Abstract
Background:
The effects of ketofol (a combination of ketamine and propofol) on systemic hemodynamics and requirement of opioids/Propofol have already been studied and published. However, there is paucity in the literature on the effects of ketofol on cerebral oxygenation. This study aims to compare the effects of ketofol (ketamine + propofol [1:5]) with propofol on cerebral oxygenation using jugular venous oxygen saturation (SjVO2), in patients undergoing surgical clipping of intracranial aneurysms.
Materials and Methods:
A total of 40 World Federation of Neurosurgeons I and II patients were randomized into ketofol (n = 20) and propofol (n = 20) groups. Postinduction, SjVO2 catheter was inserted, and anesthesia was maintained with propofol and fentanyl in the propofol group and ketofol and fentanyl in the ketofol group. Jugular venous oxygen saturation (SjVO2) was obtained at baseline, 1 h and 2 h intraoperatively, and at 6 h after the surgery. Intraoperative hemodynamics and brain relaxation scores were also noted.
Results:
Entire SjVO2 values in both groups were within the normal limits. Higher SjVO2 values were observed in ketofol group compared to propofol at 1 and 2 h after starting of the drug and at 6 h after surgery (P < 0.05). In propofol group, a significant fall in SjVO2 was recorded at 2 h after beginning the drug as compared to the baseline (P = 0.001). More than 20% fall in mean arterial pressure (MAP) compared to baseline MAP was noted in 75% of patients in propofol group and 15% of patients in ketofol group (P = 0.002). In propofol group, 55% of patients required rescue drug phenylephrine to treat hypotension, whereas only 15% of patients required it in ketofol group (P = 0.02). Fentanyl requirement in ketofol group was less as compared to the propofol group (P = 0.022). Brain relaxation scores were comparable in both the study groups (P = 0.887).
Conclusion:
Maintenance of anesthesia with ketofol provides better cerebral oxygenation and hemodynamic stability compared to propofol in neurosurgical patients.
Keywords: Brain relaxation, cerebral oxygenation, hemodynamics, ketofol
INTRODUCTION
In neurosurgical patients, goals of anesthesia include the maintenance of adequate cerebral oxygenation, cerebral perfusion pressure (CPP), stable hemodynamics, and relaxed brain to facilitate surgical procedures.[1] Among several inhalational and intravenous agents, propofol is presently being preferred over other drugs for the maintenance of anesthesia in neurosurgical patients. Although it produces dose-dependent decrease in both cerebral blood flow and cerebral metabolic rate of oxygen, it causes hypotension, especially in hypovolemic patients, thus affecting perfusion and oxygenation of the brain.[2] On the other hand, despite several advantages have been attributed to ketamine, it is not frequently used as an anesthetic agent of choice because of its sympathomimetic effects.[3]
Since propofol causes dose-dependent hypotension and ketamine prevents hypotension by its sympathomimetic effect, it was postulated that combination of both the drugs will result in a mixture (termed as ketofol) which will have additive effects, so that the dose of individual drug can be decreased and benefit of both the drugs such as amnesia, analgesia, hypnosis, and hemodynamic stability can be achieved.[4]
Cerebral oxygenation can be indirectly assessed by jugular venous oxygen saturation (SjVO2), which reflects the global cerebral balance between oxygen demand and supply.[5] The SjVO2 monitoring during the perioperative period helps in guiding oxygenation, hyperventilation therapy, and optimizing perfusion pressure. It provides an early diagnosis of cerebral ischemia resulting from either intracranial or systemic causes.[6,7]
Although numerous studies have been published on the effects of ketofol on hemodynamics and opioid consumption intraoperatively, there is a lack of evidence on its effects on cerebral oxygenation. Hence, in the present study, the primary aim was to compare the effects of ketofol with propofol alone on cerebral oxygenation using SjVO2, in patients undergoing surgical clipping of intracranial aneurysm. We chose patients of aneurysmal clipping with the objective that the maintenance of CPP and oxygenation is the pivotal aim during the intraoperative period. We hypothesize that the ketofol may achieve the aim of maintaining adequate CPP and oxygenation though a better balance between MAP and intracranial pressure (ICP) as compared to propofol alone.
MATERIALS AND METHODS
After approval from the Institutional Ethical Committee and written informed consent from the patient or next of kin, this prospective randomized study was conducted in patients undergoing aneurysmal clipping following spontaneous rupture of intracranial aneurysm. American Society of Anesthesiologists IE/IIE, between the age group of 18 and 65 years, Hunt and Hess (H and H) Grade I–II, World Federation of Neurosurgeons (WFNS) Grade I–II patients were included in the study. Patients with a diameter of aneurysm >25 mm, known coronary artery disease, psychiatric disease, and poor-grade aneurysm (H and H IV–V, WFNS IV–V) were excluded from the study.
Forty patients were randomized into ketofol (KP) (n = 20) and propofol (P) (n = 20) groups using computer-generated random tables, and concealment was done using opaque sealed envelope method. Doubleblindedness was ensured as neither the investigator nor the patient knew of the drug being administered. The sample size of total 40 patients was calculated with an α error of 0.05, and the power of the study was 80% considering SjVO2 values <50% as significant cerebral hypoperfusion based on the previous study.[8] The patients in propofol group received propofol as maintenance agent, and the patients in ketofol group received combination of ketamine and propofol in a ratio of 1:5 for the maintenance of anesthesia.
Initially, few pilot cases were done using incremental concentrations of ketamine from 1:6–1:3 with propofol. Ketamine-propofol combination in a ratio of 1:5 was chosen to be used, as it provided better hemodynamic stability as well as reduced airway secretions when compared with other concentrations used.
The study drug was prepared in a 50 mL syringe, 48 ml of propofol (10 mg.mL-1) was loaded in the propofol (P) group, and for ketofol group (KP), 40 mL of propofol and 8 ml of ketamine (10 mg.mL-1) were added by an anesthesiologist who was not directly involved in the study to ensure double-blindedness. Thus, 1 ml of ketofol contained propofol 8.3 mg and ketamine 1.7 mg. These study drug infusions were used only for the maintenance of anesthesia after the measurement of baseline SjVO2.
A standard anesthesia protocol was followed for the induction of anesthesia. Patients in both the groups were induced with thiopentone (4–6 mg.kg-1) with the dose titrated to loss of eyelash response and intubated after the administration of vecuronium (0.1 mg.kg-1). Fentanyl was administered in a dose of 2 μg.kg-1 followed by 1 μg.kg-1 as infusion for intraoperative analgesia. Lignocaine 1.5 mg.kg-1 was administered before laryngoscopy to attenuate the hemodynamic stress response. PaCO2 was kept between 32 and 35 mmHg; ventilation was maintained with 50% oxygen and air in all the patients.
For beat-to-beat monitoring of blood pressure and blood gas analysis, an arterial catheter was placed in the radial artery before the induction of anesthesia. Patients were monitored for heart rate (H), mean arterial pressure (MAP), pulse oximetry (SpO2), end-tidal carbon dioxide, neuromuscular monitoring, temperature, pulse pressure variation, and systolic pressure variation along with urine output. The patients were maintained with sevoflurane till the insertion of SjVO2 catheter.
Jugular bulb oximetry was done using a single-lumen 5 Fr, 20 cm long central venous catheter under ultrasound guidance was inserted on the side of aneurysm using the standard puncture as for internal jugular venous cannulation but in the retrograde direction. The catheter was inserted by Seldinger's technique. Confirmation of correct placement was done using C-arm by the skull and neck radiography. The tip should lie at the C1-C2 level or at the level of mastoid, just medial to it for appropriate sampling. The blood samples were withdrawn slowly at a rate of not ≥2 ml/min to avoid extracerebral contamination. Baseline samples were taken immediately after the insertion of catheter. After the baseline sampling, sevoflurane was turned off, and anesthesia was maintained with study drug infusion at 0.3 mL.kg-1.h-1. Further, sampling of jugular bulb blood was done every hourly after the starting of the test drug till the end of the surgery, followed by 6 h after the stopping of drug.
The study drug infusion was titrated to maintain hemodynamic stability throughout the period of surgery. Hemodynamic parameters such as H and MAP were recorded at different time periods such as preinduction, baseline value (before the start of study drug), after pin insertion, during making burr hole, at starting of craniotomy, at bone flap removal, at opening of dura mater, during temporary clipping, during permanent clipping of aneurysm, at dural closure, at muscle suturing, at skin closure, at extubation, and 6 h after completion of surgery.
Whenever MAP fell to <20% of the baseline value, an intravenous fluid bolus of 200 ml was infused, and the study drug infusion was decreased by 10%. If hypotension still continued, boluses of phenylephrine 50 μg were administered at an interval of 5 min. If hypotension persisted even after three consecutive phenylephrine boluses, then nor adrenaline infusion was started at the rate of 0.1 μg.kg-1.min-1. If MAP increased >20% of the baseline value, the study drug infusion was increased by 10%. If hypertension continued, then boluses of esmolol 0.5–1 mg.kg-1 IV over 30 s were given and repeated at 5-min interval. The number of boluses of rescue drugs and total dose of rescue drugs used were noted.
Brain relaxation was assessed on a grading system after craniotomy before the opening of dura mater by the blinded operating neurosurgeon who had at least 5 years of experience. Brain condition was assessed by a four grade scales: Grade 1 – brain surface lies below the surface of craniotomy margin and well retracted into the cranial cavity with good brain pulsations, Grade 2 – brain surface lies just below the surface of craniotomy region margin and brain pulsations are well seen, Grade 3 – brain surface lies at the level of craniotomy margins and brain pulsations are observed faintly, and Grade 4 – the brain surface is jutting out or expanding beyond the craniotomy margin and brain pulsation are not clearly defined.[9]
Fentanyl infusion was stopped at the beginning of scalp closure, whereas anesthetic agents were stopped after the removal of headpins. Patients were shifted to the neurosurgical intensive care unit (ICU) for further neurocritical care. Total duration of anesthesia (from induction to stoppage of drug infusions) and total duration of surgery (from incision to last skin suture) were also noted.
Statistical analysis
Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA, version 21.0). The continuous data were presented as a mean ± standard deviation for normally distributed data; two groups were compared using the Student's t-test. Categorical and nominal data were described as proportions, and Chi-square test or Fisher's exact test was used to look at significant associations. Paired sample t-test was also used for baseline comparison. P < 0.05 was considered statistically significant.
RESULTS
The patient characteristics were comparable in both the groups [Table 1]. All the values of SjVO2 in both the groups were within the normal limits (50%–70%). Higher values of SjVO2 were observed in ketofol group compared to propofol group at 1 and 2 h after starting of the study drug, and at 6 h after surgery which was statistically significant (P < 0.05). In the propofol group, a statistically significant fall in SjVO2 was recorded at 2 h after the starting of the study drug as compared to the baseline (P = 0.001), whereas in ketofol group, all the recordings were compared with baseline (P > 0.05) [Table 2].
Table 1.
Patient demographics
| Parameters | KP (n=20) | P (n=20) | P |
|---|---|---|---|
| Age (year) | 48.05±8.287 | 49.25±12.523 | 0.723β |
| Sex (male/female) | 10/10 | 12/8 | 0.404π |
| Weight | 68.15±4.715 | 70.25±6.356 | 0.076β |
| ASA Grade IE/IIE | 6/14 | 7/13 | |
| Site of ruptured aneurysm ACOM/ | 6/5/1/3/2/3 | 6/5/2/2/3/2 | |
| MCA/ACA/PCOM/ICA/DACA | |||
| Hunt and Hess (1/2) | 17/3 | 16/4 | 0.453π |
| WFNS (1/2) | 5/15 | 5/15 | 1.000π |
| Baseline hemoglobin | 11.04±2.10 | 11.67±2.24 | 0.364 |
πVariables analyzed by Fisher’s exact test, βVariables analyzed by independent t-test, P<0.05 was considered as statistically significant. ASA=American Society of Anesthesiologist, ACOM=Anterior communicating artery, MCA=Middle cerebral artery, ACA=Anterior cerebral artery, PCOM=Posterior communicating artery, ICA=Internal carotid artery, DACA=Distal anterior cerebral artery, WFNS=World Federation of Neurosurgeon, KP=Ketofol, P=Propofol
Table 2.
Jugular venous oximetry parameters
| SjVO2 | |||
|---|---|---|---|
| KP (n=20) | P (n=20) | P | |
| Baseline | 65.13±2.40 | 63.80±3.44 | 0.164 |
| At 1 h | 65.56±2.98 | 62.58±3.35 | 0.005* |
| At 2 h | 63.61±3.50 | 60.41±3.48 | 0.006* |
| At 6 h postsurgery | 64.78±1.65 | 63.05±3.16 | 0.036* |
*P value < 0.05 is considered significant. SjVO2=Jugular venous oximetry, KP=Ketofol, P=Propofol
Brain relaxation scores were comparable in both the study groups (P = 0.887). Brain relaxation in ketofol group was as good as that of propofol group [Table 3]. Among intraoperative characteristics, ketamine decreased the requirement of fentanyl in ketofol group as compared to the propofol group, and the difference was statistically significant (P = 0.022) [Table 3]. The addition of ketamine to propofol decreased the total requirement of propofol in ketofol group when compared to the propofol group, and the difference was statistically significant (P = 0.020) [Table 3]. In propofol group, 15 patients (75%) had ≥20% fall in MAP compared to five patients (25%) in ketofol group (P = 0.002). In propofol group, 11 patients (55%) required rescue drug phenylephrine to treat hypotension, whereas only three patients (15%) required it in ketofol group (P = 0.02) [Table 3]. Other intraoperative characteristics such as total intravenous fluids given, total urine output, anesthesia duration, and surgery duration were comparable in both groups [Table 3].
Table 3.
Intaoperative characterstics
| Parameters | KP (n=20) | P (n=20) | P |
|---|---|---|---|
| Total fentanyl used (μg)/per patient | 271.9±29.8 | 307.15±35.6 | 0.022 |
| Total propofol used (mg)/per patient | 725.95±31.1 | 971±34.8 | 0.020 |
| Brain relaxation grade, 1/2/3/4 | 2/13/5/0 | 3/12/5/0 | 0.887π |
| MAP >20% fall (number of patients), n (%) | 5 (15) | 15 (75) | 0.002* |
| Phenylephrine required (number of patients), n (%) | 3 (15) | 11 (55) | 0.020* |
| MAP >20% rise (number of patients), n (%) | 5 (25) | 3 (15) | 0.693 |
| Esmolol required (number of patients), n (%) | 4 (20) | 3 (15) | 1.000 |
| Crystalloids (ml) | 2282.5±246.128 | 2197.5±271.679 | 0.306β |
| Urine output (ml) | 865±202.029 | 854±123.391 | 0.836β |
| Anesthesia time (min) | 186.75±20.15 | 185.75±19.49 | 0.874β |
| Surgery time (min) | 157.25±19.09 | 155.75±19.89 | 0.809β |
πVariables analyzed by Fisher’s exact test, *P value < 0.05 is considered significant, βVariables analyzed by independent t-test, P<0.05 was considered as statistically significant. KP=Ketofol, P=Propofol, MAP=Mean arterial pressure
Throughout the drug infusion period, MAP and H were better maintained, and hemodynamic fluctuations were less in patients receiving ketofol compared to those receiving propofol. Patients receiving propofol had a statistically significant lower recordings of H and MAP compared to those receiving ketofol (P < 0.05) [Table 4].
Table 4.
Hemodynamic parameters
| MAP | Hemodynamic | |||||
|---|---|---|---|---|---|---|
| KP (n=20) | P (n=20) | P | KP (n=20) | P (n=20) | P | |
| Preinduction | 103.25±5.17 | 100.60±5.42 | 0.122 | 80.95±4.55 | 81.10±3.04 | 0.903 |
| Baseline (prior to start of drug) | 95.75±8.40 | 97.00±8.65 | 0.646 | 82.00±7.13 | 82.30±6.59 | 0.891 |
| Head pin application | 102.85±6.18 | 102.55±6.67 | 0.883 | 89.75±5.93 | 89.05±6.43 | 0.722 |
| Burr hole | 98.20±7.08 | 67.90±4.17 | 0.0001* | 86.95±5.43 | 71.75±6.70 | 0.0001* |
| Starting craniotomy | 97.15±6.32 | 69.00±4.63 | 0.0001* | 89.40±6.18 | 69.40±4.41 | 0.0001* |
| Bone flap removal | 98.90±6.11 | 69.95±4.95 | 0.0001* | 88.15±3.53 | 69.80±5.63 | 0.0001* |
| Dural opening | 94.70±4.97 | 70.70±5.29 | 0.0001* | 83.80±7.37 | 69.00±6.29 | 0.0001* |
| Temporary clipping | 96.90±3.91 | 73.85±7.65 | 0.0001* | 74.75±7.54 | 77.20±8.43 | 0.339 |
| Permanent clipping | 90.85±6.30 | 71.10±4.52 | 0.0001* | 85.25±5.48 | 73.25±8.50 | 0.0001* |
| Dural closure | 99.65±7.08 | 76.20±7.89 | 0.0001* | 86.35±4.55 | 74.40±8.05 | 0.0001* |
| Muscle suture | 101.6±6.40 | 77.05±6.44 | 0.0001* | 85.30±4.69 | 71.90±7.31 | 0.0001* |
| Skin suture | 103.45±6.31 | 101.25±6.81 | 0.0001* | 83.70±6.32 | 83.20±8.22 | 0.830 |
| 6 h postoperation | 107.00±10.84 | 99.71±12.20 | 0.0001* | 80.65±15.19 | 80.25±10.76 | 0.924 |
*P value < 0.05 is considered significant. KP=Ketofol, P=Propofol, MAP=Mean arterial pressure
DISCUSSION
Both intravenous, as well as inhalational agents, have been used for the maintenance of anesthesia during neurosurgery.[10,11,12] Ketamine is less prevalent in neurosurgery because of its presumed adverse effects on cerebral hemodynamics.[3,13] The effects of each individual drug propofol and ketamine on cerebral oxygenation have been demonstrated in many studies; however, there is paucity in literature about the effects of ketofol on cerebral oxygenation. The present study is the first prospective randomized clinical trial which compares the effects of ketofol with propofol on cerebral oxygenation.
Jugular venous oxygen saturation
We observed a statistically significant lower SjVO2 recording in patients who received propofol as compared to those who received ketofol at all-time points. It was statistically significant at 1-h, 2-h, and 6-h time points. Within the propofol group, the dip in SjVO2 at 2 h after starting the study drug infusion was significantly lower as compared to baseline (P = 0.001). We observed the lesser fall and lesser fluctuation in SjVO2 values in patients who received ketofol.
Similar to our study, many studies have shown that maintenance of anesthesia with propofol is associated with significantly lower levels of SjVO2 compared to inhalational anesthetic agents.[8,9,14] Various studies had observed that there was no significant change in SjVO2 when different doses of ketamine was administered to patients who were on propofol sedation.[8] Hence, results of our study with respect to effect of ketofol on SjVO2 is comparable with previous studies.
Propofol per se decreases the SjVO2, and temporary clipping of parent aneurysmal vessel in our study could have aggravated this fall in SjVO2 in propofol group, thus explaining a significant fall in SjVO2 within the propofol group at 2 h after the start of test drug which usually coincides with temporary clipping time. The above observation of SjVO2 can be explained by the pharmacological action of ketamine. Sympathomimetic action of ketamine prevents the hypotension and bradycardia induced by propofol. Thus, a combination of ketamine and propofol maintains cerebral oxygenation.
Although there is a statistical difference in SjVO2 in between the groups, all the recordings of SjVO2 were within a normal range (50%–70%) in both the groups. The lowering of SjVO2 observed in propofol group may further risk the patients for cerebral ischemia, especially in patients vulnerable to insults such as prolonged temporary clipping, raised ICP, hypovolemia, and hemodynamic instability.
Bhardwaj et al.[9] compared the effect of propofol and desflurane in SAH patients who underwent aneurysm clipping. Similar to our result, they observed lower values of SjVO2 in propofol group. However, the recordings were within a normal range (50%–70%) in either of the groups. Iwata et al.[14] studied the effects of increasing concentration of propofol on jugular venous bulb oxygen saturation in neurosurgical patients under normothermic and mild hypothermic conditions. SjVO2 was measured at predicted propofol concentrations of 3, 5, and 7 μg/ml using a target-controlled infusion system in both groups. Although the authors concluded that under normothermic and hypothermic conditions, the increasing concentrations of propofol did not affect SjVO2 values in neurosurgical patients, there was a significant fall in SjVO2 (<50%) at 3 μg/ml of propofol in normothermic patients (31% vs. 13%). Thus, under normothermic condition, the results are similar to our study.
Albanèse et al.[8] analyzed the effect of intermittent doses of ketamine in traumatic brain injury patients who were receiving propofol sedation in ICU. They observed significant decrease in ICP and without any change in SjVO2 and CPP. Similarly, a study by Caricato et al.[15] reported that no significant variation of SjVO2, MAP, CPP, and middle cerebral artery flow velocity were observed during endotracheal tube suctioning in traumatic brain injury patients sedated with propofol and remifentanil infusion, when ketamine infusion was added to prevent cough reflex during endotracheal suctioning. The study findings of ketamine are comparable with our study drug ketofol.
Brain relaxation
We found the quality of brain relaxation was similar in both the study groups (P = 0.887). Maximum number of patients had good brain condition in both the groups (grade II) [Figure 1]. Many studies have shown that ICP reduction was more in intravenous anesthetics compared to inhalational anesthetics,[16,17,18] but various anesthetic techniques have not shown any difference in brain relaxation.[19] To the best of our knowledge, there is no study which has evaluated the effect of ketofol on brain relaxation.
Figure 1.
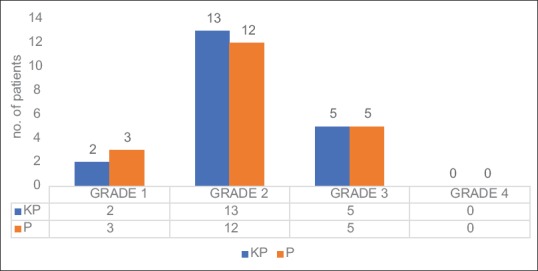
Brain relaxation grade
Hemodynamic stability
Heart rate (HR) and MAP recordings during test drug infusion were lower in propofol group as compared to ketofol group. Statistically significant lower MAP values were recorded at most of the time points in patients receiving propofol [Figure 2].
Figure 2.
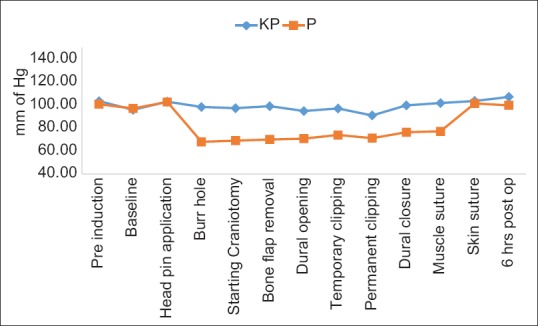
Mean arterial pressure
In our study, fall in HR and MAP levels were more, and the hemodynamic fluctuations were higher in the propofol group as compared to ketofol. This observation can be explained by the sympathomimetic action of ketamine which alleviates the hypotension and bradycardia induced by propofol. A study by Aouad et al.[20] conducted on pediatric patients undergoing procedural anesthesia, tried to evaluate the cumulative dose requirement of propofol/fentanyl as compared to ketofol/fentanyl. Similar to our study, they also concluded that better hemodynamic stability is achieved, and there is reduced consumption of propofol and fentanyl in patients receiving ketofol. This is due to analgesic property of ketamine which decreases the need for fentanyl/propofol in ketofol group.
A study from Smischney et al.[21] witnessed a greater proportion of patients induced with propofol (48.8%) had fallen in MAP ≥20% from baseline value as compared to ketofol (12%). The author observed that improved hemodynamics during the first 10-min after induction was attained with ketofol as an induction agent. This effect of ketofol on hemodynamics during the first 10 min after induction is comparable to the present study observation during intraoperative period. Similar to our study, Sharma et al.[22] observed better hemodynamic homeostasis along with optimum sedoanalgesia during TIVA with ketofol vis-à-vis propofol/fentanyl combination in minor orthopedic procedures.
Our study proposes the use of ketofol for the maintenance of anesthesia. This may alleviate the necessity for rescue vasopressors during the procedure and helps to maintain a better hemodynamic stability, thereby maintaining adequate cerebral perfusion and preventing cerebral ischemia. We have few limitations in this study. First, the monitoring of SjVO2 was not performed continuously. Hence, real-time correlation of SjVO2 with cerebral oxygenation could not be recorded. Second, the study cohort included only good grade aneurysms. Hence, the outcome of the study cannot be generalized to all grades of patients of SAH. Finally, the depth of anesthesia was titrated according to hemodynamics only and not by using any specialized monitoring like bispectral index, or entropy as ketamine is known to interfere with such monitoring and give inappropriate results.
CONCLUSION
This study concludes that maintenance of anesthesia in patients undergoing clipping of aneurysm after SAH with ketofol provides better cerebral oxygenation and hemodynamic stability compared to propofol. There is no difference in quality of brain relaxation while using either ketofol or propofol. Therefore, ketofol is preferred over propofol as maintenance anesthetic agent in neurosurgical patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Juul N, Morris GF, Marshall SB, Marshall LF. Intracranial hypertension and cerebral perfusion pressure: Influence on neurological deterioration and outcome in severe head injury. The executive committee of the international selfotel trial. J Neurosurg. 2000;92:1–6. doi: 10.3171/jns.2000.92.1.0001. [DOI] [PubMed] [Google Scholar]
- 2.Hans P, Bonhomme V. Why we still use intravenous drugs as the basic regimen for neurosurgical anaesthesia. Curr Opin Anaesthesiol. 2006;19:498–503. doi: 10.1097/01.aco.0000245274.69292.ad. [DOI] [PubMed] [Google Scholar]
- 3.Akin A, Guler G, Esmaoglu A, Bedirli N, Boyaci A. A comparison of fentanyl-propofol with a ketamine-propofol combination for sedation during endometrial biopsy. J Clin Anesth. 2005;17:187–90. doi: 10.1016/j.jclinane.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Andolfatto G, Abu-Laban RB, Zed PJ, Staniforth SM, Stackhouse S, Moadebi S, et al. Ketamine-propofol combination (ketofol) versus propofol alone for emergency department procedural sedation and analgesia: A randomized double-blind trial. Ann Emerg Med. 2012;59:504–120. doi: 10.1016/j.annemergmed.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 5.De Deyne C, Van Aken J, Decruyenaere J, Struys M, Colardyn F. Jugular bulb oximetry: Review on a cerebral monitoring technique. Acta Anaesthesiol Belg. 1998;49:21–31. [PubMed] [Google Scholar]
- 6.Thiagarajan A, Goverdhan PD, Chari P, Somasunderam K. The effect of hyperventilation and hyperoxia on cerebral venous oxygen saturation in patients with traumatic brain injury. Anesth Analg. 1998;87:850–3. doi: 10.1097/00000539-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Schaffranietz L, Heinke W. The effect of different ventilation regimes on jugular venous oxygen saturation in elective neurosurgical patients. Neurol Res. 1998;20(Suppl 1):S66–70. doi: 10.1080/01616412.1998.11740613. [DOI] [PubMed] [Google Scholar]
- 8.Albanèse J, Arnaud S, Rey M, Thomachot L, Alliez B, Martin C. Ketamine decreases intracranial pressure and electroencephalographic activity in traumatic brain injury patients during propofol sedation. Anesthesiology. 1997;87:1328–34. doi: 10.1097/00000542-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Bhardwaj A, Bhagat H, Grover VK, Panda NB, Jangra K, Sahu S, et al. Comparison of propofol and desflurane for postanaesthetic morbidity in patients undergoing surgery for aneurysmal SAH: A randomized clinical trial. J Anesth. 2018;32:250–8. doi: 10.1007/s00540-018-2474-z. [DOI] [PubMed] [Google Scholar]
- 10.Todd MM, Warner DS, Sokoll MD, Maktabi MA, Hindman BJ, Scamman FL, et al. A prospective, comparative trial of three anesthetics for elective supratentorial craniotomy.Propofol/fentanyl, isoflurane/nitrous oxide, and fentanyl/nitrous oxide. Anesthesiology. 1993;78:1005–20. doi: 10.1097/00000542-199306000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Petersen KD, Landsfeldt U, Cold GE, Petersen CB, Mau S, Hauerberg J, et al. Intracranial pressure and cerebral hemodynamic in patients with cerebral tumors: A randomized prospective study of patients subjected to craniotomy in propofol-fentanyl, isoflurane-fentanyl, or sevoflurane-fentanyl anesthesia. Anesthesiology. 2003;98:329–36. doi: 10.1097/00000542-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Bastola P, Bhagat H, Wig J. Comparative evaluation of propofol, sevoflurane and desflurane for neuroanaesthesia: A prospective randomised study in patients undergoing elective supratentorial craniotomy. Indian J Anaesth. 2015;59:287–94. doi: 10.4103/0019-5049.156868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kay B, Rolly G. I.C.I 35868 – The effect of a change of formulation on the incidence of pain after intravenous injection. Acta Anaesthesiol Belg. 1977:28, 317–22. [PubMed] [Google Scholar]
- 14.Iwata M, Kawaguchi M, Inoue S, Takahashi M, Horiuchi T, Sakaki T, et al. Effects of increasing concentrations of propofol on jugular venous bulb oxygen saturation in neurosurgical patients under normothermic and mildly hypothermic conditions. Anesthesiology. 2006;104:33–8. doi: 10.1097/00000542-200601000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Caricato A, Tersali A, Pitoni S, De Waure C, Sandroni C, Bocci MG, et al. Racemic ketamine in adult head injury patients: Use in endotracheal suctioning. Crit Care. 2013;17:R267. doi: 10.1186/cc13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bundgaard H, von Oettingen G, Larsen KM, Landsfeldt U, Jensen KA, Nielsen E, et al. Effects of sevoflurane on intracranial pressure, cerebral blood flow and cerebral metabolism. A dose-response study in patients subjected to craniotomy for cerebral tumours. Acta Anaesthesiol Scand. 1998;42:621–7. doi: 10.1111/j.1399-6576.1998.tb05292.x. [DOI] [PubMed] [Google Scholar]
- 17.Talke P, Caldwell JE, Richardson CA. Sevoflurane increases lumbar cerebrospinal fluid pressure in normocapnic patients undergoing transsphenoidal hypophysectomy. Anesthesiology. 1999;91:127–30. doi: 10.1097/00000542-199907000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Talke P, Caldwell J, Dodsont B, Richardson CA. Desflurane and isoflurane increase lumbar cerebrospinal fluid pressure in normocapnic patients undergoing transsphenoidal hypophysectomy. Anesthesiology. 1996;85:999–1004. doi: 10.1097/00000542-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Chui J, Mariappan R, Mehta J, Manninen P, Venkatraghavan L. Comparison of propofol and volatile agents for maintenance of anesthesia during elective craniotomy procedures: Systematic review and meta-analysis. Can J Anaesth. 2014;61:347–56. doi: 10.1007/s12630-014-0118-9. [DOI] [PubMed] [Google Scholar]
- 20.Aouad MT, Moussa AR, Dagher CM, Muwakkit SA, Jabbour-Khoury SI, Zbeidy RA, et al. Addition of ketamine to propofol for initiation of procedural anesthesia in children reduces propofol consumption and preserves hemodynamic stability. Acta Anaesthesiol Scand. 2008;52:561–5. doi: 10.1111/j.1399-6576.2008.01584.x. [DOI] [PubMed] [Google Scholar]
- 21.Smischney NJ, Beach ML, Loftus RW, Dodds TM, Koff MD. Ketamine/propofol admixture (ketofol) is associated with improved hemodynamics as an induction agent: A randomized, controlled trial. J Trauma Acute Care Surg. 2012;73:94–101. doi: 10.1097/TA.0b013e318250cdb8. [DOI] [PubMed] [Google Scholar]
- 22.Sharma R, Jaitawat SS, Partani S, Saini R, Sharma N, Gupta S. A randomised controlled trial to compare TIVA infusion of mixture of ketamine propofol (ketofol) and fentanyl-propofol (fentofol) in short orthopaedic surgeries. Indian J Clin Anaesth. 2016;3:404–10. [Google Scholar]


