Abstract
The in vivo intervertebral range of motion (ROM) is an important predictor for spinal disorders. While the subaxial cervical spine has been extensively studied, the motion characteristics of the occipito-atlantal (C0-1) and atlanto-axial (C1-2) cervical segments were less reported due to technical difficulties in accurate imaging of these two segments. In this study, we investigated the intervertebral ROMs of the entire cervical spine (C0-T1) during in vivo functional neck motions of asymptomatic human subjects, including maximal flexion-extension, left-right lateral bending, and left-right axial torsion, using previously validated dual fluoroscopic imaging and model registration techniques. During all neck motions, C0-1, similar to C7-T1, was substantially less mobile than other segments and always contributed less than 10% of the cervical rotations. During the axial rotation of the neck, C1-2 contributed 73.2±17.3% of the cervical rotation, but each of other segments contributed less than 10% of the cervical rotation. During both lateral bending and axial torsion neck motions, regardless of primary or coupled motions, the axial torsion ROM of C1-2 was significantly greater than its lateral bending ROM (p<0.001), whereas the opposite differences were consistently observed at subaxial segments. This study reveals that there are distinct motion patterns at upper and lower cervical segments during in vivo neck motions. The reported data could be useful for the development of new diagnosis methods of cervical pathologies and new surgical techniques that aim to restore normal cervical segmental motion.
Keywords: Occipito-atlantal cervical segment, Atlanto-axial cervical segment, Subaxial cervical segments, Intervertebral range of motion, In vivo neck motions, Cervical spine, Spinal kinematics
1. Introduction
The human cervical spine comprises three markedly different intervertebral anatomies, located at the occipito-atlantal (C0-1) segment, the atlanto-axial (C1-2) segment, and subaxial (C2-T1) segments, respectively (Middleditch and Oliver, 2005). The spinal pathologies are distinctly different among these segments (Dickman et al., 1991; Malcolm, 2002). For example, basilar invagination and dislocations are often related to the instability of the occipito-atlanto-axial cervical region (Goel, 2009; Lu et al., 2009; Yang et al., 2014), and disc degeneration at subaxial segments is a major cause of neck pain (Friedenberg and Miller, 1963; Kelly et al., 2012). Accurate knowledge of the intervertebral motions of the entire cervical spine is critical for the evaluation of spinal disorders induced by trauma or degenerative spinal diseases at single or multiple segments (Passias et al., 2011), and for minimization of iatrogenic alterations at the adjacent segments to reduce the risk of secondary surgical interventions (Chung et al., 2014; Hilibrand and Robbins, 2004). Such information can also be useful for planning rehabilitation regimens for patients after conservative/surgical treatments of spinal pathologies (MacWilliams et al., 2014).
In vivo neck / cervical segment motion of asymptomatic subjects has been extensively investigated using various techniques, such as skin marker-based motion analysis (Henmi et al., 2006; Wu et al., 2007), computed tomography (CT)- / magnetic resonance imaging (MRI)-based static imaging (Ishii et al., 2006, 2004; Kang et al., 2019; Zhai et al., 2019), as well as single-/bi-planar X-ray/fluoroscopic imaging (Anderst et al., 2017, 2015, 2013; Iai et al., 1993; Lin et al., 2014; Yu et al., 2019). Most previously reported studies focused on the intervertebral kinematics of the subaxial cervical spine, and showed that each segment has specific motion characteristics (Lin et al., 2014; Yu et al., 2019). However, only a few in vivo studies involved the evaluation of the intervertebral ROMs at upper cervical segments (C0-1 and C1-2) using conventional CT/MRI (Ishii et al., 2006, 2004; Kang et al., 2019; Zhai et al., 2019) and using biplanar X-ray/fluoroscopic imaging (Anderst et al., 2017, 2015; Iai et al., 1993) in one or multiple neck motions. Therefore, for the normal cervical spine, it is generally known that an upper cervical segment (C0-1/C1-2) allows a different ROM from that of a lower cervical segment (C2-T1) in certain neck motions (Anderst et al., 2015; Iai et al., 1993; Kang et al., 2019). A holistic investigation of the physiological segment ROM distribution across the entire cervical spine (throughout C0 to T1) during various in vivo neck motions has not been reported in the literature.
The objective of this study was to determine the intervertebral ROMs at each cervical segment from C0 to T1 between two maximal positions captured quasi-statically during in vivo flexion-extension (FE), left-right lateral bending (LB), and left-right axial torsion (AT) of the neck, using previously validated dual fluoroscopic (DF) imaging and model registration techniques (Mao et al., 2016; Wang et al., 2008). Because the adjacent vertebrae at the C0-1 and C1-2 segments articulate through cartilaginous interface joints without intervertebral discs (Middleditch and Oliver, 2005), it was hypothesized that the ROMs of these two segments differ from those of the subaxial cervical segments.
2. Methods
2.1. Collection of image data
The study was approved by the institutional review board (protocol number: K-2017-008) at Tongji Hospital in Shanghai, China. Ten asymptomatic subjects without prior diagnosed spinal disorders were recruited. Each signed an informed consent form before participating in the study. The skull (C0) and the entire cervical spine (C1-T1) of each subject were scanned using a CT scanner (SOMATOM Definition AS+, Siemens, Forchheim, Germany) in a supine position. The acquired CT images of each subject had a slice thickness of 0.60 mm, an image resolution of 512 pixels × 512 pixels, and a voxel size of 0.86 mm × 0.86 mm × 0.60 mm. After CT imaging, two subjects were excluded, with one subject suffering from congenital block vertebrae causing fusion at the C2-3 segment and one with CT images not qualified for model construction due to the loss of image data in internet transmission. Therefore, eight asymptomatic female subjects (mean age: 33.4±5.7 years; age range: 28~46 years; mean weight: 52.7±7.3 kg; mean height: 1.56±0.06 m; mean body mass index: 21.9±3.4 kg/m2; smoking status: none) were tested and analyzed in the subsequent data processing. Detailed demographic information was presented in Table 1.
Table 1.
The demographic information of all the eight female asymptomatic subjects in this study (BMI: body mass index; SD: standard deviation).
| Subject # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean ± SD |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 33 | 34 | 46 | 29 | 28 | 34 | 34 | 29 | 33.4 ± 5.7 |
| Weight (kg) | 46.8 | 55.5 | 64 | 45.5 | 56.6 | 42.2 | 58.2 | 52.7 | 52.7 ± 7.3 |
| Height (m) | 1.62 | 1.55 | 1.52 | 1.60 | 1.51 | 1.45 | 1.64 | 1.56 | 1.56 ± 0.06 |
| BMI (kg/m2) | 17.8 | 23.2 | 27.7 | 17.8 | 24.8 | 20.2 | 21.6 | 21.7 | 21.9 ± 3.4 |
| Smoking | No | No | No | No | No | No | No | No | N/A |
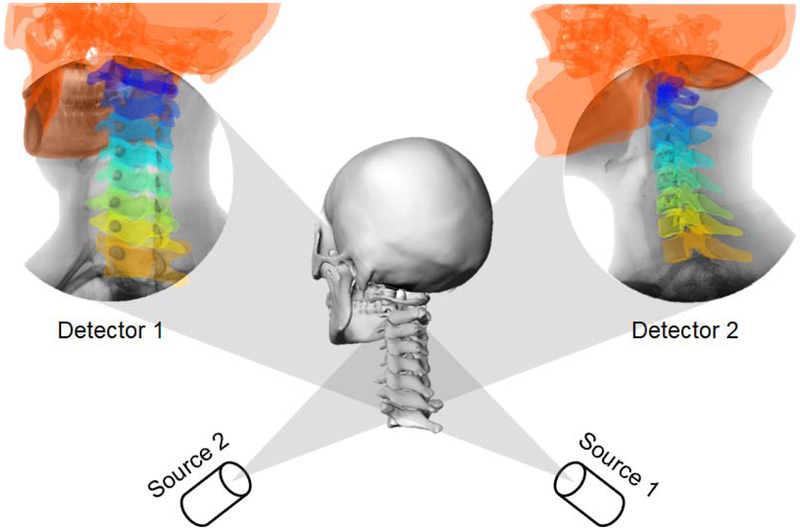
Following the CT scanning, each subject was asked to perform three functional neck motions, including maximal FE, left-right LB, and left-right AT, in their comfortable manners while they stood upright. That is, we did not attempt to impose any specific requirement on the cervical motion performed by the subjects. The cervical spine (C0-T1) of each subject was imaged in each neck position using a DF imaging system according to previously reported protocols (Wang et al., 2008). Two fluoroscopes (BV Pulsera, Philips, Amsterdam, Netherlands) were positioned with their image intensifiers perpendicular to each other. Subjects’ maximal neck postures during each neck motion were quasi-statically captured at a frequency of 30 frames per second with a pulse-width of 8 mili-seconds. The high acquisition frequency of the fluoroscopes allowed us to capture neck maximal postures held by subjects no more than one second. It was ensured that all cervical vertebrae and at least the occiput of the skull could be visualized within the field-of-view of both image intensifiers; if any component (vertebra/skull) completely disappeared on an intensifier, we asked the subject to change the standing location until all components could be viewed on both intensifiers. The total exposure dose of CT and DF imaging for each subject was estimated to be 4 mSv, approximately 20% of the average dose limit for occupational exposure in a year (Wrixon, 2008).
2.2. Processing of image data
The CT images were segmented in Amira 6 (Thermo Fisher Scientific, Waltham, MA) to create three-dimensional (3D) anatomical bone models of the skull and cervical spine using a protocol established in our laboratory (Mao et al., 2016; Wang et al., 2008). These 3D skull and vertebral models were imported into a virtual DF imaging environment constructed in MATLAB R2012a (MathWorks, Inc., Natick, MA) that replicated the actual experimental setup (Tsai et al., 2013; Wang et al., 2008). The quality of DF images in this study has been enhanced to enable the 2D-3D registration of the skull and upper cervical vertebrae. As illustrated in Fig. 1, each 3D model was independently translated and rotated in six degrees of freedom (6DOFs), until their projections matched the osseous outlines/features captured on the orthogonal DF images. The resulting 3D models, therefore, represented the in vivo positions/orientations of vertebrae in 3D space during various neck motions, as shown in Fig. 2.
Fig. 1.
Illustration of a C0-T1 cervical spine 3D model, which was registered to the dual fluoroscopic images captured in the neutral position.
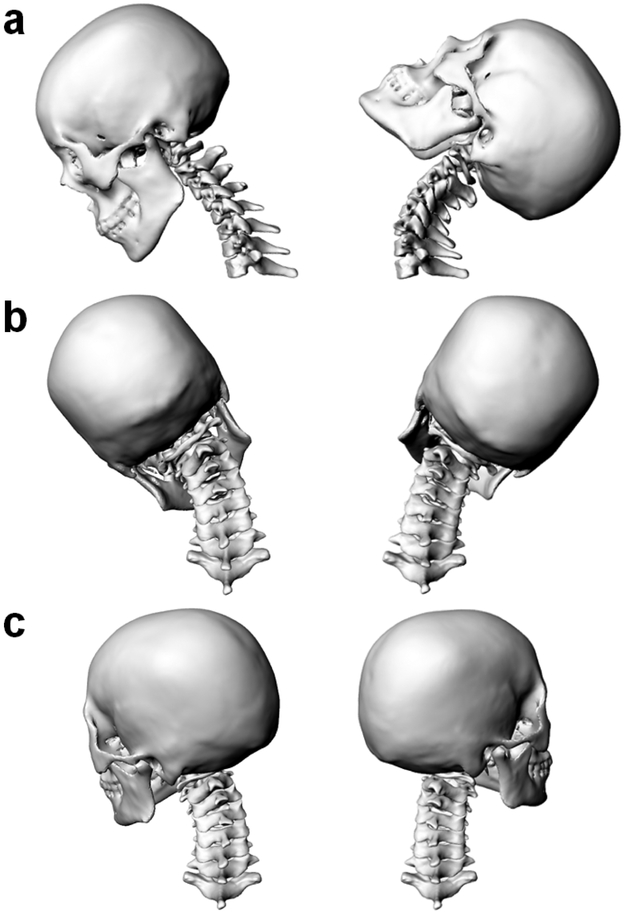
Fig. 2.
Registered 3D C0-T1 cervical spine models of a subject in different static positions. (a) flexion (left) and extension (right); (b) left (left) and right (right) lateral bending; (c) left (left) and right (right) axial torsion.
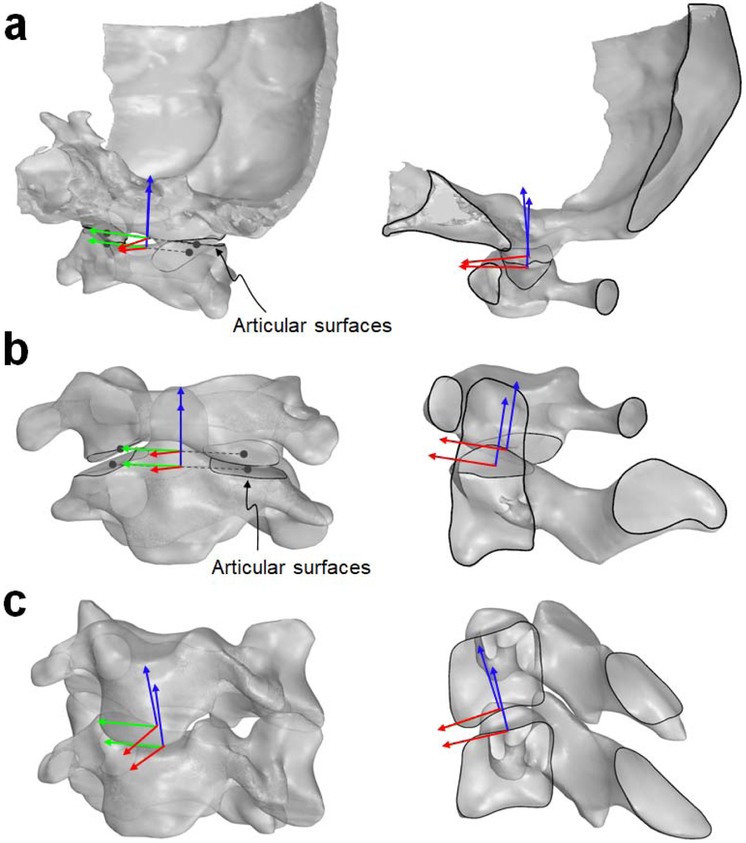
To measure 6DOF intervertebral rotations and translations in each cervical spine position, local coordinate systems (CSs) were established at the superior and inferior intervertebral joint articulations or disc endplates using the CT-derived 3D vertebral models in Rhinoceros 5.0 (Robert McNeel & Associates, Seattle, WA). Subsequently, these local CSs were mapped to the registered 3D vertebral models in different neck positions (Fig. 2). For the C0-1 and C1-2 intervertebral articulations (Fig. 3a,b), new definitions of the local CSs based on anatomic landmarks were introduced. The origins of the C0, C1, and C2 CSs were located at the middle point of the line connecting the centroids of the left and right articular surfaces. Within the sagittal plane of the occiput, the superior direction of the C0 CS was perpendicular to the foramen magnum. Within the sagittal planes of C1 and C2 vertebrae, their superior directions were parallel to the C1 articular surface of the anterior arch and to the pivot axis of the C2 odontoid process, respectively. Compared to the CS definitions of the upper cervical segments adopted in previous studies (Anderst et al., 2017; Ishii et al., 2006, 2004; Kang et al., 2019), our definition resulted in different locations of the coordinate origins, but the orientations of the coordinate axes were consistent. For the subaxial segments (Fig. 3c), a well-established definition of the local CSs was adopted from previous works of our group (Mao et al., 2016; Yu et al., 2019). The origins of the CSs were defined at the centroids of the superior and inferior disc footprints. Within the sagittal plane of each subaxial vertebra, the anterior direction was tangent to the convex disc endplate profile.
Fig. 3.
The definitions of intervertebral local coordinate systems for the C0-1 segment (a), the C1-2 segment (b) and subaxial segments (c) graphically described in the oblique views (left) and sagittal cross-section views (right). For a better visualization, the occiput separated from the skull was shown in (a). Without loss of generality, only the C3-4 segment, representing a subaxial segment, was presented in (c).
This study focused on the investigation of maximal primary and coupled rotational ranges of all cervical segments during three typical motions of the neck, i.e., FE, LB, and AT rotations, as shown in Fig. 2. Besides the rotational ROMs, we also measured the ROMs of coupled anterior-posterior, left-right, and superior-inferior translations during each neck motion, and presented these data in Appendix A. According to a previously reported validation study (Wang et al., 2008), the accuracy of DF registration in measuring cadaveric ovine vertebrae was 0.4 mm. The repeatability in reproducing in vivo 6DOF kinematics of human lumbar vertebrae using the DF technique was 0.3 mm in translations and 0.7° in orientations (Wang et al., 2008). It is should be noted that the accuracy and repeatability of in vivo DF measurements depend on the resolution of DF images (Bingham and Li, 2006).
A repeated-measures analysis of variance (ANOVA) with Tukey post hoc tests was conducted using the R 3.5.1 software (http://www.r-project.org) to compare intervertebral ROMs in each of the 6DOFs between any two segments in FE, LB, and AT tasks, and compare any two rotational/translational ROMs at each segment in FE, LB, and AT tasks. A statistical significance was determined when the p-value is less than 0.05. The results of the statistical analysis can be found in Appendix A.
3. Results
3.1. Cervical intervertebral ROMs in flexion-extension neck motion
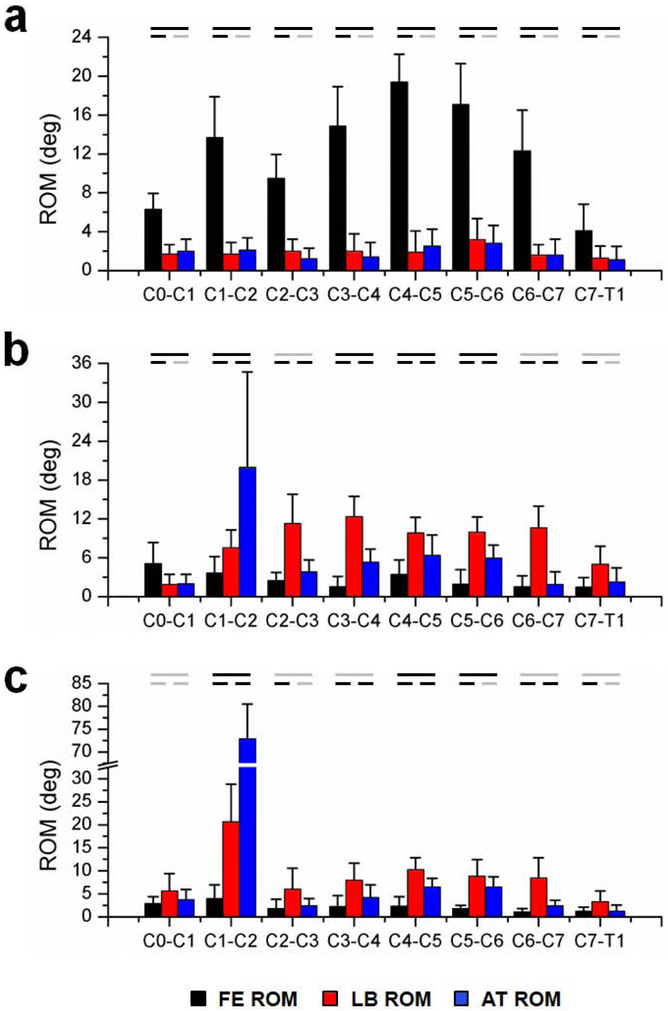
In FE neck motion (Table 2), the overall FE ROM measured between C0 and T1 was 94.5±13.8°. The primary intervertebral FE ROMs were 6.3±1.6° at C0-1, 13.7±4.2° at C1-2, and 4.1±2.7° at C7-T1. Correspondingly, they contributed 6.6±1.4%, 14.5±4.4%, and 4.5±2.9% of the overall FE ROM, respectively. The FE ROM of C0-1 was similar to that of C7-T1 (p=0.113>0.05), and both were significantly smaller than those of all other segments (p<0.05). The FE ROM of C1-2 was significantly smaller than that of C4-5 (19.4±2.9°, p=0.023) and significantly greater than that of C2-3 (9.5±2.4°, p=0.006) and C7-T1 (p=0.001), but no significant difference existed compared to other segments (p>0.05). For subaxial segments, each contributed more than 10% of the overall cervical FE ROM expect for C7-T1; the maximal rotation was 19.4±2.9° occurring at C4-5.
Table 2.
Comparison of the primary intervertebral rotational ROMs (º) at each cervical segment of the entire cervical spine (C0-T1) in FE, LB and AT tasks (mean ± standard deviation values are presented).
| Primary FE ROM | Primary LB ROM | Primary AT ROM | |
|---|---|---|---|
| C0-C1 (a) | 6.3 ± 1.6 b-g | 1.9 ± 1.5 b-h | 3.7 ± 2.2 b,e,f |
| C1-C2 (b) | 13.7 ± 4.2 a,c,e,h | 7.6 ± 2.7 a,d | 72.9 ± 7.6 a,c-h |
| C2-C3 (c) | 9.5 ± 2.4 a,b,d-f,h | 11.3 ± 4.5 a,h | 2.4 ± 1.6 b,e,f |
| C3-C4 (d) | 14.9 ± 4.0 a,c,e,h | 12.4 ± 3.1 a,b,h | 4.2 ± 2.7 b |
| C4-C5 (e) | 19.4 ± 2.9 a-d,g,h | 9.8 ± 2.4 a,h | 6.5 ± 1.9 a-c,g,h |
| C5-C6 (f) | 17.1 ± 4.2 a,c,g,h | 10.0 ± 2.3 a,h | 6.5 ± 2.1 a-c,g,h |
| C6-C7 (g) | 12.3 ± 4.2 a,e,f,h | 10.6 ± 3.3 a,h | 2.4 ± 1.2 b,e,f |
| C7-T1 (h) | 4.1 ± 2.7 b-g | 5.0 ± 2.8 a,c-g | 1.2 ± 1.4 b,e,f |
| Overall (C0-T1) | 94.5 ± 13.8 | 60.5 ± 14.4 | 103.1 ± 20.1 |
Note:
The letters (“a – h”) represent the eight cervical segments from proximal to distal, respectively. The letter superscripts of the primary rotational ROM at a segment indicate that there are the significant differences (p < 0.05) when comparing to the ROMs of the segments represented by these letters.
As shown in Fig. 4a, the coupled LB and AT rotations were significantly smaller than the primary FE rotation at each cervical segment (p<0.05). There were no significant differences between the coupled LB and AT rotations at all cervical segments (p>0.05).
Fig. 4.
The primary/coupled FE, LB, and AT intervertebral rotational ROMs of each cervical segment in FE (a), LB (b), and AT (c) neck tasks. Error bars represent one standard deviation. The black lines indicate that there are significant differences (p < 0.05) between two rotational ROMs at each segment (the gray lines indicate no significant differences).
3.2. Cervical intervertebral ROMs in lateral bending neck motion
In LB neck motion (Table 2), the overall LB ROM measured between C0 and T1 was 60.5±14.4° The primary LB intervertebral ROMs were 1.9±1.5° at C0-1, 7.6±2.7° at C1-2, and 5.0±2.8° at C7-T1, which contributed 2.9±1.8%, 13.2±6.3%, and 8.1±3.8% of the overall LB ROM, respectively. The LB ROMs of C0-1 were significantly smaller than all other segments (p<0.05). The LB ROM of C1-2 was similar to those of the other subaxial segments (p>0.05), and only significantly smaller than that of C3-4 (12.4±3.1°, p=0.041). For subaxial segments, all segments had similar ROMs (p>0.05), except for C7-T1 whose ROM was significantly smaller than others (p<0.05).
As shown in Fig. 4b, the primary LB ROMs were significantly greater than the coupled FE ROMs throughout all segments (p<0.05), except for C0-1 where the opposite significant difference existed (p=0.032). On average, the coupled AT ROM (2.0±1.5°) of C0-1 was almost equal to its primary LB ROM (1.9±1.5°). The primary LB ROM (7.6±2.7°) of C1-2 was significantly smaller than its coupled AT ROM (20.0±14.7°, p=0.037). In contrast, the primary LB ROMs of subaxial segments were consistently larger than their coupled AT ROMs, although there was no significant difference for C7-T1 (p=0.125>0.05).
3.3. Cervical intervertebral ROMs in axial torsion neck motion
In AT neck motion (Table 2), the overall AT ROM measured between C0 and T1 was 103.1±20.1°. The primary AT intervertebral ROMs were 3.7±2.2° at C0-1,72.9±7.6° at C1-2, and 1.2±1.4° at C7-T1, which contributed 3.6±2.0%, 73.2±17.3% and 1.2±1.3% of the overall AT ROM, respectively. The AT ROM of C1-2 was approximately ten-folds as much as the maximum ROMs of all other segments (p<0.001), occurring at C4-5 and at C5-6. On average, the AT ROMs of C4-5 (6.5±1.9°) and C5-6 (6.5±2.1°) were almost identical; both were significantly greater than the AT ROMs of C0-1 and other subaxial segments (p<0.05), except for C3-4 (p>0.05).
As shown in Fig. 4c, the AT ROM of C0-1 was not significantly different from its FE and LB ROMs (p>0.05). At C1-2, the AT neck task caused a large coupled LB ROM (21.3±10.1°). For C0-1 and subaxial segments, it was noted that their coupled LB ROMs were always larger than their primary AT ROMs, despite the significances only found for C3-4, C4-5, and C6-7 (p<0.05). Except for C0-1, the coupled FE ROMs were significantly lower than the coupled LB ROMs at all segments (p<0.05).
4. Discussion
In this study, the intervertebral ROMs of C0-1, C1-2, and subaxial cervical segments during in vivo maximal FE, LB, and AT neck motions were investigated using a combined CT and DF imaging technique. Our measurements indicated that during the three neck motions, both C0-1 and C7-T1 contributed much less rotation (< 10%) to the overall cervical rotation compared to other segments. During the axial rotation of the neck, all segments similarly contributed less than 10% of the overall cervical rotation, except for C1-2, which contributed 73.2±17.3% of the overall cervical rotation. During both LB and AT neck motions, regardless of primary or coupled motions, the AT ROM of C1-2 was significantly greater than its LB ROM (p<0.001), whereas the opposite differences were observed at subaxial segments. These data partially support our hypothesis that there are unique intervertebral motion behaviors at C0-1 and C1-2 during various neck motions.
Most previous studies on cervical spine kinematics focused on intervertebral motion of only upper or lower cervical segments during specific in vivo neck motions (Tables 3-5). For FE neck motion (Table 3), Anderst et al. (2015) and Yu et al. (2019) indicated that the maximal ROMs occurred at C4-5 or C5-6, in agreement with our data. For LB neck motion (Table 4), our data regarding LB ROMs of C3-C7 subaxial segments were only similar to those reported by Lin et al. (2014). For AT neck motion (Table 5), our data only compared favorably with the AT ROMs of C1-2 (3.4°) and C1-2 (72.6°) reported by Ishii et al. (2004). In general, there are variations between our data and others’ data on the ROMs of cervical segments and the overall cervical ROMs in each neck motion. The discrepancies in ROMs with other studies may be caused by the differences in experimental setups/procedures (e.g., how to control neck motion), subjects’ demographics (e.g., gender and age), and DF image resolutions which affect the accuracy of model registration.
Table 3.
Comparison of the primary FE rotational ROMs (º) at each cervical segment in the FE task measured in the current study with those in previously reported in vivo DF measurements of cervical dynamic motion (mean ± standard deviation values are presented). The demographic information in different studies, including gender and age, is also presented (M: males; F: females; y: years).
| Current study | Anderst et al. (2015) | Yu et al. (2019) | |
|---|---|---|---|
| 8 F 33.4 ± 5.7 y 28-46 y |
15M, 14F 27.3 ± 4.4 y 20-35 y |
6 M, 4 F 40.3 ± 10.9 y |
|
| C0-C1 | 6.3 ± 1.6 | ||
| C1-C2 | 13.7 ± 4.2 | 15.6 ± 5.8 | |
| C2-C3 | 9.5 ± 2.4 | 12.7 ± 2.6 | |
| C3-C4 | 14.9 ± 4.0 | 17.1 ± 3.3 | 13.8 ± 4.3 |
| C4-C5 | 19.4 ± 2.9 | 19.5 ± 3.4 | 15.1 ± 5.1 |
| C5-C6 | 17.1 ± 4.2 | 19.7 ± 3.7 | 14.5 ± 7.0 |
| C6-C7 | 12.3 ± 4.2 | 15.8 ± 4.8 | 9.2 ± 4.3 |
| C7-T1 | 4.1 ± 2.7 | 8.3 ± 3.5 | |
| Overall | 94.5 ± 13.8 | 119.0 ± 13.8 |
Note:
The overall ROM reported by Anderst et al. (2015) was measured between the head and torso using an optical track system, different from our measurement using the 2D-3D registration technique.
Table 5.
Comparison of the primary AT rotational ROMs (º) at each cervical segment in the AT task measured in the current study with those in previously reported in vivo X-ray/DF/MRI measurements of cervical dynamic motion (mean ± standard deviation values are presented). The demographic information in different studies, including gender and age, is also presented (M: males; F: females; y: years).
| Current study |
Iai et al. (1993) | Ishii et al. (2004) | Lin et al. (2014) | Anderst et al. (2015) | Anderst et al. (2017) | Yu et al. (2019) | |
|---|---|---|---|---|---|---|---|
| 8 F 33.4 ± 5.7 y 28-46 y |
20 M 27.7 y 27-50 y |
8 M, 7 F 24.3 y 22-31 y |
6 M, 4 F 22.6 ± 2.6 y |
15 M, 14 F 27.3 ± 4.4 y 20-35 y |
13 M, 7 F 28.0 ± 4.2 y 21-35 y |
6 M, 4 F 40.3 ± 10.9 y |
|
| C0-C1 | 3.7 ± 2.2 | 8.0 | 3.4 | ||||
| C1-C2 | 72.9 ± 7.6 | 76.0 | 72.6 | 73.6 (36.8 ± 6.7) | |||
| C2-C3 | 2.4 ± 1.6 | ||||||
| C3-C4 | 4.2 ± 2.7 | 8.4 (4.2 ± 1.3) | 11.8 ± 2.1 | 3.5 ± 1.7 | |||
| C4-C5 | 6.5 ± 1.9 | 9.2 (4.6 ± 1.3) | 11.3 ± 1.7 | 6.9 ± 3.8 | |||
| C5-C6 | 6.5 ± 2.1 | 6.0 (3.0 ± 1.0) | 9.3 ± 1.9 | 9.6 ± 4.1 | |||
| C6-C7 | 2.4 ± 1.2 | 2.6 (1.3 ± 0.8) | 6.5 ± 1.7 | 2.6 ± 2.5 | |||
| C7-T1 | 1.2 ± 1.4 | 3.8 ± 1.2 | |||||
| Overall | 103.1 ± 20.1 | 142.8 ± 13.1 |
Note:
The ROMs in parentheses represent reported one-sided ROMs, which were converted to the two-sided ROMs by multiplying 2, since no significant difference was found in the left and right axial torsion in the corresponding study. The overall ROM reported by Anderst et al. (2015) was measured between the head and torso using an optical track system, different from our measurement using the 2D-3D registration technique.
Table 4.
Comparison of the primary LB rotational ROMs (º) at each cervical segment in the LB task measured in the current study with those in previously reported in vivo DF/MRI measurements of cervical dynamic motion (mean ± standard deviation values are presented). The demographic information in different studies, including gender and age, is also presented (M: males; F: females; y: years).
| Current study | Ishii et al. (2006) | Lin et al. (2014) | Anderst et al. (2015) | |
|---|---|---|---|---|
| 8 F 33.4 ± 5.7 y 28-46 y |
6 M, 6 F 23.6 y 20-30 y |
6 M, 4 F 22.6 ± 2.6 y |
15M, 14F 27.3 ± 4.4 y 20-35 y |
|
| C0-C1 | 1.9 ± 1.5 | 3.8 (1.9 ± 0.9) | ||
| C1-C2 | 7.6 ± 2.7 | 3.2 (1.6 ± 1.3) | ||
| C2-C3 | 11.3 ± 4.5 | 7.4 (3.7 ± 2.0) | ||
| C3-C4 | 12.4 ± 3.1 | 7.0 (3.5 ± 1.4) | 12.8 (6.4 ± 2.3) | 14.3 ± 2.8 |
| C4-C5 | 9.8 ± 2.4 | 6.6 (3.3 ± 1.0) | 10.4 (5.2 ± 1.4) | 13.1 ± 3.2 |
| C5-C6 | 10.0 ± 2.3 | 8.6 (4.3 ± 1.4) | 12.2 (6.1 ± 1.8) | 12.3 ± 3.2 |
| C6-C7 | 10.6 ± 3.3 | 11.4 (5.7 ± 1.9) | 12.2 (6.1 ± 1.8) | 14.5 ± 3.9 |
| C7-T1 | 5.0 ± 2.8 | 8.2 (4.1 ± 2.7) | 5.6 ± 2.4 | |
| Overall | 60.5 ± 14.4 | 83.9 ± 14.5 |
Note:
The ROMs in parentheses represent reported one-sided ROMs, which were converted to the two-sided ROMs by multiplying 2, since no significant difference was found in the left and right lateral bending in the corresponding study. The overall ROM reported by Anderst et al. (2015) was measured between the head and torso using an optical track system, different from our measurement using the 2D-3D registration technique.
In this study, we measured the ROMs of all cervical segments from a sample of asymptomatic subjects, which enabled us to conduct a consistent comparison of intervertebral motion patterns of the entire cervical spine. Compared to subaxial segments, we found the distinct kinematic characteristics of C0-1 and C1-2, which can be attributed to their unique anatomies. For C0-1 (Fig. 3a), there is a pair of bilateral condyloid joints in which the C0 inferior oval-shaped articular processes fit into the C1 superior articular hollows (Middleditch and Oliver, 2005). The C0-1 articulation anatomy favors FE motion but constrains LB and AT motions. However, our data showed that the primary FE ROM at C0-1 was only 6.3±1.6° during in vivo FE neck motion, in contrast to the allowable FE ROM of approximately 24.5° at C0-1 measured in vitro (Panjabi et al., 1988); it implies that soft tissues (ligaments and muscles) surrounding the upper cervical spine may provide additional constraints during neck FE. For C1-2 (Fig. 3b), the median pivot joint formed by the anterior atlas arch and the front odontoid process allows a large range of axial rotation (Middleditch and Oliver, 2005). Correspondingly, the AT ROM of C1-2 accounts for 73.2±17.3% of the overall C0-T1 ROM in AT neck motion, as measured in this study. The bilateral arthrodial joints of C1-2 are composed of a relatively flat C1 inferior articular surfaces and saddle-shaped C2 superior articular surfaces (Middleditch and Oliver, 2005). Hence, the AT of C1 relative to C2 may facilitate further LB of C1 relative to C2. This behavior should be distinguished from that in AT, where a large coupled contralateral bending occurring at C1-2 compensates for coupled ipsilateral bending generated by all subaxial segments, in order to maintain the horizontal head position (Ishii et al., 2004).
Our data on cervical intervertebral ROMs offer compelling clinical implications into the neck injury mechanisms and the etiologies of neck pathologies. For example, a majority of head/neck injuries during football sports, have been observed during the lateral impact of the head (Banerjee et al., 2004). The ROM of the neck in LB is approximately a half of that in neck FE or AT, and C0-1, C1-2, and C7-T1 are the least mobile segments during the neck LB. Consequently, the head/neck may be less tolerable to lateral impact loads, with C0-1, C1-2, and C1-T1 more vulnerable to injury than other segments. In the case of the stiff neck symptom, neck mobility (primarily in AT) is substantially reduced, but there has been no consensus about the etiology. As a normal C1-2 segment can provide more than 70% of the neck AT, a stiff neck may be associated with a significant limitation to the C1-2 rotation, to mitigate the impingement against inflamed surrounding soft tissues (Gubin et al., 2009). However, it should be acknowledged that the association of injuries/diseases with the allowable neck/segment ROMs requires further investigations by collecting the injury/disease patterns from a large cohort of patients.
The selection of spinal fusion or total disc replacement (TDR) to treat spinal disorders at a subaxial cervical segment markedly influences postoperative performance, including radiological parameters, spinal kinematics, and adjacent segment degeneration (Daniels et al., 2012; Li et al., 2018; Zechmeister et al., 2011). Our data showed that during all functional neck motions, C7-T1 allows the smallest ROMs, indicating that C7-T1 could be more suitable for the fusion treatment; the segments above C7-T1 may favor TDR treatments. Recently, hybrid surgery has been thought to be a promising solution to multi-segment cervical diseases (Grasso, 2015; Hung et al., 2018; Zang et al., 2015). Clinical studies generally suggested that hybrid surgeries, in which the fusions were commonly performed near C7-T1, can be comparable with and even superior to multi-segment fusions or TDRs (Grasso, 2015; Hung et al., 2018; Zang et al., 2015), consistent with the implication of this study.
Several limitations in this study should be addressed in future investigations. Only eight female participants’ image data were available to analyze cervical intervertebral ROMs. It have been demonstrated that cervical spine / segment ROMs are associated with gender (Trott et al., 1996), age (Simpson et al., 2008), or both simultaneously (Dvorak et al., 1991; Lind et al., 1989). To address this limitation requires a large cohort of subjects in future work. Consequently, it could be insufficient to perform clinical diagnoses and provide patient-specific treatment recommendations only based on the data of this study. Furthermore, this study focused on the maximal intervertebral ROMs during various in vivo neck motions, whereas the dynamic motion characteristics of C0-1 and C1-2 were not evaluated. Future work should investigate the dynamic intervertebral motion of upper segments and their dynamic contributions to the motion of the entire cervical spine.
In conclusion, we determined the intervertebral ROMs of the cervical spine from C0 to T1 during in vivo maximal FE, LB, and AT neck motions using a DF imaging system. For the eight asymptomatic female subjects tested in this study, we demonstrated that the C0-1 and C1-2 featured distinct motion characteristics during these neck motions. Future work should further evaluate the effects of gender and age on neck motion using a larger cohort of subjects. The reported cervical motion measurements in this study could be used to improve the understanding of the cervical function, benchmark post-operative spinal kinematics, and guide patients’ rehabilitation to prevent dangerous activity.
Supplementary Material
Acknowledgements
This study was sponsored by National Institutes of Health (1R03AG056897), Shanghai Pujiang Program (17PJ1405000), the National Natural Science Foundation of China (31771017), and Shanghai Jiao Tong University (YG2017MS09, ZH2018QNA06, ZXGF082101/050). The funding sources had no role in the study design, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Appendix A: Cervical Intervertebral Range of Motion Data and Statistical Analysis Results
References
- Anderst W, Rynearson B, West T, Donaldson W, Lee J, 2017. Dynamic in vivo 3D atlantoaxial spine kinematics during upright rotation. J. Biomech 60, 110–115. 10.1016/j.jbiomech.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Anderst WJ, Donaldson WF, Lee JY, Kang JD, 2015. Three-dimensional intervertebral kinematics in the healthy young adult cervical spine during dynamic functional loading. J. Biomech 48, 1286–1293. 10.1016/j.jbiomech.2015.02.049 [DOI] [PubMed] [Google Scholar]
- Anderst WJ, Donaldson WF, Lee JY, Kang JD, 2013. Cervical motion segment percent contributions to flexion-extension during continuous functional movement in control subjects and arthrodesis patients. Spine (Phila. Pa. 1976) 38, 533–539. 10.1097/BRS.0b013e318289378d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Palumbo MA, Fadale PD, 2004. Catastrophic cervical spine injuries in the collision sport athlete, part 1: Epidemiology, functional anatomy, and diagnosis. Am. J. Sports Med 32, 1077–1087. 10.1177/0363546504265605 [DOI] [PubMed] [Google Scholar]
- Bingham J, Li G, 2006. An optimized image matching method for determining in-vivo TKA kinematics with a dual-orthogonal fluoroscopic imaging system. J. Biomech. Eng 128, 588–595. 10.1115/1.2205865 [DOI] [PubMed] [Google Scholar]
- Chung J-Y, Kim S-K, Jung S-T, Lee K-B, 2014. Clinical adjacent-segment pathology after anterior cervical discectomy and fusion: results after a minimum of 10-year follow-up. Spine J. 14, 2290–2298. 10.1016/j.spinee.2014.01.027 [DOI] [PubMed] [Google Scholar]
- Daniels AH, Paller DJ, Feller RJ, Thakur NA, Biercevicz AM, Palumbo MA, Crisco JJ, Madom IA, 2012. Examination of cervical spine kinematics in complex, multiplanar motions after anterior cervical discectomy and fusion and total disc replacement. Int. J. Spine Surg 6, 190–194. 10.1016/j.ijsp.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman CA, Mamourian A, Sonntag VKH, Drayer BP, 1991. Magnetic resonance imaging of the transverse atlantal ligament for the evaluation of atlantoaxial instability. J. Neurosurg 75, 221–227. 10.3171/jns.1991.75.2.0221 [DOI] [PubMed] [Google Scholar]
- Dvorak J, Panjabi MM, Novotny JE, Antinnes JA, 1991. In vivo flexion/extension of the normal cervical spine. J. Orthop. Res 9,828–834. 10.1002/jor.1100090608 [DOI] [PubMed] [Google Scholar]
- Friedenberg ZB, Miller WT, 1963. Degenerative Disc Disease of the Cervical Spine: A Comparative Study of Asymptomatic and Symptomatic Patients. J. Bone Joint Surg. Am 45, 1171–8. [PubMed] [Google Scholar]
- Goel A, 2009. Treatment of basilar invagination by atlantoaxial joint distraction and direct lateral mass fixation. J. Neurosurg. Spine 1, 281–286. 10.3171/spi.2004.1.3.0281 [DOI] [PubMed] [Google Scholar]
- Grasso G, 2015. Clinical and radiological features of hybrid surgery in multilevel cervical degenerative disc disease. Eur. Spine J 24, 842–848. 10.1007/s00586-015-4281-7 [DOI] [PubMed] [Google Scholar]
- Gubin AV, Ulrich EV, Taschilkin AI, Yalfimov AN, 2009. Etiology of child acute stiff neck. Spine (Phila. Pa. 1976). 34, 1906–1909. 10.1097/BRS.0b013e3181abbf3d [DOI] [PubMed] [Google Scholar]
- Henmi S, Yonenobu K, Masatomi T, Oda K, 2006. A biomechanical study of activities of daily living using neck and upper limbs with an optical three-dimensional motion analysis system. Mod. Rheumatol 16, 289–293. 10.1007/s10165-006-0499-x [DOI] [PubMed] [Google Scholar]
- Hilibrand AS, Robbins M, 2004. Adjacent segment degeneration and adjacent segment disease: The consequences of spinal fusion? Spine J. 4, 190–194. 10.1016/j.spinee.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Hung C-W, Wu M-F, Yu G-F, Ko C-C, Kao C-H, 2018. Comparison of sagittal parameters for anterior cervical discectomy and fusion, hybrid surgery, and total disc replacement for three levels of cervical spondylosis. Clin. Neurol. Neurosurg 168, 140–146. 10.1016/j.clineuro.2018.03.003 [DOI] [PubMed] [Google Scholar]
- lai H, Moriya H, Goto S, Takahashi K, Yamagata M, Tamaki T, 1993. Three-dimensional motion analysis of the upper cervical spine during axial rotation. Spine (Phila. Pa. 1976) 18, 2388–92. [DOI] [PubMed] [Google Scholar]
- Ishii T, Mukai Y, Hosono N, Sakaura H, Fujii R, Nakajima Y, Tamura S, Iwasaki M, Yoshikawa H, Sugamoto K, 2006. Kinematics of the cervical spine in lateral bending: in vivo three-dimensional analysis. Spine (Phila. Pa. 1976) 31, 155–60. [DOI] [PubMed] [Google Scholar]
- Ishii T, Mukai Y, Hosono N, Sakaura H, Nakajima Y, Sato Y, Sugamoto K, Yoshikawa H, 2004. Kinematics of the Upper Cervical Spine in Rotation. Spine (Phila. Pa. 1976) 29, E139–E144. 10.1097/01.brs.0000116998.55056.3c [DOI] [PubMed] [Google Scholar]
- Kang J, Chen G, Zhai X, He X, 2019. In vivo three-dimensional kinematics of the cervical spine during maximal active head rotation. PLoS One 14, 1–16. 10.1371/journal.pone.0215357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JC, Groarke PJ, Butler JS, Poynton AR, O’Byrne JM, 2012. The Natural History and Clinical Syndromes of Degenerative Cervical Spondylosis. Adv. Orthop 2012, 1–5. 10.1155/2012/393642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wu H, Chu J, Liu M, Hou S, Yu S, Hou T, 2018. Motion analysis of dynamic cervical implant stabilization versus anterior discectomy and fusion: a retrospective analysis of 70 cases. Eur. Spine J 27, 2772–2780. 10.1007/s00586-018-5755-1 [DOI] [PubMed] [Google Scholar]
- Lin CC, Lu TW, Wang TM, Hsu CY, Hsu SJ, Shih TF, 2014. In vivo three-dimensional intervertebral kinematics of the subaxial cervical spine during seated axial rotation and lateral bending via a fluoroscopy-to-CT registration approach. J. Biomech 47, 3310–3317. 10.1016/j.jbiomech.2014.08.014 [DOI] [PubMed] [Google Scholar]
- Lind B, Sihlbom H, Nordwall A, Malchau H, 1989. Normal range of motion of the cervical spine. Arch. Phys. Med. Rehabil 70, 692–695. [PubMed] [Google Scholar]
- Lu B, He XJ, Zhao CG, Li HP, Wang D, 2009. Artificial atlanto-odontoid joint replacement through a transoral approach. Eur. Spine J 18,109–117. 10.1007/s00586-008-0835-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacWilliams BA, Rozumalski A, Swanson AN, Wervey R, Dykes DC, Novacheck TF, Schwartz MH, 2014. Three-dimensional lumbar spine vertebral motion during running using indwelling bone pins. Spine (Phila. Pa. 1976) 39, E1560–E1565. 10.1097/BRS.0000000000000646 [DOI] [PubMed] [Google Scholar]
- Malcolm GP, 2002. Surgical disorders of the cervical spine: presentation and management of common disorders. J. Neurol. Neurosurg. Psychiatry 73 Suppl 1, i34–41. 10.1136/JNNP.73.SUPPL_1.I34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Driscoll SJ, Li JS, Li G, Wood KB, Cha TD, 2016. Dimensional changes of the neuroforamina in subaxial cervical spine during in vivo dynamic flexion-extension. Spine J. 16, 540–546. 10.1016/j.spinee.2015.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleditch A, Oliver J, 2005. Functional anatomy of the spine, 2nd ed, Elsevier Butterworth-Heinemann. Elsevier Butterworth-Heinemann. [Google Scholar]
- Panjabi M, Dvorak J, Duranceau J, Yamamoto I, Gerber M, Rauschning W, Bueff HU, 1988. Three-dimensional movements of the upper cervical spine. Spine (Phila. Pa. 1976). 13, 726–30. [DOI] [PubMed] [Google Scholar]
- Passias PG, Wang S, Kozanek M, Xia Q, Li W, Grottkau B, Wood KB, Li G, 2011. Segmental lumbar rotation in patients with discogenic low back pain during functional weight-bearing activities. J. Bone Jt. Surg. - Ser. A 93, 29–37. 10.2106/JBJS.I.01348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson AK, Biswas D, Emerson JW, Lawrence BD, Grauer JN, 2008. Quantifying the Effects of Age, Gender, Degeneration, and Adjacent Level Degeneration on Cervical Spine Range of Motion Using Multivariate Analyses. Spine (Phila. Pa. 1976) 33, 183–186. 10.1097/BRS.0b013e31816044e8 [DOI] [PubMed] [Google Scholar]
- Trott PH, Pearcy MJ, Ruston SA, Fulton I, Brien C, 1996. Three-dimensional analysis of active cervical motion: The effect of age and gender. Clin. Biomech 11, 201–206. 10.1016/0268-0033(95)00072-0 [DOI] [PubMed] [Google Scholar]
- Tsai TY, Li JS, Wang S, Lin H, Malchau H, Li G, Rubash H, Kwon YM, 2013. A novel dual fluoroscopic imaging method for determination of THA kinematics: In-vitro and in-vivo study. J. Biomech 46, 1300–1304. 10.1016/j.jbiomech.2013.02.010 [DOI] [PubMed] [Google Scholar]
- Wang S, Passias P, Li Gang, Li Guoan, Wood K, 2008. Measurement of vertebral kinematics using noninvasive image matching method-validation and application. Spine (Phila. Pa. 1976) 33, E355–E361. 10.1097/BRS.0b013e3181715295 [DOI] [PubMed] [Google Scholar]
- Wrixon AD, 2008. New ICRP recommendations. J. Radiol. Prot 28, 161–168. 10.1088/0952-4746/28/2/R02 [DOI] [PubMed] [Google Scholar]
- Wu SK, Lan HHC, Kuo LC, Tsai SW, Chen CL, Su FC, 2007. The feasibility of a video-based motion analysis system in measuring the segmental movements between upper and lower cervical spine. Gait Posture 26, 161–166. 10.1016/j.gaitpost.2006.07.016 [DOI] [PubMed] [Google Scholar]
- Yang SY, Boniello AJ, Poorman CE, Chang AL, Wang S, Passias PG, 2014. A Review of the Diagnosis and Treatment of Atlantoaxial Dislocations. Glob. Spine J 4, 197–210. 10.1055/s-0034-1376371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Li JS, Guo T, Lang Z, Kang JD, Cheng L, Li G, Cha TD, 2019. Normal intervertebral segment rotation of the subaxial cervical spine: An in vivo study of dynamic neck motions. J. Orthop. Transl 1–8. 10.1016/j.jot.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Mao H, Li J-S, Tsai T-Y, Cheng L, Wood KB, Li G, Cha TD, 2017. Ranges of Cervical Intervertebral Disc Deformation During an In Vivo Dynamic Flexion–Extension of the Neck. J. Biomech. Eng 139, 064501 10.1115/1.4036311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang L, Ma M, Hu J, Qiu H, Huang B, Chu T, 2015. Comparison of Hybrid Surgery Incorporating Anterior Cervical Discectomy and Fusion and Artificial Arthroplasty versus Multilevel Fusion for Multilevel Cervical Spondylosis: A Meta-Analysis. Med. Sci. Monit 21, 4057–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmeister I, Winkler R, Mad P, 2011. Artificial total disc replacement versus fusion for the cervical spine: a systematic review. Eur. Spine J 20, 177–184. 10.1007/s00586-010-1583-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai X, Kang J, Chen X, Dong J, Qiu X-W, Ding X-A, Liu J, He X-J, 2019. [In vivo measurement of three-dimensional motion of the upper cervical spine using CT three-dimensional reconstruction]. Zhongguo Gu Shang 32, 658–665. 10.3969/j.issn.1003-0034.2019.07.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.