Abstract
Allium tripedale (A. tripedale) is a species of wild Allium native to northwest Iran that its hepatoprotective effects have not yet been confirmed. This study investigated the effect of A. tripedale plant against acetaminophen (APAP)-induced acute liver damage. After preliminary studies, the A. tripedale methanol fraction (ATMF) was selected for in vivo study. Thirty-six rats were divided into six groups of 6 each and treated by gavage as follows: groups 1 and 2 received normal saline; group 3 received 400 mg/kg of ATMF; and groups 4-6 were treated with 100, 200, and 400 mg/kg of ATMF, respectively. After two consecutive weeks, except groups 1 and 3, rats were administered with an oral single dose of APAP (2 g/kg). After 48 h, blood and liver samples were collected for histological and biochemical examinations. The results showed that APAP caused a significant increase in alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase and alkaline phosphatase serum levels, lipid peroxidation (all with P < 0.001) and hepatic nitric oxide (P < 0.01). In addition, APAP led to the depletion of the total antioxidant capacity, total thiol group (both with P < 0.001), and structural alterations in the hepatic tissue. Following administration of ATMF extract, a significant improvement was observed in the functional and oxidative stress markers of hepatic tissue alongside histopathologic changes. In conclusion, the present study showed that the administration of ATMF might prevent hepatic oxidative damage by improving oxidant/antioxidant balance in animals exposed to APAP.
Keywords: Acetaminophen, Allium tripedale, Hepatoprotective, Oxidative stress
INTRODUCTION
Acetaminophen (APAP) is one of the main over-the-counter medicines, which is used for its analgesic and antipyretic effects. Overdose of APAP is one of the most common causes of drug-related liver injury, whereas its hepatotoxicity is a reason for liver transplantation in the world (1,2). According to the National Poison Data System annual report in 2016, APAP and its combination products were listed as the fifth and seventh highest causes of fatalities related to substances poisoning (3).
In APAP overdose (more than 150 mg/kg), increasing toxic metabolites like N-acetyl-p-benzoquinone imine and subsequently decreasing hepatocellular glutathione levels can lead to oxidative damages. In addition, mitochondrial membrane permeability transition pore opening, matrix swelling, cessation of ATP production, and DNA damage can be effective in acute hepatic failure induced by APAP (1,2).
Allium is a large genus of onion or garlic-scented bulbous herbs which belongs to the Amaryllidaceae family. Several studies have shown therapeutic effects of Allium on various pathologic statuses such as cardiovascular diseases, cancer, diabetes, and hepatic failure (4,5,6,7,8).
For instance, Obioha et al. showed that onion could improve liver function alongside increase the liver content of glutathione in the cadmium-induced oxidative damage in rats (7). Also, Ogunmodede et al. found that onion exerts hepatoprotective effects through improving oxidant/antioxidant balance in diabetic rabbits (8). There are also evidences in support of hepatoprotective effects of other Allium species such as A. hirtifolum, A. sativum, and A. tuberosum in various animal models (9,10,11).
A. tripedale is a species of wild Allium native to northwest Iran. The leaves which have strong and somewhat unpleasant taste are used as a spice vegetable by local people (12). According to local evidence, this plant traditionally is used for rheumatic and joint pains, and treating bladder and kidney stones in Lorestan province, I.R. Iran. Additionally, few studies have proved its antifungal and antibacterial activities (13,14).
The aim of the present study was to evaluate the hepatoprotective effects of A. tripedale on the hepatocellular oxidative damage induced by APAP in the liver tissue of rats.
MATERIAL AND METHODS
All chemicals were purchased from Merck (Darmstadt, Germany) unless otherwise stated. APAP (99% purity, CAS#: 103-90-2), 2-thiobarbituric acid, Coomassie brilliant blue G-250, and 5,5’-dithiobis (2-nitrobenzoic acid) were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA).
Collection and fractionation of Allium tripedale plant
A. tripedale was collected in May 2018 from Sanandaj, Kurdistan province, western Iran. The herb identified by a botanist and a specimen (No. 404) was deposited in the Hamadan Research and Education Center for Agricultural and Natural Resources. Fifteen g of the shade-dried plant was crushed, extracted with ethanol 50%, and filtered through a Whatman No.1 filter paper. The solution was concentrated by rotary evaporator under vacuum. The hydroalcoholic extract was suspended in methanol and filtered using Whatman No. 1 filter paper for separation of methanol soluble compounds. The extract was fractionated on a silica gel column eluted sequentially with hexane, ethyl acetate, and methanol. The antioxidant capacity of all fractions (hexane, ethyl acetate, methanolic, and hydroalcoholic fractions) was determined by ferric reducing antioxidant power (FRAP) method to choose the best fraction for further studies. High quantity of selected fraction was prepared and kept at 4 °C until in vivo studies.
Animal experiments
Thirty-six male albino rats (250-300 g) were provided from the animal house of Hamadan University of Medical Science, I.R. Iran. They were kept in the animal laboratory of Faculty of Pharmacy of Hamadan University of Medical Science at 23 ± 2 °C and relative humidity 50% and 12/12-h light/dark cycle for one week prior and during the study. The study protocol was approved by the Institutional Animal Ethics Committee of Hamadan University of Medical Science with the ethical number: IR.UMSHA.REC.1396.491.
Preliminary studies
The possible acute toxicity of A. tripedale methanol fraction (ATMF) was studied on rats at doses of 5, 50, 300, 2000, and 4000 mg/kg. After 24 h, liver function was evaluated by measuring serum alanine aminotransferase (ALT) and histological examination. We observed no toxicity in all doses tested in this study. Therefore, the maximum safe dose of 400 mg/kg was selected as 1/10 of the maximum acute dose (4000 mg/kg). Subsequently, doses of 100, 200, and 400 mg/kg were selected as therapeutic doses.
Experimental protocol
Rats were divided into six groups of 6 animal each and treated with gavage for 14 days as follows: groups 1 and 2 received normal saline; group 3 received ATMF (400 mg/kg); groups 4-6 received 100, 200, and 400 mg/kg of ATMF, respectively.
After 14 consecutive days, except for groups 1 (control) and 3, animals were administered with a single dose of APAP (2 g/kg). Forty-eight h after administration of APAP, the animals were anaesthetized by ether and blood sample was taken through cardiac puncture. The serum was separated and kept at -20 °C for biochemical analysis. After isolation of liver, a portion of hepatic tissue was homogenized in phosphate buffer (10% w/v; pH = 7.4) and centrifuged at 3000 g for 10 min at 4 °C. Then, its supernatant was kept at -80 °C for biochemical analysis. The other parts of liver tissue kept in formalin 10% for histopathological examinations.
Serum enzymes analysis
ALT, aspartate aminotransferase (AST), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) activities in serum samples were determined using commercial kits (Pars Azmoon, Tehran, I.R. Iran) (15).
Evaluation of hepatic oxidative damage
Lipid peroxidation (LPO) and total antioxidant capacity (TAC) were respectively determined by thiobarbituric acid reactive substances and FRAP methods (16). Total thiol groups (TTG) were determined spectrophotometrically with 5,5’-dithiobis (2-nitrobenzoic acid) reagent, and the tissue level of nitric oxide (NO) was determined using Griess reagent (16).
Protein assay
Total protein content was assessed by the Bradford method in the crude homogenate of hepatic tissues. This method is based on the absorbance shift of the dye Coomassie brilliant blue G250 at 595 nm (17).
Histopathologic examination
Liver samples were fixed in formalin 10% solution. After preparing paraffin-embedded block by automatic tissue processor, the tissue was cut into sections of 4-6 μm thickness by a rotary microtome. The samples were dyed with hematoxylin and eosin (H&E) and pictured by the camera under a microscope for histopathological examination.
Statistical analysis
Quantitative variables were analyzed by Graph Pad Prism (version 6.0) and reported as the mean ± standard error of the mean (SEM). One way ANOVA followed by Tukey’s post hoc was used for determination of statistical differences between mean values. A P-value ≤ 0.05 was considered statistically significant.
RESULTS
The effects of ATMF on the animal body and tissue weight
According to our findings, gaining weight was not significantly different between control and treatment groups. The liver weight/body weight index did not show significant changes as compared with that of control (Table).
Table.
Body and liver weight changes in studied groups. Values are expressed as mean ± SEM, n = 6 for each group.
| Groups | D1W | D14W | WG | LW | LW/D14W |
|---|---|---|---|---|---|
| Control | 250.6 ± 3.6 | 285.5 ± 3.1 | 34.8 ± 2.2 | 11.5 ± 0.2 | 0.040 ± 0.0007 |
| ATMF (400 mg/kg) | 256.7 ± 3.3 | 288.1 ± 3.5 | 31.4 ± 4.1 | 12.1 ± 0.3 | 0.042 ± 0.0011 |
| APAP (2 g/kg) | 249.3 ± 6.3 | 285.6 ± 5.2 | 36.3 ± 3.5 | 12.5 ± 0.6 | 0.044 ± 0.0018 |
| APAP + ATMF (100 mg/kg) | 253.9 ± 3.9 | 282.1 ± 7.7 | 28.2 ± 6.5 | 12.1 ± 0.4 | 0.046 ± 0.0016 |
| APAP + ATMF (200 mg/kg) | 253.3 ± 4.7 | 289.5 ± 7.1 | 36.2 ± 6.1 | 13.0 ± 0.6 | 0.045 ± 0.0018 |
| APAP + ATMF (400 mg/kg) | 250.1 ± 1.3 | 280.9 ± 4.7 | 30.8 ± 3.6 | 11. 5 ± 0.4 | 0.041 ± 0.0018 |
ATMF, Allium tripedale methanol fraction; APAP, acetaminophen; D1W, day 1 weight; D14W, day 14 weight; WG, weight gain; LW, liver weight.
The effects of ATMF on liver biomarkers
The average serum ALT activity was determined to be 51.7 ± 6.3, 55.8 ± 6.9, 50.7 ± 8.3, 48.7 ± 7.7, 61.4 ± 8.7, 58.3 ± 6.1 U/L at doses of 0, 5, 50, 300, 2000, and 4000 mg/kg, respectively. Average serum ALT did not significantly differ amongst various given doses. Mortality or pathological changes in liver tissues were not observed up to 4000 mg/kg in the animals.
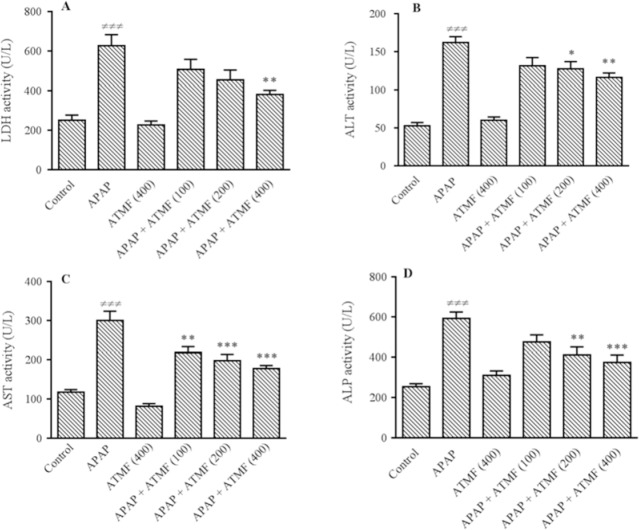
Our findings revealed that administration of 400 mg/kg of ATMF produced no sign of toxicity after 14 consecutive days of treatment. Administration of APAP could significantly increase serum LDH (Fig. 1A), ALT (Fig. 1B), AST (Fig. 1C), and ALP (Fig. 1D) activities in comparison with the control (P < 0.001). ATMF could improve the serum levels of aminotransferase enzymes (ALT, AST) as well as ALP and LDH in animals exposed to APAP.
Fig. 1.
Effects of ATMF (100, 200, 400 mg/kg) on hepatic serum enzymes levels, (A-D) LDH, ALT, AST, and ALP, respectively, in APAP-exposed Wistar rats. Values are expressed as means ± SEM, n = 6 for each group. ≠≠≠P < 0.001 Indicates significant differences compared with control group; *P < 0.05, **P < 0.01 and ***P < 0.001 show significant differences vs APAP group. LDH, lactate dehydrogenase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; APAP, acetaminophen (equal 2 g/kg); ATMF, Allium tripedale methanol fraction.
The effects of ATMF on hepatic oxidative damage
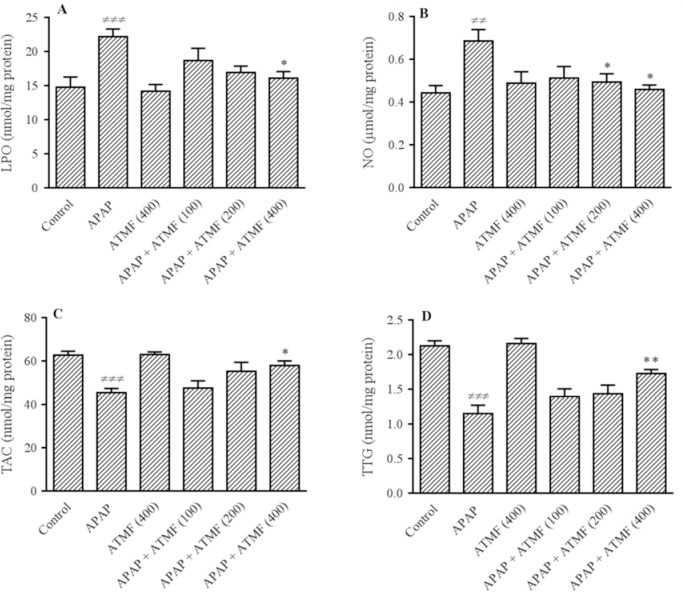
Following APAP administration, the levels of LPO (Fig. 2A; P < 0.001) and NO (Fig. 2B; P < 0.01) were increased and TAC (Fig. 2C; P < 0.001), as well as TTG (Fig. 2D; P < 0.001) levels were decreased in liver tissues compared to those of control group. In this study, a remarkable decrease was observed in LPO and NO levels following pretreatment with different doses of ATMF compared to APAP group. In addition, a concomitant improve was observed in TAC and TTG hepatic levels.
Fig. 2.
Effects of ATMF (100, 200, 400 mg/kg) on hepatic oxidative stress biomarkers (A-D) LPO, NO, TAC, and TTG, respectively, in APAP-exposed Wistar rats. Values are expressed as means ± SEM, n=6 for each group. ≠≠P < 0.01 and ≠≠≠P < 0.001 indicate significant differences vs control group; *P < 0.05 and **P < 0.01 indicate significant differences compared to APAP group. LPO, lipid peroxidation; NO, nitric oxide; TAC, total antioxidant capacity; TTG, total thiol groups; APAP, acetaminophen (equal 2 g/kg); ATMF, Allium tripedale methanol fraction.
The effects of ATMF on histopathological changes
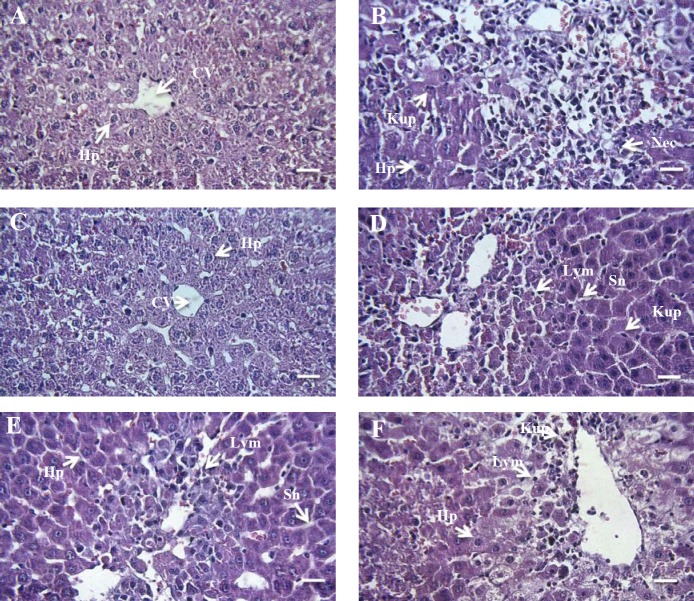
As shown in Fig. 3, pathological changes such as necrosis, inflammation, hyperplasia of kupffer cells, disruption of hepatocytes sinusoidal dilatation, and infiltration of mononuclear cells were observed in liver tissues of APAP group. ATMF could prevent some of these changes such as necrosis and inflammation.
Fig. 3.
Photomicrographs of hepatic tissue in different groups using hematoxylin and eosin method. (A) Negative control group, (B) APAP, (C) ATMF (400 mg/kg), (D) APAP + ATMF (100 mg/kg), (E) APAP + ATMF (200 mg/kg), (F) APAP + ATMF (400 mg/kg). Original magnification of all images is × 40 (scale bar = 100 μm). APAP, acetaminophen (equal 2 g/kg); ATMF, Allium tripedale methanol fraction; Cv, central vein; Hp, hepatocyte; Nec, necrosis; Sn, sinusoid; Kup, kupffer cells; Lym, lymphocyte.
DISCUSSION
In this study, the possible effects of ATMF in the dosage range 100-400 mg/kg were evaluated, which confirmed the safety of fraction at this dosage range. Previously, Kiani et al. confirmed safety dose of ATMF up to 4000 mg/kg, which indicates the high safety of this fraction (18).
Hepatic aminotransferases such as ALT and AST are known as critical markers for hepatocellular damage. In addition, ALP elevation is known to be related to the impairment of biliary tract function and raise of biliary pressure (16). Following administration of APAP at 2 g/kg, levels of hepatic serum enzymes ALT, AST, ALP, and LDH were increased, which is in line with other reports (19,20). This is related to disruption of liver cells integrity and leakage of cytosolic contents into the blood circulation (21). ATMF administration reduced the levels of these enzymes as compared to the control group, which may be due to stabilization of cell membrane and repairing hepatic tissue damage caused by APAP.
Since oxidative stress plays a key role in APAP-induced hepatotoxicity, the oxidant/antioxidant indices such as LPO, NO, TAC, and TTG were measured in this study. A remarkable increase in LPO and NO and a significant decrease in TAC and TTG hepatic levels were observed in the positive control group, indicating the induction of oxidative stress due to APAP toxicity (22).
In this regards, hepatic oxidative damage induced by APAP has been reported in several studies (1,2,22). Increased NO may be associated with APAP-inducing effects on the NO synthase (2). The excess NO reacts with superoxide and produces a reactive intermediate called peroxynitrite, which leads to protein nitration in the absence of glutathione (16). On the other hand, peroxynitrite and hydrogen peroxide are known as the most important causes of lipid peroxidation (23). LPO is a radical reaction that leads to the destruction of polyunsaturated fatty acids in lipid membranes and cell death (24). After administration of ATMF, LPO and NO hepatic levels decreased, which may be related to the improvement of the thiol molecules and the antioxidant capacity of the liver tissues.
It seems that high levels of sulfur-containing compounds in the Allium species play an important role in the recovery of thiol groups and prevention of APAP hepatic damages as well, which explains the increase of TTG level (23). There are several studies, which show antioxidant effects of sulfhydryl groups on different animal models (25,26,27). In addition to sulfur-containing compounds, the phytochemical studies of A. tripedale plant revealed the presence of natural compounds such as flavonoids, phenols, alkaloids, and tannins, which might have protective effects against liver oxidative injury (14,28). However, more studies are still required to evaluate the biological properties of the active constituents of A. tripedale plant.
CONCLUSION
In conclusion, this data suggest that ATMF can prevent hepatic APAP toxicity by improving hepatic tissue oxidant/antioxidant balance. Our findings may offer a new approach for clinical trials using A. tripedale as an herbal remedy for the treatment of liver diseases.
ACKNOWLEDGMENTS
The financial support for conducting the present study was provided by the Vice Chancellor of Research and Technology, Hamadan University of Medical Sciences, Hamadan, I.R. Iran (Grant No. 9608024843).
REFERENCES
- 1.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122(4):1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lancaster EM, Hiatt JR, Zarrinpar A. Acetaminophen hepatotoxicity: an updated review. Arch Toxicol. 2015;89(2):193–199. doi: 10.1007/s00204-014-1432-2. [DOI] [PubMed] [Google Scholar]
- 3.Gummin DD, Mowry JB, Spyker DA, Brooks DE, Fraser MO, Banner W. 2016 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th annual report. Clin Toxicol (Phila) 2017;55(10):1072–1252. doi: 10.1080/15563650.2017.1388087. [DOI] [PubMed] [Google Scholar]
- 4.Chu CC, Wu WS, Shieh JP, Chu HL, Lee CP, Duh PD. The anti-inflammatory and vasodilating effects of three selected dietary organic sulfur compounds from Allium species. J Funct Biomater. 2017;8(1) doi: 10.3390/jfb8010005. pii: E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khazaei S, Esa NM, Ramachandran V, Hamid RA, Pandurangan AK, Etemad A, et al. In vitro antiproliferative and apoptosis inducing effect of Allium atroviolaceum bulb extract on breast, cervical, and liver cancer cells. Front Pharmacol. 2017;8:5–20. doi: 10.3389/fphar.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabiu S, Madende M, Ajao AA, Aladodo RA, Nurain IO, Ahmad JB. The Genus Allium (Amaryllidaceae: Alloideae): Features, Phytoconstituents, and Mechanisms of Antidiabetic Potential of Allium cepa and Allium sativum. In: Watson RR, Preedy VR, editors. Bioactive Food as Dietary Interventions for Diabetes. 2nd ed. Academic Press; 2019. pp. 137–154. [Google Scholar]
- 7.Obioha UE, Suru SM, Ola-Mudathir KF, Faremi TY. Hepatoprotective potentials of onion and garlic extracts on cadmium-induced oxidative damage in rats. Biol Trace Elem Res. 2009;129(1-3):143–156. doi: 10.1007/s12011-008-8276-7. [DOI] [PubMed] [Google Scholar]
- 8.Ogunmodede OS, Saalu LC, Ogunlade B, Akunna GG, Oyewopo AO. An evaluation of the hypoglycemic, antioxidant and hepatoprotective potentials of onion (Allium cepa L.) on alloxan-induced diabetic rabbits. Int J Pharmacol. 2012;8(1):21–29. [Google Scholar]
- 9.Javad H, Seyed-Mostafa HZ, Farhad O, Mehdi M, Ebrahim AO, Nader RG, et al. Hepatoprotective effects of hydroalcoholic extract of Allium hirtifolium (Persian shallot) in diabetic rats. J Basic Clin Physiol Pharmacol. 2012;23(2):83–87. doi: 10.1515/jbcpp-2012-0017. [DOI] [PubMed] [Google Scholar]
- 10.Usmani S, Qureshi HJ, Zaheer A. Hepatoprotective and antioxidative effects of Allium sativum var lehsun gulabi on acetaminophen induced acute hepatitis in male albino rats. Pak J Physiol. 2019;15(1):32–36. [Google Scholar]
- 11.Tang X, Olatunji OJ, Zhou Y, Hou X. Allium tuberosum: Antidiabetic and hepatoprotective activities. Food Res Int. 2017;102:681–689. doi: 10.1016/j.foodres.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 12.Kusterer J, Keusgen M. Cysteine sulfoxides and volatile sulfur compounds from Allium tripedale. J Agric Food Chem. 2010;58(2):1129–1137. doi: 10.1021/jf903581f. [DOI] [PubMed] [Google Scholar]
- 13.Shirani M, Samimi A, Kalantari H, Madani M, Kord Zanganeh A. Chemical composition and antifungal effect of hydroalcoholic extract of Allium tripedale (Tvautv.) against OCandida species. Curr Med Mycol. 2017;3(1):6–12. doi: 10.29252/cmm.3.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezatpour B, Azami M, Motamedi M, Rashidipour M, Mahmoudvand H, Alirezaei M, et al. Chemical composition, in vitro antibacterial and cytotoxicity effect of Nectaroscordum tripedale extract. Herb Med J. 2016;1(1):29–36. [Google Scholar]
- 15.Mirmohammadlu M, Hosseini SH, Kamalinejad M, Esmaeili Gavgani M, Noubarani M, Eskandari MR. Hypolipidemic, hepatoprotective and renoprotective effects of Cydonia oblonga Mill. fruit in streptozotocin-induced diabetic rats. Iran J Pharm Res. 2015;14(4):1207–1214. [PMC free article] [PubMed] [Google Scholar]
- 16.Nili-Ahmadabadi A, Alibolandi P, Ranjbar A, Mousavi L, Nili-Ahmadabadi H, Larki-Harchegani A, et al. Thymoquinone attenuates hepatotoxicity and oxidative damage caused by diazinon: an in vivo study. Res Pharm Sci. 2018;13(6):500–508. doi: 10.4103/1735-5362.245962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassani S, Maqbool F, Salek-Maghsoudi A, Rahmani S, Shadboorestan A, Nili-Ahmadabadi A, et al. Alteration of hepatocellular antioxidant gene expression pattern and biomarkers of oxidative damage in diazinon-induced acute toxicity in Wistar rat: a time-course mechanistic study. Excli J. 2018;17:57–71. doi: 10.17179/excli2017-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiani AA, Ezatpour B, Niazi M, Jahanbakhsh S. Toxicity Effect of Nectaroscordum tripedale Extract on Hematological and Biochemical Parameters in Mice. Ent Appl Sci Let. 2018;5(2):22–25. [Google Scholar]
- 19.Prabu K, Kanchana N, Sadiq AM. Hepatoprotective effect of Eclipta alba on paracetamol induced liver toxicity in rats. J Microbiol Biotech Res. 2011;1(3):75–79. [Google Scholar]
- 20.Shakya AK, Shukla S. Protective effect of Sharbat-e-Deenar against acetaminophen-induced hepatotoxicity in experimental animals. J Tradit Chin Med. 2017;37(3):387–392. [PubMed] [Google Scholar]
- 21.Zeinvand-Lorestani H, Nili-Ahmadabadi A, Balak F, Hasanzadeh Gh, Sabzevari O. Protective role of thymoquinone against paraquat-induced hepatotoxicity in mice. Pestic Biochem Physiol. 2018;148:16–21. doi: 10.1016/j.pestbp.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Wu Q, Liu A, Anadón A, Rodriguez JL, Martinez-Larranaga MR, et al. Paracetamol: overdose-induced oxidative stress toxicity, metabolism, and protective effects of various compounds in vivo and in vitro. Drug Metab Rev. 2017;49(4):395–437. doi: 10.1080/03602532.2017.1354014. [DOI] [PubMed] [Google Scholar]
- 23.Dastan D, Karimi S, Larki-Harchegani A, Nili-Ahmadabadi A. Protective effects of Allium hirtifolium Boiss extract on cadmium-induced renal failure in rats. Environ Sci Pollut Res Int. 2019;26(18):18886–18892. doi: 10.1007/s11356-019-04656-7. [DOI] [PubMed] [Google Scholar]
- 24.Jaeschke H, Ramachandran A. Oxidant stress and lipid peroxidation in acetaminophen hepatotoxicity. React Oxyg sSpecies (Apex) 2018;5(15):145–158. [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu CK, Chen TY, Lin JH, Wang CY, Wang BS. Protective effects of five Allium derived organosulfur compounds against mutation and oxidation. Food Chem. 2016;197(Pt A):829–835. doi: 10.1016/j.foodchem.2015.11.064. [DOI] [PubMed] [Google Scholar]
- 26.Shaik IH, George JM, Thekkumkara TJ, Mehvar R. Protective effects of diallyl sulfide, a garlic constituent, on the warm hepatic ischemia-reperfusion injury in a rat model. Pharm Res. 2008;25(10):2231–2242. doi: 10.1007/s11095-008-9601-8. [DOI] [PubMed] [Google Scholar]
- 27.Annamalai S, Mohanam L, Raja V, Dev A, Prabhu V. Antiobesity, antioxidant and hepatoprotective effects of Diallyl trisulphide (DATS) alone or in combination with Orlistat on HFD induced obese rats. Biomed Pharmacother. 2017;93:81–87. doi: 10.1016/j.biopha.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 28.Chehri Z, Zolfaghari B, Dinani Sadeghi M. Isolation of cinnamic acid derivatives from the bulbs of Allium tripedale. Adv Biomed Res. 2018;7:60–81. doi: 10.4103/abr.abr_34_17. [DOI] [PMC free article] [PubMed] [Google Scholar]