Abstract
Context:
In in vitro fertilization (IVF) cycles, the recommended dose of recombinant human chorionic gonadotropin (r-hCG), for triggering final follicular maturation is 250 μg, although there is some disagreement.
Aims:
The aim of our study was to assess the effect on the number of mature oocytes retrieved after triggering ovulation in IVF cycles using 250 μg or 500 μg of r-hCG.
Settings and Design:
Prospective, single-center, randomized study.
Subjects and Methods:
100 women undergoing IVF with embryo transfer. The primary outcome measure was the total number of oocytes retrieved per follicle, number of mature oocytes, and number of embryos generated. The secondary outcomes included clinical and biochemical pregnancy rates and incidence of ovarian hyperstimulation syndrome.
Results:
Mean number of oocytes retrieved (6.5 ± 4.0 vs. 6.4 ± 3.9, P = 0.3) and mean number of mature oocytes (4.0 ± 2.3 vs. 3.2 ± 2.3, P = 0.09) were similar in the two groups; however, mean number of oocytes retrieved per follicle was found to be higher with 500 μg r-hCG (67.4 ± 23.9 vs. 77.5 ± 23.3, P = 0.04). In the subgroup of poor responder women, there was a significant increase in the number of mature oocytes retrieved with double dose of r-hCG (2.2 ± 1.8 vs. 3.7 ± 1.9, P = 0.06), leading to improvement in fertilization and clinical pregnancy rates.
Conclusions:
Double dose of r-hCG for final follicular maturation in IVF cycles resulted in improvement in mean number of oocytes per follicle but did not result in improved pregnancy rates in the women. In the subset of poor responders, 500 μg r-hCG seems to be more advantageous than the lower dose, although larger randomized trials are needed to generalize this strategy.
KEYWORDS: In vitro fertilization, in vitro fertilization outcomes, mature oocytes, poor responder, recombinant-human chorionic gonadotropin trigger
INTRODUCTION
Human chorionic gonadotropin (hCG), a natural analog of luteinizing hormone (LH), is used to trigger final oocyte maturation in in vitro fertilization (IVF) cycles.[1] The recommended dose of r-hCG is 250 μg[2] because it provides the best outcomes and the lower complication rate,[3] although there is some disagreement.[4] When a higher dose of recombinant hCG (r-hCG) (500 μg) was used, the mean number of oocytes retrieved was the same, but the numbers of two pronuclear fertilized (metaphase II [MII]) oocytes and cleaved embryos were significantly higher with 500 μg r-hCG in one study.[3] Therefore, the aim of our study was to assess the effect on the number of mature MII oocytes retrieved after triggering ovulation in IVF cycles using 250 μg or 500 μg of r-hCG. We also studied the effect of double dose of r-hCG as compared to the conventional dose in the subgroup of poor responder women.[5,6]
SUBJECTS AND METHODS
Selection and description of participants
This was a prospective, single-center, randomized, open-label study performed between June 2017 and August 2017 at a tertiary care hospital. We included 100 women between the ages of 21–40 years with normal or poor ovarian reserve undergoing IVF- embryo transfer (ET) as eligible for the trial.
For sample size calculation, from our own statistics, the mean percentage of MII oocytes from a cohort of 120 patients treated with IVF over a period of 3 months was 78.0% with an standard deviation (SD) of 13.8%. It was assumed that 250 μg r-hCG was as effective as 500 μg r-hCG in inducing oocyte maturity if the percentage of MII oocytes per patient fell within 1 SD of the original proportion (i.e., 13.8%) when the patients were treated with 500 μg r-hCG. To fulfill such an assumption, the number of patients required in each group was 50 to give a power of 90% at the 5% significance level. Hence, 100 women between the ages of 21 and 40 years were included into the study. Poor ovarian responders were defined as patients with antral follicle count (AFC) <7 and anti-Mullerian hormone (AMH) ≤1.1 ng/ml as per the Bologna criteria given by ESHRE.[7]
We excluded women who were hyperresponders, defined as those with (1) history of ovarian hyperstimulation syndrome (OHSS), (2) high basal ovarian reserve (AMH >10 ng/ml or AFC >20), and (3) those showing hyperresponse (more than 15 follicles with average diameter of 16 mm or greater). We also excluded women who were planned for embryo cryopreservation and ET in subsequent cycles, exclusion was performed at the time of randomization.
Outcome measures
The primary outcome measure was the total number of oocytes retrieved per follicle, number of mature MII oocytes, and number of embryos generated. The secondary outcomes included clinical and biochemical pregnancy rates and incidence of OHSS.
Technical information
Women included in the study underwent treatment with standard long gonadotropin-releasing hormone (GnRH) agonist, antagonist, or microdose-flare regimen according to the basal ovarian reserve. Treatment was performed with recombinant-follicle-stimulating hormone (r-FSH) (Gonal F®, Merck-Serono Inc., Spain) according to the patient's age, BMI, antral follicular count, AMH, and previous responses to ovarian stimulation. In GnRH antagonist cycles, Cetrorelix (Cetrotide®, Merck-Serono, Spain) was added when at least 1 follicle reached a diameter of 12 mm. Follicular development was monitored by regular transvaginal ultrasonography performed every 48 h. r-hCG (Ovitrelle®, Serono, Spain) was given when two or more follicles reached 18 mm in diameter.
Patients were randomly allocated into either the 250 or 500 μg r-hCG group according to a computer-generated randomization table, with the allocation group number presented to the staff nurse administering the drug. The clinicians and laboratory staff involved as well as the patient herself were blinded as to the dosage of r-hCG used. Transvaginal ultrasound-guided oocyte retrieval was performed 36 h after administration of r-hCG injection. After retrieval, oocytes were cultured in G-1 version 3 medium for 2 h and examined, MII oocytes were identified by the presence of a polar body. This was followed by IVF/ICSI after which fertilization was checked 14–18 h after the procedure. An oocyte was considered to be normally fertilized when two pronuclei (PN) were visible. The embryos were cultured for 2–5 days before the transfer followed by ET under ultrasound guidance. The day of transfer and number of embryos transferred were decided by the physician based on the patient and cycle characteristics.
Patients were monitored for signs and symptoms of OHSS, if present, and categorized as mild, moderate, or severe, embryo transfer was deferred in these patients. Luteal phase support was provided by natural micronized progesterone 100 mg intramuscular daily for 15 days followed by vaginal micronized progesterone 200 mg twice daily till 14 weeks of gestation if pregnancy occurred. Serum beta-HCG was measured 16 days after ET and pelvic ultrasound was performed to confirm intrauterine pregnancy. Clinical pregnancy was defined as the presence of fetal cardiac activity on transvaginal ultrasonography. Biochemical pregnancy was defined as the presence of positive beta-HCG (≥5 mIU/ml) but the absence of gestational sac/cardiac activity on ultrasonography.
Serum E2, P, and hCG concentrations were measured using commercially available kits (Automated Chemiluminescence ACS-180 System, Bayer Corp., Tarrytown, NY, USA). The sensitivity of the E2 assay was 10.0 pg/ml, and the intra- and inter-assay coefficients of variation were 8.1% and 8.7%, respectively. The sensitivity of the P assay was 0.11 ng/ml, and the intra- and inter-assay coefficients of variation for P were 5.0% and 7.8%, respectively. The sensitivity of the hCG assay was 2 IU/L, and the intra- and inter-assay coefficients of variation for hCG were 1.8% and 4.9%, respectively.
Ethics
All patients signed informed written consent, and the study was approved by a duly constituted departmental review board. All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as revised in 2000.
Statistical analysis
Continuous variables were expressed as the mean ± SD and were compared between the two groups using Student's t-test. Categorical variables were expressed as percentage and compared using X-test or Fisher's exact test where appropriate. Statistical significance was established if P < 0.05.
RESULTS
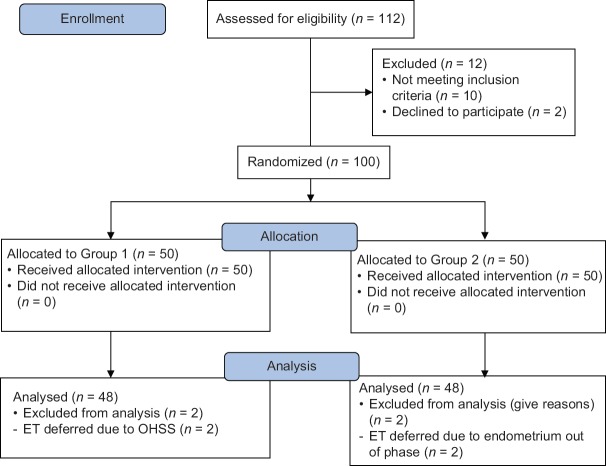
One hundred and twelve women undergoing IVF-ET over a 3-month period were assessed for eligibility, out of which 100 women were included in the study. Fifty women (Group 1) received 250 mcg r-hCG and 50 women (Group 2) received 500 mcg r-hCG. Four women were excluded from the final analysis as ET was deferred in the present cycle due to endometrium being out of phase or due to patient developing signs of OHSS [Figure 1].
Figure 1.
CONSORT diagram
Baseline demographic characteristics of patients treated with 250 mcg or 500 mcg r-hCG has been shown in Table 1. There were no significant differences in age, BMI, duration of infertility, causes of infertility, basal FSH, and basal LH between groups. The basal serum AMH level (5.3 ± 2.2 ng/ml in group 1 versus 3.1 ± 1.8 ng/ml in Group 2, P < 0.001) and basal AFC (17.0 ± 7.2 vs. 12.2 ± 4.0 in Group 1 and 2, respectively, P = 0.001) were significantly lower in Group 2 as compared to Group 1. Women in Group 2 also had longer duration of gonadotropin stimulation (10.1 ± 1.0 vs. 11.0 ± 1.5 days in Group 1 and 2, respectively, P = 0.008) and required higher dose of drug for adequate cycle response (1696.3 ± 170.0 IU in Group 1 vs. 2031.1 ± 276.9 IU in Group 2, P < 0.001). However, there were no differences in the IVF protocol used, level of estradiol (E2) and progesterone (P) on the day of hCG trigger and level of serum LH and P on the day of oocyte retrieval between the groups [Table 1].
Table 1.
Baseline demographic and cycle characteristics
| r-hCG 250 µg (n=48) | r-hCG 500 µg (n=48) | P | |
|---|---|---|---|
| Age (years) | 30.1±4.2 | 31.5±3.7 | 0.08 |
| BMI (kg/m2) | 23.7±3.7 | 24.1±2.9 | 0.55 |
| Type of infertility (%) | |||
| Primary | 42 (87.5) | 39 (81.3) | 0.39 |
| Secondary | 6 (12.5) | 9 (18.7) | |
| Duration of infertility (years) | 5.7±3.1 | 5.3±2.9 | 0.54 |
| Cause of infertility | |||
| Tubal | 28 (58.3) | 22 (45.8) | 0.57 |
| Male factor | 7 (14.5) | 7 (14.5) | |
| Endometriosis | 7 (14.5) | 9 (18.7) | |
| Unexplained | 6 (12.5) | 10 (20.8) | |
| Previous failed IVF (%) | 16 (33.3) | 12 (25) | 0.37 |
| Protocol | |||
| Long | 18 (37.5) | 24 (50) | 0.35 |
| Short | 28 (58.3) | 21 (43.7) | |
| Microflare | 2 (4.1) | 3 (6.2) | |
| Basal FSH (mIU/ml) | 6.0±1.5 | 6.6±2.3 | 0.13 |
| Basal LH (mIU/ml) | 4.5±2.3 | 5.0±3.0 | 0.36 |
| Serum AMH (ng/ml) | 5.3±2.2 | 3.1±1.8 | <0.001 |
| Antral follicle count | 17.0±7.2 | 12.2±4.0 | 0.001 |
| Total dose of gonadotropins (IU) | 1696.3±170.0 | 2031.1±276.9 | <0.001 |
| Total days of stimulation | 10.1±1.0 | 11.0±1.5 | 0.008 |
| Serum E2 (pg/ml) | 3364.2±293.0 | 3242.6±315.4 | 0.06 |
| Serum P (ng/ml) | 0.8±0.1 | 0.8±0.1 | 1.0 |
| Serum LH on day of oocyte retrieval (mIU/ml) | 0.6±1.7 | 1.0±1.2 | 0.18 |
| Serum P on day of oocyte retrieval (ng/ml) | 11.9±6.5 | 13.2±7.2 | 0.35 |
BMI=Body mass index, IVF=In vitro fertilization, FSH=Follicle-stimulating hormone, LH=Luteinizing hormone, AMH=Anti-Mullerian hormone, E2=Estradiol, P=Progesterone, r-hCG=Recombinant-human chorionic gonadotropin
The number of follicles aspirated, oocytes retrieved, MII oocytes, fertilization rate, and number of produced embryos were not significantly different between groups. When we compared the mean number of oocytes retrieved per follicle, this was found to be significantly higher with 500 μg r-hCG (67.4 ± 23.9 in Group 1 vs. 77.5 ± 23.3 in Group 2, P = 0.04). Cycle outcomes of patients treated with 250 mcg r-hCG or 500 mcg r-hCG have been presented in Table 2. No significant differences were noted between the two groups in terms of biochemical and clinical pregnancy rates. OHSS occurred in two patients, both of whom received 250 mcg r-hCG. Both the cases were categorized as mild and managed conservatively; embryo transfer was not performed in these patients.
Table 2.
Treatment outcome
| r-hCG 250 µg (n=48) | r-hCG 500 µg (n=48) | P | |
|---|---|---|---|
| Number of aspirated follicles | 9.6±4.3 | 8.0±4.1 | 0.06 |
| Number of retrieved oocytes | 6.5±4.0 | 6.4±3.9 | 0.3 |
| Number of oocytes retrieved/follicle | 67.4±23.9 | 77.5±23.3 | 0.04 |
| Number of MII oocytes | 4.0±2.3 | 3.2±2.3 | 0.09 |
| Fertilized oocytes (n) | 6.3±3.5 | 6.1±3.4 | 0.77 |
| Fertilization rate (%) | 79.3±23.2 | 80.4±32.2 | 0.84 |
| Number of embryos (n) | 5.4±2.7 | 4.7±3.0 | 0.23 |
| Transferred embryos (n) | 1.8±0.8 | 1.9±0.7 | 0.51 |
| Biochemical pregnancy rate (%) | 41.6 (20/48) | 39.5 (19/48) | 0.83 |
| Clinical pregnancy rate (%) | 35.4 (17/48) | 35.4 (17/48) | 1.0 |
| OHSS (%) | 4.1 (2/48) | 0 (0/48) |
MII=Metaphase II, OHSS=Ovarian hyperstimulation syndrome, r-hCG=Recombinant-human chorionic gonadotropin
There was heterogeneity between the two groups with regard to basal ovarian reserve, hence, to account for this confounder, we performed subgroup analysis on women with poor ovarian reserve, defined as patients with AFC <11 and AMH ≤1.1 ng/ml. This included 26 women in the study; 9 women in Group 1 (250 μg r-hCG) and 17 women in Group 2 (500 μg r-hCG). Although there was no difference in the mean number of oocytes retrieved among the groups (4.0 ± 2.8 in Group 1 vs. 6.6 ± 3.3, P = 0.06), the percentage of MII oocytes was significantly higher in those receiving the higher dose of r-hCG (33.7% ± 26.4% vs. 51.9% ± 28.2% in Group 1 and 2, respectively, P = 0.03). The mean number of oocytes retrieved per follicle was also higher in the 500 μg r-hCG group (56.6 ± 31.2 vs. 83.4 ± 23.4 in Group 1 and 2, respectively, P = 0.02), indicating improved efficacy of the higher dose in the poor responder subgroup. There was also a trend toward improvement in the fertilization rate from 63.4% to 69.4% and the clinical pregnancy rate from 22.2% to 23.5%, although this was not significant due to the small numbers [Table 3].
Table 3.
Treatment outcome in poor responder subgroup
| r-hCG 250 µg (n=9) | r-hCG 500 µg (n=17) | P | |
|---|---|---|---|
| Number of retrieved oocytes | 4.0±2.8 | 6.6±3.3 | 0.06 |
| Number of oocytes retrieved/follicle | 56.6±31.2 | 83.4±23.4 | 0.02 |
| Number of MII oocytes | 2.2±1.8 | 3.7±1.9 | 0.06 |
| Percentage of MII oocytes/total oocytes | 33.7±26.4 | 51.9±28.2 | 0.03 |
| Fertilization rate (%) | 63.4±34.4 | 69.4±20.4 | 0.57 |
| Number of embryos | 3.2±3.0 | 3.8±2.7 | 0.6 |
| Biochemical pregnancy rate (%) | 22.2 (2/9) | 29.4 (5/17) | 0.69 |
| Clinical pregnancy rate (%) | 22.2 (2/9) | 23.5 (4/17) | 0.94 |
MII=Metaphase II, r-hCG=Recombinant-human chorionic gonadotropin
DISCUSSION
One of the most important predictive factors for the success of IVF is the number and quality of oocytes available for fertilization. For this reason, researchers are investigating the best way of obtaining the highest number of mature oocytes in in vivo stimulation. For final oocyte maturation and development of other periovulatory events such as softening of the connective tissue of follicle for easy detachment of oocyte cumulus complex from the follicle wall, the physiological LH surge is essential, and this is brought about in IVF cycles by hCG.[7]
In our study, we demonstrated an equivalence of the 250 μg and 500 μg of r-hCG for induction of final follicular maturation and luteinization in patients undergoing IVF in terms of number of mature oocytes retrieved, fertilization rate, and the number of embryos. However, we found an increase in the number of oocytes retrieved per follicle when final maturation was induced by higher dose, i.e., 500 μg of r-hCG as compared to the conventional dose of 250 μg r-hCG. However, this did not translate to an increase in the number of embryos or improvement in clinical pregnancy rate.
In the subgroup of poor responder women, we demonstrated an increase in the number of mature oocytes from a mean of 2.2 to 3.7, leading to an improvement in the fertilization rate from 63.4% to 69.4%. There was also a trend toward improvement in the biochemical and clinical pregnancy rates in the poor responders. Although these differences were not significant, and it was not our primary end point, it seemed that oocyte competence could be improved by increasing the dose of r-hCG at the time of triggering. This could be due to a diminished number of LH/hCG receptors in the ovarian follicles of this subset of women, thereby requiring a higher dose for initiation of the cascade of events leading to resumption of meiosis and release of the oocyte. Moreover, an increase in the absolute number of mature oocytes retrieved would improve IVF results by increasing the number of good quality embryos.
Chang et al. were the first to demonstrate that numbers of 2PN fertilized oocytes and cleaved embryos were significantly higher with 500 μg r-hCG than the lower dose without enhancement of OHSS incidence.[3] Similar to our study, the luteal phase serum progesterone (P) concentration was higher in patients receiving the higher dose of r-hCG.[3] However, they found that both doses were equally efficacious in terms of oocytes retrieved per follicle (250 mg of r-hCG, 13.6; 500 mg of r-hCG, 14.6) or the implantation rate (18.7%, 21.3%, respectively). Our results are consistent with that of Madani et al. who demonstrated that mean number of retrieved oocytes per follicles were 69.84 ± 17.44 and 77.16 ± 17.61 in 250 μg r-hCG and 500 μg r-hCG, respectively, which was significantly higher with 500 μg r-hCG than the lower dose (P = 0.04). Other cycles and clinical outcomes were comparable between groups.[8] Another recent study by Fabris et al. found an improvement in the number of mature oocytes retrieved (5.3 + 3.6 [4.4–6.1] vs. 2.4 + 2.2 [2–2.9], P < 0.001) in women who had high immature oocyte rate in previous treatment cycle.[9] They used a dual trigger of 250 μg r-hCG along with GnRH agonist triptorelin unlike our study in which we used double dose of standard r-hCG trigger.
In contrast, Chan et al. found that percentage of MII oocytes was similar in the two groups (89.3% vs. 86.0%; P = 0.326) despite higher serum and follicular fluid hCG levels on day 2 and day 4 post-hCG injection. There was no difference in the implantation and pregnancy rates between the two groups.[4] Clua et al. also found no significant differences in the total number of oocytes and MII oocytes among oocyte donors in whom ovulation was triggered with 250 μg or 500 μg of r-hCG. The pregnancy rate per embryo transfer in the corresponding recipients was similar for both the groups (58.2% vs. 56.1%).[10] Both Chang et al.[3] and Chan et al.[4] found a higherP level in the luteal phase in women receiving 500 μg r-hCG, similar to the results of our study. Whether such a difference in the serum P level confers a more favorable luteal environment for implantation is unclear.
In the present study, the incidence of OHSS was comparable between groups. Surprisingly, in the study of Chan et al.,[4] there was a trend toward higher incidence of OHSS with 250 μg r-hCG compared to 500 μg r-hCG, probably due to the patients recruited. Both Ludwig et al.[11] and Chang et al.[3] observed a higher incidence of OHSS with 500 μg than with 250 μg, but this was not statistically significant. In our study, no cases of severe OHSS were observed, probably due to our strict exclusion criteria. Donors with OHSS risk were either cancelled or received GnRH agonist to trigger instead of hCG.
CONCLUSION
A double dose of r-hCG for final follicular maturation in IVF cycles resulted in improvement of mean number of oocytes per follicle but did not result in higher number of MII oocytes retrieved or higher pregnancy rates in the women. However, in the subset of poor responders, double dose of r-hCG resulted increased number of mature oocytes obtained, fertilization rate, and improved pregnancy rates. Further randomized studies with a larger number of patients are required to confirm the results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Abdelmassih V, Oliveira FG, Goncalves SP, Varella AD, Diamond MP, Abdelmassih R. A prospective, randomized and blinded comparison between 10,000 IU urinary and 250 microg recombinant human chorionic gonadotropin for oocyte maturation in in vitro fertilization cycles. J Assist Reprod Genet. 2005;22:149–53. doi: 10.1007/s10815-005-4911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driscoll GL, Tyler JP, Hangan JT, Fisher PR, Birdsall MA, Knight DC. A prospective, randomized, controlled, double-blind, double-dummy comparison of recombinant and urinary HCG for inducing oocyte maturation and follicular luteinization in ovarian stimulation. Hum Reprod. 2000;15:1305–10. doi: 10.1093/humrep/15.6.1305. [DOI] [PubMed] [Google Scholar]
- 3.Chang P, Kenley S, Burns T, Denton G, Currie K, DeVane G, et al. Recombinant human chorionic gonadotropin (rhCG) in assisted reproductive technology: Results of a clinical trial comparing two doses of rhCG (Ovidrel) to urinary hCG (Profasi) for induction of final follicular maturation in in vitro fertilization-embryo transfer. Fertil Steril. 2001;76:67–74. doi: 10.1016/s0015-0282(01)01851-9. [DOI] [PubMed] [Google Scholar]
- 4.Chan CC, Ng EH, Tang OS, Yeung WS, Lau EY, Ho PC. A prospective, randomized, double-blind study to compare two doses of recombinant human chorionic gonadotropin in inducing final oocyte maturity and the hormonal profile during the luteal phase. J Clin Endocrinol Metab. 2005;90:3933–8. doi: 10.1210/jc.2004-2169. [DOI] [PubMed] [Google Scholar]
- 5.Peñarrubia J, Balasch J, Fábregues F, Creus M, Cívico S, Vanrell JA. Recurrent empty follicle syndrome successfully treated with recombinant human chorionic gonadotrophin. Hum Reprod. 1999;14:1703–6. doi: 10.1093/humrep/14.7.1703. [DOI] [PubMed] [Google Scholar]
- 6.Littman ED, Milki AA. The combination of urinary and recombinant HCG improves outcome in patients with decreased oocyte/follicle ratio in previous cycles. Eur J Obstet Gynecol Reprod Biol. 2003;109:60–2. doi: 10.1016/s0301-2115(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 7.Meniru GI, Craft IL. Evidence from a salvaged treatment cycle supports an aetiology for the empty follicle syndrome that is related to terminal follicular developmental events. Hum Reprod. 1997;12:2385–7. doi: 10.1093/humrep/12.11.2385. [DOI] [PubMed] [Google Scholar]
- 8.Madani T, Mohammadi Yeganeh L, Ezabadi Z, Hasani F, Chehrazi M. Comparing the efficacy of urinary and recombinant HCG on oocyte/follicle ratio to trigger ovulation in women undergoing intracytoplasmic sperm injection cycles: A randomized controlled trial. J Assist Reprod Genet. 2013;30:239–45. doi: 10.1007/s10815-012-9919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabris AM, Cruz M, Legidos V, Iglesias C, Muñoz M, García-Velasco JA. Dual triggering with gonadotropin-releasing hormone agonist and standard dose human chorionic gonadotropin in patients with a high immature oocyte rate. Reprod Sci. 2017;24:1221–5. doi: 10.1177/1933719116682873. [DOI] [PubMed] [Google Scholar]
- 10.Clua E, Martínez F, Tur R, Sanmartín P, Chueca A, Barri PN. Triggering ovulation with 250 μg or 500 μg of r-hCG in oocyte donors treated with antagonist protocol has no effect on the number of mature oocytes retrieved: A randomized clinical trial. Gynecol Endocrinol. 2012;28:678–81. doi: 10.3109/09513590.2011.652244. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig M, Doody KJ, Doody KM. Use of recombinant human chorionic gonadotropin in ovulation induction. Fertil Steril. 2003;79:1051–9. doi: 10.1016/s0015-0282(03)00173-0. [DOI] [PubMed] [Google Scholar]