Abstract
Background:
The postoperative settings in cardiothoracic intensive care unit (ICU) patients pose a certain risk with pulmonary dysfunction causing morbidity and mortality. Lung ultrasound (LUS) has a potential to supplant or replace Chest X-rays (CXR) in these subset of patients, who will require bed side pulmonary pathology diagnosis and interventions.
Aims and Objectives:
Aim of the study is to compare the diagnosis predicted from LUS to the diagnosis made from routine bedside CXR and to find the degree of agreement in diagnosis made by both modalities in different cardiopulmonary pathologies in ICUs.
Materials and Methods:
Prospective observational study involving 250 postoperative patients, admitted in cardio-thoracic and vascular ICU of a tertiary referral centre. LUS was done in the study patients after the scheduled CXR in the immediate postoperative period and postoperative day one. Findings of pulmonary pathologies by each imaging modality were independently interpreted by two different team of specialist investigators. The findings were evaluated for the degree of agreement between the two imaging modalities using Cohen's kappa statistical test.
Results:
CXR and LUS imaging showed substantial agreement in the diagnosing cardiopulmonary pathologies (κ = 0.652) in the immediate postoperative period as well as on the postoperative day one (κ = 0.740). For specific cardiopulmonary pathologies, the degree of agreement was moderate for pleural effusion (κ = 0.561), substantial for atelectasis (κ = 0.673) and interstitial edema (κ = 0.707) and perfect for pneumothorax (κ = 0.931).
Conclusions:
LUS can effectively replace CXR with reduction in radiation exposure in the immediate postoperative period and also in the follow up period. It can be used as a bedside diagnostic and monitoring tool in postoperative cardiothoracic and ICUs for diagnosing pneumothorax, pleural effusion, atelectasis and interstitial edema.
Keywords: Bedside lung ultrasound, cardiothoracic intensive care unit, diagnostic value and comparison with bedside chest roentgenogram, postoperative lung conditions
INTRODUCTION
Ultrasound of the lung was once considered not possible because ultrasonic waves did not penetrate well in organ filled with air. Air and fluid composition of the lung changes with pathological changes in the lung tissue. Many authors reported that these artifacts can be interpreted into a clinical meaningful diagnosis.[1,2] It is presently known that analyzing a combination of sonographic artifacts and real images makes it possible to diagnose interstitial, alveolar, and pleural abnormalities in the emergency and critical care setting. Protocols based on bedside lung ultrasound (LUS) (BLUE-protocol, RUSH-protocol, and FALLS-protocol) were introduced for rapid and accurate diagnosis of life-threatening pathologies in emergency care.[3,4] In most of the cardiothoracic and vascular surgical intensive care units (ICUs), bedside chest X-ray (CXR) is considered as the standard of care. Recent literature illustrate that bedside LUS is as good as computerized tomographic scan for diagnosing and monitoring pathologies in surgical and medical ICU patients.[5,6] To the best of our knowledge, there are not many studies on diagnostic efficacy of bedside LUS in postoperative cardiothoracic ICUs. Hence, we designed this study to find whether LUS can be used instead of CXR in postoperative cardiothoracic ICUs for diagnosing lung pathologies. The primary objective of the study is to compare the diagnosis obtained from bedside LUS with the diagnosis from routine bedside CXR. The secondary objective of the study is to compare the diagnostic ability of bedside LUS against CXR for various cardiopulmonary pathologies in cardiothoracic ICU patients.
MATERIALS AND METHODS
After obtaining approval from the Institutional Ethics Committee (IEC No: SCT/IEC/804 September 21, 2015) and written informed consent from the patients, this prospective observational study was conducted. A total of 250 consecutive patients, between 18 and 75 years of age, undergoing elective cardiothoracic and vascular surgery during September 2015–June 2017, were enrolled for the study. The exclusion criteria were as follows: (1) patients with open sternum, (2) patients on intra-aortic balloon pump support, (3) preexisting lung pathologies, and (4) patients undergoing emergency surgery. After transferring the enrolled study patients from the operation room to postoperative cardiothoracic ICU, bedside CXR was done. Bedside LUS was performed in all the study patients soon after the CXR according to the study protocol. LUS was done in all postoperative cardiac surgical patients in cardiac surgery ICU by the principle investigator soon after bedside CXR. CXR was interpreted by an independent team in the ICU. A provisional diagnosis was made, and intervention, if any required, was carried out after the imaging. The primary investigator who interpreted the LUS was blinded to the finding of CXR, which was interpreted by a different team. The study was repeated similarly in the postoperative day 1 morning. Bedside LUS was performed and interpreted in all the patients by the same investigator, and the CXR was analyzed by another independent investigator. Both the investigators were blinded to each other findings.
Bedside lung ultrasound study protocol
Bedside LUS was done with the patient in a semi-recumbent position. For imaging posterior thorax, the patient was rolled over to semi-lateral decubitus position. We used a linear probe with frequency range of 6–12 MHz (Esaote Mylab one, Genova, Italy) for studying superficial structures such as pleura and a convex probe with a frequency of 1–4 MHz (Philips, ClearVue 350, USA) for studying pathologies such as pericardial effusion and minimal pleural effusion at costophrenic angle. Each hemithorax was arbitrarily divided into six regions [Figure 1a and b]. Anterior region extends from parasternal line to anterior axillary line. Axillary region extends from anterior axillary line to posterior axillary line. Posterior region extends from posterior axillary line to paravertebral line. Every region was further divided equally into upper and lower by an imaginary line. Each LUS study region was examined for the following findings [Figures 2 and 3]: (1) Bat sign, (2) A-line, (3) Lung sliding, (4) Seashore sign, (5) Bar codesign, (6) Lung point, (7) Lung pulse, (8) B-line, (9) Sinusoidal sign, (10) Quad sign, (11) Distance between visceral and parietal pleura if interpleural area was found to be anechoic in horizontal cross section, (12) Tissue like echo texture of the lungs, and (13) Dynamic or static air bronchogram. In both mechanically ventilated and spontaneously breathing patients (after extubation on postoperative day 1), diaphragmatic excursion was noted by imaging the anterior subcostal region. Transthoracic echocardiography was done to look for any pericardial effusion. The following lung pathologies were diagnosed with per the international evidence-based recommendation for the point of care LUS:[7] (1) pneumothorax, (2) pleural effusion, (3) interstitial edema, (4) lung atelectasis, (5) pericardial effusion, (6) endobronchial intubation, and (7) diaphragmatic palsy.
Figure 1.
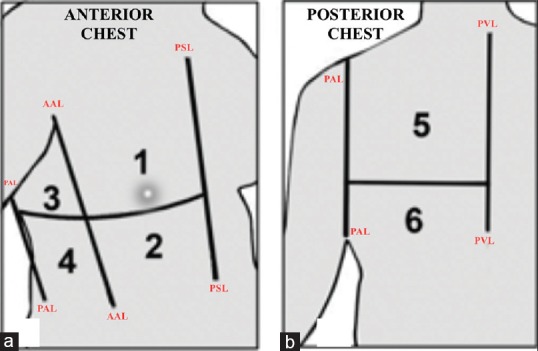
Illustrative sketch showing the division of hemithorax into six regions. (a) Anterior chest is divided into anterior region and axillary region; (b) posterior chest comprises the posterior region of hemithorax. AAL = Anterior axillary line, PAL = Posterior axillary line, PSL = Parasternal line, PVL = Paravertebral line
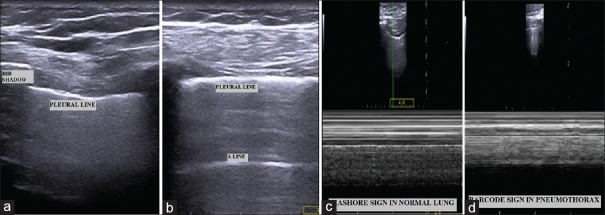
Figure 2.
Lung ultrasound findings. (a) Bat sign; (b) A-line; (c) Sea shore sign in normal lung (d) Bar code sign in pneumothorax
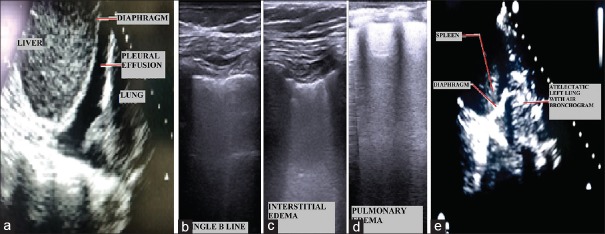
Figure 3.
Lung ultrasound findings. (a) Pleural effusion in right hemithorax; (b) Single B-line (c) B-lines ≥3 showing minimal interstitial edema; (d) More B-lines showing frank pulmonary edema; (e) Atelectatic left lower lobe showing air bronchogram
Bedside chest X-ray study protocol
An anteroposterior CXR was taken at bedside in the postoperative ICU using a portable X-ray machine (SIEMENS, Multimobil, Goa, India) with the patient in a semi-recumbent position. Technical factors for optimal imaging were adjusted according to the American College of Radiology recommendations.[8] The diagnosis of pleural effusion, pneumothorax, consolidation, atelectasis, and interstitial edema was made according to the recommendations of the Nomenclature Committee of Fleischner Society.[9] Endobronchial intubation was diagnosed in CXR if the tip of the endotracheal tube is in the bronchus. Abnormal elevation of the diaphragm in relation to opposite side diaphragm was considered to indicate diaphragm weakness. An increased cardiothoracic ratio in CXR in comparison to that of preoperative CXR was considered mediastinal widening, suggesting pericardial effusion.
Statistical analysis
The results are expressed as mean and standard deviation (SD), counts, or percentages. Based on the previous study,[10] the sample size was calculated before the patient recruitment by taking into account an expected higher degree of agreement (κ >0.80) between LUS and CXR imaging in detecting the lung abnormalities. A sample of at least 250 patients was required to achieve 85% power and Type I alpha error of 0.05. Continuous variables were expressed as mean ± SD, and qualitative data were expressed as numbers (n) and percentages (%). Agreement between CXR and LUS in detecting pathological findings was done using Cohen's Kappa statistics (44). κ value between 0.41 and 0.60 is moderate agreement, 0.61–0.80 is substantial agreement, and 0.81–1.0 is almost perfect agreement. The Statistical Package for the Social Sciences Version 21.0 (SPSS Inc., IBM, Chicago, IL, USA) was used to analyze the data.
RESULTS
A total of 250 patients were studied during the study time period. A total of 500 LUS and corresponding 500 CXR studies were done. The demographic profile and surgical details of the patient are given in Table 1. Overall, there is a substantial degree of agreement in diagnosis between the LUS and CXR imaging (κ = 0.652) [Table 2]. Furthermore, the degree of agreement was good, when the imaging diagnosis was compared between the LUS and CXR in the immediate postoperative period (κ = 0.602) and 1st postoperative day (κ = 0.740) [Table 2].
Table 1.
Demographic profile and surgical details of patients
| Parameters | Mean±SD |
|---|---|
| Age (years) | 55.45±13.81 |
| Weight (kg) | 58.65±14.92 |
| Height (cm) | 159.45±6.13 |
| Duration of surgery (min) | 295.64±48.43 |
| Sex (male:female) | 195:55 |
| Surgery | Number of patients n (%) |
| CABG | 141 (56.4) |
| Heart valve surgery | 47 (18.8) |
| Congenital heart disease surgery | 14 (5.6) |
| Combined CABG and valve surgery | 9 (3.6) |
| Vascular surgery | 27 (10.8) |
| Thoracic surgery | 12 (4.8) |
CABG=Coronary artery bypass surgery, SD=Standard deviation
Table 2.
Degree of agreement between lung ultrasound and chest X-ray imaging in diagnosing normal and abnormal lung conditions
| Total number of study | LUS (n) | Kappa coefficient | |
|---|---|---|---|
| Normal | Abnormal | ||
| Overall, degree of agreement between LUS and CXR imaging | |||
| CXR (n=500) | |||
| Normal | 404 | 33 | 0.652 |
| Abnormal | 11 | 52 | |
| Degree of agreement between LUS and CXR imaging in the immediate postoperative period | |||
| CXR (n=250) | |||
| Normal | 180 | 26 | 0.602 |
| Abnormal | 7 | 37 | |
| Degree of agreement between LUS and CXR imaging in the first postoperative period | |||
| CXR (n=250) | |||
| Normal | 224 | 7 | 0.740 |
| Abnormal | 3 | 16 | |
CXR=Chest X-ray, LUS=Lung ultrasound
The degree of agreement between LUS and CXR imaging in diagnosing various cardiothoracic pathological conditions was moderate for pleural effusion (κ = 0.561), substantial for atelectasis (κ = 0.673), and interstitial edema (κ = 0.707) and perfect for pneumothorax (κ = 0.931) [Table 3]. None of the patients in the study was diagnosed to have endobronchial intubation, pericardial effusion, and diaphragmatic palsy by either LUS or CXR imaging.
Table 3.
Degree of agreement between lung ultrasound and chest X-ray imaging in diagnosing various pathological lung conditions
| Degree of agreement between LUS and CXR imaging in diagnosing pleural effusion | |||
|---|---|---|---|
| Total number of study (n=500) | Pleural effusion diagnosed by LUS (n) | Kappa coefficient | |
| Present | Absent | ||
| Pleural effusion diagnosed by CXR (n) | |||
| Present | 8 | 5 | 0.561 |
| Absent | 7 | 480 | |
| Degree of agreement between LUS and CXR imaging in diagnosing atelectasis | |||
| Total number of study (n=500) | Atelectasis diagnosed by CXR (n) | Kappa coefficient | |
| Present | Absent | ||
| Atelectasis diagnosed by CXR (n) | |||
| Present | 5 | 3 | 0.673 |
| Absent | 3 | 489 | |
| Degree of agreement between LUS and CXR imaging in diagnosing interstitial edema | |||
| Total number of study (n=500) | Interstitial edema diagnosed by CXR (n) | Kappa coefficient | |
| Present | Absent | ||
| Interstitial edema diagnosed by CXR (n) | |||
| Present | 39 | 0 | 0.707 |
| Absent | 67 | 433 | |
| Degree of agreement between LUS and CXR imaging in diagnosing pneumothorax | |||
| Total number of study (n=500) | Pneumothorax diagnosed by CXR (n) | Kappa coefficient | |
| Present | Absent | ||
| Pneumothorax diagnosed by CXR (n) | |||
| Present | 7 | 0 | 0.931 |
| Absent | 1 | 492 | |
CXR=Chest X-ray, LUS=Lung ultrasound
DISCUSSION
LUS is not a routine point of care diagnostic modality in cardiothoracic ICUs, though many literatures are available supporting its role in multiple diagnostic and therapeutic procedures.[11,12] Postoperative cardiothoracic patients with inotropic supports and chest drains were difficult to shift outside the ICUs for imaging the thorax. LUS is radiation-free, and portable ultrasound machines are now available in most of the ICUs. Although the learning curve for LUS imaging is steep, it can be performed quickly and safely at the bedside in the ICUs.[13]
In our study, among 500 CXRs and LUS studies of 250 patients, 12.6% of CXRs showed abnormalities; whereas in LUS studies, 17% were abnormal. Among 85 LUS studies with pathological diagnosis, 61.2% had the same diagnosis as the corresponding CXRs. Among 415 normal LUS studies, 97.3% had normal CXR findings showing good agreement between CXR and LUS imaging. The same degree of agreement was observed between CXR and LUS imaging at different time periods (immediate postoperative period and 1st postoperative day) Therefore, LUS can be used as an effective alternative for CXRs, and thereby reducing radiation exposure to the patients.[10]
For diagnosing pleural effusion, LUS showed a fair agreement with CXR. Minimal pleural effusions which needed no intervention were diagnosed by LUS in seven studies. However, this was not diagnosed by CXR, because in a supine postoperative bedside CXR imaging, only pleural effusion of ≥ 200 ml can be detected.[14] Furthermore, bedside CXR has poor accuracy for differentiating mild pleural effusion from parenchymal disease such as atelectasis.[15] In the immediate postoperative period, both LUS and CXR imaging showed basal atelectasis in four patients and whole lung atelectasis in one patient. Lung recruitment maneuvers in the above five patients expanded the atelectatic segments and showed normal study in both LUS and CXR imaging on the postoperative day 1. Hence, LUS is as effective as CXR for the recruitment of the lung tissue in patients with atelectasis.[16]
In the immediate postoperative LUS examination, one patient revealed mild pneumothorax, whereas the corresponding CXR study showed no pneumothorax. In the same patient, LUS study on the postoperative day 1 showed a massive pneumothorax with the parallel CXR revealing the same. We found that mild pneumothorax could be detected by LUS earlier than the CXR, whereas the CXR was useful only if the pneumothorax was more than mild severity.[17]
Since the endotracheal tube position was accurate for all the patients, no endobronchial intubation was detected by either LUS or CXR imaging. Endobronchial intubation can be ruled out by LUS, but the exact position of endotracheal tube tip in relation to vertebrae can be detected by only CXR. Bedside CXR shows a static image of diaphragm either in expiration or inspiration, whereas LUS can be used to see the range movement of diaphragm during inspiration and expiration over two or more respiratory cycles.[18] In our study, neither CXR nor LUS revealed the diaphragmatic weakness in any patient. Thus, the agreement between LUS and CXR for detecting normal diaphragmatic function was good (κ =1.0).[19] Since all the cardiac surgical patients had a mediastinal drain in the immediate and 1st postoperative day, neither CXR nor LUS revealed pericardial effusion in any patient.
Although our study favors the bedside LUS as a substitute for CXR in postoperative cardiothoracic ICUs, it has certain limitations. Hilum and deeper lung tissue could not be easily studied by LUS. There existed a restricted field of study in postoperative patients due to dressings and drains. Subcutaneous emphysema, when present, it does not allow the underlying pleura and lung parenchyma for proper evaluation.
The overall outcome of our study on patients in postoperative cardiothoracic ICUs adds strength and evidence to the current literature by Peris et al.[5] and Xirouchaki et al.[6] which showed that bedside LUS significantly reduced the number of chest radiographs and computed tomography scans, and it can be used as a surrogate for bedside imaging in critically ill patients.
In future, randomized controlled trails should be carried out by comparing the LUS as a sole imaging modality for diagnosis, decision-making, and intervention with the gold standard imaging techniques, so that bedside LUS can permanently replace the radiation risks of patients in the ICUs.
CONCLUSION
The diagnostic ability of bedside LUS and CXR in the postoperative cardiothoracic ICU patients is correlating well. Hence, LUS can supplement or even replace the bedside CXR as a point of care diagnostic modality in the postoperative cardiothoracic ICUs with the added advantage of assessing the response to therapy immediately without any radiation exposure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Volpicelli G. Lung sonography. J Ultrasound Med. 2013;32:165–71. doi: 10.7863/jum.2013.32.1.165. [DOI] [PubMed] [Google Scholar]
- 2.Kiley S, Cassara C, Fahy BG. Lung ultrasound in the intensive care unit. J Cardiothorac Vasc Anesth. 2015;29:196–203. doi: 10.1053/j.jvca.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein DA. BLUE-protocol and FALLS-protocol: Two applications of lung ultrasound in the critically ill. Chest. 2015;147:1659–70. doi: 10.1378/chest.14-1313. [DOI] [PubMed] [Google Scholar]
- 4.Seif D, Perera P, Mailhot T, Riley D, Mandavia D. Bedside ultrasound in resuscitation and the rapid ultrasound in shock protocol. Crit Care Res Pract. 2012;2012:503254. doi: 10.1155/2012/503254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peris A, Tutino L, Zagli G, Batacchi S, Cianchi G, Spina R, et al. The use of point-of-care bedside lung ultrasound significantly reduces the number of radiographs and computed tomography scans in critically ill patients. Anesth Analg. 2010;111:687–92. doi: 10.1213/ANE.0b013e3181e7cc42. [DOI] [PubMed] [Google Scholar]
- 6.Xirouchaki N, Magkanas E, Vaporidi K, Kondili E, Plataki M, Patrianakos A, et al. Lung ultrasound in critically ill patients: Comparison with bedside chest radiography. Intensive Care Med. 2011;37:1488–93. doi: 10.1007/s00134-011-2317-y. [DOI] [PubMed] [Google Scholar]
- 7.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–91. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 8.Amorosa JK, Bramwit MP, Mohammed TL, Reddy GP, Brown K, Dyer DS, et al. ACR appropriateness criteria routine chest radiographs in intensive care unit patients. J Am Coll Radiol. 2013;10:170–4. doi: 10.1016/j.jacr.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Tuddenham WJ. Glossary of terms for thoracic radiology: Recommendations of the nomenclature committee of the fleischner society. AJR Am J Roentgenol. 1984;143:509–17. doi: 10.2214/ajr.143.3.509. [DOI] [PubMed] [Google Scholar]
- 10.Vezzani A, Manca T, Brusasco C, Santori G, Valentino M, Nicolini F, et al. Diagnostic value of chest ultrasound after cardiac surgery: A comparison with chest X-ray and auscultation. J Cardiothorac Vasc Anesth. 2014;28:1527–32. doi: 10.1053/j.jvca.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4:1. doi: 10.1186/2110-5820-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolaou S, Talsky A, Khashoggi K, Venu V. Ultrasound-guided interventional radiology in critical care. Crit Care Med. 2007;35:S186–97. doi: 10.1097/01.CCM.0000260630.68855.DF. [DOI] [PubMed] [Google Scholar]
- 13.Miller A. Practical approach to lung ultrasound. BJA Educ. 2016;16:39–45. [Google Scholar]
- 14.Woodring JH. Recognition of pleural effusion on supine radiographs: How much fluid is required? AJR Am J Roentgenol. 1984;142:59–64. doi: 10.2214/ajr.142.1.59. [DOI] [PubMed] [Google Scholar]
- 15.Kitazono MT, Lau CT, Parada AN, Renjen P, Miller WT., Jr Differentiation of pleural effusions from parenchymal opacities: Accuracy of bedside chest radiography. AJR Am J Roentgenol. 2010;194:407–12. doi: 10.2214/AJR.09.2950. [DOI] [PubMed] [Google Scholar]
- 16.Tusman G, Acosta CM, Costantini M. Ultrasonography for the assessment of lung recruitment maneuvers. Crit Ultrasound J. 2016;8:8. doi: 10.1186/s13089-016-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sartori S, Tombesi P, Trevisani L, Nielsen I, Tassinari D, Abbasciano V, et al. Accuracy of transthoracic sonography in detection of pneumothorax after sonographically guided lung biopsy: Prospective comparison with chest radiography. AJR Am J Roentgenol. 2007;188:37–41. doi: 10.2214/AJR.05.1716. [DOI] [PubMed] [Google Scholar]
- 18.Sim SS, Lien WC, Chou HC, Chong KM, Liu SH, Wang CH, et al. Ultrasonographic lung sliding sign in confirming proper endotracheal intubation during emergency intubation. Resuscitation. 2012;83:307–12. doi: 10.1016/j.resuscitation.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Houston JG, Fleet M, Cowan MD, McMillan NC. Comparison of ultrasound with fluoroscopy in the assessment of suspected hemidiaphragmatic movement abnormality. Clin Radiol. 1995;50:95–8. doi: 10.1016/s0009-9260(05)82987-3. [DOI] [PubMed] [Google Scholar]