Abstract
Evidence suggests that estrogen signaling is involved in sex differences in the prevalence rates and control of asthma, but the expression patterns of estrogen receptor variants and estrogen function in the lung are not well established. We investigated the expression of major estrogen receptor variants occurring naturally and after the development of allergen-induced airway hyperreactivity in a murine model of allergic asthma, along with the role of estrogen signaling in small-airway ciliary motion and smooth muscle contraction. Female BALB/c mice were sensitized with ovalbumin, and estrogen receptor expression patterns were examined by immunofluorescence and Western blot analysis. Time-lapse video and photodiode-based displacement measurement systems were used to assess the effects of estrogen signaling on airway ciliary beat frequency and smooth muscle contraction. We found that a novel variant of estrogen receptor (ER)–α, ER-α36, is expressed in airway epithelial and smooth muscle cells. ER-α36 was predominately localized on the plasma membranes of airway cells. After sensitization to allergen, the expression levels of ER-α36 increased significantly (P < 0.01), whereas the expression of ER-β and ER-α66 did not significantly change. Estrogen treatment in vitro resulted in a rapid increase in airway cilia motion in a dose-dependent fashion, but did not exert any effect on airway smooth muscle contraction. We speculate that the up-regulation of estrogen receptor expression associated with allergen-induced airway hyperresponsiveness may constitute a protective mechanism to facilitate the clearance of mucus. The identification and localization of specific estrogen receptor subtypes in the lung could lead to newer therapeutic avenues aimed at addressing sex differences of asthma susceptibility.
Keywords: asthma, estrogen receptor, ovalbumin sensitization, airway hyperresponsiveness, ciliary beat frequency
Asthma is characterized by airway inflammation and airway hyperresponsiveness (AHR), which lead to airway obstruction, wheezing, and dyspnea. The prevalence and hospitalization rates for asthma are higher in prepubescent boys versus girls, but become greater among females of reproductive age (1, 2). The available epidemiologic and experimental evidence indicates that estrogens may contribute to sex differences in the rates of prevalence and severity of asthma. The effects of estrogen on airway function are not clearly defined. Study results have been contradictory, possibly reflecting different experimental conditions. For example, previous reports indicated that treatment with estrogen inhibits the cholinergic constriction of asthmatic tracheal rings in vitro (3), decreases acetylcholine (Ach)–induced airway responsiveness, and protects against both the AHR and inflammation associated with allergen-induced asthma in ovariectomized animals (4, 5). The estrogen antagonist ICI182780, however, was shown to enhance methacholine (MCh)–induced changes in airway function in allergen-challenged female mice (6). Estrogen was also reported to inhibit the proliferation of lung myofibroblasts through the nongenomic effects of estrogen (7). High concentrations of estrogen during pregnancy appear to exert anti-inflammatory effects, whereas low concentrations of estrogen are associated with inflammatory events (8). These later findings are consistent with the observation that postmenopausal women with asthma have lower concentrations of estrogen than do postmenopausal healthy female subjects, and hormone replacement therapy in these women with asthma leads to a diminution of asthma symptoms (9).
Estrogen signaling is mediated by its cognate receptor, the estrogen receptor (ER). Two major isoforms of ER, ER-α and ER-β, are known to occur. They share common protein structures, and each possesses three independent but interacting functional domains: the N-terminal domain (A/B domain), the central DNA-binding domain (C domain), and the C-terminal ligand-binding domain (D/E/F domain). The N-terminal domain has a ligand-independent activation function (AF-1) involved in interactions with co-activators and the transcriptional activation of target genes in the absence of ligands. The DNA-binding domain plays important roles in receptor dimerization and in binding to specific DNA sequences. The C-terminal mediates ligand-binding through its ligand-binding domain, and also induces gene transcription through a ligand-dependent activation function (AF-2) (10). Full-length ER-α is a 66-kD protein (ER-α66) that contains all three functional domains.
The available evidence demonstrates the existence of two major estrogen-signaling pathways, the genomic and nongenomic signaling pathways. Through the genomic pathway, ERs are well known to act via transcription factors to regulate gene expression and elicit their effects. However, a different estrogen-signaling pathway mediates the rapid effects of estrogens within seconds to minutes. This nongenomic estrogen signaling pathway is involved in rapid responses to both physiologic and nonphysiologic estrogens (11), which is particularly important in so-called estrogen “nontarget” tissues, such as the cardiovascular system (12).
Recently, a 36-kD novel variant of ER-α66, ER-α36, was identified and cloned. ER-α36 lacks both transcriptional activation domains (AF-1 and AF-2), but retains the DNA-binding (C) domain (13, 14). ER-α36 primarily localizes in the cytoplasm and on the plasma membrane, and responds to both estrogens and anti-estrogens by transducing membrane-initiated signaling cascades (14). ER-α36 is expressed in both ER-α66–positive and ER-α66–negative breast cancer cells (15, 16), indicating that ER-α36 is subjected to totally different transcriptional regulation from that of ER-α66. The transcription of ER-α36 is initiated from a promoter located in the first intron of the ER-α66 gene (17), suggesting that ER-α36 may be expressed in previously identified “non–estrogen-targeting” tissues.
Here, we examined the expression patterns of ER variants in murine airways, and investigated the association between the expression of ER and allergen-induced AHR. In addition, we assessed the influences of estrogen on ciliary beat frequency and smooth muscle contraction.
Materials and Methods
Animals
Female BALB/c mice, aged 5–8 weeks (Harlan laboratories, Inc., Indianapolis, IN), were maintained on an ovalbumin (OVA)–free diet. Male mice were used as controls for ER localization studies. The animals in this study were used according to a protocol approved by the Institutional Animal Care and Use Committee of Creighton University.
Precision-Cut Lung Slices
Mice were killed with an overdose of ketamine. Lung slices were cut as previously described by Bai and colleagues (18).
Indirect Immunofluorescence
Lung slices were fixed with 4% paraformaldehyde, and incubated overnight at 4°C with 5% goat serum (plus 0.25% Triton-100X) to block nonspecific antibody binding. Slices were then incubated with polyclonal primary antibodies against ER-α36 (1:50; Alpha Diagnostic International, San Antonio, TX), ER-α66 (1:100; NeoMarkers, now Thermo Fisher Scientific Anatomical Pathology, Fremont, CA), and ER-β (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. The negative control for ER-α36 staining was performed with 20 amino acids peptides from which the ER-α36 antibody was raised. Alexa Fluor 488–labeled secondary antibody and phalloidin (both 1:500; Invitrogen, Carlsbad, CA) were applied to slices for 4 hours at room temperature. Finally, the slices were mounted with antifade mounting medium plus 4′,6-diamidino-2-phenylindole (DAPI), and inspected using an LSM 510 META Confocal Microscope (Carl Zeiss Advanced Imaging Microscopy, Jena, Germany).
Ovalbumin Sensitization
BALB/c mice were injected intraperitoneally with 20 μg OVA (Sigma Chemical Co., St. Louis, MO) diluted in 2 mg Imject alum adjuvants (total volume, 0.1 ml; Thermo Fisher Scientific Anatomical Pathology) on Days 1 and 14. Sensitized mice received 1% aerosolized OVA, delivered via ultrasonic nebulizer, for 30 minutes on Days 28, 29, and 30. On Day 32, the mice were challenged with 5% aerosolized OVA for 30 minutes. Methacholine (MCh) challenges were performed with a Buxco System (Buxco Research Systems, Wilmington, NC).
Western Blot Analysis
Lung lobes or airways were excised 48 hours after challenge with 5% OVA. Protein was extracted from lung tissue or airway homogenates using 1 × complete lysis buffer (Santa Cruz Biotech), and analyzed by Western blot analysis. Student paired t tests were used to determine significant changes.
Measurement of Ciliary Beat Frequency
Lung slices from naive (nonsensitized) mice were used for measurements of ciliary beat frequency (CBF). Epithelial cells (ECs) with a CBF of 10–22 Hz were selected for recording by using a photodiode-based displacement measurement system (19, 20). Small areas of interest (slits) with a few cilia were selected toward the ciliary tip. 17β-estradiol (E2; Sigma-Aldrich, St. Louis, MO) was delivered to the chamber after the spontaneous CBF was measured. The power spectrum of the frequency domain was analyzed using Clampfit software (Molecular Devices, Sunnyvale, CA). Dose-dependent effects of E2 on CBF were calculated and represented by the average percentage change before versus after different concentrations of E2.
Measurement of Airway Contraction
Healthy lung slices from naive mice with active cilia on airway ECs and elongated normal smooth muscle cells (SMCs) were selected. Phase-contrast images were captured by a CoolSNAP HQ2 camera (Photometrics, Tucson, AZ) on a Nikon (Melville, NY) microscope, using Image-Pro Plus (Media Cybernetics, Inc., Bethesda, MD). Frames were captured in time lapse, and changes in cross-sectional area of the airway were measured with respect to time using Image analysis software (ImageJ; National Institutes of Health, Bethesda, MD).
Results
Immunofluorescence Staining of ER Variants in Murine Lungs
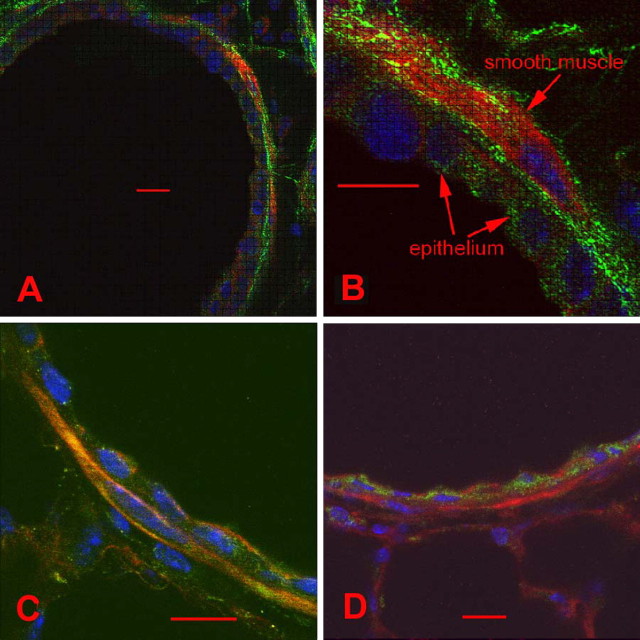
To assess the expression pattern and spatial distribution of ER-α36 in murine airways, we performed indirect immunofluorescence with antibodies specific for ER-α36. The ER-α36–specific antibody was custom-made against the 20 unique amino acids at the C-terminal of ER-α36. No staining was evident with the negative control for ER-α36 (i.e., the 20 amino acids peptides from which the ER-α36 antibody was raised). Because all three primary antibodies to the ERs were raised in rabbits, we used the same secondary antibody with rabbit IgG as the primary antibody, and did not observe any staining. Phalloidin and DAPI were used to stain actin and DNA, respectively. Smooth muscle cells (SMCs) were identified by their location and shape. SMCs were often separated from the airway lumen by just one layer of ECs. As shown in Figure 1, ER-α36 was mainly present on the plasma membrane of the airway luminal ECs and spindle-shaped SMCs (Figures 1A and 1B). However, other variants of the estrogen receptors, ER-α66 (Figure 1C) and ER-β (Figure 1D), were mainly located in the nuclear and cytoplasmic compartments of ECs and SMCs. No differences in ER-α36 staining patterns were evident between female and male mice (data not shown). ER-α36 was also expressed on the membrane and in the cytoplasm of cultured human airway SMCs (data not shown).
Figure 1.
Immunofluorescence staining of estrogen receptor (ER) variants in murine small airways. Murine small airways were stained separately with anti-ER-α36–specific, ER-α66–specific, and ER-β–specific antibodies (green). Cytoskeletons were labeled with phalloidin (red), and nuclei were labeled with DAPI (blue). (A and B) ER-α36 immunostaining (green) was evident mainly on the plasma membrane and partly in the cytoplasms of epithelial cells and smooth muscle cells. (C) ER-α66 (green) was stained in the cytoplasms and nuclei of epithelial cells and smooth muscle cells. (D) The expression of ER-β was detected in the cytoplasms and nuclei of small airway tissue. Scale bar = 10 μm for all images.
Expression of ER-α36 Is Elevated in Airways of OVA-Sensitized Mice
We next investigated whether antigen sensitization affects the protein expression of ERs. Western blot analysis was used to examine the expression of ERs in murine lungs and the association with sensitization to allergen.
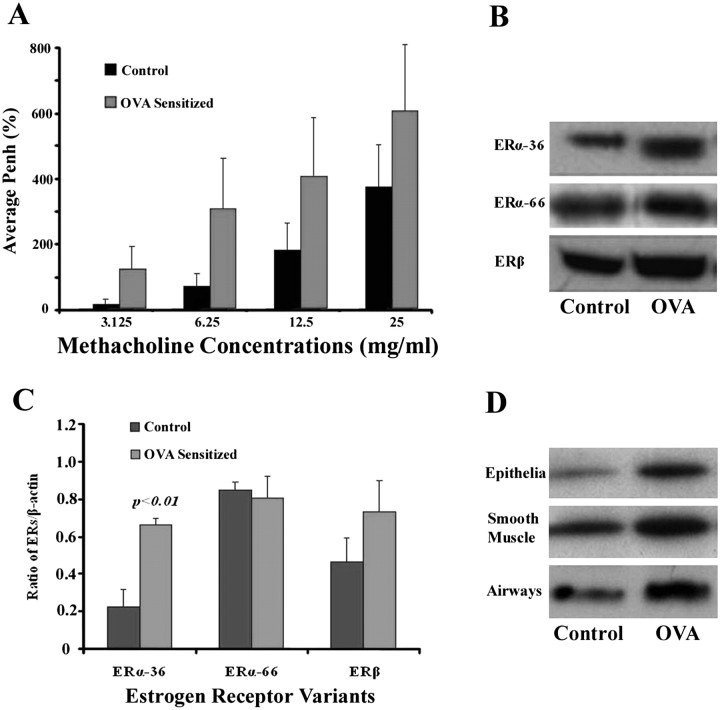
To verify the antigen sensitization protocol, AHR was measured and showed greater enhanced pause (PenH) values with gradient-dose MCh in sensitized versus control mice (Figure 2A). The presence of ER-α36, ER-α66, and ER-β was also demonstrated in the lung by Western blot analysis (Figure 2B). Furthermore, the expression of ER-α36 increased after sensitization to allergen (Figure 2B). To minimize individual variations, the expression of ER was quantified and normalized to the expression of β-actin by analyzing the ratio of ERs/β-actin band density in each sample with a densitometer (FluorChem FC2 System; Bucher Biotech AG, Santa Clara, CA). The average ratios of three mice in each group are shown in Figure 2C. The concentration of ER-α36 was significantly elevated after ovalbumin sensitization (P < 0.01). In contrast to ER-α36, the expression of neither ERβ nor ER-α66 was significantly different after sensitization to antigen. ECs and SMCs were dissected manually from the tracheae and bronchi. To acquire enough samples for Western blot analysis, tissues were collected and mixed from four mice in each group. An up-regulation of ER-α36 expression related to allergen exposure was also demonstrated in isolated ECs and SMCs from the upper airways (Figure 2D).
Figure 2.
Effect of ovalbumin (OVA) sensitization on airway hyperresponsiveness (AHR) and the expression of ERs. (A) Each bar represents the average values of enhanced pause (PenH) with different doses of methacholine. (B) Representative Western blot analysis of ER-α36, ER-α66, and ER-β in whole lung tissues of control and OVA-sensitized mice. (C) Each bar represents the average ratios of ERs/β-actin (three mice in each group) of control versus OVA-sensitized groups. Only the expression of ERα-36 increased significantly after ovalbumin sensitization (P < 0.01). (D) The elevation of ERα-36 associated with ovalbumin sensitization is confirmed in murine epithelia, smooth muscle, and whole airways. Each band represents the ERα-36 level, which was extracted from four mice in each group.
Effects of Estrogen on Airway CBF
Lung slices were harvested from unsensitized mice. Only the slices in which the airways were visibly lined with ciliated cells were collected and cultured by floating the slices in DMEM, supplemented with 10% FBS and 1% antibiotic–antimycotic solution in Petri dishes at 37°C in an incubator with 10% CO2. The target airway lined with beating cilia was placed under an upright microscope. Subsequently, a small area of interest (slit) was selected for the measurement of CBF (Figure 3A). The magnitude and beat frequency of ciliary motion were measured using a photodiode-based displacement measurement system that had previously been used to measure the stereociliary motion of cochlear hair cells (19, 20). This system can measure ciliary motion down to an amplitude of 20 nm and up to a frequency of 1,200 Hz. This technique greatly reduces the complicating effects of the metachronal activity of closely packed cilia on intensity waveforms. To simplify the frequency analysis, the frequency domain of ciliary motion is represented by a power spectrum (squared magnitude) analyzed according to fast Fourier transform analysis. Because a pair of CBFs had to be recorded from the same population of the cilia inside the tiny slit before and after the application of E2, each individual slice was used for only one pair of measurements. The effects of E2 concentrations at 10−14–10−6 M were tested on CBF, and the data from each concentration of E2 were collected from 8–10 specimens. The average CBF percent changes were calculated from the CBF after treatment with E2, compared with spontaneous CBF.
Figure 3.
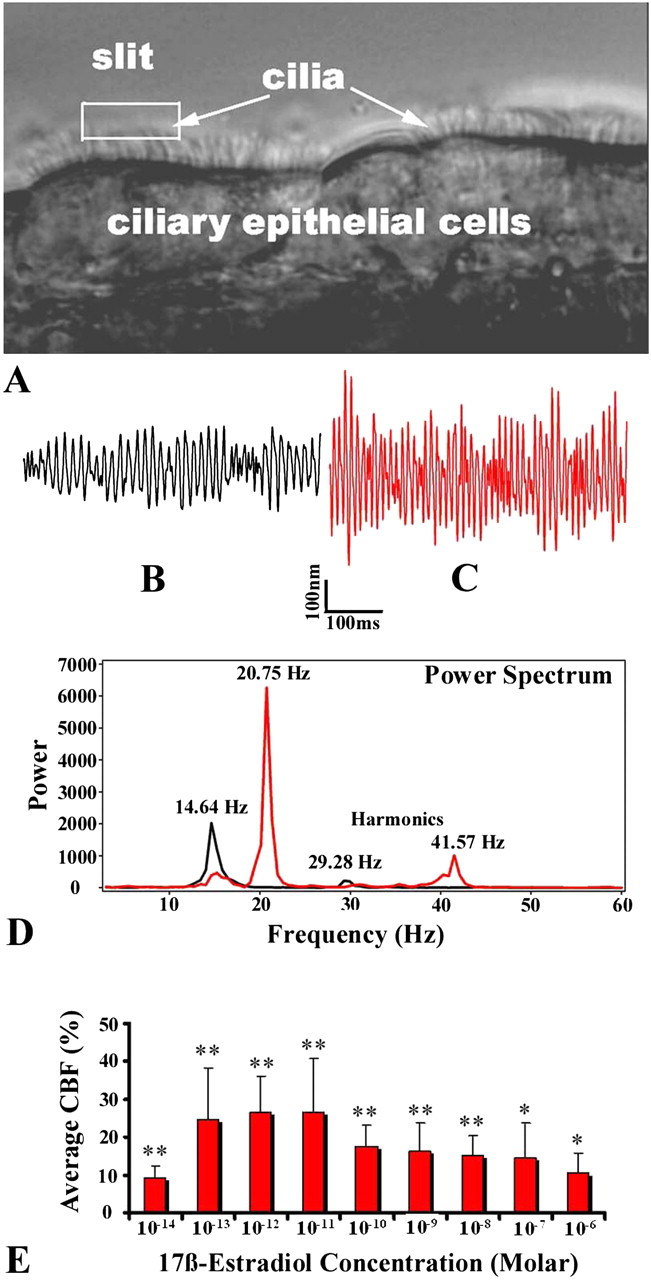
Effect of estrogen on small airway ciliary motion. (A) Magnitude and beat frequency of ciliary motion were measured with a photodiode-based displacement measurement system. The slit shows the target area with several beating cilia. (B and C) Representative responses of spontaneous ciliary motion recorded before (black) and 3 minutes after (red) treatment with 10−11 M 17β-estradiol (E2). (D) The power spectra (peaks) of the two upper traces were analyzed using fast Fourier transform analysis. Treatment with E2 increased the ciliary beat frequency (CBF) from 14.64 Hz to 20.75 Hz. (E) Bell-shaped, dose-dependent effects of E2 on small airway CBF. The range of resting CBF was 10–19 Hz. The average CBF percent changes represent the percent changes of each targeted spot before versus after treatment with E2, averaged for the same dose of E2. Differences before and after treatment with E2: *P < 0.05 and **P < 0.01.
The results showed that the addition of E2 significantly increased the magnitude of small airway ciliary beating (Figures 3B and 3C) and dominant frequency (Figure 3D). The elevation of CBF usually persisted for 5–10 minutes without washout. Treatment with E2 accelerated CBF to a statistically significant extent at all doses tested in a bell-shaped, dose-dependent fashion (Figure 3E). Under our current conditions, the concentrations of 10−13–10−11 M E2 were most effective for increasing the CBF of airway ECs.
Effects of Estrogen on Airway Smooth Muscle Contraction
To examine the acute effects of estrogen on airway smooth muscle contractility, E2 (10−4–10−12 M) was applied to lung slices. E2 did not significantly affect lung slice contractility at the doses tested. This lack of effect is illustrated in Figure 4, using a representative dose of 10−6 M of E2, and in the video (in the online supplement), comparing two doses of E2 versus two doses of Ach. To test whether E2 might affect the contractility noted with ACh (see the video in the online supplement), we added E2 before and after ACh-induced contraction. Treatment with E2 did not significantly inhibit (Figure 4, top trace) or reverse (Figure 4, bottom trace) ACh-induced airway contraction. To explore the further effects of E2 on ACh-induced contraction, we preincubated lung slices with E2 (10−9–10−6 M) for 4–12 hours before adding ACh. No significant inhibition of smooth muscle contraction was evident (data not shown).
Figure 4.
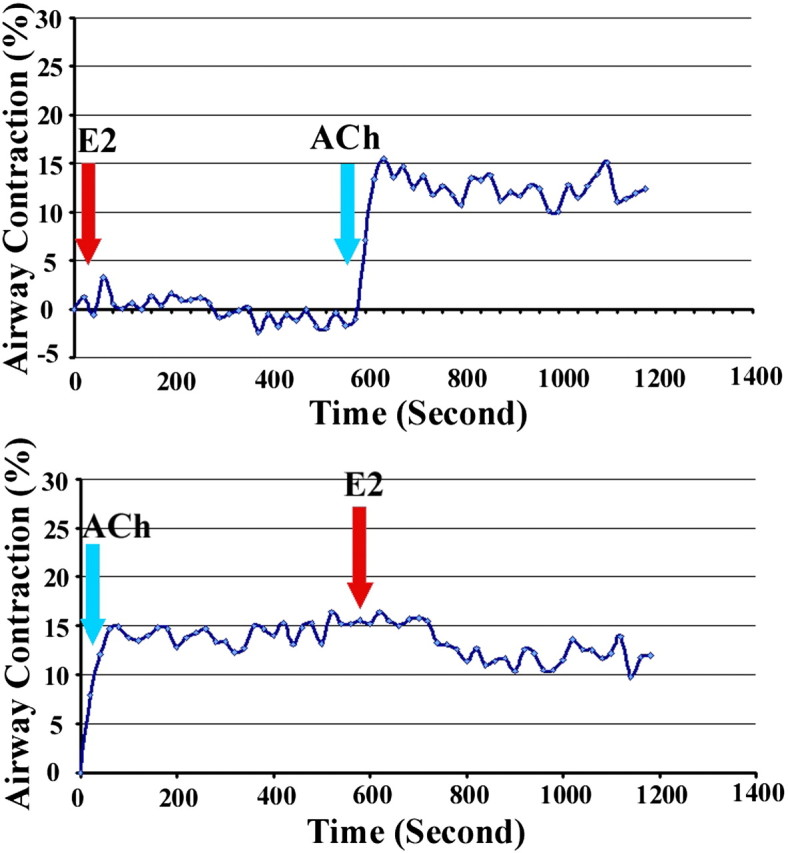
Acute effects of E2 on airway smooth muscle contraction. The data represent effects of E2 on acetylcholine (ACh)–induced smooth muscle contraction. Top trace: E2 (1 μM) did not promote or inhibit ACh (0.1 μM)–induced small airway contraction. Bottom trace: E2 did not reverse Ach-induced contraction.
Discussion
Our study contains several novel findings of importance in understanding the biologic effects of estrogens in airway disease. First, we demonstrated the expression and location of a new variant of ER-α, ER-α36, in both the ECs and SMCs of murine airways. Second, we showed that sensitization to allergen significantly increases the expression of ER-α36 in airways. Third, we demonstrated that estrogen increases airway ciliary motion in a dose-dependent fashion. Finally, we demonstrated that estrogen does not exert an acute effect on airway contraction in lung-slice preparations. Exactly how these data fit with previous reports is unclear, because previous data on the effects of estrogen in airway biology are disparate.
At least two major factors may account for the sex differences found in allergic asthma: the plasma concentration of estrogen and the reactivity of ERs. First, variations in plasma concentrations of estrogen are believed to affect asthmatic exacerbations and the severity of asthma. In general, postmenarcheal women manifest more severe asthma than men (21–23). Research indicates that allergic asthma in women is often triggered or intensified by natural body transitions and cycles such as puberty, pregnancy, and menopause, which are associated with varying concentrations of estrogen (24, 25). However, estrogen concentrations in mice are difficult to measure, and we therefore have no data on the possible association between blood estrogen and our findings.
Second, estrogens exert their effects by binding to two major ERs, ER-α and ER-β. Estrogen deficiency leads to a change in receptor expression. Estrogen signaling is mediated via the classic nuclear transcriptional activation pathways, as well as the nonclassic membrane-initiated signaling pathways. This study provides evidence that a novel membrane-based ER variant, ER-α36, is expressed on the plasma membrane and in the cytoplasm of airway ciliary ECs and SMCs. The expression of ER-α36 was up-regulated after sensitization to allergen. To our knowledge, this is the first study to evaluate the expression of ER-α36 systematically, as related to allergen sensitization in airways and lung. A high level of ER activation via endogenous estrogen was reported to be pro-asthmatic (21). Higher levels of ER-α36 expression were found in osteoblasts and osteoclasts from postmenopausal women, and E2 induces a significant decrease in the expression levels of ER-α36 in a concentration-dependent manner (26). If, as shown in some studies, estrogen exerts a protective effect against AHR, the increased expression of ER-α36 could constitute a compensating mechanism against allergen-induced AHR. Therefore, we propose that exposure to allergens may trigger a physiological reduction in estrogen level linked with airway inflammation, and the depletion of endogenous estrogen could affect the negative feedback mechanism in the hypothalamic–pituitary axis by increasing the allergen-induced expression of ER-α36. The consequences of high expression of ER-α36 could involve elevating estrogen efficiency to modulate pulmonary function. However, this notion is speculative, because we did not assess estrogen concentrations during these studies. A better understanding of the effects of estrogen and the role of ER-α36 expression in the sensitization and elicitation of allergic reactions may initiate new strategies for the therapy and prevention of asthma.
Another putatively important effect of estrogen on lung biology involves its influence on airway cilia motion. Mucociliary clearance is a critical host defense mechanism, and plays a crucial role in the removal of foreign materials within the airways. Under normal conditions, cilia beat at a low frequency. However, airway CBF increases in response to a variety of receptor-mediated stimuli, enabling the mucociliary system to transport relatively large particles at remarkable velocities (27–29). On the other hand, a consequence of high-frequency beating involves a loss of an appreciable amount of energy by the ciliated cell. Our experiments provide strong evidence that E2 significantly increased the airway CBF in a concentration-dependent manner. Concentrations of E2 from 10−13–10−11 M induced the maximum effect on CBF, and these overlap with concentrations found during menstrual cycles in women. The acceleration of CBF by E2 was detected in seconds to minutes. This rapid E2 response potentially occurs via membrane-initiated signaling pathways. High levels of ER-α36 expression in cytoplasmic and plasma membranes of airway ECs may participate in this rapid regulation of CBF. ER-α36 was reported to mediate membrane-initiated mitogenic estrogen signaling (13, 14). Because ER-α36 lacks both the AF-1 and AF-2 transcriptional activation domains, it usually functions as a dominant-negative inhibitor of ER-α66 and ER-β, to inhibit both the AF-1 and AF-2 functions of ER-α66 and ER-β. In a transgenic murine model, Pedram and colleagues (30) demonstrated that although rapid signaling occurs because of steroid hormone action at the membrane, the chronic regulation of signal transduction may involve nuclear ER-α and the transcription of genes coding for kinases, phosphatases, and modulating proteins. Unfortunately, no accepted ER-α36–specific agonists and antagonists are readily available. Thus, we did not characterize which ER subtype might mediate the changes in CBF. The exact underlying mechanisms involved in increasing CBF are not elucidated. Estrogen may modulate CBF in a relatively rapid, nongenomic manner by enhancing the production of nitric oxide and the activity of intracellular calcium in airway epithelia. Of foremost importance, the faster CBF induced by E2 in a physiological range may help mucociliary clearance. These novel data strongly support the idea of the rapid, nongenomic effects of estrogens involved in murine airway functions. Lastly, a number of mechanisms were proposed for the possible inhibitory effects of estrogen on AHR, including decreased ACh-induced airway reactivity because of increased epithelial acetylcholinesterase activity, and the relaxation of tracheal SMCs by the opening of large conductance Ca2+-dependent K+ channel (BKCa) through the activation of the nitric oxide–cyclic guanosine monophosphate (cGMP)–protein kinase G pathway (3, 4). Membrane-initiated estrogen signaling was linked to rapid responses to estrogen, and it generally activates signaling pathways such as mitogen-activated protein kinase/extracellular regulated kinase, phosphatidylinositol-3–kinase, and protein kinase C pathways (31). However, in the present study, we did not observe any acute changes in airway contraction via estrogen alone, or any inhibitory effect on ACh-induced contraction with lung-slice preparations in vitro. E2 was also unable to reverse preexisting ACh-induced contraction.
The chronic effects of E2 and tamoxifen at several doses and times were tested by intranasal delivery, followed by assessments of AHR in vivo. We could not show any consistent effects of E2 on methacholine-induced changes in PenH (data not shown). E2 could conceivably combine with all variants of the estrogen receptors, and the different expressions and functions of these receptors may lead to a lack of observable effects. The literature supports the concept that the effects of estrogen can vary significantly, depending on the dose and timing of administration (32). Disparate reported results may result from different model systems (mice versus human) and locations of airway preparations (trachea versus small airways).
The orphan G protein–coupled receptor 30 (GPR30) was believed to mediate nongenomic responses to estrogen. However, Kang and colleagues (33) found that the activities of GPR30 in response to estrogen occurred through its ability to induce the expression of ER-α36. The selective GPR30 agonist, G1, actually interacts with ER-α36. Thus, they suggested that ER-α36, and not GPR30, is involved in nongenomic estrogen signaling.
Finally, it is important to recognize that sex hormones include testosterone and progesterone. Each of them may independently affect pulmonary function and pathophysiological conditions in the lung to varying degrees. A natural hormone balance should be considered a key element in defining their role in allergic respiratory diseases.
In conclusion, estrogen and ERs are involved in mediating lung biologic functions. In the present study, we were unable to comment specifically on the potential biologic effects of the ER subtypes that we identified in airway ECs and SMCs. Nonetheless, the localization of ER-α36 to airway epithelia is consistent with the finding that estrogen is capable of rapidly increasing airway ciliary motion. We did not find any immediate effects of estrogen on airway smooth muscle contraction. This, however, does not rule out an effect that may occur after longer periods of exposure or under other conditions. Why allergen sensitization increases the expression of ER-α36 is unknown. If, as shown in some studies, estrogen exerts a protective effect against AHR, we speculate that the increased expression of ER may constitute a protective mechanism against the associated allergen-induced AHR. To elucidate fully the role of estrogen and ER subtypes in airway biology, further data are needed, especially in terms of receptor-specific agonists and antagonists in human or other relevant preclinical models.
Footnotes
This work was supported by Nebraska Tobacco Settlement Biomedical Research Development Program grants LB692 and LB595.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0268OC on June 3, 2011
References
- 1.Skobeloff EM, Spivey WH, St. Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA 1992;268:3437–3440. [PubMed] [Google Scholar]
- 2.Becklake MR, Kauffmann F. Sex difference in airway behaviour over the human life span. Thorax 1999;54:1119–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimitropoulou C, White RE, Ownby DR, Catravas JD. Estrogen reduces carbachol-induced constriction of asthmatic airways by stimulating large-conductance voltage and calcium dependent potassium channels. Am J Respir Cell Mol Biol 2005;32:239–247. [DOI] [PubMed] [Google Scholar]
- 4.Degano B, Prevost MC, Berger P, Molimard M, Pontier S, Rami J, Escamilla R. Estradiol decreases the acetylcholine-elicited airway reactivity in ovariectomized rats through an increase in epithelial acetylcholinesterase activity. Am J Respir Crit Care Med 2001;164:1849–1854. [DOI] [PubMed] [Google Scholar]
- 5.Dimitropoulou C, Drakopanagiotakis F, Chatterjee A, Snead C, Catravas JD. Estrogen replacement therapy prevents airway dysfunction in a murine model of allergen-induced asthma. Lung 2009;187:116–127. [DOI] [PubMed] [Google Scholar]
- 6.Matsubara S, Swasey CH, Loader JE, Dakhama A, Joetham A, Ohnishi H, Balhorn A, Miyahara N, Takeda K, Gelfand EW. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am J Respir Cell Mol Biol 2008;38:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flores-Delgado G, Bringas P, Buckley S, Anderson KD, Warburton D. Nongenomic estrogen action in human lung myofibroblasts. Biochem Biophys Res Commun 2001;283:661–667. [DOI] [PubMed] [Google Scholar]
- 8.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28:521–574. [DOI] [PubMed] [Google Scholar]
- 9.Kos-Kudla B, Ostrowska Z, Marek B, Ciesielska-Kopacz N, Sieminska L, Kajdaniuk D, Nowak M, Kudla M. Hormone replacement therapy in postmenopausal asthmatic women. J Clin Pharm Ther 2000;25:461–466. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev 2001;81:1535–1565. [DOI] [PubMed] [Google Scholar]
- 11.Bulayeva NN, Wozniak AL, Lash LL, Watson CS. Mechanisms of membrane estrogen receptor–alpha–mediated rapid stimulation of Ca2+ levels and prolactin release in a pituitary cell line. Am J Physiol Endocrinol Metab 2005;288:E388–E397. [DOI] [PubMed] [Google Scholar]
- 12.Simoncini T, Mannella P, Genazzani AR. Rapid estrogen actions in the cardiovascular system. Ann N Y Acad Sci 2006;1089:424–430. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZY, Zhang XT, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor–alpha36, a novel variant of human estrogen receptor–alpha66. Biochem Biophys Res Commun 2005;336:1023–1027. [DOI] [PubMed] [Google Scholar]
- 14.Wang ZY, Zhang XT, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor–alpha, hER-alpha36: transduction of estrogen- and antiestrogen-dependent membrane–initiated mitogenic signaling. Proc Natl Acad Sci USA 2006;103:9063–9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee L, Cao J, Deng H, Chen P, Gatalica Z, Wang ZY. ER-α36, a novel variant of ER-α, is expressed in ER-positive and -negative human breast carcinomas. Anticancer Res 2008;28:479–483. [PMC free article] [PubMed] [Google Scholar]
- 16.Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J, Wang ZY, Wang T, Fan Z, Fan T, Lin B, et al. Expression of ER-{alpha}36, a novel variant of estrogen receptor {alpha}, and resistance to tamoxifen treatment in breast cancer. J Clin Oncol 2009;27:3423–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou Y, Ding L, Coleman M, Wang Z. Estrogen receptor–alpha (ER-alpha) suppresses expression of its variant ER-alpha 36. FEBS Lett 2009;583:1368–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai Y, Zhang M, Sanderson MJ. Contractility and Ca2+ signaling of smooth muscle cells in different generations of mouse airways. Am J Respir Cell Mol Biol 2007;36:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He DZ, Jia S, Dallos P. Mechanoelectrical transduction of adult outer hair cells studied in a gerbil hemicochlea. Nature 2004;429:766–770. [DOI] [PubMed] [Google Scholar]
- 20.Jia S, He DZ. Motility-associated hair bundle motion of outer hair cells. Nat Neurosci 2005;8:1028–1034. [DOI] [PubMed] [Google Scholar]
- 21.Melgert BN, Ray A, Hylkema MN, Timens W, Postma DS. Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep 2007;7:143–150. [DOI] [PubMed] [Google Scholar]
- 22.Roorda RJ, Gerritsen J, van Aalderen WM, Schouten JP, Veltman JC, Weiss ST, Knol K. Follow-up of asthma from childhood to adulthood: influence of potential childhood risk factors on the outcome of pulmonary function and bronchial responsiveness in adulthood. J Allergy Clin Immunol 1994;93:575–584. [DOI] [PubMed] [Google Scholar]
- 23.Fagan JK, Scheff PA, Hryhorczuk D, Ramakrishnan V, Ross M, Persky V. Prevalence of asthma and other allergic diseases in an adolescent population: association with sex and race. Ann Allergy Asthma Immunol 2001;86:177–184. [DOI] [PubMed] [Google Scholar]
- 24.Tan KS. Premenstrual asthma: epidemiology, pathogenesis and treatment. Drugs 2001;61:2079–2086. [DOI] [PubMed] [Google Scholar]
- 25.Vrieze A, Postma DS, Kerstjens HA. Perimenstrual asthma: a syndrome without known cause or cure. J Allergy Clin Immunol 2003;112:271–282. [DOI] [PubMed] [Google Scholar]
- 26.Xie H, Sun M, Liao XB, Yuan LQ, Sheng ZF, Wang D, Yu ZY, Zhang LY, Zhou HD, Luo XH, et al. Estrogen receptor α36 mediates a bone-sparing effect of 17β-estrodiol in postmenopausal women. J Bone Miner Res 2011;26:156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gheber L, Priel Z. Synchronization between beating cilia. Biophys J 1989;55:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gheber L, Priel Z. Metachronal activity of cultured mucociliary epithelium under normal and stimulated conditions. Cell Motil Cytoskeleton 1994;28:333–345. [DOI] [PubMed] [Google Scholar]
- 29.Zagoory O, Braiman A, Priel Z. The mechanism of ciliary stimulation by acetylcholine roles of calcium, PKA, and PKG. J Gen Physiol 2002;119:4329–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedram A, Razandi M, Kim JK, O'Mahony F, Lee EY, Luderer U, Levin ER. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem 2009;284:3488–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warner M, Gustafsson JA. Nongenomic effects of estrogen: why all the uncertainty? Steroids 2006;71:91–95. [DOI] [PubMed] [Google Scholar]
- 32.Townsend EA, Thompson MA, Pabelick CM, Prakash YS. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2010;298:L521–L530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang LG, Zhang XT, Xie Y, Tu YP, Wang D, Liu ZM, Wang ZY. Involvement of estrogen receptor variant ER-α36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol 2010;24:709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]