Abstract
Background
Persons who are infected with human immunodeficiency virus (HIV) are at high risk of human papillomavirus (HPV)-associated cancers. The objectives are to compare antibody titers to HPV 6, 11, 16, and 18 and rate of abnormal cytology between perinatally HIV-infected (PHIV) and perinatally HIV-exposed, uninfected (PHEU) youth.
Methods
This is a prospective observational cohort study of HPV4 vaccinated youth performed as part of the multicenter Pediatric HIV/AIDS Cohort Study Adolescent Master Protocol. Seroconversion and geometric mean titer (GMT) against HPV types 6, 11, 16, and 18 were calculated. Vaccine effectiveness included rates of abnormal cervical cytology and genital warts.
Results
Seroconversion to HPV 6, 11, 16, and 18 occurred in 83%, 84%, 90%, and 62% of 310 vaccinated PHIV youth compared to 94%, 96%, 99%, and 87% of 148 vaccinated PHEU youth, respectively (P < .05 for all comparisons). GMTs were lower in the PHIV vs PHEU within each category of HPV4 doses received. Higher GMTs were associated with younger age, lower HIV type 1 RNA viral load, and higher CD4% at first HPV4 vaccination, as well as shorter duration between last vaccine dose and antibody specimen. Abnormal cytology occurred in 33 of 56 PHIV and 1 of 7 PHEU sexually active vaccinated females, yielding incidence rates per 100 person-years of 15.0 (10.9 to 20.6) and 2.9 (0.4 to 22.3), respectively.
Conclusion
Antibody titers to HPV4 were lower for all serotypes in PHIV compared to PHEU youth. Protection against abnormal cytology was also diminished in sexually active PHIV females.
Keywords: HPV vaccine, perinatally HIV infected youth, antibody titers, abnormal cytology
Antibody titers for human papillomavirus (HPV) 6, 11, 16 and 18 were lower in HPV vaccinated perinatally human immunodeficiency virus-infected (PHIV) youth compared to those HIV-exposed, uninfected youth. HPV vaccine effectiveness was reduced in PHIV girls, as 33 of 56 development abnormal cytology during observation.
The efficacy of the quadrivalent human papillomavirus (HPV4) vaccine (Merck) reached nearly 100% in clinical trials [1, 2]. Several studies have shown the durability of antibodies in children induced by HPV vaccine, but most were performed over relatively short periods of time [3]. Population-based studies using national registries suggest that the HPV vaccine is already having an impact in cohorts vaccinated at the target ages 11–14 years [4–6].
Antibody durability and efficacy in immunocompromised populations, including those with perinatal human immunodeficiency virus infection (PHIV), is less understood. This population has experienced higher rates of antibody decay and loss of protective antibody levels over time for measles, mumps, and rubella (MMR), varicella, as well as other vaccines, compared to uninfected groups [7–9]. Furthermore, human immunodeficiency virus (HIV) infection in adults increases the risk for development of all HPV-associated cancers including cervix, vaginal, vulvar, anal, and oropharyngeal, prompting additional concern for PHIV children [10, 11].
In an analysis of vaccinated PHIV children receiving a 3-dose schedule of HPV4, seroconversion at month 1 after the third dose showed no difference to historic controls of healthy children [12]. However, follow-up at 72 weeks demonstrated a more rapid decay than seen in healthy children for HPV 6 and 18 [13]. In further follow-up of this group, there was a 50–70% decline in antibodies to HPV 6, 11, and 16 and an 89% decline to HPV 18 between year 2 and years 4–5 [14]. The clinical relevance of this decline in antibody titer is not clear because it is believed that the antibody response generated by the vaccine is well above what is required for protection [15].
Limited antibody titer data are available for PHIV children receiving fewer than 3 doses, and no HPV vaccine efficacy data have been published for PHIV children of any age. The purpose of the study was to examine: (1) antibody response to HPV 6, 11, 16, and 18 in PHIV children receiving the HPV4 vaccine compared to perinatally HIV-exposed but uninfected (PHEU) youth; (2) the factors associated with lower antibody titers among PHIV youth; and (3) the incidence of abnormal cytology and genital warts among those receiving at least 1 dose of HPV4.
METHODS
Study Population
We studied participants enrolled in the Pediatric HIV/AIDS Cohort Study (PHACS) Adolescent Master Protocol, composed of 451 PHIV and 227 PHEU youth ages 7 to 16 years enrolled between March 2007 and November 2009 at 15 US (including Puerto Rican) clinical research sites [16, 17]. These protocols were approved by the institutional review boards (IRB) of each participating site and the Harvard T. H. Chan School of Public Health. Written informed consent was obtained from all participants or parents/guardians, and written assent was obtained for minors according to local IRB guidelines.
Eligibility Criteria
Participants were required to have received between 1 and 3 HPV4 vaccine doses. We also included sexually active unvaccinated youth to examine natural seroconversion among those exposed. Sexual activity information was collected annually via audio computer-assisted self-interview—starting at 10 years of age—and starting at age approximately 18 years via an online survey [17, 18]. Sexual activity was defined as vaginal, anal, or oral sex, and date of sexual debut was self-reported. Participants also must have had an available serum specimen drawn at least 20 days after their most recent HPV vaccine dose; unvaccinated participants must have had their specimen drawn after their sexual debut. The most recent specimen (furthest from last vaccine dose) was chosen for each participant.
Laboratory and Clinical Data
All analyses used follow-up data through 1 July 2014, with the exception of the genital warts/abnormal cervical cytology analysis, which used follow-up through 1 January 2017 to increase analytic power for vaccine effectiveness estimates.
Clinical data and laboratory specimen collection and storage have been previously described [16, 17]. Sera were analyzed by Merck laboratories using 2 distinct antibody assays. The featured assay of this analysis was the competitive Luminex immunoassay (cLIA), which was designed to detect neutralizing immunoglobulin G (IgG) antibodies to the 4 HPV types targeted by the Gardasil quadrivalent vaccine [19, 20]. Due to sensitivity issues with the cLIA to detect HPV 18 antibodies, results were also obtained using a direct binding anti-HPV IgG enzyme immunoassay (EIA) [21].
Outcome Measures
Average titers to HPV types 6, 11, 16, and 18 by group were expressed as geometric mean titers (GMTs) of milli-Merck units per milliliter of sera (mMU/mL) based on all samples. We set assay values to the detection limit when samples tested below it. Seropositivity cutoffs for the quadrivalent cLIA and IgG EIA were 20, 16, 20, and 24 mMU/mL and 15, 15, 7, and 10 mMU/mL for HPV types 6, 11, 16, and 18, respectively [19, 22].
Genital warts and abnormal cervical cytology events were identified using the annual medical record abstraction protocol. Abnormal cytology was defined as atypical squamous cells of undetermined significance (ASCUS) or worse.
Statistical Analysis
Demographic and clinical characteristics between PHIV and PHEU participants were compared using the Wilcoxon rank sum or Fisher exact tests as appropriate. We estimated seropositive proportions by cohort and vaccine doses received using 95% exact binomial confidence intervals (CIs) and tested for differences between groups using Fisher exact test. We further examined the effect of cohort on vaccine immune response using least squares means regression on log-transformed titer to predict GMT as a function of cohort and doses received, adjusting for years from last dose to specimen date. We also compared HPV GMTs by Rubella serostatus using data from a previous PHACS analysis; the Wilcoxon test was used for inference [8].
Among PHIV youth, we identified independent predictors of antibody titer by first building bivariable models to test the association of various demographic and HIV characteristics on log antibody titer; estimates were then exponentiated back to the original scale for reporting. Variables significant at alpha <.10 in the bivariable models for any HPV type were included in the multivariable models for each type. Number of HPV vaccine doses received was forced into the multivariable models.
We compared incidence rates of first genital warts or abnormal cervical cytology by cohort in the entire study population and among various subpopulations defined by sex and timing of vaccine initiation relative to sexual debut. Follow-up began at the date of sexual debut and continued until first event or end of observation. Incidence rate ratios by cohort and estimated cohort-specific incidence rates were obtained by fitting Poisson models using generalized estimating equations with the robust variance estimators. Fisher exact statistics were used for zero events. To identify correlates of abnormal cytology among PHIV females who received at least 1 vaccine dose prior to sexual debut, we built a series of bivariable models to estimate risk by vaccine- and HIV-related factors.
All analyses were conducted using SAS version 9.4 and SAS/STAT version 14.1 (SAS Institute, Cary, North Carolina).
RESULTS
Vaccination Coverage and Demographics
Antibody titer data were available on 310 PHIV and 148 PHEU youth. HPV vaccine coverage was better in the PHIV population, with only 10% unvaccinated compared to 22% for PHEU youth; 40% vs. 16%, respectively, received at least 2 vaccine doses (Table 1). Females were more likely to receive all 3 doses (46% and 14% of PHIV and PHEU females compared with 6% and 0% of PHIV and PHEU males, respectively). PHIV youth, compared to PHEU youth, were older, more likely black, and their caregivers were more educated. PHIV youth were also older at first dose (mean [standard deviation] age: 13.7 [2.5] years vs 12.4 [2.1] years; P < .001), had a lower body mass index Z-score at first dose (0.2 [1.2] vs 0.6 [1.4]; P = .01) and older at time of specimen collection (17.2 [2.3] years vs 15.7 [2.4] years; P < .001).
Table 1.
Descriptive Characteristics of the Study Population
| Total (N = 458)a | Cohort | P Valueb | ||
|---|---|---|---|---|
| PHIV (N = 310)a | PHEU (N = 148)a | |||
| Birth cohort <1998 | 310 (68) | 242 (78) | 68 (46) | <.001 |
| Female sex | 259 (57) | 179 (58) | 80 (54) | .48 |
| Black race | 318 (69) | 224 (72) | 94 (64) | .02 |
| Missing | 22 (5) | 18 (6) | 4 (3) | |
| Hispanic ethnicity | 125 (27) | 77 (25) | 48 (32) | .07 |
| Missing | 3 (1) | 0 (0) | 3 (2) | |
| Caregiver highest education level | ||||
| Did not graduate high school | 185 (40) | 116 (37) | 69 (47) | .01 |
| High school | 137 (30) | 100 (32) | 37 (25) | |
| Some college or 2-year degree | 75 (16) | 45 (15) | 30 (20) | |
| 4-year college degree or higher | 59 (13) | 48 (15) | 11 (7) | |
| Missing | 2 (0) | 1 (0) | 1 (1) | |
| Income >$20 000/year | 201 (44) | 155 (50) | 46 (31) | <.001 |
| Missing | 22 (5) | 17 (5) | 5 (3) | |
| Ever sexually active | 257 (56) | 173 (56) | 84 (57) | 1.00 |
| Missing | 13 (3) | 11 (4) | 2 (1) | |
| Age at sexual debut | ||||
| Mean (SD) | 13.2 (3.1) | 13.4 (3.1) | 12.8 (3.0) | .11 |
| Median | 14 | 14 | 14 | |
| Q1, Q3 | 12, 15 | 12, 16 | 11, 15 | |
| Missing | 201 (44) | 137 (44) | 64 (43) | |
| Number of HPV vaccine doses received | ||||
| Unvaccinated | 65 (14) | 32 (10) | 33 (22) | |
| 1 dose | 245 (53) | 154 (50) | 91 (61) | <.001 |
| 2 doses | 47 (10) | 34 (11) | 13 (9) | |
| 3 doses | 101 (22) | 90 (29) | 11 (7) | |
| Age at first vaccine dose (years) | ||||
| Mean (SD) | 13.3 (2.5) | 13.7 (2.5) | 12.4 (2.1) | <.001 |
| Median | 13.0 | 13.4 | 11.9 | |
| Q1, Q3 | 11.4, 14.7 | 11.8, 15.4 | 11.2, 13.5 | |
| Never vaccinated | 65 (14) | 32 (10) | 33 (22) | |
| BMI Z-score at first vaccine dose | ||||
| Mean (SD) | 0.3 (1.2) | 0.2 (1.2) | 0.6 (1.4) | .01 |
| Median | 0.3 | 0.3 | 0.7 | |
| Q1, Q3 | −0.5, 1.2 | −0.6, 1.0 | −0.3, 1.7 | |
| Never vaccinated | 65 (14) | 32 (10) | 33 (22) | |
| Missing | 28 (6) | 23 (7) | 5 (3) | |
| Years from last vaccine dose to date of specimen collection | ||||
| Mean (SD) | 2.9 (1.5) | 3.0 (1.6) | 2.8 (1.4) | .14 |
| Median | 2.8 | 2.9 | 2.6 | |
| Q1, Q3 | 1.8, 4.1 | 1.9, 4.3 | 1.6, 3.9 | |
| Never vaccinated | 65 (14) | 32 (10) | 33 (22) | |
| Age at specimen collection | ||||
| Mean (SD) | 16.7 (2.4) | 17.2 (2.3) | 15.7 (2.4) | <.001 |
| Median | 16.9 | 17.5 | 15.7 | |
| Q1, Q3 | 14.9, 18.5 | 15.5, 18.9 | 13.9, 17.3 | |
| BMI Z-score at specimen collection | ||||
| Mean (SD) | 0.4 (1.2) | 0.3 (1.2) | 0.6 (1.3) | .01 |
| Median | 0.5 | 0.4 | 0.7 | |
| Q1, Q3 | −0.5, 1.4 | −0.5, 1.2 | −0.2, 1.7 |
Abbreviations: BMI, body mass index; HPV, human papillomavirus; PHEU, perinatally HIV-exposed, uninfected; PHIV, perinatally HIV-infected; SD, standard deviation; Q1, first quartile; Q3, third quartile.
aData are expressed as no. (%) unless otherwise indicated.
bCategorical data are compared using Fisher exact test and continuous data with the Wilcoxon test.
Seropositivity and GMT by Number of Doses Received and HIV Status
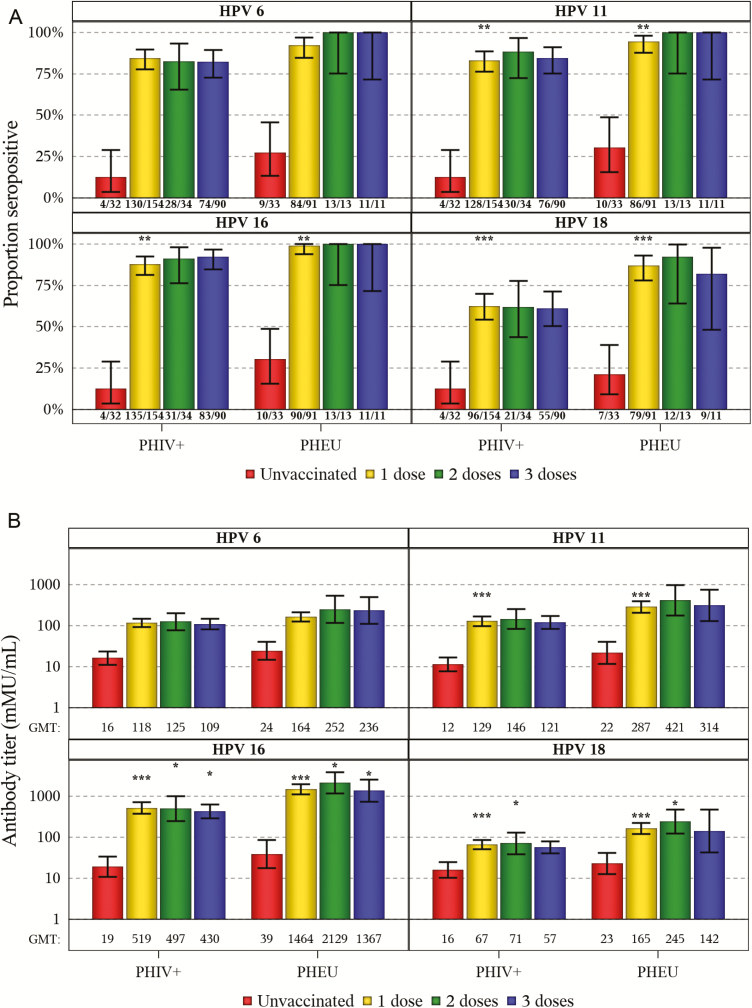
Using cLIA, 83%, 84%, 90%, and 62% of vaccinated PHIV were seropositive for HPV 6, 11, 16. and 18 compared to 94%, 96%, 99%, and 87% of vaccinated PHEU (all significant at P < .01), respectively. Unvaccinated youth in both cohorts had markedly lower seropositivity than their vaccinated peers (Figure 1A). Fewer vaccinated PHIV than PHEU were seropositive across all dose categories and HPV types; this comparison was statistically significant among those receiving 1 dose for HPV 11, 16, and 18. Percent seropositive increased in both cohorts for HPV 18 at all dose categories when using the total IgG assay (Supplemental Figure 1).
Figure 1.
A, Seropositive proportions with 95% confidence intervals (CIs) according to human papillomavirus (HPV) type, by dose and cohort. B, Antibody geometric mean titers with 95% CIs according to HPV type, by dose and cohort. Abbreviations: GMT, geometric mean titer; HIV, human immunodeficiency virus; HPV, human papillomavirus; PHEU, perinatally HIV-exposed, uninfected; PHIV, perinatally HIV-infected. *P < .05, **P < .01, ***P < .001 for PHIV vs. PHEU.
The GMTs for unvaccinated youth of each cohort were substantially lower than for those with any vaccine dose for all HPV types (Figure 1B). Within each cohort, GMTs were similar whether they received 1, 2, or 3 doses. Compared with PHEU, PHIV had lower GMTs regardless of dose. PHIV youth had statistically significantly lower GMTs across all dose categories for HPV 16, for 1 and 2 doses for HPV 18, and for 1 dose for HPV 11. Age at vaccine initiation and time since vaccination are known to influence GMT; we therefore restricted the population to youth who received their first dose before their 15th birthday and adjusted for time from last dose to specimen draw date (Supplemental Figure 2). Significant differences between cohorts remained for HPV 11, 16, and 18.
Factors Associated With GMT Among PHIV Youth
The lower observed seropositivity and GMTs among PHIV youth prompted us to examine factors that may affect titer. We performed bivariable analyses among PHIV youth who received at least 1 vaccine dose (Supplemental Table 1). Higher GMTs were associated with later birth cohort; number of vaccine doses received while on ≥3 months of consecutive combination antiretroviral therapy (cART); younger age; higher CD4%; lower viral load; on ≥3 months of consecutive cART at first dose; and fewer years from last dose to antibody specimen.
In multivariable analysis (Table 2), we found for all 4 HPV types that higher GMTs were associated with younger age and lower HIV RNA at first HPV4 vaccination, as well as fewer years between last vaccine dose and antibody specimen for HPV types 6, 11, and 16. A higher CD4 count at time of first vaccination was associated with higher levels of HPV 11 and 16 and, although not statistically significant, trended in the same direction for HPV 6 and 18.
Table 2.
Multivariable Models for Fold-changes in Antibody Titer for Predictors of Interest Among Perinatally Human Immunodeficiency Virus–infected Participants Who Received at Least One Vaccine Dose, by Human Papillomavirus Type
| Characteristic | HPV 6 | HPV 11 | HPV 16 | HPV 18 | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | |
| Birth cohort ≥1998 | −1.29 (−2.22 to 1.33) |
.35 | −1.04 (−1.98 to 1.82) |
.90 | −1.11 (−2.29 to 1.85) |
.78 | −1.16 (−2.18 to 1.61) |
.64 |
| HPV vaccine doses received | ||||||||
| 1 dose | Ref | Ref | Ref | Ref | ||||
| 2 doses | 1.38 (−1.23 to 2.34) |
.35 | 1.64 (−1.14 to 3.07) |
.23 | 1.55 (−1.30 to 3.15) |
.35 | 1.52 (−1.21 to 2.80) |
.14 |
| 3 or more doses | −1.06 (−1.72 to 1.52) |
−1.00 (−1.77 to 1.76) |
−1.06 (−2.01 to 1.79) |
−1.24 (−2.16 to 1.41) |
||||
| Age at first vaccine dose (per 1 year increase) | −1.16 (−1.27 to −1.05) |
.003 | −1.17 (−1.31 to −1.05) |
.01 | −1.20 (−1.36 to −1.06) |
.01 | −1.16 (−1.29 to −1.04) |
.01 |
| CD4% ≥ 25% at first vaccine dose | 1.49 (−1.04 to 2.29) |
.07 | 1.87 (1.12 to 3.11) |
.02 | 1.85 (1.04 to 3.29) |
.04 | 1.43 (−1.16 to 2.35) |
.16 |
| HIV RNA (copies/mL) at first vaccine dose | ||||||||
| <400 | Ref | Ref | Ref | Ref | ||||
| 400 to <1000 | −2.00 (−3.75 to −1.07) |
<.001 | −2.28 (−4.80 to −1.08) |
<.001 | −3.70 (−8.55 to −1.60) |
<.001 | −2.23 (−4.61 to −1.07) |
<.001 |
| ≥1000 | −2.17 (−3.29 to −1.43) |
−2.95 (−4.83 to −1.80) |
−4.09 (−7.12 to −2.34) |
−2.91 (−4.72 to −1.80) |
||||
| On 3+ months continuous cART at first vaccine dose | 1.17 (−1.35 to 1.83) |
.51 | −1.05 (−1.79 to 1.63) |
.86 | −1.07 (−1.95 to 1.71) |
.83 | 1.60 (−1.05 to 2.71) |
.08 |
| Time from last vaccine dose to antibody specimen (per 1 year increase) | −1.23 (−1.40 to −1.08) |
.002 | −1.22 (−1.43 to −1.05) |
.01 | −1.33 (−1.57 to −1.12) |
.001 | −1.15 (−1.34 to 1.01) |
.07 |
Modeling was performed using the natural log transformed antibody titer outcomes. Parameter estimates and 95% CIs were then exponentiated back to the original scale for reporting. An effect of 1.00 represents no influence of the independent variable on antibody titer in the raw scale, an effect of 2 represents a doubling in titer (ie, 200 to 400 or 1000 to 2000), and an effect of −2 is a halving of titer (ie, 100 to 50). The scale of effect measure ranges from −∞ to −1, then to 1 onward up to ∞. Selection of independent variables to include in the multivariable models was based on univariable results, in which a variable was considered for inclusion in each of the multivariable models for the 4 HPV types if significant at alpha <0.10 in any of the univariable models for these HPV types. Among these selected variables, if certain sets of variables were then judged to be too collinear or causally related for inclusion together in a single multivariable model, the variable with the highest clinical importance was chosen for a given set. Additionally, number of HPV vaccine doses received was forced into the 4 multivariable models. Reported P values are based on Type III sum of squares when variables are >2 categories.
Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HPV, human papillomavirus; Ref, reference.
To examine overall ability to make antibodies in response to vaccination, we compared rubella serostatus to HPV GMT among PHIV youth who received ≥2 MMR doses and 1 to 3 HPV4 doses. The GMT for all 4 HPV types was significantly higher if rubella seropositive than seronegative (Supplemental Table 2).
Incidence Rates of Abnormal Cervical Cytology and Genital Warts
We then assessed the clinical impact of HPV4 vaccination. Among 56 PHIV and 7 PHEU females who were sexually active, cervical cytology-tested, and HPV4 vaccinated, 33 PHIV and 1 PHEU had abnormal cervical cytology (Table 3). Sixteen PHIV females had ASCUS, 13 had low grade (L) squamous intraepithelial lesions (SIL), 1 had high grade (H) SIL, and 3 had SIL of unknown grade; the 1 PHEU case was ASCUS. Compared with PHEU, PHIV had a 5-fold higher incidence of abnormal cytology (IRR 5.2, 95% CI 0.7 to 41.7, P = .12). Restricting to those who initiated vaccination prior to sexual debut attenuated the IRR, but PHIV females still remained at higher risk (IRR 3.0, 95% CI 0.4 to 25.7, P = .32). Three PHIV females in our study population were sexually active, cervical cytology-tested, and unvaccinated, 2 of whom had abnormal cytology. Comparatively, 16 females received all 3 doses prior to sexual debut, 8 of whom were diagnosed with abnormal cytology.
Table 3.
Incidence Rates of Abnormal Cervical Cytology and Genital Warts Among Sexually Active, Vaccinated Participants, by Cohort
| PHIV+ | PHEU (Reference) | ||||
|---|---|---|---|---|---|
| Analysis Sample | # Participants # Events PY |
IR per 100 PY (95% CI) |
# Participants # Events PY |
IR per 100 PY (95% CI) |
IRR (95% CI) P value |
| Pap-tested females abnormal cervical cytology outcomea | |||||
| Crude | n = 56 33 219.7 |
15.0 (10.9–20.6) |
n = 7 1 34.9 |
2.9 (0.4–22.3) |
5.2 (0.7–41.7) .12 |
| Restricted to those initiating vaccine prior to sexual debut | n = 39 23 125.6 |
18.3 (12.5–26.8) |
n = 4 1 16.4 |
6.1 (0.7–50.4) |
3.0 (0.4–25.7) .32 |
| Females and males genital warts outcomeb | |||||
| Restricted to females | n = 110 9 541.8 |
1.7 (0.9–3.2) |
n = 40 1 159.3 |
0.6 (0.1–4.4) |
2.6 (0.3–20.7) .35 |
| Restricted to males | n = 88 0 530.4 |
0.0 (0.0–0.7) |
n = 34 1 152.4 |
0.7 (0.0–3.7) |
NA |
| Restricted to females initiating vaccine prior to sexual debut | n = 81 8 333.9 |
2.4 (1.2–4.7) |
n = 35 1 121.9 |
0.8 (0.1–5.7) |
2.9 (0.4–22.9) .31 |
| Restricted to males initiating vaccine prior to sexual debut | n = 27 0 91.2 |
0.0 (0.0–4.0) |
n = 16 0 40.8 |
0.0 (0.0–9.0) |
NA |
Abbreviations: ASCUS, atypical squamous cells of undetermined significance; CI, confidence interval; CIN 1, cervical intraepithelial neoplasia 1; HGSIL, high grade squamous intraepithelial lesion; HPV, human papillomavirus IR, incidence rate; IRR, incidence rate ratio; LGSIL, low grade squamous intraepithelial lesion; NA, not available; PHEU, perinatally HIV-exposed, uninfected; PHIV, perinatally HIV-infected; PY, person-years.
aFollow-up began at date of sexual debut, estimated from the earliest integer age that participants reported vaginal, anal, or oral sex and adding half a year to the date participants turned that integer age, and extended through until abnormal cytology was detected, or if no event, then the date of most recent Pap test. Events that occurred in this study population included cervical dysplasia, LGSIL, HGSIL, CIN 1, ASCUS – high-risk HPV positive, ASCUS – high-risk HPV negative, and ASCUS – HPV not tested.
bFollow-up began at date of sexual debut, estimated from the earliest integer age that participants reported vaginal, anal, or oral sex and adding half a year to the date participants turned that integer age, and extended through until the last seen date between the Adolescent Master Protocol (AMP) and AMP Up protocol. Events that occurred in this study population only included genital or anal warts.
Incidence of genital warts for PHIV males and PHEU males and females was low. For vaccinated males, 0 of 88 PHIV and 1 of 34 PHEU had genital warts; for vaccinated females, 9 of 110 PHIV and 1 of 40 PHEU had genital warts (Table 3).
Risk of Abnormal Cytology Among PHIV Females in Bivariate Analysis
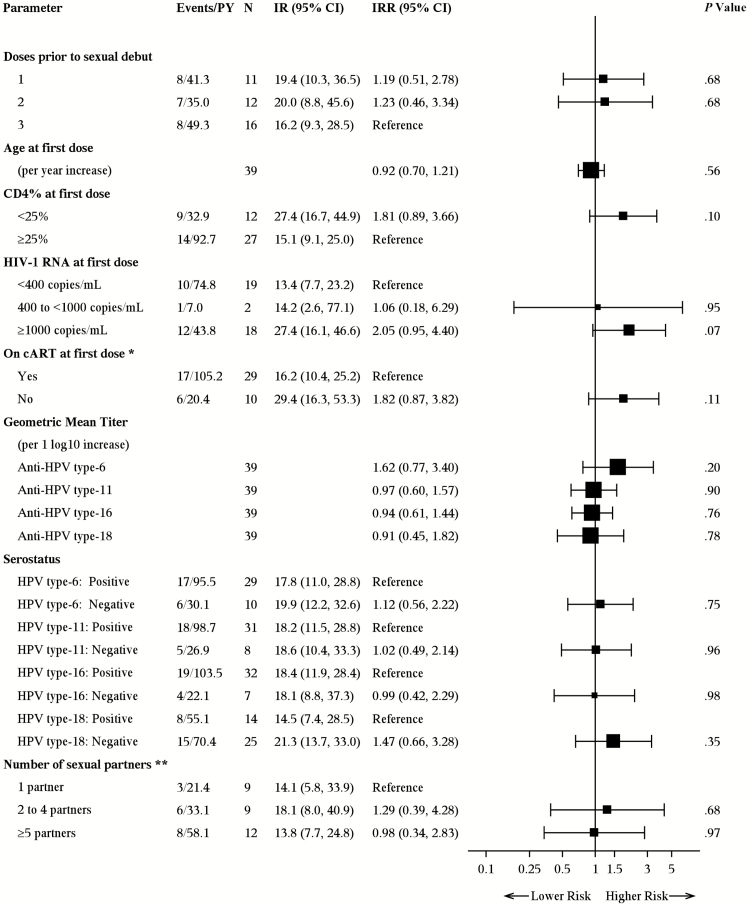
With the high risk of abnormal cytology observed in PHIV females, we sought to identify whether that risk varied by anti-HPV antibody production among other factors. In the 39 sexually active, cervical cytology-tested PHIV females who were vaccinated prior to sexual debut, CD4 <25%, HIV RNA ≥1000 c/mL, and not on ≥3 months of consecutive cART at first vaccination tended to predict higher risk of abnormal cytology, but estimates were not statistically stable (Figure 2). The number of doses prior to sexual debut, age at first dose, HPV titer or serostatus, and number of sexual partners were not associated with abnormal cytology.
Figure 2.
Bivariate analysis of incidence rates of abnormal cervical cytology incidence rate per 100 person-years among sexually active female perinatally HIV-infected participants who were Pap tested and vaccinated prior to sexual debut. Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; HIV-1, human immunodeficiency virus type 1; IR, incidence rate per 100 person-years; IRR, incidence rate ratio; PHIV, perinatally HIV-infected; PY, person-years. *At the time of first vaccine dose, cART must have been both currently prescribed and extended back at least 3 consecutive months prior from that point. **Highest number of reported lifetime sexual partners across vagina, anal, and oral sex acts, per the most recent report prior to the end of follow-up, ie, date of outcome event or last seen date between the Adolescent Master Protocol (AMP) and AMP Up protocols. Nine participants never reported number of sexual partners and were therefore excluded.
DISCUSSION
This population of HPV4-vaccinated PHIV youth had lower rates of seropositivity and GMTs for HPV 6, 11, 16, and 18 antibodies than vaccinated PHEU youth. Importantly, PHIV youth had significantly lower GMT for HPV 16, independent of doses received. Although the level of protection defined by antibody titer remains unknown, these findings are alarming as HPV 16 is more likely to cause disease than the other types [23].
Somewhat surprisingly, we found no differences in GMT by number of doses received within PHIV or PHEU youth. A study in Costa Rica, which was not intended to compare doses, demonstrated good durability of the bivalent vaccine in those receiving only 1 dose [24]. Four years after vaccination, GMTs remained well above those in natural infections and appeared to have plateaued. In contrast, Sankaranarayanan et al [25] reported on an HPV trial that was prematurely halted, resulting in a cohort with 1, 2, and 3 doses. Antibody titers in those receiving a single dose or 2 doses within a 2-month interval were inferior to those receiving 2 doses at a 6-month interval or 3 doses. Our findings suggest that a 3rd dose in HIV-infected individuals does not give the additional boost seen in healthy individuals [26]. Among PHIV youth, 82% to 92% were seropositive after 3 doses for HPV 6, 11, and 16 compared to 100% in the PHEU cohort.
Similar to previous reports in healthy populations [2], HPV 18 seropositivity, relative to the other HPV types, was low for both cohorts with slightly greater than half of PHIV youth testing seropositive. Although this lower seropositivity for HPV 18 has been demonstrated in other studies [20, 27], this does not explain the lower GMT in PHIV since the assay should perform similarly in both cohorts.
As seen with other vaccines including MMR [7, 28], lower GMT in our population was associated with evidence of immunosuppression, including lower CD4 percent and higher viral load at first vaccine dose. In the trial by Levin et al [18], titers for PHIV children showed significant decay after 4–5 years and lower GMT were associated with lower CD4% and high viral load at all time points measured. In our analysis, the lower GMT associated with longer time since vaccination may indicate ongoing decay.
The increased risk of HPV-associated cancers among HIV-infected adults has triggered an interest in vaccine efficacy trials in this population. Although these trials have shown good seroconversion in adolescents and adults with HIV [27, 29, 30], there are no published efficacy trials for the prevention of cervical disease. Because HPV is primarily sexually transmitted, the need to vaccinate PHIV children prior to sexual debut is apparent. Alarmingly, less than half of our PHIV girls and even fewer boys received all 3 HPV vaccine doses prior to sexual debut.
The most striking observation in our study was the high rate of abnormal cytology in PHIV females, regardless of the number of doses received or timing of doses relative to sexual debut. Brogly et al [31] reported a cumulative incidence of 48% in similar aged PHIV adolescents prior to availability of the HPV vaccine, whereas our cumulative incidence was almost 60% among sexually active females with at least 1 HPV4 dose prior to sexual debut. One potential explanation is that these girls may already have been HPV-infected prior to onset of sexual activity. Transmission of HPV DNA has been documented to occur perinatally as well as during infant hygiene care in healthy children [32–35]. It is possible that in HIV-infected children, as observed in HIV-infected adults, HPV acquisition is more likely to occur and clearance less likely [36]. In a small cross-sectional study, 30.4% of PHIV girls with no history of sexual activity or abuse had genital HPV compared to 7.4% in the HIV-uninfected group, underscoring the possibility of nonsexual transmission [37]. The vulnerability of the cervical transformation zone to HPV infection and cancer development is well described and may explain the higher prevalence of abnormal cytology compared to genital warts, which predominantly infects squamous tissue in PHIV girls [38]. The lower number of cases of external genital warts in PHIV boys compared to girls is not well explained but may be due to differences in condom use as well as greater self-surveillance in girls. Another possibility is that sexual activity was underreported.
The association of abnormal cytology with low CD4%, high viral load, and <3 months of consecutive cART at time of initial vaccination also suggests that antibodies generated during times of immunosuppression may be less effective [39, 40]. HPV type replacement is another possible explanation for the abnormal cytology observed.
Our comparison of abnormal cytology by HIV status was limited because very few PHEU girls had reached 21 years, the age at which cervical cancer screening is recommended to start. In contrast, cervical cancer screening in HIV-infected persons is recommended to start within 1 year of sexual debut, resulting in a greater number of screened PHIV girls. Another limitation was the observational nature of the study. We were unable to identify participants who should have been screened but did not have cytology. The small number of participants with cytology and small number that received all 3 vaccine doses prior to sexual debut precluded a sufficiently powered vaccine effectiveness analysis.
In conclusion, the association between level of immunosuppression and lower GMT, as well as observed reduced effectiveness, suggests that PHIV children vaccinated at a time of immunosuppression should be revaccinated when immunocompetent. Alternatively, mother-to-infant HPV transmission may have occurred as HIV-infected mothers have high rates of HPV shedding from genital/oral fluids, and HPV vaccine trials in PHIV infants could be considered. As HPV4 vaccine effectiveness was much lower in PHIV girls than expected, further studies are needed to assess HPV type associated with these cytologic abnormalities and the natural history of these lesions because their progression potential remains unknown.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
The following institutions, clinical site investigators, and staff participated in conducting Pediatric HIV/AIDS Cohort Study (PHACS) Adolescent Master Protocol (AMP) and AMP Up in 2016, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Mirza Baig, Alma Villegas; Children’s Diagnostic & Treatment Center: Ana Puga, Sandra Navarro, Patricia A. Garvie, James Blood; Boston Children’s Hospital: Sandra K. Burchett, Nancy Karthas, Betsy Kammerer; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Ray Shaw, Raphaelle Auguste; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Juliette Johnson; St. Christopher’s Hospital for Children: Janet S. Chen, Maria Garcia Bulkley, Latreaca Ivey, Mitzie Grant; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins, Jamie Russell-Bell; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University School of Medicine: Margarita Silio, Medea Gabriel, Patricia Sirois; University of California, San Diego: Stephen A. Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Eric Cagwin, Emily Barr, Alisa Katai; University of Miami: Gwendolyn Scott, Grace Alvarez, Gabriel Fernandez, Anai Cuadra.
Acknowledgments. The authors thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. Data management services were provided by Frontier Science and Technology Research Foundation (Principal Investigator [PI]: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
Disclaimer. The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or US Department of Health and Human Services or Merck Sharp & Dohme Corp.
Financial support. The PHACS was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant numbers are HD02102 and HD052104) with cofunding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T. H. Chan School of Public Health (HD052102) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-principal Investigator: Ellen Chadwick; Project Director: Patrick Davis). Supported in part by a research grant from Investigator Initiated Studies Program of Merck Sharp & Dohme Corp.
Potential conflicts of interest. A.-B. M. is a member of Merck Global Human Papillomavirus Vaccine Advisory Board. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Pediatric HIV/AIDS Cohort Study:
Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter, William Shearer, Mary Paul, Norma Cooper, Lynnette Harris, Murli Purswani, Mahboobullah Mirza Baig, Alma Villegas, Ana Puga, Sandra Navarro, Patricia A Garvie, James Blood, Sandra K Burchett, Nancy Karthas, Betsy Kammerer, Andrew Wiznia, Marlene Burey, Ray Shaw, Raphaelle Auguste, Arry Dieudonne, Linda Bettica, Juliette Johnson, Janet S Chen, Maria Garcia Bulkley, Latreaca Ivey, Mitzie Grant, Katherine Knapp, Kim Allison, Megan Wilkins, Jamie Russell-Bell, Midnela Acevedo-Flores, Heida Rios, Vivian Olivera, Margarita Silio, Medea Gabriel, Patricia Sirois, Stephen A Spector, Kim Norris, Sharon Nichols, Elizabeth McFarland, Eric Cagwin, Emily Barr, Alisa Katai, Gwendolyn Scott, Grace Alvarez, Gabriel Fernandez, and Anai Cuadra
References
- 1. Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis 2009; 199:926–35. [DOI] [PubMed] [Google Scholar]
- 2. Joura EA, Kjaer SK, Wheeler CM, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine 2008; 26:6844–51. [DOI] [PubMed] [Google Scholar]
- 3. Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201–9. [DOI] [PubMed] [Google Scholar]
- 4. Drolet M, Bénard É, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crowe E, Pandeya N, Brotherton JM, et al. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ 2014; 348:g1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benard VB, Castle PE, Jenison SA, et al. ; New Mexico HPV Pap Registry Steering Committee Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol 2017; 3:833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abzug MJ. Vaccination in the immunocompromised child: a probe of immune reconstitution. Pediatr Infect Dis J 2009; 28:233–6. [DOI] [PubMed] [Google Scholar]
- 8. Siberry GK, Patel K, Bellini WJ, et al. ; Pediatric HIV AIDS Cohort Study (PHACS); Pediatric HIV AIDS Cohort Study PHACS Immunity to measles, mumps, and rubella in US children with perinatal HIV infection or perinatal HIV exposure without infection. Clin Infect Dis 2015; 61:988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Purswani MU, Karalius B, Yao TJ, et al. ; Pediatric HIV/AIDS Cohort Study (PHACS) Prevalence and persistence of varicella antibodies in previously immunized children and youth with perinatal HIV-1 infection. Clin Infect Dis 2016; 62:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frisch M, Biggar RJ, Engels EA, Goedert JJ; AIDS-Cancer Match Registry Study Group Association of cancer with AIDS-related immunosuppression in adults. JAMA 2001; 285:1736–45. [DOI] [PubMed] [Google Scholar]
- 11. Wang CJ, Sparano J, Palefsky JM. Human immunodeficiency virus/AIDS, human papillomavirus, and anal cancer. Surg Oncol Clin N Am 2017; 26:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levin MJ, Moscicki AB, Song LY, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr 2010; 55:– 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinberg A, Song LY, Saah A, et al. ; IMPAACT/PACTG P1047 Team Humoral, mucosal, and cell-mediated immunity against vaccine and nonvaccine genotypes after administration of quadrivalent human papillomavirus vaccine to HIV-infected children. J Infect Dis 2012; 206:1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levin MJ, Huang S, Moscicki AB, et al. ; IMPAACT P1085 Protocol Team Four-year persistence of type-specific immunity after quadrivalent human papillomavirus vaccination in HIV-infected children: effect of a fourth dose of vaccine. Vaccine 2017; 35:1712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jit MCY, Brisson M.. 2-dose vs. 3-dose HPV vaccination: can a 2-dose strategy be the best use of resources? Prague: Eurogin Congress; 2012. [Google Scholar]
- 16. Van Dyke RB, Patel K, Siberry GK, et al. Antiretroviral treatment of US children with perinatally acquired HIV infection: temporal changes in therapy between 1991 and 2009 and predictors of immunologic and virologic outcomes. J Acquir Immune Defic Syndr 2011; 57:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tassiopoulos K, Patel K, Alperen J, et al. ; Pediatric HIV/AIDS Cohort Study Following young people with perinatal HIV infection from adolescence into adulthood: the protocol for PHACS AMP Up, a prospective cohort study. BMJ Open 2016; 6:e011396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tassiopoulos K, Moscicki AB, Mellins C, et al. ; Pediatric HIV/AIDS Cohort Study Sexual risk behavior among youth with perinatal HIV infection in the United States: predictors and implications for intervention development. Clin Infect Dis 2013; 56:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dias D, Van Doren J, Schlottmann S, et al. Optimization and validation of a multiplexed Luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol 2005; 12:959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Opalka D, Lachman CE, MacMullen SA, et al. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol 2003; 10:108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Opalka D, Matys K, Bojczuk P, et al. Multiplexed serologic assay for nine anogenital human papillomavirus types. Clin Vaccine Immunol 2010; 17:818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 2006; 24:5571–83. [DOI] [PubMed] [Google Scholar]
- 23. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017; 141:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kreimer AR, Rodriguez AC, Hildesheim A, et al. ; CVT Vaccine Group Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst 2011; 103:1444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sankaranarayanan R, Prabhu PR, Pawlita M, et al. ; Indian HPV Vaccine Study Group Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol 2016; 17:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309:1793–802. [DOI] [PubMed] [Google Scholar]
- 27. Kahn JA, Xu J, Kapogiannis BG, Sleasman JW. Brief report: antibody responses to quadrivalent HPV vaccination in HIV-infected young women as measured by total IgG and competitive Luminex immunoassay. J Acquir Immune Defic Syndr 2017; 75:241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moss WJ, Clements CJ, Halsey NA. Immunization of children at risk of infection with human immunodeficiency virus. Bull World Health Organ 2003; 81:61–70. [PMC free article] [PubMed] [Google Scholar]
- 29. Giacomet V, Penagini F, Trabattoni D, et al. Safety and immunogenicity of a quadrivalent human papillomavirus vaccine in HIV-infected and HIV-negative adolescents and young adults. Vaccine 2014; 32:5657–61. [DOI] [PubMed] [Google Scholar]
- 30. Kojic EM, Kang M, Cespedes MS, et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis 2014; 59:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brogly SB, Watts DH, Ylitalo N, et al. Reproductive health of adolescent girls perinatally infected with HIV. Am J Public Health 2007; 97:1047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rintala MA, Grénman SE, Puranen MH, et al. Transmission of high-risk human papillomavirus (HPV) between parents and infant: a prospective study of HPV in families in Finland. J Clin Microbiol 2005; 43:376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith EM, Parker MA, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP. Evidence for vertical transmission of HPV from mothers to infants. Infect Dis Obstet Gynecol 2010; 2010:326369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castellsagué X, Drudis T, Cañadas MP, et al. Human papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: a prospective study in Spain. BMC Infect Dis 2009; 9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rintala MA, Grénman SE, Järvenkylä ME, Syrjänen KJ, Syrjänen SM. High-risk types of human papillomavirus (HPV) DNA in oral and genital mucosa of infants during their first 3 years of life: experience from the Finnish HPV Family Study. Clin Infect Dis 2005; 41:1728–33. [DOI] [PubMed] [Google Scholar]
- 36. Krishnamurti U, Unger ER. Pathobiology of human papillomaviruses in human immunodeficiency virus-infected persons. Semin Diagn Pathol 2017; 34:364–70. [DOI] [PubMed] [Google Scholar]
- 37. Moscicki AB, Puga A, Farhat S, Ma Y. Human papillomavirus infections in nonsexually active perinatally HIV infected children. AIDS Patient Care STDS 2014; 28:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Herfs M, Yamamoto Y, Laury A, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A 2012; 109:10516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gunn BM, Alter G. Modulating antibody functionality in infectious disease and vaccination. Trends Mol Med 2016; 22:969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang Y, Ferrari G, Alter G, et al. Diversity of antiviral IgG effector activities observed in HIV-infected and vaccinated subjects. J Immunol 2016; 197:4603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.