Abstract
The purpose of this investigation was to compare ibuprofen versus an ibuprofen/acetaminophen combination for postoperative pain control in a patient model specific to teeth diagnosed with symptomatic irreversible pulpitis and symptomatic apical periodontitis. One hundred and two patients presenting with moderate to severe pain from a maxillary or mandibular posterior tooth diagnosed with symptomatic irreversible pulpitis and symptomatic apical periodontitis were included. Following local anesthetic administration, complete endodontic cleaning and shaping was performed. Patients were randomly assigned to receive identically appearing tablets of ibuprofen 200 mg or a combination of ibuprofen 200 mg/acetaminophen 216.7 mg with instructions to take 3 tablets every 6 hours as needed for pain. Patients were also given a prescription for an escape medication to take if the study medications did not adequately control their pain. A 4-day diary was used to record pain ratings and medication use. Moderate to severe pain was experienced by 59–61% of the patients on postoperative day 1 and 50–57% of the patients on day 2, with the pain ratings decreasing over the next 2 days. There were no statistically significant differences between the 2 groups in postoperative pain, percussion pain, or medication use. There was no difference between ibuprofen and the combination of ibuprofen/acetaminophen in the reduction of postoperative pain following endodontic debridement in patients with symptomatic irreversible pulpitis and symptomatic apical periodontitis.
Key Words: Ibuprofen, Acetaminophen, Symptomatic patients, Symptomatic irreversible pulpitis, Symptomatic apical periodontitis, Postoperative pain
Systematic and Cochrane reviews have found superior postoperative pain control with combinations of ibuprofen/acetaminophen versus use of either medication alone.1–4 However, these reviews were largely based on third molar extraction models in young adults who have no pain or pre-existing infections. As stated by Moore and Hersh,5 the efficacies of ibuprofen combined with other nonsteroidal anti-inflammatory drugs differ depending on the model being studied. They further stated that additional research evaluating postoperative pain management with combined use of ibuprofen and acetaminophen is needed for endodontic procedures.
Ibuprofen is commonly recommended for postoperative pain management following endodontic therapy and dosing with 600 mg every 6 hours is recommended for reducing inflammation found with vital endodontic conditions.6,7 Menhinick et al8 is often quoted to support the recommendation of combination dosing (ibuprofen 600 mg/acetaminophen 1000 mg every 6 hours) because the combination was more effective at reducing postoperative endodontic pain than ibuprofen alone. However, that study had only 18–20 patients per group, included varying pulpal and periapical diagnoses (symptomatic irreversible pulpitis, pulpal necrosis, no symptomatic apical periodontitis), and had a maximum follow-up time of 8 hours. Even though a combination of ibuprofen and acetaminophen was found to be more effective than ibuprofen alone, ibuprofen was not found to be significantly better than a placebo.
A key predictor of postoperative pain is the intensity of the patient's preoperative pain.9 Another important factor is the preoperative status or diagnosis of the pulpal and periapical tissues.9 Law et al9 found that a diagnosis of symptomatic apical periodontitis was a factor in predicting postoperative pain. Gotler et al10 compared postoperative pain in patients who underwent endodontic therapy in teeth with differing initial pulpal diagnoses including vital healthy pulp tissues, necrotic pulp tissues, or previous endodontically treated teeth requiring retreatment. The results demonstrated that vital teeth induced a significantly higher incidence and severity of postoperative pain (64%) compared with necrotic pulps (38%) or retreated teeth (49%). The authors thought the pain was higher in vital teeth because of endodontic treatment causing inflammation periapically. However, in the Gotler et al10 study, canal obturation was performed, which could have added to the effect of periapical inflammation. This information supports the idea that postoperative pain management of symptomatic irreversible pulpitis with symptomatic apical periodontitis would be a good model because postoperative pain would be expected.
Most previous recommendations for postoperative pain have been based on studies using acetaminophen 1000 mg every 6 hours, but in 2011 the producer of Tylenol® voluntarily lowered the maximum daily dose to 3000 mg/d in both single dose and opioid-containing medications. This was in response to a Food and Drug Administration suggestion to lower the daily dose in an effort to reduce risk of liver damage. Even though the 1000-mg dose of acetaminophen can still be used as a prescribed dose, patients using over-the-counter medications as recommended, following endodontic therapy, should be taking the 650-mg dose as directed.
Smith et al11 completed a systematic review and meta-analysis of nonsteroidal anti-inflammatory drugs for managing postoperative endodontic pain in patients presenting with preoperative pain. The meta-analysis showed that ibuprofen 600 mg and combined ibuprofen 600 mg/acetaminophen 1000 mg were more effective than placebo but were not significantly different from one another. This meta-analysis concluded that with the limited number of endodontic postoperative pain studies, there was insufficient data to recommend the most effective postoperative pain medication, dose amount, or dose interval.11
The risks that come with taking any medication should be weighed against any potential benefits. Recommending or prescribing a 2-medication dose regimen over a solo medication regimen warrants consideration of each individual patient and their clinical condition rather than a routine recommendation given to all. Therefore, the purpose of this investigation was to compare ibuprofen versus an ibuprofen/acetaminophen combination for postoperative pain control in a patient model specific to teeth diagnosed with symptomatic irreversible pulpitis and symptomatic apical periodontitis.
MATERIALS AND METHODS
One hundred twenty-four (124) adult patients participated in this study. All were emergency patients and were in good health as determined by a health history and oral questioning. Six (6) patients were disqualified due to the presence of some necrotic tissue upon access. Sixteen (16) patients failed to return their completed surveys and were subsequently dismissed from the study. Data analysis was completed on 102 patients with mandibular and maxillary posterior teeth diagnosed with symptomatic irreversible pulpitis and symptomatic apical periodontitis.
Exclusion criteria were as follows: patients younger than 18 years of age and older than 65 years of age; history of significant medical problems (American Society of Anesthesiologists class II or higher); allergies or contraindications to ibuprofen, acetaminophen, and/or local anesthetics; use of any central nervous system depressants or any analgesic medication within the last 6 hours; pregnancy; and inability to give informed consent. The Ohio State University Human Subjects Review Committee approved the study. Written informed consents and research authorizations were obtained from all patients.
Each patient had a vital mandibular or maxillary posterior tooth (molar or premolar) causing moderate to severe spontaneous pain that also was noted to have had a prolonged response to cold testing with Green Endo-Ice® (1,1,1,2 tetrafluoroethane; Hygenic Corp, Akron, OH) at the time of the appointment. All patients had a periapical diagnosis involving the same tooth of symptomatic apical periodontitis identified by a positive response to percussion. Patients were excluded if there was a normal response to percussion. No patient exhibited radiographic periradicular pathosis other than a widened periodontal ligament. Therefore, each patient included in the study had only 1 tooth that fulfilled the criteria for a clinical diagnosis of symptomatic irreversible pulpitis and symptomatic apical periodontitis and were experiencing spontaneous moderate to severe pain.
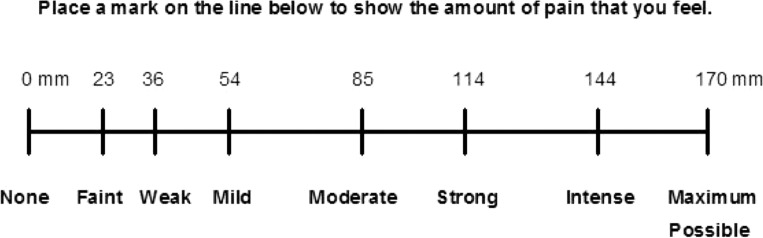
Patients completed a Corah Dental Anxiety Scale to rate their level of anxiety.12 Each patient was also required to rate presenting pain on a Heft-Parker visual analogue scale, which has been used extensively in dental anesthesiology and dental pain research.13 It was developed to be a hybrid alternative to traditional visual analog scales (VASs) with no guides since it is a graphic rating scale with a horizontal line with category word designations (Figure 1). The VAS was divided into 4 categories to be consistent with other studies.14–18 No pain corresponded to 0 mm. Mild pain was defined as greater than 0 mm and less than or equal to 54 mm. Mild pain included the descriptors of faint, weak, and mild pain. A score greater than 54 mm and less than 114 mm indicated moderate pain and included the descriptor of moderate pain. Severe pain was defined as equal to or greater than 114 mm. Severe pain included the descriptors of strong, intense, and maximum possible. Only patients who recorded their presenting pain levels as moderate or severe on the VAS were included in the study.
Figure 1. .
Heft-Parker VAS for pain (the numbers across the top were not included in the patient's VAS).
Local anesthesia was administered in a standardized manner depending on if the tooth being treated was in the mandibular or maxillary arch. Patients received 3 mL of 2% lidocaine with 1:100,000 epinephrine (Xylocaine, AstraZeneca LP, Dentsply, York, PA), for a total dose of 60 mg of lidocaine and 30 μg of epinephrine, using a conventional inferior alveolar nerve block (IANB).19 It was assumed the lingual nerve was anesthetized with the IANB, but this was not confirmed clinically. The anesthetic was delivered using a Computer Controlled Local Anesthetic Delivery system (CCLAD; Milestone Scientific, Deerfield, IL) unit. Because a buccal infiltration of articaine was added to the mandibular teeth, no long buccal nerve block was used initially. For maxillary teeth, all patients received 2.45 mL of 2% lidocaine with 1:100,000 epinephrine via buccal infiltration and 0.35 mL via palatal infiltration using the CCLAD system. The total dose was 56 mg of lidocaine and 28 μg of epinephrine. The principal investigator (A.S.) performed all the injections.
The patients were questioned every 5 minutes for a total of 15 minutes for lip (IANB) or cheek numbness (maxilla), with all patients noted to have had lip/cheek numbness. Patients with a mandibular tooth received an additional 1.4 mL of 4% articaine with 1:100,000 epinephrine (Septocaine, Septodont, Dentsply) via buccal infiltration at the apex of the tooth being treated using the CCLAD. The total dose was 56 mg of articaine and 14 μg of epinephrine. After 5 minutes following the buccal infiltration to allow the articaine to work,20 an intraosseous injection was delivered using the Stabident (Fairfax Dental, Inc, Miami, FL) intraosseous anesthesia delivery system. The maxillary teeth also received a Stabident intraosseous injection, but no maxillary buccal infiltration of 4% articaine with 1:100,000 epinephrine was administered. All subjects received 1.8 mL of 2% lidocaine with 1:100,000 epinephrine via the intraosseous injection distal to the tooth to be treated as described by Nusstein et al,21 unless a second molar was being treated in which the injection was delivered mesial to the tooth. The total dose was 36 mg of lidocaine and 18 μg of epinephrine.
All patients received an intraosseous injection to standardize the anesthetic technique in order to achieve initial pulpal anesthesia. The buccal infiltration success rate (no or mild pain upon endodontic access) in patients with symptomatic irreversible pulpitis using 4% articaine with 1:100,000 epinephrine following an IANB has ranged from 42 to 48% in molars.22 Authors have found success rates (no or mild pain upon endodontic treatment in patients with symptomatic irreversible pulpitis) of maxillary molar buccal infiltration ranged from 54 to 85%, with an average rate of 64%.23–27 Although not everyone may require an intraosseous injection for pulpal anesthesia with symptomatic irreversible pulpitis, a number of patients will. Therefore, local anesthesia was administered in this fashion to all patients to remove that as a potential confounding variable.
K-type hand files (Patterson Dental, Saint Paul, MN) and rotary Vortex files (Dentsply Tulsa, Tulsa, OK) were used for canal preparation. An apex locator (Root ZX II, J. Morita USA, Irvine, CA) was then used to determine the working length approximately 1.0 mm from the apex and confirmed with a digital radiograph. The minimum canal preparation was a size 30 with a .04 taper because it was important that complete cleaning and shaping was performed. Canals were irrigated with 3.0% sodium hypochlorite (Clorox Company, Oakland, CA) following the use of every third hand and rotary file. The canals were not obturated. A cotton pellet was placed, and the tooth was temporized with Cavit (Cavit G, 3M ESPE, Seefeld, Germany). Complete removal of pulpal tissue and canal preparation was performed by the senior author (A.S.).
Before the experiment, the ibuprofen and ibuprofen/acetaminophen groups were assigned 6-digit random numbers. The number assignment determined which drug regimen would be administered postoperatively for each patient. Only the random numbers were recorded on the data collection sheet in order to maintain blinding of the experiment.
The blinding of the ibuprofen and ibuprofen/acetaminophen medications was done as follows. A registered pharmacist compounded identically appearing tablets of ibuprofen 200 mg (I) and tablets of ibuprofen 200 mg/acetaminophen 216.7 mg (IA). Sixty tablets of either the I or the IA combination were placed in identically appearing bottles. The pharmacist prepared the master code sheet and assigned the random numbers to the bottles to blind the patient and the operator. A copy of the master list of random numbers was supplied by the compounding pharmacist solely to the lead researcher (M.D.); it was not made available to anyone else during the data collection period.
At the end of the debridement appointment, the patients received a bottle containing 60 tablets from either the I or the IA group. The patients were instructed to take 3 tablets equating to ibuprofen 600 mg or ibuprofen 600 mg/acetaminophen 650 mg every 6 hours as needed for pain. The patients were instructed not to take any other pain medications during this investigation. The patients were given a prescription for an escape pain medication to be used only if the initial study medication did not manage the pain. The prescription had instructions to the pharmacist as follows “Void until the pharmacy calls ###-###-####” (cellular study phone) prior to filling the prescription,” so the patients could not fill the prescription until the investigator was notified by the pharmacist. If that occurred, the patients were then instructed to stop taking all study medications once starting the escape medication, to avoid potential for acetaminophen toxicity. Either hydrocodone 5 mg/acetaminophen 325 mg, 12 tablets, 1–2 tablets every 6 hours or codeine 30 mg/acetaminophen 300 mg, 12 tablets, 1–2 tablets every 6 hours was prescribed to patients as the escape pain medications.
The patients received a diary to record findings for 4 days posttreatment for pain, percussion pain, and the amount and type of study medications taken. Patients received specific instructions on how to tap on the experimental tooth that had emergency endodontic treatment and record the findings as their percussion pain. Starting on the morning after treatment, patients recorded their pain levels on a VAS as described earlier for postoperative treatment pain. Patients also recorded the number of study medications taken within each 24-hour period. The patient scheduled their follow-up appointment for 4 days. When patients returned, the investigator went over the pain diary and collected all unused medications to verify diary results. Endodontic treatment was completed after 4 days, at a time determined by the patient's schedule.
All results were collected and statistically analyzed. The patient's age and presenting pain were assessed preoperatively using the randomization test. The Mann-Whitney Wilcoxon test was used to assess differences in the Corah Dental Anxiety Scale. The chi-square test (or the Fisher exact test if expected frequencies were <5) was used to evaluate preoperative group differences in gender, tooth location, and upper versus lower arch. Postoperative pain ratings were evaluated using multiple randomization tests with p-values adjusted for multiple comparisons using the step-down Bonferroni method of Holm. Separate power analyses for every variable being measured were not calculated. The statistical analysis was based on the study by Wells et al28 using the Heft-Parker VAS measurement of postoperative pain. With a nondirectional alpha risk of 0.05 and assuming a SD of 50 mm, a difference of ±35 mm could be detected with a power of 0.88 with 50 patients per group.
RESULTS
Table 1 shows the preoperative variables. There were no statistically significant differences between the 2 study groups with regard to age, gender, presenting pain, Corah Dental Anxiety Score, specific tooth type, or arch (ie, upper vs lower arch).
Table 1. .
Initial Preoperative Variables for the Ibuprofen (I) and Ibuprofen/Acetaminophen (IA) Groups*
|
Variable |
I Group |
IA Group |
P Value |
| Age, y | 34 ± 11 | 35 ± 12 | .6929 |
| Total # patients analyzed | 49 | 53 | |
| Gender | |||
| Female | 29 | 39 | .6861 |
| Male | 20 | 14 | |
| Presenting initial pain† | 129 ± 22 | 128 ± 23 | .7103 |
| Initial Corah Anxiety Scale ratings‡ | 9 | 9 | .9893 |
| Tooth type | .6569 | ||
| Mandibular | |||
| Molars | 45% (22/49) | 40% (21/53) | |
| Premolars | 4% (2/49) | 11% (6/53) | |
| Maxillary | |||
| Molars | 35% (18/49) | 41% (22/53) | |
| Premolars | 14% (7/49) | 7% (4/53) | |
| Arch location | .6861 | ||
| Mandible | 49% (24/49) | 51% (27/53) | |
| Maxilla | 51% (25/49) | 49% (26/53) | |
There were no significant differences (p > .05) between the 2 groups.
Mean ± SD, Heft-Parker VAS ratings (mm).
Median.
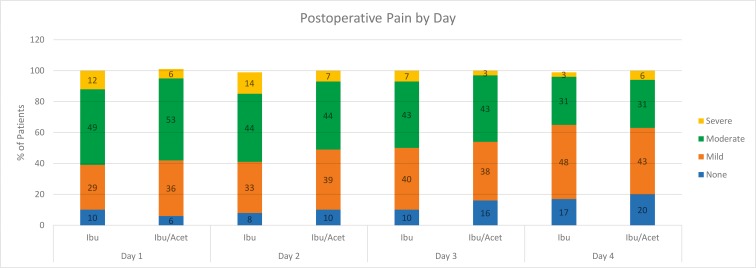
Table 2 and Figure 2 present pain ratings by day for the I and IA groups. On postoperative day 1, moderate to severe pain was experienced by 61% of the patients in the I group versus 59% of the patients in the combination IA group. On postoperative day 2, 58% had moderate to severe pain in the I group compared with 51% in the combination IA group. Pain ratings decreased in both groups over days 3 and 4. The mean pain scores between the 2 study groups failed to demonstrate statistically significant differences for any postoperative day.
Table 2. .
Percentages and Discomfort Ratings of Postoperative Pain for the Ibuprofen (I) and Ibuprofen/Acetaminophen (IA) Groups by Day*
|
Study Group |
None, % # |
Mild, % # |
Moderate, % # |
Severe, % # |
Mean, mm ± SD |
Difference in Means, mm ± SD |
P Value for Mean |
|
| Day 1 | I (n = 49) | 10% 5 | 29% 14 | 49% 24 | 12% 6 | 63 ± 41 | 3 ± 41 | 1.000 |
| IA (n = 53) | 6% 3 | 36% 19 | 53% 28 | 6% 3 | 60 ± 42 | |||
| Day 2 | I (n = 36) | 8% 3 | 33% 12 | 44% 16 | 14% 5 | 53 ± 44 | 3 ± 40 | 1.000 |
| IA (n = 41) | 10% 4 | 39% 16 | 44% 18 | 7% 3 | 50 ± 37 | |||
| Day 3 | I (n = 30) | 10% 3 | 40% 12 | 43% 13 | 7% 2 | 38 ± 41 | 3 ± 38 | 1.000 |
| IA (n = 37) | 16% 6 | 38% 14 | 43% 16 | 3% 1 | 41 ± 35 | |||
| Day 4 | I (n = 29) | 17% 5 | 48% 14 | 31% 9 | 3% 1 | 26 ± 28 | 6 ± 33 | 1.000 |
| IA (n = 35) | 20% 7 | 43% 15 | 31% 11 | 6% 2 | 32 ± 39 |
Patients requiring opioids were excluded on days 2–4.
Figure 2. .
Percentages and discomfort ratings of postoperative pain for the ibuprofen and ibuprofen/acetaminophen groups by day. patients requiring opioids were excluded on days 2–4.
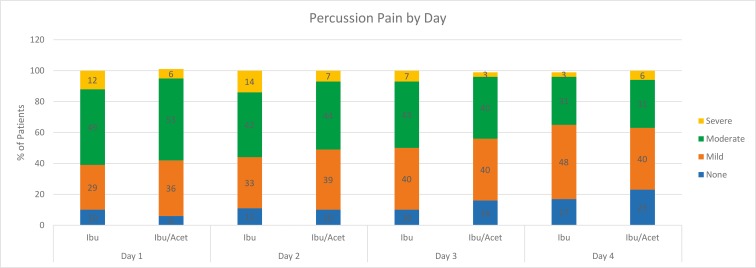
Table 3 and Figure 3 illustrate percussion pain by day between the I and IA groups. On postoperative day 1, moderate to severe percussion pain was experienced by 61% of the patients in the I group compared with 59% of the patients in the combination IA group. On postoperative day 2, 56% had moderate to severe percussion pain in the I group versus 51% in the combination IA group. Percussion pain ratings decreased in both groups over days 3 and 4. The mean percussion pain scores between the 2 study groups failed to demonstrate statistically significant differences for any postoperative day.
Table 3. .
Percentages and Discomfort Ratings of Percussion Pain for the Ibuprofen (I) and Ibuprofen/Acetaminophen (IA) Groups by Day*
|
Study Group |
None, % # |
Mild, % # |
Moderate, % # |
Severe, % # |
Mean, mm ± SD |
Difference in Means, mm ± SD |
P Value for Mean |
|
| Day 1 | I (n = 49) | 10% 5 | 29% 14 | 49% 24 | 12% 6 | 63 ± 41 | 3 ± 41 | 1.000 |
| IA (n = 53) | 6% 3 | 36% 19 | 53% 28 | 6% 3 | 60 ± 42 | |||
| Day 2 | I (n = 36) | 11% 4 | 33% 12 | 42% 15 | 14% 5 | 60 ± 39 | 10 ± 38 | 1.000 |
| IA (n = 41) | 10% 4 | 39% 16 | 44% 18 | 7% 3 | 50 ± 37 | |||
| Day 3 | I (n = 30) | 10% 3 | 40% 12 | 43% 13 | 7% 2 | 40 ± 34 | 7 ± 34 | 1.000 |
| IA (n = 37) | 16% 6 | 40% 15 | 40% 15 | 3% 1 | 47 ± 35 | |||
| Day 4 | I (n = 29) | 17% 5 | 48% 14 | 31% 9 | 3% 1 | 26 ± 28 | 16 ± 33 | 1.000 |
| IA (n = 35) | 23% 8 | 40% 14 | 31% 11 | 6% 2 | 42 ± 38 |
Patients requiring opioids were excluded on days 2–4.
Figure 3. .
Percentages and discomfort ratings of postoperative percussion pain for the ibuprofen and ibuprofen/acetaminophen groups by day. Patients requiring opioids were excluded on days 2–4.
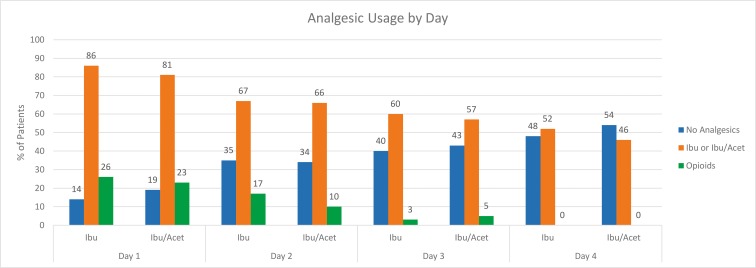
Table 4 and Figure 4 demonstrate the percentage and mean number of tablets of I and IA taken by day. The mean number of tablets taken was divided by the number of participants (excluding the patients taking opioids) for each day (Table 4). On day 1, 86 and 81% took I or IA, respectively. The percentage of medications use decreased over days 2–4. Some patients did not require analgesic medications because presumably they had none or mild pain (Table 4). The percentage of medications use decreased over days 2–4. Some patients did not require analgesic medications because presumably they had mild or no pain (Table 4).
Table 4. .
Patients Requiring No Analgesics and Those Taking Ibuprofen or Ibuprofen/Acetaminophen, Plus Those Requiring Opioids, As Well As the Mean Number of Ibuprofen or Ibuprofen/Acetaminophen Combination Tablets Taken by Day
|
Study Group |
Requiring No Analgesics, # (%) |
Taking I or IA, # (%) |
Requiring Opioids, # (%) |
Average # of I or IA Tablets Taken/Day, Mean ± SD |
|
| Day 1 | I (n = 49) | 7 (14) | 42 (86) | 13 (26) | 4.5 ± 3.0 |
| IA (n = 53) | 10 (19) | 43 (81) | 12 (23) | 5.2 ± 4.0 | |
| Day 2 | I (n = 36) | 12 (35) | 24 (67) | 6 (17) | 3.8 ± 3.4 |
| IA (n = 41) | 14 (34) | 27 (66) | 4 (10) | 3.9 ± 3.8 | |
| Day 3 | I (n = 30) | 12 (40) | 18 (60) | 1 (3) | 3.6 ± 3.7 |
| IA (n = 37) | 16 (43) | 21 (57) | 2 (5) | 3.0 ± 3.6 | |
| Day 4 | I (n = 29) | 14 (48) | 15 (52) | 0 (0) | 2.9 ± 3.3 |
| IA (n = 35) | 19 (54) | 16 (46) | 0 (0) | 2.7 ± 3.8 | |
| Total Number of Patients Requiring Opioids | |||||
|
Study Group |
# |
% |
P Value |
||
| I (n = 49) | 20 | 41% | .8149 | ||
| IA (n = 53) | 18 | 34% | |||
Figure 4. .
Percentage of patients taking ibuprofen, ibuprofen/acetaminophen, and opioid. Opioid percentages are presented on top of the ibuprofen and ibuprofen/acetaminophen percentages.
The total number of patients requiring the opioid-containing rescue medication was 41% (20/49) in the I group and 34% (18/53) in the IA group, with no significant difference (p = .8149) noted between the 2 groups. Because pain medication use was evaluated in the morning of day 1, patients with pain started taking the ibuprofen or combination ibuprofen/acetaminophen the day of the appointment following the conclusion of the endodontic procedure. When their pain was not managed effectively by the provided study medications (I or IA), patients converted to taking the escape medication (Table 4). Because of the escape medication use, there was a decrease in overall number of study participants for days 2, 3, and 4. The number of patients taking the rescue/escape medication each day for the I and IA groups was as follows: day 1: 26% (13/49) versus 23% (12/53); day 2: 17% (6/36) versus 10% (4/41); day 3: 3% (1/30) versus 5% (2/37); and day 4: 0% (0/29) versus 0% (0/35) (Table 4). Because of the use of opioid medications, the total N for each group fell below the line established by the power analysis for days 2–4. As such, there may have been statistically significant differences between the 2 study groups with regard to pain ratings for days 2–4 that remained undetected due to the lower number of study subjects available at those times.
DISCUSSION
Differences in the preoperative parameters of age, gender, presenting pain, Corah Dental Anxiety Scale, tooth type, and arch location were minimized as no statistically significant differences were demonstrated between the 2 groups (Table 1). This study sampled a middle-aged population (mean age 34–35 years). The presenting initial severe pain (128–129 mm) is well representative of emergency patients with symptomatic irreversible pulpitis as previously shown (Table 1).14–18 The Corah Dental Anxiety Scale ratings averaged 9, which would indicate moderate anxiety (Table 1).12 Because the current study evaluated emergency patients in pain, the occurrence of moderate anxiety would be expected. Previous studies of patients with symptomatic irreversible pulpitis found similar anxiety scores.14–18 The influence of tooth type and arch location was minimized because the teeth were evenly distributed (Table 1).
For both the I and IA groups, moderate to severe pain was reduced from presenting pain levels (Tables 1, 2, and 3). A portion of this pain reduction would be associated with debridement of the tooth.29 Moderate to severe pain was experienced by 59–61% of the patients on postoperative day 1 and 50–57% of the patients on day 2, with the pain ratings continuing to decline over the next 2 days. Hargreaves and coauthors30 measured the amount of mechanical allodynia that was present in patients diagnosed with irreversible pulpitis and symptomatic apical periodontitis. The authors found a 77% pain threshold reduction in teeth diagnosed with irreversible pulpitis compared with the contralateral side.30 This mechanical allodynia may be related to the sensitization of pulpal and periapical mechanoreceptors occurring while the patient is experiencing a toothache and possibly central sensitization.30 These theories may offer an explanation why patients may still report pain associated with a symptomatic vital tooth that has undergone endodontic debridement.
For the I and IA groups, moderate to severe percussion pain was experienced by 60–63% of the patients on postoperative day 1 and 47–59% of the patients on day 2, with the percussion pain ratings decreasing over the next 2 days (Table 3). There was no statistically significant difference between treatment groups for percussion pain. Percussion pain ratings mimicked the pain ratings (Tables 2 and 3). Postoperative percussion pain may be experienced due to the inflamed periapical tissues, mechanical allodynia (diagnosis of symptomatic apical periodontitis), and/or the result of the cleaning and shaping procedure.
Regarding postoperative pain of the intraosseous injection, a previous study found approximately a 7–15% potential for moderate pain associated with either a primary (used as the only injection) maxillary or mandibular intraosseous injection using the Stabident system.31 Regarding the tooth “feeling high,” Gallatin et al31 reported a 5–15% incidence. While these 2 factors (postoperative moderate pain and the tooth “feeling high”) may have contributed to postoperative and percussion pain, they would not account for the high moderate to severe postoperative pain ratings on day 1 and 2. As such, it was felt the pain was most likely due to the periapical diagnosis of symptomatic apical periodontitis (inflamed, allodynic periapical tissue).
Acetaminophen's proposed mechanisms of action are through inhibition of COX, inhibition of the opioid cannabinoid and serotonergic systems, and AM404 TRPV1 inhibition.32 While the acetaminophen mechanisms may be different and complimentary to ibuprofen, the complex interaction of peripheral mediators and central effects may not be superior in all pain models.5,28 Wells et al28 demonstrated no difference between ibuprofen and an ibuprofen/acetaminophen combination in postoperative pain control in symptomatic teeth with pulpal necrosis and spontaneous pain.
In teeth diagnosed with symptomatic irreversible pulpitis, the pulpal tissues are inflamed, with the inflammation spreading to the periodontal ligament resulting in a diagnosis of symptomatic apical periodontitis. While the inflamed pulpal tissue is removed during endodontic debridement, the periapical tissue is basically left untreated, with the periapical inflammation persisting. The continued periapical presence of various inflammatory mediators, and their possible central nociceptive effects, is the mostly likely reason why the study medications (I/IA) were not totally effective.
There are many endodontic studies evaluating postoperative pain.33–40 It is difficult to correlate their results with the current study because of the inclusion of necrotic and vital healthy teeth, use of a single medication dose, and the limited evaluation time of 8 or 24 hours.
Most ibuprofen and ibuprofen/acetaminophen use was in the first several days and decreased over the 3 days paralleling the decreasing pain ratings (Table 4). Patients were instructed to take the I or IA every 6 hours as needed for pain. Because the mean number of tablets taken reflects an overall mean, some patients may have taken more tablets than other patients who had less pain. While possible, it was felt highly unlikely that the study patients were poor at following the analgesic instructions. However, the exact cause of the overall mean number of tablets taken being low in both groups remains unknown. Perhaps, this pattern simply reflects the clinical course of postoperative analgesic use by these patients in the specific groups being studied.
The analgesic medications were prescribed to be taken “as needed for pain” rather than requiring the dose be taken every 6 hours for the duration of the study. One problem with giving medications in a required manner (every 6 hours) clinically is that patients may be required to take medications when no longer indicated thereby increasing the risk of side effects. While a required regimen may make sense for a drug trial lasing only 3–12 hours, medications for this study were prescribed “as needed for pain.” This gave patients the option to take the analgesic medications when they felt it was needed.
The percentage of patients requiring the escape opioid medication was 41% (20/49) in the I group and 34% (18/53) in the IA group, which was not found to be a statistically significant difference. Therefore, even though ibuprofen or the combination of ibuprofen/acetaminophen was available, 34–41% of the patients still required rescue therapy with the opioid-containing escape medication. It is possible that patients opted to use the escape medication thinking it would give superior pain relief, since it was a prescription and also contained an opioid. Wells et al,28 in a study comparing ibuprofen versus ibuprofen/acetaminophen use for postoperative endodontic pain in symptomatic patients with a diagnosis of pulpal necrosis, found that while there was no statistically significant difference between the 2 groups in terms of analgesic use, approximately 20% of patients in both groups required opioid medication to control their pain. As stated by Moore and Hersh,5 “Patients having pain before treatment and severe postoperative pain may need alternative analgesic strategies that include opioids.” While opioid analgesics can be prescribed for the treatment of acute pain, the objective is to ensure prescribing the lowest dosage for the shortest period of time. Currently, the abuse of opioids further emphasizes that effective, alternative nonopioid medications need to be developed for clinical use.
Further studies should address the most effective medications, dose, timing, and future drugs to control postoperative endodontic pain. The risks that come with taking any medication should be weighed against any potential benefits.41 Combination dosing versus alternative dosing schedules is a key topic and likely next step in endodontic pain management research. While alternating dosing may be considered an ideal topic for future studies, the first step was to assess if there was any advantage to using ibuprofen in combination with acetaminophen over use of ibuprofen alone. Comparing the effectiveness of ibuprofen 600 mg with and without acetaminophen 1000 mg every six hours is another topic that warrants consideration for future studies.
Conclusion
In conclusion, there was no statistically significant difference between ibuprofen 600 mg versus the combination of ibuprofen 600 mg/acetaminophen 650 mg as prescribed every 6 hours for the reduction of postoperative pain following endodontic debridement in patients with symptomatic irreversible pulpitis and symptomatic apical periodontitis.
ACKNOWLEDGMENT
The authors deny any conflicts of interest related to this study.
Footnotes
Dr Stamos completed this study for a Master of Science degree. The other authors contributed to the study design, IRB submission, statistical analysis, and were actively involved in the execution of the study, financial funding, served on the Master's Examination Committee, and critically revised and approved the version as submitted.
Dr Stamos was awarded sixth place in the Oral Scientific competition at the AAE International meeting in Denver, CO, April 2018.
REFERENCES
- 1.Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal anti-inflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110:1170–1179. doi: 10.1213/ANE.0b013e3181cf9281. [DOI] [PubMed] [Google Scholar]
- 2.Bailey E, Worthington HV, van Wijk A, Yates JM, Coulthard P, Afzal Z. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth. Cochrane Database Syst Rev. 2010;12(12) doi: 10.1002/14651858.CD004624.pub2. :CD0004624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derry CJ, Derry S, Moore RA. Single dose oral ibuprofen plus paracetamol (acetaminophen) for acute postoperative pain. Cochrane Database Syst Rev. 2013;24(6) doi: 10.1002/14651858.CD010210.pub2. :CD010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore RA, Derry S, Aldington D, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;(9) doi: 10.1002/14651858.CD008659.pub3. :CD008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore PA, Hersh EV. Combining ibuprofen and acetaminophen for acute pain management after third-molar extractions: translating clinical research to dental practice. J Am Dent Assoc. 2013;144:898–908. doi: 10.14219/jada.archive.2013.0207. [DOI] [PubMed] [Google Scholar]
- 6.Mickel AK, Wright AP, Chogle S, Jones JJ, Kantorovich I, Curd F. An analysis of current analgesic preferences for endodontic pain management. J Endod. 2006;32:1146–1154. doi: 10.1016/j.joen.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Hargreaves KM, Berman LH. Cohen's Pathways of the Pulp 11th ed. St. Louis, MO: Mosby; 2015. 117. [Google Scholar]
- 8.Menhinick KA, Gutmann JL, Regan JD, Taylor SE, Buschang PH. The efficacy of pain control following nonsurgical root canal treatment using ibuprofen or a combination of ibuprofen and acetaminophen in a randomized, double-blind, placebo-controlled study. Int Endod J. 2004;37:531–541. doi: 10.1111/j.1365-2591.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 9.Law AS, Nixdorf DR, Aguirre AM et al. Predicting severe pain after root canal therapy in the National Dental PBRN. J Dent Res. 2015;94(suppl 3):37S–43S. doi: 10.1177/0022034514555144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotler M, Bar-Gil B, Ashkenazi M. Postoperative pain after root canal treatment: a prospective cohort study. Int J Dent. 2012;2012:310467. doi: 10.1155/2012/310467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith EA, Marshall JG, Selph SS, Barker DR, Sedgley CM. Nonsteroidal anti-inflammatory drugs for managing postoperative endodontic pain in patients who present with preoperative pain: a systematic review and meta-analysis. J Endod. 2017;43:7–15. doi: 10.1016/j.joen.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Corah NL. Development of a dental anxiety scale. J Dent Res. 1969;48:596. doi: 10.1177/00220345690480041801. [DOI] [PubMed] [Google Scholar]
- 13.Heft M, Parker SR. An experimental basis for revising the graphic rating scale for pain. Pain. 1984;19:153–161. doi: 10.1016/0304-3959(84)90835-2. [DOI] [PubMed] [Google Scholar]
- 14.Simpson M, Drum M, Nusstein J, Reader A, Beck M. Effect of preoperative ibuprofen/acetaminophen on the success of the inferior alveolar nerve block in patients with symptomatic irreversible pulpitis. J Endod. 2011;37:593–597. doi: 10.1016/j.joen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Stanley W, Drum M, Nusstein J, Reader A, Beck M. Effect of nitrous oxide on the efficacy of the inferior alveolar nerve block in patients with symptomatic irreversible pulpitis. J Endod. 2012;38:565–569. doi: 10.1016/j.joen.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Fullmer S, Drum M, Reader A, Nusstein J, Beck M. Effect of preoperative acetaminophen/hydrocodone on the efficacy of the inferior alveolar nerve block in patients with symptomatic irreversible pulpitis: a prospective, randomized, double-blind, placebo-controlled study. J Endod. 2014;40:1–5. doi: 10.1016/j.joen.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Click V, Drum M, Reader A, Nusstein J, Beck M. Evaluation of the Gow-Gates and Vazirani-Akinosi techniques in patients with symptomatic irreversible pulpitis: a prospective randomized study. J Endod. 2015;41:16–21. doi: 10.1016/j.joen.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Schellenberg J, Drum M, Reader A, Nusstein J, Fowler S, Beck M. Effect of buffered 4% lidocaine on the success of the inferior alveolar nerve block in patients with symptomatic irreversible pulpitis; A prospective, randomized, double-blind study. J Endod. 2015;41:791–796. doi: 10.1016/j.joen.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen NB, Hayden J., Jr . Local and General Anesthesia in Dentistry 2nd ed. Philadelphia, PA: Lea & Febiger; 1967. [Google Scholar]
- 20.Robertson D, Nusstein J, Reader A, Beck M, McCartney M. The anesthetic efficacy of articaine in buccal infiltration of mandibular posterior teeth. J Am Dent Assoc. 2007;138:1104–1112. doi: 10.14219/jada.archive.2007.0324. [DOI] [PubMed] [Google Scholar]
- 21.Nusstein J, Reader A, Nist R, Beck M, Meyers WJ. Anesthetic efficacy of the supplemental intraosseous injection of 2% lidocaine with 1:100,000 epinephrine in irreversible pulpitis. J Endod. 1998;24:487–491. doi: 10.1016/S0099-2399(98)80053-8. [DOI] [PubMed] [Google Scholar]
- 22.Fowler S, Drum M, Reader A, Beck M. Anesthetic success of an inferior alveolar nerve block and supplemental articaine buccal infiltration for molars and premolars in patients with symptomatic irreversible pulpitis. J Endod. 2016;42:390–392. doi: 10.1016/j.joen.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal V, Singla M, Miglani S, Ansari I, Kohli S. A prospective, randomized, single-blind comparative evaluation of anesthetic efficacy of posterior superior alveolar nerve blocks, buccal infiltrations, and buccal plus palatal infiltrations in patients with irreversible pulpitis. J Endod. 2011;37:1491–1494. doi: 10.1016/j.joen.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Kanaa MD, Whitworth JM, Meechan JG. A comparison of the efficacy of 4% articaine with 1:100,000 epinephrine and 2% lidocaine with 1:80,000 epinephrine in achieving pulpal anesthesia in maxillary teeth with irreversible pulpitis. J Endod. 2012;38:279–282. doi: 10.1016/j.joen.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Atasoy Ulusoy ÖĪ, Alaçam T. Efficacy of single buccal infiltrations for maxillary first molars in patients with irreversible pulpitis: a randomized controlled clinical trial. Int Endod J. 2014;47:222–227. doi: 10.1111/iej.12129. [DOI] [PubMed] [Google Scholar]
- 26.Moradi Askari E, Parirokh M, Nakhaee N, Hosseini HR, Abbott PV. The effect of maxillary first molar root length on the success rate of buccal infiltration anesthesia. J Endod. 2016;42:1462–1466. doi: 10.1016/j.joen.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Hosseini HR, Parirokh M, Nakhaee N, V Abbott P, Samani S. Efficacy of articaine and lidocaine for buccal infiltration of first maxillary molars with symptomatic irreversible pulpitis: a randomized double-blinded clinical trial. Iran Endod J. 2016;11:79–84. doi: 10.7508/iej.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells LK, Drum M, Nusstein J, Reader A, Beck M. Efficacy of ibuprofen and ibuprofen/acetaminophen on postoperative pain in symptomatic patients with a pulpal diagnosis of necrosis. J Endod. 2011;37:1608–1612. doi: 10.1016/j.joen.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Sebastian R, Drum M, Reader A, Nusstein J, Fowler S, Beck M. What is the effect of no endodontic debridement on postoperative pain for symptomatic teeth with pulpal necrosis? J Endod. 2016;42:378–382. doi: 10.1016/j.joen.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Khan AA, Owatz CB, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. Measurement of mechanical allodynia and local anesthetic efficacy in patients with irreversible pulpitis and acute periradicular periodontitis. J Endod. 2007;33:796–799. doi: 10.1016/j.joen.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Gallatin J, Nusstein J, Reader A, Beck M, Weaver J. A comparison of injection pain and postoperative pain of two intraosseous anesthetic techniques. Anesth Prog. 2003;50:111–120. [PMC free article] [PubMed] [Google Scholar]
- 32.Aminoshariae A, Khan A. Acetaminophen: old drug new issues. J Endod. 2015;41:588–593. doi: 10.1016/j.joen.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Torabinejad M, Cymerman JJ, Frankson M, Lemon RR, Maggio JD, Schilder H. Effectiveness of various medications on postoperative pain following complete instrumentation. J Endod. 1994;20:345–354. doi: 10.1016/S0099-2399(06)80098-1. [DOI] [PubMed] [Google Scholar]
- 34.Menke E, Jackson C, Bagby M, Tracy T. The effectiveness of prophylactic etodolac on postendodontic pain. J Endod. 2000;26:712–715. doi: 10.1097/00004770-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Gopikrishna V, Parameswaran A. Effectiveness of prophylactic use of rofecoxib in comparison with ibuprofen on postendodontic pain. J Endod. 2003;29:62–64. doi: 10.1097/00004770-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Nekoofar MH, Sadeghipanah M, Dehpour AR. Evaluation of meloxicam (A cox-2 inhibitor) for management of postoperative endodontic pain: a double-blind placebo-controlled study. J Endod. 2003;29:634–637. doi: 10.1097/00004770-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Arslan H, Topcuoglu HS, Aladag H. Effectiveness of tenoxicam and ibuprofen for pain prevention following endodontic therapy in comparison to placebo: a randomized double-blind clinical trial. J Oral Science. 2011;53:157–161. doi: 10.2334/josnusd.53.157. [DOI] [PubMed] [Google Scholar]
- 38.Mehrvarzfar P, Abbott PV, Saghir MA, et al. Effects of three oral analgesics on postoperative pain following root canal preparation: a controlled clinical trial. Int Endod J. 2012;45:76–82. doi: 10.1111/j.1365-2591.2011.01950.x. [DOI] [PubMed] [Google Scholar]
- 39.Elzaki WM, Abubakr NH, Ziada HM, Ibrahim YE. Double-blind randomized placebo-controlled clinical trial of efficiency of nonsteroidal anti-inflammatory drugs in the control of post-endodontic pain. J Endod. 2016;42:835–842. doi: 10.1016/j.joen.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Mokhtari F, Yazdi K, Mahabadi AM, Modaresi SJ, Hamzeheil Z. Effect of premedication with indomethacin and ibuprofen on postoperative endodontic pain: a clinical trial. Iran Endod J. 2016;11:57–62. doi: 10.7508/iej.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore PA, Ziegler KM, Lipman RD, Aminoshariae A, Carrasco-Labra A, Mariotti A. Benefits and harms associated with analgesic medications used in the management of acute dental pain. J Am Dent Assoc. 2018;149:256–265. doi: 10.1016/j.adaj.2018.02.012. [DOI] [PubMed] [Google Scholar]