Abstract
Contagious agalactia (CA) is a disease caused equally by four Mycoplasma species, in single or mixed infections. Clinical signs are multiple, including mastitis, arthritis, keratoconjunctivitis, pneumonia, and septicemia, non-specific, and expressed differently depending whether sheep or goats are affected, on causative mycoplasmas as well as type of husbandry. CA has been reported worldwide and its geographic distribution maps to that of small ruminant breeding areas. However, as current diagnostic tests are expensive and difficult to implement, it is certainly underdiagnosed and prevalence data are only available for a few countries. CA control relies on vaccines, chemotherapy and good herd management practices. It requires long-term commitment but is often unsuccessful, with frequent clinical relapses. The persistence of the etiological agents, despite their overall susceptibility to antimicrobials, comes from their genetic plasticity and capacity to escape the host immune response. The existence of asymptomatic carriers and the numerous sources of infections contribute to rapid spread of the disease and complicate the control and prevention efforts. Here we review all these aspects in order to highlight recent progress made and identify gaps in knowledge or tools needed for better disease management. Discussion also underlines the detrimental effect of contagious agalactia on small ruminant welfare.
Keywords: contagious agalactia, mycoplasma, disease prevention and control, diagnosis, pathogenicity and infection course, epidemiology
Introduction
Known for more than 200 years, contagious agalactia (CA) of sheep and goats was first reported back in 1816 in Italy, where it was quickly dubbed “mal di sito” (“disease of the place”) in reference to its ability to persist in an environment and contaminate newly introduced herds.1 Its contagious nature resulted in the official designation of the name CA in 1871 by Brusasco.2 Despite being termed “agalactia”, which refers to a marked drop or even a complete loss of milk production, its clinical outcome is not restricted to lactating females nor to only the udder/mammary glands. CA has multiple clinical signs that are often gathered under the acronym MAKePS, for mastitis, arthritis, keratoconjunctivitis, pneumonia, and septicemia.3 It primarily results in a drop in milk production, followed by an increased general morbidity and mortality. It should be considered a serious threat to animal welfare in its acute phase as well as in its chronic form.
Mycoplasma (M.) agalactiae (Ma), the “historical” etiological agent, was successfully isolated for the first time in 1923 and characterized as “filterable but not invisible”, similarly to the agent of bovine pleuropneumonia.4 At that time, although the Mycoplasma genus was not yet established, this type of bacteria was already known to be parasitic and to cause chronic and generally difficult-to-eradicate diseases. Ma is still the main etiological agent in sheep, while three other (sub)species, namely M. mycoides subsp. capri (Mmc), M. capricolum subsp. capricolum (Mcc), and M. putrefaciens (Mp), are considered equally causative agents in goats, as their infection results in a similar clinical picture (Conclusion of the EC COST action 826).5 These three (sub)species are phylogenetically distant from Ma and belong or are close to a phylogenetically homogeneous group named the M. mycoides cluster.6–8 They share many genetic and antigenic traits making it more complicated to specifically segregate and identify them.7–10 Furthermore, mixed mycoplasmal infections in goats are regularly reported,11–13 which could complicate diagnosis and control measures. As clinical signs are non-specific, a CA outbreak cannot be confirmed without laboratory testing. These difficulties have prompted a huge field of research and development for diagnosis and control tools over the past years.14–17 However, the situation today is still not fully satisfactory and CA remains a significant burden in countries where small ruminant dairy production is important. Causative agents of CA are considered non-zoonotic, despite a report in 2014 of one case of human Mcc infection with recurrent fever, septicemia, and suspected meningitis in the absence of promoting factors like immunocompromised response or prolonged contact with animals.18 Isolates have sometimes been reported in unusual animal hosts, such as cattle and wild fauna.19–30
There are still not enough data to accurately estimate the economic consequences of CA in sheep and goats, and the only figure – a mean annual loss of US$30 million in European countries around the Mediterranean rim – dates from 17 years ago.31 A more recent study stated that the cost of one outbreak in mixed sheep/goat farms can range from 7 to 130 k€ depending on herd size and onset of disease in relation to the lactation period.32 CA is already geographically widespread and is poised to become even more important as small ruminant milk production increases worldwide (Table 1).
Table 1.
Production Of Milk From Sheep And Goats Worldwide And Its Evolution Between 2005 And 2017 For The First 15 Producers In 2017.
| Milk Production In Tonnes | ||||||
|---|---|---|---|---|---|---|
| Goats | Sheep | |||||
| Year | 2017 | 2005 | 2017 | 2005 | ||
| Total worldwide | 18,894,731 (+28%) | 14,791,746 | 11,567,441 (+13%) | 10,267,917 | ||
| Fifteen biggest producers in 2017 | India | 6,165,500 | 3,790,000 | Turkey | 1,344,779 | 789,877 |
| Bangladesh | 1,113,849 | 864,389 | China | 1,166,803 | 1,114,930 | |
| Sudan | 1,109,112 | 1,519,000 | China, mainland | 1,166,803 | 1,114,930 | |
| Pakistan | 842,036 | 675,000 | Greece | 732,095 | 752,171 | |
| France | 590,000 | 553,691 | Syrian Arab Republic | 644,561 | 765,851 | |
| Greece | 562,491 | 511,373 | Romania | 527,503 | 544,400 | |
| Turkey | 523,395 | 253,759 | Spain | 514,198 | 407,800 | |
| Spain | 491,374 | 471,900 | Iran (Islamic Republic of) | 449,718 | 307,943 | |
| South Sudan | 468,000 | nk | Italy | 410,380 | 532,049 | |
| Mali | 400,487 | 400,000 | Sudan | 403,394 | 487,000 | |
| Somalia | 373,828 | 412,600 | Somalia | 380,000 | 475,000 | |
| Niger | 362,129 | 236,004 | Mali | 364,330 | 179,470 | |
| Indonesia | 361,793 | 284,459 | Algeria | 284,684 | 203,000 | |
| Iran (Islamic Republic of) | 311,625 | 396,242 | France | 270,000 | 255,355 | |
| Russian Federation | 256,483 | 253,692 | Afghanistan | 206,675 | 167,059 | |
Notes: Countries that are among the 15 biggest producers in 2017 for both sheep and goat milk are in bold characters. Data extracted from Food and Agriculture Organization of the United Nations (FAO). http://www.fao.org/faostat/en/#data/QL.33
The prevalence of CA varies hugely between regions across the world, as does the relative importance of each etiological agent. It is important to distinguish sporadic cases, i.e., single outbreaks rapidly and successfully controlled, from endemic areas where effective control measures quickly become very expensive due to too many animals affected or herds mixed during the grazing season in upland farm systems, for instance. Once introduced, CA is always difficult to get rid of. For instance, in the Canary Islands (Spain), CA was first reported in the early 1990s and is still one of the major small ruminant health problems there today.34 There are only limited data on the relative importance of CA as a health concern in small ruminants as general surveys about mastitis rarely include mycoplasmosis.35–37 With respect to clinical or subclinical mastitis, in countries where CA is suspected as endemic, Ma is less prevalent than Staphylococcus or Streptococcus spp. but it remains the second or third etiology to consider, with a more dramatic and longer-term impact on production than other pathogens.38–42
This review aims to update current knowledge of CA and its etiological agents taking into account the most recent developments in research on diagnosis, epidemiology, and control.
Clinical Aspects
Etiology
The Mycoplasma species involved in CA syndrome depend on the host animal. Ma causes disease in both sheep and goats whereas the three other agents are primarily goat pathogens, although they are sporadically evidenced in sheep, especially in mixed breeding environments.43–46 Mixed infections involving several Mycoplasma species have been reported,12–14,47 and the general balance between Ma and other members of the M. mycoides cluster infection is highly region-dependent.
Clinical Forms
Since the early works, studies monitoring CA clinical features in endemic areas have been scarce.48 Prevalence studies are often conducted without taking into account whether there is or not some clinical aspects associated with isolation of mycoplasmas, see for instance,46,49–52 while case reports are infrequent and often limited to narrow areas.43,53–55 The main trend is that the classical overall picture of clinical CA has changed little, outside a few severe respiratory cases of Mmc infection in goats, initially suspected as contagious caprine pleuropneumonia.56–59
In acute CA episodes, mastitis, arthritis, pneumonia, and keratoconjunctivitis are the most frequently reported clinical signs with variations at individual and herd level in terms of presence, association and intensity, depending on whether sheep or goats are affected and on herd size, structure, and husbandry practices.48,60 The relative frequency and combination of disorders at different stages of infection are seldom reported but seem to vary strongly,61,62 with some outbreaks featuring only one type of sign.63,64
Clinical outcome is influenced by but not strictly correlated with the etiological agent involved.65 Overall mortality can be high (up to 100%) in young animals48,66 but less so in adults, from nil (generally with Ma) to 40–50%.16,48 Acute and severe forms are considered more frequent with species of the M. mycoides group than with Ma.5,48 Dramatic cases are more frequent in goats whatever the mycoplasma species involved.3,5,43,56,58,59
CA primarily affects lactating females and young animals.15 Lactating females with CA mainly develop mastitis at an early stage, but the intensity is variable whatever the Mycoplasma species involved (Ma, Mmc, Mcc, or Mp).1,3,48,67 General signs, such as weakness, pyrexia, or anorexia, that precede focal localizations often go unnoticed in adults whereas they can rapidly lead to mortality in young animals.48 Arthritis occurs frequently in young animals as a polyarthritis form causing recumbence, especially with Mcc1,48 and Mmc,54,55,64,68 but only occasionally in adults, where it causes lameness and swollen joints affecting mainly the tarsus and carpus.69 Mp was long thought to cause only mammary disorders until arthritis was also evidenced with Mp isolates.63,69 Conjunctivitis can occur with Ma and less frequently with Mmc and Mcc1,48 but is not often reported as it does not affect herd performance. Pneumonia is less frequent, at least in adults,48 but severe pneumonic forms with Ma, Mmc, and Mcc have been described, especially in young goats.13,55,69,71 There are sporadic reports of genital,12,72 like vaginitis, salpingitis, metritis, testicular degeneration, and neurological disorders.73–75
Clinical signs are frequent during the post-partum period or onset of lactation61,76 or against a background of any immune-depressed conditions such as farm transfer,15 breakdown in sanitary conditions, or concurrent infection.11
Disease Course
Infection generally onsets with a rapid spread of acute disorders over weeks or months11,61 that either recede with time, more or less rapidly depending on the measures implemented, or become recurrent.48,77,78 Clinical cases may also appear more gradually or remain sporadic.15 Course towards chronicity, with attenuated or sporadic disorders, is considered a natural evolution of the disease.15,48 It has been reproduced in experimental Ma infections where an increase in immune response fails to eliminate the mycoplasmas due to their capacity for immune-evasion.79,80 There are also extensive reports of asymptomatic mycoplasmal circulation in goat herds, evidenced mainly in bulk tank milk or ear canal samples, that can persist for years without manifesting any clinical sign, see for instance, Refs. 47,81–85 It can result from past history of the disease in the herd and lead to new clinical CA outbreaks, as carriage strains are as pathogenic as those isolated from acute CA cases.82,86
Transmission
The long persistence of mycoplasmas in clinically CA-infected organs (udders, eyes, respiratory tract) and other biological niches (ear canals) makes the main routes for direct within-herd transmission oral (e.g., colostrum feeding), respiratory (e.g., nasal discharge), and mammary (e.g., kid feeding).53,54 The environment, without being a sustainable reservoir, may also play an indirect role in transmission through bedding, the milking parlor, shared feeders, and troughs.48 Milking practices and devices are thought to play a highly significant role in indirect transmission.11,70,80 Disease transmission between farms frequently occurs with the introduction of asymptomatic carriers.11,44,54 As evidenced in other mycoplasmas infecting cattle,87 reproduction should also be regarded as a route of introduction or transmission, as semen shedding has been largely demonstrated in goats88–94 and appears possible in sheep.95,96
Diagnosis
Clinical Suspicion And Differential Diagnosis
Despite its economic impact worldwide, CA is still under-investigated due to under-awareness by animal health stakeholders, varied clinical impacts, and a lack of financial, technical, and training means. In typical acute forms, different signs of CA syndrome observed within a herd, i.e., mastitis, arthritis, pneumonia, and keratoconjunctivitis, may prompt a mycoplasma diagnosis.1,5 If the disease course remains staggered or if mild signs are observed, then a differential diagnosis is necessary. Several other non-CA agents need to be ruled out, such as (i) Staphylococcus and Streptococcus spp. in mastitis,97 (ii) caprine arthritis encephalitis virus in goat joint lesions,98 (iii) Pasteurellaceae, parainfluenza, Visna-Maëdi, and “peste des petits ruminants” viruses for respiratory signs,99 and (iv) other Mycoplasma spp. like M. conjunctivae in conjunctivitis alone, M. capricolum subsp. capripneumoniae the causative agent of contagious caprine pleuropneumonia3 and M. ovipneumoniae in respiratory diseases in both sheep and goats.57,100,101
It is often a first-line failure to evidence other pathogens that prompts a mycoplasma investigation. The high variability and low specificity of clinical signs mean clinical suspicion and specific diagnosis may be delayed or even never performed. The problem is compounded by the fact that physiological monitoring as a herd health management tool does not apply for CA. Indeed, in active outbreaks, somatic cell counts (SCC) may increase in correlation with mammary signs80,102,103 but remain in normal ranges in chronically infected herds.47,85,104,105 Other milk quality traits are affected similarly by mastitis, whatever the etiological agent.106
Consequently, confirmatory CA diagnosis still relies on conventional laboratory analyses, some of which require specific methods and expertise to rule out other commensal or opportunistic mycoplasmas commonly found in biological samples (e.g., M. arginini).
Relevant Samples For Direct Diagnosis
Milk (individual/pooled/tank), joint fluids, and eye swabs are relevant samples in diseased herds, and it is recommended practice to sample several animals in the same herd, due to inter-individual variability in shedding.1,107 Excretion in milk is higher in the clinical phase and might become intermittent with time,49,83,85,108 necessitating repeated samplings. Eye swabs and joint fluids tend to contain less mycoplasma (especially if lesions are not recent) and do not make the best samples in extensive surveys as they require animal containment or post-mortem sampling.43,51,84 Lungs, lymph nodes, and mammary glands can also be sampled post-mortem.1 Ear canal swabs are not recommended for CA diagnosis as ear canals are a frequent habitat for mycoplasmas in both healthy and diseased goat herds, and they are difficult to sample reproducibly.82,109–111
Diagnostic Methods
Mycoplasma species involved in CA are relatively easy to cultivate, through liquid-medium enrichment (2–3 days) followed by streaking on agar plates and another 2–3 days incubation to isolate colonies harboring a distinctive morphology.1 Several media have been developed that contain selective components to inhibit potential contamination of mycoplasma cultures by the sample flora.17 Identification uses serological (dot immunobinding on membrane filtration, MF-dot or immunofluorescence assay, IFA) or molecular (PCR) methods or MALDI-TOF, as older biochemical testing methods have been abandoned due to over variability.17,112 Several CA-agent-specific PCR assays exist and have already been extensively reviewed.15 However, to date, there is still no one-step technique that can individually identify the four subspecies. A multiplex real-time-PCR method targeting all CA agents (M. agalactiae on one hand and the M. mycoides cluster on the other hand) has recently been developed and brought to market but it may prove expensive, especially for large-scale screening studies.85,113 Performances of all-round PCR diagnosis remain highly dependent on the DNA extraction methods used.85,108 With bulk tank milk samples, a combination of culturing and PCR may increase the sensitivity of detection.49,108 The high mutation rate in mycoplasma genomes makes it prudent to regularly re-validate available PCRs on newly circulating strains.
Another molecular detection method called Loop-mediated Isothermal Amplification (LAMP) requires less time and equipment than PCR and is used for several human and veterinary pathogens.114 It is portable and usable as a pen-side test for Ma detection.115,116 Although it holds promise with detection in under 60 mins, it requires further validation on field samples, with special emphasis on the high risk of cross-contamination and subjective reading.114
Indirect methods for diagnosis are also usable in the absence of vaccination at the herd level.17,117,118 Commercial ELISAs are currently limited to Ma serodiagnosis. They have proven to be more sensitive than complement fixation tests but their performances still require regular testing and validation to adapt to different epidemiological contexts.117,119,120 Some are not suitable for early infection detection due to the delayed onset of a serological response.120,121 Immunoblotting tests can be used as confirmatory methods but require cumbersome reagent preparation and expertise for reading.17,120,122 The recent development of a lateral flow assay as a serological pen-side test warrants further validation and, to our knowledge, is not yet available as a kit.123
Epidemiology Worldwide (Including Surveillance)
CA counts as one of the 117 notifiable animal diseases, infections, and infestations listed by the World Organization for Animal Health (OIE) in 2019 and has done for years. The Terrestrial Animal Health Code consequently (i) issues recommendations on sheep and goat imports, that include, for instance, the absence of clinical signs on the day of shipment, 6 months of husbandry in a CA-free establishment and in a quarantine station for the 21 days prior to shipment, (ii) repeatedly reviews its protocols for diagnosis and control, and (iii) appoints reference laboratories, which are currently the Istituto Zooprofilattico Sperimentale della Sicilia in Italy alongside the Animal and Plant Health Agency, in the UK. However, as CA is not shortlisted among the six diseases that hold official recognition of status by the OIE, as mandated by the World Trade Organization, no official map of CA prevalence worldwide is published. CA is believed to occur wherever pastoralism and small ruminant dairy production are common, but it is almost certainly underdiagnosed and underreported. Indeed, if we examine data from small ruminant milk production worldwide, reports of the disease should have increased with the increased tonnage and the presence of several newcomers to the market (Table 1). However, officially (OIE reports), CA has never been reported in India and Bangladesh, is absent from Sudan, and there is no information for Turkey and China, even though these countries make up the big five producers of goat and sheep milk. CA has been regularly reported in Europe, particularly in Mediterranean regions, as well as the Middle East, Asia, North Africa, and South America, through OIE notifications or scientific reports.17,62,124,125 It is more sporadic in other countries, like the USA with recent reports124 or New Zealand, with no evidence for CA in the last decade. Note that wild fauna may spread the disease or at least act as a reservoir for the etiological agents, but is not specifically surveyed.29,126
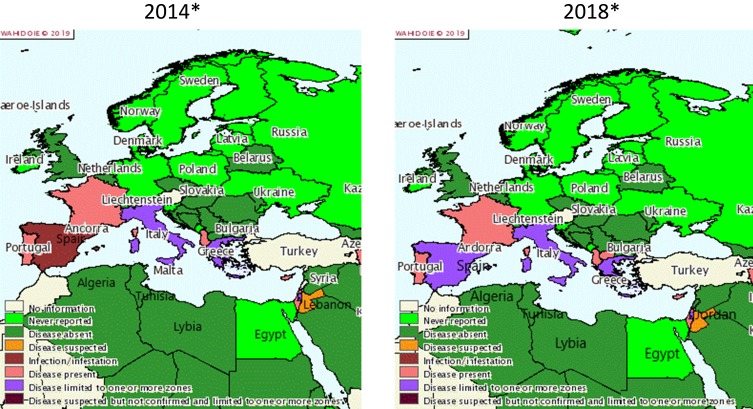
Historically and regularly since the 1980s, CA has been described in Mediterranean countries.1 Figure 1 proposes a snapshot on CA distribution in these countries according to OIE reporting. In the past five years (2014–2018), the disease has been annually reported to the OIE in Greece, Italy, Spain, France, Portugal, Cyprus, Albania, Israel, North Macedonia (until 2015), the Palestinian territories, and Jordan (suspected). For Italy, Greece, and Spain, there are partial quantitative data available, and some regions appear particularly affected as they report more often (Sardinia, Andalusia and Castile and Leon, Central Greece and Central, Eastern and Western Macedonia), but the OIE data are insufficient to calculate accurate prevalence. Other countries from Southeast Europe (Bulgaria, Croatia, Slovenia, Montenegro, Bosnia and Herzegovina, and Serbia) and the Maghreb (Tunisia, Algeria, Libya) did not report any CA and some Eastern Mediterranean countries (Lebanon, Egypt, Turkey) either gave no information or no reports. However, the scientific literature does feature clinical cases or surveys of CA for several of these countries: (i) Bosnia and Herzegovina where a survey on respiratory and ocular samples evidenced a few cases of Mmc, Mcc, and Mp in both sheep and goats,46 (ii) North Macedonia, where several Ma CA cases in sheep and goats were reported,127 and (iii) Turkey, where Ma appeared to be dominant in both sheep and goats (15% Ma-positive in 234 individual samples).45,128 This clearly underlines how, despite mandatory notification, CA remains overlooked by national veterinary services.
Figure 1.
Distribution of contagious agalactia in 2014 and 2018 in Mediterranean countries according to OIE reporting.
Notes: Reproduced with the kind authorisation of the World Organisation for Animal Health [OIE] www.oie.int.129 World Organisation for Animal Health (OIE).
Furthermore, the official OIE figures do not distinguish the different etiological agents, which have to be investigated through scientific publications. In Spain, the situation depends on the region. In north and central Spanish provinces, Ma is widely present in sheep (37% PCR positive bulk tank milk), but Mmc, Mcc, and Mp remain undetected.83 In Murcia, a goat survey evidenced a high prevalence of CA (67% of the tested farms) dominated by Ma (75%) compared to Mmc (4%).49 In contrast, in the Canary Islands, the prevalence of Mmc in goats reached or exceeded Ma figures117 as it was documented locally in Gran Canaria where Mmc was present in 90% of the CA-positive flocks accounting for 38% of the tested herds.34 In Jordan, a small ruminant survey found mostly M. mycoides cluster species and few Ma cases.43 In France, the “Vigimyc” surveillance network monitors the CA situation at a national level, except for the Pyrénées-Atlantiques region.44,57 Vigimyc is a voluntary-basis network for collecting and identifying isolates from clinical cases, so it cannot serve to assess prevalence but it can give an idea of the relative proportions of each species in CA cases: Mmc (42%), Mcc (26%), and Mp (15%).44 Unlike in Spain, Ma is rarely isolated in goats with clinical signs. These trends have remained relatively stable since 2010 (unpublished data). Most ovine clinical cases covered by the network concern respiratory disorders. In the Pyrénées-Atlantiques, which is a major French sheep-breeding region, CA is endemic in sheep but the prevalence of Ma infection has fallen below 5% after several years of control measures (see http://www.gds64.fr/maladies-actions-sanitaires/ovins-caprins/agalactie-contagieuse/les-actions/). In other countries, in the absence of surveys simultaneously targeting all CA mycoplasma species, their current relative distributions remain unknown. In Italy, for instance, studies have focused mostly on Ma where it is considered as dominant, in Sicily and Sardinia.130 Ma strains have been indeed isolated from sheep in several regions including Sardinia, Lazio, Sicily, and Puglia,103,131,132 whereas Mmc strains were also isolated in goats in Sardinia and Sicily.54,133,134 In 2016, a random Ma survey (PCR on bulk tank milk) conducted in Sardinia in both sheep and goats found relatively low prevalence rates in sheep 4.8% (n=1064 farms) and goats 4.5% (n=66 farms).135 Likewise, in Greece and Cyprus, all the available studies in both sheep and goats have focused exclusively on Ma.40,53,136,137 Consequently, the statement that Ma is the dominant CA etiology in the Mediterranean area130 should be revised, as while it may be true for sheep (in which Ma is the only etiological agent), it is not the case in goats. Potential differences in breed susceptibilities versus one or the other etiological agent have yet to be explored although they are poorly probable due to previously described clinical cases in herds with mixed breed.15,54
Recent Advances In The Biology Of The Etiological Agents
The etiological agents of CA all belong to the Mycoplasma genus, a group of bacteria that has evolved from a Gram-positive ancestor, through successive and severe gene reduction, resulting in their current form, which are bacteria with small genomes (ca. 0.5–1.5 Mbp),138 no cell wall,139 and reduced metabolic pathways.140 These characteristics have forged a parasitic life-style with huge dependence on the animal-host for nutrients.
Taxonomy
The taxonomic status of the Ma species has remained unchanged since it was first established in the Mycoplasma genus in the 1950s. There used to be a subspecies, named M. agalactiae subsp. bovis that rapidly became the M. bovis species, which is phylogenetically very close to Ma and involved in bovine pneumonia, arthritis, and mastitis.141 In contrast, the taxonomy and phylogeny of the other etiological agents have been revised extensively over the years with for instance M. mycoides subsp. mycoides Large Colony biotype (MmmLC) and M. mycoides subsp. capri (Mmc) being grouped under one unique subspecies, Mmc.8 Mmc, like Mcc, is part of the M. mycoides cluster, a group of closely related species that also includes the etiological agents of contagious bovine and caprine pleuropneumonia and a potential fourth member, M. feriruminatoris subsp. nov.7,8,142 However, M. putrefaciens the fourth etiological agent of CA does not strictly belong to the cluster, and its relative positioning has been debated several times.7,142
Genomic Plasticity
The theory of a reductive evolution of mycoplasmas with genetic erosion leading to extinction, clonality, or intracellularity has been challenged in recent decades, and, today, at least one genome has been sequenced for each species (Table 2). Contrary to other bacteria genomes, plasmids are poor contributors to genome diversity and dissemination of beneficial traits, such as antimicrobial resistance, as they are found only in the M. mycoides cluster and have a small size with no cargo gene.143 In silico comparative genomic analysis has pointed out massive horizontal gene transfers between different species sharing the same host.144,145 Around 130 genes in M. agalactiae, for example, are thought to originate from the phylogenetically remote M. mycoides cluster. This gene transfer was later confirmed in vitro, using M. agalactiae as a model organism, and associated with the presence of self-transmissible mobile genetic elements called Integrative Conjugative Elements (ICEs).146,147 ICEs encode their own excision, transfer by conjugation, and integration into the recipient-cell chromosome. Once in contact, the recipient and donor cells can also exchange through an unconventional conjugative mechanism of chromosomal transfers (CTs) which involves large chromosomal regions, whatever their genomic locations.147 These CTs, which can comprise up to 10% of the genome, generate highly mosaic genomes, leading to a concept from Citti et al that
mycoplasma populations can be seen as a dynamic gene pool compensating genome erosion as well as clonality, allowing horizontal dissemination of genetic traits that may in turn result in the emergence of new strains, with new properties.148,149
Table 2.
List Of Strains With A Sequenced Genome, Among The Etiological Agents Of Contagious Agalactia, With Main Characteristics Of The Genomes. All Strains Were Originally Isolated From Goats
| (Sub)Species | Strain | Genome | ||||||
|---|---|---|---|---|---|---|---|---|
| Name | Isolation Place, Date | Clinical Signs | RefSeq Accession And Ref | Size (bp) | GC Content | Total Number Of CDS | Mobile Genetic Elements | |
| M. agalactiae | PG2 | Spain, 1952 | nk, type strain | NC_009497144 | 877,438 | 29.7 | 752 | No plasmid, one vestigial ICE |
| M. mycoides subsp. capri | 95010 | France, 1995 | Arthritis | NC_8015431221 | 1,153,998 | 23.8 | 924 | One plasmid of 1,840 bp (pMmc-95010), ICEM, 2 copies |
| M. capricolum subsp. capricolum | California Kid | USA, 1955 | Arthritis, type strain | NC_007633222 | 1,010,023 | 23.8 | 827 | No plasmid, ICEC, 1 copy |
| M. putrefaciens | KS1 | USA, nk | nk, type strain | NC_015946150 | 832,603 | 26.9 | 650 | No plasmid, nor ICE |
Abbreviations: ICE, integrative conjugative elements; nk, not known.
All this has been demonstrated in vitro and mainly for M. agalactiae, but the same process can reasonably be hypothesized in other mycoplasma species, as ICEs are relatively prevalent in ruminant mycoplasmas.151 Conjugative transfers of ICEs or CTs between M. mycoides and Ma have never yet been demonstrated in vitro or in vivo. Further investigation is warranted and would be key to understanding the evolution of strains within the goat host, which might be favorable to horizontal gene transfers between M. mycoides and M. agalactiae, in contrast to the sheep host where Ma the sole CA agent.152 Indeed, clonal spread of Ma in sheep has been reported several times at different scales, from an endemic area up to country-wide level,29,153–155 whereas caprine isolates are described as more variable.152,156 The development of new molecular genotyping methods such as multiple locus variable number of tandem repeats analysis (MLVA) or multilocus sequence typing (MLST) with a better discriminatory power than restriction endonuclease-based techniques, size variation in vpma genes repertoire, Insertion Sequence (IS) typing or random amplified polymorphic DNA patterns has brought clearer insight into M. agalactiae diversity.154,157,158 In contrast, there is still no approved MLST or MLVA scheme for members of the M. mycoides cluster despite one proposal for an MLST scheme.7,159 With the decrease of whole-genome sequencing costs, core genome MLST, as developed for the poultry pathogen M. synoviae, should become a more accurate alternative to MLST.160
Another feature of mycoplasma genomes is their fast evolution, with one of the highest bacterial mutation rates due to their degenerated DNA repair systems. M. gallisepticum, for example, has a nucleotide substitution rate of 0.5–1.2×10−5 per site per year whereas Staphylococcus aureus has a rate of 1.2–2.6×10−6.161 This has implications for PCR-based diagnosis since mismatches can occur in the primer hybridization sequence and thus erode the specificity of the PCR assays, as illustrated by several studies.9,162
Virulence
The pathological effects of mycoplasma infections are deemed to result more from an inappropriate response of the animal host following colonization rather than production of mycoplasmal virulence factors with cell-toxic effects.163 Efficient colonization and persistence in the host are key steps in mycoplasmosis, and they are mainly mediated by efficient nutrient scavenging, adhesion to the host cells, and immune evasion. Intracellular invasion has been documented in various species from the avian pathogen M. gallisepticum164 to more recently the porcine agent M. hyopneumoniae,165 but its role in pathogenesis is currently unknown. In Ma, the immune evasion is largely mediated by variable surface proteins, called “Vpma”, and phase variations of capsular coating.166,167 The Vpma expression profiles at different stages and different sites of ewes experimentally infected with Ma changed very rapidly, preventing efficient immune response.168 Furthermore, Vpma were shown to play a role in cell adhesion and invasion; hence, their variation is important in pathogenesis.169 In Ma, adhesion is also mediated by other well-described adhesins, like P40.170 Another original but complex system of immune evasion has recently been evidenced in mycoplasmas, the so-called MIB–MIP system able to cleave off the VH domain of the host immunoglobulin G.171
For years, the lack of genetic tools to engineer mycoplasma genomes has delayed formal characterization of virulence traits in vivo. However, recent advances in synthetic biology and in editing of mycoplasma genomes cloned in yeasts have opened new possibilities.172,173 Very recently, Jores et al succeeded in excising 68 non-essential genes accounting for ca. 100 kbp from the JCVI-Syn3.0 mutant of Mmc GM12 and generate a fully attenuated strain.174 Cumulative deletion of 5 candidate virulence traits, like the glycerol-dependent hydrogen metabolic pathway, 9 lipoproteins including major antigens (LppA/P72), mycoplasma-specific F1-like X0 ATPase, the MIB-MIP system, an ICE, and other Lpps including lppQ failed to specifically designate which deletion is responsible for attenuation in a challenge goat model.
All this host-mycoplasma interplay has a huge impact on clinical expression of the disease, resulting in long-term persistence within a host or a herd, intermittent excretion and an immune response that fluctuates over time. All these parameters also influence the efficiency of diagnostic tools.
Means And Relative Effectiveness Of Control
Control of CA relies on culling the affected flocks, chemotherapy and/or vaccination, or combinations of all three measures, but there is practically no international coordination on the issue, with each country opting for its own way depending on pattern of infective spread (enzootic area versus sporadic cases). Good herd hygiene management practices and biosecurity measures are also key, as once introduced in a herd, the disease may spread fast.15,54 Eradication of CA requires sustained financial resources and long-term commitment from the stakeholders, which creates another additional cost that is rarely taken into account.15,32,130
Vaccines
For a number of years, relatively ineffective mycoplasma vaccines were produced directly from milk or other tissues of infected small ruminants.175 They were then banned as they were suspected to be responsible for increase in scrapie outbreaks in Italy. From then on, several assays were performed to prepare safe and effective vaccines, mainly against Ma CA.176–183 Live attenuated vaccines, such as the ones used in Turkey, were reported to be more efficient in the long term than inactivated vaccines.31,184 However, they are not authorized in many countries and should not be used during the lactation period. Hence, most developments have focused on inactivated vaccines either for local use (autogenous vaccine) or for licensing. However, even today there is still no effort to standardize an assessment method, nor to harmonize regulatory constraints worldwide, and still there is not a single universally recognized vaccine.17
The strain included in the vaccine as well as the inactivation method and the adjuvant used are all key factors for immunogenic efficacy against potentially very variable infective strains.184–186 Mineral-oil adjuvant-inactivated vaccines induce higher and longer-lasting protective immunity than the aluminium-hydroxide-absorbed vaccines, but they can also induce lesions at the injection site.176,179,182,187 Inactivated vaccines remain sub-optimal, as most of the time they reduce clinical severity rather than preventing new infections or even milk excretion.49,180,184 The quick decrease in antibody titers, frequently followed by a generic ELISA test that gives no indication on their protective nature, imposes repeated vaccinations every 6 months. Consequently, vaccination should be combined with chemotherapy to improve the chance of both clinical and microbiological cure.
There has been little attempt to develop vaccines against the M. mycoides cluster species except one in India against Mmc.188 Licensed vaccines can be polyvalent with proven efficacy in an endemic area180 or monovalent but actually claiming to cross-protect against different Mycoplasma spp.189 The development of synthetic biology to customize strains might be a promising approach for tomorrow's vaccines.174
Autogenous vaccines against CA have attracted little attention in the literature, and case studies or experimental challenge reports are scarce.184,190 As a rule, autogenous vaccines are considered a useful addition or feasible substitutes to licensed vaccines,191 but only bring local solutions, in farms that have already suffered an outbreak. They are especially considered in M. mycoides CA cases as there is no commercial vaccine.
Antimicrobials And Resistance
Tetracyclines, macrolides, and fluoroquinolones are currently the top recommended drugs against CA,14,15 while other molecules, such as florfenicol and tiamulin, were more popular in the past.48 Papers have long underlined the importance of early, collective and repeat administrations for therapeutic success at the herd scale.1 Unfortunately, actual use of antimicrobials and the clinical and microbiological outcomes are rarely reported. There are few antimicrobials available with a market authorization specific for small ruminants. Most of these shortfalls are tackled using products authorized in cattle, rationalized on the cascade principle. Antimicrobials do allow clinical recovery but rarely complete bacteriological clearance,48 as nicely illustrated by two recent reports of bitherapy with tetracycline and macrolides.54,69 A study on M. bovis, another ruminant mycoplasma very closely related to Ma, showed that the level of resistance for almost all antimicrobials except fluoroquinolones has increased dramatically in contemporary isolates.192 In this context, antimicrobial susceptibility testing (AST) of CA agents is vital, but has only been tackled in a few studies.152,193–200
High-throughput AST techniques such as the disk diffusion method cannot be used for Mycoplasma spp. due to their slow growth and the need for complex medium. AST thus hinges on minimum inhibitory concentration (MIC) testing, either by agar or micro-broth dilution, for which specific recommendations have been issued.201 The lack of harmonized procedures and quality controls for veterinary mycoplasma AST rules out good data comparison, and, within veterinary mycoplasmas, species affecting small ruminants are often neglected.202 A further barrier is the absence of clinical breakpoints for interpretation of MICs in terms of therapeutic efficacy in vivo (resistant intermediary, or susceptible). For some species harboring a single tissue tropism, breakpoints for other pathogens with similar biological niches have been used (e.g., Pasteurellaceae for M. bovis),192,203 but this approach is not feasible with CA agents due to the diversity of their body localization.
Table 3 summarizes MIC values from different studies for each etiological agent of CA stratified by the main antimicrobials used in small ruminants. The general picture is a dominant low-MIC population of strains with a secondary, limited (especially for phenicols and fluoroquinolones) population exhibiting moderate MIC increases for most antimicrobial families. There are slight differences between CA species, but overall the shift to high MIC only concerns a few strains, which is a very different picture to M. bovis for which most current strains are resistant.202 For oxytetracycline, the most widely used tetracycline, tylosin, the most widely used macrolide, and lincomycin, the most widely used lincosamide, regardless of mycoplasma species, the dominant population has low MICs (≤1 µg/mL). A shift toward higher values (MIC90 between 2 and 16 µg/mL) was evidenced in Ma, Mcc, and Mp except for Mp with tylosin, see, for instance, Refs. 152, 159, 204. For oxytetracycline, MIC values were higher for Ma than for the M. mycoides cluster.195,198 Poumarat et al also reported higher MICs with oxytetracycline but lower MICs with tylosin for Ma in goats versus sheep, which may reflect the different use of the molecules.152 The bimodal distribution of MICs with higher values (8 ≤ MIC90 ≤ 32 µg/mL) is also observed for other 16-membered ring macrolides like spiramycin in all species except Mp and tilmicosin for Ma and Mcc.152,159,196,197,199 For the latest generation macrolides, represented by the long-acting tulathromycin, some increased MICs have been documented with Ma but they remained limited ([2–8] µg/mL).152,196 No increased-MIC population has been observed yet with the less-used second-generation tetracycline, doxycycline, as there is no MIC above 1 µg/mL, see, for instance, Refs.198 and.199 All MICs for aminosides were high for species of the “M. mycoides” cluster (MIC90 > 16 µg/mL), which is consistent with a suspected intrinsic resistance of other species within this group.205 For Ma, MICs with aminosides are broadly distributed, with infrequent reports of increased MICs.152,195,196,199 MIC values with phenicols mostly range between 1 and 8 µg/mL with a limited MIC increase (MIC90 reaching 4 or 8 µg/mL) whatever the mycoplasma species.152,196,200 Finally, for third-generation fluoroquinolones, there is a less marked-shift towards increased MIC values, with MIC remaining low (≤0.5 µg/mL) except in a few Spanish Mcc and Ma strains with values between 1 and 4 µg/mL.159,196
Table 3.
Overview Of MIC Distribution Of CA Mycoplasma Species (Microbroth Or Agar Dilution Methods Depending On The Study) And Main Molecular Resistance Mechanisms When Known
| Mycoplasma Species | Fluoroquinolones | Phenicols | Tetracyclines | Macrolides | Lincosamides | Aminosides | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ENRO | FFC | OXY | TYL | LIN | SPT | |||||||
| MIC Range MIC90 Range µg/mL | Mec. | MIC Range MIC90 Range µg/mL | Mec. | MIC Range MIC90 Range µg/mL | Mec. | MIC Range MIC90 Range µg/mL | Mec. | MIC Range MIC90 Range µg/mL | Mec. | MIC Range MIC90 Range µg/mL | Mec. | |
|
Ma 152,196,198,199,204, 195 |
<0.125–0.8 0.1–0.5 |
Mutations in QRDR | 1–8 2–8 |
ns | 0.06–8 0.5–8 |
rrs mutations | 0.025–16 0.25–8 |
rrn and L22 coding gene mutations | 0.125–4 1–4 |
rrn and L22 coding gene mutations | 0.4–12.8 2–8 |
ns |
|
Mcc 200,206,207 |
0.025–3.2 0.25–0.4 |
Mutations in QRDR | 4–8 8 |
ns | 0.25–4 0.5–2 |
na | 0.05–12.8 ≤0.03–12.8 |
rrn and L22 coding gene mutations | 0.2–12.8 12.8 |
rrn and L22 coding gene mutations | 16–128 128 |
ns |
|
Mmc 194,198,200, 197 |
0.025–0.2 0.1–0.125 |
na | 2–4 4 |
ns | 0.125–4 0.25–0.5 |
na | 0.025–1 0.06–1 |
na | 0.125–12.8 0.5–0.8 |
rrn and L22 coding gene mutations | 8–128 16–64 |
ns |
|
Mp 193,198,200 |
0.06–0.25 0.125–0.25 |
na | 0.5–4 1 |
ns | 0.250–8 0.5–4 |
na | 0.015–2 0.015–0.12 |
na | 0.250–16 1–2 |
rrn and L22 coding gene mutations | 8–32 16–32 |
ns |
Abbreviations: Mec., mechanism; ns, not studied so far; na, not applicable; ENRO, enrofloxacin; FFC, florfenicol; OXY, oxytetracycline, TYL, tylosin; LIN, lincomycin; SPT, spectinomycin.
As described in other species,202 CA mycoplasma resistance hinges mostly on point mutations affecting the antimicrobial binding target. Macrolides and lincosamides resistance correlate with mutations in domain V of the 23S rRNA and ribosomal protein L22 in Ma and Mcc isolates.204,206 Increased MIC values with third-generation fluoroquinolones are associated with mutation in the quinolone resistance determining regions of gyrA (Glu87Lys), gyrB (Pro343Leu), and parC (Ser80Thr or Asp84Gly) in Ma and Mcc field isolates.207 For oxytetracycline, the situation is more complex. In some Ma strains, but not all, the classical hot-spot mutations in the Tet-1 domain in one or both rrs allele(s) have been associated with resistance.198 In the M. mycoides cluster, strains with increased MICs do not harbor any mutation in or outside Tet-1,198 suggesting the existence of other resistance mechanisms. Mechanisms for phenicol and aminoside resistances remain unexplored.
Other Compounds With Antimicrobial Effect
Several recent studies have assessed the in vitro antimycoplasmal efficacy of plant extracts against Mcc and Mmc as an alternative to antimicrobials208–213 including one field trial with promising results.214 These studies focused on endemic plants generally used by the local populations for medicinal purposes, which is also a promising approach given how access to veterinary drugs is critical in many small ruminant breeding regions of the world. Studies have evidenced antimycoplasmal activities of several plant extracts using recommended AST methods.208,209,211 MIC ranged between 3.1–12.5 mg/mL and 0.05–0.6 mg/mL for Mcc,208,211 and 0.001 −1 mg/mL and 0.13–0.6 mg/mL for Mmc.209,211 The efficacy of plant extracts appears to be highly dependent on extraction process and storage conditions.209 Given their high inhibitory concentrations compared to conventional drugs, further investigations are needed to gauge their safety for animals and their bioavailability.
Good Herd Management Practices And Animal Welfare
In addition to antimicrobial treatment and vaccines, herd management practices are helpful in confining the disease within an infected herd and preventing the introduction of infected animals.
As demonstrated experimentally with Ma and Mmc, colostrum heating (56°C, 60 mins) reduces mycoplasma load and thus limits offspring oral infection.215 The reduction of mammary transmission between adults requires checking milking equipment and process hygiene (e.g., milking order, parlor, cleaning devices and milkers’ hands, discarding foremilk, thorough teat cleaning and dipping).118 Isolating clinically affected animals is generally recommended but is rarely practicable when clinical forms are widespread. Regular disinfection of the farm environment (beddings, feeders, troughs), and especially the milking device, can help limit indirect transmission. Several disinfectants have proven to be efficient in vitro against Mycoplasma spp.216,217 but their in-field implementation has yet to be validated. A control and slaughter strategy aiming to eliminate infected and/or debilitated animals could be a good complementary measure to recover production capacity or further achieve eradication. The detection of shedders requires repeated individual or bulk direct testing due to the intermittency of excretion, which is also applicable to introduced animals. All introduced animals should be monitored, at least in quarantine.50 Rather than individual analyses, pre-movement testing in supplier herds needs to be repeated to reduce the risk of introduction.85 As semen shedding maybe an issue (see paragraph on transmission routes), these measures should also be applied to males temporarily lent out for mating or to semen suppliers.90,91
Stress-limiting breeding practices could promote clinical recovery (quality of feeding and health management, environmental conditions). A recent assessment of small ruminant welfare in current breeding systems challenges some of the commonly recommended herd management practices and control measures as they run counter to natural behaviors.218,219 For instance, goats are naturally more reactive, aggressive and exploratory than sheep, and hence their space provided per animal is critical. Goats need to maintain vocal, visual and olfactory contacts with other individuals, so any isolation (kids from their mothers or bucks from does) or regrouping practices will be stressful. Intensive indoor housing systems provide control of feeding regimes, climate, and parasite loads, but they also put constraints on naturalness. A good compromise between animal needs and natural social organization and management systems can reduce stress experienced by the animals, which could in turn limit their tendency to get sick, especially from mycoplasmosis that often results from stress-driven non-adapted immune response, as observed in small ruminants13 as well as in cattle.220
Conclusions And Knowledge Gaps
Small ruminant livestock is distributed worldwide, including in many low-income countries, some of which are currently switching from traditional, extensive, familial systems to semi-intensive or intensive farming. This trajectory raises the likelihood of increasing CA risk with animal grouping. Better follow-up of the true prevalence of the disease worldwide and its associated economic burden is needed to support this transition, and should ultimately help raise awareness on CA of the regulatory authorities.
CA has been known for a long time, yet it is still a neglected disease worldwide. It is difficult to prevent and control due to its rapid spread, multiple sources of infection with both horizontal and vertical transfer, multiple potential etiological agents with different antimicrobial susceptibilities, and different forms of the disease from acute to subacute, and chronic or asymptomatic.
Diagnostic tests are underperformed due to the cost and expertise required, creating a need to develop affordable new pen-side tests. Although the etiological agents remain susceptible to most antimicrobial families, surveillance of resistance is important, as some resistant clinical isolates have emerged that will require efforts to harmonize the techniques and develop easy-to-perform tests. There is still no universal vaccine available, despite huge efforts to develop one, at least against Ma. Hence, in 2019, there is still a pressing need for validated low-cost vaccines and a proper vaccination scheme suitable for low and middle-income countries. With recent progress in genome manipulation, tailoring genetically attenuated strains should be reconsidered as an option.
Concerning the agents per se, there are still a number of unresolved questions concerning circulation in the host, interplay with the immune system, shedding, and infectious dose for various transmission routes. The development of genome editing tools might help to decipher the virulence determinant of different strains, and all these data are expected to have a tangible impact on CA prevention and control.
Last but not least, CA poses a set of challenges in terms of growing consumer concern for the livestock welfare. The disease affects all three pillars of welfare: the animal’s physical state (whatever its clinical form), affective state (with chronic debilitation), and behavioral needs which are compromised by the herd management practices imposed by CA. So far, there has been little if any effort to frame CA as an animal welfare issue.
In conclusion, the old CA disease that emerged in the early 19th century will continue to require collective commitment from breeders, veterinarians, scientists, and regulatory authorities through the next coming decades.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lambert M. Contagious agalactia of sheep and goats. Rev Sci Tech. 1987;6:699–711. doi: 10.20506/rst.6.3.308 [DOI] [PubMed] [Google Scholar]
- 2.Madanat A, Zendulkova D, Pospísil Z. Contagious agalactia of sheep and goats. A review. Acta Vet Brno. 2001;70:403–412. doi: 10.2754/avb200170040403 [DOI] [Google Scholar]
- 3.Thiaucourt F, Bolske G. Contagious caprine pleuropneumonia and other pulmonary mycoplasmoses of sheep and goats. Rev Sci Tech. 1996;15(4):1397–1414. doi: 10.20506/rst.issue.15.4.2493 [DOI] [PubMed] [Google Scholar]
- 4.Bridré J, Donatien A. Le microbe de l’agalaxie contagieuse et sa culture in vitro. C R Acad Des Sci. 1923;177:841–843. [Google Scholar]
- 5.Nicholas RAJ, Ayling RD, McAuliffe L. Contagious agalactia In: Mycoplasma Diseases of Ruminants. Oxfordshire, UK: CAB International; 2008:98–113. [Google Scholar]
- 6.Cottew GS, Breard A, DaMassa AJ, et al. Taxonomy of the Mycoplasma mycoides cluster. Isr J Med Sci. 1987;23(6):632–635. [PubMed] [Google Scholar]
- 7.Manso-Silvan L, Perrier X, Thiaucourt F. Phylogeny of the Mycoplasma mycoides cluster based on analysis of five conserved protein-coding sequences and possible implications for the taxonomy of the group. Int J Syst Evol Microbiol. 2007;57(10):2247–2258. doi: 10.1099/ijs.0.64918-0 [DOI] [PubMed] [Google Scholar]
- 8.Manso-Silvan L, Vilei EM, Sachse K, Djordjevic SP, Thiaucourt F, Frey J. Mycoplasma leachii sp. nov. as a new species designation for Mycoplasma sp. bovine group 7 of Leach, and reclassification of Mycoplasma mycoides subsp. mycoides LC as a serovar of Mycoplasma mycoides subsp. capri. Int J Syst Evol Microbiol. 2009;59(6):1353–1358. doi: 10.1099/ijs.0.005546-0 [DOI] [PubMed] [Google Scholar]
- 9.Le Grand D, Saras E, Blond D, Solsona M, Poumarat F. Assessment of PCR for routine identification of species of the Mycoplasma mycoides cluster in ruminants. Vet Res. 2004;35(6):635–649. doi: 10.1051/vetres:2004037 [DOI] [PubMed] [Google Scholar]
- 10.Poumarat F, Longchambon D, Martel JL. Application of dot immunobinding on membrane filtration (MF dot) to the study of relationships within “M. mycoides cluster” and within “glucose and arginine-negative cluster” of ruminant mycoplasmas. Vet Microbiol. 1992;32(3–4):375–390. doi: 10.1016/0378-1135(92)90159-Q [DOI] [PubMed] [Google Scholar]
- 11.Kinde H, DaMassa AJ, Wakenell PS, Petty R. Mycoplasma infection in a commercial goat dairy caused by Mycoplasma agalactiae and Mycoplasma mycoides subsp. mycoides (caprine biotype). J Vet Diagn Invest. 1994;6(4):423–427. doi: 10.1177/104063879400600404 [DOI] [PubMed] [Google Scholar]
- 12.Gil MC, Pena FJ, Hermoso De Mendoza J, Gomez L. Genital lesions in an outbreak of caprine contagious agalactia caused by Mycoplasma agalactiae and Mycoplasma putrefaciens. J Vet Med B Infect Dis Vet Public Health. 2003;50(10):484–487. doi: 10.1046/j.0931-1793.2003.00709.x [DOI] [PubMed] [Google Scholar]
- 13.De La Fe C, Gutiérrez A, Poveda JB, Assunção P, Ramirez AS, Fabelo F. First isolation of Mycoplasma capricolum subsp capricolum, one of the causal agents of caprine contagious agalactia, on the island of Lanzarote (Spain). Vet J. 2005;173(2):440–442. doi: 10.1016/j.tvjl.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Martin A, Amores J, Paterna A, De la Fe C. Contagious agalactia due to Mycoplasma spp. in small dairy ruminants: epidemiology and prospects for diagnosis and control. Vet J. 2013;198(1):48–56. doi: 10.1016/j.tvjl.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 15.Corrales JC, Esnal A, De la Fe C, et al. Contagious agalactia in small ruminants. Small Rumin Res. 2007;68(1–2):154–166. doi: 10.1016/j.smallrumres.2006.09.010 [DOI] [Google Scholar]
- 16.Kumar A, Rahal A, Chakraborty S, Verma AK, Dhama K. Mycoplasma agalactiae, an etiological agent of contagious agalactia in small ruminants: a review. Vet Med Int. 2014;2014:286752. doi: 10.1155/2014/286752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Organization for Animal Health (OIE). Volume 1, Chapter 3.7.3 Contagious agalactia In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Terrestrial Manual), 8th Edition, 2018; Paris: OIE; 2019:1430–1440. https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.07.03_CONT_AGALACT.pdf [Google Scholar]
- 18.Heller M, Schwarz R, Noe G, et al. First human case of severe septicaemia associated with Mycoplasma capricolum subsp. capricolum infection. JMM Case Rep. 2015;2(5). doi: 10.1099/jmmcr.0.000101. [DOI] [Google Scholar]
- 19.Truscott RB, Finley GG. Studies on Mycoplasma mycoides subsp. mycoides (LC) in lambs and calves. Can J Comp Med. 1985;49(2):233–234. [PMC free article] [PubMed] [Google Scholar]
- 20.Taoudi A, Kirchhoff H. Isolation of Mycoplasma capricolum from cows with mastitis. Vet Rec. 1986;119(10):247. doi: 10.1136/vr.119.10.247 [DOI] [PubMed] [Google Scholar]
- 21.Kapoor PK, Garg DN, Mahajan SK. Isolation of Mycoplasma mycoides subsp. mycoides (LC variant, Y-Goat) from naturally aborted bovine fetuses. Theriogenology. 1989;32(4):683–691. doi: 10.1016/0093-691X(89)90289-6 [DOI] [PubMed] [Google Scholar]
- 22.Hung AL, Alvarado A, Lopez T, Perales R, Li O, Garcia E. Detection of antibodies to mycoplasmas in South American camelids. Res Vet Sci. 1991;51(3):250–253. doi: 10.1016/0034-5288(91)90072-V [DOI] [PubMed] [Google Scholar]
- 23.Nicolas MM, Stalis IH, Clippinger TL, et al. Systemic disease in Vaal rhebok (Pelea capreolus) caused by mycoplasmas in the mycoides cluster. J Clin Microbiol. 2005;43(3):1330–1340. doi: 10.1128/JCM.43.3.1330-1340.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ur-Rahman S, Siddique M, Rasool M. Seroprevalence of Mycoplasma mycoides subspecies capri in ruminants and camel. Small Rumin Res. 2006;63(1–2):28–31. doi: 10.1016/j.smallrumres.2005.01.012 [DOI] [Google Scholar]
- 25.Verbisck-Bucker G, Gonzalez-Candela M, Galian J, Cubero-Pablo MJ, Martin-Atance P, Leon-Vizcaino L. Epidemiology of Mycoplasma agalactiae infection in free-ranging Spanish ibex (Capra pyrenaica) in Andalusia, southern Spain. J Wildl Dis. 2008;44(2):369–380. doi: 10.7589/0090-3558-44.2.369 [DOI] [PubMed] [Google Scholar]
- 26.De La Fe C, Assunção P, Gutiérrez C, et al. Survey of antibodies to Mycoplasma agalactiae and Mycoplasma mycoides subsp. mycoides (large colony type) in dromedaries (Camelus dromedarius) in close contact with an infected goat population. J Arid Environ. 2009;73(4–5):594–595. doi: 10.1016/j.jaridenv.2008.09.022 [DOI] [Google Scholar]
- 27.Giangaspero M, Orusa R, Nicholas RA, et al. Characterization of Mycoplasma isolated from an ibex (Capra ibex) suffering from keratoconjunctivitis in northern Italy. J Wildl Dis. 2010;46(4):1070–1078. doi: 10.7589/0090-3558-46.4.1070 [DOI] [PubMed] [Google Scholar]
- 28.Ostrowski S, Thiaucourt F, Amirbekov M, et al. Fatal outbreak of Mycoplasma capricolum pneumonia in endangered markhors. Emerg Infect Dis. 2011;17(12):2338–2341. doi: 10.3201/eid1712.110187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tardy F, Baranowski E, Nouvel LX, et al. Emergence of atypical Mycoplasma agalactiae strains harboring a new prophage and associated with an alpine wild ungulate mortality episode. Appl Environ Microbiol. 2012;78(13):4659–4668. doi: 10.1128/AEM.00332-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catania S, Gobbo F, Schiavon E, Nicholas RAJ. Severe otitis and pneumonia in adult cattle with mixed infection of Mycoplasma bovis and Mycoplasma agalactiae. Vet Rec Case Rep. 2016;4(2):e000366. doi: 10.1136/vetreccr-2016-000366 [DOI] [Google Scholar]
- 31.Nicholas RAJ. Improvements in the diagnosis and control of diseases of small ruminants caused by mycoplasmas. Small Rumin Res. 2002;45(2):145–149. doi: 10.1016/S0921-4488(02)00095-0 [DOI] [Google Scholar]
- 32.Loria GR, Puleio R, Nicholas RAJ. Contagious Agalactia: economic losses and good practice. J Bacteriol Mycol. 2018;5(5):1076. [Google Scholar]
- 33.FAOSTAT Statistical Database. Data extracted from FAOSTAT, Livestock Primary. Rome, Italy: Food and Agriculture Organization of the United Nations. 2019. Available from: http://www.fao.org/faostat/en/#data/QL. Accessed July 4, 2019. [Google Scholar]
- 34.De La Fe C, Assunção P, Antunes T, Rosales RS, Poveda JB. Microbiological survey for Mycoplasma spp. in a contagious agalactia endemic area. Vet J. 2005;170(2):257–259. doi: 10.1016/j.tvjl.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 35.Dore S, Liciardi M, Amatiste S, et al. Survey on small ruminant bacterial mastitis in Italy, 2013–2014. Small Rumin Res. 2016;141:91–93. doi: 10.1016/j.smallrumres.2016.07.010 [DOI] [Google Scholar]
- 36.Lima MC, Souza MCC, Espeschit IF, et al. Mastitis in dairy goats from the state of Minas Gerais, Brazil: profiles of farms, risk factors and characterization of bacteria. Pesqui Vet Bras. 2018;38:1742–1751. doi: 10.1590/1678-5150-pvb-5698 [DOI] [Google Scholar]
- 37.Mishra AK, Sharma N, Singh DD, et al. Prevalence and bacterial etiology of subclinical mastitis in goats reared in organized farms. Vet World. 2018;11(1):20–24. doi: 10.14202/vetworld. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Contreras A, Corrales JC, Sierra D, Marco J. Prevalence and aetiology of non-clinical intramammary infection in Murciano-Granadina goats. Small Rumin Res. 1995;17(1):71–78. doi: 10.1016/0921-4488(95)00651-Z [DOI] [Google Scholar]
- 39.Contreras A, Sierra D, Sánchez A, et al. Mastitis in small ruminants. Small Rumin Res. 2007;68(1):145–153. doi: 10.1016/j.smallrumres.2006.09.011 [DOI] [Google Scholar]
- 40.Giadinis ND, Arsenos G, Tsakos P, et al. “Milk-drop syndrome of ewes”: investigation of the causes in dairy sheep in Greece. Small Rumin Res. 2012;106(1):33–35. doi: 10.1016/j.smallrumres.2012.04.018 [DOI] [Google Scholar]
- 41.Paterna A, Contreras A, Gómez-Martín A, et al. The diagnosis of mastitis and contagious agalactia in dairy goats. Small Rumin Res. 2014;121(1):36–41. doi: 10.1016/j.smallrumres.2013.12.002 [DOI] [Google Scholar]
- 42.Dore S, Amatiste S, Bergagna S, et al. Survey on contagious agalactia of small ruminants in Italy, 2014–2017. IDF Mastitis Conference 2019; May 16 2019; Copenhagen. [Google Scholar]
- 43.Al-Momani W, Halablab MA, Abo-Shehada MN, Miles K, McAuliffe L, Nicholas RAJ. Isolation and molecular identification of small ruminant mycoplasmas in Jordan. Small Rumin Res. 2006;65(1):106–112. doi: 10.1016/j.smallrumres.2005.05.022 [DOI] [Google Scholar]
- 44.Chazel M, Tardy F, Le Grand D, Calavas D, Poumarat F. Mycoplasmoses of ruminants in France: recent data from the national surveillance network. BMC Vet Res. 2010;6:32. doi: 10.1186/1746-6148-6-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goçmen H, Rosales R, Ayling R, Ülgen M. Comparison of PCR tests for the detection of Mycoplasma agalactiae in sheep and goats. Turk J Vet Anim Sci. 2016;40:421–427. doi: 10.3906/vet-1511-65 [DOI] [Google Scholar]
- 46.Maksimovic Z, Bacic A, Rifatbegovic M. Mycoplasmas isolated from ruminants in Bosnia and Herzegovina between 1995 and 2015. Veterinaria. 2016;65:27–-31 [Google Scholar]
- 47.De La Fe C, Sánchez A, Gutierrez A, et al. Effects on goat milk quality of the presence of Mycoplasma spp. in herds without symptoms of contagious agalactia. J Dairy Res. 2009;76(1):20–23. doi: 10.1017/S002202990800366X [DOI] [PubMed] [Google Scholar]
- 48.Bergonier D, Berthelot X, Poumarat F. Contagious agalactia of small ruminants: current knowledge concerning epidemiology, diagnosis and control. Rev Sci Tech. 1997;16(3):848–873. doi: 10.20506/rst.issue.16.3.6 [DOI] [PubMed] [Google Scholar]
- 49.Amores J, Sánchez A, Gómez-Martín Á, Corrales JC, Contreras A, de la Fe C. Surveillance of Mycoplasma agalactiae and Mycoplasma mycoides subsp. capri in dairy goat herds. Small Rumin Res. 2012;102(1):89–93. doi: 10.1016/j.smallrumres.2011.09.008 [DOI] [Google Scholar]
- 50.Dos Santos SB, De Melo RPB, Da Silva LTR, et al. Epidemiology of Mycoplasma agalactiae and Mycoplasma mycoides cluster in flocks of northeastern Brazil. Cienc Rural. 2018;48:4. [Google Scholar]
- 51.Hajizadeh A, Ghaderi R, Ayling RD. Species of mycoplasma causing contagious agalactia in small ruminants in northwest Iran. Vet Ital. 2018;54(3):205–210. doi: 10.12834/VetIt.831.4072.2 [DOI] [PubMed] [Google Scholar]
- 52.Matos RAT, Santos SB, Alves RV, et al. Occurrence and risk factors associated with Mycoplasma agalactiae infection in dairy goat herds of Paraíba State, Brazil. Pesqui Vet Bras. 2019;39(2):93–98. doi: 10.1590/1678-5150-pvb-5538 [DOI] [Google Scholar]
- 53.Filioussis G, Giadinis ND, Petridou EJ, Karavanis E, Papageorgiou K, Karatzias H. Congenital polyarthritis in goat kids attributed to Mycoplasma agalactiae. Vet Rec. 2011;169(14):364. doi: 10.1136/vr.d4627 [DOI] [PubMed] [Google Scholar]
- 54.Agnello S, Chetta M, Vicari D, et al. Severe outbreaks of polyarthritis in kids caused by Mycoplasma mycoides subspecies capri in Sicily. Vet Rec. 2012;170(16):416. doi: 10.1136/vr.100481 [DOI] [PubMed] [Google Scholar]
- 55.Johnson GC, Fales WH, Shoemake BM, et al. An outbreak of Mycoplasma mycoides subspecies capri arthritis in young goats: a case study. J Vet Diagn Invest. 2019;31(3):453–457. doi: 10.1177/1040638719835243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hernandez L, Lopez J, St-Jacques M, Ontiveros L, Acosta J, Handel K. Mycoplasma mycoides subsp. capri associated with goat respiratory disease and high flock mortality. Can Vet J. 2006;47(4):366–369. [PMC free article] [PubMed] [Google Scholar]
- 57.Poumarat F, Jarrige N, Tardy F. Purpose and overview of results of the Vigimyc Network for the epidemiological surveillance of mycoplasmoses in ruminants in France. EuroRéférence. 2014;12:24–28. [Google Scholar]
- 58.Shah MK, Saddique U, Ahmad S, et al. Isolation rate and antimicrobial susceptibility profiles of Mycoplasma mycoides subspecies capri field isolates from sheep and goats in Pakistan. Small Rumin Res. 2017;153:118–122. doi: 10.1016/j.smallrumres.2017.06.002 [DOI] [Google Scholar]
- 59.Rahman H, Saddique U, Hassan Z, et al. The predominant incidence of Mycoplasma mycoides subsp. capri in suspected cases of contagious caprine pleuropneumonia in sheep and goats of northern Pakistan. Pak J Zool. 2018;50:50. [Google Scholar]
- 60.Bergonier D, Poumarat F. Contagious agalactia of small ruminants: epidemiology, diagnosis and control. Rev Sci Tech. 1996;15(4):1431–1475. doi: 10.20506/rst.15.4.988 [DOI] [PubMed] [Google Scholar]
- 61.Szeredi L, Tenk M, Dan A. Infection of two goatherds with Mycoplasma mycoides subsp. capri in Hungary, evidence of a possible faecal excretion. J Vet Med B Infect Dis Vet Public Health. 2003;50(4):172–177. doi: 10.1046/j.1439-0450.2003.00654.x [DOI] [PubMed] [Google Scholar]
- 62.Azevedo EOD, Alcântara MDBD, Nascimento ERD, et al. Contagious agalactia by Mycoplasma agalactiae in small ruminants in Brazil: first report. Braz J Microbiol. 2006;37:576–581. doi: 10.1590/S1517-83822006000400033 [DOI] [Google Scholar]
- 63.Rodriguez J, Poveda J, Gutierrez C, Acosta B, Fernandez A. Polyarthritis in kids associated with Mycoplasma putrefaciens. Vet Rec. 1994;135(17):406–407. doi: 10.1136/vr.135.17.406 [DOI] [PubMed] [Google Scholar]
- 64.Bajmocy E, Turcsanyi I, Bolske G, Bacsadi A, Kiss I. Disease caused by Mycoplasma mycoides subspecies mycoides LC in Hungarian goat herds. Acta Vet Hung. 2000;48(3):277–283. doi: 10.1556/AVet.48.2000.3.4 [DOI] [PubMed] [Google Scholar]
- 65.DaMassa AJ, Holmberg CA, Brooks DL. Comparison of caprine mycoplasmosis caused by Mycoplasma capricolum, Mycoplasma mycoides subsp. mycoides, and Mycoplasma putrefaciens. Isr J Med Sci. 1987;23(6):636–640. [PubMed] [Google Scholar]
- 66.DaMassa AJ, Brooks DL, Adler HE. Caprine mycoplasmosis: widespread infection in goats with Mycoplasma mycoides subsp mycoides (Large-Colony type). Am J Vet Res. 1983;44(2):322–325. [PubMed] [Google Scholar]
- 67.Gil MC, Hermoso de Mendoza M, Alonso JM, Rey J, Poveda JB, Hermoso de Mendoza J. Mastitis caused by Mycoplasma mycoides subspecies mycoides (Large Colony Type) in goat flocks in Spain. J Vet Med B Infect Dis Vet Public Health. 1999;46(10):741–743. doi: 10.1046/j.1439-0450.1999.00303.x [DOI] [PubMed] [Google Scholar]
- 68.Kwantes LJ, Harby HAM. Caprine mycoplasmal arthritis in the Sultanate of Oman. Small Rumin Res. 1995;16(3):287–289. doi: 10.1016/0921-4488(95)00619-V [DOI] [Google Scholar]
- 69.Giadinis ND, Petridou EJ, Sofianidis G, et al. Mortality in adult goats attributed to Mycoplasma capricolum subspecies capricolum. Vet Rec. 2008;163(9):278–279. doi: 10.1136/vr.163.9.278 [DOI] [PubMed] [Google Scholar]
- 70.DaMassa AJ, Brooks DL, Holmberg CA, Moe AI. Caprine mycoplasmosis: an outbreak of mastitis and arthritis requiring the destruction of 700 goats. Vet Rec. 1987;120(17):409–413. doi: 10.1136/vr.120.17.409 [DOI] [PubMed] [Google Scholar]
- 71.Mondal D, Srivastava MK. Ovine pneumonia due to Mycoplasma agalactiae. Indian J Anim Sci. 2004;74(3):253–255. [Google Scholar]
- 72.Kidanemariam A, Gouws J, van Vuuren M, Gummow B. Ulcerative balanitis and vulvitis of dorper sheep in South Africa: a study on its aetiology and clinical features. J S Afr Vet Assoc. 2005;76(4):197–203. doi: 10.4102/jsava.v76i4.426 [DOI] [PubMed] [Google Scholar]
- 73.Loria GR, Casalone C, Sparacino L, et al. Mycoplasma agalactiae in sheep brains: a new site of infection? Large Anim Rev. 2007;13:65–68. [Google Scholar]
- 74.Schumacher VL, Hinckley L, Liao X, Tulman E, Geary SJ, Smyth JA. Meningitis caused by Mycoplasma mycoides subspecies capri in a goat. J Vet Diagn Invest. 2011;23(3):565–569. doi: 10.1177/1040638711403413 [DOI] [PubMed] [Google Scholar]
- 75.Rosales RS, Puleio R, Loria GR, Catania S, Nicholas RAJ. Mycoplasmas: brain invaders? Res Vet Sci. 2017;113:56–61. doi: 10.1016/j.rvsc.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 76.Zavagli V. L’agalactie contagieuse des brebis et des chèvres. Bull Off Int Epizoot. 1951;36:336–362. [Google Scholar]
- 77.Levisohn S, Davidson I, Caro Vergara MR, Rapoport E. Use of an ELISA for differential diagnosis of Mycoplasma agalactiae and M. mycoides subspecies mycoides (LC) in naturally infected goat herds. Res Vet Sci. 1991;51(1):66–71. doi: 10.1016/0034-5288(91)90033-K [DOI] [PubMed] [Google Scholar]
- 78.Villalba EJ, Poveda JB, Fernandez A, Rodriguez JL, Gutierrez C, Gomez-Villamandos J. An outbreak caused by Mycoplasma mycoides species in goats in the Canary Islands. Vet Rec. 1992;130(15):330–331. doi: 10.1136/vr.130.15.330 [DOI] [PubMed] [Google Scholar]
- 79.Castro-Alonso A, Rodríguez F, De la Fé C, et al. Correlating the immune response with the clinical–pathological course of persistent mastitis experimentally induced by Mycoplasma agalactiae in dairy goats. Res Vet Sci. 2009;86(2):274–280. doi: 10.1016/j.rvsc.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 80.Todaro M, Puleio R, Sabelli C, Scatassa ML, Console A, Loria GR. Determination of milk production losses in Valle del Belice sheep following experimental infection of Mycoplasma agalactiae. Small Rumin Res. 2015;123(1):167–172. doi: 10.1016/j.smallrumres.2014.10.005 [DOI] [Google Scholar]
- 81.Kheirabadi KH, Ebrahimi A. Investigation of Mycoplasma agalactiae in milk and conjunctival swab samples from sheep flocks in west central, Iran. Pak J Biol Sci. 2007;10(8):1346–1348. doi: 10.3923/pjbs.2007.1346.1348 [DOI] [PubMed] [Google Scholar]
- 82.Tardy F, Mercier P, Solsona M, Saras E, Poumarat F. Mycoplasma mycoides subsp. mycoides biotype large colony isolates from healthy and diseased goats: prevalence and typing. Vet Microbiol. 2007;121(3–4):268–277. doi: 10.1016/j.vetmic.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 83.Ariza-Miguel J, Rodriguez-Lazaro D, Hernandez M. A survey of Mycoplasma agalactiae in dairy sheep farms in Spain. BMC Vet Res. 2012;8:171. doi: 10.1186/1746-6148-8-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Olaogun OM, Kanci A, Barber SR, et al. Survey of Victorian small ruminant herds for mycoplasmas associated with contagious agalactia and molecular characterisation of Mycoplasma mycoides subspecies capri isolates from one herd. Aust Vet J. 2017;95(10):392–400. doi: 10.1111/avj.2017.95.issue-10 [DOI] [PubMed] [Google Scholar]
- 85.Tardy F, Treilles M, Gay E, et al. Contagious agalactia monitoring in caprine herds through regular bulk tank milk sampling. J Dairy Sci. 2019;102(6):5379–5388. doi: 10.3168/jds.2018-15889 [DOI] [PubMed] [Google Scholar]
- 86.Tardy F, Maigre L, Tricot A, Poumarat F, Nguyen L, Le Grand D. Comparison of isolates of Mycoplasma mycoides subspecies capri from asymptomatic and septicaemic goats. J Comp Pathol. 2011;144(1):70–77. doi: 10.1016/j.jcpa.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 87.Haapala V, Pohjanvirta T, Vahanikkila N, et al. Semen as a source of Mycoplasma bovis mastitis in dairy herds. Vet Microbiol. 2018;216:60–66. doi: 10.1016/j.vetmic.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 88.De la Fe C, Amores J, Martín AG, Sánchez A, Contreras A, Corrales JC. Mycoplasma agalactiae detected in the semen of goat bucks. Theriogenology. 2009;72(9):1278–1281. doi: 10.1016/j.theriogenology.2009.07.024 [DOI] [PubMed] [Google Scholar]
- 89.De la Fe C, Martín AG, Amores J, et al. Latent infection of male goats with Mycoplasma agalactiae and Mycoplasma mycoides subspecies capri at an artificial insemination centre. Vet J. 2010;186(1):113–115. doi: 10.1016/j.tvjl.2009.07.010 [DOI] [PubMed] [Google Scholar]
- 90.Amores J, Gómez-Martín A, Corrales JC, Sánchez A, Contreras A, De la Fe C. Presence of contagious agalactia causing mycoplasmas in Spanish goat artificial insemination centres. Theriogenology. 2011;75(7):1265–1270. doi: 10.1016/j.theriogenology.2010.11.040 [DOI] [PubMed] [Google Scholar]
- 91.Gómez-Martín A, Corrales JC, Amores J, et al. Controlling contagious agalactia in artificial insemination centers for goats and detection of Mycoplasma mycoides subspecies capri in semen. Theriogenology. 2012;77(6):1252–1256. doi: 10.1016/j.theriogenology.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 92.Gómez-Martín A, Uc N, Vieira LA, et al. Survival capacity of Mycoplasma agalactiae and Mycoplasma mycoides subsp capri in the diluted semen of goat bucks and their effects on sperm quality. Theriogenology. 2015;83(5):911–919. doi: 10.1016/j.theriogenology.2014.11.029 [DOI] [PubMed] [Google Scholar]
- 93.Paterna A, Gómez-Martín Á, Van Der Ham MP, et al. Mycoplasma excretion in reproductive male and female goats. Jpn J Vet Res. 2017;65(2):83–88. [Google Scholar]
- 94.Pourbakhsh SA, Abtin A, Ashtari A, Kheirkhah B, Bayatzadeh MA, Ahangaran S. Isolation and detection of Mycoplasma agalactiae from semen samples of goats. Arch Razi Inst. 2017;72(3):159–164. doi: 10.22092/ari.2017.111610 [DOI] [PubMed] [Google Scholar]
- 95.Gregory L, Rizzo H, Gaeta NC, et al. Interference of Mycoplasma spp. or Ureaplasma spp. in ovine semen quality. J Microbiol Res. 2012;2(5):118–122. doi: 10.5923/j.microbiology.20120205.01 [DOI] [Google Scholar]
- 96.Prats-van der Ham M, Tatay-Dualde J, de la Fe C, et al. Detecting asymptomatic rams infected with Mycoplasma agalactiae in ovine artificial insemination centers. Theriogenology. 2017;89:324–328. doi: 10.1016/j.theriogenology.2016.09.014 [DOI] [PubMed] [Google Scholar]
- 97.Bergonier D, Crémoux R, Rupp R, Lagriffoul G, Berthelot X. Mastitis of dairy small ruminants. Vet Res. 2003;34(5):689–716. doi: 10.1051/vetres:2003030 [DOI] [PubMed] [Google Scholar]
- 98.Crawford TB, Adams DS. Caprine arthritis-encephalitis: clinical features and presence of antibody in selected goat populations. J Am Vet Med Assoc. 1981;178(7):713–719. [PubMed] [Google Scholar]
- 99.Chakraborty S, Kumar A, Tiwari R, et al. Advances in diagnosis of respiratory diseases of small ruminants. Vet Med Int. 2014;2014:508304. doi: 10.1155/2014/508304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nicholas RAJ, Ayling RD, Loria GR. Ovine mycoplasmal infections. Small Rumin Res. 2008;76(1):92–98. doi: 10.1016/j.smallrumres.2007.12.014 [DOI] [Google Scholar]
- 101.Rifatbegovic M, Maksimovic Z, Hulaj B. Mycoplasma ovipneumoniae associated with severe respiratory disease in goats. Vet Rec. 2011;168(21):565. doi: 10.1136/vr.d886 [DOI] [PubMed] [Google Scholar]
- 102.Contreras A, Miranda RE, Sánchez A, et al. Presence of Mycoplasma species and somatic cell counts in bulk-tank goat milk. Small Rumin Res. 2008;75(2):247–251. doi: 10.1016/j.smallrumres.2007.11.007 [DOI] [Google Scholar]
- 103.Tolone M, Sutera AM, Borrello S, et al. Effect of Mycoplasma agalactiae mastitis on milk production and composition in Valle dell Belice dairy sheep. Ital J Anim Sci. 2019;18(1):1067–1072. doi: 10.1080/1828051X.2019.1617044 [DOI] [Google Scholar]
- 104.Corrales JC, Sanchez A, Luengo C, Poveda JB, Contreras A. Effect of clinical contagious agalactia on the bulk tank milk somatic cell count in Murciano-Granadina goat herds. J Dairy Sci. 2004;87(10):3165–3171. doi: 10.3168/jds.S0022-0302(04)73451-7 [DOI] [PubMed] [Google Scholar]
- 105.Gonzalo C, Carriedo JA, Blanco MA, et al. Factors of variation influencing bulk tank somatic cell count in dairy sheep. J Dairy Sci. 2005;88(3):969–974. doi: 10.3168/jds.S0022-0302(05)72764-8 [DOI] [PubMed] [Google Scholar]
- 106.Gelasakis AI, Angelidis A, Giannakou R, Arsenos G. Bacterial subclinical mastitis and its effect on milk quality traits in low-input dairy goat herds. Vet Rec. 2018;183(14):449. doi: 10.1136/vr.104804 [DOI] [PubMed] [Google Scholar]
- 107.Marogna G, Rolesu S, Lollai S, Tola S, Leori G. Clinical findings in sheep farms affected by recurrent bacterial mastitis. Small Rumin Res. 2010;88(2):119–125. doi: 10.1016/j.smallrumres.2009.12.019 [DOI] [Google Scholar]
- 108.Tatay-Dualde J, Sánchez A, Prats-van der Ham M, et al. Sensitivity of two methods to detect Mycoplasma agalactiae in goat milk. Ir Vet J. 2015;68(1):21. doi: 10.1186/s13620-015-0049-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cottew GS, Yeats FR. Occurrence of mycoplasmas in clinically normal goats. Aust Vet J. 1981;57(1):52–53. doi: 10.1111/j.1751-0813.1981.tb07094.x [DOI] [PubMed] [Google Scholar]
- 110.Cottew GS, Yeats FR. Mycoplasmas and mites in the ears of clinically normal goats. Aust Vet J. 1982;59(3):77–81. doi: 10.1111/j.1751-0813.1982.tb02731.x [DOI] [PubMed] [Google Scholar]
- 111.Gil MC, Hermoso De Mendoza M, Rey J, et al. Isolation of mycoplasmas from the external ear canal of goats affected with contagious agalactia. Vet J. 1999;158(2):152–154. doi: 10.1053/tvjl.1999.0383 [DOI] [PubMed] [Google Scholar]
- 112.Pereyre S, Tardy F, Renaudin H, et al. Identification and subtyping of clinically relevant human and ruminant mycoplasmas by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2013;51(10):3314–3323. doi: 10.1128/JCM.01573-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Becker CA, Ramos F, Sellal E, Moine S, Poumarat F, Tardy F. Development of a multiplex real-time PCR for contagious agalactia diagnosis in small ruminants. J Microbiol Methods. 2012;90(2):73–79. doi: 10.1016/j.mimet.2012.04.020 [DOI] [PubMed] [Google Scholar]
- 114.Wong YP, Othman S, Lau YL, Radu S, Chee HY. Loop-mediated isothermal amplification (LAMP): a versatile technique for detection of micro-organisms. J Appl Microbiol. 2018;124(3):626–643. doi: 10.1111/jam.13647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rekha V, Rana R, Thomas P, et al. Development of loop-mediated isothermal amplification test for the diagnosis of contagious agalactia in goats. Trop Anim Health Prod. 2015;47(3):581–587. doi: 10.1007/s11250-015-0767-x [DOI] [PubMed] [Google Scholar]
- 116.Loria GR, Puleio R, Nicholas R, Arcoleo G, Drago C. A novel LAMP field test for the diagnosis of contagious agalactia caused by Mycoplasma. agalactiae. Anim Husb Dairy Vet Sci. 2018;2(3):1076. [Google Scholar]
- 117.Assunção P, De la Fe C, Ramirez AS, Poveda JB, Andrada M. Serological study of contagious agalactia in herds of goats in the Canary Islands. Vet Rec. 2004;154(22):684–687. doi: 10.1136/vr.154.22.684 [DOI] [PubMed] [Google Scholar]
- 118.Al-Momani W, Nicholas RA, Abo-Shehada MN. Risk factors associated with Mycoplasma agalactiae infection of small ruminants in northern Jordan. Prev Vet Med. 2008;83(1):1–10. doi: 10.1016/j.prevetmed.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 119.Lambert M, Calamel M, Dufour P, Cabasse E, Vitu C, Pepin V. Detection of false-positive sera in contagious agalactia with a multiantigen ELISA and their elimination with a protein G conjugate. J Vet Diagn Invest. 1998;10(4):326–330. doi: 10.1177/104063879801000403 [DOI] [PubMed] [Google Scholar]
- 120.Poumarat F, Le Grand D, Gaurivaud P, et al. Comparative assessment of two commonly used commercial ELISA tests for the serological diagnosis of contagious agalactia of small ruminants caused by Mycoplasma agalactiae. BMC Vet Res. 2012;8:109. doi: 10.1186/1746-6148-8-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hasso SA, Al-Omran AH. Antibody response patterns in goats experimentally infected with Mycoplasma agalactiae. Small Rumin Res. 1994;14(1):79–81. doi: 10.1016/0921-4488(94)90014-0 [DOI] [Google Scholar]
- 122.Nicholas RAJ, Ayling RD, McAuliffe L. Antigenic Analysis of Mycoplasmas In: Mycoplasma Diseases of Ruminants. Oxfordshire, UK: CAB International; 2008:98–113. [Google Scholar]
- 123.Arun TR, Rana R, Singh P, et al. Development of a gold nanoparticle based lateral flow assay for rapid diagnosis of contagious agalactia in goats. Asian J Anim Vet Adv. 2014;9:405–413. doi: 10.3923/ajava.2014.405.413 [DOI] [Google Scholar]
- 124.OIE. Animal health information WAHIS interface; 2005–2019. Available from: https://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/Diseasetimelines/index/newlang/en?header_disease_type_hidden=0&header_disease_id_hidden=0&header_selected_disease_name_hidden=0&header_disease_type=0&header_disease_id_terrestrial=47&header_disease_id_aquatic=−999&header_firstyear=2005&header_lastyear=2019. Accessed November 26, 2019.
- 125.Hosein Abadi E, Saadati D, Najimi M, Hassanpour M. A Study on Mycoplasma agalactiae and chlamydophila abortus in aborted ovine fetuses in Sistan and Baluchestan region, Iran. Arch Razi Inst. 2019;74(3):295–301. doi: 10.22092/ari.2018.120393.1193 [DOI] [PubMed] [Google Scholar]
- 126.Verbisck G, Gonzalez-Candela M, Cubero MJ, Leon L, Serrano E, Perales A. Mycoplasma agalactiae in Iberian ibex (Capra pyrenaica) in Spain. Vet Rec. 2010;167(11):425–426. doi: 10.1136/vr.c4908 [DOI] [PubMed] [Google Scholar]
- 127.Cokrevski S, Crcev D, Loria GR, Nicholas RAJ. Outbreaks of contagious agalactia in small ruminants in the Republic of Macedonia. Vet Rec. 2001;148(21):667. doi: 10.1136/vr.148.21.667 [DOI] [PubMed] [Google Scholar]
- 128.Hanedan B, Kirbas A, Kandemir FM, Aktas MS, Yildiz A. Evaluation of arginase activity, nitric oxide and oxidative stress status in sheep with contagious agalactia. Acta Vet Hung. 2017;65(3):394–401. doi: 10.1556/004.2017.037 [DOI] [PubMed] [Google Scholar]
- 129.World Organisation for Animal Health (OIE). Available from: https://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/Diseasedistributionmap/index/newlang/en?disease_type_hidden=0&disease_id_hidden=47&selected_disease_name_hidden=Contagious+agalactia+%28-+-%29+&disease_type=0&disease_id_terrestrial=47&species_t=0&disease_id_aquatic=-999&species_a=0&sta_method=semesterly&selected_start_year=2014&selected_report_period=1&selected_start_month=1&date_submit=OK. Accessed September 9, 2019.
- 130.Loria GR, Nicholas RA. Contagious agalactia: the shepherd’s nightmare. Vet J. 2013;198(1):5–6. doi: 10.1016/j.tvjl.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 131.Fusco M, Corona L, Onni T, et al. Development of a sensitive and specific enzyme-linked immunosorbent assay based on recombinant antigens for rapid detection of antibodies against Mycoplasma agalactiae in sheep. Clin Vaccine Immunol. 2007;14(4):420–425. doi: 10.1128/CVI.00439-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Loria GR, Puleio R, Agnello S, Marogna G, Nicholas RAJ. Can vaccines for contagious agalactia reduce disease progression in infected animals: a preliminary study? Vet Rec Case Rep. 2018;6:4. doi: 10.1136/vetreccr-2018-000715 [DOI] [Google Scholar]
- 133.Corona L, Cillara G, Tola S. Proteomic approach for identification of immunogenic proteins of Mycoplasma mycoides subsp. capri. Vet Microbiol. 2013;167(3):434–439. doi: 10.1016/j.vetmic.2013.08.024 [DOI] [PubMed] [Google Scholar]
- 134.Cillara G, Manca MG, Longheu C, Tola S. Discrimination between Mycoplasma mycoides subsp. capri and Mycoplasma capricolum subsp. capricolum using PCR-RFLP and PCR. Vet J. 2015;205(3):421–423. doi: 10.1016/j.tvjl.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 135.Carta T, Mannu F, Fadda M, et al. Development of a real-time PCR for detection of Mycoplasma agalactiae in bulk tank milk samples and epidemiology of infection in Sardinia [Conference poster]. XVII Congresso Nazionale S.I.Di.L.V.; September 28–30, 2016; Parma. [Google Scholar]
- 136.Filioussis G, Ioannou I, Petridou E, Avraam M, Giadinis ND, Kritas SK. Isolation and analysis of tetracycline-resistant Mycoplasma agalactiae strains from an infected goat herd in Cyprus. Acta Vet Hung. 2013;61(3):291–296. doi: 10.1556/AVet.2013.018 [DOI] [PubMed] [Google Scholar]
- 137.Filioussis G, Petridou E, Giadinis ND, Kritas SK. In vitro susceptibilities of caprine Mycoplasma agalactiae field isolates to six antimicrobial agents using the E test methodology. Vet J. 2014;202(3):654–656. doi: 10.1016/j.tvjl.2014.08.024 [DOI] [PubMed] [Google Scholar]
- 138.Lo WS, Gasparich GE, Kuo CH. Convergent evolution among ruminant-pathogenic mycoplasma involved extensive gene content changes. Genome Biol Evol. 2018;10(8):2130–2139. doi: 10.1093/gbe/evy172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brown DR, Whitcomb RF, Bradbury JM. Revised minimal standards for description of new species of the class mollicutes (division Tenericutes). Int J Syst Evol Microbiol. 2007;57(Pt 11):2703–2719. doi: 10.1099/ijs.0.64722-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yus E, Maier T, Michalodimitrakis K, et al. Impact of genome reduction on bacterial metabolism and its regulation. Science. 2009;326(5957):1263–1268. doi: 10.1126/science.1177263 [DOI] [PubMed] [Google Scholar]
- 141.Askaa G, Erno H. Elevation of Mycoplasma agalactiae subsp. bovis to Species Rank: Mycoplasma bovis (Hale et al.) comb. nov. Int J Syst Bacteriol. 1976;26(3):323–325. doi: 10.1099/00207713-26-3-323 [DOI] [Google Scholar]
- 142.Ambroset C, Pau-Roblot C, Game Y, Gaurivaud P, Tardy F. Identification and characterization of mycoplasma feriruminatoris sp. nov. Strains isolated from alpine ibex: a 4th Species in the Mycoplasma mycoides cluster hosted by non-domesticated ruminants? Front Microbiol. 2017;8:939. doi: 10.3389/fmicb.2017.00939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Breton M, Tardy F, Dordet-Frisoni E, et al. Distribution and diversity of mycoplasma plasmids: lessons from cryptic genetic elements. BMC Microbiol. 2012;12:257. doi: 10.1186/1471-2180-12-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sirand-Pugnet P, Lartigue C, Marenda M, et al. Being pathogenic, plastic, and sexual while living with a nearly minimal bacterial genome. PLoS Genet. 2007;3(5):e75. doi: 10.1371/journal.pgen.0030075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pereyre S, Sirand-Pugnet P, Beven L, et al. Life on arginine for Mycoplasma hominis: clues from its minimal genome and comparison with other human urogenital mycoplasmas. PLoS Genet. 2009;5(10):e1000677. doi: 10.1371/journal.pgen.1000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dordet Frisoni E, Marenda MS, Sagne E, et al. ICEA of Mycoplasma agalactiae: a new family of self-transmissible integrative elements that confers conjugative properties to the recipient strain. Mol Microbiol. 2013;89(6):1226–1239. doi: 10.1111/mmi.2013.89.issue-6 [DOI] [PubMed] [Google Scholar]
- 147.Dordet-Frisoni E, Sagne E, Baranowski E, et al. Chromosomal transfers in mycoplasmas: when minimal genomes go mobile. MBio. 2014;5(6). doi: 10.1128/mBio.01958-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Citti C, Dordet-Frisoni E, Nouvel LX, Kuo CH, Baranowski E. Horizontal gene transfers in Mycoplasmas (Mollicutes). Curr Issues Mol Biol. 2018;29:3–22. doi: 10.21775/cimb.029.003 [DOI] [PubMed] [Google Scholar]
- 149.Faucher M, Nouvel L-X, Dordet-Frisoni E, et al. Mycoplasmas under experimental antimicrobial selection: the unpredicted contribution of horizontal chromosomal transfer. PLoS Genet. 2019;15(1):e1007910. doi: 10.1371/journal.pgen.1007910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Calcutt MJ, Foecking MF. Genome sequence of Mycoplasma putrefaciens type strain KS1. J Bacteriol. 2011;193(21):6094. doi: 10.1128/JB.06051-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tardy F, Mick V, Dordet-Frisoni E, et al. Integrative conjugative elements are widespread in field isolates of Mycoplasma species pathogenic for ruminants. Appl Environ Microbiol. 2015;81(5):1634–1643. doi: 10.1128/AEM.03723-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Poumarat F, Gautier-Bouchardon AV, Bergonier D, Gay E, Tardy F. Diversity and variation in antimicrobial susceptibility patterns over time in Mycoplasma agalactiae isolates collected from sheep and goats in France. J Appl Microbiol. 2016;120(5):1208–1218. doi: 10.1111/jam.2016.120.issue-5 [DOI] [PubMed] [Google Scholar]
- 153.Tola S, Idini G, Manunta D, et al. Comparison of Mycoplasma agalactiae isolates by pulsed field gel electrophoresis, SDS-PAGE and immunoblotting. FEMS Microbiol Lett. 1996;143(2–3):259–265. doi: 10.1111/fml.1996.143.issue-2-3 [DOI] [PubMed] [Google Scholar]
- 154.Nouvel L-X, Marenda MS, Glew MD, et al. Molecular typing of Mycoplasma agalactiae: tracing European-wide genetic diversity and an endemic clonal population. Comp Immunol Microbiol Infect Dis. 2012;35(5):487–496. doi: 10.1016/j.cimid.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 155.Ariza-Miguel J, Rodriguez-Lazaro D, Hernandez M. Molecular characterization of Mycoplasma agalactiae reveals the presence of an endemic clone in Spain. J Clin Microbiol. 2013;51(2):656–660. doi: 10.1128/JCM.02835-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.De la Fe C, Amores J, Tardy F, Sagne E, Nouvel LX, Citti C. Unexpected genetic diversity of Mycoplasma agalactiae caprine isolates from an endemic geographically restricted area of Spain. BMC Vet Res. 2012;8:146. doi: 10.1186/1746-6148-8-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pilo P, Fleury B, Marenda M, Frey J, Vilei EM. Prevalence and distribution of the insertion element ISMag1 in Mycoplasma agalactiae. Vet Microbiol. 2003;92(1–2):37–48. doi: 10.1016/S0378-1135(02)00311-5 [DOI] [PubMed] [Google Scholar]
- 158.McAuliffe L, Churchward CP, Lawes JR, Loria G, Ayling RD, Nicholas RA. VNTR analysis reveals unexpected genetic diversity within Mycoplasma agalactiae, the main causative agent of contagious agalactia. BMC Microbiol. 2008;8:193. doi: 10.1186/1471-2180-8-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tatay-Dualde J, Prats-Van Der Ham M, De La Fe C, et al. Antimicrobial susceptibility and multilocus sequence typing of Mycoplasma capricolum subsp. capricolum. PLoS One. 2017;12:3. doi: 10.1371/journal.pone.0174700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ghanem M, El-Gazzar M. Development of Mycoplasma synoviae (MS) core genome multilocus sequence typing (cgMLST) scheme. Vet Microbiol. 2018;218:84–89. doi: 10.1016/j.vetmic.2018.03.021 [DOI] [PubMed] [Google Scholar]
- 161.Delaney NF, Balenger S, Bonneaud C, et al. Ultrafast evolution and loss of CRISPRs following a host shift in a novel wildlife pathogen, Mycoplasma gallisepticum. PLoS Genet. 2012;8(2). doi: 10.1371/annotation/b5608bc6-aa54-40a7-b246-51fa7bc4a9db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marenda MS, Sagne E, Poumarat F, Citti C. Suppression subtractive hybridization as a basis to assess Mycoplasma agalactiae and Mycoplasma bovis genomic diversity and species-specific sequences. Microbiology. 2005;151(2):475–489. doi: 10.1099/mic.0.27590-0 [DOI] [PubMed] [Google Scholar]
- 163.Browning GF, Noormohammadi AH, Markham PF. Identification and characterization of virulence Genes in Mycoplasmas In: Browning GF, Citti C, editors. Mollicutes: Molecular Biology and Pathogenesis. Caister Academic Press; 2014:77–90. [Google Scholar]
- 164.Vogl G, Plaickner A, Szathmary S, Stipkovits L, Rosengarten R, Szostak MP. Mycoplasma gallisepticum invades chicken erythrocytes during infection. Infect Immun. 2008;76(1):71–77. doi: 10.1128/IAI.00871-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Raymond BBA, Turnbull L, Jenkins C, et al. Mycoplasma hyopneumoniae resides intracellularly within porcine epithelial cells. Sci Rep. 2018;8(1):17697. doi: 10.1038/s41598-018-36054-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Citti C, Nouvel LX, Baranowski E. Phase and antigenic variation in mycoplasmas. Future Microbiol. 2010;5(7):1073–1085. doi: 10.2217/fmb.10.71 [DOI] [PubMed] [Google Scholar]
- 167.Gaurivaud P, Ganter S, Villard A, et al. Mycoplasmas are no exception to extracellular vesicles release: revisiting old concepts. PLoS One. 2018;13(11):e0208160. doi: 10.1371/journal.pone.0208160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Baranowski E, Bergonier D, Sagné E, et al. Experimental infections with Mycoplasma agalactiae identify key factors involved in host-colonization. PLoS One. 2014;9(4):e93970. doi: 10.1371/journal.pone.0093970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hegde S, Zimmermann M, Rosengarten R, Chopra-Dewasthaly R. Novel role of Vpmas as major adhesins of Mycoplasma agalactiae mediating differential cell adhesion and invasion of Vpma expression variants. Int J Med Microbiol. 2018;308(2):263–270. doi: 10.1016/j.ijmm.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 170.Fleury B, Bergonier D, Berthelot X, Peterhans E, Frey J, Vilei EM. Characterization of P40, a cytadhesin of Mycoplasma agalactiae. Infect Immun. 2002;70(10):5612–5621. doi: 10.1128/IAI.70.10.5612-5621.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Arfi Y, Minder L, Di Primo C, et al. MIB-MIP is a mycoplasma system that captures and cleaves immunoglobulin G. Proc Natl Acad Sci USA. 2016;113(19):5406–5411. doi: 10.1073/pnas.1600546113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lartigue C, Vashee S, Algire MA, et al. Creating bacterial strains from genomes that have been cloned and engineered in yeast. Science. 2009;325(5948):1693–1696. doi: 10.1126/science.1173759 [DOI] [PubMed] [Google Scholar]
- 173.Gibson DG, Glass JI, Lartigue C, et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329(5987):52–56. doi: 10.1126/science.1190719 [DOI] [PubMed] [Google Scholar]
- 174.Jores J, Ma L, Ssajjakambwe P, et al. Removal of a subset of non-essential genes fully attenuates a highly virulent Mycoplasma strain. Front Microbiol. 2019;10:664. doi: 10.3389/fmicb.2019.00664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Caramelli M, Ru G, Casalone C, et al. Evidence for the transmission of scrapie to sheep and goats from a vaccine against Mycoplasma agalactiae. Vet Rec. 2001;148(17):531–536. doi: 10.1136/vr.148.17.531 [DOI] [PubMed] [Google Scholar]
- 176.León Vizcaíno L, Garrido Abellán F, Cubero Pablo MJ, Perales A. Immunoprophylaxis of caprine contagious agalactia due to Mycoplasma agalactiae with an inactivated vaccine. Vet Rec. 1995;137(11):266–269. doi: 10.1136/vr.137.11.266 [DOI] [PubMed] [Google Scholar]
- 177.Buonavoglia D, Fasanella A, Sagazio P, Tempesta M, Iovane G, Buonavoglia C. Persistence of antibodies to Mycoplasma agalactiae in vaccinated sheep. New Microbiol. 1998;21(2):209–212. [PubMed] [Google Scholar]
- 178.Tola S, Manunta D, Rocca S, et al. Experimental vaccination against Mycoplasma agalactiae using different inactivated vaccines. Vaccine. 1999;17(22):2764–2768. doi: 10.1016/S0264-410X(99)00070-5 [DOI] [PubMed] [Google Scholar]
- 179.Greco G, Corrente M, Buonavoglia D, Aliberti A, Fasanella A. Inactivated vaccine induces protection against Mycoplasma agalactiae infection in sheep. New Microbiol. 2002;25(1):17–20. [PubMed] [Google Scholar]
- 180.De La Fe C, Assunção P, Saavedra P, Tola S, Poveda C, Poveda JB. Field trial of two dual vaccines against Mycoplasma agalactiae and Mycoplasma mycoides subsp. mycoides (large colony type) in goats. Vaccine. 2007;25(12):2340–2345. doi: 10.1016/j.vaccine.2006.11.050 [DOI] [PubMed] [Google Scholar]
- 181.Buonavoglia D, Greco G, Quaranta V, Corrente M, Martella V, Decaro N. An oil-emulsion vaccine induces full-protection against Mycoplasma agalactiae infection in sheep. New Microbiol. 2008;31(1):117–123. [PubMed] [Google Scholar]
- 182.Buonavoglia D, Greco G, Corrente M, et al. Long-term immunogenicity and protection against Mycoplasma agalactiae induced by an oil adjuvant vaccine in sheep. Res Vet Sci. 2010;88(1):16–19. doi: 10.1016/j.rvsc.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 183.Abo El-Yazid H, Soliman R, Wassif I, et al. Protective efficacy of the inactivated adjuvant vaccines against Mycoplasma agalactiae infection in goats. Int J Vet Sci Med. 2018;8:14–19. [Google Scholar]
- 184.Agnone A, Manna M, Sireci G, et al. A comparison of the efficacy of commercial and experimental vaccines for contagious agalactia in sheep. Small Rumin Res. 2013;112:230–234. doi: 10.1016/j.smallrumres.2012.12.022 [DOI] [Google Scholar]
- 185.Lacasta D, Ferrer LM, Ramos JJ, Gonzalez JM, Ortin A, Fthenakis GC. Vaccination schedules in small ruminant farms. Vet Microbiol. 2015;181(1–2):34–46. doi: 10.1016/j.vetmic.2015.07.018 [DOI] [PubMed] [Google Scholar]
- 186.Kabiri K, Pourbakhsh SA, Norouzi J, Sekhavati M, Tadayon K. Homogeneity of Mycoplasma agalactiae vaccine strains in an agalaxy- high-burden environment. Iran J Microbiol. 2019;11(1):48–54. [PMC free article] [PubMed] [Google Scholar]
- 187.Campos AC, Azevedo EO, Alcântara MDB, et al. Efficiency of inactive vaccines against contagious agalactia in Brazil. Arq Bras Med Vet Zootec. 2013;65:1394–1402. doi: 10.1590/S0102-09352013000500018 [DOI] [Google Scholar]
- 188.Sunder J, Srivastava NC, Singh VP. Preliminary trials on development of vaccine against Mycoplasma mycoides subsp. mycoides type LC infection in goats. J Appl Anim Res. 2002;21(1):75–80. doi: 10.1080/09712119.2002.9706359 [DOI] [Google Scholar]
- 189.Ramirez AS, De La Fe C, Assunçao P, Gonzales M, Poveda C. Preparation and evaluation of an invactivated polyvalent vaccine against Mycoplasma spp. on infected goats In: Eur 19693–COST Action 826 Mycoplasmas of Ruminants : Pathogenicity, Diagnostics, Epidemiology and Molecular Genetics. Brussels: European Commission; 2001:154–157. [Google Scholar]
- 190.Marogna G, A. B, A. F, Schianchi G. Produzione, uso ed efficacia sul campo di un vaccino stabulogeno contro Mycoplasma mycoides subsp. capri nelle capre. Large Anim Rev. 2015;Suppl. 1(5):104–106. [Google Scholar]
- 191.Saléry M. Autogenous vaccines in Europe: national approaches to authorisation. Regul Rapporteur. 2017;14(6):27–32. [Google Scholar]
- 192.Gautier-Bouchardon AV, Ferre S, Le Grand D, Paoli A, Gay E, Poumarat F. Overall decrease in the susceptibility of Mycoplasma bovis to antimicrobials over the past 30 years in France. PLoS One. 2014;9(2):e87672. doi: 10.1371/journal.pone.0087672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Antunes NT, Tavio MA, Mercier P, et al. In vitro susceptibilities of Mycoplasma putrefaciens field isolates. Antimicrob Agents Chemother. 2007;51(9):3452–3454. doi: 10.1128/AAC.00420-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Antunes NT, Tavío MM, Assunção P, et al. In vitro susceptibilities of field isolates of Mycoplasma mycoides subsp. mycoides large colony type to 15 antimicrobials. Vet Microbiol. 2007;119(1):72–75. doi: 10.1016/j.vetmic.2006.08.013 [DOI] [PubMed] [Google Scholar]
- 195.Antunes NT, Tavío MM, Assunção P, et al. In vitro susceptibilities of field isolates of Mycoplasma agalactiae. Vet J. 2008;177(3):436–438. doi: 10.1016/j.tvjl.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 196.De Garnica ML, Rosales RS, Gonzalo C, Santos JA, Nicholas RAJ. Isolation, molecular characterization and antimicrobial susceptibilities of isolates of Mycoplasma agalactiae from bulk tank milk in an endemic area of spain. J Appl Microbiol. 2013;114(6):1575–1581. doi: 10.1111/jam.2013.114.issue-6 [DOI] [PubMed] [Google Scholar]
- 197.Paterna A, Tatay-Dualde J, Amores J, et al. In vitro assessment of the antimicrobial susceptibility of caprine isolates of Mycoplasma mycoides subsp. capri. Vet J. 2016;214:96–101. doi: 10.1016/j.tvjl.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 198.Prats-van der Ham M, Tatay-Dualde J, Ambroset C, De la Fe C, Tardy F. The moderate drift towards less tetracycline-susceptible isolates of contagious agalactia causative agents might result from different molecular mechanisms. Vet Microbiol. 2018;220:39–46. doi: 10.1016/j.vetmic.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 199.Paterna A, Sanchez A, Gomez-Martin A, et al. In vitro antimicrobial susceptibility of Mycoplasma agalactiae strains isolated from dairy goats. J Dairy Sci. 2013;96(11):7073–7076. doi: 10.3168/jds.2012-6492 [DOI] [PubMed] [Google Scholar]
- 200.Al-Momani W, Nicholas RAJ, Janakat S, Abu-Basha E, Ayling RD. The in vitro effect of six antimicrobials against Mycoplasma putrefaciens, Mycoplasma mycoides subsp. mycoides LC and Mycoplasma capricolum subsp. capricolum isolated from sheep and goats in Jordan. Trop Anim Health Prod. 2006;38(1):1–7. doi: 10.1007/s11250-006-4334-3 [DOI] [PubMed] [Google Scholar]
- 201.Hannan PC. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. International Research Programme on Comparative Mycoplasmology. Vet Res. 2000;31(4):373–395. doi: 10.1051/vetres:2000100 [DOI] [PubMed] [Google Scholar]
- 202.Gautier-Bouchardon AV. Antimicrobial Resistance in Mycoplasma spp. Microbiol Spectr. 2018;6:4. doi: 10.1128/microbiolspec.ARBA-0030-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Khalil D, Becker CAM, Tardy F. Monitoring the decrease in susceptibility to ribosomal RNAs targeting antimicrobials and its molecular basis in clinical mycoplasma bovis isolates over time. Microb Drug Resist. 2017;23(6):799–811. doi: 10.1089/mdr.2016.0268 [DOI] [PubMed] [Google Scholar]
- 204.Prats-van der Ham M, Tatay-Dualde J, de la Fe C, et al. Molecular resistance mechanisms of Mycoplasma agalactiae to macrolides and lincomycin. Vet Microbiol. 2017;211:135–140. doi: 10.1016/j.vetmic.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 205.Ayling RD, Bisgaard-Frantzen S, March JB, Godinho K, Nicholas RA. Assessing the in vitro effectiveness of antimicrobials against Mycoplasma mycoides subsp. mycoides small-colony type to reduce contagious bovine pleuropneumonia infection. Antimicrob Agents Chemother. 2005;49(12):5162–5165. doi: 10.1128/AAC.49.12.5162-5165.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Prats-van der Ham M, Tatay-Dualde J, Gómez-Martín Á, et al. 23S rRNA and L22 ribosomal protein are involved in the acquisition of macrolide and lincosamide resistance in Mycoplasma capricolum subsp. capricolum. Vet Microbiol. 2018;216:207–211. doi: 10.1016/j.vetmic.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 207.Tatay-Dualde J, Prats-van der Ham M, de la Fe C, et al. Resistance mechanisms against quinolones in Mycoplasma capricolum subsp. capricolum. Vet J. 2017;223:1–4. doi: 10.1016/j.tvjl.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 208.Al-Momani W, Abu-Basha E, Janakat S, Nicholas RAJ, Ayling RD. In vitro antimycoplasmal activity of six Jordanian medicinal plants against three Mycoplasma species. Trop Anim Health Prod. 2007;39(7):515–519. doi: 10.1007/s11250-007-9033-1 [DOI] [PubMed] [Google Scholar]
- 209.Arjoon AV, Saylor CV, May M. In Vitro efficacy of antimicrobial extracts against the atypical ruminant pathogen Mycoplasma mycoides subsp. capri. BMC Complement Altern Med. 2012;12. doi: 10.1186/1472-6882-12-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lopez A, de Tangil MS, Vega-Orellana O, Ramirez AS, Rico M. Phenolic constituents, antioxidant and preliminary antimycoplasmic activities of leaf skin and flowers of Aloe vera (L.) Burm. f. (syn. A. barbadensis Mill.) from the Canary Islands (Spain). Molecules. 2013;18(5):4942–4954. doi: 10.3390/molecules18054942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kama-Kama F, Midiwo J, Nganga J, et al. Selected ethno-medicinal plants from Kenya with in vitro activity against major African livestock pathogens belonging to the “Mycoplasma mycoides cluster”. J Ethnopharmacol. 2016;192:524–534. doi: 10.1016/j.jep.2016.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kama-Kama F, Omosa LK, Nganga J, et al. Antimycoplasmal Activities of Compounds from Solanum aculeastrum and Piliostigma thonningii against Strains from the Mycoplasma mycoides Cluster. Front Pharmacol. 2017;8:920. doi: 10.3389/fphar.2017.00920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shah MK, Sadique U, Ahmad S, et al. Molecular identification and comparative antimycoplasmal activity of three indigenous medicinal plants against Mycoplasma putrefaciens isolated from sheep. J Anim Plant Sci. 2017;27(5):1543–1551. [Google Scholar]
- 214.Silva NS, Marinho ML, Azevedo EO, Campos AC, Carvalho MGX. Allopathic and homeopathic treatment with contagious agalactia in goats: a comparative study. Arch Vet Sc. 2013;18(4):57–64. [Google Scholar]
- 215.Paterna A, Sanchez A, Amores J, et al. Survival of Mycoplasma agalactiae and Mycoplasma mycoides subspecies capri in heat treated goat colostrum. Vet J. 2013;196(2):263–265. doi: 10.1016/j.tvjl.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 216.Justice-Allen A, Trujillo J, Corbett R, Harding R, Goodell G, Wilson D. Survival and replication of Mycoplasma species in recycled bedding sand and association with mastitis on dairy farms in Utah. J Dairy Sci. 2010;93(1):192–202. doi: 10.3168/jds.2009-2474 [DOI] [PubMed] [Google Scholar]
- 217.Eterpi M, McDonnell G, Thomas V. Decontamination efficacy against Mycoplasma. Lett Appl Microbiol. 2011;52(2):150–155. doi: 10.1111/j.1472-765X.2010.02979.x [DOI] [PubMed] [Google Scholar]
- 218.Miranda-de la Lama GC, Mattiello S. The importance of social behaviour for goat welfare in livestock farming. Small Rumin Res. 2010;90(1):1–10. doi: 10.1016/j.smallrumres.2010.01.006 [DOI] [Google Scholar]
- 219.Zobel G, Neave HW, Webster J. Understanding natural behavior to improve dairy goat (Capra hircus) management systems. Transl Anim Sci. 2019;3(1):212–224. doi: 10.1093/tas/txy145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Calcutt MJ, Lysnyansky I, Sachse K, Fox LK, Nicholas RAJ, Ayling RD. Gap analysis of Mycoplasma bovis disease, diagnosis and control: an aid to identify future development requirements. Transbound Emerg Dis. 2018;65(1):91–109. doi: 10.1111/tbed.2018.65.issue-S1 [DOI] [PubMed] [Google Scholar]
- 221.Thiaucourt F, Manso-Silvan L, Salah W. et al. Mycoplasma mycoides, from “mycoides Small Colony” to “capri”. A microevolutionary perspective. BMC Genomics. 2011;12:114. doi: 10.1186/1471-2164-12-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wise KS, Foecking MF, Roske K. et al. Distinctive repertoire of contingency genes conferring mutation- based phase variation and combinatorial expression of surface lipoproteins in Mycoplasma capricolum subsp. capricolum of the Mycoplasma mycoides phylogenetic cluster. J Bacteriol. 2006;188:(13):4926–4941. doi: 10.1128/JB.00252-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- OIE. Animal health information WAHIS interface; 2005–2019. Available from: https://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/Diseasetimelines/index/newlang/en?header_disease_type_hidden=0&header_disease_id_hidden=0&header_selected_disease_name_hidden=0&header_disease_type=0&header_disease_id_terrestrial=47&header_disease_id_aquatic=−999&header_firstyear=2005&header_lastyear=2019. Accessed November 26, 2019.