Abstract
Purpose
Cold therapy on the operated area after surgery is often used as an analgesic and to reduce pain, swelling, and increase range of motion. However, evidence to support the results of cold therapy is still scarce and the mechanism underlying its effectiveness remains unclear. The present study aimed to investigate whether a pleasant sensation evoked by icing the treated knee or a site distant from the treated site (the hand) influenced the acute effect on pain intensity in patients who have undergone total knee arthroplasty (TKA).
Patients and methods
A total of 37 patients with knee OA who underwent TKA were enrolled in this study. This prospective, randomized, cross-over study was performed for 2 days consecutively between days 8 and 15 postoperatively. Cold pack was placed on the anterior surface of the treated knee and palm for 10 mins, respectively. The main primary outcomes were the intensity of knee pain during maximal passive knee flexion.
Results
The two-way ANOVA showed significance only in the main effect of a pleasant sensation (F = 11.3, p = 0.001), but not in the icing site (F = 0.005, p = 0.94) and interaction (F = 0.65, p = 0.42).
Conclusion
This study shows that a pleasant sensation evoked by knee or hand icing influenced the effect on pain intensity during maximal knee flexion in patients after TKA. Even if knee icing has no effect on pain and evokes no pleasant sensation, it may be worthwhile to conduct hand icing to reduce pain.
Keywords: total knee arthroplasty, Icing, pleasant sensation, descending pain inhibition system
Introduction
Total knee arthroplasty (TKA) has played an important role in the management of end-stage knee osteoarthritis (OA).1 Range of motion (ROM) is one of the indicators of successful TKA2 and is directly related to function.3 Knee joint kinematics data have demonstrated that 110° of knee flexion is required to achieve rehabilitation of the knee movement.3 Therefore, early recovery of ROM is crucial after TKA, and adequate analgesia during ROM exercises is necessary to achieve this aim.
Cold therapy on the operated area after surgery is often used as an analgesic4,5 and to reduce pain,6,8,9 swelling,7 and increase ROM.10,11 The effectiveness of analgesia induced by cold therapy, such as cryotherapy, ice pack, and ice massage, has been reported to reduce tissue temperature12,13 and nerve conduction velocity.14–16 However, evidence to support the results of cold therapy is still scarce17,18 and the mechanism underlying its effectiveness remains unclear.
Pleasant sensation such as music,19,20 pictures,21 odors,22 or hypnosis23 have achieved a reduced response to pain stimulation. The effects of such pleasant sensations were partly mediated by activating the descending modulation pain pathway of the spinal cord20,24 and opioid analgesia-related brain regions.25 It is well known that a pleasant sensation is evoked by cooling. The pleasantness of cold stimuli on capsaicin-treated skin has been associated with the activation of the prefrontal cortex and periaqueductal gray matter that constitutes the descending pain inhibition-related area.26 Cold application to the unaffected skin on the contralateral side has been reported to reduce pain induced by capsaicin in healthy volunteers.27,28 These findings might indicate that the efficacy of cooling may be produced via the descending pain inhibition system as well as cooling-mediated decreased peripheral nociceptive input. However, these studies were performed in healthy volunteers; therefore, it is unclear whether the response to cooling may be influenced by the existence of pleasant sensations in patients after surgery. We hypothesized that cold application to a site distant from the treated knee would be as effective for pain reduction as cold application directly to the treated knee and inducing a pleasant sensation would influence the efficacy of pain reduction by icing. We investigated whether a pleasant sensation evoked by icing the treated knee or a site distant from the treated site (the hand) influenced the acute effect on pain intensity during maximal knee flexion in patients who have undergone TKA.
Materials and Methods
Participants
A total of 37 patients with knee OA who underwent TKA were enrolled in a university hospital and a general hospital. For a two-treatment crossover design, we assumed an alpha error of 0.05, a statistical power of 0.95, and a treatment effect of 19.9 mm29 on a 100-mm pain intensity visual analog scale (VAS), with an estimated standard deviation of 20 mm, a within-subject correlation of 0.50, and a necessary sample size of n = 27. As this study was conducted in a clinical setting with more experimental noise than a laboratory setting, we assumed a lower effect size and a need for more participants. Therefore, we decided on n = 37 as the sample size.
The inclusion criteria were patients who underwent primary TKA, ≥40 years of age, and exhibiting ambulation before surgery. Exclusion criteria were revision TKA, nonambulatory before surgery, presence of severe inflammation, and evidence of deep vein thrombosis after surgery.
This study was conducted in compliance with the Declaration of Helsinki. This study followed the CONSORT recommendations concerning randomized trial reporting. This trial is registered with UMIN Clinical Trial Registry: UMIN000024596.
Surgical and Postoperative Procedures
TKA was conducted using a minimally invasive surgical approach with a posterior-stabilized prosthesis. A midline skin incision was made via a medial mini-midvastus arthrotomy, and the patella was subluxated laterally but not everted.
All patients received epidural anesthesia during surgery and patient-controlled epidural analgesia for 48 h postoperatively. Postoperative rehabilitation included strengthening and range of motion exercises and mobilization with a high walker from day 1 postoperatively. Postoperative analgesics were standardized for all patients. Nonsteroidal anti-inflammatory drugs (Loxoprofen sodium) were administered postoperatively twice a day for 4 weeks. The patients were discharged or transferred from the hospital after at least 3 weeks postoperatively. Continuous flow cold compression therapy was provided to all participants using the Icing System CE4000 (Nippon Sigmax Co., Ltd.) from the day of surgery until day 2 postoperatively.
Knee icing was offered to subjects from day 3 to day 7 as usual care.
Intervention
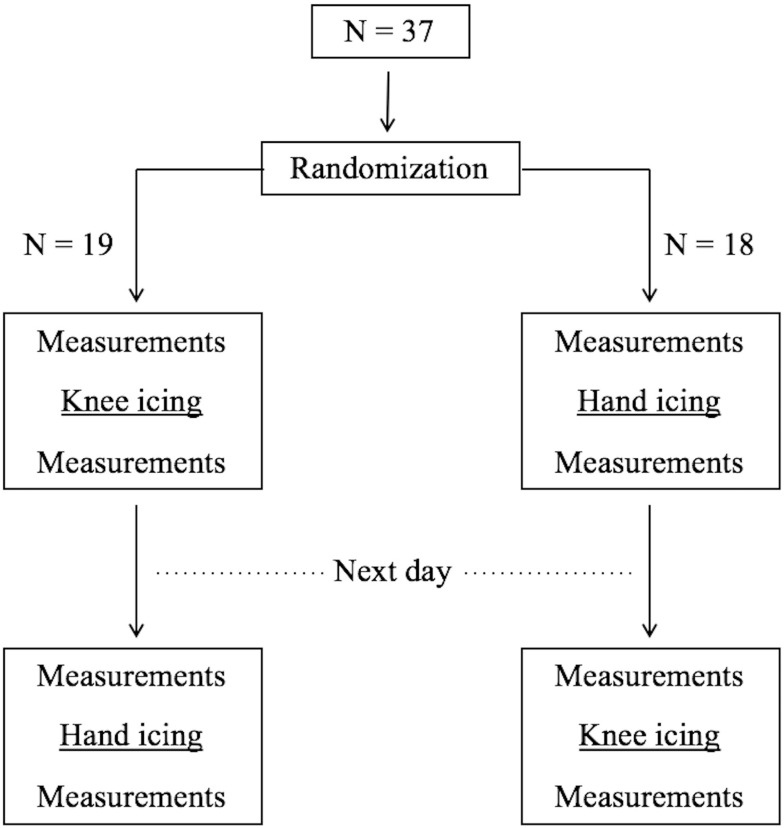
This prospective, randomized, cross-over study was performed for 2 days consecutively between days 8 and 15 postoperatively (Figure 1). This study began 8 days after the operation on the basis of the fact that the effect of inflammation was intense 1 week postoperatively, and the effect of icing could not be adequately evaluated. The order of treatments was randomized using block randomization. The patients were made to rest in the supine position with pillows under their head and knees during both treatments. Icing was conducted using an aqua gel cold pack (Hisamitsu Pharmaceutical Co., Inc. Tokyo, Japan). The cold pack was covered with a cloth to protect the skin from potential thermal damage and placed on the anterior surface of the treated knee or palm on separate days, respectively. The cold pack temperature before treatment was 1–1.5°C. Patients were instructed to apply the cold pack for 10 min.
Figure 1.
Trial profile.
Outcome Measures
All assessments were performed by physical therapists with an average of 7 years of working experience. The primary outcomes were the intensity of knee pain during maximal passive knee flexion that was measured by the physical therapist. The outcome assessor was blind to the intervention assignment. The angle of passive knee flexion was measured using a universal goniometer. Patients were asked about the pain intensity after maintaining maximal knee flexion for 10 s. Pain intensity was measured using VAS, ranging from 0 mm (no pain) to 100 mm (worst pain). Pain intensity was assessed before and after icing. The angle of maximal passive knee flexion after icing was set to the same angle before icing by the same physiotherapist. This method, in which the physiotherapist measured passive knee flexion using a universal goniometer, has high intra-tester and intra-day reliability.30 Change in score induced by icing was calculated by subtracting pain intensity measured before icing from that measured after icing. Immediately after each icing, the patients were asked, “Did you feel a pleasant sensation during icing?” and they were classified into two groups based on whether they did or did not experience a pleasant sensation evoked by icing.
Statistical Analysis
All statistical analyses were performed using the Statistical Package for Social Sciences Version 22 (IBM SPSS Statistics for Windows, Version 22.0; IBM Corp., Armonk, NY, USA). Data distribution was tested for homoscedasticity using the Levene’s test. The differences in knee pain intensity measured before knee and hand icing, after knee and hand icing, and before and after treatment for knee and hand icing were assessed using paired t-tests, respectively. Moreover, the differences in pain intensity score change between knee and hand icing were assessed using paired t-tests. A two-way factorial analysis of variance (ANOVA) was used to evaluate the effect on the icing site (knee, hand) and effect of the sensation (pleasant, no pleasant) in pain intensity score change. The sphericity assumption was analyzed using Mendoza’s multi-sample sphericity test. Value of p <0.05 was considered statistically significant. Effect size of the icing site, sensation, and interaction were calculated on the basis of η2 (a large effect was defined as >0.14, a moderate effect between 0.06 and 0.14, and a small effect <0.06).
Results
Table 1 summarizes the patients’ characteristics. A pleasant sensation was evoked in 24 patients (64.8%) during knee icing and in 26 (70.2%) during hand icing. Eleven patients (29.7%) felt a pleasant sensation during knee icing but no pleasant sensation during hand icing, 13 (35.1%) felt a pleasant sensation during hand icing but no pleasant sensation during knee icing, and 13 (35.1%) felt a pleasant sensation during both knee and hand icing.
Table 1.
Demographic and Clinical Information
| Characteristics | Mean (SD) or N (%) |
|---|---|
| Gender (female) | 32 (86.4%) |
| Age (years) | 75.3 (5.9) |
| Pleasant sensation during knee (number) | 24 (64.8%) |
| Pleasant sensation during hand (number) | 26 (70.2%) |
| ROM before knee icing (degree) | 102.4 (13.1) |
| ROM before hand icing (degree) | 105.0 (12.4) |
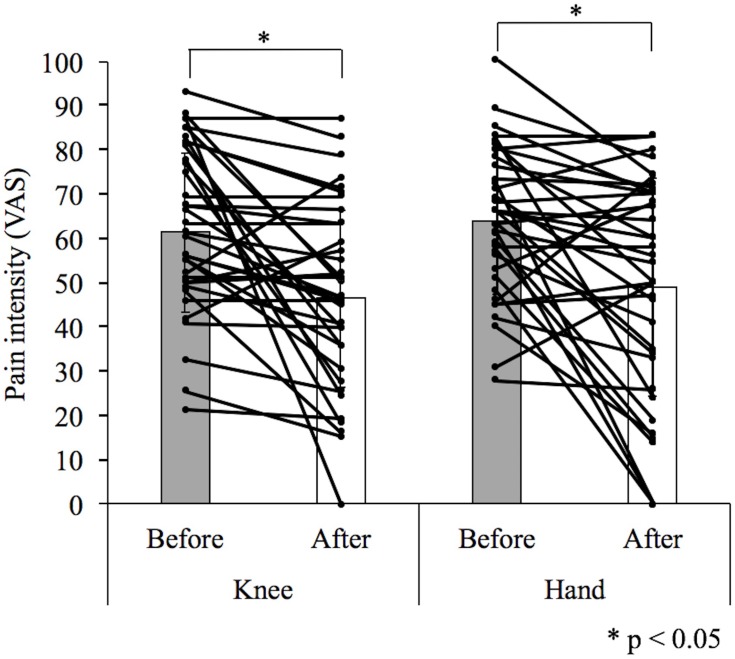
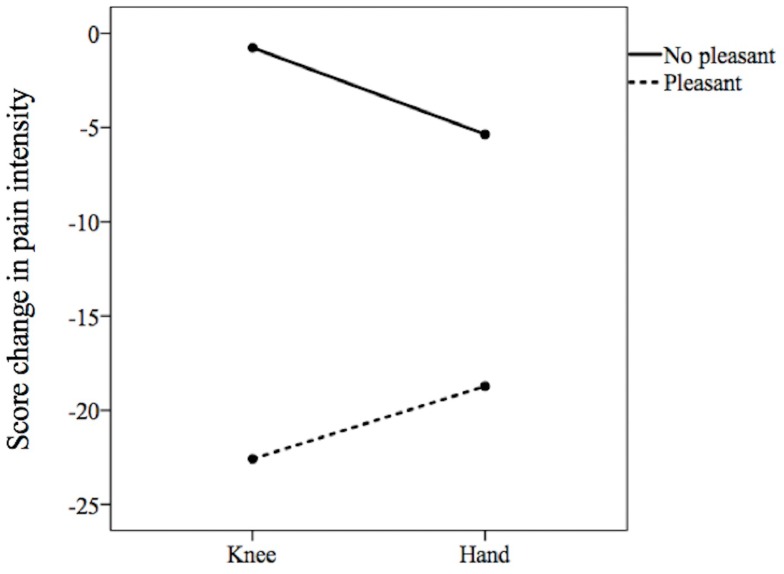
No significant difference was found between pain intensity measured before knee icing (61.3 ± 17.8 mm) and hand icing (63.7 ± 16.1 mm; difference, −2.4; 95% confidence interval [CI], −6.6–1.7; p = 0.25) and between that measured after knee icing (46.4 ± 19.8 mm) and hand icing (48.9 ± 24.6 mm; difference, −2.5; 95% CI, −11.3–6.2; p = 0.55; Table 2). The pain intensity reduced from 61.3 ± 17.8 mm before knee icing to 46.4 ± 19.8 mm after knee icing (difference, 14.9; 95% CI, 7.6–22.2; p = 0.0002) and from 63.7 ± 16.1 mm before hand icing to 48.9 ± 24.6 mm after hand icing (difference, 14.7; 95% CI, 7.1–22.3; P = 0.0003, Figure 2). There was no significant difference in pain intensity score change between knee icing (−14.9 ± 21.7 mm) and hand icing (−14.7 ± 22.4 mm; difference, −0.16; 95% CI, −9.3–9.0; p = 0.97; Table 2). The two-way ANOVA showed significance only in the main effect of a pleasant sensation (F = 11.3, p = 0.001, η2 = 0.14) but not in the icing site (F = 0.005, p = 0.94, η2 = 0.001) and interaction (F = 0.65, p = 0.42, η2 = 0.01, Figure 3). Mendoza’s multi-sample sphericity test revealed that the sphericity assumption was satisfied.
Table 2.
Pain Intensity Before and After Knee and Hand Icing, and Change Score in Knee and Hand Icing
| Knee | Hand | Mean Difference (95% CI) | p value | |
|---|---|---|---|---|
| Before icing | 61.3±17.8 | 63.7±16.1 | −2.4 (−6.6 to 1.7) | 0.25 |
| After icing | 46.4±19.8 | 48.9±24.6 | −2.5 (−11.3 to 6.2) | 0.55 |
| Change score | −14.9±21.7 | −14.7±22.4 | −0.16 (−9.3 to 9.0) | 0.97 |
Figure 2.
Comparison of the differences between before and after treatment in knee or hand icing.
Figure 3.
Comparisons of the differences in the change score of pain intensity on the icing site between pleasant versus no pleasant sensation.
Notes: The two-way analysis of variance showed significance only in the main effect of a pleasant sensation (p=0.001), but not the icing site (p=0.94) and interaction (p=0.42).
Discussion
Our findings showed that both knee and hand icing had an effect on pain intensity during maximal knee flexion, and the effect of icing was influenced by the existence of a pleasant sensation and not the icing site. This was a randomized cross-over study in which the patients received knee or hand icing on two consecutive days. The sequence of testing was randomized to control for an order effect. Moreover, there was no difference in pain intensity before knee and hand icing. This result suggested that there was no carry-over effect.
The present study result that knee or hand icing had an acute effect on pain intensity during maximal knee flexion is inconsistent with the results of a previous report stating that knee or elbow joint icing for 30 min after TKA had no acute effect on knee extension strength or knee pain.31 We evaluated pain intensity during maximal knee flexion, but Holm et al31 evaluated pain intensity during knee extension strength and knee pain, and our mean pain intensity score before treatment was higher than that reported by Holm et al31 Patients with higher pain intensity score may react more easily to treatment than those with lower pain intensity. Moreover, Holm et al31 suggested that the reason why knee icing had no effect on knee pain was insufficient cooling. In the present study, the cold pack temperature before treatment was 1–1.5°C, and this temperature may have been enough to cool the skin.
A surprising result in our study was the finding of a similar effect in knee and hand icing. Several studies have stated that the effects of cold application were produced by a change in the peripheral tissue, such as decreased tissue temperature12,13 and sensory fiber conduction.14–16 However, these reasons cannot only explain that hand icing reduced pain intensity. A possible reason is that hand icing activated the descending pain inhibition system and, as a result, pain during maximal knee flexion may be reduced. Diffuse noxious inhibitory controls (DNIC), which constitute one of the main descending pain inhibition systems, are supraspinal mechanisms that control pain via the subnucleus reticularis,32 which suppresses spinal neuron activity and reduces pain by nociceptive stimulation. Noxious cold application to the arm has been reported to reduce response to nociceptive stimulation in the leg.33,34 However, our result showed that a pleasant sensation led to a higher reduction in pain than no pleasant sensation, in which case DNIC might not influence the efficacy of icing. Therefore, we hypothesized that a pleasant sensation due to icing may be as effective as other sensations, such as music,19,20 pictures,21 odors.22 Mohr et al26 reported that cold stimuli to capsaicin-treated skin evoked a pleasant sensation and the sensation was correlated with activation of the descending pain inhibition system-related cortical areas, but the untreated skin elicited an unpleasant perception. This inconsistence in response to icing conducted to a distant site may result from a difference in experimental setting (laboratory versus clinical setting).
This study began 8 days postoperatively. Icing immediately after surgical intervention could be an effective strategy. However, peak levels of serum C-reactive protein (an inflammatory marker), are usually observed on day 2 or 3 postoperatively, and these levels rapidly decline over the rest of the first week postoperatively.35,36 In our view, a stable peripheral condition of the patient can be considered the most appropriate period to perform this study to avoid the risk of introduction of bias during periods of intense inflammation.
Our data suggested a new clinical implication. In approximately one-third of the patients, it is likely that cold application to the hand resulted in more reduced pain than that to the knee. Several studies have reported that cold application to the knee was not effective in pain reduction.17,37,38 Even if knee icing has no effect on pain and evokes no pleasant sensation, it might be worthwhile to conduct hand icing to reduce pain during maximal knee flexion after TKA.
The current study has some limitations. First, there might be potential bias because neither the patients nor the outcome assessors could be blinded due to the nature of the intervention. Second, this study did not include assessment of skin temperature and nerve conduction velocity (NCV) in the sensory nerves. Reduction in sensory NCV was associated with an increase in the threshold and tolerance of pain after cooling.14 Therefore, we cannot conclude that the efficacy of knee icing was produced by reduction in NCV or activation of a pleasant sensation via the descending pain inhibition system. Third, our findings may not be generalizable to patients after revision TKA, or those with severe inflammation and other cooling methods, such as continuous cryotherapy and ice massage. Fourth, the other pleasant sensations, such as music and pictures, were not evaluated in this study. Therefore, it is unclear whether a pleasant sensation evoked by icing has a specific effect on pain intensity. It is possible that the pleasant sensations evoked by other modalities are as effective as cold application for pain intensity after TKA. Fifth, the patients were stratified on the basis of the “pleasant” sensation of icing. This is a subjective measure because the magnitude of the “pleasant” response cannot be quantified.
Conclusion
This study shows that a pleasant sensation evoked by knee or hand icing influenced the effect on pain intensity during maximal knee flexion in patients after TKA. Hand icing may be an optional treatment for patients after undergoing TKA.
Acknowledgments
We thank Tomonari Okada and Yoshiteru Akezaki for help with date collecting.
Ethics Approval and Informed Consent
Ethics approval was obtained from the institutional ethics committee of Konan Women’s University. Written informed consent was obtained from all subjects prior to the study.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Felson DT, Lawrence RC, Hochberg MC, et al. Osteoarthritis: new insights. Part 2: treatment approaches. Ann Intern Med. 2000;133(9):726–737. [DOI] [PubMed] [Google Scholar]
- 2.MA R, Campbell ED. Effect of range of motion on the success of a total knee arthroplasty. J Arthroplasty. 1987;2(2):95–97. doi: 10.1016/S0883-5403(87)80015-3 [DOI] [PubMed] [Google Scholar]
- 3.Rowe PJ, Myles CM, Walker C, et al. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: how much knee motion is sufficient for normal daily life? Gait Posture. 2000;12(2):143–155. doi: 10.1016/S0966-6362(00)00060-6 [DOI] [PubMed] [Google Scholar]
- 4.Martin SS, Spindler KP, Tarter JW, et al. Does cryotherapy affect intraarticular temperature after knee arthroscopy? Clin Orthop Relat Res. 2002;400:184–189. doi: 10.1097/00003086-200207000-00023 [DOI] [PubMed] [Google Scholar]
- 5.Woolf SK, Barfield WR, Merrill KD, et al. Comparison of a continuous temperature-controlled cryotherapy device to a simple icing regimen following outpatient knee arthroscopy. J Knee Surg. 2008;21(1):15–19. doi: 10.1055/s-0030-1247786 [DOI] [PubMed] [Google Scholar]
- 6.Kullenberg B, Ylipaa S, Soderlund K, et al. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21(8):1175–1179. [DOI] [PubMed] [Google Scholar]
- 7.Schroder D, Passler HH. Combination of cold and compression after knee surgery. A prospective randomized study. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):158–165. doi: 10.1007/BF01467918 [DOI] [PubMed] [Google Scholar]
- 8.Singh H, Osbahr DC, Holovacs TF, et al. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective, randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522–525. doi: 10.1067/mse.2001.118415 [DOI] [PubMed] [Google Scholar]
- 9.Webb JM, Williams D, Ivory JP, et al. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopedics. 1998;21(1):59–61. doi: 10.3928/0147-7447-19980101-14 [DOI] [PubMed] [Google Scholar]
- 10.Cohn BT, Draeger RI, Jackson DW. The effects of cold therapy in the postoperative management of pain in patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17(3):344–349. doi: 10.1177/036354658901700306 [DOI] [PubMed] [Google Scholar]
- 11.Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174–178. [PubMed] [Google Scholar]
- 12.Knight KL. Cryotherapy in Sport Injury Management. Champaign, IL: Human Kinetics; 1995. [Google Scholar]
- 13.Nadler SF, Weingand K, Kruse RJ. The physiological basis and clinical applications of cryotherapy and thermotherapy for the pain practitioner. Pain Physician. 2004;7(3):395–399. [PubMed] [Google Scholar]
- 14.Algafly AA, George KP. The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br J Sports Med. 2007;41(6):365–369. doi: 10.1136/bjsm.2006.031237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chesterton LS, Foster NE, Ross L. Skin temperature response to cryotherapy. Arch Phys Med Rehabil. 2002;83(4):543–549. doi: 10.1053/apmr.2002.30926 [DOI] [PubMed] [Google Scholar]
- 16.Herrera E, Sandoval MC, Camargo DM, et al. Motor and sensory nerve conduction are affected differently by ice pack, ice massage, and cold water immersion. Phys Ther. 2010;90(4):581–591. doi: 10.2522/ptj.20090131 [DOI] [PubMed] [Google Scholar]
- 17.Markert SE. The use of cryotherapy after a total knee replacement: a literature review. Orthop Nurs. 2011;30(1):29–36. doi: 10.1097/NOR.0b013e318205749a [DOI] [PubMed] [Google Scholar]
- 18.McMaster W, Liddle S. Cryotherapy influence on posttraumatic limb edema. Clin Orthop Relat Res. 1980;150:283–287. [PubMed] [Google Scholar]
- 19.Roy M, Peretz I, Rainville P. Emotional valence contributes to music-induced analgesia. Pain. 2008;134(1–2):140–147. doi: 10.1016/j.pain.2007.04.003 [DOI] [PubMed] [Google Scholar]
- 20.Roy M, Lebuis A, Hugueville L, et al. Spinal modulation of nociception by music. Eur J Pain. 2012;16(6):870–877. doi: 10.1002/ejp.2012.16.issue-6 [DOI] [PubMed] [Google Scholar]
- 21.Meagher MW, Arnau RC, Rhudy JL. Pain and emotion: effects of affective picture modulation. Psychosom Med. 2011;63(1):79–90. doi: 10.1097/00006842-200101000-00010 [DOI] [PubMed] [Google Scholar]
- 22.Villemure C, Slotnick BM, Bushnell MC. Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain. 2003;106(1–2):101–108. doi: 10.1016/S0304-3959(03)00297-5 [DOI] [PubMed] [Google Scholar]
- 23.Rainville P, Bao QVH, Chretien P. Pain-related emotions modulate experimental pain perception and autonomic responses. Pain. 2005;118(3):306–318. doi: 10.1016/j.pain.2005.08.022 [DOI] [PubMed] [Google Scholar]
- 24.Rhudy JL, Williams AE, McCabe KM, et al. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology. 2005;42(5):579–587. doi: 10.1111/j.1469-8986.2005.00313.x [DOI] [PubMed] [Google Scholar]
- 25.Kut E, Candia V, von Overbeck J, et al. Pleasure-related analgesia activates opioid-insensitive circuits. J Neurosci. 2011;31(11):4148–4453. doi: 10.1523/JNEUROSCI.3736-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohr C, Leyendecker S, Mangels I, et al. Central representation of cold-evoked pain relief in capsaicin induced pain: an event-related fMRI study. Pain. 2008;139(2):416–430. doi: 10.1016/j.pain.2008.05.020 [DOI] [PubMed] [Google Scholar]
- 27.Pud D, Sprecher E, Yarnitsky D. Homotopic and heterotopic effects of endogenous analgesia in healthy volunteers. Neurosci Lett. 2005;380(3):209–213. doi: 10.1016/j.neulet.2005.01.037 [DOI] [PubMed] [Google Scholar]
- 28.Pud D, Yarnitsky D, Eisenberg E, et al. Effects of cold stimulation on secondary hyperalgesia (HA) induced by capsaicin in healthy volunteers. Exp Brain Res. 2006;170(1):22–29. doi: 10.1007/s00221-005-0185-9 [DOI] [PubMed] [Google Scholar]
- 29.Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64(1):29–33. doi: 10.1136/ard.2004.022905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakobsen TL, Christensen M, Christensen SS, et al. Reliability of knee joint range of motion and circumference measurements after total knee arthroplasty: does tester experience matter? Physiother Res Int. 2010;15(3):126–134. doi: 10.1002/pri.450 [DOI] [PubMed] [Google Scholar]
- 31.Holm B, Husted H, Kehlet H, et al. Effect of knee joint icing on knee extension strength and knee pain early after total knee arthroplasty: a randomized cross-over study. Clin Rehabil. 2012;26(8):716–723. doi: 10.1177/0269215511432017 [DOI] [PubMed] [Google Scholar]
- 32.Bouhassira D, Villanueva L, Bing Z, et al. Involvement of the subnucleus reticularis in diffuse noxious inhibitory controls in the rat. Brain Res. 1992;595(2):353–357. doi: 10.1016/0006-8993(92)91071-L [DOI] [PubMed] [Google Scholar]
- 33.Arendt-Nielsen L, Sluka KA, Nie HL. Experimental muscle pain impairs descending inhibition. Pain. 2008;140(3):465–471. doi: 10.1016/j.pain.2008.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe S, Kakigi R, Hoshiyama M, et al. Effects of noxious cooling of the skin on pain perception in man. J Neurol Sci. 1996;135(1):68–73. doi: 10.1016/0022-510X(95)00253-X [DOI] [PubMed] [Google Scholar]
- 35.Niskanen RO, Korkala O, Pammo H, et al. Serum C-reactive protein levels after total hip and knee arthroplasty. J Bone Joint Surg. 1996;78-B:431–433. doi: 10.1302/0301-620X.78B3.0780431 [DOI] [PubMed] [Google Scholar]
- 36.White J, Kelly M, Dunsmuir R. C-reactive protein level after total hip and total knee replacement. J Bone Joint Surg Br. 1998;80:909–911. doi: 10.1302/0301-620X.80B5.0800909 [DOI] [PubMed] [Google Scholar]
- 37.Insall JN, Dorr LD, Scott RD, et al. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 38.Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty? Clin Orthop Relat Res. 2014;472(11):3417–3423. doi: 10.1007/s11999-014-3810-8 [DOI] [PMC free article] [PubMed] [Google Scholar]