Abstract
The soluble guanylyl cyclase (sGC)–cyclic guanosine monophosphate signaling pathway evokes vascular smooth muscle relaxation; whether this pathway mediates airway smooth muscle relaxation remains controversial. We posit that sGC activators are equi-effective as β-agonists in reversing contractile agonist-induced airway smooth muscle shortening. To provide clarity, we tested the efficacy of sGC stimulator and activator drugs, BAY 41-2272 and BAY 60-2270, respectively, in reversing bronchoconstriction of human small airways using human precision-cut lung slices (hPCLS). Both BAY drugs reversed carbachol-induced bronchoconstriction to a maximal degree comparable to that of formoterol. Moreover, the sGC drugs remained effective bronchodilators despite formoterol-induced desensitization of the airways. Analysis of the hPCLS after their activation by sGC or β2-adrenergic receptor agonist showed distinct cyclic nucleotide accumulation in the hPCLS. Collectively, these data suggest that cAMP and cyclic guanosine monophosphate pathways are equi-effective for reversing carbachol-induced bronchoconstriction in the human airway via separate and distinct second messenger pathways. This should open the door for future studies to test whether sGC-targeted drugs alone or in combination can serve as effective bronchodilators in asthma and chronic obstructive pulmonary disease.
Keywords: asthma, smooth muscle, relaxation, remodeling
Clinical Relevance
The data presented in this article provide an alternative therapeutic pathway that induces bronchodilation similar to that induced by a β2-adrenergic receptor agonist and is still functional in the face of desensitization of the β2-adrenergic receptor.
Soluble guanylyl cyclase (sGC), a hemeprotein, modulates the nitric oxide (NO)-dependent signal cascade that promotes smooth muscle relaxation, particularly in the vasculature (1). Activated by NO binding to its heme, the sGC enzyme increases production of cGMP as a second messenger (2, 3) that in turn activates cyclic guanosine monophosphate (cGMP)-dependent protein kinase. As a consequence, agonist-induced cytosolic calcium transients decrease, thereby inhibiting actin–myosin cross-bridge cycling. The NO–sGC signaling pathway underlies the majority of physiological actions attributed to NO that mediate myriad effects across the cardiovascular, gastrointestinal, urogenital, nervous, and immune systems.
Although dilation in the vasculature is primarily NO–sGC dependent, the role of the NO–sGC pathway in reversing bronchoconstriction remains controversial (4–7). Despite dramatic improvements in the treatment of airway inflammation in asthma with novel monoclonal antibodies, there has been little progress in the development of novel classes of bronchodilators for nearly 50 years. Furthermore, bronchodilators that require generation of cAMP have limitations due to β2-adrenergic receptor (β2AR) desensitization, as well as having diminished efficacy in the presence of T-helper cell type 2 inflammation (8–10). Recently, direct-acting sGC agonists that bypass NO generation have been developed (11, 12). The sGC agonists BAY 41-2272 (BAY 41) and BAY 60-2770 (BAY 60) stimulate the heme-containing sGC and directly activate the heme-free form of sGC, respectively. Analogs of these drugs, riociguat and cinaciguat, are clinically indicated for treating pulmonary hypertension (13). Using such agonists, we explored whether activating the sGC–cGMP signaling pathway would evoke bronchodilation in human precision cut lung slices (hPCLS). Our data suggest that sGC agonists can effectively reverse airway constriction in humans and thus may offer therapeutic value as bronchodilators in asthma or chronic obstructive pulmonary disease.
Methods
Reagents
The PDE inhibitor 3-isobutyl-1-methylxanthine and formoterol fumarate dihydrate were purchased from Sigma-Aldrich. BAY 60 and BAY 41 were obtained from Bayer AG. Diethylenetriamine NONOate (NOC-18) was obtained from Cayman Chemical Co. cGMP and cAMP ELISA kits were obtained from Cell Signaling Technology.
hPCLS Generation and Bronchodilation Assays
hPCLS were prepared as previously described (8, 9, 14). Briefly, whole human lungs from donors without asthma were dissected and inflated using 2% (wt/vol) low–melting-point agarose. Once the agarose set, the lobe was sectioned, and cores of 8-mm diameter were made. The cores that contained noncartilaginous airways of 1–3 mm in diameter by visual inspection were sliced at a thickness of 350 μm (VF300 Vibratome; Precisionary Instruments) and collected in wells containing supplemented Ham’s F-12 medium. In some cases, hPCLS were incubated overnight with formoterol (100 nM) to induce β2AR desensitization in the slices. To study bronchoconstriction and subsequent bronchodilation, the hPCLS were contracted with carbachol (CCh; 10−5 M) and then bronchodilated with formoterol or either BAY compound (BAY 41 or BAY 60) at indicated concentrations (10−10 M to 10−5 M). In Figure 1D, hPCLS were incubated with NOC-18 (500 μM; 24 h). Airways were contracted to CCh, then dilated to BAY 41, all of which was performed in the presence of NOC-18 (500 μM). The human lung tissue samples were commercially obtained from anonymous donors (National Disease Research Interchange or the International Institute for the Advancement of Medicine) and are therefore exempt from requiring institutional review board approval. Although these samples have demographic information about the donors, there is no information linking the subject’s identification to the tissue sample.
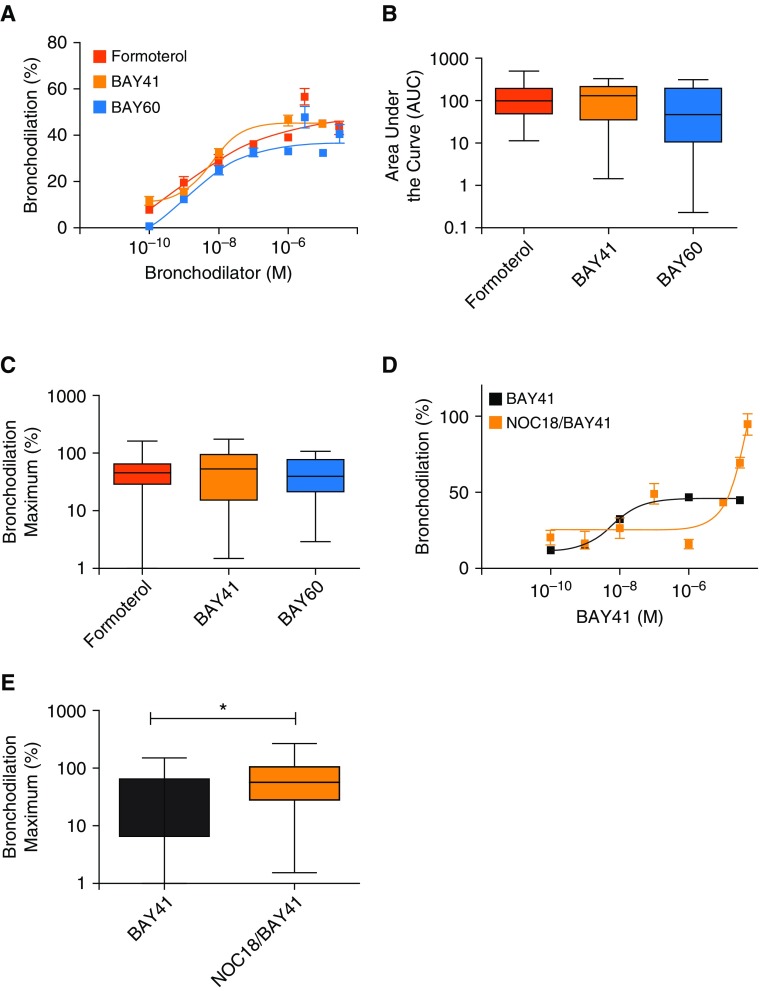
Figure 1.
Soluble guanylyl cyclase (sGC) agonists and formoterol similarly bronchodilate human precision cut lung slices (hPCLS). Small airways in hPCLS were contracted with carbachol, followed by addition of the indicated compounds and image collection and processing to determine bronchiolar lumen area, expressed as a percentage of the baseline value. (A) Concentration response curves (100 pM–30 μM) illustrating dilation to formoterol, BAY 41-2272 (BAY 41), or BAY 60-2770 (BAY 60). (B) Integrated area under the curve of the concentration–response curves. (C) Maximal amount of bronchodilation induced by each bronchodilator. (D) hPCLS were incubated overnight in media with or without the nitric oxide (NO) donor diethylenetriamine NONOate (NOC-18; 500 μM) before the contraction and relaxation experiments. (E) Maximal amount of bronchodilation induced by BAY 41 in the presence and absence of NOC-18. (A–C) Data derived from n = 15 donor lungs, showing mean ± SEM with 18–27 slices tested per condition; (D and E) data derived from n = 8 donor lungs, showing mean ± SEM with 5–20 slices tested per condition. *P < 0.05.
cAMP and cGMP ELISA
Standard protocols were followed as previously mentioned (15). The hPCLS were washed with cold PBS, resuspended in cGMP/cAMP lysis buffer (obtained from respective ELISA kits), and homogenized manually with tissue grinders by keeping on ice for 30 minutes. To preserve the cGMP/cAMP accumulated in the tissue, 500 μM of 3-isobutyl-1-methylxanthine was added to the lysis buffer. The suspended, homogenized hPCLS tissues were then lysed by three freeze–thaw cycles (in liquid nitrogen and at 37°C, respectively). The lysates were centrifuged for 30 minutes at 4°C, and the supernatants were collected. The cAMP and cGMP concentrations in various generated hPCLS tissue lysates (that either had or had not been earlier preconstricted before bronchodilation with formoterol or sGC activators) were estimated using the cAMP or cGMP ELISA kits.
Results
sGC Agonists (BAY 41 and BAY 60) and Formoterol Reverse CCh-induced Luminal Diameter Narrowing
We compared the efficacy of the sGC agonists and formoterol to reverse CCh-induced bronchoconstriction in human small airways obtained from donors without asthma (Figure 1 and Table E1 in the data supplement). Slices were constricted to 80% with CCh and then exposed to increasing concentrations of BAY 41, BAY 60, or formoterol (10−10 M to 10−5 M). As shown in Figures 1A–1C, both sGC agonists were as effective as formoterol in terms of their concentration–response curve, their area under the curve (formoterol vs. BAY 41, 125.7 + 19.8 vs. 155.2 + 32.7; P = n.s.; formoterol vs. BAY 60, 125.7 + 19.8 vs. 101 ± 26.7; P = n.s.), and the maximal bronchodilation achieved (formoterol vs. BAY 41, 52.1 + 5.7% vs. 57.8 + 8.3%; P = n.s.; formoterol vs. BAY 60, 52.1 + 5.7% vs. 47.4 + 5.7%; P = n.s.). Treating the hPCLS overnight with the NO donor NOC-18 significantly enhanced the maximal bronchodilation to BAY 41 (BAY 41 vs. NOC-18/BAY, 39.4 ± 8.3% vs. 75.07 + 14.5%) (Figures 1D and 1E), an observation that is consistent with our previous data illustrating that NO has an additive effect on sGC activation (16). Overall, our results are consistent with the notion that sGC agonists engaged their target to induce bronchodilation.
Bronchodilation by Formoterol and sGC Agonists Is Mediated through Two Distinct Signaling Pathways
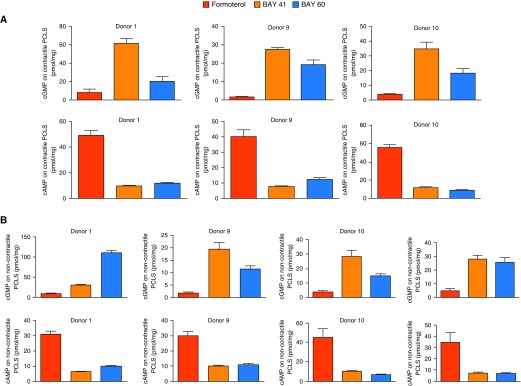
To investigate mechanisms by which dilation occurs in response to the BAY compounds, we measured downstream generation of second messengers cAMP and cGMP in the dilated hPCLS (Figure 2A), as well as in noncontractile, nonconstricted hPCLS that were treated with formoterol or sGC agonists (Figure 2B). Assessment of cyclic nucleotide concentrations by ELISA revealed that elevated cGMP concentrations were induced in the hPCLS treated with sGC agonists BAY 41 or BAY 60, with negligible concentrations of cAMP induced by these agonists (Figure 2A). Conversely, hPCLS dilated with formoterol had elevated cAMP concentrations and negligible concentrations of cGMP that were induced (Figure 2A). We observed similar results in experiments using noncontractile slices (Figure 2B).
Figure 2.
Basal cyclic guanosine monophosphate (cGMP) and cAMP measures in hPCLS after dilation of hPCLS by formoterol or sGC activators. (A) cGMP and cAMP assessment of hPCLS that had airways which were preconstricted, then dilated with the agonists as indicated. (B) cGMP and cAMP measurement in hPCLS after exposure to bronchodilators only, with no preconstriction of airways as noted in A. Each measure is mean ± SD of n = 3 slices per condition. Each set of figures, top (cGMP) and below (cAMP), is from hPCLS derived from individual lungs. n = 3 and n = 4 lungs are used in these measures for A and B, respectively.
Activation of sGC Induces Bronchodilation Despite Formoterol-induced Desensitization of β2AR
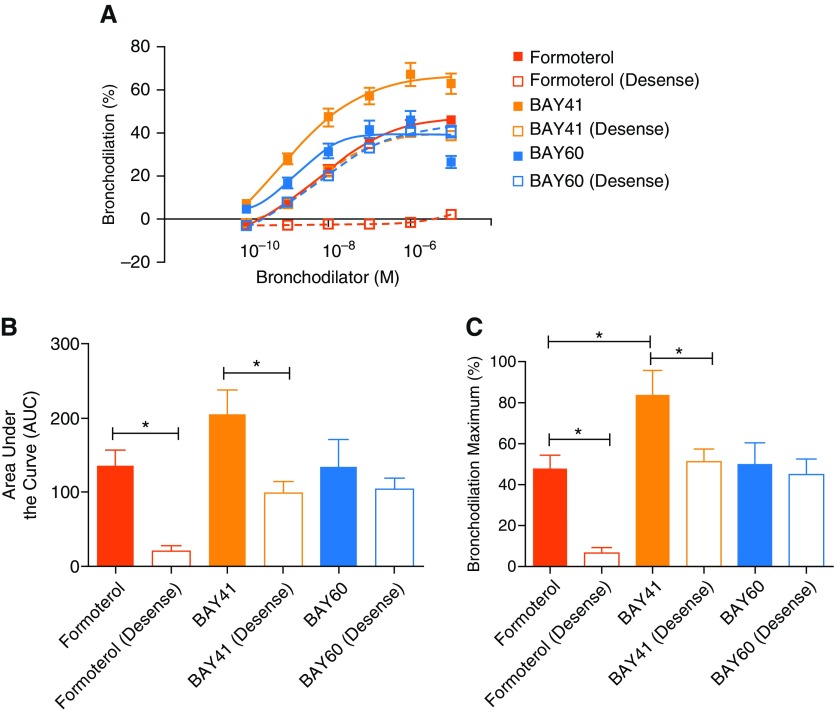
Desensitization of the β2AR renders β2-agonists ineffective in reversing contractile agonist-induced luminal diameter narrowing in hPCLS (9, 10, 14). Because sGC agonists increased cGMP with little effect on cAMP, we posited that formoterol-induced desensitization would likely not induce heterologous desensitization of sGC-activated pathways evoking bronchodilation. Accordingly, we incubated hPCLS with formoterol overnight to desensitize the slices to β2AR agonists, and then we determined whether sGC activators retained the ability to reverse luminal diameter narrowing induced by CCh. As shown in Figure 3, formoterol-induced bronchodilation was markedly attenuated in the desensitized hPCLS, whereas the bronchodilation in response to either sGC agonist remained intact. In these experiments, BAY 41 induced greater bronchodilation than formoterol in the normal slices, but the greater response was attenuated to a degree similar to that with formoterol in the desensitized hPCLS. In comparison, for BAY 60, there was no significant change seen between the normal and desensitized hPCLS for any of the three dilation response parameters. Although both compounds directly activate sGC, BAY 41 and BAY 60 act on different forms of sGC to induce signaling through a cGMP pathway.
Figure 3.
BAY 41 and BAY 60 induce bronchodilation of hPCLS despite desensitization of the β2-adrenergic receptor (β2AR) pathway. Slices were treated overnight with formoterol (100 nM) to desensitize the β2AR pathway, then were washed, preconstricted, and subjected to titration with the indicated agonists to assess bronchodilation. (A) Concentration–response curves are shown for each of the bronchodilators in the presence and absence of formoterol desensitization. (B) Area under the curve and (C) maximal bronchodilation are also shown. Shaded squares/solid lines/solid bars illustrate slices dilated to formoterol, BAY 41, or BAY 60. Open squares/dashed lines/open bars illustrate slices desensitized to formoterol before dilation to formoterol, BAY 41, or BAY 60. Data are derived from n = 4 donor lungs showing mean ± SEM, with 9–14 slices tested per condition with *P < 0.05.
Discussion
Our findings demonstrate that NO-independent, direct-acting sGC agonists are equally as effective as an industry standard, the β2-adrenergic agonist formoterol, in promoting bronchodilation in hPCLS. This challenges a conventional notion that the sGC–cGMP signaling pathway is ineffective as a bronchodilator in humans, which was based on preclinical studies conducted before direct sGC agonists became available. NO inhalation studies performed with adult and pediatric healthy volunteers or subjects with asthma showed that NO is at best a weak bronchodilator relative to albuterol in humans, either at baseline or in subjects induced toward bronchospasm with methacholine (17–20). Regarding sGC involvement, no studies have been performed in human subjects. However, pharmacologic inhibition of sGC in naive mice was reported to make them hyperreactive toward methacholine (6), and our recent study was the first to suggest that preconstricted hPCLS undergoes bronchodilation in response to sGC agonists such as BAY 41 and BAY 60 (15).
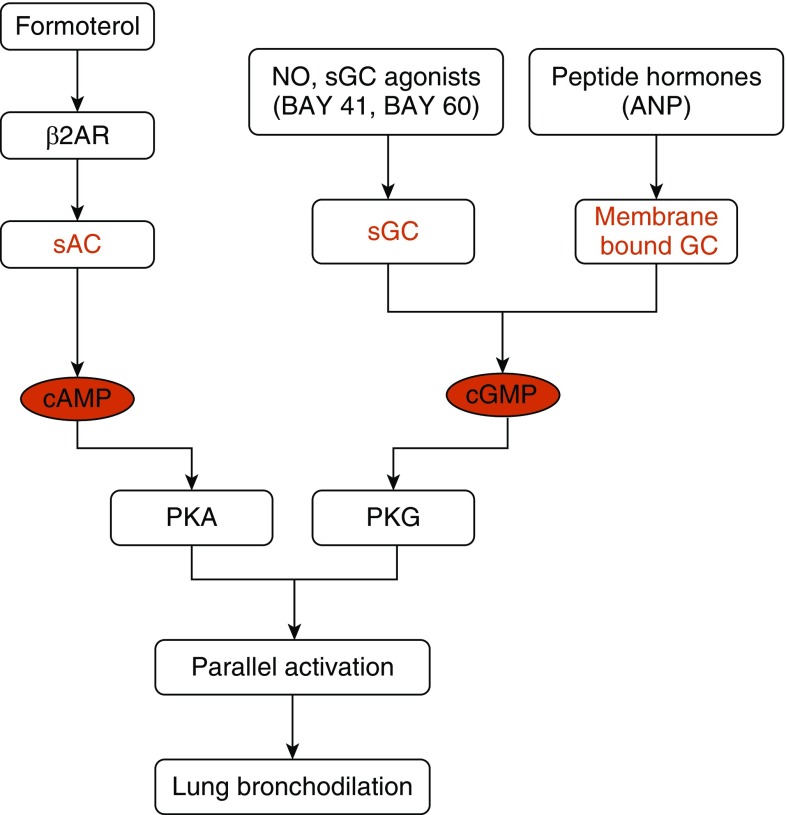
Our cyclic nucleotide measures in the hPCLS suggest that bronchodilation via the sGC–cGMP pathway is mechanistically independent of the β2AR–soluble adenylyl cyclase–cAMP signaling pathway (Figure 4), and there exists little cross-talk between the two cyclic nucleotide pathways to achieve bronchodilation. The equivalent efficacy and mechanistic independence of the two pathways shifts the view of their relative importance and implies that sGC agonists, either alone or in combination with established β2-agonists, could be beneficial in promoting bronchodilation in asthma, particularly in the subset of patients whose respiratory disorders exhibit or develop resistance toward β2 agonists. We found BAY 41 and BAY 60 to be equally effective despite their acting on two different forms of sGC. Their similar efficacy is indicative of and consistent with tissues containing two forms of sGC under both normal and pathophysiological conditions, namely sGC’s heme-containing and heme-free forms, which respond to BAY 41 or to BAY 60, respectively (12). Increased oxidant stress, such as that which occurs in the inflamed airway, can damage sGC and increase the sGC subpopulation that is responsive to BAY 60 and insensitive to BAY 41 or to the physiologic activator NO (15, 21). This may help explain why the efficacy of NO therapy was found to be poor in early preclinical studies of asthma. Indeed, we recently showed that the proportion of heme-free, BAY 60–responsive sGC increases in the airway in inflammatory asthma mouse models (15). A trend in prevalence toward heme-free sGC in asthma was also suggested by a recent disease cluster-based analysis (22). Because our present findings imply that both sGC forms are present in hPCLS made from healthy donors, they suggest that both types of sGC agonist could be broadly effective in patients with asthma and other respiratory diseases, particularly when conventional therapies with β2-adrenergic agonists are found to be attenuated.
Figure 4.
Flow chart depicting parallel activation of the β2AR–soluble adenylyl cyclase (sAC)–cAMP and NO–sGC–cGMP signaling pathways. ANP = atrial natriuretic peptide; PKA = protein kinase A; PKG = protein kinase G.
Cross-talk between the cAMP and cGMP signals has been documented in cardiac myocytes and occurs at the level of PDEs to provide a dual means to trigger downstream functions. For example, the cross-talk occurring in the regulation of cardiac contractility suggests that cGMP, produced by stimulation of natriuretic peptide receptor B for C-type natriuretic peptide, inhibits cAMP degradation by PDE3, thereby enhancing the cAMP-mediated signaling from β-adrenoceptors (23). PDE2 repression by cGMP also leads to enhancement of cAMP signaling in other circumstances (24), including in the peripheral nervous system (25). Our present study with hPCLS showed no evidence of cross-talk between the pathways that lead to the generation of either cAMP or cGMP (as depicted in Figure 4). Any cyclic nucleotide cross-talk that may occur downstream from their production might be expected to elevate lung bronchodilation by a similar mechanism whereby cGMP inhibition of PDEs increases cAMP buildup. This type of cross-talk would be particularly beneficial in combinatorial therapies. Conversely, in instances in which the β2ARs are dysfunctional, dilation can still be achieved via sGC agonists, as our present study suggests.
Although cross-talk mechanisms similar to those in cardiac myocytes may propagate excitation–contraction coupling in airways, recent studies on zebrafish larvae (26) suggest that endogenous NO reduces calcium transients in an sGC-dependent manner, thereby abrogating excitation–contraction coupling. Perez-Zoghbi and colleagues demonstrated that NO diminishes calcium oscillations through inhibition of the inositol 1,4,5-trisphosphate receptor with generation of cGMP (27). Traditional β2AR agonists such as formoterol decrease airway constriction through diminishing calcium sensitivity at low concentrations and decreasing calcium oscillations at higher concentrations (28, 29). Although these findings still need to be documented in airway smooth muscle cells, if replicated in the lung, they would suggest that activation of the NO–sGC–cGMP pathway may be timed differently or might even remain dormant under physiological conditions, but that it could still be efficiently activated to achieve bronchodilation by bypassing NO through the use of the sGC agonists. In addition, combined therapy with a β2AR agonist and an sGC activator may augment the effects of either one alone, given the attenuation of calcium oscillations by both classes of compounds.
Although these studies have been performed in intact lung tissue, our model does have limitations. The effects we observed were at relatively high concentrations, so it may be difficult to attain these concentrations. Alternatively, using an inhalation route alleviates some worries about systemic adverse side effects such as hypotension. In addition, our studies solely used tissue from donors without disease. Although tissue derived from donors with asthma would have provided greater insight into the effects of these compounds in the context of a disease state, technical issues prevented us from generating hPCLS of sufficient quality from lungs of donors with asthma.
Collectively, our studies suggest that alternative pathways to those induced by the β2AR elicit bronchodilation and can be used in the treatment of lung disorders such as asthma and chronic obstructive pulmonary disease. Furthermore, these pathways are less susceptible to G protein–coupled receptor desensitization than are β2AR agonists, the current industry standard. Use of therapeutics targeting sGC directly may serve as a novel therapeutic approach to reverse airway hyperreactivity.
Acknowledgments
Acknowledgment
The authors thank William Jester, Gaoyuan Cao, and Brian Deeney for expert technical assistance.
Footnotes
Supported by National Institutes of Health grants HL081064 (A.G., S.E.E., and D.J.S.) and HL114471 (C.J.K.-W. and R.A.P.).
Author Contributions: C.J.K.-W.: study design, data acquisition, data analysis and interpretation, drafting and editing of the manuscript, and final approval for publication. A.G.: study design, data acquisition, data analysis and interpretation, drafting and editing of the manuscript, and final approval for publication. P.S.: study design, drafting and editing of the manuscript, and final approval for publication. S.E.E.: study design, drafting and editing of the manuscript, and final approval for publication. D.J.S.: study design, drafting and editing of the manuscript, and final approval for publication. R.A.P.: study design, drafting and editing of the manuscript, and final approval for publication.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0001OC on July 24, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Moncada S, Higgs EA.The discovery of nitric oxide and its role in vascular biology Br J Pharmacol 2006147Suppl 1):S193–S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derbyshire ER, Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem. 2012;81:533–559. doi: 10.1146/annurev-biochem-050410-100030. [DOI] [PubMed] [Google Scholar]
- 3.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 4.Ellis JL. Role of soluble guanylyl cyclase in the relaxations to a nitric oxide donor and to nonadrenergic nerve stimulation in guinea pig trachea and human bronchus. J Pharmacol Exp Ther. 1997;280:1215–1218. [PubMed] [Google Scholar]
- 5.Nijkamp FP, Folkerts G. Nitric oxide and bronchial hyperresponsiveness. Arch Int Pharmacodyn Ther. 1995;329:81–96. [PubMed] [Google Scholar]
- 6.Papapetropoulos A, Simoes DC, Xanthou G, Roussos C, Gratziou C. Soluble guanylyl cyclase expression is reduced in allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2006;290:L179–L184. doi: 10.1152/ajplung.00330.2005. [DOI] [PubMed] [Google Scholar]
- 7.Uray FP, de Alfonzo RG, de Becemberg IL, Alfonzo MJ. Muscarinic agonists acting through M2 acetylcholine receptors stimulate the migration of an NO-sensitive guanylyl cyclase to the plasma membrane of bovine tracheal smooth muscle. J Recept Signal Transduct Res. 2010;30:10–23. doi: 10.3109/10799890903325585. [DOI] [PubMed] [Google Scholar]
- 8.Cooper PR, Lamb R, Day ND, Branigan PJ, Kajekar R, San Mateo L, et al. TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. Am J Physiol Lung Cell Mol Physiol. 2009;297:L530–L537. doi: 10.1152/ajplung.00133.2009. [DOI] [PubMed] [Google Scholar]
- 9.Koziol-White CJ, Yoo EJ, Cao G, Zhang J, Papanikolaou E, Pushkarsky I, et al. Inhibition of PI3K promotes dilation of human small airways in a Rho kinase-dependent manner. Br J Pharmacol. 2016;173:2726–2738. doi: 10.1111/bph.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinett KS, Koziol-White CJ, Akoluk A, An SS, Panettieri RA, Jr, Liggett SB. Bitter taste receptor function in asthmatic and nonasthmatic human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2014;50:678–683. doi: 10.1165/rcmb.2013-0439RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123:2263–2273. doi: 10.1161/CIRCULATIONAHA.110.981738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H S AK, Meurer S, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006;116:2552–2561. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mittendorf J, Weigand S, Alonso-Alija C, Bischoff E, Feurer A, Gerisch M, et al. Discovery of riociguat (BAY 63-2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. ChemMedChem. 2009;4:853–865. doi: 10.1002/cmdc.200900014. [DOI] [PubMed] [Google Scholar]
- 14.Cooper PR, Panettieri RA., Jr Steroids completely reverse albuterol-induced β2-adrenergic receptor tolerance in human small airways. J Allergy Clin Immunol. 2008;122:734–740. doi: 10.1016/j.jaci.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh A, Koziol-White CJ, Asosingh K, Cheng G, Ruple L, Groneberg D, et al. Soluble guanylate cyclase as an alternative target for bronchodilator therapy in asthma. Proc Natl Acad Sci USA. 2016;113:E2355–E2362. doi: 10.1073/pnas.1524398113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandner P, Becker-Pelster EM, Stasch JP. Discovery and development of sGC stimulators for the treatment of pulmonary hypertension and rare diseases. Nitric Oxide. 2018;77:88–95. doi: 10.1016/j.niox.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Högman M, Frostell CG, Hedenström H, Hedenstierna G. Inhalation of nitric oxide modulates adult human bronchial tone. Am Rev Respir Dis. 1993;148:1474–1478. doi: 10.1164/ajrccm/148.6_Pt_1.1474. [DOI] [PubMed] [Google Scholar]
- 18.Kacmarek RM, Ripple R, Cockrill BA, Bloch KJ, Zapol WM, Johnson DC. Inhaled nitric oxide: a bronchodilator in mild asthmatics with methacholine-induced bronchospasm. Am J Respir Crit Care Med. 1996;153:128–135. doi: 10.1164/ajrccm.153.1.8542105. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer KD, Ellison G, Robertson D, Day RW. The effect of inhaled nitric oxide in pediatric asthma. Am J Respir Crit Care Med. 1996;153:747–751. doi: 10.1164/ajrccm.153.2.8564128. [DOI] [PubMed] [Google Scholar]
- 20.Sanna A, Kurtansky A, Veriter C, Stănescu D. Bronchodilator effect of inhaled nitric oxide in healthy men. Am J Respir Crit Care Med. 1994;150:1702–1704. doi: 10.1164/ajrccm.150.6.7952636. [DOI] [PubMed] [Google Scholar]
- 21.Baldissera L, Jr, Squebola-Cola DM, Calixto MC, Lima-Barbosa AP, Rennó AL, Anhê GF, et al. The soluble guanylyl cyclase activator BAY 60-2770 inhibits murine allergic airways inflammation and human eosinophil chemotaxis. Pulm Pharmacol Ther. 2016;41:86–95. doi: 10.1016/j.pupt.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Langhauser F, Casas AI, Dao VT, Guney E, Menche J, Geuss E, et al. A diseasome cluster-based drug repurposing of soluble guanylate cyclase activators from smooth muscle relaxation to direct neuroprotection. NPJ Syst Biol Appl. 2018;4:8. doi: 10.1038/s41540-017-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavlaki N, Nikolaev VO. Imaging of PDE2- and PDE3-mediated cGMP-to-cAMP cross-talk in cardiomyocytes. J Cardiovasc Dev Dis. 2018;5:E4. doi: 10.3390/jcdd5010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy FO. Cardiac PDEs and crosstalk between cAMP and cGMP signalling pathways in the regulation of contractility. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:665–670. doi: 10.1007/s00210-013-0874-z. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Yu Y, Ruan L, Wang C, Pan J, Klabnik J, et al. The roles of phosphodiesterase 2 in the central nervous and peripheral systems. Curr Pharm Des. 2015;21:274–290. doi: 10.2174/1381612820666140826115245. [DOI] [PubMed] [Google Scholar]
- 26.Xiyuan Z, Fink RHA, Mosqueira M. NO-sGC pathway modulates Ca2+ release and muscle contraction in zebrafish skeletal muscle. Front Physiol. 2017;8:607. doi: 10.3389/fphys.2017.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Zoghbi JF, Bai Y, Sanderson MJ. Nitric oxide induces airway smooth muscle cell relaxation by decreasing the frequency of agonist-induced Ca2+ oscillations. J Gen Physiol. 2010;135:247–259. doi: 10.1085/jgp.200910365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delmotte P, Sanderson MJ. Effects of formoterol on contraction and Ca2+ signaling of mouse airway smooth muscle cells. Am J Respir Cell Mol Biol. 2010;42:373–381. doi: 10.1165/rcmb.2008-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ressmeyer AR, Bai Y, Delmotte P, Uy KF, Thistlethwaite P, Fraire A, et al. Human airway contraction and formoterol-induced relaxation is determined by Ca2+ oscillations and Ca2+ sensitivity. Am J Respir Cell Mol Biol. 2010;43:179–191. doi: 10.1165/rcmb.2009-0222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]