Abstract
BACKGROUND.
Persons with low socioeconomic status and nonwhite persons in the United States have high rates of cardiovascular disease. The use of combination pills (also called “polypills”) containing low doses of medications with proven benefits for the prevention of cardiovascular disease may be beneficial in such persons. However, few data are available regarding the use of polypill therapy in underserved communities in the United States, in which adherence to guideline-based care is generally low.
METHODS.
We conducted a randomized, controlled trial involving adults without cardiovascular disease. Participants were assigned to the polypill group or the usual-care group at a federally qualified community health center in Alabama. Components of the polypill were atorvastatin (at a dose of 10 mg), amlodipine (2.5 mg), losartan (25 mg), and hydrochlorothiazide (12.5 mg). The two primary outcomes were the changes from baseline in systolic blood pressure and low-density lipoprotein (LDL) cholesterol level at 12 months.
RESULTS.
The trial enrolled 303 adults, of whom 96% were black. Three quarters of the participants had an annual income below $15,000. The mean estimated 10-year cardiovascular risk was 12.7%, the baseline blood pressure was 140/83 mm Hg, and the baseline LDL cholesterol level was 113 mg per deciliter. The monthly cost of the polypill was $26. At 12 months, adherence to the polypill regimen, as assessed on the basis of pill counts, was 86%. The mean systolic blood pressure decreased by 9 mm Hg in the polypill group, as compared with 2 mm Hg in the usual-care group (difference, −7 mm Hg; 95% confidence interval [CI], −12 to −2; P = 0.003). The mean LDL cholesterol level decreased by 15 mg per deciliter in the polypill group, as compared with 4 mg per deciliter in the usual-care group (difference, −11 mg per deciliter; 95% CI, −18 to −5; P<0.001).
CONCLUSIONS.
A polypill-based strategy led to greater reductions in systolic blood pressure and LDL cholesterol level than were observed with usual care in a socioeconomically vulnerable minority population. (Funded by the American Heart Association Strategically Focused Prevention Research Network and the National Institutes of Health; ClinicalTrials.gov number, .)
INTRODUCTION
Cardiovascular disease remains the leading cause of death and disability in the United States.1 Persons with low socioeconomic status and nonwhite persons are particularly vulnerable and have high cardiovascular mortality.2 There is wide geographic variation, with disproportionate disease burden in the southeastern United States and rural areas.3
Two leading risk factors for cardiovascular disease are elevated blood pressure and elevated low-density lipoprotein (LDL) cholesterol. Nearly two thirds of adults in the United States have high blood pressure as defined by the 2017 American College of Cardiology (ACC)–American Heart Association (AHA) guidelines regarding hypertension.4 Nonetheless, fewer than half the adults with hypertension are treated and have their hypertension controlled.5 Similarly, approximately one-third of U.S. adults are eligible for statin therapy based on the 2013 ACC-AHA cholesterol guidelines, but only a minority receive therapy.6,7 Hypertension and hypercholesterolemia are particularly common in groups with low socioeconomic status, in which treatment rates are strikingly low.8–11
Although pharmacologic measures are frequently used to manage cardiovascular risk factors, there are differing opinions regarding implementation. The traditional strategy identifies high-risk persons on the basis of clinical prediction algorithms, an approach that is endorsed in major guidelines. In contrast, a population-based strategy focuses on shifting the entire risk distribution by means of broadly applied, low-cost interventions that involve relatively few side effects.12 A consideration that favors the population- based approach is the recognition that many persons who have a cardiovascular event would be classified by conventional algorithms as being at low or intermediate risk.13,14 There are additional challenges with a risk-based approach in resource-limited settings. It is unclear whether traditional prediction algorithms are applicable to persons with low socioeconomic status. Furthermore, a risk-based strategy may be difficult to implement owing to the need for frequent testing and follow-up visits and complex medication regimens.
The “polypill” is a fixed-dose combination of medications with proven benefits for the prevention of cardiovascular disease.15 In population-based strategies for the prevention of cardiovascular disease, the polypill offers potential advantages over conventional pharmacotherapy. First, the simplicity of using a daily pill may improve adherence to therapy. Second, the elimination of requirements for dose adjustment may be useful in settings in which frequent follow-up visits are impractical. Third, for blood-pressure control, the combination of multiple low-dose medications rather than the use of one or two higher-dose medications may improve the safety profile, given that side effects are typically dose dependent.16
Although there have been previous trials of polypills for the prevention of cardiovascular disease,17–25 this approach has not been extensively studied in underserved minority populations. Therefore, we undertook a randomized, clinical trial to assess the effectiveness of a polypill-based strategy in an underserved population of persons with low socioeconomic status.
METHODS
Clinical trial design.
We designed a two-group, open-label, randomized, controlled, clinical trial comparing polypill therapy with usual care. The trial protocol, which is available with the full text of this article at NEJM.org, was approved by the Vanderbilt University institutional review board, the Oversight Advisory Committee of the AHA Strategically Focused Prevention Research Network, and two committees of the Southern Community Cohort Study (SCCS). The trial was monitored by an independent data and safety monitoring board and by the Food and Drug Administration under a noncommercial Investigational New Drug application. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol.
Setting and Recruitment.
The SCCS was initiated in 2001 to examine root causes of health disparities in cancer.26 The study enrolled 85,000 participants, predominantly from minority populations, across a network of community health centers in the southeastern United States; enrollment was completed in 2009. Potentially eligible participants for the polypill trial were identified among previously enrolled SCCS participants living within 50 miles of the Franklin Primary Health Center in Mobile, Alabama, or among current non-SCCS patients or residents near the center.
Eligibility criteria and screening examinations were identical for both the SCCS and non-SCCS participants. Potentially eligible persons 45 to 75 years of age who had no reported history of coronary heart disease, stroke, cancer, liver disease, or insulin-dependent diabetes were sent a prescreening questionnaire. Respondents who were taking no more than two antihypertensive medications were invited to the Franklin Primary Health Center for a clinical examination, which included blood pressure, fasting lipid, and blood chemical measurements. Eligible participants met each of the following criteria: a systolic blood pressure between 120 and 160 mm Hg, an LDL cholesterol level of less than 190 mg per deciliter (4.90 mmol per liter), an estimated glomerular filtration rate of at least 60 ml per minute per 1.73 m2 of body-surface area, normal potassium levels, hepatic aminotransferase levels of less than three times the upper limit of the normal range, no contraindications to any polypill component, status of not being pregnant, and current use of no more than two antihypertensive medications. In June 2016, the criterion for the upper boundary of systolic blood pressure (160 mm Hg) was removed after consultation with the institutional review board and the data and safety monitoring board. Eligible participants who provided written informed consent were randomly assigned to receive either the polypill or usual care.
Treatments.
Participants who were assigned to the polypill group received 90-day refillable supplies of daily trial medication prepared by the Vanderbilt Investigational Drug Service. The polypill consisted of four low-dose medications: atorvastatin (10 mg), amlodipine (2.5 mg), losartan (25 mg), and hydrochlorothiazide (12.5 mg). Generic versions were placed securely in sealed gelatin capsules and bottled in 90-day supplies. The trial pills were produced at a cost to the investigators of $26 per month per participant. The initial dispensation of the polypill supply was shipped overnight to the individual participants, with subsequent refills shipped to the Franklin Primary Health Center pharmacy for distribution to participants.
Participants who were assigned to the usual care group were offered routine care at the Franklin Primary Health Center, in conjunction with any ongoing care that they were receiving from a primary care physician. For participants in either group, our trial team engaged in consistent communication with each participant’s primary care physician, including a standard letter conveying clinical data and the reminder that the physician was free to implement any additional therapies that were deemed to be appropriate.
Follow-up visits.
All participants were scheduled for follow-up visits at 2 months and 12 months after randomization. A clinical examination was conducted, blood pressure measured, and a fasting blood sample obtained. Adherence to the polypill regimen was assessed by means of pill counts performed by the trial coordinator at each trial related visit. Participants received $50 remuneration for each clinic visit that was completed.
Outcome measures.
The two primary outcomes were the changes in systolic blood pressure and LDL cholesterol level from baseline to 12 months. Blood-pressure data were obtained by calculating the mean of two resting, manual, in-clinic measurements of blood pressure by a trial nurse. An appropriately sized blood-pressure cuff was selected on the basis of the size of the patient. Lipid profiles were obtained by a trained phlebotomist and sent to a single, local laboratory facility. The Martin–Hopkins equation was used to calculate the LDL cholesterol level, with a direct measurement of the LDL cholesterol level when the triglyceride level exceeded 400 mg per deciliter (4.52 mmol per liter). In the polypill group, adherence to therapy was assessed by means of pill count and participant report. Secondary outcomes included changes from baseline to 12 months in the diastolic blood pressure, total cholesterol level, high-density lipoprotein cholesterol level, triglyceride level, and predicted 10-year risk of cardiovascular disease. The 2013 ACC–AHA risk estimator was used to predict the 10-year risk of cardiovascular disease on the basis of the pooled cohort equations; the risk score indicates the likelihood of a person having an atherosclerotic cardiovascular event in the next 10 years.27,28 Safety outcomes, including serious adverse events, were assessed in both trial groups. Specific side effects were assessed, including the incidence of myalgias, hypotension, and light-headedness.
Statistical analyses.
We estimated the power for our trial on the basis of assumptions about the degree of correlation between the baseline and 12-month values for both systolic blood pressure and LDL cholesterol level. Estimates of baseline variability were based on data from the SCCS and the Jackson Heart Study.29,30 Assuming a correlation (r) of 0.7 between the two measurements for the two primary outcomes, we calculated that the enrollment of 150 participants in each group would provide the trial with 80% power to detect a between-group difference of 5.3 mm Hg for the systolic blood pressure and 9.8 mg per deciliter (0.25 mmol per liter) for the LDL cholesterol level. Details are provided in the trial protocol.
For the primary analyses, we evaluated changes in systolic blood pressure and LDL cholesterol level from baseline to 12 months. Similar analyses were conducted for changes from baseline to 2 months. Crude differences were calculated and tested for significance with the use of Student’s t-test, followed by multivariable regression models with the difference as the dependent variable and treatment group as the primary exposure variable; additional covariates were age, sex, body-mass index, presence or absence of diabetes, presence or absence of hypertension of stage 2 or higher (baseline systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg), and cardiovascular risk score.28 In sensitivity analyses, we used multiple imputation to account for outcome data that were missing because of death or discontinuation. Two-sided P values of less than 0.05 were considered to indicate statistical significance for the two primary outcomes.
For secondary outcomes, between-group differences and 95% confidence intervals are reported. The 95% confidence intervals were not adjusted for multiplicity, and therefore inferences drawn from these intervals may not be reproducible. To assess adherence to the polypill regimen, we completed pill counts at each refill and computed the percentage of participants reporting at the 2-month and 12-month visits that they had taken the polypill the day before. Prespecified analyses of subgroups according to sex, hypertension of stage 2 or higher (yes or no), baseline prescription antihypertensive therapy (yes or no), baseline LDL cholesterol level (<130 or ≥130 mg per deciliter [<3.5 or ≥3.5 mmol per liter]), and baseline statin therapy (yes or no) were performed.
RESULTS
Characteristics of the Participants.
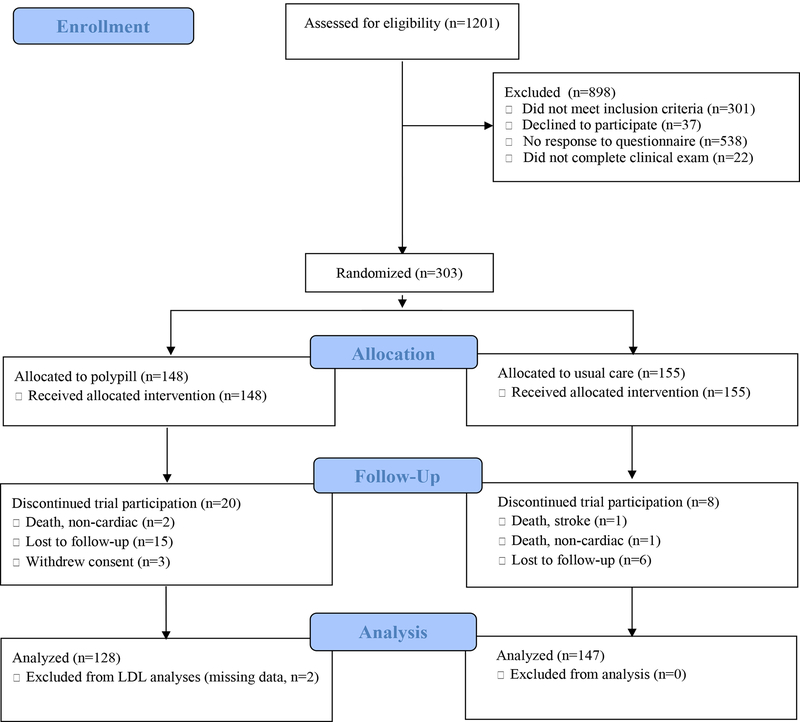
From December 2015 through July 2017, a total of 977 SCCS participants met the age criteria for trial eligibility, and 439 returned prescreening questionnaires. A total of 279 potentially eligible SCCS participants underwent screening examinations, and 150 (54%) were enrolled. In addition, 224 Franklin Primary Health Center patients or residents of the surrounding community underwent screening examinations; from this group, 176 persons were found to be eligible, and 153 (68%) were enrolled. Thus, a total of 303 participants were enrolled and underwent randomization, 148 to the polypill group and 155 to the usual-care group (Fig. 1).
Figure 1:
CONSORT Diagram
A total of 60% of the participants were women. The mean age of the participants was 56 years, and the trial population was predominantly black (96%) (Table 1). Approximately three quarters of the participants reported having an annual household income below $15,000. Obesity was common; the mean body-mass index (the weight in kilograms divided by the square of the height in meters) of the participants exceeded 30, and 43% of the participants had hypertension of stage 2 or higher. The mean estimated 10-year cardiovascular risk was 12.7% overall (12.4% in the polypill group and 13.0% in the usual-care group). Overall, the baseline blood pressure was 140/83 mm Hg, and the baseline LDL cholesterol level was 113 mg per deciliter (2.90 mmol per liter). None of the baseline characteristics differed significantly (P<0.05) between the groups.
Table 1:
Baseline Characteristics
| Characteristic* | Polypill (n=148) | Usual Care (n=155) |
|---|---|---|
| Mean age, years | 56 ± 6 | 56 ± 6 |
| Male sex | 65 (44%) | 56 (36%) |
| African-American | 141 (95%) | 151 (97%) |
| Mean BMI, kg/m2 | 31.3 ± 8.5 | 30.4 ± 8.4 |
| Stage 2+ hypertension | 62 (42%) | 67 (43%) |
| Diabetes mellitus | 17 (11%) | 22 (14%) |
| Mean predicted 10-year risk of CVD | 12.4% ± 8.9 | 13.0% ± 10.1 |
| Current smoking | 65 (44%) | 80 (52%) |
| Taking any anti-hypertensive med | 78 (53%) | 84 (54%) |
| Taking statin | 26 (18%) | 27 (17%) |
| Taking amlodipine | 31 (21%) | 35 (23%) |
| Taking losartan | 6 (4%) | 14 (9%) |
| Taking HCTZ | 27 (18%) | 26 (17%) |
| Mean systolic blood pressure, mmHg | 140 ± 18 | 140 ± 17 |
| Mean diastolic blood pressure, mm Hg | 83 ± 8 | 83 ± 8 |
| Mean LDL cholesterol, mg/dL | 114 ± 32 | 112 ± 37 |
| Mean HDL cholesterol, mg/dl | 61 ± 21 | 64 ± 23 |
| Mean triglycerides, mg/dl | 116 ± 86 | 110 ± 74 |
| Annual household income** | ||
| < $15,000 | 107 (72%) | 120 (77%) |
| $15,000 to <$25,000 | 28 (19%) | 21 (14%) |
| $25,000 to <$50,000 | 7 (5%) | 11 (7%) |
| $50,000 to <$100,000 | 6 (4%) | 3 (2%) |
No baseline characteristics differ at P<0.05
Annual household income based on self-report.
Medication use.
At the final visit, 80% of the participants who had received a prescription for polypills reported having taken the pill the day before. On the basis of counts of unused pills that were conducted at each refill visit, the median adherence to the polypill regimen was 86% (interquartile range, 79 to 93).
In the polypill group, clinicians reduced doses of other antihypertensive or lipid-lowering medications or discontinued their use in 44% of the patients. A total of 2% of the participants in the polypill group had an escalation in therapy. In the usual-care group, none of the participants had a deescalation of therapy and 10% had an escalation of therapy.
Outcomes.
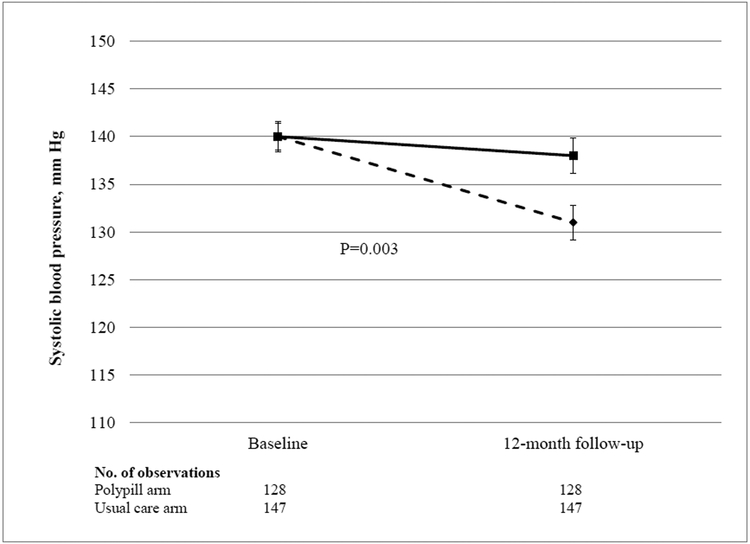
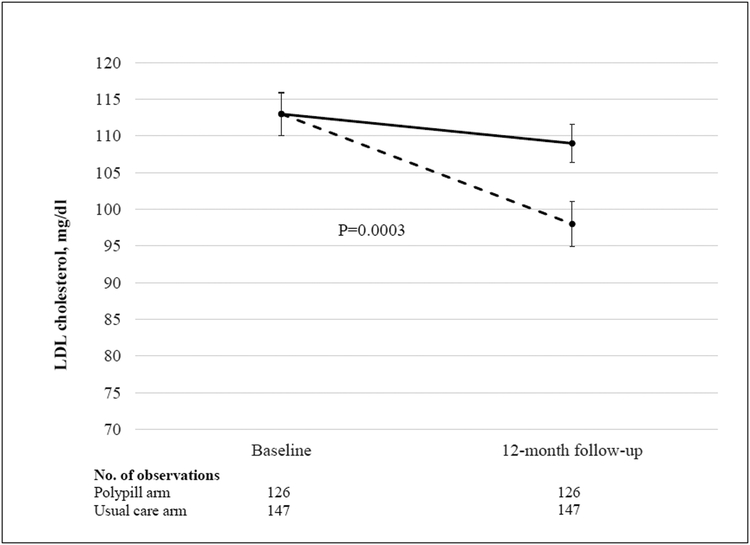
A total of 275 participants (91%) completed the 12-month trial visit. Data for the two primary outcomes are shown in Table 2 and Figure 2. The mean systolic blood pressure decreased by 9 mm Hg in the polypill group, as compared with 2 mm Hg in the usual-care group (difference, −7 mm Hg; 95% confidence interval [CI], −12 to −2; P = 0.003). The mean LDL cholesterol level decreased by 15 mg per deciliter (0.40 mmol per liter) in the polypill group, as compared with 4 mg per deciliter (0.10 mmol per liter) in the usual-care group (difference, −11 mg per deciliter; 95% CI, −18 to −5 [−0.30 mmol per liter; 95% CI, −0.45 to −0.10]; P<0.001). To account for patients with missing data on the systolic blood pressure or LDL cholesterol level at 12 months, we performed a sensitivity analysis using multiple imputation. The multivariable-adjusted differences in the changes from baseline to 12 months remained significant for both systolic blood pressure (between group difference, −7 mm Hg; 95% CI, −11 to −2; P = 0.002) and LDL cholesterol level (between group difference, −11 mg per deciliter; 95% CI, −17 to −5 [−0.30 mmol per liter; 95% CI, −0.45 to −0.10]; P<0.001
Table 2:
Primary and secondary end points, by study arm
| Polypill | Usual Care | Difference (95%, CI)* | P | |||
|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | |||
| Primary End Points | ||||||
| Systolic BP, mmHg | 140 (19) | 131 (21) | 140 (17) | 138 (23) | −7 (−12, −2) | 0.003 |
| LDL cholesterol, mg/dl | 113 (33) | 98 (35) | 113 (37) | 109 (32) | −11 (−18, −5) | 0.0003 |
| Secondary End Points | ||||||
| Diastolic BP, mmHg | 83 (8) | 78 (9) | 83 (8) | 81 (10) | −3 (−5, −1) | - |
| TC, mg/dl | 198 (37) | 183 (47) | 199 (42) | 194 (37) | −11 (−19, −3) | - |
| HDL cholesterol, mg/dl | 62 (21) | 60 (21) | 64 (23) | 63 (21) | −1 (−4, 2) | - |
| TG, mg/dl | 116 (88) | 118 (104) | 110 (76) | 115 (71) | −2 (−20, 15) | - |
| CVD risk estimate | 12.0 (8.8) | 9.4 (8.0) | 12.8 (9.9) | 13.3 (11.5) | −3.1 (−4.6, −1.6) | - |
Values shown are mean (SD). BP, blood pressure; TC, total cholesterol; TG, triglycerides; CVD, cardiovascular disease. Sample size for systolic blood pressure (at both baseline and 12 months): polypill arm (n=128), usual care (n=147). Sample size for LDL cholesterol (at both baseline and 12 months): polypill arm (n=126), usual care (n=147). Twenty participants in the polypill arm and 8 participants in the usual care arm discontinued trial participation and did not have data at 12 months. In addition, two participants in the polypill arm had missing LDL data, due to insufficient blood sample (n=1) or missing baseline direct LDL cholesterol measurement (n=1). One TG observation was censored due to value > 1000.
95% confidence intervals presented in this report have not been adjusted for multiplicity, and therefore inferences drawn from these intervals may not be reproducible.
Figure 2:
Changes in systolic blood pressure (Figure 2A) and LDL cholesterol (Figure 2B) at 12 months, in participants randomized to polypill (dashed line) or usual care (solid line).
Data for the secondary outcomes are shown in Table 2. At 12 months, the net difference in the diastolic blood pressure between the polypill group and the usual-care group was −3 mm Hg (95% CI, −5 to −1), and the net difference in the total cholesterol level was −11 mg per deciliter (95% CI, −19 to −3 [−0.30 mmol per liter; 95% CI, −0.50 to −0.10]). Changes in the systolic blood pressure and LDL cholesterol level in the two treatment groups, according to prespecified subgroups, are shown in Table 3. We also examined systolic blood pressure and LDL cholesterol level at the 2-month visit, with data available for 291 participants (96%). At the 2-month visit, the net between-group difference in the systolic blood pressure was −5 mm Hg (95% CI, −9 to −2), and the net between-group difference in the LDL cholesterol level was −18 mg per deciliter (95% CI, −24 to −12 [−0.45 mmol per liter; 95% CI, −0.60 to −0.30]).
Table 3:
Changes in systolic blood pressure and LDL cholesterol, in pre-specified subgroups
| End Point | Subgroup | Polypill | Usual Care | |||
|---|---|---|---|---|---|---|
| Baseline | 12 mos | Baseline | 12 mos | Interaction P | ||
| SBP, mmHg | Male (n=103) | 144 (20) | 136 (23) | 140 (16) | 135 (20) | 0.15 |
| Female (n=172) | 138 (17) | 128 (19) | 140 (18) | 139 (25) | ||
| Stage 2 HTN (n=119) | 157 (16) | 141 (21) | 156 (14) | 151 (25) | 0.21 | |
| No stage 2 HTN (n=156) | 127 (6) | 123 (16) | 128 (6) | 129 (17) | ||
| On baseline therapy (n=146) | 139 (17) | 131 (19) | 142 (18) | 142 (25) | 0.82 | |
| No baseline therapy (n=129) | 142 (20) | 132 (22) | 137 (16) | 133 (20) | ||
| LDL cholesterol, mg/dl | Male (n=102) | 105 (36) | 93 (35) | 101 (38) | 100 (34) | 0.84 |
| Female (n=171) | 118 (30) | 101 (35) | 119 (35) | 114 (29) | ||
| LDL ≥ 130 (n=81) | 151 (17) | 122 (33) | 157 (19) | 137 (26) | 0.55 | |
| LDL < 130 (n=192) | 98 (23) | 88 (30) | 94 (24) | 97 (26) | ||
| On baseline statin (n=43) | 108 (33) | 101 (31) | 102 (30) | 102 (25) | 0.53 | |
| No baseline statin (n=230) | 114 (33) | 98 (35) | 115 (38) | 110 (33) | ||
Values shown are mean (SD). SBP, systolic blood pressure; HTN, hypertension. Twenty participants in the polypill arm and 8 participants in the usual care arm discontinued trial participation and did not have data at 12 months. In addition, two participants in the polypill arm had missing LDL cholesterol data, due to insufficient blood sample (n=1) or missing baseline direct LDL cholesterol measurement (n=1). For SBP, ‘baseline therapy’ refers to ongoing pre-enrollment use of any prescription anti-hypertensive therapy.
Safety.
During the trial, there were five serious adverse events: three noncardiac deaths (one death due to complications after urologic surgery and one death due to acute alcohol intoxication in the polypill group and one death due to a motor vehicle accident in the usual-care group), one death from stroke (in the usual-care group), and one case of coronary-artery bypass surgery (in the usual-care group). None of the events were judged by the data and safety monitoring board to be related to the trial. In the polypill group, the reported incidence of myalgias was 1% and the incidence of hypotension or light-headedness was 1%. No participants in the polypill group had abnormal results on liver-function tests.
DISCUSSION
In this randomized trial, the use of a polypill yielded greater reductions from baseline in systolic blood pressure and LDL cholesterol level than were observed with usual care in a socioeconomically vulnerable, minority population. There are several distinctive aspects of this trial. First, the trial showed the feasibility and effectiveness of a polypill-based strategy in a real-world clinical setting in which most patients reported an annual household income of less than $15,000. Second, there are limited data on the use of a dedicated polypill in the United States, especially in black patients, in whom patterns of cardiovascular risk factors may differ from those in white patients. Third, the trial was conducted entirely at a federally qualified community health center. These centers provide an important safety net in medically underserved communities, but these populations of patients are poorly represented in clinical trials.
The observed reductions in systolic blood pressure and LDL cholesterol level were statistically and clinically significant. On the basis of meta-analyses of cardiovascular-outcomes trials in primary prevention,31–37 we estimate that such changes, if sustained, would lead to an approximate 25% reduction in the incidence of cardiovascular events. This figure is consistent with the 25% relative reduction in the estimated cardiovascular risk that was observed among the participants who had been randomly assigned to receive the polypill, as compared with those assigned to receive usual care (Table 2).
Retention in the trial was high, with 91% of participants completing the final trial visit. Notwithstanding the limitations of participant reports and pill counts, adherence in the polypill group appeared to be high, a finding that is noteworthy given that approximately half the patients in the United States stop their prescribed cardiovascular medications within 1 year.5 The simplicity of taking a single daily pill may be an important contributor to adherence.
Participants in the trial were free to continue or discontinue their nontrial medications. One potential concern is undertreatment if patients substitute the polypill for more potent regimens. It is reassuring, then, that the subgroup of participants who had been taking statins or antihypertensive medications before trial enrollment still had reductions in blood pressure and LDL cholesterol level with the polypill.
Several limitations of the trial warrant comment. We used an open-label design to preserve clinician flexibility to adjust medications in either trial group and to assess the real-world effectiveness of the polypill approach. We cannot rule out the possibility that the treatment of participants in one or both groups was influenced by trial involvement or trial-group assignment. We also acknowledge that the trial was conducted in a single community health center and therefore may not be generalizable to other settings.
The primary outcomes could be ascertained only in participants who completed the 12-month visit. Thus, between-group differences in loss to follow-up could have influenced the results. However, the results were similar in sensitivity analyses in which multiple imputation was used for missing outcome data. In addition, even if the results for each participant in the polypill group who did not complete the 12-month visit did not differ from those for patients who received usual care, the overall mean reductions in systolic blood pressure and LDL cholesterol level would be attenuated only slightly (to between group differences of −6 mm Hg and −10 mg per deciliter [−0.25 mmol per liter], respectively).
Participants in the polypill group were not charged for the trial medication, which introduced the possibility that reduced drug cost contributed to the results. However, our trial site had a 340B pharmacy program that provided medications free of charge or nearly free of charge to all uninsured patients. The only expense would have been a copayment of $3 or less for a 90-day supply of medication. Thus, drug cost was probably not a substantial barrier in the usual-care group.
We recognize that a “one size fits all” approach to cardiovascular disease prevention runs counter to current trends in precision medicine, in which clinical, genomic, and lifestyle factors are used for the development of individualized treatment strategies.38,39 Although the precision approach has clear virtues, a broader approach may benefit patients who face barriers to accessing the full advantages of precision medicine. Challenges in implementing cardiovascular disease prevention that are due to lack of income, underinsurance, and multiple visits for testing and drug-dose adjustment may be especially problematic in populations with low socioeconomic status. Thus, the simplicity and low cost of the polypill regimen make this approach attractive when such barriers are common. It is important to emphasize that use of the polypill does not preclude individualized, add-on therapies for residual elevations in blood-pressure or cholesterol levels, as judged by a patient’s physician.
In conclusion, in this randomized trial in a low-income, minority population, a polypill-based strategy led to reductions from baseline in systolic blood pressure and LDL cholesterol level that were significantly greater than those observed with usual care.
Supplementary Material
ACKNOWLEDGMENTS
We thank Michael Blaha (chair), Harvey Murff, Chang Yu, and Martha Shrubsole for serving on the data and safety monitoring board; Robert Dittus, Wei Zheng, MacRae Linton, David Maron, and Deborah Friedman for input on the trial design; Michelle Rohlsen Karp and Anne Woodhouse for contributions to field work and trial recruitment; and key staff members, especially Judy Mitchell at the Franklin Primary Health Center, for operational assistance with this trial.
FUNDING
Supported by grants from the American Heart Association Strategically Focused Prevention Research Network (to Drs. Blot and Wang) and the National Institutes of Health (U01-CA202979, to Dr. Blot).
REFERENCES
- 1.Roth GA, Johnson C, Abajobir A, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mensah GA, Cooper RS, Siega-Riz AM, et al. Reducing Cardiovascular Disparities Through Community-Engaged Implementation Research: A National Heart, Lung, and Blood Institute Workshop Report. Circ Res 2018;122:213–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, et al. Trends and Patterns of Geographic Variation in Cardiovascular Mortality Among US Counties, 1980–2014. JAMA 2017;317:1976–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 5.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA 2003;290:199–206. [DOI] [PubMed] [Google Scholar]
- 6.Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr., et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014;370:1422–31. [DOI] [PubMed] [Google Scholar]
- 7.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- 8.Ahluwalia JS, McNagny SE, Rask KJ. Correlates of controlled hypertension in indigent, inner-city hypertensive patients. J Gen Intern Med 1997;12:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill MN, Bone LR, Kim MT, Miller DJ, Dennison CR, Levine DM. Barriers to hypertension care and control in young urban black men. Am J Hypertens 1999;12:951–8. [DOI] [PubMed] [Google Scholar]
- 10.Kotchen JM, Shakoor-Abdullah B, Walker WE, Chelius TH, Hoffmann RG, Kotchen TA. Hypertension control and access to medical care in the inner city. Am J Public Health 1998;88:1696–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shea S, Misra D, Ehrlich MH, Field L, Francis CK. Predisposing factors for severe, uncontrolled hypertension in an inner-city minority population. N Engl J Med 1992;327:776–81. [DOI] [PubMed] [Google Scholar]
- 12.Rose G. Sick individuals and sick populations. Int J Epidemiol 1985;14:32–8. [DOI] [PubMed] [Google Scholar]
- 13.Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 2003;290:898–904. [DOI] [PubMed] [Google Scholar]
- 14.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 2006;355:2631–9. [DOI] [PubMed] [Google Scholar]
- 15.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ 2003;326:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 2003;326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow CK, Thakkar J, Bennett A, et al. Quarter-dose quadruple combination therapy for initial treatment of hypertension: placebo-controlled, crossover, randomised trial and systematic review. Lancet 2017;389:1035–42. [DOI] [PubMed] [Google Scholar]
- 18.Group PC, Rodgers A, Patel A, et al. An international randomised placebo-controlled trial of a four-component combination pill (“polypill”) in people with raised cardiovascular risk. PLoS One 2011;6:e19857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Indian Polycap S, Yusuf S, Pais P, et al. Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trial. Lancet 2009;373:1341–51. [DOI] [PubMed] [Google Scholar]
- 20.Patel A, Cass A, Peiris D, et al. A pragmatic randomized trial of a polypill-based strategy to improve use of indicated preventive treatments in people at high cardiovascular disease risk. Eur J Prev Cardiol 2015;22:920–30. [DOI] [PubMed] [Google Scholar]
- 21.Selak V, Elley CR, Bullen C, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. BMJ 2014;348:g3318. [DOI] [PubMed] [Google Scholar]
- 22.Soliman EZ, Mendis S, Dissanayake WP, et al. A Polypill for primary prevention of cardiovascular disease: a feasibility study of the World Health Organization. Trials 2011;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wald DS, Morris JK, Wald NJ. Randomized Polypill crossover trial in people aged 50 and over. PLoS One 2012;7:e41297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster R, Salam A, de Silva HA, et al. Fixed Low-Dose Triple Combination Antihypertensive Medication vs Usual Care for Blood Pressure Control in Patients With Mild to Moderate Hypertension in Sri Lanka: A Randomized Clinical Trial. JAMA 2018;320:566–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yusuf S, Lonn E, Pais P, et al. Blood-Pressure and Cholesterol Lowering in Persons without Cardiovascular Disease. N Engl J Med 2016;374:2032–43. [DOI] [PubMed] [Google Scholar]
- 26.Signorello LB, Hargreaves MK, Blot WJ. The Southern Community Cohort Study: investigating health disparities. J Health Care Poor Underserved 2010;21:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ASCVD Risk Estimator Plus. 2016. at http://tools.acc.org/ASCVD-Risk-Estimator-Plus/.)
- 28.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S49–73. [DOI] [PubMed] [Google Scholar]
- 29.Sampson UK, Edwards TL, Jahangir E, et al. Factors associated with the prevalence of hypertension in the southeastern United States: insights from 69,211 blacks and whites in the Southern Community Cohort Study. Circ Cardiovasc Qual Outcomes 2014;7:33–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor HA Jr., Akylbekova EL, Garrison RJ, et al. Dyslipidemia and the treatment of lipid disorders in African Americans. Am J Med 2009;122:454–63. [DOI] [PubMed] [Google Scholar]
- 31.Blood Pressure Lowering Treatment Trialists C, Ninomiya T, Perkovic V, et al. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta-analysis of randomised controlled trials. BMJ 2013;347:f5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–67. [DOI] [PubMed] [Google Scholar]
- 34.Force USPST, Bibbins-Domingo K, Grossman DC, et al. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2016;316:1997–2007. [DOI] [PubMed] [Google Scholar]
- 35.Silverman MG, Ference BA, Im K, et al. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016;316:1289–97. [DOI] [PubMed] [Google Scholar]
- 36.Sundstrom J, Arima H, Jackson R, et al. Effects of blood pressure reduction in mild hypertension: a systematic review and meta-analysis. Ann Intern Med 2015;162:184–91. [DOI] [PubMed] [Google Scholar]
- 37.Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: a critical reappraisal. Circ Res 2015;116:1058–73. [DOI] [PubMed] [Google Scholar]
- 38.Khoury MJ, Galea S. Will Precision Medicine Improve Population Health? JAMA 2016;316:1357–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Psaty BM, Dekkers OM, Cooper RS. Comparison of 2 Treatment Models: Precision Medicine and Preventive Medicine. JAMA 2018;320:751–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.