Abstract
Background
Nimotuzumab (NTZ) is an anti-EGFR monoclonal antibody. However,the effect of targeted drugs combined with induction therapy in locally advanced nasopharyngeal carcinoma remains unclear. The aim of this study is to investigate the safety and efficacy of NTZ combined with cisplatin plus 5-fluorouracil (PF) as induction regimen in locally advanced nasopharyngeal carcinoma (NPC) patients receiving concurrent radiochemotherapy.
Methods
This was a multicenter randomized controlled study performed in eight Guangxi hospitals in 2015–2017. Eligible patients with NPC were randomized into nimotuzumab/PF (NPF group) and docetaxel/PF (DPF group) regimens, respectively, as induction therapy. After 2 cycles of induction therapy, all patients received cisplatin and concurrent intensity modulated radiation therapy (IMRT). Then, the two groups were compared for safety and efficacy.
Results
A total of 118 patients with stage III-IVa NPC were assessed, with 58 and 60 in the NPF and DPF groups, respectively. Compared with DPF treatment, NPF induction therapy showed a more pronounced effect on cervical lymph nodes (P = 0.036), with higher response rate (RR) (81% vs 60%). Compared with the DPF group, the NPF group showed significantly reduced leukopenia, neutropenia and gastrointestinal reactions (all P < 0.05); rash only appeared in the NPF group, but all cases were grade 1. During concurrent treatment with radiotherapy and chemotherapy, the NPF group showed better tolerance to radiotherapy and chemotherapy; neutropenia, anemia, gastrointestinal reactions, oral mucositis and radiation dermatitis in the NPF group were significantly reduced (P < 0.05). The expression rate of EGFR was 94.9% (112/118). Compared with the DPF group, patients with EGFR expression in the NPF group showed better response (77.8% vs 63.0%, P = 0.033).
Conclusion
For locally advanced NPC patients receiving follow-up cisplatin and IMRT, nimotuzumab/PF for induction therapy has better lymph node response rate and milder adverse reactions than the DPF regimen. In addition, the patients have better tolerance in subsequent concurrent radiotherapy and chemotherapy; however, long-term efficacy needs further follow-up evaluation.
Trial registration
The registration number of the clinical trial is ChiCTR-OIC-16008201 and retrospectively registered on March 31, 2016.
Keywords: Locally advanced nasopharyngeal carcinoma, EGFR monoclonal antibody, Induction therapy, Curative effect, Adverse reaction
Background
Nasopharyngeal carcinoma (NPC) is a head and neck cancer with a unique biological behavior; unlike other head and neck tumors, NPC has higher sensitivity to radiotherapy and chemotherapy [1–3]. Therefore, radiochemotherapy is the main treatment option for locally advanced NPC. With the application of intensity modulated radiation therapy (IMRT), distant metastasis has become a major factor affecting prognosis in NPC [3]. Induction therapy may reduce the micrometastasis of NPC, better radiotherapeutic conditions for locally advanced nasopharyngeal carcinoma (especially in patients with giant lesions), and improve patient survival and prognosis [4]. Based on concurrent radiotherapy and chemotherapy (CCRT), induction chemotherapy (IC) could increase the 5-year absolute benefit rates of progression-free survival (PFS) and distance control (DC) by 4.2 and 8.7%, respectively reduce the risk of cancer-related death by 4.8% [5], and improve the overall survival (OS) rate of patients [6]. IC-CCRT is the most effective DC regimen (HR = 0.44, 95%CI 0.27–0.71; P-score 95%) in the comprehensive treatment of NPC. It improves OS (HR = 0.81, 95%CI 0.63–1.04; P-score 63%) and PFS (HR = 0.68, 95%CI 0.54–0.85; P-score 95%) [5]. Therefore, induction chemotherapy combined with concurrent radiochemotherapy is considered an effective therapeutic mode for locally advanced NPC [4, 7], but the optimal induction therapy remains uncertain.
The DPF regimen consisting of docetaxel, cisplatin and 5-fluorouracil shows a higher objective remission rate (ORR) compared with the cisplatin plus 5-fluorouracil (PF) regimen. It is recommended by category I of NCCN guidelines for diagnosis and treatment of head and neck squamous cell carcinoma [8, 9], Induction therapy with the DPF regimen is also used clinically for NPC patients, in whom the ORRs of primary lesions and cervical lymph nodes after DPF induction chemotherapy could reach 92.9% [10]. Based on concurrent radiochemotherapy, DPF chemotherapy effectively improves 3-year OS (86% vs 92%, P = 0.029) and distant metastasis-free survival (DMFS) (83% vs 90%, P = 0.031) [11]. In N2–3 NPC patients, the 3-year distant metastasis rate decreases by 26% (P = 0.08) while OS increases by 25% (P = 0.21) [12]. Compared with induction chemotherapy such as administration of docetaxel combined with cisplatin (TP) or PF, the DPF regimen alone significantly increases PFS (HR = 0.70; 95%CI 0.49–0.95), OS (HR = 0.59; 95%CI 0.37–0.92) [6]. However, while the DPF regimen achieves higher efficacy, treatment-related adverse reactions also increase significantly, especially the incidence of grade 3–4 neutropenia whose rate could reach 42% [11, 13], which hampers the clinical application of DPF.
With the gradual development of molecular biology research, molecular targeted therapy has become a research hotspot in cancer therapy. The expression rate of epidermal growth factor receptor (EGFR) in nasopharyngeal carcinoma is 68–89%, which is much higher than that of other solid tumors [14]. Meanwhile, EGFR expression is closely related to prognosis in NPC [15–17]. Overexpression of EGRF significantly increases the risk of adverse prognosis of NPC OS (HR = 1.86, 95%CI 1.25–2.77; P = 0.000),disease-free survival (DFS) (HR = 2.25, 95%CI 1.66–3.04; P = 0.000), locoregional recurrence free survival (LRFS) (HR = 2.93, 95%CI 1.71–5.02; P = 0.000) [15]. In patients with locally advanced NPC receiving IMRT, combined anti-EGFR receptor therapy may be a more effective treatment strategy [18].
Nimotuzumab (NTZ) is a humanized monoclonal antibody [19, 20]. Compared with cetuximab (CTX), NTZ is human-derived and highly selective, with a long half-life. It competitively inhibits the binding of endogenous ligands to EGFR and blocks the downstream signal transduction pathway mediated by EGFR, thereby inhibiting the proliferation of tumor cells, promoting apoptosis of tumor cells, suppressing angiogenesis and increasing radiosensitivity to chemotherapy; meanwhile, it has few adverse reactions and low incidence of rash. Therefore, whether combined with CCRT or applied as an induction therapeutic, skin rash and mucosal reactions of nimotuzumab are significantly improved compared with those of cetuximab. Retrospective analysis suggested that sequential concurrent radiochemotherapy after induction chemotherapy combined with NTZ is effective and well tolerated in the treatment of locally advanced NPC [21]. In addition, it was demonstrated that NTZ combined with concurrent chemoradiotherapy is beneficial in the treatment of locally advanced NPC, with limited toxicity and good-tolerability [22]. However, there is a lack of prospective analysis of nimotuzumab for induction therapy in locally advanced NPC. Therefore, the current randomized controlled study aimed to assess the safety and efficacy of NTZ combined with PF as induction regimen in locally advanced NPC patients receiving concurrent radiochemotherapy.
Methods
Patients
This randomized controlled study assessed NPC patients receiving initial treatment in eight hospitals in Guangxi (Oncology Department of the Fourth Affiliated Hospital of Guangxi Medical University, Radiotherapy Department of Wuzhou Red Cross Hospital, Radiotherapy Department of Liuzhou People’s Hospital, Radiotherapy Department of the First Affiliated Hospital of Guangxi Medical University, Radiotherapy Department of the Second Affiliated Hospital of Guangxi Medical University, Oncology Department of Nanning Second People’s Hospital, Oncology Department of Liuzhou Traditional Chinese Medicine Hospital, Radiotherapy Department of the affiliated hospital of Guilin Medical College) from January 2015 to December 2017.
Inclusion criteria were: 1) 18–70 years old; 2) undifferentiated non-keratinizing nasopharyngeal carcinoma pathologically diagnosed by biopsy; 3) clinical stage III-IVa (08 Chinese stage); 4) KPS score ≥ 70; 5) serum hemoglobin ≥10 mg/dL, platelet ≥10,000/mu L, and absolute neutrophil count ≥1500/mu L; 6) serum creatinine ≤1.5 times UNL or creatinine clearance rate ≥ 60 ml/min; bilirubin ≤1.5 times UNL and AST (SGOT) and ALT (SGPT) ≤ 1.5 times UNL; 8) estimated survival time ≥ 6 months.
Exclusion criteria were: 1) previous diagnosis of malignant tumors; 2) previous radiotherapy, chemotherapy, or targeted therapy; 3) radiotherapy or chemotherapy contraindications; 4) allergy to any study drug.
The study was approved by the Ethics Committee of the Fourth Affiliated Hospital of Guangxi Medical University (PJK2015201). The registration number of the clinical trial is ChiCTR-OIC-16008201, and all patients provided signed informed consent obtained was written.
Treatments
In this open-label trial, allocation concealment was performed before eligible patients were randomly divided into two groups: NPF (induction therapy with nimotuzumab combined with PF; nimotuzumab 200 mg/time/week by intravenous drip, cisplatin 75 g/m2 by intravenous drip at d1, and continuous infusion of 5-fluorouracil 750 mg/m2/day at d1–5; repeated every 3 weeks) and DPF (induction chemotherapy; docetaxel 75 g/m2 by intravenous drip at d1, cisplatin 75 g/m2 by intravenous drip at d1, and continuous infusion of 5-fluorouracil 750 mg/m2/d at d1–5; repeated every 3 weeks) groups. After 2 cycles of induction therapy, cisplatin (80 g/m2 by intravenous drip at d1, repeated every 3 weeks, for a total of 3 cycles) was administered to both groups concurrently with intensity modulated radiation therapy (IMRT). G-CSF was not administered prophylactically before the first induction therapy or concurrent chemotherapy; in patients with grade 4 neutropenia, prophylactic G-CSF leucocyte-raising therapy was used during the follow-up cycle. In patients who still showed intolerability, chemotherapeutic drug dosage was reduced by 15% according to the principle of reduction of chemotherapeutic drugs. All chemotherapy cycles routinely included antiemetic treatment, and the follow-up cycle antiemetic regimen was adjusted according to gastrointestinal reactions.
All patients were treated by IMRT. The range of tumors was determined by magnetic resonance (MR), and the target area was delineated and planned by enhanced CT. According to the actual situation of each center, the planning target volume (PTV) formed by expanding 3–5 mm from each target area was administered a prescription dose: nasopharyngeal tumor volume (GTVnx) and cervical lymph node volume (GTVnd) at 69.69–70.06 Gy and 64.17–70.06 Gy, respectively; high-risk area of primary focus (CTV1) at 66.03 Gy; low-risk area of primary focus and cervical lymph node drainage area (CTV2) at 50.4–54.25 Gy. The number of segmentations was 31–33 times, 5 times a week. According to the requirements of RTOG 0615 and RTOG 0225, organ-threatening dose and planning were assessed.
Evaluation indexes
The primary endpoints were the efficacy of induction therapy and adverse reactions to induction therapy. The secondary endpoints were immediate and 3-month follow-up effects of the whole therapy and adverse reactions to concurrent chemoradiotherapy. The acute and late side effects of radiotherapy were evaluated according to RTOG acute and late radiation reaction scoring criteria, and the adverse reactions related to chemotherapy were assessed according to NCI-CTC AE3.0 classification criteria of common adverse drug reactions. The curative effects were based on Response Evaluation Criteria in Solid Tumors (RECIST 1.1) [23] and included complete response (CR; no detectable cancer after your treatment), partial response (PR; at least 30% decrease in total lesion area), stable disease (SD; no change or decrease of total lesion area below 30%) and progressive disease (PD; increase in total lesion area).
EGFR detection
EGFR expression in nasopharyngeal carcinoma tissues was evaluated by immunohistochemical staining. The tumor tissues were fixed with 10% formaldehyde, paraffin embedded and sliced into 4-μm sections. After conventional dewaxing, endogenous peroxidase activity was blocked by treatment with hydrogen peroxide, followed by antigen retrieval via heating. Then, the samples were successively incubated with primary (overnight at 4 °C) and biotinylated secondary (37 °C for 30 min) antibodies, and treated with DAB for 10 min. After dehydration with graded ethanol, counterstaining was performed with hematoxylin. The range and intensity of staining were observed and recorded under a microscope. Phosphate buffer saline was used as a negative control, and known positive samples were used as positive controls. Grading was performed according to staining intensity and the percentage of positive cells. Positive signals were dark blue-purple granules in the cell membrane or cytoplasm of the tumor cells; positive cells were grouped into 0–4%, 5–24%, 25–49%, 50–75 and > 75%. Two deputy directors of pathology interpreted the results independently.
Statistical methods
In the current non-inferiority study, the efficacy of induction therapy was the primary point for non-inferiority and RR decreases less than 5% as non-inferiority margin. We used an alpha of 0.05 and a beta of 0.20. Considering a dropout rate of 10%, we found that 60 was an appropriate sample size for each group. Therefore, a total of 120 patients were enrolled. IBM SPSS 19.0 was used for statistical analysis. Measurement data were analyzed using t test, while count data were compared by the χ2 test. Differences were considered significant at p < 0.05.
Results
Baseline patient characteristics
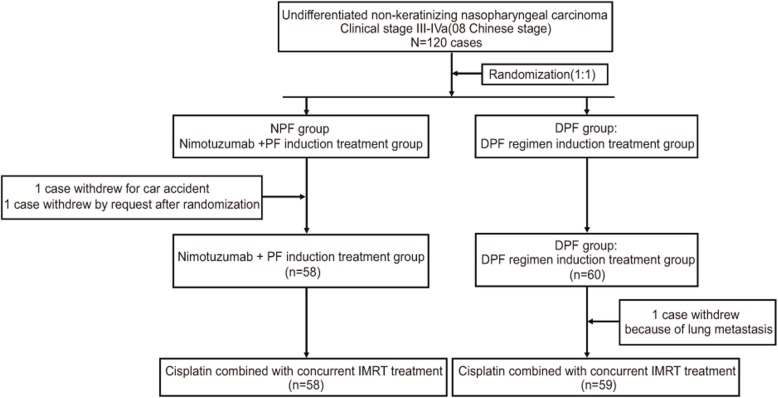
From May 2015 to November 2017, a total of 118 patients were enrolled in the current study. The study flowchart was shown in Fig. 1. The average age of all patients was 44 years (22–68 years). There were 98 males and 20 females, indicating a male to female ratio of 4.9. A total of 66 cases had stage III disease while 52 had stage IVa. The NPF and DPF groups comprised 58 and 60 cases, respectively. There were no significant differences in baseline characteristics, including age, sex, and clinical stage between the two groups (P > 0.05) (Table 1).
Fig. 1.
Study flowchart
Table 1.
Clinical characteristics of the two groups
| Characteristics | No. of patients (%) | P | ||
|---|---|---|---|---|
| Total (n = 118) |
NPF group (n = 58) |
DPF group (n = 60) |
||
| Median age (range) | 44(22–68) | 43(22–65) | 45(22–68) | 0.214 |
| Gender | ||||
| Males | 98(83.1) | 47(81.0) | 51(85.0) | 0.371 |
| Females | 20(16.9) | 11(19.0) | 9(15.0) | |
| Clinical stage (China, 2008) | ||||
| III | 66(55.9) | 34(58.6) | 32(53.3) | 0.347 |
| IVa | 52(44.1) | 24(41.4) | 28(46.7) | |
| T stage | 0.436 | |||
| T1 | 6(5.1) | 3(5.1) | 3(5.0) | |
| T2 | 20(16.9) | 12(20.7) | 8(13.3) | |
| T3 | 57(48.3) | 27(46.6) | 30(50.0) | |
| T4 | 35(29.7) | 16(27.6) | 19(31.7) | |
| N stage | 0.585 | |||
| N0 | 3(25.4) | 2(3.4) | 1(1.6) | |
| N1 | 27(22.9) | 15(25.9) | 12(20.0) | |
| N2 | 67(56.9) | 30(51.7) | 37(61.7) | |
| N3 | 21(17.8) | 11(19.0) | 10(16.7) | |
Therapeutic effects
By September 2018, all patients had completed induction therapy and therapeutic effects were evaluated. In the induction stage, compared with the DPF group, NPF induction therapy had more pronounced effects on cervical lymph nodes (P = 0.036) and RR (CR + PR) (81% vs 60%). There were no significant differences in nasopharyngeal lesions and overall efficacy (P = 0.446, P = 0.143) between the two groups. One case of lung metastasis after induction chemotherapy in the DPF group exited the study. A total of 117 patients were further treated with cisplatin and concurrent IMRT, including 58 and 59 in the NPF and DPF groups, respectively. There were no significant differences between the two groups (P = 0.449 and P = 0.409) in immediate efficacy evaluation and 3-month efficacy evaluation at the end of the whole course of treatment (Table 2).
Table 2.
Therapeutic effects in the two groups
| Therapeutic effecta | After induction therapy (n = 118) |
After concurrent radiochemotherapy (n = 117)b |
3 months after the treatment (n = 117) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nasopharyngeal lesions (%) | Lymph node lesions (%) | Total(%)(nasopharyngeal and lymph node lesions) | ||||||||
| NPF group (n = 58) | DPF group (n = 60) | NPF group (n = 58) | DPF group (n = 60) | NPF group (n = 58) | DPF group (n = 60) | NPF group (n = 58) | DPF group (n = 59) | NPF group (n = 58) | DPF group (n = 59) | |
| CR | 2(3.4) | 0(0) | 5(8.6) | 1(1.7) | 2(3.4) | 0(0) | 39(67.2) | 38(64.4) | 51(87.9) | 50(84.7) |
| PR | 33(56.9) | 31(51.7) | 42(72.4) | 35(58.3) | 39(67.2) | 37(61.7) | 19(32.8) | 21(35.6) | 7(12.1) | 9(15.3) |
| SD | 23(39.7) | 29(48.3) | 11(19.0) | 24(40.0) | 17(29.3) | 22(36.7) | 0(0) | 0(0) | 0(0) | 0(0) |
| PD | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 1(1.7) | 0(0) | 0(0) | 0(0) | 0(0) |
| P | 0.446 | 0.036 | 0.143 | 0.499 | 0.409 | |||||
aCR Complete remission, PR Partial remission, SD Stable disease, PD Progressive disease
b After induction therapy, 1 patient in the DPF group showed distant metastasis and withdrew from the study
Adverse reactions
In the induction stage, the main adverse reactions in the two groups were grade 1–2 leukopenia, neutropenia and gastrointestinal reactions. Compared with the DPF group, the NPF group showed significantly reduced leucopenia, neutropenia and gastrointestinal reactions (P = 0.037, P = 0.018 and P = 0.032, respectively). Rashes only appeared in the NPF group, and all were grade 1; after NTZ treatment, rashes could disappear spontaneously. There were no significant differences in hemoglobin decrease, thrombocytopenia, liver or kidney impairment, and oral mucositis (P > 0.05). In the concurrent radiotherapy and chemotherapy phase, the NTP group showed better treatment tolerance. Neutropenia, anemia, gastrointestinal reactions, oral mucositis and radiation dermatitis were significantly reduced in the NTP group compared with DPF group (P = 0.033, P = 0.049, P = 0.037, P = 0.020 and P = 0.035, respectively). Leukopenia, thrombocytopenia, and liver and kidney functions were also improved, but the differences were not statistically significant (P > 0.05) (Table 3).
Table 3.
Adverse reactions in the two groups
| Adverse reaction | Induction therapy stage (n = 118) (%) |
Concurrent radiochemotherapy stage (n = 117) (%) |
||||
|---|---|---|---|---|---|---|
| NPF group (n = 58) | DPF group (n = 60) | P | NPF group (n = 58) | DPF group (n = 59) | P | |
| Leukocytopenia | 0.037 | 0.090 | ||||
| 0 | 20(34.5) | 17(28.3) | 14(24.1) | 11(18.6) | ||
| 1 | 28(48.3) | 21(35.0) | 29(50.0) | 24(40.7) | ||
| 2 | 7(12.1) | 13(21.7) | 14(24.1) | 20(33.9) | ||
| 3 | 3(5.2) | 8(13.3) | 1(1.7) | 4(6.8) | ||
| 4 | 0(0) | 1(1.7) | 0(0) | 0(0) | ||
| Neutropenia | 0.018 | 0.033 | ||||
| 0 | 19(32.8) | 17(28.3) | 13(22.4) | 9(15.3) | ||
| 1 | 27(46.6) | 19(31.7) | 27(46.6) | 20(37.3) | ||
| 2 | 9(15.5) | 11(18.3) | 14(24.1) | 22(37.3) | ||
| 3 | 3(5.2) | 9(15.0) | 4(6.9) | 6(10.2) | ||
| 4 | 0(0) | 4(6.7) | 0(0) | 2(3.4) | ||
| Anemia | 0.247 | 0.049 | ||||
| 0 | 49(84.5) | 47(78.3) | 42(72.4) | 33(56.9) | ||
| 1 | 9(15.5) | 11(18.3) | 16(27.6) | 23(39.7) | ||
| 2 | 0(0) | 2(3.3) | 0(0) | 2(3.4) | ||
| Thrombocytopenia | 0.452 | 0.532 | ||||
| 0 | 53(91.4) | 53(88.3) | 55(94.8) | 55(93.2) | ||
| 1 | 5(8.6) | 6(10.0) | 3(5.2) | 3(5.1) | ||
| 2 | 0(0) | 1(0.8) | 0(0) | 1(1.7) | ||
| Liver function damage | 0.275 | 0.178 | ||||
| 0 | 49(84.5) | 43(71.7) | 46(79.3) | 41(69.5) | ||
| 1 | 7(12.1) | 15(21.6) | 12(20.7) | 17(28.8) | ||
| 2 | 1(1.7) | 2(3.3) | 0(0) | 1(1.7) | ||
| 3 | 1(1.7) | 0(0) | 0(0) | 0(0) | ||
| Renal function damage | 0.166 | 0.254 | ||||
| 0 | 55(94.8) | 53(88.3) | 55(94.8) | 53(88.3) | ||
| 1 | 3(5.2) | 6(10.0) | 3(5.2) | 6(10.0) | ||
| 2 | 0(0) | 1(1.7) | 0(0) | 0(0) | ||
| Gastrointestinal reaction | 0.032 | 0.037 | ||||
| 0 | 8(13.8) | 3(5.0) | 9(15.5) | 4(6.8) | ||
| 1 | 38(65.5) | 37(61.7) | 32(55.2) | 28(47.5) | ||
| 2 | 11(19.0) | 16(26.7) | 16(27.6) | 25(42.4) | ||
| 3 | 1(1.7) | 4(6.7) | 1(1.7) | 2(3.4) | ||
| Oral mucositis | 0.099 | 0.020 | ||||
| 0 | 55(94.8) | 51(85.0) | 12(20.7) | 6(10.2) | ||
| 1 | 2(3.4) | 6(10.0) | 27(46.6) | 23(39.0) | ||
| 2 | 1(1.7) | 3(5.0) | 19(32.8) | 28(47.5) | ||
| 3 | 0(0) | 0(0) | 0(0) | 2(3.4) | ||
| skin rash | 0.012 | |||||
| 0 | 52(89.7) | 60(100) | – | – | – | |
| 1 | 6(10.3) | 0(0) | – | – | – | |
| Radiation skin reaction | 0.035 | |||||
| 0 | – | – | 8(13.8) | 6(10.2) | ||
| 1 | – | – | 34(58.6) | 25(42.4) | ||
| 2 | – | 16(27.6) | 26(44.1) | |||
| 3 | – | – | 0(0) | 2(3.4) | ||
Association of EGFR expression with the efficacy of induction therapy
The overall expression rate of EGFR was 94.9% (112/118), including 94.8% (55/58) and 95.5% (57/60) in the NPF and DPF groups, respectively. There was no significant difference in EGFR expression levels between the two groups (P = 0.058) (Table 4). EGFR expression was not significantly correlated with the efficacy of induction chemotherapy with DPF (P = 0.090), but significantly affected the efficacy of induction therapy combined with nimotuzumab (P = 0.015); compared with chemotherapy, induction therapy combined with nimotuzumab had better response (77.8% vs 63.0%,P = 0.033)., as shown in Table 5.
Table 4.
Expression of EGFR in both patient groups
| EGFR expression | NPF group (%) | DPF group (%) | Total (%) |
|---|---|---|---|
| 0–4% | 3(5.2) | 3(5.0) | 6(5.1) |
| 5–24% | 10(17.2) | 3(5.0) | 13(17.2) |
| 25–49% | 9(15.5) | 8(13.3) | 17(14.4) |
| 50–74% | 26(44.8) | 29(48.3) | 55(46.6) |
| 75–100% | 10(17.2) | 17(28.3) | 27(22.9) |
| P | 0.058 | ||
Table 5.
Correlation between EGFR expression and the curative effect of induction therapy
| EGFR expression | NPF group (n = 58) | DPF group (n = 60) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | CR | PR | SD | PD | P | |
| 0–4% | 0 | 1 | 1 | 0 | 0 | 3 | 0 | 0 | |
| 5–24% | 0 | 4 | 6 | 0 | 0 | 1 | 2 | 0 | |
| 25–49% | 1 | 7 | 1 | 0 | 0 | 4 | 4 | 0 | 0.033 |
| 50–74% | 0 | 18 | 8 | 0 | 0 | 23 | 6 | 1 | |
| 75–100% | 1 | 9 | 1 | 0 | 0 | 6 | 10 | 0 | |
| P | 0.015 | 0.09 | \ | ||||||
Discussion
This study assessed the effectiveness of NTZ combined with PF as induction regimen in locally advanced NPC cases receiving concurrent radiochemotherapy, and demonstrated that nimotuzumab combined with PF for induction therapy has better lymph node response rate and milder adverse reactions compared with the DPF regimen. In addition, the patients showed improved tolerance in subsequent concurrent radiotherapy and chemotherapy.
In a study by Chua DT [24] EGFR was shown to be expressed in 89% of nasopharyngeal carcinoma cases, and high EGFR expression is considered an independent prognostic factor for local control, non-recurrence and disease-related survival in stage III-IV NPC. In the latter report, 72% of patients with EGFR expression (> 25%) showed significant adverse prognosis after induction chemotherapy and radiotherapy. It was therefore suggested that anti-EGFR therapy might be necessary to improve prognosis in locally advanced NPC with high EGFR expression to increase clinical benefits. In the present study, 94.9% of NPC patients expressed EGFR, including 77.7% whose EGFR expression exceeded 25%, corroborating Chua’s study [24] The efficacy of induction therapy combined with anti-EGFR was related to EGFR (P = 0.015). Meanwhile, supplementing anti-EGFR monoclonal antibody significantly affected the efficacy of induction therapy (P = 0.033), suggesting that induction therapy combined with anti-EGFR therapy is feasible.
One of the aims of induction therapy is to effectively alleviate the lesions and create improved radiotherapy conditions for NPC, especially in patients with giant lesions, achieving better prognosis. For instance, in NPC patients undergoing follow-up CCRT, 5-year OS rates in the CR, PR and SD subgroups after induction chemotherapy were shown to be 100, 79.4 and 60%, respectively. The efficacy of induction therapy may therefore affect patient survival and prognosis [25]. It was reported that induction therapy with taxanes significantly increases ORR (OR = 4.57, 95%CI 1.14–18.30, P = 0.032, z = 2.15) [26] compared with the non-taxane regimen. However, in this study, induction therapy with NPF had higher RR in lymph node lesions (81.0% vs 60%) compared with DPF. These findings suggest that induction therapy combined with EGFR is more effective in alleviating lesions, creating better radiotherapy conditions and improving survival and prognosis in NPC with high EGFR expression.
With the application of IMRT, distant metastasis has become a major factor affecting prognosis in NPC. Meanwhile, induction therapy may reduce the micrometastasis of locally advanced NPC, and is considered an effective control scheme for distant metastasis in various comprehensive treatment regimens, improving PFS and OS [5]. Based on concurrent radiochemotherapy, the DPF regimen was added to induce chemotherapy, which effectively increased the 3-year OS (P = 0.029) and DMFS (P = 0.031) [11]. Meanwhile, the 3-year distant metastasis rate of NPC patients with N2–3 disease decreased by 26% (P = 0.08), while OS increased by 25% (P = 0.21) [12]. Among the various induction therapies commonly used in clinic, the PF regimen significantly reduces the risk of adverse prognosis of PFS (HR = 0.75; 95%CI, 0.56–0.99), whereas the DPF regimen increases the risk of adverse prognosis of OS while improving PFS (HR = 0.59; 95%CI, 0.37–0.92) [6]. However, adding EGFR monoclonal antibody based on DPF does not significantly increase the survival advantage of NPC patients. NTZ combined with DPF does not improve 5-year OS (89.9% vs 93.3%) and PFS (79.3% vs 82.1%) compared with NTZ combined with PF [21]. Therefore, EGFR combined with PF may represent a more economical and effective induction therapeutic regimen for NPC. As shown above, there was no significant difference between the NPF and DPF groups during immediate and 3-month curative effect evaluation. Although long-term survival data for NPF induction therapy and DPF induction chemotherapy were not available in this study, N stage is the main prognostic factor of DMFS and OS in nasopharyngeal carcinoma. A higher regional lymph node response rate (P = 0.036) after NPF induction therapy would help control regional lymph node-related distant metastasis and improve survival. It is worth assessing long-term survival after treatment with the NPF regimen.
On the other hand, induction therapy by NTZ combined with PF reduces the intensity of chemotherapy compared with DPF, which is helpful in improving hematological toxicity that restricts the clinical application of DPF. Adverse reactions caused by induction chemotherapy, especially increased systemic toxicity, hamper the application of induction chemotherapy and follow-up concurrent radiochemotherapy to a certain extent. It was reported that the incidence of grade 3–4 neutropenia in taxane-based induction therapy is significantly higher than that of non-taxane containing regimens (39.1% vs 16.1%, OR = 3.62, 95%CI 2.42–5.40, P < 0.001, z = 6.29) [26]. The incidence rates of neutropenia and stomatitis were 42 and 41%, respectively, after addition of the DPF regimen based on CCRT, which affected compliance with IC and subsequent CCRT. Adding anti-EGFR therapy to standard therapy (radiotherapy or radiochemotherapy) significantly increases OS (HR = 0.51; 95%CI, 0.39–0.66) and DFS (HR = 0.68; 95%CI, 0.54–0.86); meanwhile, systemic toxicity significantly decreases when EGFR therapy replaces cytotoxic drugs at the time of radiotherapy [27]. In NPC patients administered IMRT, CTX/NTZ combined with induction therapy can reduce severe toxicity and achieve better survival benefit compared with CTX/NTZ applied in concurrent radiotherapy [28]. These findings suggest that anti-EGFR monoclonal antibody combined with an appropriate intensity of chemotherapy may constitute an effective induction therapy to ensure or increase efficacy while markedly alleviating toxic reactions. Because of humanization, NTZ has better tolerance in nasopharyngeal carcinoma treatment [21, 29]. Compared with CTX, NTZ significantly improves skin rash (P < 0.001), oral mucositis (P < 0.001) and weight loss (P < 0.008) [29, 30]. In this study, addition of humanized anti-EGFR monoclonal antibody created conditions to reduce the intensity of induction chemotherapy; therefore, systemic toxicity and gastrointestinal reactions during induction chemotherapy were effectively controlled. Compared with DPF, the NPF regimen effectively alleviated neutropenia (P = 0.018) and gastrointestinal reactions (P = 0.032) in the induction phase, while use of EGFR monoclonal antibody in human chemotherapy did not increase mucosal-related toxicity. Toxicity control during induction therapy could also effectively improve patient tolerance during follow-up concurrent radiochemotherapy. Therefore, neutropenia, anemia, gastrointestinal reaction, oral mucositis and radiation dermatitis in the NPF group were significantly improved compared with the DPF group (P = 0.033, P = 0.049, P = 0.037, P = 0.020 and P = 0.035, respectively), which greatly improved the tolerance of NPF patients to whole course treatment and solved key problems that restrict the clinical application of induction chemotherapy which could create conditions for improved prognosis.
The main limitation of this study is that it only performed short-term evaluation of efficacy and adverse reactions. Therefore, long-term evaluation of the NPF regimen deserves further investigation, and would further assess the clinical significance of this regimen. In addition, the latest research shows that gemcitabine and cisplatin as induction chemotherapy added to chemoradiotherapy significantly improved LRFS and OS among patients with locoregionally advanced nasopharyngeal carcinoma [31]. Therefore, a comparison of larger sample sizes with new induced chemotherapy regimens may better confirm the role of NPF schemes.
Conclusion
In conclusion, compared with DPF induction chemotherapy for locally advanced NPC cases receiving follow-up cisplatin and concurrent IMRT, nimotuzumab combined with PF as induction chemotherapy has better lymph node response rate, less adverse reactions, and better induction therapy and concurrent radiochemotherapy tolerance compared with the DPF regimen. Therefore, the NPF regimen may become a new choice for induction therapy of locally advanced nasopharyngeal cancer.
Acknowledgements
We would like to thank all the patients and the personnel involved in this study. We are also grateful to Professors Min Yi and Wenhua Li at the Pathology Department, the Fourth Affiliated Hospital of Guangxi Medical University, for help in pathologic assessment. This study adheres to CONSORT guidelines.
Abbreviations
- CCRT
Concurrent radiotherapy and chemotherapy
- CR
Complete response
- CTX
Cetuximab
- DC
Distance control
- DFS
Disease-free survival
- DMFS
Distant metastasis-free survival
- DPF
Docetaxel/PF
- EGFR
Epidermal growth factor receptor
- IC
Induction chemotherapy
- IMRT
Intensity modulated radiation therapy
- LRFS
Locoregional recurrence free survival
- MR
Magnetic resonance
- NPC
Nasopharyngeal carcinoma
- NPF
Nimotuzumab/PF
- NTZ
Nimotuzumab
- OS
Overall survival
- PD
Progressive disease
- PF
Plus 5-fluorouracil
- PFS
Progression-free survival
- PR
Partial response
- PTV
Planning target volume
- RECIST
Response Evaluation Criteria in Solid Tumors
- RR
Response rate
- SD
Stable disease
Authors’ contributions
YL conceived and coordinated the study, designed, performed and analyzed the experiments, wrote the paper. DC, JL, JG, ZL, RW, WL, CH, XN, ML and HH carried out the data collection, data analysis, and revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Funding
This work was supported by Liuzhou City Science and technology research projects (2015 J030512), Liuzhou City Science and technology research projects (2016G020203) and Department of Health of Guangxi Zhuang Autonomous Region Self-raised Funds Project (Z2016167), Department of Health of Guangxi Zhuang Autonomous Region Self-raised Funds Project (Z20170895) in the study design, collection and analysis of data.
Availability of data and materials
The data set supporting the results of this article are included within the article.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Ethics Committee of the Fourth Affiliated Hospital of Guangxi Medical University (PJK2015201). Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ying Lu, Email: 1786734840@qq.com.
Dagui Chen, Email: 813120992@qq.com.
Jinhui Liang, Email: 566jv@sina.cn.
Jianquan Gao, Email: Gjq2369712@sina.com.
Zhanxiong Luo, Email: lzrmyylzx@163.com.
Rensheng Wang, Email: 13807806008@163.com.
Wenqi Liu, Email: liuwenqigx@163.com.
Changjie Huang, Email: changjie_huang@126.com.
Xuejian Ning, Email: nxj606@sina.com.
Meilian Liu, Email: Liu.meilian@163.com.
Haixin Huang, Email: 13507726193@163.com.
References
- 1.Liang SB, Wang Y, Hu XF, He SS, Yang XL, Liu LZ, et al. Survival and toxicities of IMRT based on the RTOG protocols in patients with nasopharyngeal carcinoma from the endemic regions of China. J Cancer. 2017;8:3718–3724. doi: 10.7150/jca.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharyya T, Babu G, Kainickal CT. Current role of chemotherapy in nonmetastatic nasopharyngeal Cancer. J Oncol. 2018;2018:3725837. doi: 10.1155/2018/3725837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan H, Cao X, Wang J. Application of intensity-modulated radiation therapy in the treatment of nasopharyngeal carcinoma. Oncol Lett. 2017;14:7773–7776. doi: 10.3892/ol.2017.6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng H, Chen L, Zhang J, Li WF, Mao YP, Zhang Y, et al. Induction chemotherapy improved Long-term outcomes of patients with Locoregionally advanced nasopharyngeal carcinoma: a propensity matched analysis of 5-year survival outcomes in the era of intensity-modulated radiotherapy. J Cancer. 2017;8:371–377. doi: 10.7150/jca.16732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribassin-Majed L, Marguet S, Lee AWM, Ng WT, Ma J, Chan ATC, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol. 2017;35:498–505. doi: 10.1200/JCO.2016.67.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YP, Tang LL, Yang Q, Poh SS, Hui EP, Chan ATC, et al. Induction chemotherapy plus concurrent Chemoradiotherapy in endemic nasopharyngeal carcinoma: individual patient data pooled analysis of four randomized trials. Clin Cancer Res. 2018;24:1824–1833. doi: 10.1158/1078-0432.CCR-17-2656. [DOI] [PubMed] [Google Scholar]
- 7.Lang J, Gao L, Guo Y, Zhao C, Zhang C, Society of H et al. Comprehensive treatment of squamous cell cancer of head and neck: Chinese expert consensus 2013. Future Oncol. 2014;10:1635–1648. doi: 10.2217/fon.14.44. [DOI] [PubMed] [Google Scholar]
- 8.Skulsky SL, O'Sullivan B, McArdle O, Leader M, Roche M, Conlon PJ, et al. Review of high-risk features of cutaneous squamous cell carcinoma and discrepancies between the American joint committee on Cancer and NCCN clinical practice guidelines in oncology. Head Neck. 2017;39:578–594. doi: 10.1002/hed.24580. [DOI] [PubMed] [Google Scholar]
- 9.Kim R, Hahn S, Shin J, Ock CY, Kim M, Keam B, et al. The effect of induction chemotherapy using Docetaxel, Cisplatin, and fluorouracil on survival in locally advanced head and neck squamous cell carcinoma: a meta-analysis. Cancer Res Treat. 2016;48:907–916. doi: 10.4143/crt.2015.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng Z, Yan RN, Tu L, Wang YY, Chen PR, Luo F, et al. Assessment of concurrent Chemoradiotherapy plus induction chemotherapy in advanced nasopharyngeal carcinoma: Cisplatin, fluorouracil, and Docetaxel versus gemcitabine and Cisplatin. Sci Rep. 2018;8:15581. doi: 10.1038/s41598-018-33614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509–1520. doi: 10.1016/S1470-2045(16)30410-7. [DOI] [PubMed] [Google Scholar]
- 12.Kawahira M, Yokota T, Hamauchi S, Onozawa Y, Ogawa H, Onoe T, et al. Survival benefit of adding docetaxel, cisplatin, and 5-fluorouracil induction chemotherapy to concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma with nodal stage N2-3. Jpn J Clin Oncol. 2017;47:705–712. doi: 10.1093/jjco/hyx057. [DOI] [PubMed] [Google Scholar]
- 13.Kong L, Zhang Y, Hu C, Guo Y, Lu JJ. Effects of induction docetaxel, platinum, and fluorouracil chemotherapy in patients with stage III or IVA/B nasopharyngeal cancer treated with concurrent chemoradiation therapy: final results of 2 parallel phase 2 clinical trials. Cancer. 2017;123:2258–2267. doi: 10.1002/cncr.30566. [DOI] [PubMed] [Google Scholar]
- 14.Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94:1593–1611. doi: 10.1002/cncr.10372. [DOI] [PubMed] [Google Scholar]
- 15.Sun W, Long G, Wang J, Mei Q, Liu D, Hu G. Prognostic role of epidermal growth factor receptor in nasopharyngeal carcinoma: a meta-analysis. Head Neck. 2014;36:1508–1516. doi: 10.1002/hed.23355. [DOI] [PubMed] [Google Scholar]
- 16.Ma X, Huang J, Wu X, Li X, Zhang J, Xue L, et al. Epidermal growth factor receptor could play a prognostic role to predict the outcome of nasopharyngeal carcinoma: a meta-analysis. Cancer Biomark. 2014;14:267–277. doi: 10.3233/CBM-140401. [DOI] [PubMed] [Google Scholar]
- 17.Ma BB, Poon TC, To KF, Zee B, Mo FK, Chan CM, et al. Prognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma--a prospective study. Head Neck. 2003;25:864–872. doi: 10.1002/hed.10307. [DOI] [PubMed] [Google Scholar]
- 18.Peng H, Tang LL, Liu X, Chen L, Li WF, Mao YP, et al. Anti-epidermal growth factor receptor therapy concurrently with induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Cancer Sci. 2018;109:1609–1616. doi: 10.1111/cas.13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Pan L, Sheng XF, Chen S, Dai JZ. Nimotuzumab, a humanized monoclonal antibody specific for the EGFR, in combination with temozolomide and radiation therapy for newly diagnosed glioblastoma multiforme: first results in Chinese patients. Asia Pac J Clin Oncol. 2016;12:e23–e29. doi: 10.1111/ajco.12166. [DOI] [PubMed] [Google Scholar]
- 20.Chong DQ, Toh XY, Ho IA, Sia KC, Newman JP, Yulyana Y, et al. Combined treatment of Nimotuzumab and rapamycin is effective against temozolomide-resistant human gliomas regardless of the EGFR mutation status. BMC Cancer. 2015;15:255. doi: 10.1186/s12885-015-1191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, Jiang C, Ye Z, Sun Q, Liu T, Xu M, et al. Efficacy and safety of nimotuzumab with neoadjuvant chemotherapy followed by concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma. Oncotarget. 2017;8:75544–75556. doi: 10.18632/oncotarget.17357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu ZG, Zhao Y, Tang J, Zhou YJ, Yang WJ, Qiu YF, et al. Nimotuzumab combined with concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a retrospective analysis. Oncotarget. 2016;7:24429–24435. doi: 10.18632/oncotarget.8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Zou Q, Qian D, Zhou L, Yang B, Chu J, et al. Intensity-modulated radiotherapy plus nimotuzumab with or without concurrent chemotherapy for patients with locally advanced nasopharyngeal carcinoma. Onco Targets Ther. 2017;10:5835–5841. doi: 10.2147/OTT.S151554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chua DT, Nicholls JM, Sham JS, Au GK. Prognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:11–20. doi: 10.1016/j.ijrobp.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 25.Ou D, Blanchard P, El KC, De FF, Even C, Levy A, et al. Induction chemotherapy with docetaxel, cisplatin and fluorouracil followed by concurrent chemoradiotherapy or chemoradiotherapy alone in locally advanced non-endemic nasopharyngeal carcinoma. Oral Oncol. 2016;62:114–121. doi: 10.1016/j.oraloncology.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Tian R, Ye HX, Zhang BG, Gu DY, Zhang BW, Teng ZP, et al. Use of taxane-containing induction chemotherapy in combination with concurrent chemoradiotherapy in Chinese patients with locally advanced nasopharyngeal carcinoma: a meta-analysis. Onco Targets Ther. 2015;8:3255–3263. doi: 10.2147/OTT.S92109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng L, Liu ZL, Xu C, Tang LL, Liu X, Lin AH, et al. The efficacy and safety of anti-epidermal growth factor receptor monoclonal antibodies in nasopharyngeal carcinoma: literature-based meta-analyses. J Cancer. 2018;9:4510–4520. doi: 10.7150/jca.27611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng H, Tang LL, Liu X, Chen L, Li WF, Mao YP, et al. Anti-EGFR targeted therapy delivered before versus during radiotherapy in locoregionally advanced nasopharyngeal carcinoma: a big-data, intelligence platform-based analysis. BMC Cancer. 2018;18:323. doi: 10.1186/s12885-018-4268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin M, You R, Liu YP, Zhang YN, Zhang HJ, Zou X, et al. Beneficial effects of anti-EGFR agents, Cetuximab or Nimotuzumab, in combination with concurrent chemoradiotherapy in advanced nasopharyngeal carcinoma. Oral Oncol. 2018;80:1–8. doi: 10.1016/j.oraloncology.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 30.You R, Hua YJ, Liu YP, Yang Q, Zhang YN, Li JB, et al. Concurrent Chemoradiotherapy with or without anti-EGFR-targeted treatment for stage II-IVb nasopharyngeal carcinoma: retrospective analysis with a large cohort and Long follow-up. Theranostics. 2017;7:2314–2324. doi: 10.7150/thno.19710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and Cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381:1124–1135. doi: 10.1056/NEJMoa1905287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set supporting the results of this article are included within the article.