Abstract
Aim:
Lifestyle is an important risk factor for increasing the prevalence of diabetes in the Indian population. In this study, we evaluate the effects of naturopathy treatment, salt-restricted low-calorie diets, and yoga in long-term glycemic control in patients with type 2 diabetes mellitus.
Methods:
In this prospective, longitudinal, two-arm cohort study, patients with type 2 diabetes mellitus referred from a tertiary care center undergoing a 3-month residential naturopathy treatment were compared with those undergoing only conventional management on glycemic control. Both fasting and postprandial blood glucose (PPBG) levels were assessed at baseline, 3 months following intervention, at 6 months, and 12 months from the study start. Data were analyzed using repeated-measures ANOVA with post hoc Bonferroni correction.
Results:
Naturopathy and yoga intervention significantly reduced PPBG levels (P < 0.001), glycated hemoglobin levels (P < 0.001), and reduced requirement for antidiabetic medications (P < 0.008) in the intervention group compared to controls. The effects were more profound immediately following intervention and lasted up to 6 months from the start of the study.
Conclusion:
The results suggest benefit with an intensive residential naturopathy-based lifestyle intervention program. Randomized controlled trials are needed to further validate the findings.
Keywords: Diabetes, fasting, glycemic control, low-salt diet, naturopathy, yoga
Introduction
Type 2 diabetes mellitus is a major global health problem. The World Health Organization estimates that diabetes, primarily type 2, affects 5.2% of the world's adult population.[1] India now has the largest population with type 2 diabetes mellitus internationally, with a 10.4% prevalence and totaling about 19 million. India is also expected to show the highest increase in the prevalence internationally by 2045 (84% increase).[2] This increase is attributed to increase in life expectancy and changes in lifestyle such as fewer healthy diets and physical inactivity. Countries need to strengthen prevention to prevent increase in the prevalence of type 2 diabetes mellitus. Several factors such as low awareness, ignorance about complications, poor adherence to medications and lifestyle interventions, bad health behaviors such as smoking and alcohol, physical inactivity, and obesity contribute to the increasing prevalence of this problem.[3]
Results from the Diabetes Prevention Program showed that a 7% weight loss in the 1st year through increased physical activity (150 min of brisk walking per week) reduced the 4-year incidence of type 2 diabetes by 58% in men and women with impaired glucose tolerance and was twice as effective as metformin therapy.[4] Moreover, improvement in fasting blood glucose (FBG) is directly related to the relative amount of weight lost.[5] Moderate weight loss may not improve glycemic control in all obese patients who have diabetes;[6] however, it is possible that patients with long-standing disease or severe pancreatic β-cell dysfunction are not as responsive to weight loss as those with less extensive disease. Guidelines and recommendations suggest 150 min of aerobic moderate exercise (50%–70% of maximum heart rate) or 75 min of vigorous-intensity exercise or a combination of both over at least 3 days in a week for glycemic control.[7,8]
Several components in naturopathic intervention such as raw diets, calorie restriction (fasting), massage, yoga, physical exercise, and hot baths are independently known to confer weight reduction and bring glycemic control.[9,10,11,12,13] Several studies have shown acute effects of these interventions using raw vegetable juices with or without yoga on glycemic control.[14,15] Our earlier study using naturopathy interventions showed good glycemic control in patients with type 2 diabetes mellitus.[16] However, there is a paucity of controlled studies with structured naturopathy intervention in patients with type 2 diabetes mellitus. In India, several people flock to residential naturopathy centers for weight loss, cardioprotective effects, and glycemic control. These interventions have been shown beneficial in the short term.[9,10,11,12,13,14,15] However, the long-term effects of such acute calorie restriction and weight loss following a residential naturopathic intervention have not been studied. In this open-label parallel cohort study, we aim to study the effect of an intensive residential naturopathic intervention versus usual care in type 2 diabetes mellitus patients immediately following intervention and long-term effects at 6 and 12 months.
Methods
Subjects
Patients with type 2 diabetes mellitus attending outpatient clinics in endocrinology department of a tertiary medical teaching hospital were invited to participate in a residential naturopathy and yoga-based lifestyle intervention over a 3-month period in a comprehensive Naturopathy Centre, Manthena Satyanarayana Raju Arogyalayam in Vijayawada, India. A convenience sampling approach was used, Patients were recruited into the study if they satisfied selection criteria and gave written inform consent to participate. Patients who agreed to undergo intensive residential naturopathy and yoga-based life style intervention were recruited as cases. Those who agreed to participate in the study but were not undergo residential naturopathy and yoga intervention were recruited as controls. The participants were required to give blood samples and fill in questionnaires and undergo periodic clinical assessments over 1 year. The intervention was conducted in a residential naturopathy and yoga center near to the hospital. The study was supervised by endocrinologists and physicians of the referral medical teaching hospital. The study was cleared by the institutional review board of both the institutions. The participants were recruited only if they satisfied the selection criteria and gave written consent.
Selection criteria
Inclusion criteria
Patients age range between 18 and 60 of both genders, with a history of type 2 diabetes mellitus for the past 1 year or more along with glycated hemoglobin (HbA1c) >7% levels; those who are depending on oral or parenteral hypoglycemic agents; those with Zubrod's performance status 0–2; and willing to give consent to participate in the study were included in the study.
Exclusion criteria
Patients with secondary complications of type 2 diabetes mellitus such as Grade III nephropathy, end-stage renal disease, and diabetic ulcers. Patients with a history of recent myocardial infarction or transient ischemic attacks, hot water epilepsy, exercise-induced asthma, uncontrolled blood pressure (systolic blood pressure >160 mmHg and diastolic blood pressure >110 mmHg), anemia (Hb <10 g%), hyponatremia (sodium <136 mg/dl), neutropenia, pancytopenia or thrombocytopenia, major depressive disorders, or psychiatric or neurological illness. Those who participated in regular exercise, yoga, nutrition, or lifestyle modification program in the preceding six months were excluded. Patients with active infections or fever, those with New York Heart Association Class III cardiac failure, or chronic obstructive pulmonary disease resulting in dyspnea or orthopnea were excluded from the study.
Sample size
The sample size was calculated using Open source Epi Info Software (version 3.1, CDC, USA) by Fleiss method with continuity correction with 95% confidence interval (CI) and 80% power, for a 15-point difference in an outcome between the control (unexposed, n = 5) and intervention (exposed, n = 20) groups. The total sample size was 176 with allocation ratio of 1:1, i.e., 88 patients in each arm. Considering 20% attrition, we recruited 106 patients in each arm.
Study procedure
A total of 102 patients in naturopathy group and 109 participants in the control group provided data at the baseline. Those in naturopathy group underwent residential naturopathy and yoga treatment in a naturopathy center. Written and signed informed consent was obtained at the time of enrolment at baseline. Briefly, sociodemographic characteristics of the patients, their personal and medical history, and details of various medications taken by the patient were abstracted on the case report form. Blood draws were carried out under aseptic conditions by a trained phlebotomist and 10 ml of blood was collected in sterile vacationers.
FBG and serum insulin 2-h postprandial blood glucose (PPBG), HbA1c and lipid profile, liver function tests, and renal function tests were carried out. Samples were collected between 7 am and 9 am on all days. Follow-up assessments were done at 3, 6, and 12 months from the start date. Medication score was calculated by assigning a score of 0 or 1 (No/Yes) for common diabetes oral medications and a score of 0 or 2 (No/Yes) for insulin, a more demanding regimen which has been used in the Diabetes Medication Satisfaction Tool earlier.[17]
At 3 months, 102 and 92 participants provided data in naturopathy and control group. Thereafter, dropouts in naturopathy group were due to failure to adhere to diet and physical activity regimen (n = 12), defaulted on tests or assessments (n = 5), not available for follow-up (n = 14). In control group, dropouts were mainly because of not available for follow-up (n = 20), increase in blood glucose levels, uncontrolled diabetes (n = 3), defaulted on tests or assessments (n = 4), and death (n = 1) due postoperative renal failure following lower limb amputation for gangrene with uncontrolled diabetes [see Figure 1: Trial profile].
Figure 1.
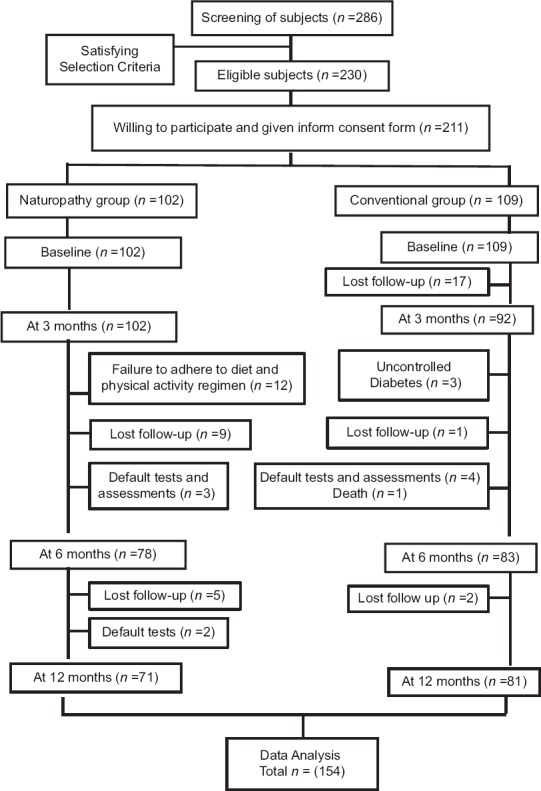
Trial profile
Outcome measures
Primary outcomes
HbA1c was measured using high-performance liquid chromatography (HPLC) with Biorad D10 Analyzer and Biorad reagents were used.
The level of HbA1c is proportional to both the average glucose concentration and the life span of the red blood cell in the circulation. The measurement of HbA1c has therefore been accepted for the clinical management of type 2 diabetes mellitus through routine monitoring. Methods for the determination of HbA1c include electrophoresis, immunoassays, and chromatography. HbA1c determination with the D-10 Dual Program has been optimized to eliminate interferences from hemoglobin variants, labile A1c, and carbamylated hemoglobin. The D-10 Dual Program is based on chromatographic separation of the analytes by ion-exchange HPLC.
Secondary outcomes
Blood glucose (fasting/postprandial) was measured by spectrophotometry (Hexokinase method) using an auto-analyzer (Beckman Unicel DxC 800 Chemistry Analyzer) with Beckman reagents
Medication score – Both oral hypoglycemic agents and parenteral insulin were calculated by using the Diabetes Medication Satisfaction Tool.
Intervention
Naturopathy and yoga-based lifestyle intervention
In this group, the participants were enrolled in a 3-month residential naturopathy intervention program comprising diet, yoga, hydriatic treatments, massage, and didactic and interactive lectures on lifestyle modification and type 2 diabetes mellitus self-management. Patients underwent a structured routine which involved physical activity, yoga program with asanas, pranayama, meditation and relaxation, calorie restriction, and salt-restricted diet. The diet prescribed was a low glycemic index, low-salt high-fiber plant-based diet containing whole grains, legumes, vegetables, and fruits with no added oil, sugar, or salt. They also underwent short intermittent juice fasting with calorie restriction over 3–4 days a month. They had to undergo a structured routine of hydriatic treatments such as hip bath, immersion bath, jets, sprays, douche, and mud and steam bath apart from partial and full-body Swedish massages. The goal of treatment was weight reduction if overweight, stress reduction, and dietary intervention to manage glycemic index. The patients were in this facility for a period of 3 months and were supervised by doctors and diabetologists over the course of their intervention. The oral antidiabetic medication was constantly monitored and tapered based on their blood glucose and HbA1c levels.
Control intervention
The control group received conventional antidiabetic treatment as per the standard guidelines. The pharmacological management of type 2 diabetes mellitus patients included both oral hypoglycemic agents, parenteral insulin, and other medicines to manage their comorbid conditions and supportive care. The control group also received a diabetes self-management program by a diabetes educator during their hospital visits.
They were also counseled on the diet and physical activity by a clinical nutritionist. Both HbA1c levels, blood glucose, and other biochemical tests were routinely conducted and monitored. Dose escalation or tapering was done based on these levels by a diabetologist.
Data analysis
Data were analyzed using Statistical Package for the Social Sciences (spss) software (version 18, IBM, USA) for Windows. Data were found to be normally distributed. A repeated-measures ANOVA was done with assessments being time and group being independent variables. The group by time interaction effects was computed using post hoc Bonferroni correction for four time points and two group measures. Therefore, 95% CI of the adjusted P value was fixed at P ≤ 0.008 for post hoc correction. Both within- and between-group effects were analyzed using intention-to-treat analysis. The missing values were imputed using the mean of the respective group for that assessment interval. Changes from the baseline between groups were assessed using independent samples t-test.
Results
The mean age of the study population was 51 years in naturopathy group and 48.8 years in control group. Participants in the naturopathy group had a long-standing type 2 diabetes mellitus, mean 10.27 years compared to control group mean 5.83 years. Gender distribution, comorbid illness, and other sociodemographic variables were similar across groups [Table 1]. There was a significant weight loss in the naturopathy group compared to the conventional group at 3 (P < 0.01) and 6 (P < 0.01) months, but not in 12th month, whereas there was significant weight loss within the group at 3 (P < 0.001) and 6 (P < 0.001) months when compared with baseline.
Table 1.
Sociodemographic and clinical characteristics of the study population
| Particulars | Mean±SD | |
|---|---|---|
| Naturopathy | Conventional | |
| Age, mean±SD | 51.0±8.1 | 48.8±8.1 |
| Duration of type 2 diabetes mellitus | 10.27±6.6 | 5.83±5.7 |
| Gender, n (%) | ||
| Male | 43 (42.2) | 50 (45.9) |
| Female | 59 (57.8) | 59 (54.1) |
| No comorbidity | 35 (34.3) | 31 (28.4) |
| Comorbidity 1 | 48 (47.1) | 65 (59.6) |
| Comorbidity 2 | 18 (17.6) | 12 (11.0) |
| Comorbidity 3 | 1 (1.0) | 1 (0.9) |
| Comorbidity obesity | 28 (27.5) | 37 (33.9) |
| Comorbidity hypertension | 46 (45.1) | 47 (43.1) |
| Comorbidity hypothyroidism | 3 (2.9) | 5 (4.6) |
| Comorbidity CAD | 3 (2.9) | 0 (0.0) |
| Comorbidity nephropathy Grade 1 | 7 (6.9) | 3 (2.8) |
| Comorbidity others | 36 (35.3) | 16 (14.7) |
| Occupation, n (%) | ||
| Government employee | 1 (1.0) | 2 (1.8) |
| Nongovernment employee/private | 9 (8.8) | 10 (9.2) |
| Homemaker | 56 (54.9) | 40 (36.7) |
| Retired employee | 11 (10.8) | 5 (4.6) |
| unemployed | 5 (4.9) | 2 (1.8) |
| Business | 3 (2.9) | 2 (1.8) |
| Others | 17 (16.7) | 48 (44.0) |
| Education, n (%) | ||
| Illiterate | 5 (4.9) | 31 (28.4) |
| Less than primary | 4 (3.9) | 8 (7.3) |
| Primary | 24 (23.5) | 24 (22.0) |
| High school | 31 (30.4) | 30 (27.5) |
| Intermediate | 12 (11.8) | 8 (7.3) |
| Graduate | 22 (21.6) | 5 (4.6) |
| Postgraduate | 4 (3.9) | 3 (2.8) |
SD=Standard deviation, CAD=Coronary artery disease
Fasting blood glucose and postprandial blood glucose levels
Multivariate analysis showed that there is no significant group by time interaction effects for fasting blood sugar between naturopathy and conventional treatments. However, the between-subject's effects showed a significant difference between groups F (1, 208) = 4.62, P = 0.03. Post hoc Bonferroni correction showed significant decrease in fasting blood sugar within the naturopathy group between baseline and postintervention at 3 months (P < 0.001), 6 months (P < 0.001), and 12 months (P = 0.006). There was no significant decrease in fasting blood sugar in naturopathy group compared to the conventional group postintervention at 3, 6, and 12 months [Table 2].
Table 2.
Comparison of changes in fasting and postprandial blood sugar over time following naturopathy and conventional treatment using repeated-measures ANOVA
| Group | FBG (mg/dl), mean±SD | FBG change score (mg/dl), mean±SD | |||||
|---|---|---|---|---|---|---|---|
| BL | 3 M | 6 M | 12 M | 3 M | 6 M | 12 M | |
| Naturopathy | 190.66±65.51 | 136.10±44.93** | 144.13±53.40** | 167.09±58.45* | 54.56±65.51 | 46.53±72.48 | 23.57±75.70 |
| Conventional | 148.54±46.62 | 136.82±38.71 | 150.00±57.48 | 157.33±62.60 | 10.35±49.64 | −0.35±49.64 | −0.35±49.640 |
| Group | Postprandial blood sugar (mg/dl) | Postprandial blood sugar change score (mg/dl) | |||||
| Naturopathy | 250.69±104.13 | 172.31±56.15** | 184.10±63.90** | 273.13±81.69 | 73.47±114.02 | 61.67±108.99 | −1.67±108.99* |
| Conventional | 212.40±77.04 | 183.73±55.14* | 195.64±63.49 | 202.19±87.50 | 26.72±81.04 | 14.81±87.08 | 8.96±96.88 |
P<0.008, P<0.01 for between groups; *P<0.008, **P<0.001 for within group effects using post hoc Bonferroni correction. BL=Baseline, 3 M=3 months, 6 M=6 months, 12 M=12 months, FBG=Fasting blood sugar, SD=Standard deviation
Multivariate analysis showed that there is a significant group by time interaction effect for PPBG between naturopathy versus conventional treatment F (3, 204) = 21.78, P < 0.001. The between-subject's effects showed a significant difference between groups F (1, 206) = 9.4, P = 0.003. Post hoc Bonferroni correction showed significant decrease in postprandial blood sugar within the naturopathy group between baseline and postintervention that is at 3 months (P < 0.001), 6 months (P < 0.001), but not in 12 months. There was no significant decrease in postprandial blood sugar in naturopathy group compared to the conventional group postintervention at 3 and 6 months. Whereas, there is a significant increase postprandial blood sugar in naturopathy group compared with conventional group at 12 months (P < 0.001) [Table 2].
Glycemic control at 3, 6, and 12 months from baseline
There was a significant decrease in FBG at 3 months from baseline in naturopathy group compared to conventional group on independent samples t-test (t = 5.0, P < 0.001, 95% CI = 26.8–61.6), at 6 months from baseline in naturopathy group compared to conventional group on independent samples t-test (t = 5.1, P < 0.001, 95% CI = 30.4–68.3), and at 12 months from baseline in naturopathy group compared to conventional group on independent samples t-test (t = 3.4, P = 0.001, 95% CI = 14.2–53.1). There was a significant decrease in PPBG at 3 months from baseline in naturopathy group compared to conventional group on independent samples t-test (t = 3.4, P = 0.001, 95% CI = 20.0–73.4), at 6 months from baseline in naturopathy group compared to conventional group on independent samples t-test (t = 3.4, P = 0.001, 95% CI = 20.1–73.5), and at 12 months from baseline in naturopathy group compared to conventional group on independent samples t-test (t = −2.3, P = 0.021, 95% CI = −65.7–−5.4) [Table 2].
Glycated hemoglobin levels
Multivariate analysis showed that there is a significant group by time interaction effect for HbA1c between naturopathy versus conventional medicine F (3, 207) = 11.83, P < 0.001. However, the between-subject's effects showed that there was no significant difference between groups. Post hoc Bonferroni correction showed significant decrease in HbA1c within the naturopathy group between baseline and postintervention, that is, at 3 (P < 0.001), 6 (P < 0.001), and 12 months (P < 0.001). Post hoc Bonferroni correction showed significant decrease in HbA1c within the conventional group between baseline and postintervention, that is, at 3 (P < 0.001), 6 (P = 0.006), and 12 months (P = 0.019). There was significant decrease in HbA1c in naturopathy group compared to the conventional group postintervention at 3 (P < 0.001) and 6 months (P = 0.035) but not at 12 months [Table 3].
Table 3.
Comparison of changes in glycated hemoglobin over time following naturopathy and conventional treatment using repeated-measures ANOVA with post hoc Bonferroni correction
| HbA1c (%), mean±SD | HbA1c change score (%), mean±SD | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 3 M | 6 M | 12 M | 3 M | 6 M | 12 M | |
| Naturopathy group | 9.6 (81)±1.8 (19) | 7.5 (58)±0.8 (8)** | 7.9 (63)±1.5 (16)** | 8.5 (69)±1.7 (18)** | 2.0 (21)±1.6 (17) | 1.6 (17)±2.0 (21) | 1.1 (12)±2.0 (21) |
| Conventional group | 9.0 (75)±1.7 (18) | 8.2 (66)±1.2 (13)** | 8.4 (68)±1.6 (17)* | 8.4 (68)±1.7 (18) | 0.7 (7)±1.7 (18) | 0.6 (6)±1.8 (19) | 0.5 (5)±1.8 (19) |
P<0.008, P<0.001, for between groups; *P<0.008, **P<0.01 for within group effects using post hoc Bonferroni correction, HbA1c values in mmol/mol is in parentheses. SD=Standard deviation, BL=Baseline, 3 M=3 months, 6 M=6 months, 12 M=12 months, HbA1c=Glycated hemoglobin
There was a significant decrease in HbA1c at 3 months from baseline in naturopathy group compared to conventional group on independent samples t-test (t = 5.7, P < 0.001, 95% CI = 0.86–1.76), at 6 months from baseline in naturopathy group compared to conventional group on independent samples t-test (t = 3.9, P < 0.001, 95% CI = 0.5–1.6), and at 12 months from baseline in naturopathy group compared to conventional group on independent samples t-test (t = 2.2, P = 0.028, 95% CI = 0.06–1.11) [Table 3].
Medication score
There was a significant decrease in number medication score using Diabetes Medication Satisfaction Tool in naturopathy group compared to conventional group at 6 months (P < 0.008) only but not at 3 and 12 months using Mann–Whitney U-test for between-group comparison [Table 4].
Table 4.
Comparison of medication score of the study population at baseline, 3, 6, and 12 months using Diabetes Medication Satisfaction Tool
| Total score, mean±SD | ||||
|---|---|---|---|---|
| Base line | 3 M | 6 M | 12 M | |
| Naturopathy group | 1.62±0.90 | 1.16±0.95 | 0.79±0.90ł | 0.75±0.93 |
| Conventional group | 1.55±0.83 | 1.24±1.02 | 1.18±1.08 | 0.82±1.06 |
P<0.008 for between groups using Mann–Whitney U-test. BL=Baseline, 3 M=3 months, 6 M=6 months, 12 M=12 months, SD=Standard deviation
Parenteral insulin
There was a significant increase in insulin usage at 6 months in conventional group compared to naturopathy group on Pearson Chi-square test for intergroup comparison (Chi-square = 4.15, P = 0.04) but not at 3 and 12 months [Table 5].
Table 5.
Parenteral medication (insulin) score at baseline, 3, 6, and 12 months
| Parenteral insulin | BL, n (%) | 3 M,n (%) | 6 M, n (%) | 12 M, n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Naturopathy group | 36 (35.5) | 66 (64.7) | 23 (22.5) | 79 (77.5) | 18 (17.6) | 84 (82.4) | 15 (14.7) | 87 (85.3) |
| Conventional group | 36 (33.3) | 72 (66.7) | 34 (31.5) | 74 (68.5) | 32 (29.6) | 76 (70.4) | 21 (19.4) | 87 (80.6) |
| P | 0.76 | 0.14 | 0.04 | 0.36 | ||||
P<0.05, P<0.01, P<0.001 for between groups using Chi-square test of proportions. BL=Baseline, 3 M=3 months, 6 M=6 months, 12 M=12 months
Discussion
The results suggest that the 3-month residential naturopathy and yoga intervention significantly reduced blood glucose levels, glycated hemoglobin levels, and reduced need for antidiabetic medications in the intervention group compared to controls. The effects were more profound immediately following intervention and lasted up to 6 months from the start of the study. However, the effects were not sustained over a longer period of 1 year from the study start in the intervention.
The results from our study are similar to earlier studies that have shown reductions in blood glucose levels and HbA1C levels with structured physical activity and dietary interventions.[9,10,11,12,15,16,18,19] While the earlier studies have shown benefit finding with physical activity, dietary intervention, and naturopathy intervention in the short term,[11,14,15,19,20] our study showed that the effects can be sustained over a 6 months period. The effect size of the intervention was small for change in PPBG levels from baseline (Effective size (ES) = −0.32 [95% CI − 0.12– −0.51]), moderate for FBG levels (ES = −0.52 [95% CI − 0.33–−0.71]), and large for HbA1C levels (ES = −1.0 [95% CI − 0.12–−0.51]) at 3 months following intervention. While the effects were maintained at 6 months, they tended to decrease at the end of 1 year.
The patients in the study underwent intermittent fasting ranging from 3 to 5 days and were on a naturopathy based plant diet rich in fruits, vegetables, sprouts, nuts, salads, and juices.
Basically, intervention group received a high-fiber, high-protein, low-carbohydrate, and nonketogenic diet. This is different from earlier studies, wherein ketogenic diets have been used to improve insulin sensitivity and reduce resistance in overweight and obese populations.
Fasting, in addition to lowering insulin levels, also improves insulin resistance significantly.[21] A low-salt diet devoid of added salt can further potentiate weight loss and bring better glycemic control. Moreover, hot baths, yoga, fruit and vegetable diets, and active lifestyle also exert anti-inflammatory effects that also lead to glycemic control.
Patients with type 2 diabetes mellitus have two–fourfold increased risk for cardiovascular disease than nondiabetic patients.[22,23,24] Therefore, using interventions that reduce weight and bring glycemic control is needed to mitigate this risk. Naturopathy and yoga-based intervention promises better glycemic control that could also reduce risk for cardiovascular diseases.[11,16,18,25,26,27] Diets rich in whole grains, fruits, vegetables, nuts, with a moderate alcohol intake, a lower intake of red meat, processed refined foods, sweets, dairy products with high fat, and soft drinks have been correlated with a reduced risk of type 2 diabetes mellitus, better glycemic control, and lipid profile in patients with type 2 diabetes mellitus.[15,28,29,30,31,32,33,34] Our diet is a salt-restricted plant-based vegan diet shown to have better glycemic control than seen with the earlier studies. The residential program was an intensive self-management intervention in type 2 diabetes mellitus populations the effects of which led to better control of type 2 diabetes mellitus in the long-term than type 2 diabetes mellitus patients undergoing standard of care. The effects are similar to structured self-management programs that have improved glycemic control in type 2 diabetes mellitus.[35]
One of the major findings of the study was the follow-up and long-term effects conferred by naturopathy intervention in type 2 diabetes mellitus patients unlike earlier studies that have only looked at acute short-term effects of these interventions. The study highlights the potential issues with adherence to these interventions in a field setting. Furthermore, the results also suggest that the effects of this intensive intervention are sustained for a longer duration (3 months after the end of intervention). The biochemistry parameters were carried out in external accredited laboratory and laboratory personnel were blinded to the study allocation. Unlike earlier studies, the pharmacological management of the study population was closely followed up.
One of the major limitations of the study was that this was not a randomized controlled study. Second, it was also not possible to have a waitlist design as the intervention and follow-up was for over a year. Third, since patients received multicomponent interventions such as low-salt diet, plant-based diet, yoga, and physical activity, it is difficult to delineate the effects to any single intervention in our study. This is a prospective cohort study, and the findings from this study are hypotheses generating and cannot be deemed confirmatory.
Overall, the residential naturopathy and yoga intervention shows promising effects in bringing glycemic control in type 2 diabetes mellitus patients. The short-term effects of these interventions are profound and last for a longer time. However, the effects wane with time as seen with 1-year follow-up of these patients. More randomized controlled studies with active control arm are needed to validate the effects of low-salt diet in these populations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. Global Report on Diabetes. France: World Health Organization; 2016. [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas 8th Edition. 8th ed. Brussels: International Diabetes Federation; 2017. [Google Scholar]
- 3.Joshi SR. Diabetes care in India. Ann Glob Health. 2015;81:830–8. doi: 10.1016/j.aogh.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 5.Watts NB, Spanheimer RG, DiGirolamo M, Gebhart SS, Musey VC, Siddiq YK, et al. Prediction of glucose response to weight loss in patients with non-insulin-dependent diabetes mellitus. Arch Intern Med. 1990;150:803–6. [PubMed] [Google Scholar]
- 6.Reisin E, Abel R, Modan M, Silverberg DS, Eliahou HE, Modan B. Effect of weight loss without salt restriction on the reduction of blood pressure in overweight hypertensive patients. N Engl J Med. 1978;298:1–6. doi: 10.1056/NEJM197801052980101. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Global Recommendations on Physical Activity for Health. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 8.Cameron F. Standards of medical care in diabetes – 2016. Aust Fam Physician. 2006;35:386–90. [PubMed] [Google Scholar]
- 9.Angadi P, Jagannathan A, Thulasi A, Kumar V, Umamaheshwar K, Raghuram N. Adherence to yoga and its resultant effects on blood glucose in type 2 diabetes: A community-based follow-up study. Int J Yoga. 2017;10:29–36. doi: 10.4103/0973-6131.186159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melchart D, Löw P, Wühr E, Kehl V, Weidenhammer W. Effects of a tailored lifestyle self-management intervention (TALENT) study on weight reduction: A randomized controlled trial. Diabetes Metab Syndr Obes. 2017;10:235–45. doi: 10.2147/DMSO.S135572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gowda S, Mohanty S, Saoji A, Nagarathna R. Integrated yoga and naturopathy module in management of metabolic syndrome: A case report. J Ayurveda Integr Med. 2017;8:45–8. doi: 10.1016/j.jaim.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mooventhan A, Shetty GB. Effect of integrative naturopathy and yoga in a patient with rheumatoid arthritis associated with type 2 diabetes and hypertension. Anc Sci Life. 2017;36:163–6. doi: 10.4103/asl.ASL_80_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar R, Mooventhan A, Manjunath NK. Immediate effect of needling at CV-12 (Zhongwan) acupuncture point on blood glucose level in patients with type 2 diabetes mellitus: A pilot randomized placebo-controlled trial. J Acupunct Meridian Stud. 2017;10:240–4. doi: 10.1016/j.jams.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Nagasukeerthi P, Mooventhan A, Manjunath NK. Corrigendum to “Short-term effect of add on bell pepper (Capsicum annuum var. Grossum) juice with integrated approach of yoga therapy on blood glucose levels and cardiovascular functions in patients with type 2 diabetes mellitus: A randomized controlled study.”[Comp. Ther. Med 34 (2017) 42-45] Complement Ther Med. 2018;37:185. doi: 10.1016/j.ctim.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Selvakumar G, Shathirapathiy G, Jainraj R, Yuvaraj Paul P. Immediate effect of bitter gourd, ash gourd, Knol-Khol juices on blood sugar levels of patients with type 2 diabetes mellitus: A pilot study. J Tradit Complement Med. 2017;7:526–31. doi: 10.1016/j.jtcme.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bairy S, Kumar AM, Raju M, Achanta S, Naik B, Tripathy JP, et al. Is adjunctive naturopathy associated with improved glycaemic control and a reduction in need for medications among type 2 diabetes patients? A prospective cohort study from India. BMC Complement Altern Med. 2016;16:290. doi: 10.1186/s12906-016-1264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson RT, Girman CJ, Pawaskar MD, Camacho FT, Calles J, Kelly WS, et al. Diabetes medication satisfaction tool: A focus on treatment regimens. Diabetes Care. 2009;32:51–3. doi: 10.2337/dc08-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley R, Sherman KJ, Catz S, Calabrese C, Oberg EB, Jordan L, et al. Adjunctive naturopathic care for type 2 diabetes: Patient-reported and clinical outcomes after one year. BMC Complement Altern Med. 2012;12:44. doi: 10.1186/1472-6882-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barreira E, Novo A, Vaz JA, Pereira AM. Dietary program and physical activity impact on biochemical markers in patients with type 2 diabetes: A systematic review. Aten Primaria. 2018;50:590–610. doi: 10.1016/j.aprim.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and diabetes study. Diabetes Care. 1997;20:537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 21.Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: Effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81:69–73. doi: 10.1093/ajcn/81.1.69. [DOI] [PubMed] [Google Scholar]
- 22.Hormigo-Pozo A, Mancera-Romero J, Perez-Unanua MP, Alonso-Fernandez M, Lopez-Simarro F, Mediavilla-Bravo JJ, et al. Consensus document on the treatment of dyslipidemia in diabetes. Semergen. 2015;41:89–98. doi: 10.1016/j.semerg.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Halter JB, Musi N, McFarland Horne F, Crandall JP, Goldberg A, Harkless L, et al. Diabetes and cardiovascular disease in older adults: Current status and future directions. Diabetes. 2014;63:2578–89. doi: 10.2337/db14-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balasubramaniam K, Viswanathan GN, Marshall SM, Zaman AG. Increased atherothrombotic burden in patients with diabetes mellitus and acute coronary syndrome: A review of antiplatelet therapy. Cardiol Res Pract. 2012;2012:909154. doi: 10.1155/2012/909154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhunia S. Can physical exercise, yoga, diet control and naturopathic treatment prevent progression of diabetes mellitus? Indian J Physiol Pharmacol. 2010;54:92–4. [PubMed] [Google Scholar]
- 26.Shantakumari N, Sequeira S, El deeb R. Effects of a yoga intervention on lipid profiles of diabetes patients with dyslipidemia. Indian Heart J. 2013;65:127–31. doi: 10.1016/j.ihj.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beena RK, Sreekumaran E. Yogic practice and diabetes mellitus in geriatric patients. Int J Yoga. 2013;6:47–54. doi: 10.4103/0973-6131.105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salas-Salvadó J, Martinez-González MÁ, Bulló M, Ros E. The role of diet in the prevention of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21(Suppl 2):B32–48. doi: 10.1016/j.numecd.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Cohen AE, Johnston CS. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A (1c) in individuals with well-controlled type 2 diabetes mellitus. Metabolism. 2011;60:1312–7. doi: 10.1016/j.metabol.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins DJ, Kendall CW, Banach MS, Srichaikul K, Vidgen E, Mitchell S, et al. Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care. 2011;34:1706–11. doi: 10.2337/dc11-0338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Nishi SK, Kendall CW, Bazinet RP, Bashyam B, Ireland CA, Augustin LS, et al. Nut consumption, serum fatty acid profile and estimated coronary heart disease risk in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2014;24:845–52. doi: 10.1016/j.numecd.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Shidfar F, Froghifar N, Vafa M, Rajab A, Hosseini S, Shidfar S, et al. The effects of tomato consumption on serum glucose, apolipoprotein B, apolipoprotein A-I, homocysteine and blood pressure in type 2 diabetic patients. Int J Food Sci Nutr. 2011;62:289–94. doi: 10.3109/09637486.2010.529072. [DOI] [PubMed] [Google Scholar]
- 34.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–79. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinsbekk A, Rygg LØ, Lisulo M, Rise MB, Fretheim A. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. A systematic review with meta-analysis. BMC Health Serv Res. 2012;12:213. doi: 10.1186/1472-6963-12-213. [DOI] [PMC free article] [PubMed] [Google Scholar]


