Abstract
Purpose
Oxidative stress plays an important role in the pathogenesis of non-alcoholic fatty liver disease (NAFLD). TRPM2 ion channel functions as a molecular sensor for oxidative stress. The aim of this study was to examine the protective effects of Salidroside, a powerful antioxidative plant, on TRPM2 in an established in vitro model of NAFLD.
Methods
NAFLD model was established by palmitic acid (PA) in hepatic L02 cell lines and was added to the media at a final concentration of 400 μM. Cells were used as normal group, PA group and PA receiving varied concentrations of Salidroside (75μg/mL, 150μg/mL, 300μg/mL). After treating 24 hrs, MTT assay was used to detect cell viability, and ALT level was measured using an appropriate kit assay. Intracellular lipid accumulation was observed by Oil red O staining. Cytosolic Ca2+ concentrations were evaluated by flow cytometer with Fluo-3/AM. Quantitative RT-PCR was used to measure the mRNA expression of TRPM2, IL-1β and IL-6, and the protein expressions of TRPM2, p-CaMKII and autophagy (LC3B, p62) were determined using Western blot.
Results
Treatment with Salidroside effectively restored liver injury and alleviated lipid droplet deposition in a dose-dependent manner, which was associated with inhibition of TRPM2/Ca2+/CaMKII pathway. Additionally, autophagic clearance was enhanced by intervention with Salidroside in a dose-dependent manner. Further investigation indicated that Salidroside down-regulated the mRNA expression of IL-1β and IL-6-pro-inflammatory cytokines.
Conclusion
These results suggest that Salidroside could alleviate inflammatory injury and steatosis via autophagy activation mediated by downregulation of the TRPM2/Ca2+/CaMKII pathway. Targeting the TRPM2 ion channel is a novel treatment strategy for oxidative stress-induced liver in NAFLD.
Keywords: non-alcoholic fatty liver disease, salidroside, TRPM2 ion channel, autophagy, cytokines
Introduction
Non-alcoholic fatty liver disease (NAFLD) is regarded as the most significant chronic liver dysfunction in Western countries. NAFLD is characterized by fat accumulation in the liver in the absence of excessive alcohol consumption.1 NAFLD is associated with insulin resistance, obesity, type II diabetes mellitus, and dyslipidemia. NAFLD is a spectrum of hepatic abnormalities extending from simple steatosis to steatohepatitis and steatofibrosis. Non-alcoholic steatohepatitis (NASH), the inflammatory condition of NAFLD characterized by neutrophil infiltration and hepatocyte ballooning, can eventually evolve to fibrosis, cirrhosis, and hepatocellular carcinoma. Approximately one-third of people with NAFLD will develop NASH.2 In addition to the elevated risk for liver disease, NAFLD is also associated with an increased risk of cardiovascular disease.3 Despite its severity and high prevalence, currently there is no pharmacological agent for the treatment of NAFLD/NASH.4
Progression from NAFLD to NASH has been modeled as a “double-hit” hypothesis.5 The first hit involves excess hepatic triglyceride and cholesterol accumulation (i.e., steatosis).6 This sensitizes the liver to the second hit, consisting of elevated hepatic oxidative stress and inflammation.7 According to the double-hit theory, oxidative stress is a major player triggering the progression of steatosis to NASH as a result of an imbalance between pro- and antioxidants that lead to liver cell damage. Although the precise mechanisms involved in the progression of NAFLD/NASH are poorly understood, blocking oxidative stress may be an effective treatment for NAFLD/NASH.2
Intracellular calcium ions (Ca2+) regulate a diverse set of cellular processes in all cells and tissues of the body, including liver diseases.8 Recently, a subset of transient receptor potential (TRP) ion channels have attracted attention because of their permeability to Ca2+. TRP channels are a superfamily of monovalent and divalent cation-permeable ion channels. Members of the melastatin subfamily, TRPM, have been shown to have important roles in cell proliferation and survival.9 TRPM2, the second member of this subfamily to be cloned, is a 1503-amino acid channel permeable to Ca2+, Na+, and K+. Most members of the TRP family of ion channels have been shown to be present in hepatocytes.10 Arguably, the most important activator of TRPM2 channel is oxidative stress.11 TRPM2 knockdown reduced acetaminophen-induced liver injury related to oxidative stress;12 thus, it has emerged as a potential therapeutic target in fighting oxidative stress-related liver diseases.13
Salidroside (Sal), a phenylpropanoid glycoside compound [2-(4-hydroxyphenyl)-ethyl-β-D-glucopyranoside], is the active ingredient of Rhodiola rosea, a plant growing at high-altitude zones that has excellent antioxidant properties.14 Multiple lines of evidence suggested that Sal could effectively improve liver diseases induced by some chemical toxins.15–18 Studies also show that Sal markedly protected against inflammatory injury of hepatocytes with NAFLD through scavenging ROS production.19
Materials and Methods
Materials
Human L02 hepatic cell lines (Wanleibio, Shenyang, China), RPMI-1640 medium and fetal bovine serum (Gibco, USA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT)(Sigma, USA), dimethyl sulfoxide (DMSO) (Sigma); Oil Red O (Sigma), fluo-3/AM kit (Beyotime Biotech, Shanghai, China), palmitic acid (Sigma), alanine aminotransferase (ALT) kit (Nanjing Jiancheng Bioengineering Institute, China), and Salidroside (purity >98%, Aladdin Biotech, Shanghai, China). The antibodies included anti-TRPM2 (Proteintech, Wuhan, China), anti-phospho-CaMKII (Proteintech), anti-LC3B (Wanleibio), anti-p62 (Wanleibio) and goat anti-rabbit secondary antibody (Wanleibio); Trizol reagent (Invitrogen, CA, USA); Reverse Transcription Kit (BioTeke, Beijing, China); Sybr Green (Solarbio, Beijing, China).
Cell Culture
Human L02 hepatic cell lines were cultured in RPMI-1640 supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C in a humidified atmosphere of 5% CO2. At logarithm growth phase, L02 cells were selected to conduct follow-up experiments.
Cell Viability Assay
L02 cells were plated in 96-well plates at a density of 3×103 cells per well and cultured for 12 hrs. After treatment with palmitic acid (PA, 400 μM)20 in the presence of different dosages of Sal (10, 30, 60, 75,150, 300 μg/mL) for 24 hrs, 50 μL of MTT were added into the media for 4 hrs. The media were removed and DMSO was added to each well. The absorbance was measured in a microplate reader (BioTek, USA) at 570 nm, and the relative cell viability was recorded as a ratio with the normal control (untreated cells, fresh medium only). Five replicate wells were counted for each condition.
NAFLD Model in L02 Cells and Sal Intervention
NAFLD model was induced by PA and was added to the media at a final concentration of 400 μM in hepatic L02 cell lines.20 Hepatic L02 cells (3×103/well) were plated into 96-well plates and were divided into five groups: normal group (untreated cells, fresh medium only), PA group and PA plus Sal intervention groups (low dose group, middle dose group and high dose group). After 24 hrs of treatment, hepatocytes were harvested for following assays.
Determination of ALT Content in the Cell Supernatant
The cultured cell supernatant was collected, and ALT content were measured using commercial kits according to the manufacturer’s protocol.
Oil Red O Staining
Cells were stained with Oil red O to examine the amount of lipid accumulation. The cultured L02 cells grown on cell plates were washed with phosphate-buffered saline (PBS) three times and fixed with 4% paraformaldehyde for 1 hr. Then, the fixed cells were washed with 60% isopropanol for 30 s and PBS three times. In darkness, the cells were stained with freshly diluted Oil Red O working solution for 1 hr at 37°C. The cells were washed with PBS two times and ultimately observed and photographed by an inverted phase-contrast microscope. The positive area for Oil Red O staining was measured with the ImagePlus 6.0 software (Media Cybernetics, Bethesda, Maryland).
Cytosolic Ca2+ Measurement
Cytosolic calcium ([Ca2+]c) was evaluated by the cell-permeable calcium-sensitive fluorescent dye Fluo-3/AM. The cells were incubated with 5 μM Fluo-3/AM for 45 mins at 37°C to load fluo-3 AM into the cell. After loading, cells were washed twice with PBS, the fluorescence intensity of [Ca2+]c was analyzed by flow cytometric analysis.
Quantitative Real-Time PCR Analysis
For the quantitative real-time PCR (qRT-PCR) analysis, total RNA was isolated from cultured cells with Trizol reagent according to the manufacturer’s instructions. Reverse transcribed into cDNA using a Reverse Transcription Kit. Quantitative PCR assays were performed with Sybr Green and real-time PCR detection system (Applied Biosystem). Amplicon expression in each sample was normalized to that of β-actin, and after normalization, gene expression was quantified using the 2−∆∆Ct method. The primer sequences are given as follows: TRPM2-forward, 5′- CCCAGAAACTGCTCACCC −3′, TRPM2-reverse, 5′- ACACGCCACAGCCCATT −3′(GenBank ID:NM_015760); IL-1β-forward, 5′- CGAATCTCCGACCACCACTA −3′, IL-1β–reverse, 5′- GCACATAAGCCTCGTTATCCC −3′(GenBank ID: NM_000454); IL-6-forward, 5′- AATAACCACCCCTGACCCAA −3′; IL-6- reverse, 5′- CCAGAAGAAGGAATGCCCATT(GenBank ID: NM_031459); β-actin-forward, 5′- GGCACCCAGCACAATGAA, β-actin- reverse, 5′- TAGAAGCATTTGCGGTGG-3′.
Western Blot Analysis
The cultured cells were lysed in cold RIPA buffer. The cells' total protein was separated by SDS–PAGE (Bio-Rad, CA, USA) and electro-transferred onto the polyvinylidene difluoride (PVDF) membranes. Furthermore, the PVDF membranes were incubated overnight at 4°C with following specific primary antibodies: TRPM2 (1:1000), p-CaMKII (1:1000), LC3B (1:500), p62 (1:500) and β-actin (1:1000). The appropriate secondary antibodies (1:5000) were used to tag primary antibody for 3 hrs and an enhanced chemiluminescence (ECL) detection system (Beyotime Biotech) was used to develop the immunoreactive bands. Finally, the protein band densities were quantified with Gel-Pro-Analyzer (Version 5.0; Media Cybernetics, Rockville, MD, USA). Targeted protein expression levels were quantitated relative to the β-actin level in the same sample and normalized to the control group, which was set as one-fold.
Statistical Analysis
All data are presented as the mean of at least three independent experiments ± S.D. Analysis of variance was conducted by one-way analysis of variance (ANOVA) using least significant difference method (LSD) on the SPSS statistical package (version 18.0 for Windows, SPSS Inc.). The level of statistical significance was set at p<0.05.
Results
Sal Alleviated PA-Induced Cell Injury and Lipid Accumulation in Hepatic L02 Cells
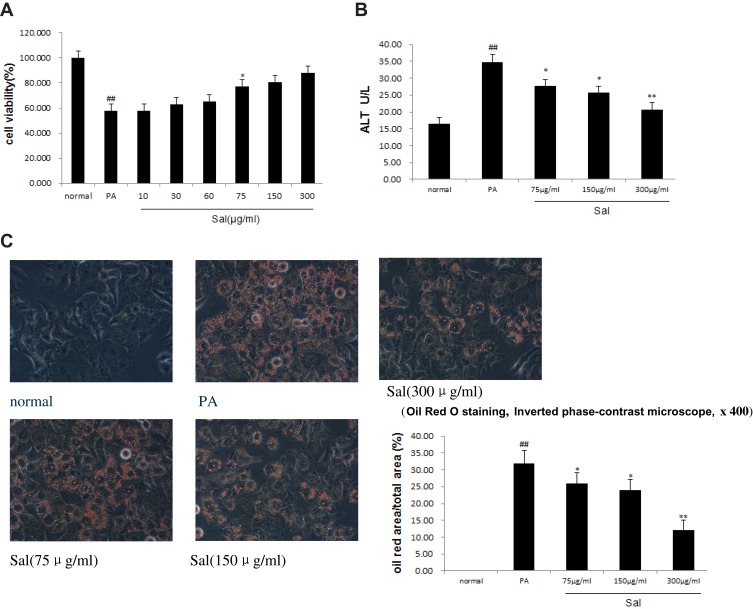
MTT results demonstrated that compared to the PA-treated cells, administration of 75μg/mL Sal to the PA-treated cells could dramatically recover cell viability (Figure 1A). We proceeded to examine Sal at 75 μg/mL (low dose group), 150 μg/mL (middle dose group), and 300 μg/mL (high dose group). Alanine aminotransferase (ALT), a sensitive indicator of hepatocyte injury, was elevated in the PA group compared with normal controls (Figure 1B), indicating that cytotoxic damage occurred in response to PA treatment. Sal prevented the adverse effects of PA-induced cell injury in a dose-dependent manner. Furthermore, Oil red O staining showed that Sal was able to reduce lipid droplet massive deposition in PA-treated L02 cells in a dose-dependent manner (Figure 1C). These results suggest that Sal has protective effects against biological symptoms of NAFLD mimicked in vitro in the hepatic L02 cell line.
Figure 1.
Sal dose-dependent alleviates PA-induced cell injury and lipid accumulation in hepatic L02 cells.
Notes: PA was added to the media at a final concentration of 400 μM in hepatic L02 cell lines and PA receiving varied concentrations of Salidroside (75μg/mL, 150μg/mL, 300μg/mL). (A) Cell viability. (B) ALT. (C) Oil red O staining (inverted phase-contrast microscope) and the positive area for Oil Red O staining. Data were shown as mean ± SD (n=3), ##P<0.01 vs normal group; *P<0.05, **P<0.01 vs PA group.
Abbreviations: PA, palmitic acid; ALT, alanine aminotransferase.
The Protective Effect of Sal Against NAFLD Involved Inhibition of TRPM2/Ca2+/CaMKII Pathway
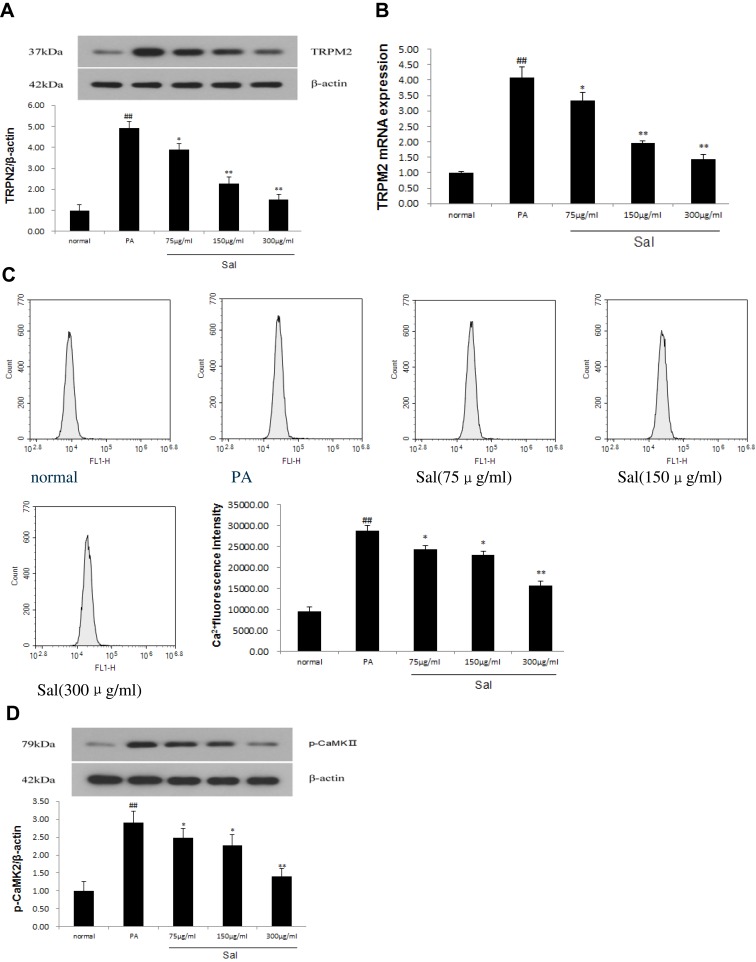
TRPM2 ion channel is a Ca2+ permeable, non-selective cation channel. As shown in Figure 2, Western blot and qPCR analysis showed that TRPM2 channel protein and mRNA expressions markedly increased in L02 cells treated with PA compared with normal controls (Figure 2A and B). Sal significantly inhibit the increased protein and mRNA expressions of TRPM2 induced by PA in a concentration-dependent manner. To explore whether TRPM2 activation is associated with increased [Ca2+]c, we examined the fluorescence intensity of [Ca2+]c by flow cytometric analysis using the fluorescent probe Fluo-3/AM (Figure 2C). The [Ca2+]c was found to be increased about three fold in cells treated with PA. Sal was able to reduce the PA-induced [Ca2+]c increase in a concentration-dependent manner. Simultaneously, p-CaMKII protein expression, a downstream target of [Ca2+]c, was up-regulated in PA-treated L02 cells, but could be progressively ameliorated by increasing dosages of Sal (Figure 2D). Our results demonstrated that Sal significantly downregulated the phosphorylation of CaMKII, suggesting that TRPM2/Ca2+/CaMKII signaling pathway was involved in hepato-protective benefits of Sal against NAFLD.
Figure 2.
Sal dose-dependent inhibited TRPM2/Ca2+/CaMKIIpathway in L02 cells treated with PA.
Notes: PA was added to the media at a final concentration of 400 μM in hepatic L02 cell lines and PA receiving varied concentrations of Salidroside (75μg/mL, 150μg/mL, 300μg/mL). (A) Western blot analysis of TRPM2 protein. (B) qPCR analysis of TRPM2 mRNA. (C) Fluorescence intensity of [Ca2+]c was detected by flow cytometer with Fluo-3/AM. (D) Western blot analysis of p-CaMKII protein. Data were shown as mean ± SD (n=3), ##P<0.01 vs normal group; *P<0.05, **P<0.01 vs PA group. Abbreviations: PA, palmitic acid; TRPM2, transient receptor potential melastatin 2; CaMKII, calmodulin-stimulated protein kinaseII.
Sal Enhanced Autophagy in L02 Cells Treated with PA
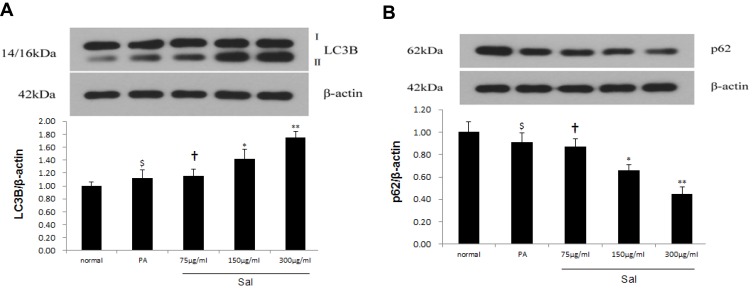
Previous studies reported that autophagy enhancement might be responsible for the protective role during NAFLD/NASH.21 As a result, we measured autophagy in hepatic cells after Sal treatment. LC3B, an autophagy indicator, which correlates with the content of autophagosomes reflects the activity of autophagosomes. We used Western blotting to examine the expression of LC3B in PA-stimulated L02 cells. Sal treatment of L02 cells increased the relative expression of LC3B-II and the LC3B-II/LC3B-I ratio compared to PA group in a dose-dependent manner (Figure 3A). To corroborate this finding, we measured another autophagy-related protein – p62. Protein degradation of p62 was increased in L02 cells treated with Sal (Figure 3B). These results confirm that Sal strengthened autophagic clearance in a cell line exogenously treated to mimic some of the cellular phenotypes of NAFLD.
Figure 3.
Sal reinforced autophagy in PA-stimulated hepatic L02 cells.
Notes: PA was added to the media at a final concentration of 400 μM in hepatic L02 cell lines and PA receiving varied concentrations of Salidroside (75μg/mL, 150μg/mL, 300μg/mL). (A) Western blot analysis showed that the dose dependence of Sal induced upregulation of the LC3B-II expression and the ratio of LC3B-II to I. (B) Western blot analysis showed that the dose dependence of Sal downregulated p62 expression. Data were shown as mean± SD (n=3), $p>0.05 vs normal group; †p>0.05 vs PA group; *p <0.05, **p <0.01 vs PA group.
Abbreviations: PA, palmitic acid; LC3B, Light chain 3B.
Effect of Sal Intervention on the Expressions of IL-1β, IL-6 mRNA in PA-Stimulated L02 Cells
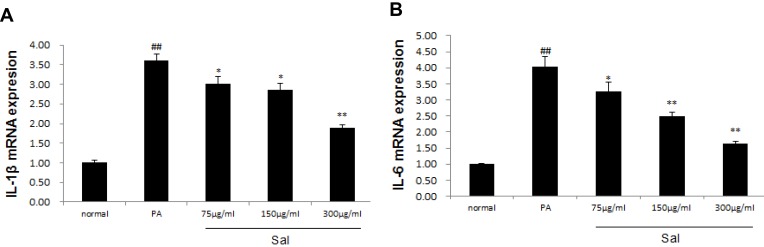
Finally, we further investigated the mRNA expression of IL-1β and IL-6 in hepatic L02 cells treated with PA. qPCR showed that the expression of IL-1β and IL-6 mRNA was significantly up-regulated in hepatic L02 cells treated with PA. Sal intervention notably down-regulated the expressions of both IL-1β and IL-6 mRNA in L02 cells (Figure 4A and B).
Figure 4.
Sal down-regulated the expressions of IL-1β, IL-6 in L02 cells treated with PA.
Notes: PA was added to the media at a final concentration of 400 μM in hepatic L02 cell lines and PA receiving varied concentrations of Salidroside (75μg/mL, 150μg/mL, 300μg/mL). (A) qPCR analysis of IL-1β mRNA. (B) qPCR analysis of IL-6 mRNA. Data were shown as mean± SD (n=3), ##P<0.01 vs normal group; *P<0.05, **P<0.01 vs PA group.
Abbreviations: PA, palmitic acid; IL-1β, Interleukin1β; IL-6, Interleukin 6.
Discussion
Lipotoxicity refers to cellular toxicity in the presence of excessive free fatty acids. Fatty acid-induced lipotoxicity in hepatocytes plays an essential role in the pathogenesis of nonalcoholic fatty liver disease.22 Fatty acids are chemically classified as saturated and unsaturated, and their structure affects cell biological functions. Palmitic acid, a saturated fatty acid, is the most toxic lipid species.23 In the current study, we investigated the direct effect of Sal on PA-induced hepato-lipotoxicity in vitro. Our data support the possibility that Sal could attenuate the progression of disease symptoms associated with NAFLD via regulation of the TRPM2/Ca2+/CaMKIIpathway and inflammatory cytokines.
Excessive formation of ROS and subsequent oxidative stress occupy an important position in the pathogenesis of NAFLD/NASH.24 Studies in vivo and in vitro showed higher free radical activity, including superoxide and hydrogen peroxide (H2O2) production, as shown by mitochondrial dysfunction and CYP2E1 up-regulation.25,26 TRPM2 ion channel is a cellular sensor of oxidative stress and is widely recognized as an ion channel with an important role in cell survival in several physiological and pathological conditions.11 H2O2 induces TRPM2 channel activation and subsequent increase of intracellular Ca2+ concentration in various cell types.27 The mode of TRPM2 channel activation by H2O2 has long been debated. Accumulating evidence suggests that H2O2 can activate TRPM2 channel either directly or indirectly.28 It has been postulated that the activation of TRPM2 channel by oxidative stress is triggered via ADP-ribose production (Citation). Mitochondria are a major source of ADP-ribose. In mitochondria, ADP-ribose is generated by the oxidative stress-induced hydrolysis of NAD+, leading to the production of nicotinamide and ADP-ribose.29 Thus, the H2O2-induced TRPM2 channel activation is often explained by formation of ADPR. However, H2O2 may also directly activate TRPM2 channel. The TRPM2 with a deletion in the C-terminus (TRPM2ΔC) is insensitive to ADPR, but still responds to H2O2, suggesting a direct effect of H2O2 on TRPM2.30,31 The activated TRPM2 ion channel further triggered Ca2+ influx of hepatocytes. Ca2+ is a critical second messenger involved in many important signaling pathways. TRPM2 channel knockout mice or pharmacological inhibition of the TRPM2 channel could protect the liver from acetaminophen toxicity.12,32 Indeed, the level of intracellular Ca2+ was increased dramatically during NAFLD stage and led to lipid accumulation, mitochondrial damage, and ER stress.33 Ca2+/calmodulin-stimulated protein kinaseII (CaMKII) is an important protein kinase in intracellular Ca2+ signaling.34 An increase in cytosolic Ca2+ induces phosphorylation of CaMKII.35 Nifedipine (NFD), a calcium channel blocker, attenuated hepatic steatosis through downregulation of p-CaMKII.36 Together with the results above, our research demonstrated that Sal inhibited TRPM2 channel activation of NAFLD-like hepatocytes in a dose-dependent manner, consequently decreasing Ca2+ influx and down-regulating of p-CaMKII.
Autophagy is a highly conserved self-digestion process, bring dispensable or potentially dangerous cytoplasmic material, such as damaged organelles and misfolded proteins, to lysosomes for degradation. Autophagy is one of the major downstream events regulated by CaMKII. The increased Ca2+ influx via TRPM2 to activate CaMKII and p-CaMKIIsubsequently phosphorylates BECN1/Beclin1 to suppress autophagy. This sequence of events ultimately renders hepatocytes more vulnerable to damage.37 Autophagy has been implicated as a key regulatory factor in the pathogenesis of NAFLD.21 Autophagy activation is considered one of the pathways in lipid clearance (lipophagy) and is associated with blocking of NASH.38,39 The expression of LC3-II and the autophagic adaptors p62 are commonly used to monitor autophagy influx. In this study, Sal stimulated LC3BII accumulation and promoted p62 protein degradation, which strengthened the initiation of autophagy. The results suggested that Sal was a reduced autophagy inducer and that autophagy activation could serve as a hepatoprotective mechanism in restoring NAFLD/NASH.
The excessive formation of ROS can drive pro-inflammatory cytokine production, which exacerbates NAFLD progression. Oxidative stress-induced TRPM2 siRNA knockdown attenuated IL-1β and IL-6 secretion.40,41 Pro-inflammatory cytokine IL-1β is regulated by NLRP3 inflammasome and Sal treatment dramatically suppressed IL-1β expression in the liver with NAFLD.19 NASH patients have higher IL-6 levels in serum than simple steatosis.42,43 STAT3 phosphorylation induced by IL-6 could accelerate high-fat diet-induced nonalcoholic steatohepatitis in mice.44 In this study, qPCR analysis showed that Sal significantly down-regulated the expression of IL-1β and IL-6 mRNA. We deduce that the protective effect of Sal on NAFLD was at least partly due to its regulation on IL-1β and IL-6 via TRPM2-mediated signaling pathway.
Conclusion
The results of this study demonstrate that inhibition of TRPM2 ion channel is a novel target that ameliorates oxidative stress-inflammatory injury in liver cells mimicking NAFLD. Sal dramatically attenuated liver injury and lipid accumulation via autophagy induction. Autophagy activation mediated by downregulation of TRPM2/Ca2+/CaMKII pathway could serve as an anti-inflammatory strategy in hepatocytes with NAFLD-like symptoms. The present results support further exploration of Sal for the treatment of NAFLD.
Acknowledgment
This research was funded by Liaoning Nature Science Fund (20180550081), China.
Abbreviations
NAFLD, non-alcoholic fatty liver disease; NASH, Non-alcoholic steatohepatitis; TRPM, transient receptor potential melastatin; Sal, Salidroside; DMSO, dimethyl sulfoxide; FBS, fetal bovine serum; MTT, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; CaMKII, calmodulin-stimulated protein kinaseII; PA, palmitic acid; ALT, alanine aminotransferase; qRT-PCR, quantitative real-time PCR; LC3B, Light chain 3B; IL, interleukin; ROS, reactive oxygen species.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis. 2016;20(2):205–214. doi: 10.1016/j.cld.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 2.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377(21):2063–2072. doi: 10.1056/NEJMra1503519 [DOI] [PubMed] [Google Scholar]
- 3.Tana C, Ballestri S, Ricci F, et al. Cardiovascular risk in non-alcoholic fatty liver disease: mechanisms and therapeutic implications. Int J Environ Res Public Health. 2019;16(17):E3104. doi: 10.3390/ijerph16173104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goedeke L, Perry RJ, Shulman GI. Emerging pharmacological targets for the treatment of nonalcoholic fatty liver disease, insulin resistance, and type 2 diabetes. Annu Rev Pharmacol Toxicol. 2019;59:65–87. doi: 10.1146/annurev-pharmtox-010716-104727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engin A. Non-alcoholic fatty liver disease. Adv Exp Med Biol. 2017;960:443–467. doi: 10.1007/978-3-319-48382-5_19 [DOI] [PubMed] [Google Scholar]
- 6.Ji Y, Gao Y, Chen H, et al. Indole-3-acetic acid alleviates nonalcoholic fatty liver disease in mice via attenuation of hepatic lipogenesis, and oxidative and inflammatory stress. Nutrients. 2019;11(9):E2062. doi: 10.3390/nu11092062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Zhang SD, Wang P, et al. Pinolenic acid ameliorates oleic acid-induced lipogenesis and oxidative stress via AMPK/SIRT1 signaling pathway in HepG2 cells. Eur J Pharmacol. 2019;861:172618. doi: 10.1016/j.ejphar.2019.172618 [DOI] [PubMed] [Google Scholar]
- 8.Bartlett PJ, Gaspers LD, Pierobon N, Thomas AP. Calcium-dependent regulation of glucose homeostasis in the liver. Cell Calcium. 2014;55(6):306–316. doi: 10.1016/j.ceca.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 9.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431 [DOI] [PubMed] [Google Scholar]
- 10.Rychkov GY, Barritt GJ. Expression and function of TRP channels in liver cells. Adv Exp Med Biol. 2011;704:667–686. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto S, Shimizu S. Significance of TRP channels in oxidative stress. Eur J Pharmacol. 2016;793:109–111. doi: 10.1016/j.ejphar.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 12.Kheradpezhouh E, Ma L, Morphett A, GJ Barritt, GY Rychkov. TRPM2 channels mediate acetaminophen-induced liver damage. Proc Natl Acad Sci USA. 2014;111(8):3176–3181. doi: 10.1073/pnas.1322657111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramírez A, Vázquez-Sánchez AY, Carrión-Robalino N, Camacho J. Ion channels and oxidative stress as a potential link for the diagnosis or treatment of liver diseases. Oxid Med Cell Longev. 2016;2016:3928714. doi: 10.1155/2016/3928714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosakowska O, Bączek K, Przybyt JL, et al. Antioxidant and antibacterial activity of roseroot (Rhodiola rosea L.) dry extracts. Molecules. 2018;23(7):E1767. doi: 10.3390/molecules23071767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Lin B, Qi S, He J, Zheng H. Protective effects of salidroside on lead acetate-induced oxidative stress and hepatotoxicity in Sprague-Dawley rats. Biol Trace Elem Res. 2019;191(2):426–434. doi: 10.1007/s12011-019-1635-8 [DOI] [PubMed] [Google Scholar]
- 16.Yuan Y, Wu SJ, Liu X, LL Zhang. Antioxidant effect of salidroside and its protective effect against furan-induced hepatocyte damage in mice. Food Funct. 2013;4(5):763–769. doi: 10.1039/c3fo00013c [DOI] [PubMed] [Google Scholar]
- 17.Sun P, Song SZ, Jiang S, et al. Salidroside regulates inflammatory response in Raw 264.7 macrophages via TLR4/TAK1 and ameliorates inflammation in alcohol binge drinking-Induced liver injury. Molecules. 2016;21(11):E1490. doi: 10.3390/molecules21111490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin SY, Dan X, Du XX, et al. Protective effects of salidroside against carbon tetrachloride (CCl4)-induced liver injury by initiating mitochondria to resist oxidative stress in mice. Int J Mol Sci. 2019;20:E3187. doi: 10.3390/ijms20133187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng T, Yang X, Li W, et al. Salidroside attenuates high-fat diet-induced nonalcoholic fatty liver disease via AMPK-dependent TXNIP/NLRP3 pathway. Oxid Med Cell Longev. 2018;2018:8597897. doi: 10.1155/2018/8597897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li JS, Wang WJ, Sun Y, Zhang YH, Zheng L. Ursolic acid inhibits the development of nonalcoholic fatty liver disease by attenuating endoplasmic reticulum stress. Food Funct. 2015;6(5):1643–1651. doi: 10.1039/c5fo00083a [DOI] [PubMed] [Google Scholar]
- 21.Khambu B, Yan S, Huda N, Liu G, Yin XM. Autophagy in non-alcoholic fatty liver disease and alcoholic liver disease. Liver Res. 2018;2(3):112–119. doi: 10.1016/j.livres.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68(2):280–295. doi: 10.1016/j.jhep.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 23.Ricchi M, Odoardi MR, Carulli L, et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24(5):830–840. doi: 10.1111/j.1440-1746.2008.05733.x [DOI] [PubMed] [Google Scholar]
- 24.Spahis S, Delvin E, Borys JM, Levy E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid Redox Signal. 2017;26(10):519–541. doi: 10.1089/ars.2016.6776 [DOI] [PubMed] [Google Scholar]
- 25.Zhang K, Kim H, Fu Z, et al. Deficiency of the mitochondrial NAD kinase causes stress-induced hepatic steatosis in mice. Gastroenterology. 2018;154(1):224–237. doi: 10.1053/j.gastro.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Liao L, Chen Y, Han F. Effects of daphnetin on lipid metabolism, insulin resistance and oxidative stress in OA-treated HepG2 cells. Mol Med Rep. 2019;19(6):4673–4684. doi: 10.3892/mmr.2019.10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozai D, Ogawa N, Mori Y. Redox regulation of transient receptor potential channels. Antioxid Redox Signal. 2014;21(6):971–986. doi: 10.1089/ars.2013.5616 [DOI] [PubMed] [Google Scholar]
- 28.Kim TK, Nam JH, Ahn WG, et al. Lys1110 of TRPM2 is critical for channel activation. Biochem J. 2013;455(3):319–327. doi: 10.1042/BJ20130303 [DOI] [PubMed] [Google Scholar]
- 29.Perraud AL, Takanishi CL, Shen B, et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem. 2005;280(7):6138–6148. doi: 10.1074/jbc.M411446200 [DOI] [PubMed] [Google Scholar]
- 30.Toda T, Yamamoto S, Yonezawa R, Mori Y, Shimizu S. Inhibitory effects of Tyrphostin AG-related compounds on oxidative stress-sensitive transient receptor potential channel activation. Eur J Pharmacol. 2016;786:19–28. doi: 10.1016/j.ejphar.2016.05.033 [DOI] [PubMed] [Google Scholar]
- 31.Shimizu T, Dietz RM, Cruz-Torres I, et al. Extended therapeutic window of a novel peptide inhibitor of TRPM2 channels following focal cerebral ischemia. Exp Neurol. 2016;283(Pt A):151–156. doi: 10.1016/j.expneurol.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kheradpezhouh E, Barritt GJ, Rychkov GY. Curcumin inhibits activation of TRPM2 channels in rat hepatocytes. Redox Biol. 2016;7:1–7. doi: 10.1016/j.redox.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao X, Guo S, Zhang S, Liu A, Shi L, Zhang Y. Matrine attenuates endoplasmic reticulum stress and mitochondrion dysfunction in nonalcoholic fatty liver disease by regulating SERCA pathway. J Transl Med. 2018;16(1):319. doi: 10.1186/s12967-018-1685-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brzozowski JS, Skelding KA. The multi-functional calcium/calmodulin stimulated protein kinase (CaMK) family: emerging targets for anti-cancer therapeutic intervention. Pharmaceuticals (Basel). 2019;12(1):E8. doi: 10.3390/ph12010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Huang L, Yue J. Oxidative stress activates the TRPM2-Ca2+-CaMKII-ROS signaling loop to induce cell death in cancer cells. Biochim Biophys Acta Mol Cell Res. 2017;1864(6):957–967. doi: 10.1016/j.bbamcr.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 36.Lee S, Han D, Kang HG, et al. Intravenous sustained-release nifedipine ameliorates nonalcoholic fatty liver disease by restoring autophagic clearance. Biomaterials. 2019;197:1–11. doi: 10.1016/j.biomaterials.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, Guo W, Hao B, et al. Mechanistic study of TRPM2-Ca2+-CAMK2-BECN1 signaling in oxidative stress-induced autophagy inhibition. Autophagy. 2016;12(8):1340–1354. doi: 10.1080/15548627.2016.1187365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Zhao H, Li X, et al. Tangshen Formula alleviates hepatic steatosis by inducing autophagy through the AMPK/SIRT1 pathway. Front Physiol. 2019;10:494. doi: 10.3389/fphys.2019.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu Q, Zhang S, Chen M, et al. Cherry anthocyanins regulate NAFLD by promoting autophagy pathway. Oxid Med Cell Longev. 2019;2019:4825949. doi: 10.1155/2019/4825949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Q, Tan Q, Ren Y, et al. Hyperosmotic stress-induced TRPM2 channel activation stimulates NLRP3 inflammasome activity in primary human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2018;59(8):3259–3268. doi: 10.1167/iovs.18-23965 [DOI] [PubMed] [Google Scholar]
- 41.Chung MK, Asgar J, Lee J, Shim MS, Dumler C, Ro JY. The role of TRPM2 in hydrogen peroxide-induced expression of inflammatory cytokine and chemokine in rat trigeminal ganglia. Neuroscience. 2015;297:160–169. doi: 10.1016/j.neuroscience.2015.03.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polyzos SA, Kountouras J, Polymerou V, Papadimitriou KG, Zavos C, Katsinelos P. Vaspin, resistin, retinol-binding protein-4, interleukin-1α and interleukin-6 in patients with nonalcoholic fatty liver disease. Ann Hepatol. 2016;15(5):705–714. doi: 10.5604/16652681.1212429 [DOI] [PubMed] [Google Scholar]
- 43.Jorge AS, Andrade JM, Paraíso AF, et al. Body mass index and the visceral adipose tissue expression of IL-6 and TNF-alpha are associated with the morphological severity of non-alcoholic fatty liver disease in individuals with class III obesity. Obes Res Clin Pract. 2018;12(Suppl 2):1–8. doi: 10.1016/j.orcp.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 44.Cai X, Fang C, Hayashi S, et al. Pu-erh tea extract ameliorates high-fat diet-induced nonalcoholic steatohepatitis,and insulin resistance by modulating hepatic IL-6/STAT3 signaling in mice. J Gastroenterol. 2016;51(8):819–829. doi: 10.1007/s00535-015-1154-0 [DOI] [PubMed] [Google Scholar]