism, and the cardiac cycle [4–6]. Encouragingly, novel compounds discovered in zebrafish have already found success in clinical trials [7]. Of particular importance here, compounds promoting regeneration have also been identified in zebrafish [8], including compounds enhancing repair of the nervous system [9,10]. Many excellent reviews cover the broader topic of how zebrafish enable drug discovery across a range of disease paradigms, see for example [3,11–14]. Here, we focus on zebrafish screening platforms and neurodegenerative disease models strategies that further the discovery of compounds which improve endogenous neuroregenerative capacities, i.e., stimulate the reparative potential of tissue-specific adult neural stem cells.
3. Bridging regenerative biology and regenerative medicine
The zebrafish (Danio rerio) has emerged as a versatile model system for regenerative biology. Paradigms ranging from epimorphic regeneration of whole body parts [15], to complex tissue restoration [16], or replacement of individual cell types [17] have been developed for this species. The latter paradigm is facilitated by a genetically-targetable inducible cell ablation methodology we developed [18,19]. This approach provides a means of bridging regenerative biology and regenerative medicine by enabling the creation of inducible physiological models of degenerative diseases, linked to the loss or functional compromise of specific cell types, in a robustly regenerative species [17]. Several additional advantages of the zebrafish system afford unique perspectives to the study of regeneration. Key among these is the amenability of zebrafish to intravital imaging, enabling direct visualization of the dynamic cellular and molecular processes governing endogenous stem cell niche activities during regeneration [10,20–22]. In addition, the rapid regenerative capacities of this model system allow direct correlations to be made between observed phenomena and functional recovery [23].
4. Zebrafish, nanomedicine, and drug optimization
Despite ever more routine use of zebrafish for drug discovery, application of this model system to the development of targeted drug delivery methods for therapeutic optimization, such as nanocarriers, remains nascent. Here we discuss advantages afforded by combining zebrafish and nanotechnology for lead drug optimization in neurodegenerative contexts. For instance, intravital imaging in zebrafish enables near real-time whole-body pharmacokinetics, allowing researchers to directly observe the biodistribution of nano-targeted drug delivery systems. One crucial barrier that the zebrafish system can address is the fact that many drug delivery systems optimized using in vitro assays do not retain efficacy in animal models [24]. Zebrafish provide a means of circumventing this issue by allowing rapid and cost-effective evaluations of large numbers of nanocarrier options directly in vertebrate models of disease and from multiple perspectives, spanning biodistribution kinetics to therapeutic efficacy, directly in vertebrate models of disease. However, the predictive value of zebrafish imaging-based nanoparticle biodistribution studies for translational endpoints—despite the exquisite temporal and spatial resolution that can be achieved—remains untested and therefore remains a major caveat until the field can mature.
5. Physiome considerations – zebrafish vs. mammals
As with any model system, the zebrafish has somewhat incomplete homology to the human physiome. With respect to the characterization of nanocarriers, these differences may impact nanoparticle transport, processing, targeting, and elimination.
Like mammals, zebrafish cerebral endothelial cells steadily develop a blood brain barrier during early development via size-dependent exclusion between 3 and 10 dpf [25–27]. Tight junctions (claudin5 and ZO-1 dependent) begin to form by 5 dpf and mature by 10 dpf, when they are associated with pericytes and astrocytes [25]. Active molecular efflux is observable by 8 dpf (via P-glyoprotein/ABCB1/4/5), and once formed, this BBB demonstrates compound-specific permeability analogous to mammals [25]. As the ideal window for zebrafish intravital imaging or microplate-based screening is between 2 and 9 dpf (after which the yolk has exhausted), nanocarriers that are CNS permeable at earlier time points should be validated in older animals with a fully developed BBB.
Zebrafish are more dependent on the innate arm of the immune system. They rely on it solely for the first ~3–4 weeks of development [28], whereupon the adaptive immune system has matured [29]. This allows interrogation of innate immune system functions in isolation. Intriguingly, depletion of adaptive immune cells (B and T cells) has little impact on viability [30] or response to secondary infection [31,32], and unlike humans, zebrafish rapidly succumb to infection in the absence of key innate signaling components [33,34]. The zebrafish lacks obvious lymph nodes, and the liver & kidney serve as alternative sites of antigen processing and presentation [35]. Evolutionary divergence has led to differences in: the complement system [36], pattern recognition receptors (PRRs) [37], immunoglobulin [38], coagulation pathway [39], and thrombocyte biology that may alter the biodistribution of, or toxicity response to administered nanocarriers. While the complement system is functionally and structurally similar, in humans it is primarily derived from the liver and circulates throughout the blood and lymph, while the zebrafish has numerous regions of extrahepatic localized expression (e.g., skin, heart, kidney, spleen, brain, intestine, gills) [36]. In addition, zebrafish lack the CD14 co-receptor necessary for the mammalian endotoxin response [37], one major cause of failed screening assays at the USA National Nanocharacterization Library (NCL, part of NCI).
The host response to nanocarrier/nanoparticle systems is largely governed by cells of the mononuclear phagocyte system, most notably, tissue-resident macrophages and other antigen presenting cells (APCs). Circulating nanocarriers quickly gain an adsorbed protein coating (i.e., “corona”) which influences recognition, phagocytosis, clearance, and subsequent immune system reactivity [40]. The content of this protein corona may be influenced by species-specific differences evident in blood/plasma composition (e.g., major presence of vitellogenins and absence of albumin in zebrafish [41]). In humans, typically <5% of administered nanocarriers reach their target [42] and most nanoparticles are sequestered by the liver and spleen, or eliminated by the kidney. Kupffer cells (tissue resident macrophages of the liver) facilitate the binding and removal of the majority of foreign materials, and their ability to clear nanocarriers is dependent on surface charge, size, and ligand chemistry [42]. Only recently, endothelial cells and macrophages of the zebrafish caudal hematopoetic tissue (and caudal vein) were characterized as functionally homologous to scavenger endothelial cells and Kupffer cells in mammals [43].
6. Zebrafish and drug discovery
6.1. Phenotypic drug discovery in vivo
Large-scale phenotypic drug discovery methods complement target-based high-throughput screening (HTS) approaches by emphasizing physiological effects in cell culture, or more recently, whole-organism disease models. Phenotypic screens can provide disease-relevant context which reductionist target-based methods often lack and enable t compound discovery in the absence of a pre-defined target. This is especially useful in cases where molecular disease etiology is unknown. Similarly, whole-organism phenotypic screens can overcome limitations when the biology underlying a disease is too complex to be captured in two-dimensional cell culture formats. In particular, neurodegenerative diseases exemplify complex etiologies which are difficult to model in cell culture, e.g., retinitis pigmentosa, Parkinson’s disease, Alzheimer’s disease.
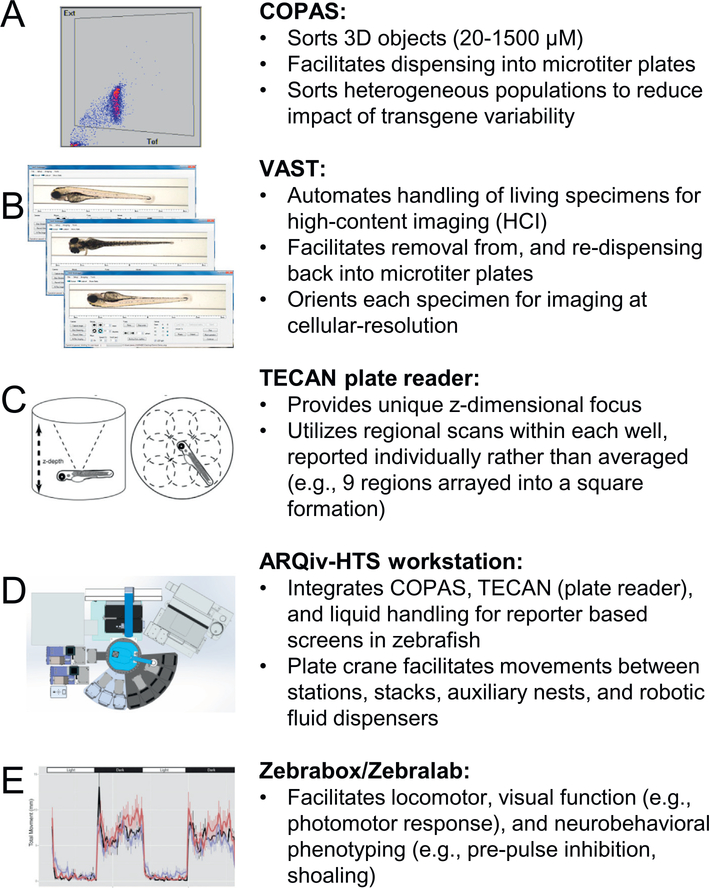
Until recently, drug discovery in living disease models was largely a process of serendipity. However, we and others have developed automated in vivo screening platforms enabling large-scale systematic phenotyping in living disease models (Fig. 1) [44,45], including observations of nanocarrier effects in zebrafish larvae. Key among technologies facilitating this approach are:
Fig. 1.
Key technologies available for evaluation of nanocarrier based enhancement of drug efficacy in vivo. Representative data format and a brief description are provided for each platform. (A) COPAS: sorts small objects based on optical density and fluorescent intensity. (B) VAST: handles and orients small objects for high-content imaging. (C) TECAN plate reader: measures fluorescence intensity in multi-well plates. (D) ARQiv-HTS workstation: integrates COPAS, TECAN, and additional automation for HTS-scale assays utilizing whole organisms. (E) Zebrabox/Zebralab: measures numerous behavioral metrics for larval zebrafish. Portions of figure (C [51], E [52]) reproduced by permission of PLOS One and Elsevier.
COPAS (Complex Object Parametric Analytic Sorter, Union Biometrica), functions as an organismal sorter, capable of fluorescent reporter detection and microplate well dispensing of living 3D model specimens ranging from 20 to 1500 μm in diameter, akin to a fluorescence activated cell sorter (FACS). Current dispense rates approach true high-throughput levels of approximately 50,000 specimens per day (i.e., 1 per second), automating incubation with small molecules and/or nanocarriers.
VAST (Vertebrate Automated Screening Technology, Union Biometrica), a bioimager platform which functions as a mid-throughput high-content imaging system for small model organisms (worms, fish, etc.) comprised of a microfluidic system that automates delivery and user-defined orientation(s) of specimens on a microscope stage. Combined with the Large Particle Sampler (Union Biometrica), VAST can aspirate samples of interest from microwell plates, deliver them for automated high-content imaging, and re-dispense samples back into microwell plates, thus facilitating longitudinal studies that can track dynamic processes such as disease progression or regeneration. This system is ideal when high-resolution imaging is required to assess phenotypes that are non-quantitative, e.g., changes in reporter distribution at organismal, tissue, or cellular levels [45].
ARQiv (Automated Reporter Quantification in vivo), a microplate reader-based system we developed which enables maximal throughput rates (i.e., tens of thousands of specimens processed per day) for reporter-based assays in small model organisms [44] and 3D cell culture models (e.g., human stem cell-derived “organoids”) [46]. To realize true high-throughput potential, we integrated a COPAS unit with ARQiv via a customized robotics-based automation system to create ARQiv-HTS [2,44].
In addition, several companies have developed platforms specifically for the zebrafish model system facilitating quantification of behavioral phenotypes (e.g., sleep/wake cycle, seizure, startle effects, etc.). Examples are the ZebraBox/ZebraLab (ViewPoint) and a suite of platforms from Noldus for quantifying changes even at the tissue level (e.g., heart rate). These platforms have been applied to drug discovery efforts focused on locomotor [47], visual function [48,49], neurobehavioral [49], and toxicity [50] phenotypes. However, high-content screening (HCS) systems such as these (and VAST) provide detailed information on complex phenotypes at the cost of throughput, limiting daily processing rates to hundreds to thousands of specimens per day. For instance, VAST is capable of processing zebrafish larvae at 20s per sample (approximately 35 min for a 96-well plate).
6.2. Pharmacokinetic and biodistribution considerations
There are important differences in the way small molecule studies are performed in zebrafish versus mammalian model systems that impact their use for drug development and optimization. For instance, during a typical zebrafish small molecule screen, larvae are bathed continuously in compounds of interest (i.e., waterborne exposure). This differs from systemic administration (e.g., intravenous, subcutaneous, oral) in mammalian models and patients which results in a pulse of compound that reaches a peak concentration before being progressively eliminated from tissue/circulation over time. Conversely, waterborne exposure results in a consistent level of compound over longer time periods, wherein the ability of the larvae to metabolize/eliminate the compound is saturated through steady absorption (via dermal, gastrointestinal, and respiratory routes) and subsequent passive diffusion throughout the larvae. Sustained equilibrium between absorbed and metabolized/excreted compound is reached in a matter of hours and despite clearance rates being comparable to mammalian models, compound accumulations differ by 2–3 orders of magnitude [53,54]). Importantly, despite differences to ‘bolus’ drug delivery methods that predominate mammalian and clinical studies, waterborne exposure in zebrafish is similar to sustained-release kinetics that is a common goal of nanomedicine-based approaches for treating chronic conditions [55].
While biodistribution has been shown to be similar in fish and mammals, minor variations [56] and major differences have been observed [57]. Thus, it is important that potential disparities in pharmacokinetics between fish and mammalian models are accounted for. MALDI-MSI (matrix assisted laser desorption/ionization mass spectrometry imaging) is a promising method of evaluating compound pharmacokinetic properties, and has been applied to monitor accumulation of psychoactive compounds in larval and adult zebrafish CNS [57,58]; this approach does not yet allow longitudinal observations. However, direct in vivo time-lapse observation of compound distribution—i.e., dynamic in vivo pharmacokinetics—is possible with auto-fluorescent or fluorescently labeled compounds tested in zebrafish [56].
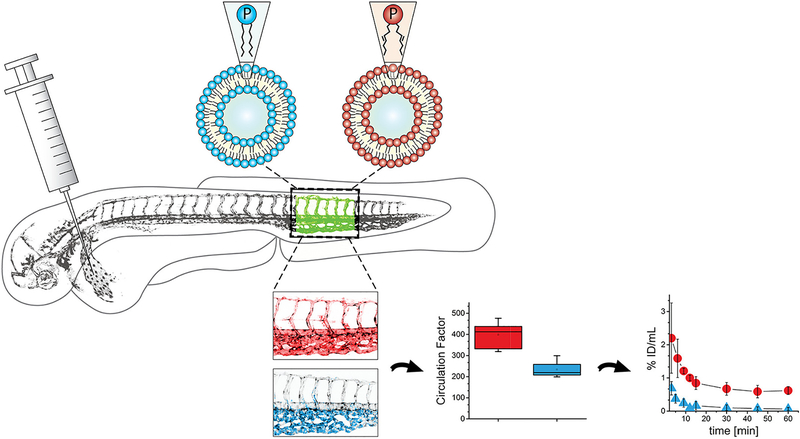
Nanocarriers are often not inherently fluorescent but are generally amenable to being loaded with or conjugated to fluorescent labels [59,60]. A recent application of fluorescently-labeled liposomes in zebrafish beautifully demonstrated their utility as a means of evaluating circulation behavior post-injection. Vascular labeling in the Tg(kdrl:GFP) line allowed quantification of both ‘circulating’ and ‘extravascular’ liposome fluorescence. Modifications to the liposome structure altered this behavior, and semi-quantitative analysis of these structural changes (e.g., PEGylation, lipid composition, cholesterol) correlated with that observed in mammalian models [61] (see Fig. 2). However, a major caveat of such studies is the possibility of incidental loss and/or transference of fluorescently labeled lipids from the liposome shell.
Fig. 2.
Zebrafish as a pre-clinical model to study the systemic circulation of nanocarrier drug delivery systems in vivo. Notably, changes in circulation patterns observed in zebrafish with various nanocarrier modifications paralleled pharmacokinetic distribution data collected from mammalian systems. Reproduced with permission of Journal of Controlled Release [60].
Extending these same principles to the CNS requires that penetration issues caused by the blood-brain barrier (BBB) and blood-retina barrier (BRB) be accounted for. In zebrafish, the BBB/BRB forms at ~3 days post-fertilization (dpf) [62] and can exclude large MW (~4 kDa) particles by 2.5 dpf, and smaller sizes (~400 Da) by 3 dpf. Although neurodegenerative conditions are often associated with disruption of the BBB/BRB, and thus may occur in genetic models of disease, more controlled methods are desirable. Several agents known to disrupt the BBB or BRB in mammalian models also do so in zebrafish. For instance, exposure to the compound penylenetetrazole (PTZ) or the oligopeptide hormone bradykinin leads to BBB/BRB breakdown and increased paracellular permeability in fish and rodents, [62–64]. However, PTZ binds the GABAA receptor and is used to induce seizure-like behaviors in zebrafish [65], complicating its usage for CNS-focused discovery efforts and behavioral screens in particular. In addition, although bradykinin treated zebrafish larvae show no deleterious changes in vasculature and no behavioral abnormalities have been reported, the observed efficacy of BBB breakdown with this agent was limited.
Finally, although drug screening in microtiter formats can be conducted with fully formed larval zebrafish, age-dependent differences in bioavailability or drug action could compromise efficacy at later adult stages. To account for age differences, a manual method of intermittent drug dosing of adult zebrafish was recently developed (ScreenCube [66]). The additional demands for screening in adults (i.e., cost, space, and labor) were considered during the development of this open-source 3D-printed device. Adults utilize more oxygen and produce more ammonia waste than their larval counterparts. Because of this, the dosing volume of 10 mL per well (per adult) may be utilized for a maximum of 3 min (either continuously or in bouts) before toxic levels of ammonia are reached. Nevertheless, researchers were able to successfully screen 520 inhibitors of epimorphic regeneration when the dosing volume had been reduced 10-fold (a marked reduction in comparison to previous efforts) [67].
7. Bridging regenerative biology and regenerative medicine
7.1. Neurodegeneration models in zebrafish
Neurodegenerative disorders are typified by the progressive loss of neurons within the CNS (e.g., of the brain, retina, spinal cord). The clinical manifestation of this diverse set of neurological disorders depends on the CNS regions involved. There are numerous neurodegenerative paradigms available in zebrafish. In each disease model, phenotypic assessment of injured/regenerating cell types is facilitated by transgenic effector/reporter vectors that are pan-neuronal [68–70], neurotransmitter-specific (e.g., dopaminergic [71,72] and monamergic [73] neurons) or cell-type specific [17,74] (e.g., photoreceptor [10,75,76], ganglion cell [77]).
Broadly, modeling of neuronal degeneration can be achieved via:1) mechanical/light lesion [78]; 2) chemical damage [73]; 3) progressive formation of protein aggregates (e.g., as in Alzheimer’s tauopathies [69,70,79] or Huntington’s [80]); 4) chemically-inducible enzyme/ prodrug ablation methodologies targeting neurons [17] and supporting glia [81]; or 5) specific deleterious mutations that result in the preferential cell loss of a given type (e.g., as in Parkinson’s [82,83] and Amotrophic Lateral Sclerosis [84]). Of the above, chemically inducible strategies offer distinct advantages for large-scale screens; cell loss can be temporally synchronized across large sample sizes, facilitating quantification of degeneration and regeneration in sample sizes appropriate for observing changes in cell loss or replacement kinetics.
In addition, cell-type specific ablation methods provide a means of bridging classic regenerative biology studies with the therapeutic ends of regenerative medicine; the latter being largely focused on diseases linked to the loss or functional compromise of individual cell types. Prior to the advent of diphtheria toxin (DT) and nitroreductase (NTR)-based cell-specific ablation techniques, the study of cellular regeneration was largely limited to mechanosensory hair cells [85], olfactory receptor neurons [86], and muscle cells [87]. Because DT receptors and the NTR enzyme can be transgenically expressed in any genetically targetable cell type, these approaches have allowed the field of cellular regeneration to expand into a wide range of cell-specific paradigms, including cells lost to neurodegenerative diseases [17]. When deployed in robustly regenerative species, such as zebrafish, such methods enable studies focused on how the replacement of individual cell types is regulated at the level of endogenous stem cells and their niche microenvironment.
Once an optimal disease modeling strategy is defined, acquiring or creating desired resources is a straightforward process. Numerous transgenic/mutant lines are available through ZIRC (Zebrafish International Resource Center) or EZRC (European Zebrafish Resource Center). Additionally, methods for the development of novel transgenic/mutant lines via Tol2-mediated transgenesis [88], or CRISPR/Cas9-based mutagenesis [89] are well-established; either approach takes approximately ~6 months to generate stable lines and another ~6 months to generate large breeding stocks.
7.2. Zebrafish screens for regeneration-modulating compounds
Though there have been numerous small molecule screens in zebrafish, only a handful have focused on identifying compounds that impact regeneration. To date, compounds implicated in modulating regenerative process act by impinging upon the reparative potential of endogenous stem cells directly or via components of the stem cell ‘niche’, such as immune cell subtypes. In particular, glucocorticoids have been shown to enhance regeneration kinetics in several regeneration paradigms. However, as a class these drugs are associated with undesirable systemic effects. In an effort to negate this limitation, nanomedicine-based targeting methods could be utilized to deliver regeneration-enhancing drugs directly to neural stem cells or to niche cell subtypes which regulate regenerative potential [90]. Below we discuss the results of some of the larger-scale zebrafish drug screens and related studies that have provided insights into the chemical biology of regeneration.
7.2.1. Fin regeneration
The zebrafish caudal fin is an ideal tissue for interrogating successful epimorphic regeneration. A 2007 compound screen utilized amputation of the larval caudal fin (posterior to the notochord) and quantification of the rate of regeneration [91] as a phenotypic metric. In a screen involving a library of 2000 compounds and evaluation of 4000 zebrafish larvae, 17 compounds (0.8% of library) were identified as inhibitors of fin regeneration. The hit compounds fell into a range of chemical space, yet through structure-function analysis, the largest cluster (5 of 17 hits) were identified as glucocorticoids. Ultimately, it was demonstrated that glucocorticoids (of which Beclamethasone was the most effective) impaired tail fin regeneration by inhibiting proliferation within the blastema. In contrast to a more recent study [92], the data suggested immune cells were not critical to tail fin regeneration, [92]. However, other studies have been shown that glucocorticoid-based modulation of the inflammatory response post-injury can accelerate regeneration kinetics in zebrafish [10,93].
7.2.2. Heart regeneration
Zebrafish have the capacity to regenerate heart muscle after injury via proliferation of pre-existing/spared cardiomyocytes. A 2013 compound screen leveraged FUCCI (fluorescent ubiquitylation-based cell cycle indicator) technology, which fluorescently labels both proliferative (S/G2/M-phase) and resting (G1-phase) cells [94]. Cardiomyocyte-specific “cmlc2:FUCCI” transgenic fish were created to label proliferative cardiomyocytes during development and regeneration. Zebrafish larvae were first screened against a small panel of compounds targeting common developmental pathways to identify those that modulate cardiomyocyte proliferation during heart formation (Hedgehog, IGF, and TGF-β); confirmatory studies demonstrated that these same pathways were active following cardiac injury in adult fish. Subsequently, pharmacological manipulation of these same pathways was shown to increase cardiomyocyte proliferation rates during heart regeneration.
7.2.3. Pancreatic beta cell regeneration
Despite mechanistic differences, both type 1 and late-stage type 2 diabetes lead to loss of insulin producing pancreatic beta cells. One potential curative therapy would be to stimulate endogenous recovery of beta-cell mass. Accordingly, a 2012 study screened 7186 compounds for enhancers of pancreatic β-cell regeneration in ~100,000 transgenic larval zebrafish in which beta cells could be selectively ablated and newly generated beat cells were differentially labeled by photoconversion of the fluorescent reporter Kaede [95]. Compounds capable of increasing the number of regenerated beta cells fell into three categories with the majority converging on the adenosine pathway. The most potent ‘hit’ compound was the adenosine agonist N-Ethylcarboxamidoadenosine (NECA). A suite of orthogonal assays subsequently confirmed the efficacy of this compound in both fish and mice.
7.2.4. Peripheral nerve regeneration
To study peripheral nerve regeneration, Granato and colleagues developed a laser mediated nerve transection assay in larval zebrafish [96,97]. However, this approach was limited to low throughput drug screening. To address this, a pectoral fin amputation assay was developed which was more amenable to large scale screening [78]. Because fin replacement is dependent on nerve regeneration, this assay serves as a proxy for peripheral nerve regeneration. A primary screen of ~350 compounds, resulted in 21 being identified as inhibitors of pectoral fin replacement, thus potential disruptors of peripheral nerve regeneration. 60% of the primary hit compounds were then confirmed in a secondary, laser-mediated nerve transection assay. Importantly, 12 validated hits were implicated in targeting molecules/pathways not previously associated with peripheral nerve regeneration, demonstrating the power of unbiased screening approaches for advancing understanding of complex processes.
7.2.5. Hair cell regeneration
Zebrafish have sensory organs, termed neuromasts, containing mechanosensory hair cells that function analogously to hair cells of the mammalian inner ear. Neuromast hair cells are exposed directly to the external environment, thus easily labeled by fluorescent dyes and susceptible to waterborne ototoxic compounds. This provides an ideal chemically-inducible cell death model system for studying the regulation of primary neuron regeneration via large-scale genetic and chemical screens [98,99]. A 2012 screen utilized aminoglycoside-induced ablation of GFP-labeled hair cells to screen a library of 1680 compounds [100]. Ultimately, six compounds were validated as inhibitors of hair cell regeneration, blocking either neural precursor or support cell proliferation. In addition, two synthetic glucocorticoids (dexamethasone and prednisolone) were confirmed as enhancers of hair cell regeneration which acted by increasing precursor proliferation. In a related effort this group performed a screen to identify improved variants of promising neuroprotective lead candidates and succeeded in identifying a new compound that was substantially more potent in fish and rat models of ototoxic injury [101]. Of note, the optimized compound was recently approved by the FDA as a new drug and has proceeded to clinical testing.
7.2.6. Retinal cell regeneration
Using a chemically-inducible model of rod photoreceptor cell ablation, we recently found dexamethasone was also capable of accelerating retinal photoreceptor regeneration kinetics in zebrafish [10]. Interestingly, dexamethasone stimulated regeneration only when applied 24 h after induction of rod cell loss, conversely pre-treatments inhibited photoreceptor regeneration. Additional data suggested that resolution of an acute inflammatory response led to enhanced neuroregeneration but that complete inhibition of immune cell reactivity disrupted the responsiveness of the retinal stem cell niche to rod cell loss. Collectively, the studies outlined exemplify the usefulness of the zebrafish model system for exploring the chemical biology of regeneration using unbiased screening approaches. Further clarification of the role that the immune system plays during the regenerative processes is clearly needed in light of intriguing evidence suggesting that immune cell-targeted therapeutic strategies can stimulate dormant reparative potential of adult stem cells in patients [102]. However, translating regeneration-promoting compounds to the clinic will require substantial investment in further drug development. Below we discuss how zebrafish afford distinct advantages to drug optimization efforts, focusing on the transformative potential of nanoparticle-based approaches for targeted drug delivery.
8. Zebrafish, nanomedicine, and drug optimization
Once lead drug candidates have been identified in a phenotypic screen, typical next steps involve investigation of the molecular mechanism of action (MoA) and follow-on screens to identify improved compounds within the same structural or functional class. Assuming a reliable degree of knowledge about MoA or site of action (e.g., target tissue), an alternative strategy would be to develop strategies for targeted drug delivery to implicated systems, tissues, or cell types. The combined strengths of zebrafish and nanomedicine are just beginning to be combined as a means to explore nanocarrier based technologies for targeted drug delivery, with the ultimate goal of supporting the development of disease-specific lead optimization strategies.
8.1. Leveraging intravital imaging for nanocarrier characterization
The advantages zebrafish afford to high-resolution intravital imaging can be leveraged to facilitate high-resolution characterization of nanocarrier distribution and function within the context of CNS injury paradigms. Rapid development and embryonic/larval stage transparency avail in vivo interrogation of mechanisms of biodistribution and pharmacokinetics via direct observation of tagged nanoparticles and their interactions with targeted tissues, microenvironments, cell types, and even subcellular compartments. In addition, optical manipulation of nanoparticles and their cargo can be used to explore inducible delivery/activation mechanisms. Moreover, zebrafish transparency can be enhanced using chemical inhibitors of pigmentation and pigmentation mutants (e.g., roy, albino, nacre) can be used to extend these types of studies beyond larval stages [103].
8.1.1. Biodistribution/pharmacokinetics
Zebrafish present known biological barriers to waterborne nanocarrier uptake and distribution to the CNS, including the chorion (with a pore canal size of 0.5–0.7 μm [104]), gastrointestinal epithelial tight junctions, and the BBB/BRB. A 2017 study evaluated how porous these barriers are using 70 and 200 nm coumarin 6-nanocrystals [105]. 70 nm nanocrystals were more effective at penetrating biological barriers during embryo/larval stages, where they accumulated in the gut, before being transported through the vasculature and accumulating within the brain and retina. FRET microscopy indicated that both intact and degraded nanocrystals accumulated within larvae over time. Further analysis in adults indicated that lipid-raft-mediated endocytosis was critical for absorption (and thus distribution) of nanocrystals. This work was consistent with prior studies indicating nanocrystals enhance the oral absorption of poorly water-soluble compounds via size-dependent improvements in saturation solubility, dissolution velocity, and adhesiveness to cell/surface membranes [106].
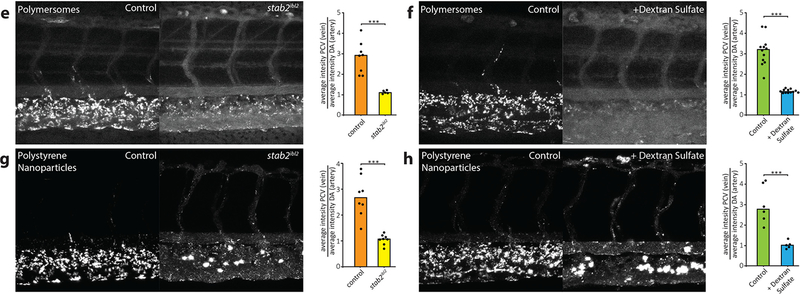
The ultimate goal of nano-delivery methodologies is cell-specific targeting, yet on-target uptake rarely surpass 1% of the total injected nanoparticle dose [107] due to off-target interactions in the liver [42] and scavenger endothelial cells (SECs) of various tissues (e.g., kidney, heart, gills). Zebrafish whole-body 4D intravital imaging was applied to monitor the distribution of fluorescent liposomes with cellular resolution in near real-time [43]. The tested liposomes accumulated on endothelial cells and co-localized with fluorescently labeled-hyaluronic acid, implicating scavenger receptors. Dextran sulfate (a competitive inhibitor of stab-2 scavenger receptors), and stab-2ibl2 mutants were used to test this. Both manipulations led to a dramatic increase in the concentration of freely circulating liposomes due to diminished stab-2 scavenger receptor binding. This study provided compelling evidence that 1) anionic liposomes are an ideal delivery mechanism for targeting cells overexpressing stab-2 (such as SECs), and 2) inhibiting nanoparticle-SEC interactions may serve to enhance bioavailability of numerous nanocarrier classes (See Fig. 3). The identity of receptors responsible for in vivo clearance of nanoparticles was previously unknown. Ultimately, in demonstrating cell- and receptor-specific binding of injected nanoparticles, Campbell et al. established a new standard for the dissection of nanoparticle biodistribution in vivo.
Fig. 3.
stab2-mediated scavenging of anionic nanoparticles in vivo. E,F) Carboxylated polystyrene nanoparticle. G,H) CCMV virus-like particle. Quantification of nanoparticle levels associated with venous versus arterial endothelial cells based on rhodamine fluorescence intensity associated with caudal vein vs dorsal aorta. Bar height represents median values, dots represent individual data points, and brackets indicate significantly different values (***p < 0.001) based on Mann–Whitney test. n = 5–12 samples per group (over two experiments). Partial figure reproduced by permission of ACS Nano [43].
8.1.2. Optical manipulation
Physical manipulation of nanocarriers is possible through application of optical tweezers (a highly focused laser beam that provides an attractive/repulsive force). This technique has been instrumental to understanding the biomechanical properties of nanoparticles designed to target specific cell types, yet this work has largely been conducted in vitro. Remarkably, a 2016 study applied near-infrared optical tweezers to trap and precisely manipulate the distribution of polystyrene nanoparticles (200 nm-1 μm size) within the zebrafish endothelium [108]. In addition, changes in affinity of polystyrene nanoparticles toward endothelial cells were measured after the addition of a polyethylene glycol coating (i.e., PEGylation). This same methodology could be applied to screen nanoparticle-cell interactions in vivo (e.g., immune, cancer, infectious, and neurodegenerative disease paradigms).
Zebrafish also provide an excellent platform for testing photoactivation-based manipulations of gene expression. Upconversion nanoparticles (UCN) excited with near-infrared light produce ultraviolet light locally and can activate UCN-loaded photo-morpholinos or photo-caged plasmids. A 2015 study demonstrated the use of UCNs as nanotransducers for light-controlled gene knockdown and gene upregulation in zebrafish [109]. Knockdown of ntla, a gene implicated in notochord formation, resulted in a robust mutant phenotype. To demonstrate efficacy in adult pigmented zebrafish, tumor cells transfected with UCNs (loaded with caged EGFP) were injected into the peritoneal cavity, where they expressed GFP following photoactivation.
Graphene has a unique combination of electronic, optical, and mechanical properties. It has been applied recently to develop graphene-based-biointerfaces as a means of addressing use-dependent drug effects (e.g.,drug-induced proarrhythmia risks) [110]. In zebrafish, reduced graphene oxide provides a method of inducing light-intensity-dependent cell stimulation during drug screening assays. Strikingly, simply injecting reduced graphene-oxide avails optical manipulation of heart rate through exogenous means (i.e., absent transgene-derived optogenetics).
8.1.3. Gaining access to the CNS
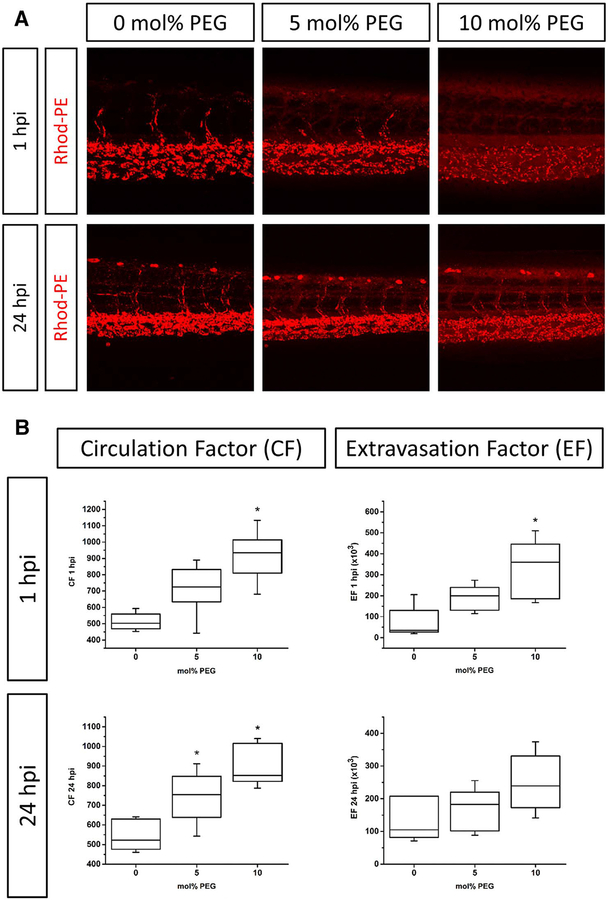
Ideally, waterborne nanocarriers would be readily absorbed and subsequent delivered to the CNS. Studies on mammalian models have tested a large number of strategies for overcoming barriers to waterborne uptake of nanoparticles and subsequent delivery to the CNS. To varying extents, modification of several key characteristics have been demonstrated to facilitate passage of nanocarriers across the BBB (e.g., shape, size, surface charge, hydrophobicity, and coating) [111]. The following aspects could be further refined using intravital imaging to assess nanoparticle accessibility to the CNS in zebrafish: 1) optimal particle size and zeta potential for BBB penetration; 2) improved PEGylation (i.e., ‘stealthing’) strategies for increasing circulation and lowering clearance rates (see Fig. 4); and 3) identify low-density surface ligands that facilitate BBB/BRB transcytosis (e.g., transferrin, α-insulin receptor/α-glucose transporter antibodies, and amphiphilic peptides). Critically, ‘stealthing’ PEGylation strategies similarly improve the circulation time in both humans and mammalian models [112,113]. Recently, dye-loaded apoferritin nanocages were successfully employed to cross the BBB in larval zebrafish via transferrin receptor binding [114]. Similarly, monosialoganglioside micelles leverage the ubiquitously expressed glycosphingolipid GM1 on endothelial cells, to transverse the zebrafish BBB [115]. In addition, it has been previously noted that the glucose transporter is expressed on both the intestine and gills of the zebrafish [116], and may serve as a means to bypass both epithelial and BBB barriers in fish (i.e., facilitating waterborne exposure).
Fig. 4.
Influence of PEGylation on liposome circulation in vivo in zebrafish. A) Liposome formulations (DSPC/cholesterol with increasing amounts of DSPE-PEG2000) were injected into transgenic zebrafish embryos and images were taken at 1 and 24 h post-injection. B) The circulation factor (CF) and extravasation factor (EF) of each lipid composition were calculated. n ≥ 5 experiments. Box plots represent median, third and first quantiles, minima and maxima. *p > 0.05 as compared to control (0 mol% PEG). Reproduced by permission of Journal of Controlled Release [60].
8.1.4. Targeting neural stem cells
Once across the BBB, targeting of endogenous stem cell niches will be paramount for regeneration-enhancing therapeutics. Though having limited inherent regenerative capacities, bone fide mammalian neural stem cells are located in the sub-ventricular zone of the lateral ventricle and the sub-granular zone of the hippocampus. In addition, retinal Müller glia can function as injury-induced stem cells if concurrently reprogramed by exogenous expression of genes such as ascl1 [117]. Nanoparticle-based targeting of mammalian neural stems has been tested almost exclusively by direct injection into or near the stem cell niche. This approach has shown promising results in models of stroke [118], Parkinson’s [119], and Alzheimer’s models [120]. Yet, when delivered systemically the biodistribution of nanoparticles is not easily quantified, thus the possibility of off-target effects remain a troubling possibility. Targeting of neural stem cells/niches in the zebrafish via nano-delivery methods has yet to be explored. However, nanoparticles have been used in zebrafish to enhance drug efficacy regarding bacterial pathogens (via macrophage targeting) [121] and in xenotransplantation-based cancer models (by limiting off-target binding via PEGylation) [122]. Again, the use of intravital imaging in zebrafish will provide significant advantages to studies aimed at defining nanoparticle formulations that successfully target neural stem cells or their niche microenvironment.
8.2. Co-opting lessons from aquatic toxicity assays
To date, nanoparticle applications in zebrafish have focused largely on toxicology. This work provides a wealth of information regarding inherent causes of toxicity—i.e. induction of reactive oxygen species (ROS) induction, necrosis, apoptosis, etc. (for which there are standardized protocols [123])—in addition to bioavailability, accumulation, and distribution. Thus, prior performance in toxicity studies may inform the choice of nanocarrier for drug optimization efforts.
Metals are sometimes utilized for the synthesis and creation of nanoparticles (e.g., quantum dots). However, metal ions can leach from cores leading to toxicity – i.e., ROS-mediated damage of the gill/ liver tissues or to DNA directly. Heavy metals are particularly toxic to zebrafish (e.g., silver- [104,124–128] and copper-based [126] nanoparticles) and their use should be approached with caution. Of note, copper ions are inherently ototoxic, causing ablation of the lateral line neuromasts [129]. Results from studies of gold nanoparticle (AuNP) toxicity are somewhat inconsistent due to a lack of standardized approaches. For instance, the toxicity of colloidal AuNPs may be dependent on the timing of exposure. When treatments were initiated at early cleavage stages (from 1–120hpf) a potential increase in embryonic malformations was observed [130]. However, when initiated at the blastula stage (~4–120hpf) no deleterious effects of AuNPs were observed across numerous sizes and concentrations [128]. Although NP particle size can impact toxicity, the majority of AuNP toxicity appears to result from surface functionalization. Positively charged AuNPs exhibit a clear concentration-dependent increase in mortality and developmental defects whereas negatively charged and neutral particles show no significant effects on survival under the same treatment conditions [131] (see [132] for a recent review of NP toxicity in zebrafish).
Similarly, metal oxides have been shown to induce differential toxicity based on size, charge, and shape [133–136], with effects ranging from induction of a pro-inflammatory immune response, ROS damage, and acute lethality [137]. Production of reactive oxygen species is the mechanism by which zinc-, titanium-, and aluminum-oxide based nanoparticles induce toxicity [124,138,139]. Conversely, Cerium-oxide based nanoparticles have been shown to be neuroprotective in zebrafish [140,141], likely by quenching oxidative stress and downstream inflammation [142], making it an attractive option for further studies in this system.
More complex nanomaterials offer a wide range of options with respect to target tissue and/or cell type, yet come with their own caveats. As with heavy metals and metal oxides, fullerenes, fullerols, and metallofullerenes induce ROS-mediated toxicity [143,144]. There is conflicting evidence as to whether the structure of carbon nanotubes (single- or multi-walled) cause toxicity [145,146]. Smaller carbon nanotubes are cleared to lymphatic tissue, whereas larger particles accumulate and disrupt tissue function. Moreover, the highly adsorptive surface area of carbon nanotubes has been shown to interfere with the bioavailability of, and thereby alter the toxicity of co-administered small molecules [147]. Quantum dots are relatively new with respect to their application to zebrafish but may be toxic [148], likely a function of the chosen surface coating. Both size and charge appear to influence dendrimer toxicity [149], with smaller G3.5 (compared to G4) dendrimers being less toxic [148]. Moreover, several studies have demonstrated that larger (i.e., high generation) PAMAM dendrimers can cause severe coagulopathies [149–151]. Though, G4.5 dendrimers complexed with anti-epileptics exhibit no toxic or teratogenic effects in zebrafish [152]. Anionic dendrimers may have issues penetrating cell membranes [153], whereas cationic dendrimer nanoparticles often destabilize the cell membrane leading to cell lysis [154] (an effect that can be attenuated by the addition of an integrin receptor recognition motif for particle internalization [155]). Importantly, as the immune system has been implicated as a direct modulator of stem cell regenerative capacity in the zebrafish CNS [10], dendrimer-based targeting reactive immune cells [156–159] may provide new therapeutic inroads toward regeneration-enhancing therapeutics.
8.3. Improving nanocarrier permeability for in vivo screens
8.3.1. Enhancing permeability/penetration
Large-scale assessments of nanocarriers for drug optimization studies in zebrafish, or other small animal modeling systems, will require methods for facile absorption, uptake, and tissue dissemination(i.e., strategies other than direct injection). For phenotypic drug discovery screens, compound absorption is facilitated by the addition of solvents that enhance penetrance into zebrafish larvae. DMSO is the most widely used solvent for small molecule drug screens, but even low amounts of DMSO (e.g., 0.5%) can lead to increased expression of heat shock pathway mediators [160], potentially confounding results. Recognizing this, one should consider application of other solvents: polyethylene glycol (PEG-400), propylene glycol, methanol, and acetone – which are generally non-toxic up to 1.5–2.5% [161].
The physiochemical properties of some nanocarriers may impede absorption through epithelial routes in teleost [162] or direct to their accumulation in off-target tissues (e.g., skin and gills) leading to toxicity [127,163–165]. In addition, solvents classically used to enhance small molecule delivery may not be effective at increasing nanocarrier uptake. Intriguingly, the use of permeation enhancers commonly applied to oral absorption (e.g., chitosan, dendrimers, peptides, etc.) provides a potential means to overcome this limitation [166–170]. With respect to the permeation of small organics in vitro, two distinct chemical categories enhance the therapeutic window: nitrogen-containing rings (1% w/v) and surfactants (anionic or zwitterionic, 0.1% w/v). Both provide potent permeation across a well-tolerated concentration range [171]; however, aquatic toxicity assays demonstrated zebrafish larvae are sensitive to anionic surfactants [172]. Nevertheless, in vitro studies provide initial concentration ranges for optimization of similar assays in vivo.
In mammals, incorporation of chitosan is one of the most common means of enhancing permeability of nanocarriers across tight junctions for oral, ocular, brain and parenteral routes; in addition, it is biodegradable, biocompatible, and stable [173]. However, while application of chitosan-containing nanoparticles improves absorption and subsequent bioavailability [168], even at sub-lethal doses (5 mg/L) it can cause increased ROS production and apoptosis in zebrafish [174]. As an alternative, Capmul MCM (glycerol monocaprylate) has been explored as an alternative for enhancing oral absorption of dendrimer-N-Acetyl-l-cysteine nanocarriers [169]. The data thus far show improved transepithelial permeability in vitro, in keeping with increased gastrointestinal stability and permeability (a 9-fold increase) in vivo. Attractively, protamine and PEG stearate have been demonstrated to facilitate penetration of double-shelled nanocapsules through both the chorion and epithelium of zebrafish larvae [175].
8.4. Microinjection routes
If solvents fail to improve nanocarriers absorption and/or distribution, microinjection may be the only viable option. Since zebrafish can rapidly metabolize and excrete injected nanocarriers, optimal injection timing will depend on the disease phenotype. In addition, the injection itself may cause injury that alters the phenotypic outcome of the assay (e.g., innate immune cell reactivity [10]). Thus, an injection site distal to the interrogated tissue is preferable. Optimal injection site locales will likely fluctuate with age. For example, between 3 and 7 dpf, pericardial injection is an excellent route for systemic administration [176]. Depending on the injection site, rapid systemic delivery or localized exposures are possible [177] with numerous options for both systemic (caudal vein, Duct of Cuvier) and local administration (hindbrain, tail muscle, otic vesicle, notochord). Manually injecting is a tedious process and not readily amenable to high-throughput screening formats. However, platforms exist for automated injection of eggs via agarose gel molds (2 k eggs per hour [178]) and image recognition (900 eggs per hour [179]), which would be ideal for toxicity and teratological testing. Initially, this was developed as an automated method of transgenesis and injection into the yolk/blastula of a fertilized embryo. Nevertheless, these methods could potentially be modified for older, hatched larvae to evaluate effects on regeneration.
Effective implementation of nanoparticles to enhance neuroregenerative capacities in zebrafish will require leveraging insights gained from prior studies in both fish and mammals. To date, the use of solvents or nanocarrier-specific modifications to enhance absorption in zebrafish remains largely unexplored, as only a small subset of available nanocarriers has proven to be both amenable to waterborne exposure and non-toxic (e.g., nanocrystals, quantum dots). Therefore it will be critical to determine the most efficient water-borne delivery of nanocarriers through the epithelia and into the CNS during assay development. By avoiding the pitfalls evident in toxicological studies, and carefully considering biological barriers present in the zebrafish model, we may expedite the design of nanocarriers that enhance biodistribution and cell-specific targeting. In doing so, the full potential of a model system enabling high-resolution insights into nano-delivery devices at the cellular level may be unlocked.
8.5. Nanoparticle-based drug optimization screen
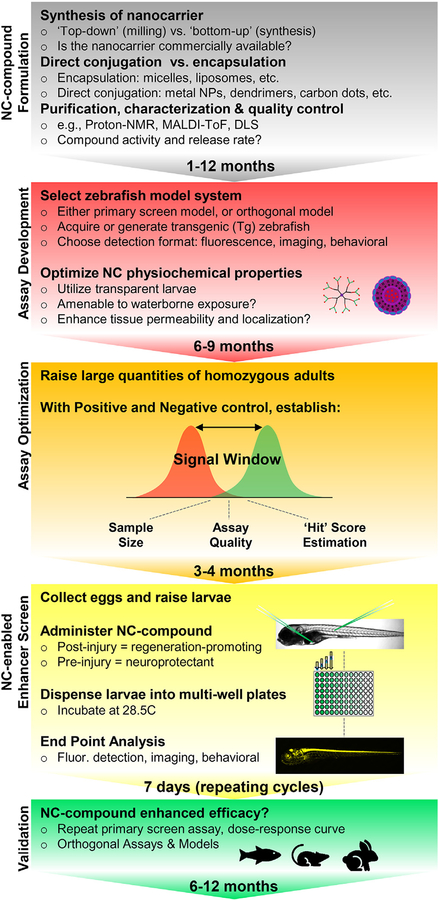
Until a method for promoting waterborne absorption of nanocarriers that enables discovery-focused HTS applications is defined, the role of nanomedicine in drug discovery and development will remain focused on the latter, i.e., drug optimization. Nevertheless, small-scale nanocarrier-based screens can provide a powerful strategy for improving the efficacy of regeneration-promoting and/or neuroprotective compounds following large-scale drug discovery assays in zebrafish models of neurodegeneration. Within this context, a small number (20–100) of primary ‘hit’ compounds would progress through five distinct phases: 1) Nanocarrier-compound formulation 2) Assay development, 3) Assay optimization, 4) Nanocarrier-compound primary screen, 5) Validation (See Fig. 5).
Fig. 5.
Graphical representation of nanocarrier-based drug optimization assay process in zebrafish. Five phases: 1) Nanocarrier-compound formulation 2) Assay development, 3) Assay optimization, 4) Nanocarrier-enabled enhancer screen, 5) Validation.
8.5.1.1. Nanocarrier-compound formulation (1–12 months).
Formulation of nanocarrier-compounds is a prerequisite to their application in zebrafish. The routes of formulation, i.e., ‘top-down’ (wet milling and high-pressure homogenization) vs. ‘bottom-up’ (precipitation and solvent evaporation) approaches have been reviewed comprehensively [180,181]. In general, the length of time required to complete formulation is dependent on the chosen nanocarrier (e.g., encapsulation vs. direct conjugation, synthesized vs. commercially available) and the number/diversity of drug compounds. Encapsulation methods are readily transferable to drugs with similar chemical properties (e.g., hydrophobicity and charge). However, in direct conjugation, the first chemical steps are unique to each compound and should be optimized to ensure: firm attachment to the nanoparticle, preservation of compound activity, and drug release the appropriate time scale. As a result, direct conjugation of compounds to the surface of a nanocarrier (e.g., metal nanoparticles, dendrimers) is more time-intensive than encapsulation of compounds (e.g., micelles and liposomes). Direct conjugation is more amenable to varied purification and characterization methods (e.g., proton nuclear magnetic resonance spectroscopy, MALDI-time of flight mass spectrometry, dynamic light scattering) as the final product is comparatively more uniform. Ultimately, the selected nanocarrier will significantly influence the time required to formulate large quantities nanocarrier-compound, or limit the size of the compound library tested (i.e., chemical space limitations), Formulation is often the rate-limiting step of any nanomedicine-based system, and thus should delineate assay throughput expectations.
8.5.1.2. Assay development (6–9 months).
Either the initial disease model used for the primary drug screen or an orthogonal model better facilitating nanocarrier-compound evaluation (but remaining representative of the disease phenotype) can be used for drug optimization screening. Orthogonal assay resources can be acquired from colleagues, international resource centers (e.g. ZIRC or EZRC), or generated via standard transgenic/mutant methods. The assay format(s) used to detect disease-relevant changes in zebrafish larvae will establish basic parameters, such as throughput rates. A microplate reader embodying key detection functionalities (see Fig. 1) facilitates rapid quantification of any reporter-based assay that can be deployed in vivo. For instance, we have shown that the loss and regeneration of fluorescently labeled neurons can be quantified at near high-throughput rates [44,51]. However, as noted above, nanocarrier-compound screens requiring injections will limit throughput to well below HTS rates anyway. Behavioral platforms can assess more subtle phenotypes and can verify functional recovery, but are by nature low-throughput. High-content imaging approaches have been developed for in vivo screens, such as VAST, which provide a powerful means of assessing complex phenotypes at mid-throughput rates, and would enable precise localization of labeled nanocarriers as an added bonus. Such assay formats may therefore be ideal for assessing nanocarrier-compounds across multiple levels of inquiry.
Concurrently, the physiochemical properties of fluorescently labeled nanocarriers should be evaluated in non-transgenic (but ideally transparent) zebrafish larvae. Using epi-fluorescent or laser-scanning confocal microscopy, changes in the following parameters may be observed: increased absorption/distribution of the nanocarrier throughout the larvae [60,122,162]; boosted localization to a region/cell of interest [121]; and/or decreased compound toxicity [149].
8.5.1.3. Assay optimization (3–4 months).
Establish the ‘signal window’ for the chosen model—i.e., the quantifiable signal difference between fish treated with positive and negative control compounds. The signal window will determine sample size, be used to assess assay quality, and provide an estimate ‘hit’ score for nanocarriers that enhance efficacy. Adjust experimental parameters to maximize the signal window and/or measurable effect size of the positive control compound in order to optimize the assay. In our experience, the Strictly Standardized Mean Difference (SSMD) is a useful measure of effect size that accounts for the inherent variability of in vivo model systems [44].
8.5.1.4. Nanocarrier-enabled enhancer Screen (time length depends on the number of nanocarriers tested).
Compare lead compound efficacy with and without nanocarriers by microinjection or waterborne exposure (using the latter if available). To evaluate phenotypic enhancement of regeneration-promoting compounds, treatments administered post-injury are recommended. To facilitate the discovery of both enhancers and inhibitors of regeneration, evaluate regenerative effects at a time point that produces half-maximal results for the normal untreated control condition (i.e., use a ‘sensitized assay’ that can show both effects). Assay options discussed above (fluorescence quantification, behavioral metrics, high-content imaging) will range in throughput from 2 min to 35 min per 96-well plate.
8.5.1.5. Validation (6–12 months).
Confirm nanocarrier-compound combinations that enhance the efficacy of regeneration-promoting lead compounds. Repeat the optimization screen for implicated nanocarrier-compound combination a minimum of three times to eliminate false positives and further validated across a broad dose-response curve to calculate a therapeutic index (i.e., the ratio of the half-maximal toxic dose to half-maximal effect dose). Finally, orthogonal assays (e.g., immunohistochemistry, other animal models) or counter-screen methods (e.g., enzyme activity assays) should be used to ensure that ‘validated hits’ perform reliably and are promising candidates for progressing to clinical trial phases.
9. Conclusion
Zebrafish have a remarkable capacity to regenerate lost neurons through endogenous neural stem cell populations, and are a firmly established vertebrate model system for interrogating the complex biology associated with neuroregeneration through large-scale genetic and chemical screens. Zebrafish are also highly amenable to in vivo examination of nanocarriers, allowing direct visualization of nanocarrier interactions at cellular and subcellular resolutions in living disease models. There are numerous physiological differences between fish and humans that impact predictive translation that thus require careful consideration (e.g., blood/plasma composition, host immune response, and nanoparticle clearance). Moreover, current technical limitations (e.g., nanocarrier-compound formulation, route of administration) underscore barriers to the large-scale application of nanocarrier-based studies in the zebrafish model. Fortunately, mounting evidence demonstrates conservation of nanocarrier properties between zebrafish and mammalian model systems.
Currently, there are few clinically approved nanoparticles [182,183] and no systemic nanoparticle therapies approved for the brain. The zebrafish is a cost-effective model system for interrogating nanoparticle drug delivery systems before moving onto more laborious pharmacokinetic studies in mammalian models, thus warranting employment during early stages of nanocarrier characterization and optimization. Several established whole-organism phenotypic screening platforms can be leveraged to assess nanoparticle-based drug optimization strategies of lead candidates that promote enhanced neuroregeneration kinetics in zebrafish. We propose here that zebrafish are a versatile and useful pre-clinical model for comprehensive mechanistic evaluation of nanocarrier-drug combinations designed to stimulate dormant regenerative capacities in patients with neurodegenerative disease.
Acknowledgements
DW and JSM prepared the manuscript. Dr. Meera Saxena edited the manuscript and provided essential insights. The work was supported by the National Institutes of Health (NIH T32EY7143 & NIH R01 EY022810 to DW and JSM, respectively).
References
- [1].Mullins MC, Hammerschmidt M, Haffter P, Nüsslein-Volhard C, Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate, Curr. Biol 4 (1994) 189–202. [DOI] [PubMed] [Google Scholar]
- [2].Wang G, Rajpurohit SK, Delaspre F, Walker SL, White DT, Ceasrine A, Kuruvilla R, Li RJ, Shim JS, Liu JO, Parsons MJ, Mumm JS, First quantitative high-throughput screen in zebrafish identifies novel pathways for increasing pancreatic β-cell mass, Elife 4 (2015) 10.7554/eLife.08261.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zon LI, Peterson RT, In vivo drug discovery in the zebrafish, Nat. Rev. Drug Discov 4 (2005) 35–44, 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- [4].Langheinrich U, Zebrafish: a new model on the pharmaceutical catwalk, BioEssays 25 (2003) 904–912, 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- [5].Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA, Drugs that induce repolarization abnormalities cause bradycardia in zebrafish, Circulation 107 (2003) 1355–1358, 10.1161/01.CIR.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- [6].Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C,Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Eliott D, Threadgold G, Harden G, Ware D, Mortimer B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P,Barker N, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K,Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J,Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P,Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A,Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Ürün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Carter NP, Harrow J,Ning Z, Herrero J, Searle SMJ, Enright A, Geisler R, Plasterk RHA, Lee C, Westerfield M, De Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJP, Crollius HR, Rogers J, Stemple DL, The zebrafish reference genome sequence and its relationship to the human genome, Nature 496 (2013) 498–503, 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, Fitzgerald GA, Daley GQ, Orkin SH,Zon LI, Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis, Nature 447 (2007) 1007–1011, 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goessling W, North TE, Repairing quite swimmingly: advances in regenerative medicine using zebrafish, Dis. Model. Mech 7 (2014) 769–776, 10.1242/dmm.016352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barreiro-Iglesias A, Mysiak KS, Scott AL, Reimer MM, Yang Y, Becker CG, Becker T, Serotonin promotes development and regeneration of spinal motor neurons in zebrafish, Cell Rep 13 (2015) 924–932, 10.1016/j.celrep.2015.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].White DT, Senguptra S, Saxena MT, Xu Q, Hanes J, Ding D, Ji H, Mumm JS, Immunomodulation-accelerated neuronal regeneration following selective rod photoreceptor cell ablation in the zebrafish retina, Proc. Natl. Acad. Sci 114 (2017) E3719–E3728, 10.1073/pnas.1617721114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wiley DS, Redfield SE, Zon LI, Chemical screening in zebrafish for novel biological and therapeutic discovery, Methods Cell Biol 138 (2017) 651–679, 10.1016/bs.mcb.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].MacRae CA, Peterson RT, Zebrafish as tools for drug discovery, Nat. Rev. Drug Discov 14 (2015) 721–731, 10.1038/nrd4627. [DOI] [PubMed] [Google Scholar]
- [13].Zheng W, Thorne N, McKew JC, Phenotypic screens as a renewed approach for drug discovery, Drug Discov. Today 18 (2013) 1067–1073, 10.1016/j.drudis.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Delvecchio C, Tiefenbach J, Krause HM, The zebrafish: a powerful platform for in vivo, HTS drug discovery, Assay Drug Dev. Technol 9 (2011) 354–361, 10.1089/adt.2010.0346. [DOI] [PubMed] [Google Scholar]
- [15].Pfefferli C, Jaźwińska A, The art of fin regeneration in zebrafish, Regeneration 2 (2015) 72–83, 10.1002/reg2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gemberling M, Bailey TJT, Hyde DRD, Poss KDK, The zebrafish as a model for complex tissue regeneration, Trends Genet 29 (2013) 611–620, 10.1016/j.tig.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].White DT, Mumm JS, The nitroreductase system of inducible targeted ablation facilitates cell-specific regenerative studies in zebrafish, Methods 62 (2013) 232–240, 10.1016/j.ymeth.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DY, Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies, Dev. Dyn 236 (2007) 1025–1035, 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- [19].Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ, Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase, Mech. Dev 124 (2007) 218–229, 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Ham TJ, Brady CA, Kalicharan RD, Oosterhof N, Kuipers J, Veenstra-Algra A, Sjollema KA, Peterson RT, Kampinga HH, Giepmans BNG, Intravital correlated microscopy reveals differential macrophage and microglial dynamics during resolution of neuroinflammation, Dis. Model. Mech 7 (2014) 857–869, 10.1242/dmm.014886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li L, Yan B, Shi YQ, Zhang WQ, Wen ZL, Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration, J. Biol. Chem 287 (2012) 25353–25360, 10.1074/jbc.M112.349126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lahne M, Hyde DR, Live-cell imaging: new avenues to investigate retinal regeneration, Neural Regen. Res 12 (2017) 1210–1219, 10.4103/1673-5374.213533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wehner D, Tsarouchas TM, Michael A, Haase C, Weidinger G, Reimer MM, Becker T, Becker CG, Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish, Nat. Commun 8 (2017) 10.1038/s41467-017-00143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Whitehead KA, Matthews J, Chang PH, Niroui F, Dorkin JR, Severgnini M, Anderson DG, In vitro - in vivo translation of lipid nanoparticles for hepatocellular siRNA delivery, ACS Nano 6 (2012) 6922–6929, 10.1021/nn301922x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fleming A, Diekmann H, Goldsmith P, Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish, PLoS One 8 (2013) 10.1371/journal.pone.0077548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stewart PA, Hayakawa EM, Interendothelial junctional changes underlie the developmental “tightening” of the blood-brain barrier, Dev. Brain Res 32 (1987) 271–281, 10.1016/0165-3806(87)90107-6. [DOI] [PubMed] [Google Scholar]
- [27].Wolburg H, Lippoldt A, Tight junctions of the blood-brain barrier: development, composition and regulation, Vasc. Pharmacol 38 (2002) 323–337, 10.1016/S1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- [28].Herbomel P, Thisse B, Thisse C, Ontogeny and behaviour of early macrophages in the zebrafish embryo, Development 126 (1999) 3735–3745. [DOI] [PubMed] [Google Scholar]
- [29].Davidson AJ, Zon LI, The “definitive” (and “primitive”) guide to zebrafish hematopoiesis, Oncogene 23 (2004) 7233–7246, 10.1038/sj.onc.1207943. [DOI] [PubMed] [Google Scholar]
- [30].Schorpp M, Bialecki M, Diekhoff D, Walderich B, Odenthal J, Maischein H-M, Zapata AG, Boehm T, Conserved functions of Ikaros in vertebrate lymphocyte development: genetic evidence for distinct larval and adult phases of T cell development and two lineages of B cells in zebrafish, J. Immunol 177 (2006) 2463–2476, 10.4049/jimmunol.177.4.2463. [DOI] [PubMed] [Google Scholar]
- [31].Petrie-Hanson L, Hohn C, Hanson L, Characterization of rag1 mutant zebrafish leukocytes, BMC Immunol 10 (2009) 10.1186/1471-2172-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hohn C, Petrie-Hanson L, Rag1−/−mutant zebrafish demonstrate specific protection following bacterial re-exposure, PLoS One 7 (2012) 10.1371/journal.pone.0044451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].van der Vaart M, van Soest JJ, Spaink HP, Meijer AH, Functional analysis of a zebrafish myd88 mutant identifies key transcriptional components of the innate immune system, Dis. Model. Mech 6 (2013) 841–854, 10.1242/dmm.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Ben Mustapha I, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor AB, Herrero-Mata MJ, Aróstegui JI, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M,Fortuny C, Yagüe J, Antón J, Pascal M, Chang HH, Janniere L, Rose Y, Garty BZ, Chapel H, Issekutz A, Maródi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova JL, Pyogenic bacterial infections in humans with MyD88 deficiency, Science (80-. ) 321 (2008) 691–696, 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Renshaw SA, Trede NS, A model 450 million years in the making: zebrafish and vertebrate immunity, Dis. Model. Mech 5 (2012) 38–47, 10.1242/dmm.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang S, Cui P, Complement system in zebrafish, Dev. Comp. Immunol 46 (2014) 3–10, 10.1016/j.dci.2014.01.010. [DOI] [PubMed] [Google Scholar]
- [37].Li YY, Li YY, Cao X, Jin X, Jin T, Pattern recognition receptors in zebrafish provide functional and evolutionary insight into innate immune signaling pathways, Cell. Mol. Immunol 14 (2017) 80–89, 10.1038/cmi.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mashoof S, Criscitiello M, Fish immunoglobulins, Biology (Basel) 5 (2016) 45, 10.3390/biology5040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Weyand AC, Shavit JA, Zebrafish as a model system for the study of hemostasis and thrombosis, Curr. Opin. Hematol 21 (2014) 418–422, 10.1097/MOH.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H, Nanoparticle up-take: the phagocyte problem, Nano Today 10 (2015) 487–510, 10.1016/j.nantod.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li C, Tan XF, Lim TK, Lin Q, Gong Z, Comprehensive and quantitative proteomic analyses of zebrafish plasma reveals conserved protein profiles between genders and between zebrafish and human, Sci. Rep 6 (2016) 10.1038/srep24329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW, Nanoparticle–liver interactions: cellular uptake and hepatobiliary elimination, J. Control. Release 240 (2016) 332–348, 10.1016/j.jconrel.2016.01.020. [DOI] [PubMed] [Google Scholar]
- [43].Campbell F, Bos F, Seiber S, Arias-Alpizar G, Koch B, Huwyler J, Kros A, Bussman J, Directing nanoparticle biodistribution through evasion and exploitation of Stab2-dependent nanoparticle uptake, ACS Nano (2018) 10.1021/acsnano.7b06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].White DT, Eroglu AU, Wang G, Zhang L, Sengupta S, Ding D, Rajpurohit SK, Walker SL, Ji H, Qian J, Mumm JS, ARQiv-HTS, a versatile whole-organism screening platform enabling in vivo drug discovery at high-throughput rates, Nat. Protoc 11 (2016) 10.1038/nprot.2016.142 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chang T-Y, Pardo-Martin C, Allalou A, Wahlby C, Yanik MF, Fully automated cellular-resolution vertebrate screening platform with parallel animal processing, Lab Chip 12 (2012) 711–716, 10.1039/C1LC20849G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vergara MN, Flores-Bellver M, Aparicio-Domingo S, McNally M, Wahlin KJ, Saxena MT, Mumm JS, Canto-Soler MV, Enabling quantitative screening in retinal organoids: 3D automated reporter quantification technology (3D-ARQ), Development (2017) 10.1242/dev.146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Saili KS, Corvi MM, Weber DN, Patel AU, Das SR, Przybyla J, Anderson KA, Tanguay RL, Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish, Toxicology 291 (2012) 83–92, 10.1016/j.tox.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Scott CA, Marsden AN, Slusarski DC, Automated, high-throughput, in vivo analysis of visual function using the zebrafish, Dev. Dyn (2016) 1–47, 10.1002/dvdy.24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hawliczek A, Nota B, Cenijn P, Kamstra J, Pieterse B, Winter R, Winkens K, Hollert H, Segner H, Legler J, Developmental toxicity and endocrine disrupting potency of 4-azapyrene, benzo[b]fluorene and retene in the zebrafish Danio rerio, Reprod. Toxicol 33 (2012) 213–223, 10.1016/j.reprotox.2011.11.001. [DOI] [PubMed] [Google Scholar]
- [50].Henriques JF, Almeida AR, Andrade T, Koba O, Golovko O, Soares AMVM, Oliveira M, Domingues I, Effects of the lipid regulator drug gemfibrozil: a toxicological and behavioral perspective, Aquat. Toxicol 170 (2016) 355–364, 10.1016/j.aquatox.2015.09.017. [DOI] [PubMed] [Google Scholar]
- [51].Walker SL, Ariga J, Mathias JR, Coothankandaswamy V, Xie X, Distel M, Köster RW, Parsons MJ, Bhalla KN, Saxena MT, Mumm JS, Automated reporter quantification in vivo: high-throughput screening method for reporter-based assays in zebrafish, PLoS One 7 (2012) 10.1371/journal.pone.0029916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Knecht AL, Truong L, Simonich MT, Tanguay RL, Developmental benzo[a]pyrene (B[a]P) exposure impacts larval behavior and impairs adult learning in zebrafish, Neurotoxicol. Teratol 59 (2017) 27–34, 10.1016/j.ntt.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Stanley KA, Curtis LR, Massey Simonich SL, Tanguay RL, Endosulfan I and endosulfan sulfate disrupts zebrafish embryonic development, Aquat. Toxicol 95 (2009) 355–361, 10.1016/j.aquatox.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kantae V, Krekels EHJ, Ordas A, González O, van Wijk RC, Harms AC, Racz PI, van der Graaf PH, Spaink HP, Hankemeier T, Pharmacokinetic modeling of paracetamol uptake and clearance in zebrafish larvae: expanding the allometric scale in vertebrates with five orders of magnitude, Zebrafish 13 (2016) 504–510, 10.1089/zeb.2016.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Natarajan JV, Nugraha C, Ng XW, Venkatraman S, Sustained-release from nanocarriers: a review, J. Control. Release 193 (2014) 122–138, 10.1016/j.jconrel.2014.05.029. [DOI] [PubMed] [Google Scholar]
- [56].Yao Y, Sun S, Fei F, Wang J, Wang Y, Zhang R, Wu J, Liu L, Liu X, Cui Z, Li Q, Yu M, Dang Y, Wang X, Screening in larval zebrafish reveals tissue-specific distribution of fifteen fluorescent compounds, Dis. Model. Mech 10 (2017) 1155–1164, 10.1242/dmm.028811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kirla KT, Groh KJ, Steuer AE, Poetzsch M, Banote RK, Stadnicka-Michalak J, Eggen RIL, Schirmer K, Kraemer T, Zebrafish larvae are insensitive to stimulation by cocaine: importance of exposure route and toxicokinetics, Toxicol. Sci 154 (2016) 183–193, 10.1093/toxsci/kfw156. [DOI] [PubMed] [Google Scholar]
- [58].Villacrez OR, Hellman K, Ono T, Sugihara Y, Rezeli M, Ek F, Marko-Varga G, Evaluation of drug exposure and metabolism in locust and zebrafish brains using mass spectrometry imaging, ACS Chem. Neurosci (2018) 10.1021/acschemneuro.7b00459. [DOI] [PubMed] [Google Scholar]
- [59].Lesniak WG, Mishra MK, Jyoti A, Balakrishnan B, Zhang F, Nance E, Romero R,Kannan S, Kannan RM, Biodistribution of fluorescently labeled PAMAM dendrimers in neonatal rabbits: effect of neuroinflammation, Mol. Pharm 10 (2013) 4560–4571, 10.1021/mp400371r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sieber S, Grossen P, Detampel P, Siegfried S, Witzigmann D, Huwyler J, Zebrafish as an early stage screening tool to study the systemic circulation of nanoparticulate drug delivery systems in vivo, J. Control. Release 264 (2017) 180–191, 10.1016/j.jconrel.2017.08.023. [DOI] [PubMed] [Google Scholar]
- [61].Semple SC, Chonn A, Cullis PR, Influence of cholesterol on the association of plasma proteins with liposomes, Biochemistry 35 (1996) 2521–2525, 10.1021/bi950414i. [DOI] [PubMed] [Google Scholar]
- [62].Xie J, Farage E, Sugimoto M, Anand-Apte B, A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development, BMC Dev. Biol 10 (2010) 10.1186/1471-213X-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Li Y, Chen T, Miao X, Yi X, Wang X, Zhao H, Lee SMY, Zheng Y, Zebrafish: a promising in vivo model for assessing the delivery of natural products, fluorescence dyes and drugs across the blood-brain barrier, Pharmacol. Res 125 (2017) 246–257, 10.1016/j.phrs.2017.08.017. [DOI] [PubMed] [Google Scholar]
- [64].Abdouh M, Talbot S, Couture R, Hassian HM, Retinal plasma extravasation in streptozotocin-diabetic rats mediated by kinin B 1 and B 2 receptors, Br. J. Pharmacol 154 (2008) 136–143, 10.1038/bjp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Baraban SC, Taylor MR, Castro PA, Baier H, Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression, Neuroscience 131 (2005) 759–768, 10.1016/j.neuroscience.2004.11.031. [DOI] [PubMed] [Google Scholar]
- [66].Monstad-Rios AT, Watson CJ, Kwon RY, ScreenCube: a 3D printed system for rapid and cost-effective chemical screening in adult zebrafish, Zebrafish (2017) 10.1089/zeb.2017.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Oppedal D, Goldsmith MI, A chemical screen to identify novel inhibitors of fin regeneration in zebrafish, Zebrafish 7 (2010) 53–60, 10.1089/zeb.2009.0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Park HC, Kim CH, Bae YK, Yeo SY, Kim SH, Hong SK, Shin J, Yoo KW, Hibi M,Hirano T, Miki N, Chitnis AB, Huh TL, Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons, Dev. Biol 227 (2000) 279–293, 10.1006/dbio.2000.9898. [DOI] [PubMed] [Google Scholar]
- [69].Tomasiewicz HG, Flaherty DB, Soria JP, Wood JG, Transgenic zebrafish model of neurodegeneration, J. Neurosci. Res 70 (2002) 734–745, 10.1002/jnr.10451. [DOI] [PubMed] [Google Scholar]
- [70].Bai Q, Garver JA, Hukriede NA, Burton EA, Generation of a transgenic zebrafish model of Tauopathy using a novel promoter element derived from the zebrafish eno2 gene, Nucleic Acids Res. 35 (2007) 6501–6516, 10.1093/nar/gkm608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bai Q, Burton EA, Cis-acting elements responsible for dopaminergic neuron-specific expression of zebrafish slc6a3 (dopamine transporter) in vivo are located remote from the transcriptional start site, Neuroscience 164 (2009) 1138–1151, 10.1016/j.neuroscience.2009.09.014. [DOI] [PubMed] [Google Scholar]
- [72].Meng S, Ryu S, Zhao B, Zhang DQ, Driever W, McMahon DG, Targeting retinal dopaminergic neurons in tyrosine hydroxylase-driven green fluorescent protein transgenic zebrafish, Mol. Vis 14 (2008) 2475–2483. [PMC free article] [PubMed] [Google Scholar]
- [73].Wen L, Wei W, Gu W, Huang P, Ren X, Zhang Z, Zhu Z, Lin S, Zhang B, Visualization of monoaminergic neurons and neurotoxicity of MPTP in live transgenic zebrafish, Dev. Biol 314 (2008) 84–92, 10.1016/j.ydbio.2007.11.012. [DOI] [PubMed] [Google Scholar]
- [74].Ariga J, Walker SL, Mumm JS, Multicolor time-lapse imaging of transgenic zebrafish: visualizing retinal stem cells activated by targeted neuronal cell ablation, J. Vis. Exp (2010) 2–7, 10.3791/2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Montgomery JE, Parsons MJ, Hyde DR, A novel model of retinal ablation demonstrates that the extent of rod cell death regulates the origin of the regenerated zebrafish rod photoreceptors, J. Comp. Neurol 518 (2010) 800–814, 10.1002/cne.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fraser B, DuVal MG, Wang H, Allison WT, Regeneration of cone photoreceptors when cell ablation is primarily restricted to a particular cone subtype, PLoS One 8 (2013) 10.1371/journal.pone.0055410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Choi JH, Law MY, Bin Chien C, Link BA, Wong ROL, In vivo development of dendritic orientation in wild-type and mislocalized retinal ganglion cells, Neural Dev 5 (2010) 10.1186/1749-8104-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bremer J, Skinner J, Granato M, A small molecule screen identifies in vivo modulators of peripheral nerve regeneration in zebrafish, PLoS One 12 (2017) 10.1371/journal.pone.0178854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Paquet D, Bhat R, Sydow A, Mandelkow E, Berg S, Hellberg S, Fälting J, Distel M, Köster RW, Schmid B, Haass C, A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation, J. Clin. Invest 119 (2009) 1382–1395, 10.1172/JCI37537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, Jahreiss L, Fleming A, Pask D, Goldsmith P, O’Kane CJ, Floto RA, Rubinsztein DC, Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway, Nat. Chem. Biol 4 (2008) 295–305, 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chung AY, Kim PS, Kim S, Kim E, Kim D, Jeong I, Kim HK, Ryu JH, Kim CH, Choi J, Seo JH, Park HC, Generation of demyelination models by targeted ablation of oligodendrocytes in the zebrafish CNS, Mol. Cells 36 (2013) 82–87, 10.1007/s10059-013-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Flinn L, Mortiboys H, Volkmann K, Kster RW, Ingham PW, Bandmann O, Complex i deficiency and dopaminergic neuronal cell loss in parkin-deficient zebrafish (Danio rerio), Brain 132 (2009) 1613–1623, 10.1093/brain/awp108. [DOI] [PubMed] [Google Scholar]
- [83].Anichtchik O, Diekmann H, Fleming A, Roach A, Goldsmith P, Rubinsztein DC, Loss of PINK1 function affects development and results in neurodegeneration in zebrafish, J. Neurosci 28 (2008) 8199–8207, 10.1523/JNEUROSCI.0979-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lemmens R, Van Hoecke A, Hersmus N, Geelen V, D’Hollander I, Thijs V, Van Den Bosch L, Carmeliet P, Robberecht W, Overexpression of mutant superoxide dismutase 1 causes a motor axonopathy in the zebrafish, Hum. Mol. Genet 16 (2007) 2359–2365, 10.1093/hmg/ddm193. [DOI] [PubMed] [Google Scholar]
- [85].Rubel EW, Dew LA, Roberson DW, Warchol ME, Corwin JT, Forge A, Li L, Nevill G, Mammalian vestibular hair cell regeneration, Science (80-. ) 267 (1995) 701–707, 10.1126/science.7839150. [DOI] [PubMed] [Google Scholar]
- [86].Graziadei PPC, Graziadei GAM, Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons, J. Neurocytol 8 (1979) 1–18, 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- [87].Chargé SBP, Rudnicki MA, Cellular and molecular regulation of muscle regeneration, Physiol. Rev 84 (2004) 209–238, 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- [88].Kawakami K, Transposon tools and methods in zebrafish, Dev. Dyn 234 (2005) 244–254, 10.1002/dvdy.20516. [DOI] [PubMed] [Google Scholar]
- [89].Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JRJ, Joung JK, Efficient genome editing in zebrafish using a CRISPR-Cas system, Nat. Biotechnol 31 (2013) 227–229, 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Carradori D, Eyer J, Saulnier P, Préat V, Des Rieux A, The therapeutic contribution of nanomedicine to treat neurodegenerative diseases via neural stem cell differentiation, Biomaterials 123 (2017) 77–91, 10.1016/j.biomaterials.2017.01.032. [DOI] [PubMed] [Google Scholar]
- [91].Mathew LK, Sengupta S, Kawakami A, Andreasen EA, Löhr CV, Loynes CA, Renshaw SA, Peterson RT, Tanguay RL, Unraveling tissue regeneration pathways using chemical genetics, J. Biol. Chem 282 (2007) 35202–35210, 10.1074/jbc.M706640200. [DOI] [PubMed] [Google Scholar]
- [92].Petrie TA, Strand NS, Yang C-T, Rabinowitz JS, Moon RT, Macrophages modulate adult zebrafish tail fin regeneration, Development 141 (2014) 2581–2591, 10.1242/dev.098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lai SL, Marín-Juez R, Moura PL, Kuenne C, Lai JKH, Tsedeke AT, Guenther S, Looso M, Stainier DYR, Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration, Elife 6 (2017) 10.7554/eLife.25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Choi W-Y, Gemberling M, Wang J, Holdway JE, Shen M-C, Karlstrom RO, Poss KD, In vivo monitoring of cardiomyocyte proliferation to identify chemical modifiers of heart regeneration, Development 140 (2013) 660–666, 10.1242/dev.088526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Andersson O, Adams BA, Yoo D, Ellis GC, Gut P, Anderson RM, German MS, Stainier DYR, Adenosine signaling promotes regeneration of pancreatic β cells in vivo, Cell Metab 15 (2012) 885–894, 10.1016/j.cmet.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rosenberg AF, Isaacman-Beck J, Franzini-Armstrong C, Granato M, Schwann cells and deleted in colorectal carcinoma direct regenerating motor axons towards their original path, J. Neurosci 34 (2014) 14668–14681, 10.1523/JNEUROSCI.2007-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Rosenberg AF, Wolman MA, Franzini-Armstrong C, Granato M, In vivo nerve-macrophage interactions following peripheral nerve injury, J. Neurosci 32 (2012) 3898–3909, 10.1523/JNEUROSCI.5225-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Owens KN, Santos F, Roberts B, Linbo T, Coffin AB, Knisely AJ, Simon JA, Rubel EW, Raible DW, Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death, PLoS Genet 4 (2008) 10.1371/journal.pgen.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pei W, Xu L, Huang S, Pettie K, Idol J, Rissone A, Jimenez E, Sinclair J, Slevin C, Varshney G, Jones M, Carrignton B, Bishop K, Huang H, Sood R, Lin S, Burgess S, Guided genetic screen to identify genes essential in the regeneration of hair cells and other tissues, NPJ Regen. Med 3 (2018) 11, 10.1038/s41536-018-0050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Namdaran P, Reinhart KE, Owens KN, Raible DW, Rubel EW, Identification of modulators of hair cell regeneration in the zebrafish lateral line, J. Neurosci 32 (2012) 3516–3528, 10.1523/JNEUROSCI.3905-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chowdhury S, Owens KN, Herr RJ, Jiang Q, Chen X, Johnson G, Groppi VE, Raible DW, Rubel EW, Simon JA, Phenotypic optimization of urea-thiophene carboxamides to yield potent, well tolerated, and orally active protective agents against aminoglycoside-induced hearing loss, J. Med. Chem . 61 (2018) 84–97, 10.1021/acs.jmedchem.7b00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R, Kannan RM, Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model, Sci. Transl. Med 4 (2012) 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Skjolding LM, Ašmonaitė G, Jølck RI, Andresen TL, Selck H, Baun A, Sturve J, An assessment of the importance of exposure routes to the uptake and internal localisation of fluorescent nanoparticles in zebrafish (Danio rerio), using light sheet microscopy, Nanotoxicology 11 (2017) 351–359, 10.1080/17435390.2017.1306128. [DOI] [PubMed] [Google Scholar]
- [104].Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Nancy Xu XH, In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos, ACS Nano 1 (2007) 133–143, 10.1021/nn700048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Li Y, Miao X, Chen T, Yi X, Wang R, Zhao H, Lee SMY, Wang X, Zheng Y, Zebrafish as a visual and dynamic model to study the transport of nanosized drug delivery systems across the biological barriers, Colloids Surf. B Biointerf 156 (2017) 227–235, 10.1016/j.colsurfb.2017.05.022. [DOI] [PubMed] [Google Scholar]
- [106].Junyaprasert VB, Morakul B, Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs, Asian J. Pharm. Sci 10 (2015) 13–23, 10.1016/j.ajps.2014.08.005. [DOI] [Google Scholar]
- [107].Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW, Analysis of nanoparticle delivery to tumours, Nat. Rev. Mater 1 (2016) 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- [108].Johansen PL, Fenaroli F, Evensen L, Griffiths G, Koster G, Optical micromanipulation of nanoparticles and cells inside living zebrafish, Nat. Commun 7 (2016) 10.1038/ncomms10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Jayakumar MKG, Bansal A, Li BN, Zhang Y, Mesoporous silica-coated upconversion nanocrystals for near infrared light-triggered control of gene expression in zebrafish, Nanomedicine 10 (2015) 1051–1061, 10.2217/nnm.14.198. [DOI] [PubMed] [Google Scholar]
- [110].Savchenko A, Cherkas V, Liu C, Braun GB, Kleschevnikov A, Miller YI, Molokanova E, Graphene biointerfaces for optical stimulation of cells, Sci. Adv (2018) 10.1126/sciadv.aat0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L, Nanoparticle-mediated brain drug delivery: overcoming blood-brain barrier to treat neurodegenerative diseases, J. Control. Release 235 (2016) 34–47, 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
- [112].Huwyler J, Drewe J, Krähenbühl S, Tumor targeting using liposomal antineoplastic drugs, Int. J. Nanomedicine 3 (2008) 21–29, 10.2147/IJN.S1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Harrington KJ, Rowlinson-Busza G, Syrigos KN, Uster PS, Abra RM, Stewart JS, Biodistribution and pharmacokinetics of 111In-DTPA-labelled pegylated liposomes in a human tumour xenograft model: implications for novel targeting strategies, Br. J. Cancer 83 (2000) 232–238, 10.1054/bjoc.1999.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Chen Z, Zhai M, Xie X, Zhang Y, Ma S, Li Z, Yu F, Zhao B, Zhang M, Yang Y, Mei X, Apoferritin nanocage for brain targeted doxorubicin delivery, Mol. Pharm 14 (2017) 3087–3097, 10.1021/acs.molpharmaceut.7b00341. [DOI] [PubMed] [Google Scholar]
- [115].Zou D, Wang W, Lei D, Yin Y, Ren P, Chen J, Yin T, Wang B, Wang G, Wang Y, Penetration of blood–brain barrier and antitumor activity and nerve repair in glioma by doxorubicin-loaded monosialoganglioside micelles system, Int. J. Nanomed 12 (2017) 10.2147/IJN.S138257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Capiotti KM, Antonioli R, Kist LW, Bogo MR, Bonan CD, Da Silva RS, Persistent impaired glucose metabolism in a zebrafish hyperglycemia model, Comp. Biochem. Physiol. Part B Biochem. Mol. Biol 171 (2014) 58–65, 10.1016/j.cbpb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- [117].Jorstad NL, Wilken MS, Grimes WN, Wohl SG, Vandenbosch LS, Yoshimatsu T, Wong RO, Rieke F, Reh TA, Stimulation of functional neuronal regeneration from Müller glia in adult mice, Nature 548 (2017) 103–107, 10.1038/nature23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kim DH, Seo YK, Thambi T, Moon GJ, Son JP, Li G, Park JH, Lee JH, Kim HH, Lee DS, Bang OY, Enhancing neurogenesis and angiogenesis with target delivery of stromal cell derived factor-1α using a dual ionic pH-sensitive copolymer, Biomaterials 61 (2015) 115–125, 10.1016/j.biomaterials.2015.05.025. [DOI] [PubMed] [Google Scholar]
- [119].Saraiva C, Paiva J, Santos T, Ferreira L, Bernardino L, MicroRNA-124 loaded nanoparticles enhance brain repair in Parkinson’s disease, J. Control. Release 235 (2016) 291–305, 10.1016/j.jconrel.2016.06.005. [DOI] [PubMed] [Google Scholar]
- [120].Tiwari SK, Agarwal S, Seth B, Yadav A, Nair S, Bhatnagar P, Karmakar M, Kumari M, Chauhan LKS, Patel DK, Srivastava V, Singh D, Gupta SK, Tripathi A, Chaturvedi RK, Gupta KC, Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway, ACS Nano 8 (2014) 76–103, 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- [121].Fenaroli F, Westmoreland D, Benjaminsen J, Kolstad T, Skjeldal FM, Meijer AH,Van Der Vaart M, Ulanova L, Roos N, Nyström B, Hildahl J, Griffiths G, Nanoparticles as drug delivery system against tuberculosis in zebrafish embryos: direct visualization and treatment, ACS Nano 8 (2014) 7014–7026, 10.1021/nn5019126. [DOI] [PubMed] [Google Scholar]
- [122].Evensen L, Johansen PL, Koster G, Zhu K, Herfindal L, Speth M, Fenaroli F, Hildahl J, Bagherifam S, Tulotta C, Prasmickaite L, Mælandsmo GM, Snaar-Jagalska E, Griffiths G, Zebrafish as a model system for characterization of nanoparticles against cancer, Nanoscale 8 (2016) 862–877, 10.1039/C5NR07289A. [DOI] [PubMed] [Google Scholar]
- [123].Dumitrescu E, Wallace K, Andreescu S, Nanotoxicity assessment using embryonic zebrafish, Nanotoxicity, Humana Press; 2018, pp. 331–343, 10.1007/978-1-4939-8916-4. [DOI] [PubMed] [Google Scholar]
- [124].Griffitt RJ, Hyndman K, Denslow ND, Barber DS, Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles, Toxicol. Sci 107 (2009) 404–415, 10.1093/toxsci/kfn256. [DOI] [PubMed] [Google Scholar]
- [125].Choi JE, Kim S, Ahn JH, Youn P, Kang JS, Park K, Yi J, Ryu DY, Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish, Aquat. Toxicol 100 (2010) 151–159, 10.1016/j.aquatox.2009.12.012. [DOI] [PubMed] [Google Scholar]
- [126].Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS, Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms, Environ. Toxicol. Chem 27 (2008) 1972–1978, 10.1897/08-002.1. [DOI] [PubMed] [Google Scholar]
- [127].Asharani PV, Lian Wu Y, Gong Z, Valiyaveettil S, Toxicity of silver nanoparticles in zebrafish models, Nanotechnology 19 (2008) 10.1088/0957-4484/19/25/255102. [DOI] [PubMed] [Google Scholar]
- [128].Bar-Ilan O, Albrecht RM, Fako VE, Furgeson DY, Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos, Small 5 (2009) 1897–1910, 10.1002/smll.200801716. [DOI] [PubMed] [Google Scholar]
- [129].Behra M, Bradsher J, Sougrat R, Gallardo V, Allende ML, Burgess SM, Phoenix is required for mechanosensory hair cell regeneration in the zebrafish lateral line, PLoS Genet 5 (2009) 10.1371/journal.pgen.1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Browning LM, Lee KJ, Huang T, Nallathamby PD, Lowman JE, Nancy Xu X-H, Random walk of single gold nanoparticles in zebrafish embryos leading to stochastic toxic effects on embryonic developments, Nanoscale 1 (2009) 138, 10.1039/b9nr00053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Harper S, Usenko C, Hutchison JE, Maddux BLS, Tanguay RL, In vivo biodistribution and toxicity depends on nanomaterial composition, size, surface functionalisation and route of exposure, J. Exp. Nanosci 3 (2008) 195–206, 10.1080/17458080802378953. [DOI] [Google Scholar]
- [132].Haque E, Ward A, Zebrafish as a model to evaluate nanoparticle toxicity, Nano 8 (2018) 561, 10.3390/nano8070561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Xu M, Fujita D, Kajiwara S, Minowa T, Li X, Takemura T, Iwai H, Hanagata N, Contribution of physicochemical characteristics of nano-oxides to cytotoxicity, Biomaterials 31 (2010) 8022–8031, 10.1016/j.biomaterials.2010.06.022. [DOI] [PubMed] [Google Scholar]
- [134].Huang X, Teng X, Chen D, Tang F, He J, The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function, Biomaterials 31 (2010) 438–448, 10.1016/j.biomaterials.2009.09.060. [DOI] [PubMed] [Google Scholar]
- [135].Limbach LK, Li Y, Grass RN, Brunner TJ, Hintermann MA, Muller M, Gunther D, Stark WJ, Oxide nanoparticle uptake in human lung fibroblasts: effects of particle size, agglomeration, and diffusion at low concentrations, Environ. Sci. Technol 39 (2005) 9370–9376, 10.1021/es051043o. [DOI] [PubMed] [Google Scholar]
- [136].Karlsson HL, Gustafsson J, Cronholm P, Möller L, Size-dependent toxicity of metal oxide particles-a comparison between nano- and micrometer size, Toxicol. Lett 188 (2009) 112–118, 10.1016/j.toxlet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- [137].Lee J, Mahendra S, Alvarez PJJ, Nanomaterials in the construction industry: a review of their applications and environmental health and safety considerations, ACS Nano 4 (2010) 3580–3590, 10.1021/nn100866w. [DOI] [PubMed] [Google Scholar]
- [138].Zhu X, Zhu L, Li Y, Duan Z, Chen W, Alvarez PJJ, Developmental toxicity in zebrafish (Danio rerio) embryos after exposure to manufactured nanomaterials: buckminsterfullerene aggregates (nC60) and fullerol, Environ. Toxicol. Chem 26 (2007) 976–979, 10.1897/06-583.1. [DOI] [PubMed] [Google Scholar]
- [139].Zhu X, Wang J, Zhang X, Chang Y, Chen Y, The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio), Nanotechnology 20 (2009) 10.1088/0957-4484/20/19/195103. [DOI] [PubMed] [Google Scholar]
- [140].Schubert D, Dargusch R, Raitano J, Chan SW, Cerium and yttrium oxide nanoparticles are neuroprotective, Biochem. Biophys. Res. Commun 342 (2006) 86–91, 10.1016/j.bbrc.2006.01.129. [DOI] [PubMed] [Google Scholar]
- [141].Bhushan B, Nandhagopal S, Rajesh Kannan R, Gopinath P, Biomimetic nanomaterials: development of protein coated nanoceria as a potential antioxidative nano-agent for the effective scavenging of reactive oxygen species in vitro and in zebrafish model, Colloids Surf. B Biointerf 146 (2016) 375–386, 10.1016/j.colsurfb.2016.06.035. [DOI] [PubMed] [Google Scholar]
- [142].Niu J, Wang K, Kolattukudy PE, Cerium oxide nanoparticles inhibits oxidative stress and nuclear factor- B activation in H9c2 cardiomyocytes exposed to cigarette smoke extract, Pharmacology 338 (2011) 53–61, 10.1124/jpet.111.179978.and. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Usenko CY, Harper SL, Tanguay RL, Fullerene C60 exposure elicits an oxidative stress response in embryonic zebrafish, Toxicol. Appl. Pharmacol 229 (2008) 44–55, 10.1016/j.taap.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Usenko CY, Harper SL, Tanguay RL, In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish, Carbon N. Y 45 (2007) 1891–1898, 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Asharani PV, Serina NGB, Nurmawati MH, Wu YL, Gong Z, Valiyaveettil S, Impact of multi-walled carbon nanotubes on aquatic species, J. Nanosci. Nanotechnol 8 (2008) 3603–3609, 10.1166/jnn.2008.432. [DOI] [PubMed] [Google Scholar]
- [146].Cheng J, Flahaut E, Cheng SH, Effect of carbon nanotubes on developing zebrafish (Danio rerio) embryos, Environ. Toxicol. Chem 26 (2007) 708–716, 10.1897/06-272R.1. [DOI] [PubMed] [Google Scholar]
- [147].Falconer JL, Jones CF, Lu S, Grainger DW, Carbon nanomaterials rescue phenanthrene toxicity in zebrafish embryo cultures, Environ. Sci. Nano 2 (2015) 645–652, 10.1039/c5en00111k. [DOI] [Google Scholar]
- [148].King-Heiden TC, Wiecinski PN, Mangham AN, Metz KM, Nesbit D, Pedersen JA, Hamers RJ, Heideman W, Peterson RE, Quantum dot nanotoxicity assessment using the zebrafish embryo, Environ. Sci. Technol 43 (2009) 1605–1611, 10.1021/es801925c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Pryor JB, Harper BJ, Harper SL, Comparative toxicological assessment of PAMAM and thiophosphoryl dendrimers using embryonic zebrafish, Int. J. Nanomedicine 9 (2014) 1947–1956, 10.2147/IJN.S60220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Jones CF, Campbell RA, Franks Z, Gibson CC, Thiagarajan G, Vieira-De-Abreu A,Sukavaneshvar S, Mohammad SF, Li DY, Ghandehari H, Weyrich AS, Brooks BD, Grainger DW, Cationic PAMAM dendrimers disrupt key platelet functions, Mol. Pharm 9 (2012) 1599–1611, 10.1021/mp2006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Jones CF, Campbell RA, Brooks AE, Assemi S, Tadjiki S, Thiagarajan G, Mulcock C, Weyrich AS, Brooks BD, Ghandehari H, Grainger DW, Cationic PAMAM dendrimers aggressively initiate blood clot formation, ACS Nano 6 (2012) 9900–9910, 10.1021/nn303472r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Igartúa DE, Martinez CS, Temprana CF, Del V. Alonso S, Prieto MJ, PAMAM dendrimers as a carbamazepine delivery system for neurodegenerative diseases: a biophysical and nanotoxicological characterization, Int. J. Pharm 544 (2018) 191–202, 10.1016/j.ijpharm.2018.04.032. [DOI] [PubMed] [Google Scholar]
- [153].Tortiglione C, Quarta A, Malvindi MA, Tino A, Pellegrino T, Fluorescent nanocrystals reveal regulated portals of entry into and between the cells of Hydra, PLoS One 4 (2009) 10.1371/journal.pone.0007698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Rittner K, Benavente A, Bompard-Sorlet A, Heitz F, Divita G, Brasseur R, Jacobs E, New basic membrane-destabilizing peptides for plasmid-based gene delivery in vitro and in vivo, Mol. Ther 5 (2002) 104–114, 10.1006/mthe.2002.0523. [DOI] [PubMed] [Google Scholar]
- [155].Pasqualini R, Koivunen E, Ruoslahti E, αv Integrins as receptors for tumor targeting by circulating ligands, Nat. Biotechnol 15 (1997) 542–546, 10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- [156].Zhang F, Mastorakos P, Mishra MK, Mangraviti A, Hwang L, Zhou J, Hanes J, Brem H, Olivi A, Tyler B, Kannan RM, Uniform brain tumor distribution and tumor associated macrophage targeting of systemically administered dendrimers, Biomaterials 52 (2015) 507–516, 10.1016/j.biomaterials.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].He H, Yuan Q, Bie J, Wallace RL, Yannie PJ, Wang J, Lancina MG, Zolotarskaya OY, Korzun W, Yang H, Ghosh S, Development of mannose functionalized dendrimeric nanoparticles for targeted delivery to macrophages: use of this platform to modulate atherosclerosis, Transl. Res 193 (2018) 13–30, 10.1016/j.trsl.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Soiberman U, Kambhampati SP, Wu T, Mishra MK, Oh Y, Sharma R, Wang J, Al Towerki AE, Yiu S, Stark WJ, Kannan RM, Subconjunctival injectable dendrimer-dexamethasone gel for the treatment of corneal inflammation, Biomaterials 125 (2017) 38–53, 10.1016/j.biomaterials.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wang B, Navath RS, Romero R, Kannan S, Kannan R, Anti-inflammatory and anti-oxidant activity of anionic dendrimer-N-acetyl cysteine conjugates in activated microglial cells, Int. J. Pharm 377 (2009) 159–168, 10.1016/j.ijpharm.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Hallare AV, Köhler HR, Triebskorn R, Developmental toxicity and stress protein responses in zebrafish embryos after exposure to diclofenac and its solvent, DMSO, Chemosphere 56 (2004) 659–666, 10.1016/j.chemosphere.2004.04.007. [DOI] [PubMed] [Google Scholar]
- [161].Maes J, Verlooy L, Buenafe OE, de Witte PAM, Esguerra CV, Crawford AD, Evaluation of 14 organic solvents and carriers for screening applications in zebrafish embryos and larvae, PLoS One 7 (2012) 10.1371/journal.pone.0043850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].van Pomeren M, Brun NR, Peijnenburg WJGM, Vijver MG, Exploring uptake and biodistribution of polystyrene (nano)particles in zebrafish embryos at different developmental stages, Aquat. Toxicol 190 (2017) 40–45, 10.1016/j.aquatox.2017.06.017. [DOI] [PubMed] [Google Scholar]
- [163].Bury NR, Grosell M, Grover AK, Wood CM, ATP-dependent silver transport across the basolateral membrane of rainbow trout gills, Toxicol. Appl. Pharmacol 159 (1999) 1–8, 10.1006/taap.1999.8706. [DOI] [PubMed] [Google Scholar]
- [164].Wood CM, Playle RC, Hogstrand C, Physiology and modeling of mechanisms of silver uptake and toxicity in fish, Environ. Toxicol. Chem 18 (1999) 71–83, 10.1897/1551-5028(1999)018b0071:PAMOMON2.3.CO;2. [DOI] [Google Scholar]
- [165].Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K, Taylor D, Barber DS, Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio), Environ. Sci. Technol 41 (2007) 8178–8186, 10.1021/es071235e. [DOI] [PubMed] [Google Scholar]
- [166].Kitchens KM, El-Sayed MEH, Ghandehari H, Transepithelial and endothelial transport of poly (amidoamine) dendrimers, Adv. Drug Deliv. Rev 57 (2005) 2163–2176, 10.1016/j.addr.2005.09.013. [DOI] [PubMed] [Google Scholar]
- [167].Yellepeddi VK, Ghandehari H, Poly(amido amine) dendrimers in oral delivery, Tissue Barr. 4 (2016) 10.1080/21688370.2016.1173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Mohammed MA, Syeda JTM, Wasan KM, Wasan EK, An overview of chitosan nanoparticles and its application in non-parenteral drug delivery, Pharmaceutics 9 (2017) 10.3390/pharmaceutics9040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Yellepeddi V, Mohammad R, Kambhampati S, Sayre C, Mishra M, Kannan R, Ghandehari H, Pediatric oral formulation of dendrimer-N-acetyl-l-cysteine conjugates for the treatment of neuroinflammation, Int. J. Pharm 545 (2018) 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Yun Y, Cho YW, Park K, Nanoparticles for oral delivery: targeted nanoparticles with peptidic ligands for oral protein delivery, Adv. Drug Deliv. Rev 65 (2013) 822–832, 10.1016/j.addr.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Whitehead K, Karr N, Mitragotri S, Safe and effective permeation enhancers for oral drug delivery, Pharm. Res 25 (2008) 1782–1788, 10.1007/s11095-007-9488-9. [DOI] [PubMed] [Google Scholar]
- [172].Vaughan M, Van Egmond R, The use of the zebrafish (Danio rerio) embryo for the acute toxicity testing of surfactants, as a possible alternative to the acute fish test, ATLA Altern. Lab. Anim 38 (2010) 231–238. [DOI] [PubMed] [Google Scholar]
- [173].Nagpal K, Singh SK, Mishra DN, Chitosan nanoparticles: a promising system in novel drug delivery, Chem. Pharm. Bull. (Tokyo) 58 (2010) 1423–1430, 10.1248/cpb.58.1423. [DOI] [PubMed] [Google Scholar]
- [174].Hu Y-L, Qi W, Han F, Shao J-Z, Gao J-Q, Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model, Int. J. Nanomedicine 6 (2011) 3351–3359, 10.2147/IJN.S25853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Teijeiro-Valiño C, Yebra-Pimentel E, Guerra-Varela J, Csaba N, Alonso MJ, Sánchez L, Assessment of the permeability and toxicity of polymeric nanocapsules using the zebrafish model, Nanomedicine 12 (2017) 2069–2082, 10.2217/nnm-2017-0078. [DOI] [PubMed] [Google Scholar]
- [176].Smith SJ, Horstick EJ, Davidson AE, Dowling J, Analysis of zebrafish larvae skeletal muscle integrity with evans blue dye, J. Vis. Exp (2015) 10.3791/53183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Benard EL, van der Sar AM, Ellett F, Lieschke GJ, Spaink HP, Meijer AH, Infection of zebrafish embryos with intracellular bacterial pathogens, J. Vis. Exp (2012) 1–8, 10.3791/3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Carvalho R, de Sonneville J, Stockhammer OW, Savage NDL, Veneman WJ, Ottenhoff THM, Dirks RP, Meijer AH, Spaink HP, A high-throughput screen for tuberculosis progression, PLoS One 6 (2011) 10.1371/journal.pone.0016779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Spaink HP, Cui C, Wiweger MI, Jansen HJ, Veneman WJ, Marín-Juez R, De Sonneville J, Ordas A, Torraca V, van der Ent W, Leenders WP, Meijer AH, Snaar-Jagalska BE, Dirks RP, Robotic injection of zebrafish embryos for high-throughput screening in disease models, Methods 62 (2013) 246–254, 10.1016/j.ymeth.2013.06.002. [DOI] [PubMed] [Google Scholar]
- [180].Zhang J, Wu L, Chan HK, Watanabe W, Formation, characterization, and fate of inhaled drug nanoparticles, Adv. Drug Deliv. Rev 63 (2011) 441–455, 10.1016/j.addr.2010.11.002. [DOI] [PubMed] [Google Scholar]
- [181].Chan HK, Kwok PCL, Production methods for nanodrug particles using the bottom-up approach, Adv. Drug Deliv. Rev 63 (2011) 406–416, 10.1016/j.addr.2011.03.011. [DOI] [PubMed] [Google Scholar]
- [182].Ventola CL, Progress in nanomedicine: approved and investigational nanodrugs, PT. 42 (2017) 742–755. [PMC free article] [PubMed] [Google Scholar]
- [183].Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR, Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date, Pharm. Res 33 (2016) 2373–2387, 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]