Abstract
Dramatic impairment of gastrointestinal (GI) function accompanies high-thoracic spinal cord injury (T3-SCI). The vagus nerve contains mechano- and chemosensory fibers as well as the motor fibers necessary for the central nervous system (CNS) control of GI reflexes. Cell bodies for the vagal afferent fibers are located within the nodose ganglion (NG) and the majority of vagal afferent axons are unmyelinated C fibers that are sensitive to capsaicin through activation of transient receptor potential vanilloid-1 (TRPV1) channels. Vagal afferent fibers also express receptors for GI hormones, including cholecystokinin (CCK). Previously, T3-SCI provokes a transient GI inflammatory response as well as a reduction of both gastric emptying and centrally-mediated vagal responses to GI peptides, including CCK. TRPV1 channels and CCK-A receptors (CCKar) expressed in vagal afferents are upregulated in models of visceral inflammation. The present study investigated whether T3-SCI attenuates peripheral vagal afferent sensitivity through plasticity of TRPV1 and CCK receptors.
Vagal afferent response to graded mechanical stimulation of the stomach was significantly attenuated by T3-SCI at 3-day and 3-week recovery. Immunocytochemical labeling for CCKar and TRPV1 demonstrated expression on dissociated gastric-projecting NG neurons. Quantitative assessment of mRNA expression by qRT-PCR revealed significant elevation of CCKar and TRPV1 in the whole NG following T3-SCI in 3-day recovery, but levels returned to normal after 3-weeks. Three days after injury, systemic administration of CCK-8s showed a significantly diminished gastric vagal afferent response in T3-SCI rats compared to control rats while systemic capsaicin infusion revealed a significant elevation of vagal response in T3-SCI vs control rats.
These findings demonstrate that T3-SCI provokes peripheral remodeling and prolonged alterations in the response of vagal afferent fibers to the physiological signals associated with digestion.
Keywords: Gastric emptying, gastrointestinal motility, vago-vagal reflexes, vagal afferents, stomach, cholecystokinin, CCK-1 receptor, CCK-A receptor, capsaicin, TRPV1, neuropathy, neurotrauma
Introduction
Widespread loss of autonomic function is a common consequence of cervical and high thoracic spinal cord injury (SCI). Clinical reports indicate reduced gastric emptying after T3-SCI (Kirshblum et al., 2002; Williams et al., 2011; Wolf and Meiners, 2003) and early satiety that may persist for years after the initial injury (Berlly and Wilmot, 1984; Cosman et al., 1991; Fealey et al., 1984; Kao et al., 1999; Kewalramani, 1979; Nino-Murcia and Friedland, 1991; Rajendran et al., 1992; Segal et al., 1995; Stinneford et al., 1993). We have demonstrated in a rat model of experimental T3-SCI that ad libitum feeding and gastric motility are diminished beginning immediately after injury (Tong and Holmes, 2009) and that chronic derangements in feeding, gastric motility and emptying persist outward to 6–18 weeks (Primeaux et al., 2007; Qualls-Creekmore et al., 2010a; Tong et al., 2009).
Gastric motility is dominated by parasympathetic vagal reflex control (reviewed in Holmes et al., 2017). The vagal afferents originating from the GI tract are composed of two distinct sensory mechanoreceptors to detect both the mechanical distension of the stomach and luminal stimuli. Mechanoreceptors with morphological specializations known as intraganglionic laminar endings (IGLEs; Powley and Phillips, 2002) and intramuscular arrays (IMAs; Berthoud and Powley, 1992) line the gastrointestinal mucosa and smooth muscle and detect both tension (stretch) exerted upon the gut wall as well as stimuli regarding composition and density of the chyme (Page et al., 2002).
One other component of the homeostatic regulation of energy intake is the reflex transmission of chemical feeding-related signals from the gastrointestinal (GI) tract to the central nervous system (CNS). Essentially, afferent fibers lining the gastric wall detect chemical mediators released by specialized cells lining the lumen of the GI tract. For example, the chemical composition of macronutrients as well as other chemicals within the GI lumen triggers the release of a wide range of peptides and classical neurotransmitters from enteroendocrine cells as part of both a paracrine and endocrine signaling cascade (Douglas et al., 1988; Liddle, 1997). These gut-derived factors (e.g., CCK, GLP-1 and ghrelin) are released during feeding or fasting and act peripherally upon receptors expressed on vagal sensory terminals in the gut wall as well as on the vagal afferent cell bodies within the NG (Broberger et al., 2001; Dockray, 2014; Helke and Hill, 1988). Specifically, the regulatory effect of the GI peptide cholecystokinin (CCK) has received the greatest attention and has been shown to modulate the firing rate of vagal afferent fibers (Okano-Matsumoto et al., 2011).
Derangements in these mechano- and chemo-sensory afferent pathways have been implicated in a wide variety of pathophysiological states (Dyavanapalli et al., 2016; Kentish and Page, 2014; Mazzone and Undem, 2016). For example, the molecular mechanism behind the GI response to pungent foods became evident with the identification of the transient receptor potential vanilloid type 1 receptor (TRPV1; Yu et al., 2016). The role of TRPV1 has frequently received attention in association with inflammatory conditions and nociceptive sensation in somatic and visceral tissues (Caterina and Julius, 2001; Jia and Lee, 2007; Lee and Gu, 2009; Zhao and Simasko, 2010; Zhao et al., 2010). In addition to activation by the ingestion of capsaicin, the active compound in pungent foods, TRPV1 is activated by noxious stimuli (Furuta et al., 2012; Nilius et al., 2007a; Nilius et al., 2007b) and TRPV1 upregulation may occur as a consequence of inflammation, including GI inflammation that accompanies SCI (Besecker et al., 2017). Physiological roles of the TRPV channel family, including TRPV1 involvement in mechano- and osmo-sensitivity are emerging (Liedtke and Kim, 2005). Furthermore, a recent report has demonstrated a functional role for TRPV1 in the GI tract. Mice deficient in the TRPV1 receptor also demonstrated attenuated response of mechanoreceptors to stretch (Kentish et al., 2015). Therefore, TRPV1 channels are in a position to modulate the satiety-inducing effects of stretch and anecdotal reports of early satiety by many individuals with SCI suggesting a potential role of TRPV1-mediated sensitivity of mechanoreceptors after injury.
The apparent reduction of GI chemosensory signaling to medullary neural circuits (Besecker et al., 2018; Tong et al., 2011) raises the question of pathophysiological remodeling of vagal afferent fibers following SCI. Using molecular techniques and in vivo electrophysiological recordings of the vagal afferent fibers innervating the stomach, we tested the hypothesis that acute and chronic high-thoracic (spinal level T3) SCI attenuate gastric mechanosensitivity. In order to establish if any previously observed reduction in sensitivity to CCK following SCI could be due to changes in receptor expression, we utilized qRT-PCR to determine the relative expression of CCK-A receptor as well as TRPV1 receptor mRNA in the gastric vagal afferent cell bodies of the NG. Finally, we recorded the afferent neural responses to systemic administration of pharmacological doses of CCK and the TRPV1 agonist, capsaicin to evaluate the functional chemosensitivity of vagal afferent fibers.
Materials and methods
Animals
All studies followed National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the Penn State Hershey College of Medicine. Male Wistar rats ≥8 weeks of age, upon entrance into the experiment, and initially weighing 175–200 g (Harlan, Indianapolis, IN, USA) were used. Animals were initially double housed in a room maintained at 21–24°C and a 12:12-h light-dark cycle with food and water provided ad libitum. After surgery animals were single housed in cages mounted on warming pads in order to assist in maintaining an adequate body temperature of 37°C. All experimental animals were observed daily, and food and body weight were recorded.
Surgical procedures and post-operative care
Animals (n=200) were randomly assigned to the control or SCI surgical group. After initial groups were assigned, the groups were further evenly divided into 3-day and 3-week post-surgical control or post-SCI study groups. Additional 1-day and 7-day groups were generated for qRT-PCR analysis. After being deeply anesthetized with 3–5% mixture of Isoflurane with oxygen (400–600ml/min), surgery for T3-contusion SCI was performed using established surgical techniques as previously described in detail (Swartz EM and Holmes GM, 2014). Briefly, upon completion of surgical exposure of the T2 laminectomy site, the adjacent T1 and T3 vertebrae were clamped into the Infinite Horizons SCI device (Precision Systems and Instrumentation, Lexington KY) and a 300 kDyne impact (15 sec dwell time) was initiated. Surgical controls underwent all procedures except for the spinal impact.
In a subset of rats (n=4/group), gastric projecting NG neurons were specifically identified in 3-day and 3-week T3-SCI or surgical control groups. In 3-day rats, immediately prior to the SCI or control laminectomy procedure, the retrograde tracer 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiI; Invitrogen/Molecular Probes, Waltham, MA) was utilized to label gastric projecting NG neurons. A midline laparotomy was performed to expose the stomach. DiI (3%), dissolved in DMSO, was microinjected at multiple points into the serosal surface of the ventral and dorsal surfaces of the corpus of the stomach (totaling 10 μL/surface). Precautions were taken to prevent the leakage of DiI from the injection sites and the visceral surface was repeatedly cleaned with a cotton swab. The wound margin was closed with absorbable suture (Vicryl 4–0) and wound clips. In the 3-week groups, DiI was injected in a separate procedure 3-days prior to tissue harvest.
Post-operatively, rats were administered supplemental fluids by injection of 5cc warmed lactated Ringer’s solution (s.c.) and stabilized in an incubator (37°C) until fully recovered from anesthesia. Afterward, animals were monitored daily for any signs of infection or complications from surgery and administered post-operative care as previously described (Swartz EM et al., 2014).
Fifteen rats were excluded from final analysis due to A) non-viable nerve recording preparations (n=14) or C); nerve recording data exceeding an outlier test (n=1).
Gastric vagal afferent nerve recording
Three-days or three-weeks following the initial surgery, T3-SCI or control animals were fasted overnight with water provided ad libitum. After being deeply anesthetized with 3–5% Isoflurane with oxygen, animals were prepared for physiological instrumentation. Animals were placed on a feedback-controlled warming pad (TCAT 2LV, Physitemp Instruments, Clifton, New Jersey) and rectal temperature monitored and maintained at 37±1 °C for the duration of the experiment. Once aseptically prepared for surgery, the animal was intubated to maintain an open airway via the trachea.
Following tracheal intubation, the femoral artery and adjacent vein or tributaries were exposed via a small incision of the proximal-medial thigh overlying the quadriceps and adductors and cleared superiorly and proximally to the inguinal ligament. An aseptic PE-50 catheter was inserted towards the abdominal aorta for arterial blood pressure and a PE-50 catheter was inserted in a direction away from the abdominal vena cava for drug administration and the wound margin was closed with wound clips. The rat was slowly weaned from Isoflurane to thiobutabarbitol (Inactin 80–100mg/kg, i.v.) which does not affect cardiovascular (Buelke-Sam et al., 1978) or gastrointestinal (Qualls-Creekmore et al., 2010b) autonomic function. The rate of Inactin infusion was monitored in conjunction with the resulting temporary drop in arterial blood pressure (SYS-BP1, World Precision Instruments, Sarasota, FL). Data from the blood pressure monitor was digitized via an analogue-to-digital converter (1401 Micro3, Cambridge Electronic Design, Cambridge, UK). All data signals were continuously digitized at 2kHz and recorded to computer using Spike2 software (Cambridge Electronic Design, Cambridge, UK). Heart rate values were calculated from the peak-to-peak mean arterial pressure (MAP) by the Spike2 software.
Once full Inactin anesthesia was achieved, a midline upper-abdominal laparotomy was performed to expose the stomach and distal esophagus. Under higher power stereoscopic magnification (45–60X), the subdiaphragmatic left vagus was isolated from the esophageal surface with fine-tipped Dumont forceps by removing the adjacent connective tissue from the nerve and esophageal surface while keeping the area moist with physiological saline. Once isolated, the gastric vagus nerve was then carefully placed on bipolar recording electrodes. The electrodes were connected to a differential amplifier (1700, A-M Systems, Inc., Sequim, WA), and whole-nerve recordings were filtered (low pass, 1–5 kHz; and high pass, 50–100 Hz with a 60-Hz notch filter) and the output signal distributed to the 1400 Micro3 A/D converter, and audio monitor (AM-8, Grass Instruments, Cambridge, MA). Digitized nerve signals were further rectified and integrated by the Spike2 software. Once the vagal nerve signal was verified, the nerve and electrodes were secured to the bipolar electrodes using KwikSil (World Precision Instruments, Sarasota, FL) and the portion of the gastric vagus proximal to the bipolar electrodes was crushed to eliminate recording vagal efferent action potentials. The stomach was carefully returned to the abdominal cavity to sufficiently maintain temperature and prevent desiccation, yet still permit access for mechanical stimulation of the stomach wall.
Baseline recordings were established after the rat had stabilized for at least 60-minutes following surgical procedures. Mechanical responses were determined using calibrated von Frey hairs (0.07g-60g). Stimulus-responses were evoked by static pressure to the gastric corpus and movement across the serosal surface at a rate of 5mm s−1 and the interval between each mechanical stimulus test was a minimum of 5-minutes of recovery. Infusions of PBS, CCK (0.06 and 0.4 μg kg−1, i.v.) or capsaicin (0.05 and 0.5 μg kg−1, i.v.) were separated by a minimum of 20-minute recovery period. Background nerve activity was normalized post-hoc to 100% after noise subtraction from the nerve recordings.
Nodose ganglia isolation
Nodose ganglia were harvested bilaterally from a cohort of animals other than those used for extended nerve recording studies. Following the appropriate time-course (1d, 3d, 1w or 3w), animals were anesthetized with 3–5% Isoflurane until non-responsive to toe pinch and corneal reflex. The rat was immediately placed dorsally and an incision was made in the skin from the superior notch of the sternum towards the midline of the mandible and both carotid arteries were exposed in order to locate the vagus nerve bilaterally. The rat was then rapidly exsanguinated and the nodose were quickly excised and placed in liquid nitrogen-cooled microcentrifuge tubes and stored at −80 degrees Celsius until all had been collected for processing.
Quantitative reverse-transcription polymerase chain reaction
Quantitative RT-PCR reactions were performed as described previously (Besecker et al., 2017). The forward and reverse primer pairs used for these studies were designed using Primer Express (Applied Biosystems) and are shown in Table 1. Quantity of mRNA for the listed primers was based on a standard curve and normalized to β-actin mRNA (ABI QuantStudio 12KFlex with available OpenArray block, Applied Biosystems). Nomenclature is presented according to Rat Genome Nomenclature Committee guidelines (http://www.informatics.jax.org/mgihome/nomen/gene.shtml) along with more common, informal, usage. For example, CCK-1r is a human protein commonly used in human research literature to represent the CCK receptor found in periphery tissue including the alimentary tract (Dufresne et al., 2006); in the rat the homolog is CCKar.
Table 1.
| Forward and reverse primer sequences for quantitative real time PCR (qRT-PCR) | ||
|---|---|---|
| Gene | Forward Primer | Reverse Primer |
| TRPV1 | 5’-CCCAGGCAACTGTGAGGGCG-3’ | 5’-GGCAGGCACAGAGTGGACCC-3’ |
| CCK | 5’-AGCTGAGGGCTGTGCTCCGA-3’ | 5’-TTCGAGGCGAGGGGTCGTGT-3’ |
| CCKar | 5’-TTCTCAGTGTGCTGGGGAAC-3’ | 5’-AGGTTGAAGGTGGAAACGCT-3’ |
Nodose ganglia dissociation
Rats were first anesthetized with CO2 and then quickly euthanized by decapitation. The ganglia were dissected in ice-cold Hank’s balanced salt solution (HBSS). Thereafter, the ganglia were enzymatically dissociated in Earle’s balanced salt solution containing 0.6 mg/ml collagenase (Roche Diagnostics Corp. Indianapolis, IN), 0.4 mg/ml trypsin (Worthington Biochemical Corp. Lakewood, NJ), 0.1 mg/ml DNAase Type I (Sigma, St. Louis, MO) in a shaking water bath at 35°C for 45-min. The neurons were then dispersed by vigorous shaking, centrifuged twice for 6-min at 500 rpm. The dispersed neurons were resuspended in minimal essential medium supplemented with 10% fetal bovine serum (VWR), 1% penicillin-streptomycin (all from Thermo-Fisher Scientific). The dissociated NG neurons were plated on multiple poly-L-lysine coated dishes or coverslips and incubated (5% CO2/95% air) at 37°C overnight until experimentation.
Fluorescence immunocytochemistry
Randomly selected coverslips with dissociated NG neurons were fixed with fresh 4% paraformaldehyde for 10-min then washed three times with 1X PBS. Neurons were blocked for 30-min with a solution of 10% normal donkey serum (NDS)/1% bovine serum albumin (BSA) (Jackson Laboratories, Bar Harbor, ME) in 1X PBS with 250 μg/mL digitonin (Sigma-Aldrich, St. Louis, MO). Cells were then incubated with primary antibody in 3% NDS/1% BSA/ 0.05% sodium azide (Acros, Thermo Fisher, NJ) in 1X PBS with 250 μg/mL digitonin (see Matsubayashi et al., 2008) for 2-h at room temperature. Primary rabbit polyclonal antibody against either CCKar (5μg/mL; Neuromics RA15049) or primary rabbit polyclonal antibody against TRPV1 (1:1000; EMD Millipore AB5370) were used to identify CCKar-positive and TRPV1-positive NG, respectively. Primary antibodies were diluted in sterile PBS (CCKar) and sterile distilled water (TRPV1). Coverslips were washed three times with 1X PBS, then incubated with fluorescein-conjugated secondary antibody (1:1000; Jackson Laboratory) in 3% NDS/1% BSA in 1X PBS with 250 μg/ml digitonin for 30-min in the dark. Lastly, coverslips were washed three times in 1X PBS then mounted with Vectashield (Vector Laboratories Inc, Burlingame, CA). To check for specificity of the secondary antibody, negative controls were prepared by omitting one primary antibody during the incubation. All antibodies were validated in rodent tissue preparations at least three times to confirm specificity and optimize concentration prior to performing any immunolabeling experiments.
Confocal imaging
Images of isolated NG neurons were acquired using a Nikon C2+ confocal microscope (Nikon Instruments, Melville, NY) with Plan Apochromat 40X/1.30 Oil DIC objective with Nikon Elements software (ver. 4.5). To identify fluorescent signals, specific band-pass filters were used to achieve proper separation of signals for each laser excitation (for double labeling 488nm laser/505–530, 543nm laser/560–590BP). Most confocal images were acquired at an approximate optical thickness of 0.5 μm or 1.0 Airy unit. Digital confocal images were saved as Nikon .ND2 format and final publication quality images were exported in the TIFF format as 300 dpi images. All images were processed and adjusted for brightness and contrast using Adobe Photoshop CC (Adobe Systems Inc., Mountain View, CA).
Histological Analysis of Lesion Centers
Following euthanasia, the spinal cord and surrounding vertebrae were removed en bloc, and immediately post-fixed in 4% paraformaldehyde. For histological staining of T3-SCI lesion extent, tissue was cryo-sectioned (40μm thick) and alternating sections were mounted on gelatin coated slides. To compare lesion severity with the spinal cords of control animals, spinal cord sections were stained with luxol fast blue (LFB) to visualize myelinated fibers. LFB-stained slides were digitally imaged on a Zeiss Axioscope light microscope and Axiocam CCD camera and analyzed as described previously (Besecker et al., 2017).
Statistical Analysis
The occurrence of outliers was tested using a Grubbs test macro written in GraphPad (GraphPad Software, La Jolla, CA). One significant outlier was identified for vagal afferent nerve recording in response to capsaicin administration and was excluded from analysis. Normality tests were performed for each analysis to determine if parametric or nonparametric statistics should be used. All statistical calculations were performed using SigmaPlot software (version 12.5; Systat Software, Inc., San Jose, CA). In the early stages of data collection, Kaplan-Meier analysis was performed to determine the lowest dose to consistently evoke an integrated, rectified vagal afferent response 10% above baseline. Effects of injury and vagal afferent nerve activity were evaluated using two-way repeated measures ANOVAs for the given postoperative time. A Tukey post-hoc test was used to determine differences in vagal afferent nerve activity between injury and stimulus parameters. Between group results from in vivo or qRT-PCR studies were compared by analysis of variance (ANOVA) and Tukey post hoc analysis. Results are expressed as means ± standard error of the mean (SEM) with significance defined as p < 0.05.
Results
Characterization of 300 kdyne injury level
The extent of the lesion epicenter was determined based upon the reduction of LFB-stained white matter at the T3 spinal cord segment. Our 300 kdyne lesion, with a 15 sec dwell time, historically produces very little white mater sparing at the lesion epicenter which is necessary to affect the diffuse distribution of descending supraspinal inputs to autonomic preganglionic centers (Besecker et al., 2017; Swartz and Holmes, 2014). The white matter calculated as percent area of total spinal cord cross-sectional area at the lesion epicenter of 3-day T3-SCI rats was significantly reduced in comparison to T3-control animals (7.0±2.7% vs. 76.2±1%, respectively; p < 0.05). At 3-weeks, the post-injury progression of the lesion epicenter is relatively complete and the lesion boundaries are more clearly defined (Hill et al., 2001), the percent area of spared white matter at the lesion epicenter of 3-week T3-SCI rats was significantly reduced in comparison to age-matched T3-control animals (1.7±0.3% vs. 74.3±0.4%, respectively; p < 0.05). These data are comparable to the injury extent reported previously and indicate the severity of our injury model (Qualls-Creekmore et al., 2010a; Swartz EM et al., 2014; Tong et al., 2009; Tong et al., 2011). Therefore, all T3-SCI rats were included for analysis based upon histological analysis.
T3-SCI reduces mechanosensitivity of vagal afferents
Our animal model of T3-SCI repeatedly provokes an immediate reduction in dietary intake of freely available chow (Holmes et al., 2008, Tong et al., 2009). Vagal afferents within the GI tract play a role in food intake and satiety signaling, in part through the transduction of mechanical distension of the stomach (Page and Kentish, 2017; Page et al., 2002). As with any reflex, the transduction of sensory information is a critical component of the stimulus-response loop. The assessment of gastric vagal mechanosensitivity is frequently the von Frey test performed using an ex vivo vagally-innervated isolated stomach preparation (c.f., Page et al., 2002). This approach permits highly controllable examination of sensory fields in response to mechanical stimulation. Previous electrophysiological characterization of gastric vagal neural activity in response to exogenous CCK-8s administration utilized an in vivo preparation to record multi-unit vagal afferent activity originating in the stomach (Okano-Matsumoto et al., 2011). While this approach also permits examination of responsiveness to mechanical stimulation, there is some reduction in precision and sensitivity since the tissue is not pinned securely. Since an in vivo approach also permits simultaneous monitoring of arterial blood pressure and heart rate, as an index of physiological integrity, we opted to use the in vivo preparation for electrophysiological studies of mechano- and chemo-sensitivity.
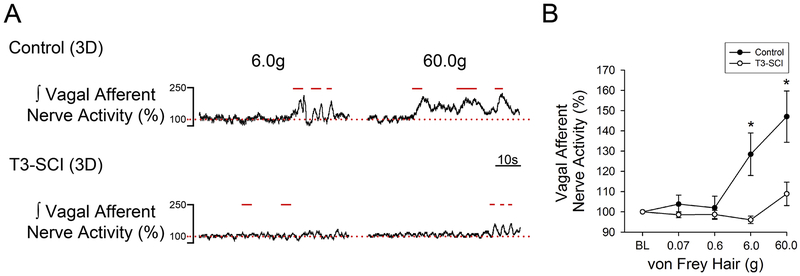
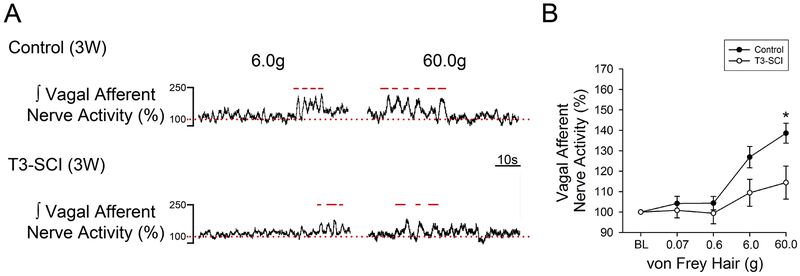
Representative gastric vagus nerve responses to mechanical stimulation from both 3-day control and T3-SCI rats are illustrated in Fig. 1A. The threshold von Frey stimulus for evoking a significant increase in vagal afferent nerve activity in surgical control rats was 6 g (p < 0.05, Fig. 1B). The resulting vagal afferent nerve response returned to baseline upon termination of the stimulus pressure. The maximal stimulus intensity for testing mechanical threshold (60 g) also provoked a significant increase in afferent firing in control rats but failed to evoke a significant vagal afferent response in T3-SCI rats (Fig. 1B). The mechanosensitivity of T3-SCI rats remained significantly reduced at 3-weeks following T3-SCI (Fig. 2), however, a subgroup of the T3-SCI rats (3/7) displayed responses to both 6g and 60g von Frey filaments that were significantly greater than their respective baseline values.
Figure 1. Gastric vagal afferent nerve mechanosensitivity in 3-day control and T3-SCI.
Mechanical stimulation (6.0g and 60.0g) of the gastric serosa with von Frey Hairs revealed a significant reduction in gastric vagal afferent sensitivity in 3-day T3-SCI rats (*p < 0.05 vs control, n = 14). Representative traces of gastric vagal afferent nerve responses to mechanical stimulation from both 3-day control and T3-SCI rats (A). Vagal nerve afferent activity percent change from baseline represented in 3-day control vs T3-SCI (B). Bars above the trace represent stimulus onset and duration. Baseline level of activity is represented with a dotted line.
Values expressed as mean ± SEM.
Figure 2. Mechanosensitivity of gastric vagal afferent nerve in 3-week control and T3-SCI.
Maximal mechanical stimulation (60.0g) of the gastric serosa with von Frey Hairs continued to reveal a significant reduction in gastric vagal afferent sensitivity in 3-week T3-SCI rats (*p < 0.05 vs control, n = 16). Gastric vagal afferent representative traces to mechanical stimulation from both 3-week control and T3-SCI rats (A). Percent change from baseline in vagal nerve afferent activity in 3-week control vs T3-SCI (B). Bars above the trace represent stimulus onset and duration. Baseline level of activity is represented with a dotted line.
Values expressed as mean ± SEM.
These data suggest that there is a prolonged desensitization of mechanoceptors in the gastric serosa following T3-SCI that lasts outwards to 3-weeks. The precise identification of these receptors as tension- or serosal-receptors remains to be determined.
T3-SCI transiently increases mRNA expression of CCKar and TRPV1 in whole nodose ganglia
Two G-protein coupled receptors for the enterocyte-secreted gut peptide CCK have been identified. The CCKa receptor (CCKar, also known as CCK1 receptor; CCK1r) has high affinity for the sulphated octapeptide CCK (CCK-8s) and is the receptor that is predominantly located within the periphery including the alimentary tract (Dufresne et al., 2006)
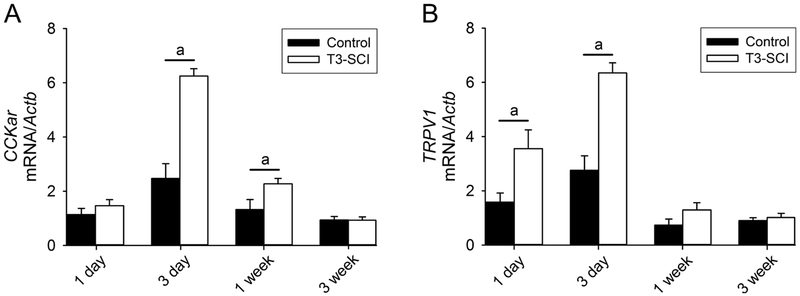
To quantify that the reduced synaptic transmission in response to CCK-8s was due to post-injury phenotypic changes in the vagal afferent neurons, total RNA was isolated to analyze expression of CCKar in NG. At 1-day, T3-SCI animals were not different from control (Fig. 3A; p > 0.05). CCKar mRNA expression was significantly increased at 3-days and 1-week post-SCI when compared to same-time controls (Fig. 3A; p < 0.05). Over time, CCKar mRNA expression returned to low levels in T3-SCI animals and was not significantly different at 3-weeks compared to controls (Fig. 3A; p > 0.05).
Figure 3. Expression levels of nodose CCKar and TRPV1 after T3-SCI.
A) Measurement of nodose CCKar mRNA expression (n=8/group) demonstrated a significant elevation in T3-SCI rats beginning at 3-day and returning to baseline 1-week post-injury (p < 0.05 vs control). Bars with lower case letters reflect significant effects: a = T3-SCI vs same-time control. B) Measurement of nodose TRPV1 mRNA expression (n=8/group) demonstrated a significant elevation in T3-SCI rats beginning at 1-day and returning to baseline 1-week post-injury (p < 0.05 vs control). Bars with lower case letters reflect significant effects: a = T3-SCI vs same-time control.
Values expressed as mean ± SEM.
Previous research has demonstrated the expression of TRPV1 channels in the NG of rats (Trancikova et al., 2018; Zhao et al., 2010) including cultured gastric vagal afferents (Zhang et al., 2004). Mice deficient for the TRPV1 receptor display attenuated gastro-esophageal mechanosensitivity (Bielefeldt and Davis, 2008) whereas pharmacological activation of TRPV1 channels is associated with hypersensitivity (Kentish et al., 2015). Our data demonstrated that TRPV1 mRNA significantly increased in NG at 1-day and 3-days following SCI when compared to same-time controls (Fig. 3B; p < 0.05). There were also significant changes within the T3-SCI group over time whereby TRPV1 mRNA expression was significantly lower after 1-week, compared to both 1-day and 3-day controls (Fig. 3B; p < 0.05).
These data reveal that T3-SCI provokes a rapid neuroplasticity in the phenotype of the NG. Since the entire ganglion was processed for qRT-PCR the organ-specific nature of these changes remains unknown. Our qRT-PCR analysis of CCKar and TRPV1 transcript expression was not followed by analysis of protein content, since analysis of protein content cannot be efficiently performed solely for GI-projecting vagal afferent neurons.
T3-SCI immunofluorescent expression of CCKar and TRPV1 in dissociated nodose ganglia
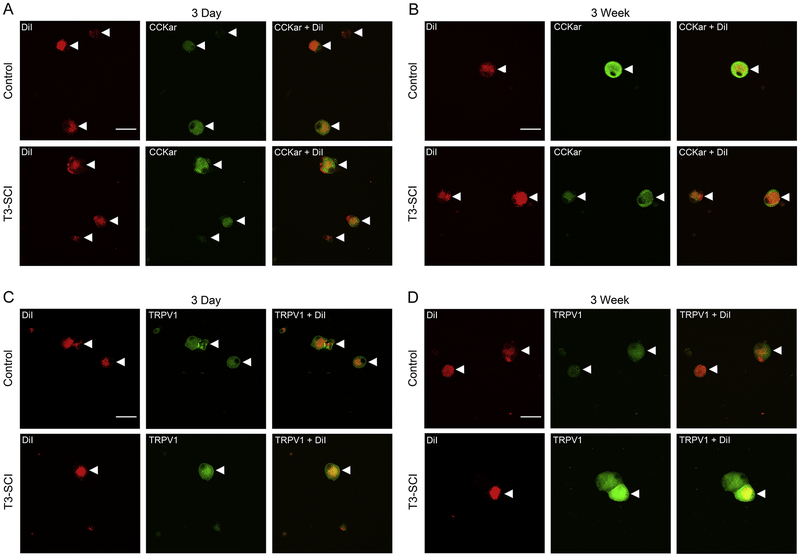
In rats, the gastric corpus is innervated by approximately 1200 neurons in each NG (Ryu et al., 2010). To visualize the specificity of our qRT-PCR data with regard to GI-projecting vagal afferent neurons from T3-SCI and surgical control animals, we performed CCKar and TRPV1 immunocytochemistry in acutely dissociated nodose neurons from animals that had also received gastric corpus injections of the retrograde tracer DiI. In order to limit the potential for any nonspecific spread of the DiI tracer, the stomach was injected three days prior to tissue harvest. DiI-positive gastric vagal afferents from both T3-SCI and surgical control animals appeared as consisting of punctate labeling when imaged with confocal microscopy (Fig. 4). Since the dissociation process results in an unpredictable loss of some cells, and samples were singly immunolabeled for either CCKar or TRPV1, we could not determine percentages of CCKar vs. TRPV1 immunopositive cells between control and injured rats. We can only indicate that the numbers of DiI-labeled cells available for analysis appeared similar between control and injured groups.
Figure 4. Immunofluorescence of gastric vagal afferent neurons of nodose ganglia in 3-day and 3-week control and T3-SCI.
Immunofluorescence of CCKar and TRPV1 in dissociated gastric-projecting NG in control and T3-SCI (n = 4/group) revealed presence of receptors at transiently levels across 3-day and 3-week time points. Images are as follows: (A) 3-day CCKar control vs SCI, (B) 3-week CCKar control vs SCI, (C) 3-day TRPV1 control vs SCI, and (D) 3-week TRPV1 control vs SCI.
These data confirm that CCKar and TRPV1 channels are present on gastric-innervating nodose afferent neurons. However, the molecular and immunocytochemical techniques do not provide a direct quantitative assessment of functional changes to vagal afferent signaling. Therefore, in vivo nerve recording, coupled with systemic administration of CCK and the TRPV1 agonist capsaicin was performed to demonstrate if functional augmentation of vagal afferent sensitivity corresponded with our bioassay and immunofluorescence data.
T3-SCI attenuates gastric vagal afferent sensitivity to CCK-8s
The gastric response to systemic and medullary CCK-8s is inhibition of phasic smooth muscle contractility (often referred to as motility) and tension (referred to as tone; Holmes et al., 2009; Raybould and Tache, 1988). Endogenous CCK and exogenous CCK-8s provoke a paracrine activation of vagal afferent fibers lining the mucosa of the GI tract (Raybould, 2007) as well as a direct action upon the vagal afferent cell bodies within the NG (Simasko and Ritter, 2003). These chemosensory actions culminate in elevated glutamatergic synaptic transmission onto second order neurons within the medulla (Baptista et al., 2005). The CCK-8s mediated synaptic transmission onto medullary neurons, presumptively from vagal afferent terminals, is diminished following SCI (Tong et al., 2011). In response to systemic administration of CCK-8s, vagal afferent fibers display a dose-dependent elevation in firing rate that is accompanied by a reduction in intragastric pressure (Okano-Matsumoto et al., 2011).
In our rat model, preliminary Kaplan-Meier analysis of the dose sufficient to evoke a gastric vagal afferent response to CCK-8s determined that neurally intact rats significantly respond to 0.4μg/kg CCK-8s. Based upon this data, subsequent analyses were performed using the 0.4μg/kg dose of CCK-8s as the maximum dose.
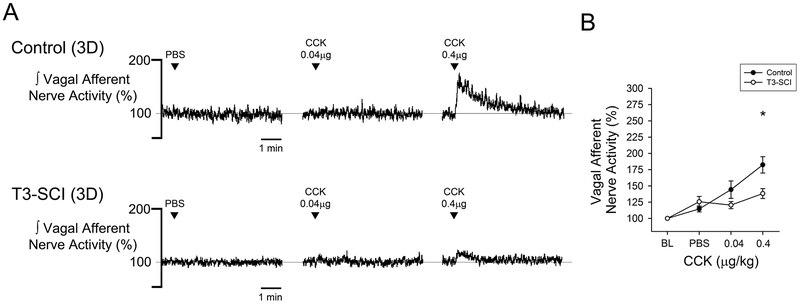
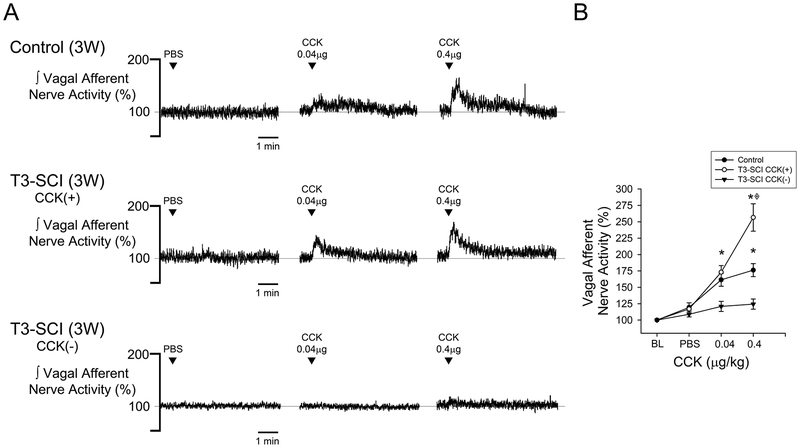
Systemic administration of vehicle did not alter vagal afferent nerve activity from baseline levels in either surgical control or T3-SCI rats (Figs. 5 & 6, p > 0.05). In animals tested at 3-days, CCK-8s increased vagal afferent discharge in surgical controls (Fig. 5A & 5B, p < 0.05). This response to CCK-8s was significantly blunted in T3-SCI rats. In surgical control rats tested at 3-weeks, CCK-8s increased vagal afferent discharge (Fig. 6A & 6B, p < 0.05). Animals that received a T3-SCI segregated into one of two distinct groups based upon their response to CCK-8s. Rats with 3-week T3-SCI that did respond to CCK-8s were significantly more sensitive to the 0.4 μg/kg dose than surgical controls (Fig. 6A & 6B, p < 0.05). Conversely, a population of T3-SCI rats remained insensitive to even the 0.4 μg/kg dose. Post hoc analysis of the lesion center histology from 3-week T3-SCI rats categorized as sensitive vs. insensitive to CCK-8s revealed no significant difference in the white mater sparing between these groups (1.7 ± 0.8% vs 1.2 ± 0.4%, respectively, p > 0.05). We conclude that the functional differences were not due to differential levels of spared fibers at the lesion center.
Figure 5. Gastric vagal afferent nerve CCK-chemosensitivity in 3-day control and T3-SCI.
Chemical stimulation with CCK (0.04μg/kg and 0.4μg/kg) via peripheral infusion revealed a significant inhibition in gastric vagal afferent response in 3-day T3-SCI rats (*p < 0.05 vs control, n = 20). Representative traces of gastric vagal afferent nerve responses to chemical stimulation from both 3-day control and T3-SCI rats (A). Vagal afferent nerve activity percent change from baseline represented in 3-day control vs T3-SCI (B).
Values expressed as mean ± SEM.
Figure 6. CCK-chemosensitivity of gastric vagal afferent nerve in 3-week control and T3-SCI.
Peripheral infusion of CCK (0.04μg/kg and 0.4μg/kg) revealed a significantly increased chemoceptor response of gastric vagal afferent nerves in 3-week T3-SCI rats (*p < 0.05 vs control, ◈p < 0.05 vs T3-SCI CCK(−); n = 19). Additionally, a bi-modal response to CCK appeared in 3-week T3-SCI rats as highly sensitive responders and desensitized responders. Gastric vagal afferent representative traces to chemical stimulation from 3-week control, T3-SCI CCK(+), and T3-SCI CCK (−) rats (A). Percent change from baseline in vagal nerve afferent activity in 3-week control vs T3-SCI CCK (+) and (−) (B). There was no significant difference in the remaining lesion center white matter between rats classified as T3-SCI CCK (+) and (−).
Values expressed as mean ± SEM.
Lidocaine nerve block verifies local action of CCK-8s
To control for potential artifacts due to peripheral administration of CCK-8s and to ensure that the effect was acting locally on gastric vagal afferents, a nerve block was administered post-infusion in a subset of animals (n = 8). Following a positive response to a 0.4 μg/kg dose of CCK-8s (response increase 137% ± 7%), lidocaine was directly applied topically to the gastric vagus nerve branch and the absence of gastric vagal response to a subsequent 0.4 μg/kg dose of CCK-8s (response 105% ± 3%) was interpreted that afferent recordings were restricted to a local effect on vagal afferent terminals.
T3-SCI transiently sensitizes gastric vagal afferents to the TRPV1 agonist capsaicin
In order to determine the dose sufficient to evoke a gastric vagal afferent response to the TRPV1 ligand, capsaicin, preliminary Kaplan-Meier analysis determined that T3-SCI rats significantly respond to 0.5μg/kg capsaicin (p < 0.05). Higher doses of capsaicin (≥1.0μg/kg) provoked profound cardiovascular, respiratory and somatic responses. Based upon this data, subsequent analyses were performed using the 0.5μg/kg dose of capsaicin as the maximum dose in order to reduce potential physiological confounds. The response to capsaicin in one rat exceeded an outlier test (Grubbs test) and was excluded from further analysis.
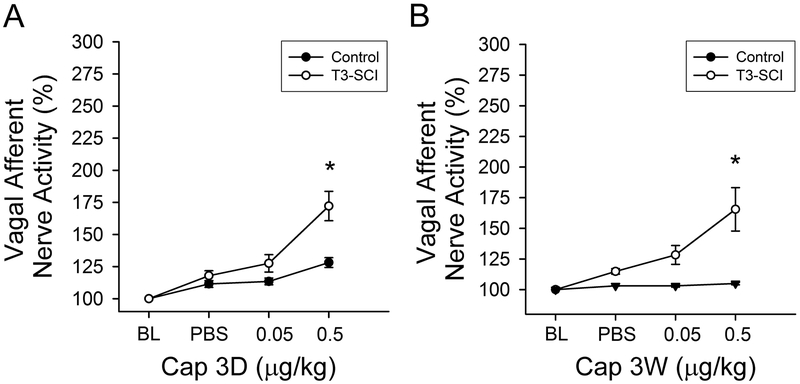
In a separate cohort of surgical control and T3-SCI rats, systemic administration of vehicle did not alter vagal afferent nerve activity from baseline levels (Fig. 7, p > 0.05). In animals tested at 3-days, the 0.5μg/kg dose of capsaicin did not increase vagal afferent discharge in surgical controls (Fig. 7A, p < 0.05). In T3-SCI rats, this same dose produced a significant increase in gastric vagal afferent nerve firing (Fig. 7A, p < 0.05). In surgical control rats tested at 3-weeks, 0.5μg/kg dose of capsaicin still failed to increase vagal afferent discharge (Fig. 7B, p < 0.05). In T3-SCI rats, the 0.5μg/kg dose of capsaicin continued to evoke a significant increase in gastric vagal afferent nerve firing (Fig. 7B, p < 0.05) despite the paradoxical reduction in TRPV1 receptor mRNA in the nodose ganglion. No differentiation between rats that could be classified as capsaicin-responders and non-responders was evident and rats were not pre-screened for their responsiveness to CCK-8s prior to capsaicin administration.
Figure 7. TRPV1-chemosensitivity of gastric vagal afferent nerve in 3-day and 3-week control and T3-SCI.
Chemoceptor physiological response to peripheral infusion of TRPV1 (0.05μg/kg and 0.5μg/kg) revealed a significant excitation in gastric vagal afferent response in 3-day and 3-week T3-SCI rats (*p < 0.05 vs control, n = 40). Graphs represent percent change from baseline in vagal afferent nerve activity in 3-day and 3-week control vs T3-SCI, respectively.
Values expressed as mean ± SEM.
These data reveal that T3-SCI provokes a rapid neuroplasticity in the physiological response of gastric vagal afferents. These functional changes only partially correspond to the molecular changes we observed, suggesting additional neuroplasticity of gastric vagal afferents following T3-SCI.
Discussion
Data from this study demonstrated that vagal afferent signaling in response to gastric mechano- and chemosensory stimuli is reduced following high-thoracic SCI. Specifically, these experimental data indicate that: 1) T3-SCI provoked a prolonged desensitization of mechanoceptors of gastric vagal afferents; 2) T3-SCI contributed to an acute chemoceptor desensitization of systemic administration of CCK-8s with differential recovery long-term; 3) T3-SCI provoked a significant amplification in the sensitivity of gastric vagal afferents to the TRPV1 agonist capsaicin; 4) T3-SCI also induced a significant up-regulation of nodose CCKar mRNA beginning at 3-days and continuing through the first week. T3-SCI caused a significant up-regulation of nodose TRPV1 over surgical control that returned to low levels by 1-week after injury; 5) T3-SCI gastric-specific NG revealed the presence of CCKar and TRPV1 receptors. These data suggest that T3-SCI provokes unparalleled structural and functional changes with variable duration. Our accumulating evidence suggests that SCI provokes impairments in the vagal afferent signaling necessary for proper GI reflexes (Besecker et al., 2018; Holmes, 2012; Swartz et al., 2014; Tong et al., 2011).
Like other visceral tissues, the vagus nerve forms a critical interface between the GI tract and the CNS. In humans and experimental rodent models, the vagus nerve is the predominant autonomic nerve supply to the upper-GI and mid-colon at approximately the hepatic flexure (reviewed in Holmes, 2012) but may extend more caudally in rats (Herrity et al., 2014). The vagus nerve is a bilateral mixed sensory and motor nerve. The somata of vagal sensory afferents reside within the nodose ganglia (NG) (Altschuler et al., 1992; Altschuler et al., 1989), and the importance of vagal afferent signaling can be inferred by the approximate 3:1 distribution of sensory to motor fibers (Prechtl and Powley, 1990). Vagal afferents are highly plastic in normal physiological conditions. Not only do vagal afferents respond to the regulatory peptides released in response to luminal contents (Date et al., 2005; Labouesse et al., 2012; Okano-Matsumoto et al., 2011) but the vagal afferent neurons differentially express receptors dependent upon this inherently cyclic peptide release (Burdyga et al., 2006a; Burdyga et al., 2010; Burdyga et al., 2006b). Evidence of pathophysiological neuronal plasticity is also beginning to accumulate in organ systems innervated by the vagus nerve (see Browning, 2010; Demir et al., 2013; Kentish et al., 2015; Kwong et al., 2008; Mawe et al., 2009; Troy et al., 2016; Yu et al., 2019). Alterations to the NG are also likely to result from inflammatory conditions associated with injury that may involve nociceptive signaling. While nociceptor cell bodies have been isolated to three primary locations, the dorsal root ganglia, trigeminal ganglia, and nodose ganglia (Caterina et al., 2001), the principal neural circuit for conducting visceral nociceptive signals is still largely regarded to pass through the dorsal root ganglia and spinal cord (see Christianson and Davis, 2010; Christianson et al., 2009; Gebhart and Bielefeldt, 2016). However, other investigators have recently proposed that vagal afferents and satellite glia located within the NG transmit or modulate nociceptive information, respectively (see Hanani, 2015; Surdenikova et al., 2012) and do so in response to inflammatory conditions (Blum et al., 2017; Feldman-Goriachnik et al., 2015).
Experimental evidence has revealed that 40% of primary gastric vagal afferent neurons express TRPV1 (Banerjee et al., 2007), and TRPV1 channels have been identified and are expressed on the vagal C-fiber afferents of the vagus nerve (Hayes, Jr. et al., 2013). Additionally, TRPV1 channels are polymodal transducers known to be activated during conditions that are likely to be present after SCI, including acidity (Strain and Waldrop, 2005), endogenous inflammatory mediators (Hayes, Jr. et al., 2013), and inflammation. Accumulating evidence supports the relationship that following SCI there is a sudden increase in systemic (Anthony and Couch, 2014; Schwab et al., 2014) and local GI inflammation (Besecker et. al., 2017); the latter occurring in conjunction with a decrease in GI blood flow. The onset of inflammation causes a release of arachidonic acid derivative, anandamide, lipoxygenase derivatives, and N-acyl dopamines known as endovanilloids (Banerjee et al., 2007) which have been shown to activate TRPV1 channels and further enhance the inflammatory process. In addition to inflammation increasing the expression of TRPV1, physiological levels of cations also contribute to activation of TRPV1 (Ahern et al., 2005) and sensitization of vagal afferents (Kang et al., 2004) specifically high levels of gastric acid (H+) and Ca2+ (Kishimoto et al., 2011). Taken together, these lines of evidence would predict a heightened visceral sensitivity in our model as a result of the increased TRPV1 mRNA in the NG. It should be noted that in this study TRPV1 mRNA content was determined from whole NG and that TRPV1 expression may not be homogeneous across all vagal afferent populations. Evidence from mouse studies indicate a disparity between gastric afferents comprising the NG (Young et al., 2008) and the total number of NG neurons that are TRPV1 positive (Bielefeldt et al., 2006). Furthermore, prior analysis of the afferent receptive fields indicated that only a small proportion of gastric vagal afferent endings are TRPV1 positive (Ward et al., 2003). Furthermore, TRPV1 channels expression was identified in relation to myenteric ganglia and other GI regions suggesting the TRPV1 modulation of GI function is extensive and complex. While determining the TRPV1 mRNA content in the whole NG does not reflect possible differences in organ-specific vagal afferent neurons, the increased neural response of the gastric vagus to the systemic administration of the TRPV1 agonist capsaicin physiologically supports our molecular data but does not reflect the diminished response to mechanical stimulation in SCI subjects. These incongruous results may not be so surprising in that NG TRPV1 mRNA expression was unaffected in mice fed a standard or high fat diet, despite reduced tension receptor responses in the high fat diet mice (Kentish et al., 2015). Clearly, additional channelopathies that diminish action potential generation must be involved in post-SCI vagal afferent dysfunction.
Vagal afferent injury induces changes in TRPV1 and cholecystokinin (CCK) within the nodose ganglion (Czaja et al., 2008; Gallaher et al., 2011). We demonstrated that SCI acutely increased mRNA expression of TRPV1 and CCKar within the NG and the increased TRPV1 mRNA expression suggests possible vagal afferent injury. The NG expresses both CCK receptors (Broberger et al., 2001; Helke et al., 1988) and in a model of vagal axotomy CCKbr and CCK are upregulated while CCKar is downregulated (Broberger et al., 2001). The elevated expression of CCKar by afferent cell bodies within the NG after T3-SCI is in contrast to the effects of vagotomy, though the pathophysiology of the two models is by no means similar. Despite this dissimilarity between these models of vagal pathophysiology, the elevated expression of CCKar would predict a potential increase in vagal afferent sensitivity to systemic CCK-8s. In fact, our results found the opposite and may reflect that while the CCKar mRNA expression increased acutely post-SCI, this may actually suggest receptor internalization or diminished receptor trafficking in response to altered GI peptide release by diminished gastric emptying.
When CCK binds to CCKar, the vagal afferent signals culminate in a slowing of gastric emptying, increase in pancreatic secretion, and promotion of satiation (Duca et al., 2013; Kinch et al., 2012). The potential exists that our T3-SCI model, with delayed gastric emptying, stops producing CCK since nutrients fail to leave the stomach to stimulate duodenal release of CCK. With a reduction in CCK release, and other putative humoral agents in nutrient signaling, this provokes a cascade of changes to homeostatic neural circuits. The possibility exists that CCKar activation leads to TRP conductance(s) which leads to an influx of calcium, with potential for cytotoxic effects OR hyperpolarization of the afferent vagus. Therefore, the gastric vagal afferent response to CCK is diminished while the response to capsaicin, the TRPV1 agonist, is augmented. These speculations require further testing.
Conclusion and Translational Perspective
We have previously shown that the efferent limb of the gastric vago-vagal reflex remained functionally capable of modulating gastric contractility (Swartz et al., 2014) and our current data support the suggestion that diminished signaling within the afferent limb may result in a net inhibition of the efferent vagus (Holmes, 2012). In a broader sense, there is considerable attention to an anti-inflammatory circuit which involves the afferent and efferent vagus nerve and α7 nicotinic acetylcholine receptor- (nAChR) expressing macrophages. This circuit has become known as the cholinergic anti-inflammatory pathway (CAP) and results in a finely tuned pro-inflammatory response (Ji et al., 2014; Martelli et al., 2014; Pavlov and Tracey, 2012). Recent evidence links activation of CCKar and the nAChR-mediated vagal reflex reduce inflammation and protect against the intestinal damage (de Haan et al., 2013). The exact mechanism of CCKar and nAChR anti-inflammatory properties are not well understood. A mechanistic explanation for the effects of electrical stimulation of the vagus nerve suppresses activation of NF-κB and diminishes the production of proinflammatory cytokines in α7 nAChR-expressing macrophages (Wu et al., 2014). This pathway may be involved in the neurochemical changes in our model of T3-SCI injury. We’ve shown that there is an increase in inflammatory markers post-SCI (Besecker et al., 2017); therefore the CAP pathway may be activated; however, the long-term anti-inflammatory effects may not be visible in the acute model (<7 days post-SCI). Therefore, our results indicate that the phenotypic changes happening acutely may not be representative of the long-term functional changes persisting after SCI. But by developing a greater understanding of the pathways involved throughout the injury-recovery process, will offer greater therapeutic targets to the SCI population.
Highlights.
Upper thoracic SCI impairs mechanosensitivity of gastric vagal afferent fibers
Vagal sensory cell soma show post-injury CCK-A receptor and TRPV1 channel plasticity
Acute gastric vagal afferent sensitivity to CCK is diminished following SCI
Gastric vagal afferents have elevated sensitivity to the TRPV1 agonist capsaicin
Chronic SCI rats display a bimodal recovery of sensitivity to CCK
Acknowledgements
The authors wish to thank Dr. Amanda Troy for providing initial guidance for nodose excision. Dr. Victor Ruiz-Velasco provided assistance in nodose ganglion dissociations. Margaret McLean provided assistance with animal care, tissue harvest, data entry and analysis. Dr. Salvatore Stella, Jr. graciously assisted with all aspects of confocal microscopy.
Funding sources
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) Grants R01-NS-049177 and F31-NS-087834.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have declared that no conflicts of interest exist.
References
- Ahern GP, Brooks IM, Miyares RL, Wang XB, 2005. Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J. Neurosci 25, 5109–5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler SM, Rinaman L, Miselis R, 1992. Viscerotopic representation of the alimentary tract in the dorsal and ventral vagal complexes in the rat In: Barnes CD, Ritter S, Ritter RC (Eds.), Neuroanatomy And Physiology Of Abdominal Vagal Afferents CRC Press, New York, pp. 21–53. [Google Scholar]
- Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR, 1989. Viscerotopic representation of the upper alimentary tract in the rat: Sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J. Comp. Neurol 283, 248–268. [DOI] [PubMed] [Google Scholar]
- Anthony DC, Couch Y, 2014. The systemic response to CNS injury. Experimental Neurology 258, 105–111. [DOI] [PubMed] [Google Scholar]
- Banerjee B, Medda BK, Lazarova Z, Bansal N, Shaker R, Sengupta JN, 2007. Effect of reflux-induced inflammation on transient receptor potential vanilloid one (TRPV1) expression in primary sensory neurons innervating the oesophagus of rats. Neurogastroenterol. Motil 19, 681–691. [DOI] [PubMed] [Google Scholar]
- Baptista V, Zheng ZL, Coleman FH, Rogers RC, Travagli RA, 2005. Cholecystokinin octapeptide increases spontaneous glutamatergic synaptic transmission to neurons of the nucleus tractus solitarius centralis. J Neurophysiol 94, 2763–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlly MH, Wilmot CB, 1984. Acute abdominal emergencies during the first four weeks after spinal cord injury. Arch. Phys. Med. Rehabil 65, 687–690. [PubMed] [Google Scholar]
- Berthoud HR, Powley TL, 1992. Vagal afferent innervation of the rat fundic stomach: Morphological characterization of the gastric tension receptor. The J. Comp. Neurol 319, 261–276. [DOI] [PubMed] [Google Scholar]
- Besecker EM, White AR, Holmes GM, 2018. Diminished gastric prokinetic response to ghrelin in a rat model of spinal cord injury. Neurogastroenterol. Motil e13258–e13n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besecker EM, Deiter GM, Pironi N, Cooper TK, Holmes GM, 2017. Mesenteric vascular dysregulation and intestinal inflammation accompanies experimental spinal cord injury. Am. J. Physiol. - Regulat. Integr. Comp. Physiol 312, 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt K, Davis BM, 2008. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am. J. Physiol. - Gastrointest. Liver Physiol 294, G130–G138. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Zhong F, Koerber HR, Davis BM, 2006. Phenotypic characterization of gastric sensory neurons in mice. Am. J. Physiol. - Gastrointest. Liver Physiol 291, G987–G997. [DOI] [PubMed] [Google Scholar]
- Blum E, Procacci P, Conte V, Sartori P, Hanani M, 2017. Long term effects of lipopolysaccharide on satellite glial cells in mouse dorsal root ganglia. Exp. Cell Res 350, 236–241. [DOI] [PubMed] [Google Scholar]
- Broberger C, Holmberg K, Shi TJ, Dockray G, Hökfelt T, 2001. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res 903, 128–140. [DOI] [PubMed] [Google Scholar]
- Browning KN, 2010. Protease-activated receptors: novel central role in modulation of gastric functions. Neurogastroenterol. Motil 22, 361–365. [DOI] [PubMed] [Google Scholar]
- Buelke-Sam J, Holson JF, Bazare JJ, Young JF, 1978. Comparative stability of physiological parameters during sustained anesthesia in rats. Lab Anim Sci 28, 157–162. [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ, 2006a. Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurones. Neuroscience 137, 1405–1415. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ, 2010. Expression of cannabinoid CB1 receptors by vagal afferent neurons: kinetics and role in influencing neurochemical phenotype. Am. J. Physiol. - Gastrointest. Liver Physiol 299, G63–G69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ, 2006b. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am. J. Physiol. - Gastrointest. Liver Physiol 290, G1289–G1297. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D, 2001. The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci 24, 487–517. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Davis BM, 2010. The Role of Visceral Afferents in Disease. Frontiers in Neuroscience 3, 1–24. [PubMed] [Google Scholar]
- Christianson JA, Bielefeldt K, Altier C, Cenac N, Davis BM, Gebhart GF, High KW, Kollarik M, Randich A, Undem B, Vergnolle N, 2009. Development, plasticity and modulation of visceral afferents. Brain Res. Rev 60, 171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman BC, Stone JM, Perkash I, 1991. Gastrointestinal complications of chronic spinal cord injury. J. Am. Paraplegia Soc 14, 175–181. [DOI] [PubMed] [Google Scholar]
- Czaja K, Burns GA, Ritter RC, 2008. Capsaicin-induced neuronal death and proliferation of the primary sensory neurons located in the nodose ganglia of adult rats. Neuroscience 154, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, Niijima A, Kangawa K, Nakazato M, 2005. Peripheral Interaction of Ghrelin with Cholecystokinin on Feeding Regulation. Endocrinology 146, 3518–3525. [DOI] [PubMed] [Google Scholar]
- de Haan JJ, Hadfoune M, Lubbers T, Hodin C, Lenaerts K, Ito A, Verbaeys I, Skynner MJ, Cailotto C, van d. V, de Jonge WJ, Greve JW, Buurman WA, 2013. Lipid-rich enteral nutrition regulates mucosal mast cell activation via the vagal anti-inflammatory reflex. Am. J. Physiol. - Gastrointest. Liver Physiol 305, G383–G391. [DOI] [PubMed] [Google Scholar]
- Demir I, Schäfer KH, Tieftrunk E, Friess H, Ceyhan GO, 2013. Neural plasticity in the gastrointestinal tract: chronic inflammation, neurotrophic signals, and hypersensitivity. Acta Neuropathol 125, 491–509. [DOI] [PubMed] [Google Scholar]
- Dockray GJ, 2014. Gastrointestinal hormones and the dialogue between gut and brain. J. Physiol 592, 2927–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas B, Woutersen R, Jansen J, de Jong AJ, Lamers C, 1988. The influence of different nutrients on plasma cholecystokinin levels in the rat. Experientia 44, 21–23. [DOI] [PubMed] [Google Scholar]
- Duca FA, Zhong L, Covasa M, 2013. Reduced CCK signaling in obese-prone rats fed a high fat diet. Horm. Behav 64, 812–817. [DOI] [PubMed] [Google Scholar]
- Dufresne M, Seva C, Fourmy D, 2006. Cholecystokinin and Gastrin Receptors. Physiol. Rev 86, 805–847. [DOI] [PubMed] [Google Scholar]
- Dyavanapalli J, Dergacheva O, Wang X, Mendelowitz D, 2016. Parasympathetic Vagal Control of Cardiac Function. Curr. Hyperten. Rep 18, 22. [DOI] [PubMed] [Google Scholar]
- Fealey RD, Szurszewski JH, Merritt JL, DiMagno EP, 1984. Effect of traumatic spinal cord transection on human upper gastrointestinal motility and gastric emptying. Gastroenterology 87, 69–75. [PubMed] [Google Scholar]
- Feldman-Goriachnik R, Belzer V, Hanani M, 2015. Systemic inflammation activates satellite glial cells in the mouse nodose ganglion and alters their functions. Glia 63, 2121–2132. [DOI] [PubMed] [Google Scholar]
- Furuta A, Suzuki Y, Hayashi N, Egawa S, Yoshimura N, 2012. Transient receptor potential A1 receptor-mediated neural cross-talk and afferent sensitization induced by oxidative stress: Implication for the pathogenesis of interstitial cystitis/bladder pain syndrome. Int. J. Urol 19, 429–436. [DOI] [PubMed] [Google Scholar]
- Gallaher ZR, Ryu V, Larios RM, Sprunger LK, Czaja K, 2011. Neural proliferation and restoration of neurochemical phenotypes and compromised functions following capsaicin-induced neuronal damage in the nodose ganglion of the adult rat. Front Neurosci 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart GF, Bielefeldt K, 2016. Physiology of Visceral Pain. Comprehensive Physiology 6, 1609–1633. [DOI] [PubMed] [Google Scholar]
- Hanani M, 2015. Role of satellite glial cells in gastrointestinal pain. Frontiers in Cellular Neuroscience 9, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D Jr., Nicol KK, Tobias JD, Chicoine LG, Duffy VL, Mansour HM, Preston TJ, 2013. Identification of the nodose ganglia and TRPV1 in swine. Lung 191, 445–447. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Hill KM, 1988. Immunohistochemical study of neuropeptides in vagal and glossopharyngeal afferent neurons in the rat. Neuroscience 26, 539–551. [DOI] [PubMed] [Google Scholar]
- Herrity AN, Rau KK, Petruska JC, Stirling DP, Hubscher CH, 2014. Identification of bladder and colon afferents in the nodose ganglia of male rats. J. Comp Neurol 522, 3667–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CE, Beattie MS, Bresnahan JC, 2001. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp. Neurol 171, 153–169. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Primeaux SD, Tong M Effects of experimental spinal cord injury on food intake, body composition and gastrointestinal expression of CD36 mRNA in rats. Appetite 51[2], 374 2008. [Google Scholar]
- Holmes GM, 2012. Upper gastrointestinal dysmotility after spinal cord injury: Is diminished vagal sensory processing one culprit? Front Physiol 3, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GM, Hudson TR, Filart R, 2017. Neurogastroenterology in Spinal Cord Dysfunction In: Weidner N, Rupp R, Tansey KE (Eds.), Neurological Aspects of Spinal Cord Injury Springer, New York, pp. 397–437. [Google Scholar]
- Holmes GM, Tong M, Travagli RA, 2009. Effects of brainstem cholecystokinin-8s on gastric tone and esophageal-gastric reflex. Am. J. Physiol. - Gastrointest. Liver Physiol 296, G621–G631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE, 2014. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal. Immunol 7, 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Lee LY, 2007. Role of TRPV receptors in respiratory diseases. Biochimica et Biophysica Acta 1772, 915–927. [DOI] [PubMed] [Google Scholar]
- Kang YM, Bielefeldt K, Gebhart GF, 2004. Sensitization of Mechanosensitive Gastric Vagal Afferent Fibers in the Rat by Thermal and Chemical Stimuli and Gastric Ulcers. J Neurophysiol 91, 1981–1989. [DOI] [PubMed] [Google Scholar]
- Kao CH, Ho YJ, Changlai SP, Ding HJ, 1999. Gastric emptying in spinal cord injury patients. Dig. Dis. Sci 44, 1512–1515. [DOI] [PubMed] [Google Scholar]
- Kentish SJ, Frisby CL, Kritas S, Li H, Hatzinikolas G, O’Donnell TA, Wittert GA, Page AJ, 2015. TRPV1 Channels and Gastric Vagal Afferent Signalling in Lean and High Fat Diet Induced Obese Mice. PLoS ONE 10, e0135892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish SJ, Page AJ, 2014. The role of gastrointestinal vagal afferent fibres in obesity. J. Physiol 593, 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kewalramani LS, 1979. Neurogenic gastroduodenal ulceration and bleeding associated with spinal cord injuries. J. Trauma 19, 259–265. [DOI] [PubMed] [Google Scholar]
- Kinch DC, Peters JH, Simasko SM, 2012. Comparative pharmacology of cholecystokinin induced activation of cultured vagal afferent neurons from rats and mice. PLoS. One 7, e34755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshblum SC, Groah SL, McKinley WO, Gittler MS, Stiens SA, 2002. Spinal cord injury medicine. 1. Etiology, classification, and acute medical management. Arch. Phys. Med. Rehabil 83, S50–S58. [DOI] [PubMed] [Google Scholar]
- Kishimoto E, Naito Y, Handa O, Okada H, Mizushima K, Hirai Y, Nakabe N, Uchiyama K, Ishikawa T, Takagi T, Yagi N, Kokura S, Yoshida N, Yoshikawa T, 2011. Oxidative stress-induced posttranslational modification of TRPV1 expressed in esophageal epithelial cells. Am. J. Physiol. - Gastrointest. Liver Physiol 301, G230–G238. [DOI] [PubMed] [Google Scholar]
- Kwong K, Carr MJ, Gibbard A, Savage TJ, Singh K, Jing J, Meeker S, Undem BJ, 2008. Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs. J. Physiol 586, 1321–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouesse MA, Stadlbauer U, Weber E, Arnold M, Langhans W, Pacheco-L+¦pez G, 2012. Vagal Afferents Mediate Early Satiation and Prevent Flavour Avoidance Learning in Response to Intraperitoneally Infused Exendin-4. J. Neuroendocrinol 24, 1505–1516. [DOI] [PubMed] [Google Scholar]
- Lee LY, Gu Q, 2009. Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr. Opin. Pharmacol 9, 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle RA, 1997. Cholecystokinin cells. Ann. Rev. Physiol 59, 221–242. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Kim C, 2005. Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon! Cell. Molec. Life Sci 62, 2985–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli D, McKinley MJ, McAllen RM, 2014. The cholinergic anti-inflammatory pathway: A critical review. Auton. Neurosci 182, 65–69. [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y, Iwai L, Kawasaki H, 2008. Fluorescent double-labeling with carbocyanine neuronal tracing and immunohistochemistry using a cholesterol-specific detergent digitonin. J Neurosci Meth 174, 71–81. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Strong DS, Sharkey KA, 2009. Plasticity of enteric nerve functions in the inflamed and postinflamed gut. Neurogastroenterol. Motil 21, 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone SB, Undem BJ, 2016. Vagal Afferent Innervation of the Airways in Health and Disease. Physiol Rev 96(3), 975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Mahieu F, Karashima Y, Voets T, 2007a. Regulation of TRP channels: a voltage-lipid connection. Biochem. Soc. Trans 35, 105–108. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA, 2007b. Transient receptor potential cation channels in disease. Physiol Rev 87, 165–217. [DOI] [PubMed] [Google Scholar]
- Nino-Murcia M, Friedland GW, 1991. Functional abnormalities of the gastrointestinal tract in patients with spinal cord injuries: Evaluation with imaging procedures. Am. J. of Roentgenology 158, 279–281. [DOI] [PubMed] [Google Scholar]
- Okano-Matsumoto S, McRoberts JA, Taché Y, Adelson DW, 2011. Electrophysiological evidence for distinct vagal pathways mediating CCK-evoked motor effects in the proximal versus distal stomach. J. Physiol 589, 371–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Kentish SJ, 2017. Plasticity of gastrointestinal vagal afferent satiety signals. Neurogastroenterol. Motil 29, e12973–e12n/a. [DOI] [PubMed] [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA, 2002. Vagal Mechanoreceptors and Chemoreceptors in Mouse Stomach and Esophagus. J Neurophysiol 87, 2095–2103. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ, 2012. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat. Rev. Endocrinol 8, 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ, 2002. Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes? I. Morphology and topography of vagal afferents innervating the GI tract. Am. J. Physiol. - Gastrointest. Liver Physiol 283, G1217–G1225. [DOI] [PubMed] [Google Scholar]
- Prechtl JC, Powley TL, 1990. The fiber composition of the abdominal vagus of the rat. Anat. Embryol. (Berl) 181, 101–115. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Tong M, Holmes GM, 2007. Effects of chronic spinal cord injury on body weight and body composition in rats fed a standard chow diet. Am. J. Physiol - Regul. Integr. Comp. Physiol 293, R1102–R1109. [DOI] [PubMed] [Google Scholar]
- Qualls-Creekmore E, Tong M, Holmes GM, 2010a. Time-course of recovery of gastric emptying and motility in rats with experimental spinal cord injury. Neurogastroenterol. Motil 22, 62–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualls-Creekmore E, Tong M, Holmes GM, 2010b. Gastric emptying of enterally administered liquid meal in conscious rats and during sustained anaesthesia. Neurogastroenterol. Motil 22, 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran SK, Reiser JR, Bauman W, Zhang RL, Gordon SK, Korsten MA, 1992. Gastrointestinal transit after spinal cord injury: Effect of cisapride. Am J Gastroenterol 87, 1614–1617. [PubMed] [Google Scholar]
- Raybould HE, Tache Y, 1988. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am. J Physiol 255, G242–G246. [DOI] [PubMed] [Google Scholar]
- Raybould HE, 2007. Mechanisms of CCK signaling from gut to brain. Curr Opin Pharmacol 7, 570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu V, Gallaher Z, Czaja K, 2010. Plasticity of nodose ganglion neurons after capsaicinand vagotomy-induced nerve damage in adult rats. Neuroscience 167, 1227–1238. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Zhang Y, Kopp MA, Brommer B, Popovich PG, 2014. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Experimental Neurology 258, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal JL, Milne N, Brunnemann SR, 1995. Gastric emptying is impaired in patients with spinal cord injury. Am J Gastroenterol 90, 466–470. [PubMed] [Google Scholar]
- Simasko SM, Ritter RC, 2003. Cholecystokinin activates both A- and C-type vagal afferent neurons. Am. J Physiol - Gastrointest. Liver Physiol 285, G1204–G1213. [DOI] [PubMed] [Google Scholar]
- Stinneford JG, Keshavarzian A, Nemchausky BA, Doria MI, Durkin M, 1993. Esophagitis and esophageal motor abnormalities in patients with chronic spinal cord injuries. Paraplegia 31, 384–392. [DOI] [PubMed] [Google Scholar]
- Strain GM, Waldrop RD, 2005. Temperature and vascular volume effects on gastric ulcerogenesis after cord transection. Dig. Dis. Sci 50, 2037–2042. [DOI] [PubMed] [Google Scholar]
- Surdenikova L, Ru F, Nassenstein C, Tatar M, Kollarik M, 2012. The neural crest- and placodes-derived afferent innervation of the mouse esophagus. Neurogastroenterol. Motil 24, e517–e525. [DOI] [PubMed] [Google Scholar]
- Swartz EM, Holmes GM, 2014. Gastric vagal motoneuron function is maintained following experimental spinal cord injury. Neurogastroenterol. Motil 27, 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz E, Holmes G, 2014. Gastric vagal motoneuron function is maintained following experimental spinal cord injury. Neurogastroenterol. Motil 27, 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Holmes GM, 2009. Gastric dysreflexia after acute experimental spinal cord injury in rats. Neurogastroenterol. Motil 21, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Qualls-Creekmore E, Browning KN, Travagli RA, Holmes GM, 2011. Experimental spinal cord injury in rats diminishes vagally-mediated gastric responses to cholecystokinin-8s. Neurogastroenterol. Motil 23, e69–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trancikova A, Kovacova E, Ru F, Varga K, Brozmanova M, Tatar M, Kollarik M, 2018. Distinct Expression of Phenotypic Markers in Placodes- and Neural Crest-Derived Afferent Neurons Innervating the Rat Stomach. Dig. Dis. Sci 63, 383–394. [DOI] [PubMed] [Google Scholar]
- Troy AE, Simmonds SS, Stocker SD, Browning KN, 2016. High fat diet attenuates glucose-dependent facilitation of 5-HT3-mediated responses in rat gastric vagal afferents. J. Physiol 594, 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR, 2003. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J. Comp. Neurol 465, 121–135. [DOI] [PubMed] [Google Scholar]
- Williams RE, Bauman WA, Spungen AM, Vinnakota RR, Farid RZ, Galea M, Korsten MA, 2011. SmartPill technology provides safe and effective assessment of gastrointestinal function in persons with spinal cord injury. Spinal Cord 50, 81–84. [DOI] [PubMed] [Google Scholar]
- Wolf C, Meiners TH, 2003. Dysphagia in patients with acute cervical spinal cord injury. Spinal Cord 41, 347–353. [DOI] [PubMed] [Google Scholar]
- Wu H, Li L, Su X, 2014. Vagus nerve through alpha7 nAChR modulates lung infection and inflammation: models, cells, and signals. Biomed. Res Int 2014, 283525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RL, Cooper NJ, Blackshaw LA, 2008. Chemical coding and central projections of gastric vagal afferent neurons. Neurogastroenterol. Motil 20, 708–718. [DOI] [PubMed] [Google Scholar]
- Yu X, Yu M, Liu Y, Yu S, 2016. TRP channel functions in the gastrointestinal tract. Sem. Immunopathol 38, 385–396. [DOI] [PubMed] [Google Scholar]
- Yu Y, Park SJ, Beyak MJ, 2019. Inducible nitric oxide synthase-derived nitric oxide reduces vagal satiety signalling in obese mice. J. Physiol 597, 1487–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jones S, Brody K, Costa M, Brookes SJ, 2004. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am. J. Physiol. - Gastrointest. Liver Physiol 286, G983–G991. [DOI] [PubMed] [Google Scholar]
- Zhao H, Simasko SM, 2010. Role of Transient Receptor Potential Channels in Cholecystokinin-Induced Activation of Cultured Vagal Afferent Neurons. Endocrinology 151, 5237–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Sprunger LK, Simasko SM, 2010. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am. J. Physiol. - Gastrointest. Liver Physiol 298, G212–G221. [DOI] [PMC free article] [PubMed] [Google Scholar]