Abstract
In the present investigation scratch wound assay was used to study the ability of several combinations of each flavonoid (chrysin, naringenin or resveratrol) with β-sitosterol to heal wounds in vitro. MTT test was performed to determine if the combination of flavonoid with β-sitosterol was toxic to fibroblasts or not. Also, superoxide dismutase (SOD) activity and interleukin-1β (IL-1β) concentrations were measured. The best closure rates were obtained with β-sitosterol combined with naringenin and β-sitosterol combined with resveratrol. The combination that produced the best closure rate namely β-sitosterol with naringenin increased SOD activity significantly. However, this combination was not better than naringenin or β-sitosterol alone in reducing IL-β concentration. The results of MTT test indicated that the combination as well as β-sitosterol alone or naringenin alone has no toxic effect on fibroblasts. In conclusion, the combination of β-sitosterol and naringenin exerted a synergistic effect on wound closure without decreasing the viability of fibroblasts, increased antioxidant defense mechanism and decreased IL-β.
Keywords: Beta-sitosterol, Interleukin-1β, Naringenin, Scratch wound assay, SOD, Synergism
INTRODUCTION
The skin is our largest organ and performs many important functions. It acts as a barrier between the body and the external environment (1). A cutaneous wound is any loss of skin integrity due to injury or illness (2). Wound healing stops bleeding and reconstitute the structural and functional barrier so that it prevents drying and microbial invasion (3).
The mammalian wound response is well understood. After injury, platelets come in contact with exposed collagen. This triggers platelet release of clotting factors, cytokines, and growth factors such as platelet-derived growth factor (PDGF) and transforming growth factor beta (TGF-ß) (4). Ultimately, a fibrin-rich clot is formed. This phase is called hemostasis (5). The second phase is called the inflammatory phase in which inflammatory cells such as neutrophils, macrophages, and mast cells are recruited to the wound area. The phagocytic cells protect against infection, clean the wounded area from debris, and secrete cytokines that direct tissue repair (5). The third phase of wound healing is the proliferative phase in which fibroblasts migrate in and deposit extracellular matrix (4). The forth phase is the remodeling phase in which new collagen matrix becomes cross-linked and organized. In this maturation phase, collagen fibers are cross-linked and strengthened while excess collagen is degraded by proteolytic enzymes. Thus, continued cycles of cell proliferation and scar degradation by proteases characterize this stage so that intact skin replaces scar tissue at the end (2,6). If any of these phases is suppressed, wound healing is prolonged.
Many drugs that promote wound healing have been widely used in medical practice. The medications of chemical origin have serious side effect potentials and costs (7). Therefore, investigation of new substances capable of promoting the wound healing process is still needed. In recent years, the use of natural products for healing wounds has increased dramatically (8,9). Although many phytochemicals have claimed to have wound healing properties, most do not have well-controlled scientific data to prove their claims (8).
Resveratrol (3,5,40-trihydroxy-trans-stilbene) is a natural polyphenolic compound found in grape skins (10) while chrysin (5,7-dihydroxyflavone) is a flavonoid contained in many plant extracts such as passion fruit (Passiflora sp.) as well as in propolis (11). Naringenin is present in citrus fruits and tomatoes (12) and β-sitosterol is a phytosterols present in many plants with a chemical structure similar to that of cholesterol (13). In the present study, the synergistic effects of each flavonoid (resveratrol, chrysin, or naringenin) with β-sitosterol was investigated in scratch wound assay in order to evaluate their wound healing potential.
The selection of the tested compounds was based on previous studies reporting that these natural products have wound healing properties (10,11,14,15).
In fact, the use of combination of wound healing active constituents is employed for the development of wound healing formulations, which may substitute the modern wound healing agents (16).
MATERIALS AND METHODS
Chemicals
β-sitosterol, chrysin, dimethyl sulfoxide (DMSO), and naringenin were obtained from Sigma, USA. PDGF was obtained from Biotechne-R&D, USA, and resveratrol was obtained from Tocris, USA. All drugs were dissolved in DMSO not exceeding 5%.
Scratch-wound migration assay
The fibroblasts migration and proliferative capabilities were measured using a scratch wound assay (17). Fibroblasts were obtained from Jordan University and were purchased originally from European collection of authenticated cell cultures (ECACC). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM) medium containing 10% fetal bovine serum (FBS) and incubated under 5% CO2 and 95% humidity. 3 × 105 cells/mL were seeded into 24-well tissue culture dishes containing cover slips pre-coated polylysine (Thermo Fisher Scientific Inc., USA) for 24 h at 37 °C. When cultured cells form confluent monolayers, a linear wound was created using a sterile 100 μL plastic pipette tip. The cover slips were washed with phosphate buffered saline (PBS) to remove any cellular debris. Two control groups were used, negative control treated with DMEM medium containing DMSO (0.25%), and positive control group treated with PDGF (10 nM) in media. The tested compounds (used in two concentrations: 50 and 100 μΜ) and their combinations were added to a set of 3 coverslips per dose and incubated for 24 h at 37 °C with 5% CO2. The surface area of the scratch was measured at zero time which is the time of performing it and also after 24 h later. Relative migration of cells under each condition were estimated through taking three representative images to the scratch area from each coverslip. Motic software version 2 (Motic China group CO, LTD) were used to analyze the data. The experiments were performed at least in triplicates. Closure rate was calculated using the formula provided by CytoSelect™ 24-well wound healing assay (Cell Biolabs, INC, USA) as follows:
Closure percent = 
Cell proliferation assay
Three-(4,5-dimethylthiazolyl)-2,5-diphenyl- tetrazolium bromide (MTT; Promega, USA) is an assay based on the mitochondrial dehydrogenase ability to reduce MTT to a purple formazan product (18). The concentration that gave the highest scratch assay closure percent for the tested compounds (50 μM) was used in MTT test. This procedure was used to assess the antiproliferative activity of β-sitosterol (50 μM), naringenin (50 μM), and their combination (1:1) in human fibroblast. The cells were suspended at a density of 2 × 104 cells/mL in media. Then 100 μL of each cell type was cultured into each well of 96-well microtiter plates and incubated at 37 °C under 5% CO2 for 24 h.
The test was performed according to the manufacturer guidelines. Briefly, 15 μL of MTT dye solution was added to each well and incubated for 4 h. Then, 100 μL of MTT stop solution were added to each well to stop the resulting MTT-formazan product, and then the absorbance was measured at 590 nm using a microplate reader (Biotech, USA).
Antioxidant assay
Cells plated at 5 × 105 cells in 1 mL final volume per well, in 24-well plates for 24 h. Then, cells were subjected to different treatments in duplicates and incubated for 24 h. Cultured cells were washed and suspended in lysis buffer then homogenized and sonicated in ice 3 cycles, 90 s/cycle using sonicator (TI-H-5 Elma, Schmidbauer GmbH, Germany). The suspension was centrifuged and the supernatant was tested for SOD concentration using Cayman’s superoxide dismutase assay kit (USA, Cat No. 706002). The test was performed according to the manufacturer directions.
Quantification of secreted interleukin-1β
Cells plated at 5 × 105 cells in 1 mL final volume per well, in 24-well plates for 24 h. Then, cells were subjected to different treatments in triplicates and incubated for 24 h. Supernatant was collected and tested for interleukin-1ß (IL-1β). Human IL-1β ELISA MAX™ Deluxe were used to measure the concentration of secreted IL-1β as per manufacturer’s instruction (Biolegend, San Diego, California, USA).
Statistical analysis
The results were stated as the mean ± SD. One-way analysis of variance (ANOVA) was performed followed by Mann Whitney U test post hoc for comparison between groups for percentage closure in scratch wound assay, SOD, and IL-1β level. Differences were considered to be statistically significant when P ≤ 0.05. Data were analyzed using Graphpad prism 6. Evaluation of the synergy was performed for β-sitosterol (50 μM) and naringenin (50 μM) combination by calculating the combination index (CI) using Compusyn software for scratch wound assay.
RESULTS
In scratch wound assay, resveratrol, naringenin, chrysin, β-sitosterol, and the combination of each flavonoid with β-sitosterol were significantly more effective than the negative control (Table 1). The best closure rate was obtained when β-sitosterol was combined with naringenin (50 μM) (Fig. 1) with combination index 0.06. The results of MTT test indicate that β-sitosterol, naringenin, and their combination were not toxic to fibroblasts (Fig. 2).
Table 1.
Percentage closure in scratch wound assay. Data are presented as mean ± SD; *P ≤ 0.05, **P ≤ 0.01, and **P ≤ 0.001 indicate significant differences with positive control. All treatments were significantly (P ≤ 0.001) different from negative control. Platelet-derived growth factor was used as a positive control.
| Treatment | Closure rate (%) |
|---|---|
| Negative control | 19.19 ± 2.31*** |
| Platelet-derived growth factor (positive control) | 82.19 ± 1.55 |
| β-sitosterol (50 μM) | 59.77 ± 5.29*** |
| β-sitosterol (100 μM) | 54.97 ± 5.23*** |
| Chrysin (50 μM) | 75.54 ± 0.88 |
| Chrysin (100 μM) | 72.35 ± 2.5 |
| Naringenin (50 μM) | 49.9 ± 4.68*** |
| Naringenin (100 μM) | 59.6 ± 7.43*** |
| Resveratrol (50 μM) | 49.71 ± 1.35*** |
| Resveratrol (100 μM) | 45.88 ± 4.55*** |
| β-sitosterol + chrysin (50 μM) | 68.86 ± 7.62* |
| β-sitosterol + chrysin (100 μM) | 65.65 ± 3.61** |
| β-sitosterol + naringenin (50 μM) | 89.01 ± 0.14 |
| β-sitosterol + naringenin (100 μM) | 73.98 ± 0.99 |
| β-sitosterol + resveratrol (50 μM) | 80.27 ± 4.16 |
| β-sitosterol + resveratrol (100 μM) | 63.56 ± 5.80*** |
Fig. 1.

Scratch wound assay. (A) Negative control at zero time, (B) negative control after 24 h, (C) β-sitosterol at 50 μM after 24 h, (D) β-sitosterol at 100 μM after 24 h, (E) naringenin at 50 μM after 24 h, (F) naringenin at 100 μM after 24 h, (G) naringenin and β-sitosterol at 50 μM after 24 h, (H) naringenin and β-sitosterol at 100 μM after 24 h and (I) platelet-derived growth factor at 10 nM after 24 h.
Fig. 2.

Evaluation of antoproliferative effects of β-sitosterol, naringenin or their combination on fibroblasts using MTT assay.
The combination that produced the best closure rate namely β-sitosterol with naringenin increased SOD activity significantly compared to untreated wound (Fig. 3). IL-1β level in untreated-wounded fibroblasts showed a significant increase compared to unwounded fibroblasts. β-sitosterol, naringenin, and their combination decreased significantly the level of IL-1β in comparison with untreated wound (Fig. 4).
Fig. 3.
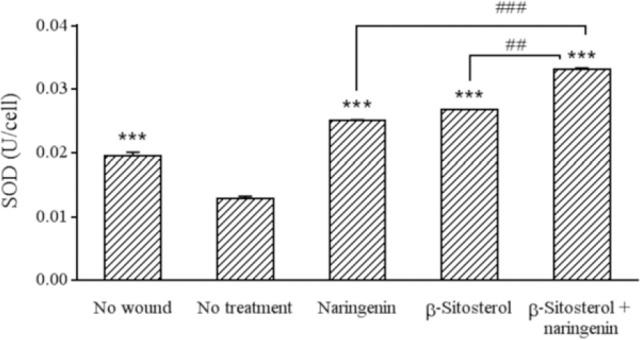
Effect of different treatments on levels of SOD. In all groups except the first one (no wound), a linear wound on monolayer cells was created using a sterile 100 μL plastic pipette tip. *** Indicates significant differences in comparison with no treatment group (P < 0.001). ##P < 0.01 and ###P < 0.001 show significant differences between indicated groups. SOD, superoxidae dismutase
Fig. 4.

Effect of different treatments on level of IL-1β. In all groups except the first one (no wound), a linear wound on monolayer cells was created using a sterile 100 μL plastic pipette tip. *** Indicates significant differences in comparison with no treatment group (P < 0.001). Interleukin-1β, IL-1β.
DISCUSSION
Wound healing is a natural process that results in repairing the injured tissue and restoring the functions of the skin (7). The first phase of healing leads to hemostasis and is followed by inflammatory phase (19). The proliferative phase follows the inflammatory phase. In this phase, granulation tissue is formed and vascular network is restored in order to supply nutrients for tissues (19).
The main players in collagen production, proliferation, migration, and granulation tissue formation are fibroblasts. Bundles of collagen fibrils produced by fibroblasts are essential for the development and migration of new blood vessels in injured area during wound repair (20).
One of the major advantages of scratch wound assay utilized in the present work is that it mimics to certain extent the proliferation and migration of fibroblast in vivo (17). The results of the present study indicated that resveratrol, naringenin, chrysin, β-sitosterol, and the combination of each flavonoid with β-sitosterol were more effective than the untreated control in increasing percentage of closure. The lower concentration (50 μM) of naringenin with β-sitosterol combination produced higher closure percent than the higher concentration (100 μM). This could be due to the fact that the higher dose (100 μM) may exert more toxic effect in normal fibroblast cell lines as demonstrated by previous studies (21). Since the studied natural products are known to be safe for normal cells, the MTT test was used primarily to check if drug combination effect was due to increase in the proliferation rate or not.
The results of this study agree with previous studies reporting wound healing activity of these compounds. Administration of resveratrol in vivo significantly increased the tensile strength and increased the hydroxyproline levels (10). Also, naringenin expressed anti-inflammatory and antioxidant effects, and resulted in significant increase in wound contraction of thermal burn induced wound in rats (14). The altered levels of oxidation stress (SOD) were significantly restored by naringenin ointment formulation. Tensile strength, hydroxyproline, and protein content were significantly increased upon wound treatment with naringin (22). Naringin stimulated angiogenesis and restrained endothelial apoptosis through modulation of inflammatory and growth factors expression in diabetic foot ulcers (22). In fact, flavonoids were proven to accelerate wound healing, which may be beneficial in controlling scar formation, slow down skin aging process, and so used for cosmetic purposes (23). On the other hand, β-sitosterol, an angiogenic plant-derived factor, has potential pharmaceutical applications in management of chronic wounds (15,24). This study represents the first report of synergistic effect between naringenin and β-sitosterol in wound healing.
Despite that the initial inflammatory response is essential for adequate wound healing; the prolonged influx of neutrophils at the wound site may result in higher concentration of produced free radicals. This results in chronic inflammation which leads to cell damage and distorted composition of extracellular matrix with subsequent failure of epithelialization (25). Wounded area contains reactive oxygen and nitrogen species. These free radicals will give rise to oxidative stress leading to lipid peroxidation, DNA damaging, and enzyme inhibition or inactivation, including free-radical scavenger enzymes (26). Therapeutic options which act on reactive oxygen species depletion during wound treatment may exhibit activity in reducing inflammation and may allow easy transition from the inflammatory to the proliferative phase. Many reported evidences suggest antioxidants of natural origin such as plant flavonoids are capable of reducing the oxidation of cellular molecules by stopping free radical chain reaction or by a scavenging of reactive oxygen species (7). In the present study, the combination that produced the best closure rate namely β-sitosterol with naringenin increased SOD activity significantly. Some wound healing agents exert a protection capacity against oxidative damage and induce the process of skin wound healing was referred to increasing the expression of the antioxidant enzymes such as SOD and catalase (27).
Most of chronic non-healing wounds are attributed to excessive inflammation. Reducing the inflammation level might aid in decreasing scar formation and enhance the healing rate. In this perspective, many in vitro and in vivo studies with animal models have demonstrated that compound of natural origin with anti-inflammatory property have revealed promising results in speeding wound healing process (7). In the present investigation, the anti-inflammatory activities of naringenin-β-sitosterol combination were investigated by examining whether the combinations inhibit IL-1β-level. Results of this study illustrated that IL-1β level in wounded, untreated fibroblasts showed a significant increase compared to wounded and treated fibroblasts. This totally agrees with the results of Chamberlain et al. (28).
The combination of β-sitosterol and naringenin was effective in lowering IL-1β level. IL-1 is a pleiotropic inflammatory cytokine, exerting manifest effects on different cell types involved in all phases of healing (28). Due to its central role in the onset of inflammation, IL-1 has been an important therapeutic target (28). IL-1β is a pro-inflammatory cytokine that has been implicated in pain and inflammation (29) as well as producing pyogenic effects (28). In addition, IL-1β reduced the expression of glioma-associated oncogene and indirectly inhibited myofibroblast formation. Also, IL-1β had anti-fibrotic effects by increasing levels of matrix metalloproteinases produced by fibroblasts exposed to TGF-β1/IL1β. Furthermore, IL-1β decreased the TGF- β1-induced upregulation of lysyl oxidase, an enzyme involved in collagen cross- linking (30).
CONCLUSION
Beta-sitosterol and naringenin exerted a synergistic effect on wound closure without decreasing the viability of fibroblasts. This combination alleviated oxidative stress via increasing SOD activity and decreased IL-1β level in wounded fibroblasts. This combination needs further in vivo investigation.
ACKNOWLEDGMENTS
This project was financially supported by the Deanship of Graduate Studies and Scientific Research/Al-Ahliyya Amman University, Jordan through the Grant number 2/7/2013-2014.
REFERENCES
- 1.Hakim N, Andersson LC, Nettelblad H, Kratz G, editors. From Basic Wound Healing to Modern Skin Engineering. London: Springer; 2009. Artificial Organs; pp. 93–105. [Google Scholar]
- 2.Cukjati D, Reberšek S, Miklavčič D. A reliable method of determining wound healing rate. Med Biol Eng Comput. 2001;39(2):263–271. doi: 10.1007/BF02344811. [DOI] [PubMed] [Google Scholar]
- 3.Toriseva M, Kähäri VM. Proteinases in cutaneous wound healing. Cell Mol Life Sci. 2009;66(2):203–224. doi: 10.1007/s00018-008-8388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diegelmann RF. From the selected works of Robert F. Diegelmann Ph.D. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Liang D, Li X, Liu HH, Zhang X, Zheng M, et al. The role of interleukin-1 in wound biology. Part I: Murine in silico and in vitro experimental analysis. Anesth Analg. 2010;111(6):1525–1533. doi: 10.1213/ANE.0b013e3181f5ef5a. [DOI] [PubMed] [Google Scholar]
- 6.Ansell DM, Holden KA, Hardman MJ. Animal models of wound repair: Are they cutting it? Exp Dermatol. 2012;21(8):581–585. doi: 10.1111/j.1600-0625.2012.01540.x. [DOI] [PubMed] [Google Scholar]
- 7.Anlas C, Bakirel T, Ustun-Alkan F, Celik B, Baran MY, Ustuner O, et al. In vitro evaluation of the therapeutic potential of Anatolian kermes oak (Quercus coccifera L.) as an alternative wound healing agent. Ind Crop Prod. 2019;137:24–32. [Google Scholar]
- 8.Davis SC, Perez R. Cosmeceuticals and natural products: wound healing. Clin Dermatol. 2009;27(5):502–506. doi: 10.1016/j.clindermatol.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Kooti W, Ghasemiboroon AAM, Harizi M, Afzalzadeh M, Afshar M, Hozeyli S, et al. Effect of compound cream, a mixture of honey, fish oil, Hypericum perforatum L. and Achilea mille folium L. on full thickness skin wound in rat. Res Pharm Sci. 2012;7(5):18. [Google Scholar]
- 10.Yaman I, Derici H, Kara C, Kamer E, Diniz G, Ortac R, et al. Effects of resveratrol on incisional wound healing in rats. Surg Today. 2013;43(12):1433–1438. doi: 10.1007/s00595-012-0455-7. [DOI] [PubMed] [Google Scholar]
- 11.Deldar Y, Pilehvar-Soltanahmadi Y, Dadashpour M, Montazer Saheb S, Rahmati-Yamchi M, Zarghami N. An in vitro examination of the antioxidant, cytoprotective and anti-inflammatory properties of chrysin-loaded nanofibrous mats for potential wound healing applications. Artif Cells Nanomed Biotechnol. 2018;46(4):706–716. doi: 10.1080/21691401.2017.1337022. [DOI] [PubMed] [Google Scholar]
- 12.Mir IA, Tiku AB. Chemopreventive and therapeutic potential of “naringenin,” a flavanone present in citrus fruits. Nutr Cancer. 2015;67(1):27–42. doi: 10.1080/01635581.2015.976320. [DOI] [PubMed] [Google Scholar]
- 13.Bin Sayeed MS, Karim SMR, Sharmin T, Morshed MM. Critical analysis on characterization, systemic effect, and therapeutic potential of beta-sitosterol: a plant-derived orphan phytosterol. Medicines (Basel) 2016;3(4) doi: 10.3390/medicines3040029. pii: E29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Roujayee AS. Naringenin improves the healing process of thermally-induced skin damage in rats. J Int Med Res. 2017;45(2):570–582. doi: 10.1177/0300060517692483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsala DE, Amadou D, Habtemariam S. Natural wound healing and bioactive natural products. Phytopharmacology. 2013;4(3):532–560. [Google Scholar]
- 16.Kumar B, Vijayakumar M, Govindarajan R, Pushpangadan P. Ethnopharmacological approaches to wound healing-exploring medicinal plants of India. J Ethnopharmacol. 2007;114(2):103–113. doi: 10.1016/j.jep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 18.Abbas MM, Abbas MA, Kandil YI. Cytotoxic activity of Varthemia iphionoides essential oil against various human cancer cell lines. Acta Pol Pharm. 2019;76(4):701–706. [Google Scholar]
- 19.Theoret C, editor. Theoret C, Physiology of wound healing. 3rd ed. Wiley & Sons, Inc; 2016. Equine Wound Management; pp. 1–13. [Google Scholar]
- 20.Shahi Z, Nakhaee M, Hasani M, Balandeh A. Effect of the powder and hydroalcoholic extract of the fruit sheath of Prosopis farcta on the fibroblasts and angiogenic process in wound healing of rat. Res Pharm Sci. 2012;7(5):S820. [Google Scholar]
- 21.Stompor M, Uram L, Podgórski R. In vitro effect of 8-prenylnaringenin and naringenin on fibroblasts and glioblastoma cells-cellular accumulation and cytotoxicity. Molecules. 2017;22(7) doi: 10.3390/molecules22071092. pii: E1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandhare AD, Alam J, Patil MV, Sinha A, Bodhankar SL. Wound healing potential of naringin ointment formulation via regulating the expression of inflammatory, apoptotic and growth mediators in experimental rats. Pharm Biol. 2016;54(3):419–432. doi: 10.3109/13880209.2015.1038755. [DOI] [PubMed] [Google Scholar]
- 23.Stipcevic T, Piljac J, Berghe DV. Effect of different flavonoids on collagen synthesis in human fibroblasts. Plant Foods For Hum Nutr. 2006;61(1):29–34. doi: 10.1007/s11130-006-0006-8. [DOI] [PubMed] [Google Scholar]
- 24.Lee ES, Shin MO, Yoon S, Moon JO. Resveratrol inhibits dimethylnitrosamine-induced hepatic fibrosis in rats. Arch Pharm Res. 2010;33(6):925–932. doi: 10.1007/s12272-010-0616-4. [DOI] [PubMed] [Google Scholar]
- 25.Sardari K, Pedram S, Zojaji V, Maleki M, Mohri M, Dehgan M, et al. Effects of Zn-7® on open wound healing in dogs. Comp Clin Path. 2006;15(4):237–243. [Google Scholar]
- 26.Thiem B, Goslmska O. Antimicrobial activity of Rubus chamaemorus leaves. Fitoterapia. 2004;75(1):93–95. doi: 10.1016/j.fitote.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez-Suarez JM, Giampieri F, Cordero M, Gasparrini M, Forbes-Hernández TY, Mazzoni L, et al. Activation of AMPK/Nrf2 signalling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing. J Funct Foods. 2016;25:38–49. [Google Scholar]
- 28.Chamberlain CS, Leiferman EM, Frisch KE, Duenwald-Kuehl SE, Brickson SL, Murphy WL, et al. Interleukin-1 receptor antagonist modulates inflammation and scarring after ligament injury. Connect Tissue Res. 2014;55(3):177–186. doi: 10.3109/03008207.2014.906408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60(1):57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mia MM, Boersema M, Bank RA. Interleukin-1β attenuates myofibroblast formation and extracellular matrix production in dermal and lung fibroblasts exposed to transforming growth factor-β1. PloS One. 2014;9(3):e91559,1–19. doi: 10.1371/journal.pone.0091559. [DOI] [PMC free article] [PubMed] [Google Scholar]


