Abstract
Chronic hypoxia augments pressure- and agonist-induced pulmonary vasoconstriction through myofilament calcium sensitization. NADPH oxidases contribute to the development of pulmonary hypertension, and both epidermal growth factor receptor and Src kinases can regulate NADPH oxidase. We tested the hypothesis that Src–epidermal growth factor receptor (EGFR) signaling mediates enhanced vasoconstrictor sensitivity after chronic hypoxia through NADPH oxidase–derived superoxide generation. Protocols employed pharmacological inhibitors in isolated, pressurized rat pulmonary arteries to examine the contribution of a variety of signaling moieties to enhanced vascular tone after chronic hypoxia. Superoxide generation in pulmonary arterial smooth muscle cells was assessed using the fluorescent indicator dihydroethidium. Indices of pulmonary hypertension were measured in rats treated with the EGFR inhibitor gefitinib. Inhibition of NADPH oxidase, Rac1 (Ras-related C3 botulinum toxin substrate 1), and EGFR abolished pressure-induced pulmonary arterial tone and endothelin-1 (ET-1)-dependent calcium sensitization and vasoconstriction after chronic hypoxia. Consistently, chronic hypoxia augmented ET-1–induced superoxide production through EGFR signaling, and rats treated chronically with gefitinib displayed reduced right ventricular pressure and diminished arterial remodeling. Src kinases were also activated by ET-1 after chronic hypoxia and contributed to enhanced basal arterial tone and vasoconstriction in response to ET-1. A role for matrix metalloproteinase 2 to mediate Src-dependent EGFR activation is further supported by our findings. Our studies support a novel role for an Src kinase–EGFR–NADPH oxidase signaling axis to mediate enhanced pulmonary vascular smooth muscle Ca2+ sensitization, vasoconstriction, and pulmonary hypertension after chronic hypoxia.
Keywords: vascular smooth muscle, NADPH oxidase, endothelin-1, pulmonary hypertension, metalloproteinases
Clinical Relevance
This study characterizes a previously undescribed signaling axis in pulmonary vascular smooth muscle involving NADPH oxidase, epidermal growth factor receptor, Src family kinases, and matrix metalloproteinases that mediates pressure- and agonist-induced myofilament Ca2+ sensitization and vasoconstriction in pulmonary arteries of rats with chronic hypoxia–induced pulmonary hypertension. We further show that chronic treatment of rats with an epidermal growth factor receptor inhibitor attenuates indices of chronic hypoxia–induced pulmonary hypertension. These findings have potential to provide new therapeutic strategies for pulmonary hypertension that target the components of this signaling pathway.
Increased pulmonary vascular resistance resulting from vasoconstriction and arterial remodeling is a hallmark of pulmonary arterial hypertension (World Health Organization group 1) as well as of hypoxia-associated pulmonary hypertensive disorders (group 3), including chronic obstructive pulmonary disease, interstitial lung disease, developmental abnormalities, and sleep apnea. Although precapillary constriction is important in the development of pulmonary hypertension (pHTN), the mechanisms involved are poorly understood. Evidence that vasodilators acutely reverse pHTN in a variety of experimental models (1–3) indicates that vasoconstrictor mechanisms provide a major contribution to pHTN. Such mechanisms include both pressure- and depolarization-induced vasoconstriction (2, 4, 5) and enhanced vasoconstrictor reactivity to endogenous G protein–coupled receptor (GPCR) agonists (6–8).
Increased vascular smooth muscle (VSM) Ca2+ sensitivity resulting from Rho kinase (ROK) activation is a primary mechanism of vasoconstriction, arterial remodeling, and associated pHTN resulting from both chronic hypoxia (CH) (2, 5, 6, 8–10) and a rat model of severe pHTN (1). ROK inhibition improves pulmonary hemodynamics in patients with pHTN (11), and pulmonary arteries from patients with idiopathic pHTN display elevated ROK activity and enhanced ROK-dependent contraction (12). Our laboratory and others have identified an important role for RhoA/ROK-mediated Ca2+ sensitization in the development of pressure-induced (myogenic) pulmonary arterial constriction, as well as enhanced GPCR- and depolarization-dependent vasoconstriction, after CH (4–6, 9). These stimuli are coupled to RhoA activation via O2− (5, 6, 10), consistent with evidence that reactive oxygen species (ROS) derived from NADPH oxidase (NOX) play an important role in the development of pHTN (7). However, neither the mechanisms that link these stimuli to O2−-dependent RhoA activation, Ca2+ sensitization, and vasoconstriction after CH nor their contribution to the development of pHTN in this setting is well understood. Interestingly, a Src kinase (Src)/epidermal growth factor receptor (EGFR) signaling pathway is activated by such stimuli in other cell types (13), thus representing a potential mechanism mediating pHTN in response to CH.
The present study tested the hypothesis that Src–EGFR signaling couples membrane stretch and ET-1 receptor stimulation to enhanced pulmonary vasoconstriction after CH through NOX-dependent O2− production. We examined the role of NOX, EGFR, and Src to mediate pressure-dependent tone and agonist-induced vasoconstriction in pulmonary arteries from CH and normoxic control rats. Because Src family kinases can activate EGFR either directly (14) or through matrix metalloproteinases (MMPs) (15, 16), a complementary goal was to examine how EGFR is activated in this setting. Our findings reveal a unique signaling mechanism in the pulmonary circulation that is induced by CH and links arterial wall stretch and receptor-mediated activation of Src/MMP/EGFR signaling to stimulation of the NOX2 isoform, myofilament Ca2+ sensitization, and vasoconstriction. These studies further support a novel role for EGFR in the development of CH-induced pHTN.
Methods
All protocols were conducted using normoxic and CH (4 wk; barometric pressure, ∼380 mm Hg) male Sprague Dawley rats and were approved by the institutional animal care and use committee of the University of New Mexico Health Sciences Center. An expanded Methods section detailing the protocols and statistics used is available in the data supplement.
Results
O2−, but Not H2O2, Mediates Pressure-Dependent Tone in Pulmonary Arteries from CH Rats
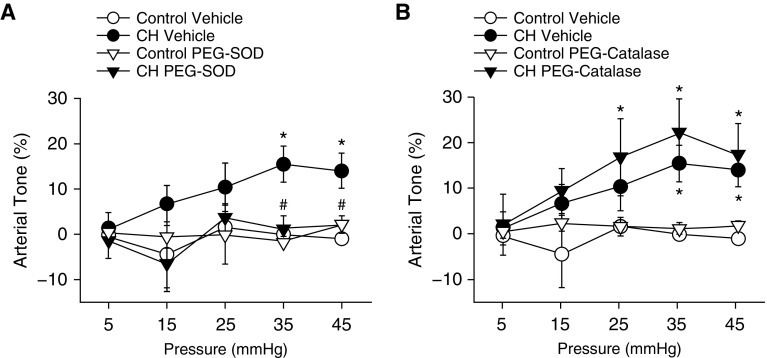
The relative contribution of O2− and H2O2 to basal tone after CH was evaluated in endothelium-disrupted small pulmonary arteries (100–200-μm inner diameter) using simultaneous measurement of vessel inner diameter and vessel wall [Ca2+]i at physiological transmural pressures (5, 6). All vessels were endothelium disrupted to isolate the influence of VSM ROS. In prior studies using this preparation, CH enabled pressure-dependent tone that was independent of L-type Ca2+ channels or changes in [Ca2+]i, but instead was mediated by ROK (4). Arteries were loaded with Fura-2 acetoxymethyl ester (Fura-2AM) to monitor vessel wall [Ca2+]i. Responses to increasing intraluminal pressure were assessed in arteries from control and CH rats in the presence of the O2− scavenger polyethylene glycol–superoxide dismutase (SOD) or the H2O2 scavenger polyethylene glycol–catalase (17, 18). Basal tone was observed in arteries from CH but not control rats (Figure 1). SOD prevented the development of CH-induced basal tone (Figure 1A), whereas catalase had no significant effect in either group (Figure 1B). There were no differences between groups in baseline diameter or vessel wall [Ca2+]i (Table 1). Vessel wall [Ca2+]i did not vary with pressure in either group (data not shown), as previously reported (4).
Figure 1.
O2− contributes to pressure-dependent pulmonary arterial tone after chronic hypoxia (CH). Arterial tone (percentage of passive inner diameter) as a function of increasing intraluminal pressure in arteries from CH and control rats in the presence of (A) polyethylene glycol–superoxide dismutase (PEG-SOD; 120 U/ml), (B) polyethylene glycol–catalase (PEG-catalase; 250 U/ml), or their vehicle. Arteries were endothelium disrupted but not permeabilized to Ca2+. Vessel inner diameter was measured in 10-mm Hg pressure steps beginning at 5 mm Hg and ending at 45 mm Hg. Passive inner diameter was determined by repeating pressure steps after removal of extracellular Ca2+ (4). Values are mean ± SE of n = 4–5 rats/group. *P < 0.05 versus control. #P < 0.05 CH drug versus CH vehicle.
Table 1.
Baseline Inner Diameter and Vessel Wall [Ca2+]i in Arteries from Control and Chronically Hypoxic Rats
| Treatment | Inner Diameter (μm) | Vessel Wall [Ca2+]i (F340/F380) | n |
|---|---|---|---|
| Control | 147 ± 5 | 0.91 ± 0.04 | 121 |
| CH | 154 ± 9 | 0.93 ± 0.02 | 123 |
| Control (Ca2+ permeabilized) | 162 ± 4 | 1.11 ± 0.04 | 49 |
| CH (Ca2+ permeabilized) | 169 ± 7 | 1.06 ± 0.04 | 52 |
Definition of abbreviations: CH = chronic hypoxia; ET-1 = endothelin-1; F340/F380 = fura-2 340/380-nm emission ratio.
Values are mean ± SE. Nonpermeabilized arteries were used for basal tone and epidermal growth factor vasoreactivity studies. Ca2+ permeabilized arteries were used for ET-1 vasoreactivity protocols. All values were measured under resting conditions at an intraluminal pressure of 12 mm Hg.
NOX and Rac1 Are Required for Basal Tone and Enhanced ET-1–induced Myofilament Ca2+ Sensitization and Vasoconstriction after CH
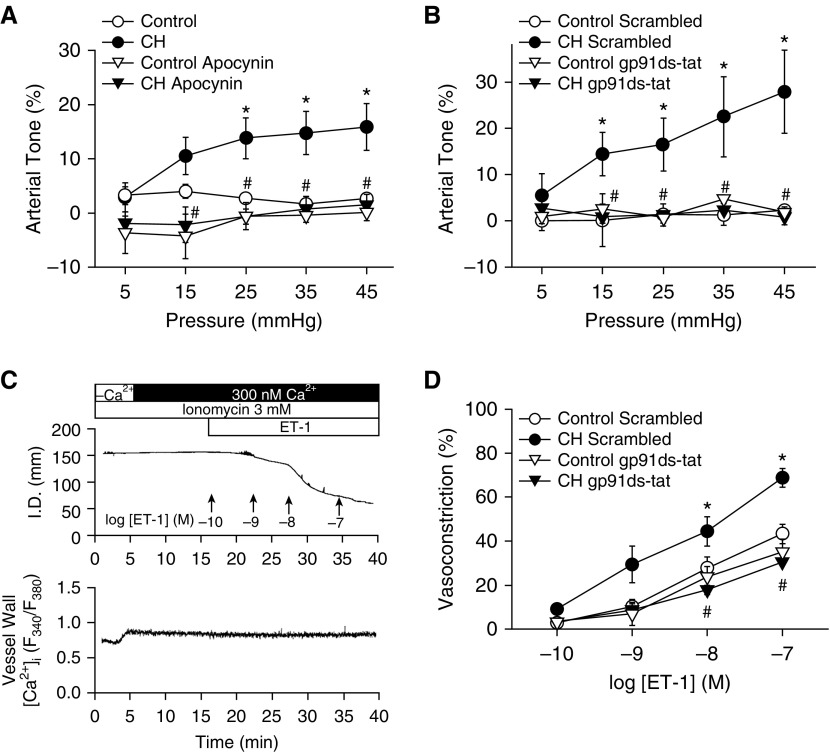
The role of NOX as a source of O2− mediating basal tone development after CH was tested in pulmonary arteries using the general NOX inhibitor apocynin and the NOX2-selective inhibitory peptide gp91 ds-tat (19). Similar to SOD (Figure 1A), apocynin and gp91 ds-tat prevented pressure-induced tone in CH arteries but had no effect in controls (Figures 2A and 2B).
Figure 2.
NADPH oxidase is required for CH-induced basal tone and augmented endothelin-1 (ET-1)–induced Ca2+ sensitization and vasoconstriction in small pulmonary arteries. (A and B) Basal arterial tone in nonpermeabilized arteries and (C and D) vasoconstrictor responses to ET-1 in Ca2+-permeabilized arteries in the presence of the NADPH oxidase inhibitor apocynin (30 μM; A) or gp91 ds-tat (50 μM; B and D) or their respective vehicles/negative controls. All arteries were endothelium disrupted. (C and D) Experiments using ET-1 were performed in arteries maintained at a transmural pressure of 12 mm Hg. Permeabilization to Ca2+ in ET-1–treated arteries was achieved using the Ca2+ ionophore ionomycin (3 μM) to directly assess mechanisms of myofilament Ca2+ sensitization independent of changes in vessel wall [Ca2+]i (6). All Ca2+-permeabilized vessels were equilibrated with physiological saline solution containing 300 nM Ca2+. Vessel wall [Ca2+]i is expressed as background-subtracted fura-2 340/380-nm emission ratios (F340/F380). Values are mean ± SE of n = 4–7 rats/group. *P < 0.05 versus control. #P < 0.05 CH drug versus CH vehicle or CH scrambled. I.D. = inner diameter; Scrambled = negative control peptide for gp91 ds-tat.
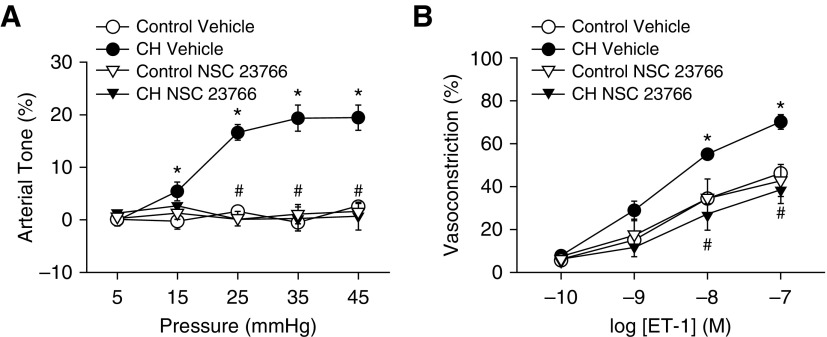
To directly evaluate the contribution of NOX-induced Ca2+ sensitization to augmented receptor-mediated vasoconstriction after CH, we examined the effect of gp91 ds-tat on ET-1–dependent vasoconstriction in endothelium-disrupted, pressurized (12 mm Hg) arteries from each group after permeabilization to Ca2+ with the Ca2+ ionophore ionomycin (6). Because vessel wall [Ca2+]i is clamped by the extracellular Ca2+ concentration (300 nM) in permeabilized vessels (confirmed using Fura-2AM) (Figure 2C), any vasoconstrictor response is a function of myofilament Ca2+ sensitization. Consistent with previous findings (6), CH exposure enhanced vasoconstriction in response to ET-1 in permeabilized arteries (Figure 2D). NOX2 inhibition attenuated vasoconstriction in response to ET-1 in arteries from CH but not control rats and normalized responses between groups (Figure 2D). There were no differences between groups in baseline diameter or vessel wall [Ca2+]i in permeabilized arteries (Table 1). Further supporting a role for NOX2 in augmented vasoreactivity after CH, inhibition of the NOX1/2 accessory protein Rac1 (Ras-related C3 botulinum toxin substrate 1) with NSC23766 prevented effects of CH to induce basal tone (Figure 3A) and augment ET-1–mediated vasoconstriction (Figure 3B).
Figure 3.
Contribution of Rac1 to basal tone and augmented ET-1–induced vasoconstriction after CH. (A and B) Basal arterial tone in nonpermeabilized arteries (A) and vasoconstrictor responses to ET-1 in Ca2+-permeabilized arteries (B) in the presence or absence of the Rac1 inhibitor NSC23766 (50 μM). All arteries were endothelium disrupted. Values are mean ± SE of n = 4–5 rats/group. *P < 0.05 versus control. #P < 0.05 CH NSC23766 versus CH vehicle.
EGFR Mediates Enhanced Vasoreactivity and ET-1–induced O2− Generation after CH
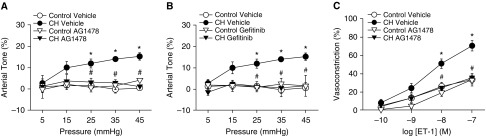
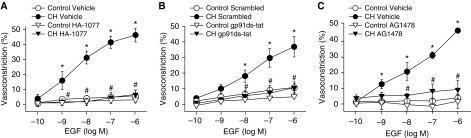
EGFR-mediated NOX2 activation provides a major source of O2− mediating enhanced depolarization-induced vasoconstriction in CH rats (10). Consistent with these results, EGFR inhibition with either AG1478 or gefitinib prevented basal tone development after CH (Figures 4A and 4B). Similarly, AG1478 prevented enhanced vasoconstriction in response to ET-1 in arteries from CH rats but had no effect in control arteries (Figure 4C).
Figure 4.
Epidermal growth factor receptor (EGFR) mediates enhanced pressure- and ET-1–dependent vasoconstrictor reactivity after CH. (A–C) Basal arterial tone in nonpermeabilized arteries (A and B) and vasoconstrictor responses to ET-1 in Ca2+-permeabilized arteries (C) in the presence of the EGFR inhibitors AG1478 (1 μM; A and C), gefitinib (50 μM; B), or their respective vehicles. All arteries were endothelium disrupted. Values are means ± SE of n = 4–6 rats/group. *P < 0.05 versus control. #P < 0.05 CH drug versus CH vehicle.
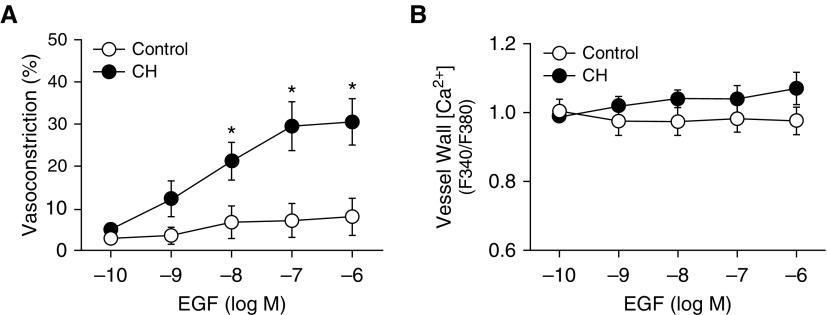
To further characterize mechanisms of EGFR-mediated vasoconstriction after CH, nonpermeabilized pulmonary arteries were stimulated with increasing concentrations of epidermal growth factor (EGF) to directly activate EGFR. Remarkably, EGF caused a robust, concentration-dependent constriction in arteries from CH rats but had no significant effect in control arteries (Figure 5A). This response occurred without a significant change in [Ca2+]i, supporting a role for Ca2+ sensitization in EGF-mediated vasoconstriction after CH (Figure 5B). Both ROK inhibition (HA-1077; Figure 6A) (20, 21) and NOX2 inhibition (Figure 6B) strongly attenuated constriction in response to EGF in CH arteries but had no effect in control vessels, consistent with effects of these agents on both pressure- and ET-1–mediated vasoconstriction. AG1478 diminished EGF-induced vasoconstriction in arteries from CH rats (Figure 6C), effectively normalizing responses to those of control vessels, thus verifying the contribution of EGFR to this response.
Figure 5.
CH augments EGF-induced vasoconstriction without altering vessel wall [Ca2+]i. (A and B) Vasoconstriction (A) and vessel wall [Ca2+]i (B) to increasing concentrations of EGF in endothelium-disrupted, pressurized, nonpermeabilized pulmonary arteries from CH and control rats. Values are mean ± SE of n = 4–5 rats/group. *P < 0.05 versus control.
Figure 6.
EGF-mediated vasoconstriction after CH is dependent on Rho kinase, NADPH oxidase 2, and EGFR. Vasoconstrictor responses to EGF in endothelium-disrupted, nonpermeabilized arteries from CH and control rats in the presence of a Rho kinase inhibitor (HA-1077; 10 μM) (A), the NADPH oxidase 2 inhibitory peptide (50 μM; gp91 ds-tat) (B), an EGFR inhibitor (1 μM; AG1478) (C), or their respective vehicles/negative controls. Values are mean ± SE of n = 4–7/group. *P < 0.05 versus control. #P < 0.05 CH drug versus CH vehicle or CH scrambled. Scrambled = negative control peptide for gp91 ds-tat.
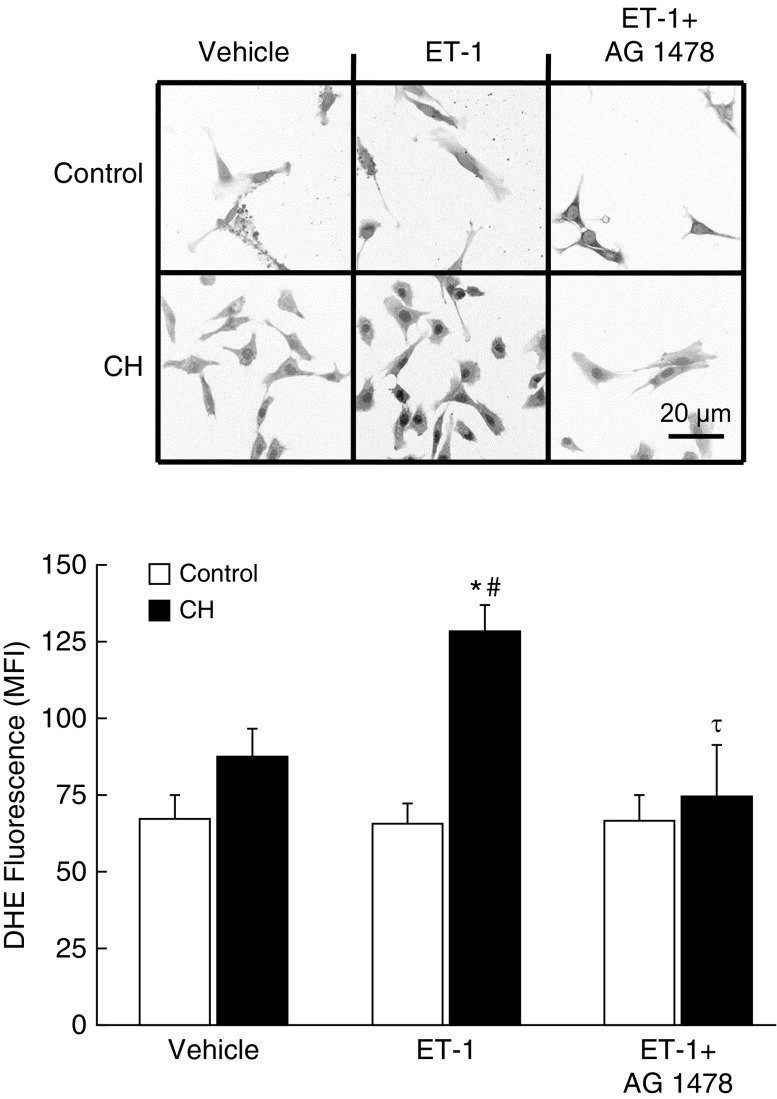
We have previously reported that ET-1 stimulates O2− generation in pulmonary arteries from CH rats (6). To evaluate the role of EGFR in this response, we measured basal and ET-1–stimulated O2− production by dihydroethidium (DHE) fluorescence in primary cultures of pulmonary arterial smooth muscle cells from CH and control rats. Basal DHE fluorescence was not different between control and CH groups (Figure 7). ET-1 selectively increased DHE fluorescence in cells from CH rats but had no significant effect in control cells. The EGFR inhibitor AG1478 prevented ET-1–induced increases in DHE fluorescence in cells from CH rats, resulting in similar fluorescence between groups. We have previously demonstrated the specificity of DHE for O2− versus H2O2 using this preparation (17, 18).
Figure 7.
CH increases ET-1–induced O2− production in pulmonary arterial smooth muscle cells through EGFR. Digitally inverted representative images and mean fluorescence intensity (MFI) of dihydroethidium (DHE) fluorescence in thresholded images of pulmonary arterial smooth muscle cells from CH and control rats. Cells were treated with vehicle, ET-1 (10−8 M), or ET-1 plus the EGFR inhibitor AG1478 (1 μM). Cells were isolated from intrapulmonary arteries (second–fifth order) and transiently cultured (3–4 d) before study (18, 19). MFI was averaged from five images/sample (one rat/sample). Each image was thresholded to select for positively stained areas above background. Values are mean ± SE of n = 4–7 rats/group. Scale bar: 20 μm. *P < 0.05 versus control ET-1. #P < 0.05 versus CH vehicle. τP < 0.05 versus CH ET-1.
Contribution of EGFR to Hypoxia-induced pHTN
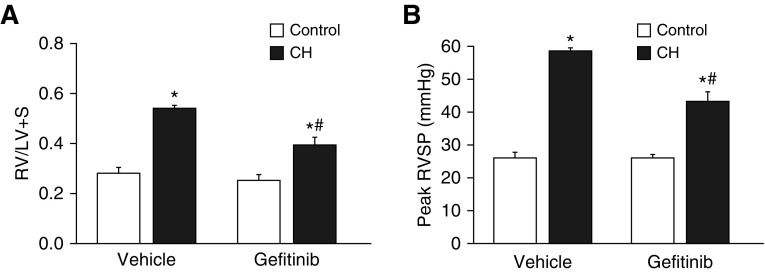
Rats were treated orally for 4 weeks with gefitinib (30 mg/kg/d) or vehicle pills to evaluate the role of EGFR signaling in the development of CH-induced pHTN. Animals exposed to CH exhibited lower body weight than normoxic rats (Table 2) (17). EGFR inhibition had no effect on body weight in either group. CH exposure increased right ventricle (RV)/left ventricle plus septum ratios (Figure 8A), demonstrating RV hypertrophy. RV/total ventricle weight and RV/body weight were similarly elevated after CH (Table 2). Gefitinib-treated CH animals displayed significantly lower indices of RV weight than vehicle-treated rats (Figure 8A and Table 2), supporting a role for EGFR in CH-induced RV hypertrophy. Left ventricle plus septum/body weight ratios were not significantly different between groups (Table 2). CH exposure produced a characteristic increase in hematocrit that was unaltered by gefitinib (Table 2).
Table 2.
Body Weight, Ventricular Weight Ratios, and Hematocrit
| Treatment | BW (g) | RV/T (mg/mg) | RV/BW (mg/g) | LV+S/BW (mg/g) | HCT (%) | HR |
|---|---|---|---|---|---|---|
| Control vehicle | 371 ± 8 | 0.22 ± 0.01 | 0.57 ± 0.02 | 2.03 ± 0.08 | 46.6 ± 0.5 | 319 ± 7 |
| CH vehicle | 303 ± 4* | 0.35 ± 0.01* | 1.08 ± 0.06* | 1.98 ± 0.07 | 70.0 ± 1.5* | 349 ± 6 |
| Control gefitinib | 359 ± 4 | 0.20 ± 0.01 | 0.52 ± 0.02 | 2.07 ± 0.09 | 45.6 ± 0.9 | 326 ± 11 |
| CH gefitinib | 306 ± 5* | 0.28 ± 0.02*† | 0.87 ± 0.06*† | 2.21 ± 0.05 | 67.8 ± 0.8* | 354 ± 15 |
Definition of abbreviations: BW = body weight; HCT = hematocrit; HR = heart rate; LV + S = left ventricle plus septum weight; RV = right ventricle weight; T = total ventricle weight.
Values are means ± SE of n = 5 rats/group.
P < 0.05 versus respective control.
P < 0.05 versus CH vehicle.
Figure 8.
EGFR contributes to CH-induced right ventricular hypertrophy and increases in right ventricular systolic pressure (RVSP). Right ventricle/left ventricle plus septum weight (RV/LV+S; A) and peak RVSP (B) from CH and control rats treated chronically with the EGFR inhibitor gefitinib (30 mg/kg/d) or vehicle pills. Heart rates were not significantly different between groups (Table 2). Values are mean ± SE of n = 4–5 rats/group. *P < 0.05 versus control. #P < 0.05 versus CH vehicle.
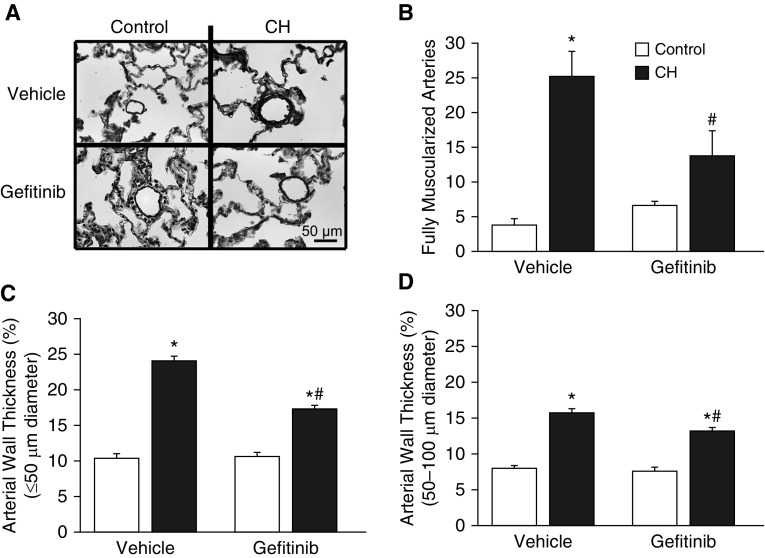
CH increased right ventricular systolic pressure (RVSP) in vehicle-treated rats, indicative of pHTN (Figure 8B). Consistent with effects of gefitinib to reduce the RV hypertrophic response after CH, EGFR inhibition lowered RVSP in CH rats but not control rats. Gefitinib similarly inhibited CH-induced pulmonary arterial remodeling (Figure 9A), as evidenced by a decreased incidence of fully muscularized small pulmonary arteries (<20 μm in diameter) (Figure 9B) and reduced arterial wall thickness (Figures 9C and 9D) compared with vehicle-treated CH rats.
Figure 9.
EGFR inhibition attenuates CH-induced pulmonary arterial remodeling. (A and B) Representative images of small pulmonary arteries (A) and number of fully muscularized arteries (<20 μm in diameter; B) from 20 images/section from CH and control rats administered daily gefitinib (30 mg/kg/d) or vehicle pills. (C and D) Arterial wall thickness (percentage outer diameter) of pulmonary arteries with diameters of less than 50 μm (C) and 50–100 μm (D). Numbers of fully muscularized arteries were assessed by fluorescence microscopy of lung sections labeled with an ACTA2 antibody. Arterial wall thickness was measured from elastin-stained sections (A). Values are mean ± SE of n = 5 rats/group. Scale bar: 50 μm. *P < 0.05 versus control. #P < 0.05 versus CH vehicle.
Src-Dependent EGFR Activation Contributes to Basal Tone and Augmented ET-1–induced Vasoconstriction after CH
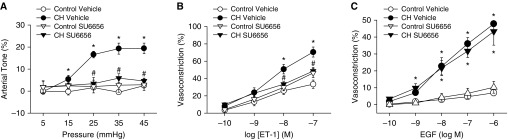
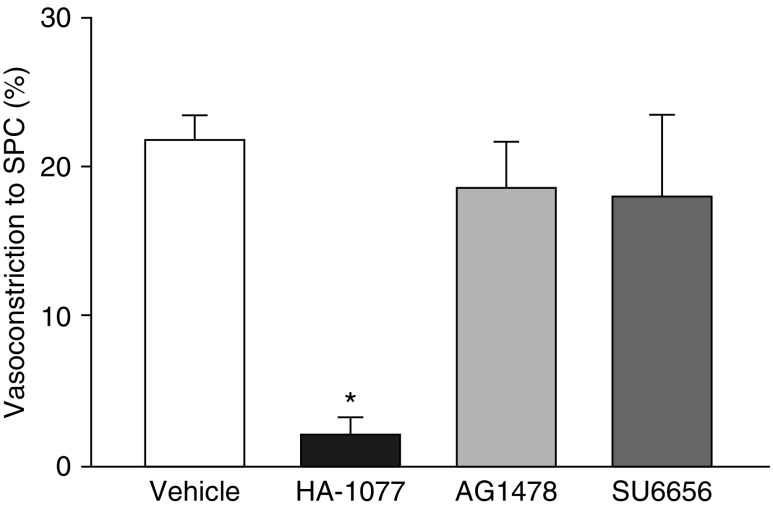
Because Src family kinases can regulate NOX and EGFR (14, 22), we examined their contribution to CH-dependent increases in vasoconstrictor reactivity. Src inhibition with SU6656 (13) prevented both pressure-dependent tone (Figure 10A) and enhanced vasoconstriction in response to ET-1 (Figure 10B) after CH. SU6656 also normalized responses to ET-1 between arteries from CH and control rats. In contrast, Src inhibition did not alter responses to EGF in either CH or control arteries (Figure 10C), suggesting that Src signals upstream of EGFR to mediate enhanced vasoreactivity after CH. We confirmed that Src kinase and EGFR inhibition did not alter ROK signaling by assessing vasoconstriction in response to the ROK activator sphingosylphosphorylcholine (10 μM) (23) in Ca2+-permeabilized arteries from control rats. Constriction in response to sphingosylphosphorylcholine was prevented by HA-1077 but not by AG1478 or SU6656 (Figure 11).
Figure 10.
Src kinases contribute to CH-induced increases in basal tone and ET-1–induced vasoconstriction, but not EGF-mediated constriction, of small pulmonary arteries. (A–C) Basal tone in nonpermeabilized arteries (A), vasoconstriction in response to ET-1 in Ca2+-permeabilized arteries (B), and vasoconstriction in response to EGF in nonpermeabilized arteries (C) from CH and control rats in the presence of the Src family kinase inhibitor SU6656 (10 μM) or vehicle. All arteries were endothelium disrupted. Values are mean ± SE of n = 4–5 rats/group. *P < 0.05 versus control. #P < 0.05 CH drug versus CH vehicle.
Figure 11.
Src and EGFR inhibitors do not alter Rho kinase signaling. Vasoconstriction in response to sphingosylphosphorylcholine (SPC; 10 μM) in Ca2+-permeabilized, endothelium-disrupted arteries treated with HA-1077 (10 μM), AG1478 (1 μM), SU6656 (10 μM), or vehicle. Values are mean ± SE of n = 4 rats/group. *P < 0.05 versus vehicle.
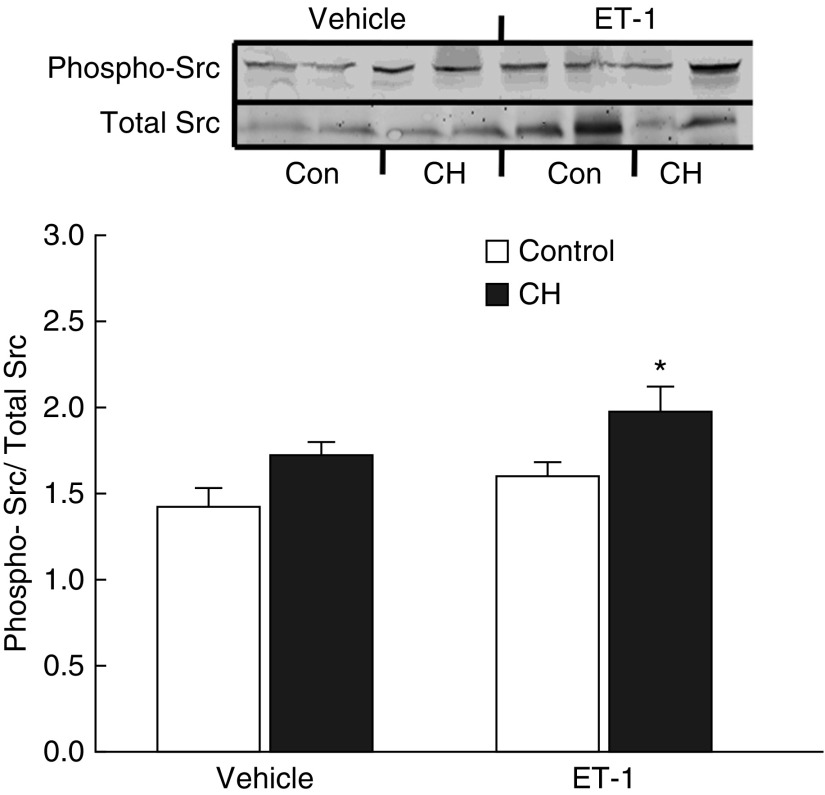
Effects of CH on basal and ET-1–induced Src activation were assessed in intrapulmonary arteries from each group by measuring levels of activated Src (phospho-Tyr416 [24]) by western blotting. Although basal levels of phospho-Src tended to be elevated after CH, this effect did not achieve statistical significance (P = 0.08) (Figure 12). However, after ET-1 stimulation, Src phosphorylation was greater in CH than in normoxic control arteries, indicative of increased activity. Total Src expression (normalized to β-actin) was not different between groups (control, 0.93 ± 0.07; CH, 0.86 ± 0.03; n = 8/group).
Figure 12.
ET-1 stimulates Src kinase in pulmonary arteries from CH rats. Representative Western blots and mean densitometric data for phosphorylated (active) Src kinase normalized to total Src kinase in homogenates from control and CH intrapulmonary arteries. Phosphorylated and total Src kinase concentrations were measured under unstimulated conditions and after treatment with ET-1 (10 nM). Values are mean ± SE of n = 4 rats/group. *P < 0.05 versus control ET-1. Con = control.
Involvement of MMPs in Hypoxia-mediated Increases in Vasoconstrictor Sensitivity to ET-1
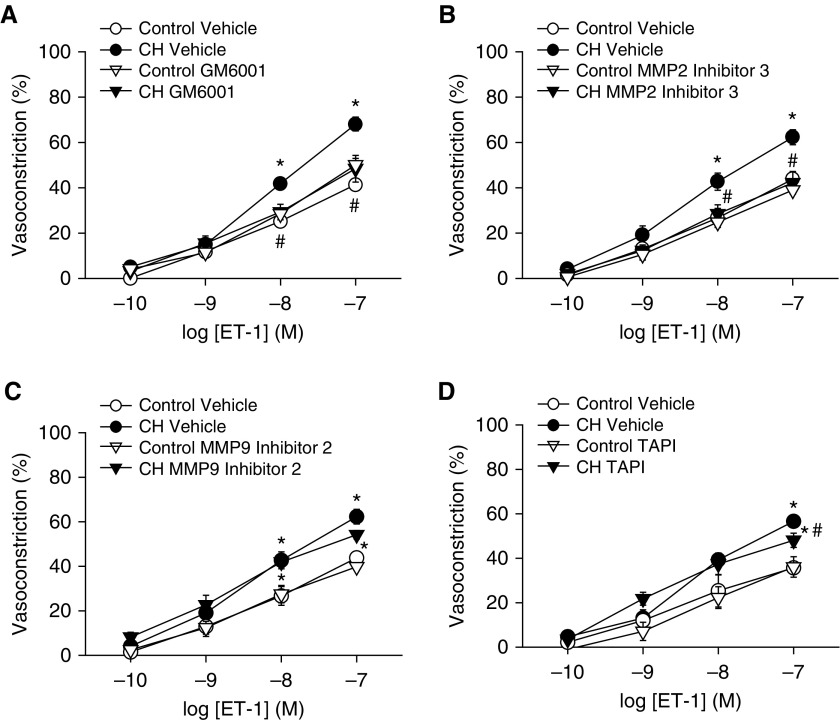
Because Src can activate EGFR through both MMP-dependent (15) and MMP-independent (14) mechanisms, we initially examined vasoconstrictor responses to ET-1 in the presence of a general MMP inhibitor, GM6001, in Ca2+-permeabilized arteries. MMP inhibition prevented the effect of CH to augment ET-1 vasoconstriction and normalized responses between groups (Figure 13A). Considering that MMP2 and MMP9 can facilitate EGFR activation (16) and that MMP2 expression is increased in rat models of pHTN (25), ET-1 vasoreactivity protocols were repeated after selective MMP2 or MMP9 inhibition. MMP2 inhibitor 3 prevented enhanced vasoconstriction in response to ET-1 in CH arteries (Figure 13B). In contrast, MMP9 inhibitor 2 was without effect on responses to ET-1 (Figure 13C). We also tested the role of ADAM17 (a disintegrin and metalloproteinase domain 17) in ET-1–induced vasoconstriction with the ADAM17 inhibitor TAPI-1 (TNF-α protease inhibitor I). TAPI-1 slightly but significantly attenuated the response to the highest concentration of ET-1 (100 nM), although augmented vasoconstriction in response to ET-1 after CH persisted (Figure 13D).
Figure 13.
Matrix metalloproteinases (MMPs) contribute to enhanced ET-1–induced vasoconstrictor reactivity after CH. Vasoconstrictor responses to increasing concentrations of ET-1 in the presence or absence of (A) general MMP inhibition (GM6001; 15 μM), (B) MMP2 inhibition (MMP2 inhibitor 3; 100 nM), (C) MMP9 inhibition (MMP9 inhibitor 2; 10 μM), and (D) ADAM17 inhibition (TAPI [TNF-α protease inhibitor I], 10 μM) in endothelium-disrupted, pressurized, Ca2+-permeabilized pulmonary arteries from CH and control rats. Values are mean ± SE of n = 4–5 rats/group. *P < 0.05 versus control; #P < 0.05 CH drug versus CH vehicle.
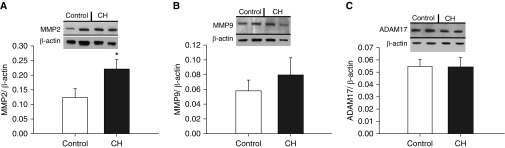
Consistent with a primary contribution of MMP2 to CH-induced increases in vasoconstrictor reactivity (Figure 12B), CH increased pulmonary arterial MMP2 expression measured by western blotting but was without effect on expression of MMP9 or ADAM17 (Figure 14).
Figure 14.
CH increases expression of pulmonary arterial MMP2 but not MMP9 or ADAM17. (A–C) Western blots and mean densitometric data for MMP2 (A), MMP9 (B), and ADAM17 (C) in homogenates of intrapulmonary arteries from normoxic and CH rats. Values are mean ± SE of n = 4/group normalized to β-actin. *P < 0.05 versus control.
Discussion
This study identified signaling mechanisms linking ET-1 receptor stimulation and intraluminal stretch to enhanced myofilament Ca2+ sensitization and vasoconstriction in the hypertensive pulmonary circulation. The major findings are as follows:
Enhanced ET-1–induced pulmonary vasoconstriction, pulmonary arterial VSM O2− generation, and basal arterial tone after CH require EGFR-dependent activation of NOX2/Rac1.
EGF constricts arteries from CH but not control rats through NOX and ROK signaling.
EGFR contributes to CH-induced RV hypertrophy, arterial remodeling, and elevated RVSP, indicative of pHTN.
Src kinases are activated by ET-1 in arteries from CH rats and mediate enhanced basal tone and vasoconstriction in response to ET-1.
MMPs contribute to augmented ET-1–induced vasoconstriction after CH.
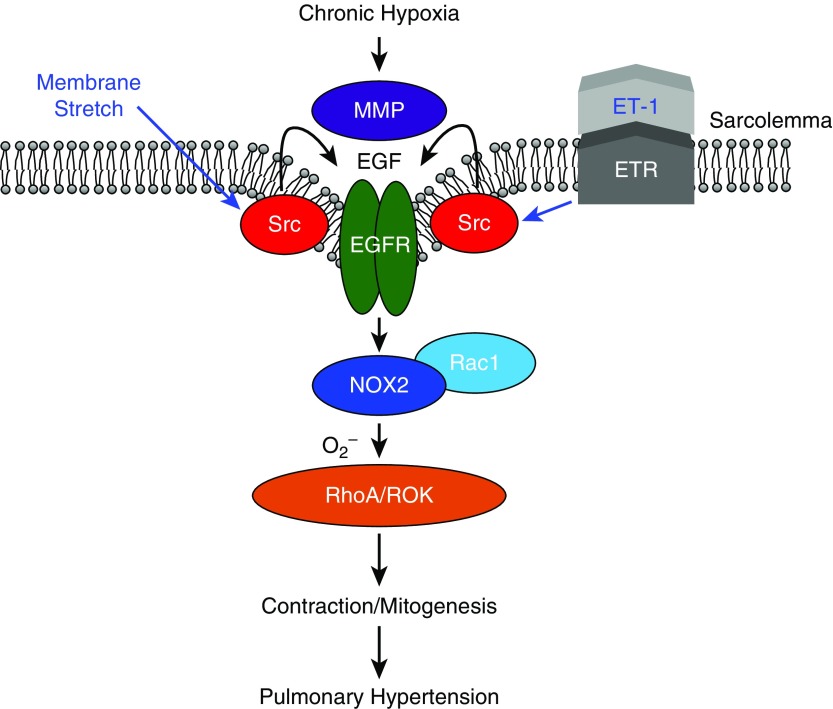
Combined, these findings demonstrate that CH facilitates pressure- and ET-1–induced activation of NOX2 through Src- and MMP-dependent EGFR signaling in pulmonary VSM (Figure 15).
Figure 15.
Summary of major findings. After CH, ET-1 receptor (ETR) stimulation and membrane stretch resulting from increases in intraluminal pressure activate EGFR via Src-dependent MMP activation. EGFR signaling leads to Rho kinase (ROK)-dependent Ca2+ sensitization through NADPH oxidase 2 (NOX2)-derived O2−. Our findings support a role for this signaling mechanism in the development of pulmonary hypertension.
Altered concentrations of O2− and H2O2 are implicated in the development of pHTN (17, 26, 27). O2− can contract pulmonary VSM through ROK activation and subsequent myofilament Ca2+ sensitization (28), whereas H2O2 can cause both contraction (29) and relaxation (30). Our current observation that CH-dependent basal tone is prevented by SOD but not catalase supports a role for O2− in this response. In addition, we have previously demonstrated that O2− contributes to enhanced ET-1–dependent (6) and KCl-dependent (10) Ca2+ sensitization and vasoconstriction in arteries from CH rats, suggesting that O2− and not H2O2 is the primary ROS associated with ROK-dependent vasoconstriction after CH.
NOX isoforms contribute to pHTN (7, 31, 32) and are a potential source of O2− mediating basal tone and enhanced ET-1–induced pulmonary VSM Ca2+ sensitization after CH. Consistent with evidence for involvement of the NOX2 isoform in CH-dependent ROS production and pHTN in mice (7), our present findings indicate a role for NOX2 in augmented pressure-dependent and ET-1–induced Ca2+ sensitization after CH (Figures 2 and 3). A similar role was observed for Rac1, an important mediator of NOX2 activation (33) that contributes to depolarization-induced Ca2+ sensitization in pulmonary arteries from CH rats (10). Given that NOX2 and Rac1 inhibition were without effect in control arteries, it appears that CH couples both membrane stretch and ET-1 receptor activation to NOX2/Rac1 signaling in the hypertensive pulmonary circulation.
EGFR is a receptor tyrosine kinase linked to membrane stretch-induced NOX activation in mesangial cells (13). EGFR is comprised of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain that mediates the tyrosine kinase activity of the receptor (34). Ligand stimulation of EGFR by EGF, transforming growth factor-α, and amphiregulin promotes receptor dimerization and autophosphorylation in the intracellular domain, thereby coupling EGFR signaling pathways involved in growth, proliferation, and cell contraction. EGFR is transactivated by a variety of stimuli, including platelet-activating factor (35), GPCR stimulation (15, 36), depolarization (37), ROS (38), and mechanical deformation of cell membranes (13, 16) and thus integrates information from distinct upstream pathways into downstream cellular responses. We tested the hypothesis that EGFR serves as a proximal mediator of NOX2 activation after CH. Consistently, we found that EGFR inhibition prevented effects of CH to induce pressure-dependent tone and augment ET-1–induced arterial constriction and VSM O2− production. This latter finding, although limited by interpretation of results with single-wavelength dyes, demonstrates that the cellular components necessary for ET-1–induced ROS production are localized to the smooth muscle cell. These results closely resemble our previous findings in isolated arteries which demonstrate that membrane depolarization-dependent increases in O2− production are also blocked by AG1478 and gp91 ds-tat (10). In addition, the EGFR agonist EGF caused constriction only in arteries from CH rats that was sensitive to inhibition of ROK, NOX2, and EGFR but independent of changes in vessel wall [Ca2+]i. These results suggest that CH selectively links EGFR stimulation to NOX2- and ROK-mediated pulmonary VSM Ca2+ sensitization and arterial constriction and are in agreement with evidence from our laboratory that EGFR inhibition prevents KCl-induced O2− production, Rac1 activation, and constriction in pulmonary arteries from rats exposed to CH (10). Collectively, these findings indicate that EGFR is a proximal activator of NOX and that this signaling pathway serves as a common mechanism that transduces a variety of contractile stimuli to NOX2-derived O2− production and ROK-mediated pulmonary vasoconstriction after CH.
Although it has been suggested that EGFR is not a promising target for treatment of pHTN, considering that lung EGFR concentrations are not altered in either human pHTN or rodent models of pHTN (39), it is alternatively possible that EGFR signaling is enhanced independent of changes in expression. Consistent with this possibility, EGFR contributes to pHTN in mice that overexpress the EGFR ligand transforming growth factor-α (40). Furthermore, CH couples depolarization-induced EGFR activation to NOX2-mediated pulmonary vasoconstriction without a change in EGFR protein concentrations but with increased tyrosine-1068 phosphorylation of EGFR (10). H2O2 causes post-translational covalent modification of EGFR resulting in tyrosine dimerization and activation after monocrotaline exposure (38). However, the primary ROS that contributes to the pathological EGFR signaling appears to differ between monocrotaline- and hypoxia-induced pHTN (H2O2 and superoxide, respectively). The physiological significance of EGFR signaling in CH-induced pHTN is further supported by effects of chronic treatment with the EGFR inhibitors gefitinib (Figures 8 and 9) and dacomitinib (41) to reduce RVSP, RV hypertrophy, and arterial remodeling in response to CH in rats. However, the relative contributions of arterial remodeling and vasomotor tone to EGFR-dependent pHTN remain to be determined. Although heart rates were not altered by gefitinib treatment, we cannot exclude the possibility that RV dysfunction contributes to lower RVSP after EGFR inhibition. Indeed, interpretation of findings from pharmacologic EGFR inhibition in vivo is limited by effects of global EGFR inhibition, including potential influences on cardiac or vascular function. Although our findings are consistent with observations that EGFR contributes to hypoxia-dependent ovine pulmonary VSM proliferation (42) and that EGFR inhibition improves survival and attenuates arterial remodeling in monocrotaline- and CH-induced pHTN in rats (41, 43), they contrast with evidence that EGFR inhibition does not significantly attenuate indices of pHTN in CH mice (39). The reason for these disparate findings is not clear but may represent a species difference.
The Src family of non–receptor tyrosine kinases are centrally involved in the transduction of GPCR (44), mechanical (13), and redox (45) stimuli to EGFR activation through both ligand-dependent and -independent mechanisms (13, 15, 16). Ligand-dependent activation of EGFR occurs through proteolytic cleavage of transmembrane ligands, including heparin-binding EGF, to release mature receptor ligands through ectodomain shedding (15, 16, 46, 47). Our results indicate that Src inhibition prevents pressure-induced tone as well as enhanced vasoconstriction in response to ET-1 after CH, similar to effects of EGFR, NOX2, and Rac1 inhibition. ET-1 further stimulated Src in intrapulmonary arteries from CH rats, as indicated by an increase in phosphorylated Src at its activation residue, tyrosine-416 (24). Our observation that Src inhibition did not alter EGF-induced vasoconstriction in arteries from CH rats further suggests that Src signals upstream of EGFR in response to membrane stretch or ET-1 stimulation. Although not directly tested in the present study, our current model (Figure 15) predicts that Src inhibition would similarly prevent ET-1–induced ROS production but would be without effect on EGF-induced ROS generation in smooth muscle cells from pHTN rats. Although mechanisms by which smooth muscle membrane stretch and ET-1 stimulate Src are not clear, Src phosphorylation can also be induced by prostaglandin F2α in the pulmonary circulation (48), suggesting that other agonists coupled to GPCRs may also evoke this signaling cascade. Interestingly, both U46619 and acute hypoxia activate Src via oxidative phosphorylation in a process that results in ROK-dependent contraction in rat pulmonary arteries (49). Whether oxidant signaling contributes to Src activation in the hypertensive pulmonary circulation, however, remains to be established.
Our evidence implicating Src in enhanced pulmonary vasoreactivity after CH is consistent with prior evidence that Src family kinases contribute to the development of monocrotaline-induced pHTN (50). Because Src kinases represent a sizable family of proteins, elucidating the specific Src isoform(s) involved remains an area for future investigation. A likely candidate is c-Src, because myogenic tone in systemic arteries requires c-Src (51), and c-Src is linked to depolarization-induced RhoA activation in mesangial cells (13).
Src can activate EGFR through ectodomain shedding of transmembrane ligands into mature ligands that are expressed on VSM cells (52). Although mechanisms of ectodomain shedding are poorly understood, several metalloproteases, including members of the MMP family (MMP2, -7, -9) (15, 16) and ADAM (46), have been implicated in this response. Interestingly, these MMP isoforms contribute to both agonist- and stretch-mediated EGFR activation and resultant VSM contraction/hypertrophy in systemic arteries (15, 16). ADAM17 is similarly involved in angiotensin II–induced proliferation and hypertrophy in rat aortic smooth muscle (46). A general MMP inhibitor prevented effects of CH to augment ET-1–mediated vasoconstriction, supporting a role for ligand-dependent activation of EGFR in this response. Of the selective MMP inhibitors used in this study, only MMP2 inhibition restored vasoreactivity to ET-1 in hypertensive arteries to the level of controls. We also detected increased MMP2 protein concentrations in CH arteries, consistent with previous reports in rats with monocrotaline-induced pHTN and patients with idiopathic pHTN (53, 54). Conversely, CH was without effect on arterial MMP9 and ADAM17 expression, and both MMP9 and ADAM17 inhibition minimally affected reactivity to ET-1 in arteries from CH rats, suggesting a primary role for MMP2 in this response. However, increased MMP2 expression alone likely does not account for the observed changes in vasoreactivity after CH, because Src/EGFR/NOX2 signaling does not occur in control arteries that exhibit basal expression of MMP2 (Figure 14). Therefore, CH appears to selectively couple ET-1 signaling to MMP stimulation, although how this occurs remains to be investigated. One possibility is that Src kinases function as redox sensors (45, 49) that link these stimuli to MMP activation after CH. Furthermore, increased MMP2 activity in pulmonary arteries from patients with idiopathic pHTN has been associated with tissue inhibitor of metalloproteinase imbalance (53). Future studies are therefore needed to address the potential role of tissue inhibitors of metalloproteinase in the response to CH and the contribution of MMPs to the development of pHTN.
Because GPCRs can activate Src (55), ET-1 receptors may directly activate these proteins in pulmonary arteries from CH rats, although this remains to be addressed. The mechanism through which membrane stretch activates Src is also unknown, but it could potentially involve VSM membrane depolarization. Indeed, membrane depolarization is capable of activating a similar EGFR/NOX2 signaling pathway in pulmonary arterial smooth muscle (10). Furthermore, CH leads to VSM membrane depolarization in small pulmonary arteries (5, 10, 56), and these arteries exhibit pressure-dependent depolarization (56). Future challenges are to address mechanisms by which CH couples ET-1 and vessel wall stretch to EGFR signaling, as well as the role of VSM membrane depolarization as a common mechanism that links these stimuli to EGFR-dependent Ca2+ sensitization and vasoconstriction after CH.
We conclude that CH unmasks an Src/EGFR/NOX2 signaling pathway leading to ROK-mediated Ca2+ sensitization and development of pulmonary vascular tone in response to receptor stimulation and membrane stretch. Our findings also suggest that MMP2 is important to this response. Studies in animals treated chronically with an EGFR inhibitor further support a novel role for EGFR in the development of CH-induced pHTN. The results of this study aid understanding of vasoconstrictor mechanisms that contribute to pHTN and have potential to provide new therapeutic strategies that target components of Src-mediated EGFR signaling.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Minerva Murphy and Tamara Howard for technical assistance.
Footnotes
Supported by National Institutes of Health (NIH) grant R01 HL111084 (N.L.J.); NIH grants R01 HL132883, R01 HL088192, and T32 HL007736 (T.C.R.); NIH grant F31 HL131334 (J.R.S.); NIH grant R01 HL095640 (B.R.W.); NIH grant K12 GM088021 (Dr. Angela Wandinger-Ness); and American Heart Association grants 13PRE14580015 (C.E.N.) and 16GRNT27700010 (T.C.R.).
Author Contributions: C.E.N. contributed to study conception and design, performed experiments, analyzed and interpreted data, and drafted and revised the manuscript. J.R.S. and S.Y. performed experiments, analyzed data, and revised the manuscript. L.W.-C., N.L.J., and B.R.W. contributed to study design, interpreted data, and revised the manuscript. T.C.R. contributed to study conception and design, supervised study execution, interpreted data, and revised the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0106OC on July 2, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, et al. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 2.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, et al. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2004;287:L665–L672. doi: 10.1152/ajplung.00050.2003. [DOI] [PubMed] [Google Scholar]
- 3.Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, et al. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med. 2005;171:494–499. doi: 10.1164/rccm.200405-637OC. [DOI] [PubMed] [Google Scholar]
- 4.Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2008;294:L797–L806. doi: 10.1152/ajplung.00253.2007. [DOI] [PubMed] [Google Scholar]
- 5.Broughton BR, Jernigan NL, Norton CE, Walker BR, Resta TC. Chronic hypoxia augments depolarization-induced Ca2+ sensitization in pulmonary vascular smooth muscle through superoxide-dependent stimulation of RhoA. Am J Physiol Lung Cell Mol Physiol. 2010;298:L232–L242. doi: 10.1152/ajplung.00276.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–L529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–L10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 8.Weigand L, Sylvester JT, Shimoda LA. Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2006;290:L284–L290. doi: 10.1152/ajplung.00449.2004. [DOI] [PubMed] [Google Scholar]
- 9.Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, et al. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L656–L664. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- 10.Norton CE, Broughton BR, Jernigan NL, Walker BR, Resta TC. Enhanced depolarization-induced pulmonary vasoconstriction following chronic hypoxia requires EGFR-dependent activation of NAD(P)H oxidase 2. Antioxid Redox Signal. 2013;18:1777–1788. doi: 10.1089/ars.2012.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukumoto Y, Yamada N, Matsubara H, Mizoguchi M, Uchino K, Yao A, et al. Double-blind, placebo-controlled clinical trial with a rho-kinase inhibitor in pulmonary arterial hypertension. Circ J. 2013;77:2619–2625. doi: 10.1253/circj.cj-13-0443. [DOI] [PubMed] [Google Scholar]
- 12.Dufour A, Sampson NS, Li J, Kuscu C, Rizzo RC, Deleon JL, et al. Small-molecule anticancer compounds selectively target the hemopexin domain of matrix metalloproteinase-9. Cancer Res. 2011;71:4977–4988. doi: 10.1158/0008-5472.CAN-10-4552. [Published erratum appears in Cancer Res 72:5141–5142.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Peng F, Gao B, Ingram AJ, Krepinsky JC. Mechanical strain-induced RhoA activation requires NADPH oxidase-mediated ROS generation in caveolae. Antioxid Redox Signal. 2010;13:959–973. doi: 10.1089/ars.2009.2908. [DOI] [PubMed] [Google Scholar]
- 14.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 15.Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94:68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- 16.Lucchesi PA, Sabri A, Belmadani S, Matrougui K. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation. 2004;110:3587–3593. doi: 10.1161/01.CIR.0000148780.36121.47. [DOI] [PubMed] [Google Scholar]
- 17.Jernigan NL, Naik JS, Weise-Cross L, Detweiler ND, Herbert LM, Yellowhair TR, et al. Contribution of reactive oxygen species to the pathogenesis of pulmonary arterial hypertension. PLoS One. 2017;12:e0180455. doi: 10.1371/journal.pone.0180455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plomaritas DR, Herbert LM, Yellowhair TR, Resta TC, Gonzalez Bosc LV, Walker BR, et al. Chronic hypoxia limits H2O2-induced inhibition of ASIC1-dependent store-operated calcium entry in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2014;307:L419–L430. doi: 10.1152/ajplung.00095.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csányi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egaña L, Lopes LR, et al. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med. 2011;51:1116–1125. doi: 10.1016/j.freeradbiomed.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. 2013;19:1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jernigan NL, Walker BR, Resta TC. Chronic hypoxia augments protein kinase G-mediated Ca2+ desensitization in pulmonary vascular smooth muscle through inhibition of RhoA/Rho kinase signaling. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1220–L1229. doi: 10.1152/ajplung.00196.2004. [DOI] [PubMed] [Google Scholar]
- 24.Taniguchi K, Xia L, Goldberg HJ, Lee KW, Shah A, Stavar L, et al. Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes. 2013;62:3874–3886. doi: 10.2337/db12-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frisdal E, Gest V, Vieillard-Baron A, Levame M, Lepetit H, Eddahibi S, et al. Gelatinase expression in pulmonary arteries during experimental pulmonary hypertension. Eur Respir J. 2001;18:838–845. doi: 10.1183/09031936.01.00084601. [DOI] [PubMed] [Google Scholar]
- 26.Chen MJ, Chiang LY, Lai YL. Reactive oxygen species and substance P in monocrotaline-induced pulmonary hypertension. Toxicol Appl Pharmacol. 2001;171:165–173. doi: 10.1006/taap.2000.9117. [DOI] [PubMed] [Google Scholar]
- 27.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, et al. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol. 2009;40:601–609. doi: 10.1165/rcmb.2008-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knock GA, Snetkov VA, Shaifta Y, Connolly M, Drndarski S, Noah A, et al. Superoxide constricts rat pulmonary arteries via Rho-kinase-mediated Ca2+ sensitization. Free Radic Biol Med. 2009;46:633–642. doi: 10.1016/j.freeradbiomed.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin MJ, Yang XR, Cao YN, Sham JS. Hydrogen peroxide-induced Ca2+ mobilization in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1598–L1608. doi: 10.1152/ajplung.00323.2006. [DOI] [PubMed] [Google Scholar]
- 30.Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol. 1987;252:H721–H732. doi: 10.1152/ajpheart.1987.252.4.H721. [DOI] [PubMed] [Google Scholar]
- 31.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, et al. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-β1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol. 2009;296:L489–L499. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L881–L888. doi: 10.1152/ajplung.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson KM. Structure-based view of epidermal growth factor receptor regulation. Annu Rev Biophys. 2008;37:353–373. doi: 10.1146/annurev.biophys.37.032807.125829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou W, Ibe BO, Raj JU. Platelet-activating factor induces ovine fetal pulmonary venous smooth muscle cell proliferation: role of epidermal growth factor receptor transactivation. Am J Physiol Heart Circ Physiol. 2007;292:H2773–H2781. doi: 10.1152/ajpheart.01018.2006. [DOI] [PubMed] [Google Scholar]
- 36.Ulu N, Gurdal H, Landheer SW, Duin M, Guc MO, Buikema H, et al. α1-Adrenoceptor-mediated contraction of rat aorta is partly mediated via transactivation of the epidermal growth factor receptor. Br J Pharmacol. 2010;161:1301–1310. doi: 10.1111/j.1476-5381.2010.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahara S, Fukuda K, Kodama H, Kato T, Miyoshi S, Ogawa S. Potassium channel blocker activates extracellular signal-regulated kinases through Pyk2 and epidermal growth factor receptor in rat cardiomyocytes. J Am Coll Cardiol. 2001;38:1554–1563. doi: 10.1016/s0735-1097(01)01558-3. [DOI] [PubMed] [Google Scholar]
- 38.Rafikova O, Rafikov R, Kangath A, Qu N, Aggarwal S, Sharma S, et al. Redox regulation of epidermal growth factor receptor signaling during the development of pulmonary hypertension. Free Radic Biol Med. 2016;95:96–111. doi: 10.1016/j.freeradbiomed.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahal BK, Cornitescu T, Tretyn A, Pullamsetti SS, Kosanovic D, Dumitrascu R, et al. Role of epidermal growth factor inhibition in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2010;181:158–167. doi: 10.1164/rccm.200811-1682OC. [DOI] [PubMed] [Google Scholar]
- 40.Le Cras TD, Hardie WD, Fagan K, Whitsett JA, Korfhagen TR. Disrupted pulmonary vascular development and pulmonary hypertension in transgenic mice overexpressing transforming growth factor-α. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1046–L1054. doi: 10.1152/ajplung.00045.2003. [DOI] [PubMed] [Google Scholar]
- 41.Yu X, Zhao X, Zhang J, Li Y, Sheng P, Ma C, et al. Dacomitinib, a new pan-EGFR inhibitor, is effective in attenuating pulmonary vascular remodeling and pulmonary hypertension. Eur J Pharmacol. 2019;850:97–108. doi: 10.1016/j.ejphar.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Sheng L, Zhou W, Hislop AA, Ibe BO, Longo LD, Raj JU. Role of epidermal growth factor receptor in ovine fetal pulmonary vascular remodeling following exposure to high altitude long-term hypoxia. High Alt Med Biol. 2009;10:365–372. doi: 10.1089/ham.2008.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merklinger SL, Jones PL, Martinez EC, Rabinovitch M. Epidermal growth factor receptor blockade mediates smooth muscle cell apoptosis and improves survival in rats with pulmonary hypertension. Circulation. 2005;112:423–431. doi: 10.1161/CIRCULATIONAHA.105.540542. [DOI] [PubMed] [Google Scholar]
- 44.Luttrell DK, Luttrell LM. Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene. 2004;23:7969–7978. doi: 10.1038/sj.onc.1208162. [DOI] [PubMed] [Google Scholar]
- 45.Giannoni E, Taddei ML, Chiarugi P. Src redox regulation: again in the front line. Free Radic Biol Med. 2010;49:516–527. doi: 10.1016/j.freeradbiomed.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 46.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, et al. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:e133–e137. doi: 10.1161/01.ATV.0000236203.90331.d0. [DOI] [PubMed] [Google Scholar]
- 47.Takaguri A, Shirai H, Kimura K, Hinoki A, Eguchi K, Carlile-Klusacek M, et al. Caveolin-1 negatively regulates a metalloprotease-dependent epidermal growth factor receptor transactivation by angiotensin II. J Mol Cell Cardiol. 2011;50:545–551. doi: 10.1016/j.yjmcc.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knock GA, Shaifta Y, Snetkov VA, Vowles B, Drndarski S, Ward JP, et al. Interaction between Src family kinases and Rho-kinase in agonist-induced Ca2+-sensitization of rat pulmonary artery. Cardiovasc Res. 2008;77:570–579. doi: 10.1093/cvr/cvm073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacKay CE, Shaifta Y, Snetkov VV, Francois AA, Ward JPT, Knock GA. ROS-dependent activation of RhoA/Rho-kinase in pulmonary artery: role of Src-family kinases and ARHGEF1. Free Radic Biol Med. 2017;110:316–331. doi: 10.1016/j.freeradbiomed.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pullamsetti SS, Berghausen EM, Dabral S, Tretyn A, Butrous E, Savai R, et al. Role of Src tyrosine kinases in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2012;32:1354–1365. doi: 10.1161/ATVBAHA.112.248500. [DOI] [PubMed] [Google Scholar]
- 51.Murphy TV, Spurrell BE, Hill MA. Cellular signalling in arteriolar myogenic constriction: involvement of tyrosine phosphorylation pathways. Clin Exp Pharmacol Physiol. 2002;29:612–619. doi: 10.1046/j.1440-1681.2002.03698.x. [DOI] [PubMed] [Google Scholar]
- 52.Stanic B, Pandey D, Fulton DJ, Miller FJ., Jr Increased epidermal growth factor-like ligands are associated with elevated vascular nicotinamide adenine dinucleotide phosphate oxidase in a primate model of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2452–2460. doi: 10.1161/ATVBAHA.112.256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lepetit H, Eddahibi S, Fadel E, Frisdal E, Munaut C, Noel A, et al. Smooth muscle cell matrix metalloproteinases in idiopathic pulmonary arterial hypertension Eur Respir J 200525834–842.15863640 [Google Scholar]
- 54.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luttrell LM, Hawes BE, van Biesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- 56.Naik JS, Earley S, Resta TC, Walker BR. Pressure-induced smooth muscle cell depolarization in pulmonary arteries from control and chronically hypoxic rats does not cause myogenic vasoconstriction. J Appl Physiol. 2005;98:1119–1124. doi: 10.1152/japplphysiol.00819.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.