Abstract
Background
We characterized associations between frailty and incident cardiovascular disease (CVD), diabetes mellitus (DM), bone disease, and mortality within a cohort of aging persons with human immunodeficiency virus (PWH).
Methods
Participants underwent frailty evaluations using the Fried frailty assessment (baseline and annually). Frailty was defined as having ≥3 frailty criteria. Clinical outcomes of mortality, CVD events, DM, and bone disease events were recorded throughout the study period (baseline to most recent study or clinic visit, or date of clinical outcome, whichever came first). Poisson regression models were used to evaluate associations between baseline frailty, change in frailty score over 48 weeks, and each clinical outcome.
Results
Among 821 men and 195 women (median age 51 years), 62 (6%) were frail at baseline. Frailty scores increased by ≥1 component among 194 participants (19%) from baseline to 48 weeks. Baseline frailty was associated with an increased risk of incident CVD and DM, with a trend toward a significant association with bone events. Among frailty components, slow gait speed was associated with incident DM and borderline associated with incident CVD. An increase in frailty from baseline to week 48 was associated with mortality but not with the other clinical outcomes.
Conclusions
Baseline frailty was associated with multiple adverse health outcomes (incident CVD, DM, and bone disease), while increase in frailty score was associated with mortality among PWH engaged in care. Incorporation of frailty assessments into the care of PWH may assist in improvement of functional status and risk stratification for age-related chronic diseases.
Keywords: human immunodeficiency virus, frailty, chronic diseases, mortality
Among persons with human immunodeficiency virus (HIV), frailty is associated with risk for cardiovascular disease, diabetes mellitus, and bone disease. An increase in frailty is associated with mortality. Routine frailty assessments may help in chronic disease risk stratification.
As potent antiretroviral therapy (ART) has markedly improved survival of persons with human immunodeficiency virus (PWH) [1], chronic age-related diseases have emerged as predominant causes of death among ART-treated persons [2]. These conditions disproportionally affect aging PWH and include cardiovascular disease (CVD), metabolic diseases, and bone demineralization [3–5]. Further compromising health among aging PWH is frailty, a syndrome of dysregulation of multiple biologic systems that leads to physical weakness and functional decline. Frailty prevalence increases with age after age 65 years in the general population [6]. However, it has been observed to occur up to a decade earlier among PWH [7] and is associated with excess burden of mortality and morbidity [8]. The frailty phenotype is a constellation of age-related symptoms that is associated with multiple adverse health consequences. Previous studies have demonstrated an association between frailty and risk of poor health outcomes among PWH (such as neurocognitive impairment, falls, and disability) [9–11]. However, the role of frailty as a predictor for subsequent development of specific age-related chronic diseases in this population is poorly understood. Here, we sought to ascertain associations between baseline frailty and changes in frailty over 48 weeks with clinical outcomes including mortality, incident CVD, diabetes mellitus (DM), and bone disease. We postulated that frailty is positively associated with the occurrence of nonfatal disease-specific clinical events and mortality and may therefore serve as a clinical predictor for these events.
METHODS
Study Population
AIDS Clinical Trials Group (ACTG) A5322 (HAILO, the Human Immunodeficiency Virus [HIV] Infection, Aging, Immune Function Long-Term Observational Study) is an ongoing, observational study of 1035 older PWH (age ≥40 years at enrollment) that longitudinally evaluates associations between ART, aging, and inflammation with incidence of non-AIDS clinical events, mortality, and functional status. Participants were recruited from a previous US longitudinal cohort, ACTG Longitudinal Linked Randomized Trials, which enrolled participants between 2000 and 2007 who were followed through 2013 [12]. HAILO participants were enrolled in 2013–2014; the 1016 participants who had a baseline frailty assessment are included in this analysis. Study visits for HAILO participants occur semiannually with medication review, chart abstractions, plasma/serum collection, fasting laboratory tests, and falls interview. Frailty assessments, body measurements, neurocognitive evaluations, additional specimen collection, and questionnaires regarding substance use, sexual behavior, insurance status, and instrumental activities of daily living are performed annually.
Frailty Assessment
Frailty was assessed among all participants using the Fried frailty assessment [6]. As previously described, the assessment includes the 5 components of weak grip; slow gait speed on a 4-meter walk; and self-reported weight loss, exhaustion, and limitations in ability to undertake vigorous physical activity [13]. Frailty at baseline (time of HAILO entry visit) was evaluated, and participants were categorized as frail if they met 3–5 criteria, pre-frail if they met 1–2 criteria, or non-frail if they did not meet any criteria. Change in frailty was defined as ≥1 component increase in frailty (vs no change or a decrease in frailty; separate assessment of decrease in frailty was not possible due to small numbers) from baseline to week 48.
Clinical Outcomes
We included clinical events that occurred after the baseline frailty assessment. Individuals with prevalent disease (any history of a diagnosis prior to baseline) with the exception of bone disease (for this outcome, history of bone disease was evaluated as a potential confounder) were excluded. Cardiovascular disease included coronary artery disease (with or without revascularization surgery), myocardial infarction, stroke/transient ischemic attack, angina, peripheral arterial disease, cardiomyopathy/heart failure, arrhythmia, deep vein thrombosis, and pulmonary embolism. Diabetes was defined as use of diabetic medication, hemoglobin A1c ≥6.5%, or a medical diagnosis of diabetes. Bone disease included fracture, avascular necrosis, osteopenia, or osteoporosis. Mortality was defined as death due to any cause.
Demographic, Behavioral, and Clinical Factors Considered as Confounders
All covariates were assessed at baseline unless otherwise indicated. Race/ethnicity was categorized as black (non-Hispanic), white (non-Hispanic), Hispanic/other; age as 40–49, 50–59, and ≥60 years; and sex as male or female. Education level was categorized as “did not complete high school,” “completed high school,” or “completed education beyond high school.” Self-reported smoking was categorized as “never smoker,” “former smoker,” or “current smoker.” Alcohol use assessment included a categorical variable for binge drinking (≥5 drinks for men, ≥4 for women within a 2-hour period), categorized as “no drinking,” “no binging drinking,” “binge drinking once/month,” and “binge drinking more than once/month.” A separate variable for self-reported frequency of alcohol use was categorized as “no drinking,” “light/moderate drinking” (1–14 drinks/week for men, 1–7 drinks/week for women, and no binge drinking), or “heavy drinking” (>14 drinks/week for men, >7 drinks/week for women, or binge drinking). Physical activity was defined as ≥3 days of moderate or vigorous activity per week. Body mass index (BMI) was categorized as underweight (BMI, <18.5 kg/m2), normal (BMI, 18.5 to <25 kg/m2), overweight (BMI, 25 to 30 kg/m2), and obese (BMI, >30 kg/m2). Waist circumference was categorized as low (≤94 cm for men, ≤80 cm for women), high (>94 cm for men, >80 cm for women), or unknown. Hypertension was defined as use of antihypertensive medications or diagnosed hypertension. Hyperlipidemia was defined as any of the following: use of lipid-lowering medications, diagnosis of hyperlipidemia, or laboratory values consistent with hyperlipidemia (low-density lipoprotein ≥160 mg/dL, total cholesterol ≥200 mg/dL, or triglycerides ≥200 mg/dL). Human immunodeficiency virus-related characteristics considered included CD4 T-lymphocyte cell count/mm3 (CD4) at time of ART initiation and at baseline, plasma HIV-RNA level at time of ART initiation, and proportion of time under observation prior to baseline with HIV RNA level <200 copies/mL. Antiretroviral therapy exposure–related factors included duration of ART; whether or not the participant remained on their initial, randomized ART regimen; history and duration of protease inhibitor use; and history and duration of tenofovir disoproxil fumarate use. Likely HIV transmission route was categorized as injection drug use, men who have sex with men, heterosexual sex, or other/unknown (assessed at enrollment into initial clinical trial). Hepatitis C virus (HCV) infection was defined by a positive HCV serology. History of CVD, non-AIDS–defining cancers, AIDS-defining events, liver disease, renal disease, bone disease, diabetes, family history of CVD, family history of diabetes, and depression were also considered as potential confounders for specific outcomes.
Statistical Analyses
For each clinical outcome, we calculated overall rates per 100 person-years and their 95% confidence intervals (CIs) using exact Poisson confidence limits. Follow-up time was time from baseline to date of the most recent visit, last clinic date for off-study participants, or date of clinical outcome, whichever occurred first.
Separate Poisson regression models were used to estimate the associations between frailty and each clinical outcome. For each frailty-outcome model, we made the a priori decision to force variables into the model that are known strong risk factors for the outcome (Figure 1). We then proceeded to assess other covariates as potential confounders. Each covariate was added individually into the model, including frailty, and the variables that were included a priori. Covariates that changed the effect estimate by ≥10% were kept in the final multivariable model. After fitting the final multivariable model for baseline frailty and the clinical outcome, we replaced frailty with (a) grip and (b) walk speed.
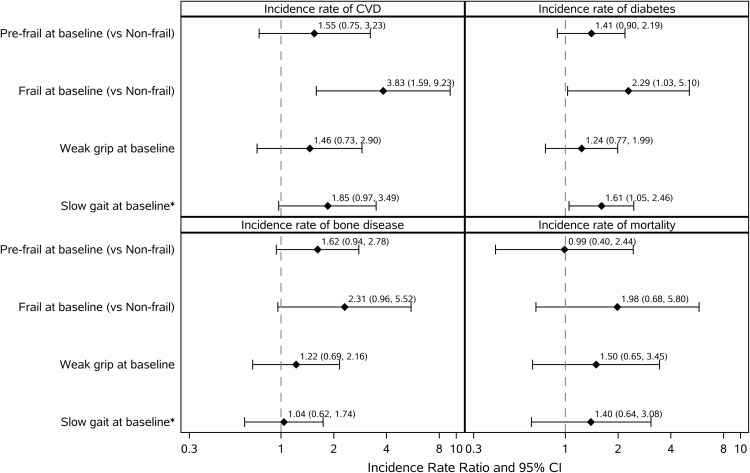
Figure 1.
Multivariable associations between baseline frailty, grip strength, gait speed, and rates of incident events. Each panel summarizes 3 separate multivariable models: frail/pre-frail and incident event, grip strength and incident event, walk speed and incident event. Model for CVD adjusted for route of human immunodeficiency virus (HIV) transmission, with age, history of diabetes, smoking, hyperlipidemia, hypertension, and family history of CVD forced in as covariates. Model for diabetes, with age, race/ethnicity, family history of diabetes, body mass index, and hyperlipidemia forced in as covariates. Model for bone disease, with age forced in as a covariate. Model for mortality adjusted for sex, history of diabetes, and route of HIV transmission, with age forced in as a covariate. Abbreviations: CI, confidence interval; CVD, cardiovascular disease. *Slow gait speed is defined as >4 seconds for a 4-meter walk.
The same model-building procedures were used to evaluate the association between frailty change from baseline to week 48 and each clinical outcome. For this evaluation, we included clinical events that occurred after the second frailty assessment at week 48. All participants lost to follow-up or who experienced the clinical outcome before week 48 were excluded (with the exception of bone disease, where history of bone disease prior to week 48 was evaluated as a potential confounder). While physical activity at baseline was not included as a potential confounder in any of the evaluations of baseline frailty and clinical outcomes (since frailty at baseline may be affected by physical activity), baseline physical activity was evaluated as a potential confounder when evaluating change in frailty from baseline to week 48.
We also summarized the time to event in months for each outcome to determine whether any outcomes occurred close to the time of the frailty assessment.
RESULTS
Among 821 men and 195 women, 48% were white, non-Hispanic and 46% were between 40 and 49 years of age at study entry. The majority (926, 91%) were virally suppressed (HIV RNA <50 copies/mL) at baseline with a median baseline CD4 of 621 cells/µL. With the exception of 7 participants, all were taking ART upon study entry (5 of those 7 started ART after study entry). Participant demographic and clinical characteristics are shown in Table 1. At baseline, 390 (38%) were pre-frail and 62 (6%) were frail. Frailty scores increased in 1 or more components among 194 (19%) participants from baseline to 48 weeks. Among these participants, increases in the following frailty components were observed: weight loss (N = 22), low physical activity (N = 53), exhaustion (N = 72), grip weakness (N = 80), and slow gait speed (N = 26).
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Characteristic | Total (N = 1016) |
|---|---|
| Age at baseline, years | |
| Median (Q1, Q3) | 51 (46, 56) |
| <50 | 446 (44%) |
| 50–59 | 412 (40%) |
| ≥ 60 | 158 (16%) |
| Sex | |
| Male | 821 (81%) |
| Female | 195 (19%) |
| Race/Ethnicity | |
| White, non-Hispanic | 486 (48%) |
| Black, non-Hispanic | 300 (29%) |
| Hispanic+other | 230 (23%) |
| Education level | |
| <High school | 152 (15%) |
| High school | 223 (22%) |
| >High school | 641 (63%) |
| Frailty status | |
| Non-frail | 564 (56%) |
| Pre-frail | 390 (38%) |
| Frail | 62 (6%) |
| 4-meter walk time (seconds) at baseline | |
| ≤4 seconds | 602 (59%) |
| >4 seconds | 414 (41%) |
| Weak grip at baseline | 224 (22%) |
| Body mass index category at baseline | |
| Underweight | 6 (1%) |
| Normal | 324 (32%) |
| Overweight | 394 (39%) |
| Obese | 292 (28%) |
| Likely route of HIV transmission | |
| Intravenous drug use | 33 (3%) |
| Men who have sex with men | 607 (60%) |
| Heterosexual | 301 (30%) |
| Other/Unknown | 75 (7%) |
| HIV RNA at baseline (copies/mL)a | |
| <50 | 926 (91%) |
| ≥50 | 87 (9%) |
| Proportion of time with HIV RNA <200 copies/mL before baseline | |
| ≤75% | 185 (24%) |
| >75% | 602 (76%) |
| CD4 count at baseline (cells/µL) | |
| Medan (Q1, Q3) | 621 (452, 827) |
| Smoking status at baseline | |
| Never | 415 (41%) |
| Prior smoker | 344 (34%) |
| Current smoker | 257 (25%) |
| Diabetes history at baseline | 113 (11%) |
| Family history of diabetes at baseline | 381 (38%) |
| History of CVD at baseline | 68 (7%) |
| Family history of CVD at baseline | 312 (31%) |
| History of hypertension at baseline | 599 (59%) |
| History of hyperlipidemia at baseline | 880 (87%) |
| Days of vigorous/moderate activities per week at baselineb | |
| <3 days | 454 (45%) |
| ≥3 days | 506 (50%) |
| History of bone disease at baseline | 181 (18%) |
Abbreviations: CVD, cardiovascular disease; HIV, human immunodeficiency virus.
aThree individuals (0%) missing baseline HIV RNA information.
bA total of 56 individuals (6%) missing physical activity information.
Median length of follow-up was 4.0 years (interquartile range = 0.3 years). Twenty-seven participants died during follow-up; the median time from their first frailty assessment to death was 22.8 months. The highest event rate was observed for diabetes with 84 events (incidence rate per 100 person-years, 2.58 [95% CI, 2.06–3.19]), followed by bone disease with 61 events (incidence rate per 100 person-years, 1.65 [95% CI, 1.26–2.12]), CVD with 43 events (incidence rate per 100 person-years, 1.23 [95% CI, 0.89–1.66]), and death with 27 events (incidence rate per 100 person-years, 0.7 [95% CI, 0.46–1.02]). Median time from first frailty assessment to incident diabetes was 23.0 months, median time to bone disease event was 23.3 months, and median time to incident CVD event was 21.1 months.
As shown in Figure 1, in the multivariable analyses, baseline frailty was associated with an increased risk of incident CVD (incidence rate ratio [IRR], 3.83 [1.59–9.23]; P = .003) and incident diabetes (IRR, 2.29 [1.03–5.10]; P = .04), with a trend toward a significant association with incident bone events (IRR, 2.31 [0.96–5.52]; P = .06). Among the components of frailty, slow gait speed was associated with incident diabetes (IRR, 1.61 [1.05–2.46]; P = .03) and borderline associated with incident CVD (IRR, 1.85 [0.97–3.49]; P = .06) but was not associated with bone events. Grip strength was not significantly associated with any of the clinical outcomes.
An increase in frailty from baseline to week 48 was significantly associated with mortality (IRR, 3.78 [1.52–9.39]; P = .004) but was not associated with incident CVD, diabetes, or bone events (Table 2). Baseline pre-frailty was not significantly associated with any of the clinical outcomes.
Table 2.
Multivariable Associations Between Frailty Change From Baseline to Week 48 and Incident Events
| Change in Frailty Score From Baseline to Week 48 (≥1 vs ≤ 0) | |||
|---|---|---|---|
| Event Type | Number of Events | Incidence Rate Ratio (95% Confidence Interval) | P Value |
| CVD | 26 | 1.16 (.47–2.84) | .8 |
| Diabetes | 51 | 1.00 (.48–2.06) | >.9 |
| Bone disease | 45 | 1.27 (.64–2.50) | .5 |
| Death | 19 | 3.78 (1.52–9.39) | .004 |
Model for cardiovascular disease (CVD) adjusted for age, family history of CVD, history of diabetes, smoking, hypertension, and hyperlipidemia. Model for diabetes adjusted for family history of diabetes, age, race/ethnicity, and body mass index. Model for bone disease adjusted for age and physical activity. Model for mortality adjusted for sex, age, and physical activity.
DISCUSSION
In this large, well-characterized cohort of PWH, the vast majority of whom were virally suppressed with CD4 count >600 cells/µL, the presence of frailty at study entry was associated with greater risk for subsequent incident CVD, diabetes, and bone disease independent of traditional risk factors for such diseases, while increase in frailty over 48 weeks was associated with increased risk for death. Furthermore, the objective frailty component of slow gait, a strong predictor of mortality among older adults without HIV [14], was associated with incident diabetes and CVD (borderline) but not with mortality or bone disease event. These observations illustrate an intimate relationship between frailty, a marker of vulnerability and physiologic dysregulation, and the development of age-related chronic diseases and death among middle-aged and older PWH.
The occurrence of the frailty phenotype prior to clinical disease onset suggests that frailty is associated with the development of these chronic diseases in our cohort. The detrimental impact of frailty on many subsequent poor health outcomes (including falls, hospitalization, disability, and mortality) has been well described among both PWH and in the general population [9–11, 15–22]. Increases in frailty score have also been shown to be associated with increased mortality risk in the general population [23], so our observed mortality association is not unexpected. Nonetheless, since PWH have a higher prevalence of frailty and progress to frailty faster than persons in the general population [7, 24], our observation underscores a potentially greater risk of mortality consequent to frailty among PWH.
Prior studies that evaluated associations between frailty and risk of chronic diseases, however, have reported variable results. Among the general population, frailty (as well as pre-frailty) has been found to be independently associated with an increased risk of CVD [25, 26], with slow gait speed, or variations of it, also associated with this increased risk [26, 27]. In the Multicenter AIDS Cohort Study, frailty was associated with subclinical atherosclerosis in men without HIV but not in PWH. An association between higher frailty score and CVD was suggested in a prior cross-sectional analysis of our cohort [13]; however, this association was not statistically significant in the final multivariable model. In this current, prospective analysis, the association between baseline frailty and risk of incident CVD, as well as the borderline association between slow gait speed and risk of incident CVD, among PWH appears to increasingly corroborate findings observed in the general population.
The association between frailty and DM is less clear. Insulin resistance and/or DM have been identified as an antecedent to frailty among elderly persons without HIV as well as PWH, but not vice versa [28–30]. Our current finding of frailty (and specifically slow gait speed) as an independent risk for incident DM presents an opposing sequence of events, further underscoring the multifarious relationship of frailty to age-related changes in physiologic processes. Conversely, frailty as a predictor of fracture has also been widely demonstrated in the general population and PWH [20, 31, 32], and our findings are consonant with these associations. Despite the low number of clinical events and relatively low prevalence of frailty (6%) in our cohort, the significant impact of frailty on risk of several chronic diseases and mortality within a single cohort is noteworthy. Further, our cohort has a high rate of durable viral suppression, which renders our observed associations between frailty and age-related, chronic diseases clinically relevant and generalizable to virally suppressed PWH in clinical care. Another strength of this analysis is that our population was recruited from a well-characterized cohort with at least several years of prior clinical data available, optimizing accuracy in our clinical and HIV-specific variables.
While our findings suggest that baseline frailty can precede certain chronic diseases among PWH, they do not establish a causal pathway between frailty and chronic disease development. Pathophysiologic mechanisms may exist, however, that are common to both. Similar to the widely studied associations between chronic immune activation and inflammation as contributors to specific chronic diseases among PWH, elevations in levels of multiple systemic markers of inflammation have been associated with the development of frailty among PWH [33–37]. Indeed, levels of specific markers of inflammation associated with CVD among PWH, such as interleukin-6, high sensitivity C-reactive protein, sCD14, and sCD163, have been independently associated with frailty [33, 35, 37–40]. Inflammation associated with frailty and age-related chronic disease may be particularly relevant to PWH since increased inflammation is fundamental to HIV pathophysiology. Additionally, accelerated sarcopenia and adiposity can occur in the setting of HIV [41], which may consequently contribute to sedentary lifestyle, thereby hastening metabolic derangements (such as insulin resistance) and contributing to chronic disease development. Functional impairments characteristic of frailty can further compound this process. These are especially important considerations in risk stratification for certain diseases, as traditional screening tools, such as the American College of Cardiology/American Heart Association pooled cohort equation and the Fracture Risk Assessment Tool (FRAX) score, underpredict disease-specific clinical events for PWH [42, 43]. Our findings support the utility of frailty assessments in the standard health maintenance of aging PWH. This may serve as a simple yet high-impact predictor of chronic, age-related diseases and better inform current predictive risk models. However, further investigation is needed to determine optimal strategies to incorporate use of frailty scores in chronic disease risk stratification. Once identified, frailty development may be halted or reduced by physical activity training. Such programs have been shown to increase strength, balance, and physical activity among elderly HIV-negative participants, ultimately leading to reduction in frailty [44–46]. For aging PWH, structured exercise programs have been shown to improve weight, strength, and cardiorespiratory fitness and to even reduce the number of frailty criteria [47, 48]. Such interventions are low risk and, at minimum, may improve health outcomes directly consequent to frailty.
Limitations to our work exist. While our median age of 51 years is consistent with the median age of PWH in the United States, our outcomes may not be generalizable to younger PWH. The majority of HAILO participants are durably virally suppressed and compliant with healthcare and research participation. Our study results may therefore not be generalizable to individuals with intermittent virologic nonsuppression or gaps in care. We observed a small number of deaths relative to the other outcomes in our study; therefore, our analysis may have been underpowered for the mortality outcome. In our final regression models, we adjusted for covariates known to be traditionally strong risk factors for each outcome of interest. It is possible, however, that there exist other risk factors we did not consider, which may have resulted in unmeasured confounding. Further, the self-reported nature of smoking and other substance use, as well as family history of specific diseases, may have led to underreporting of these entities. Three components of the frailty evaluation (weight loss, exhaustion, and low physical activity) require subjective reporting and may, in part, be consequent to clinical HIV infection itself rather than age-related frailty. Ascertaining HIV-induced functional declines, which may imply reversibility with optimization of HIV care (vs frailty associated with factors other than HIV), should be attempted when assessing frailty among PWH.
In summary, we found that the presence of frailty and increases in frailty scores over time among treated, virally suppressed PWH preceded multiple chronic disease-specific events and mortality. Routine incorporation of annual frailty assessments in the care of PWH (perhaps beginning as early as the sixth decade of life) can enhance the characterization of age-related functional declines and may thereby aid in risk stratification for the development of age-associated chronic diseases. Further, frailty may comprise a modifiable target for interventions aimed at improvement of functional status and, potentially, comorbidity avoidance.
Notes
Author contributions.All authors contributed to study design, data interpretation, manuscript revision, and approval of the final draft. K. W. and K. T. performed the data analysis. S. G. K. and F. L. P. prepared the initial manuscript.
Acknowledgments.The authors thank all study volunteers of A5001 (AIDS Clinical Trials Group Longitudinal Linked Randomized Trials [ALLRT]) and A5322 (the Human Immunodeficiency Virus Infection, Aging, Immune Function Long-Term Observational Study), the AIDS Clinical Trials Group (ACTG) clinical units, and the ACTG.
Disclaimer.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support.Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the NIH under awards UM1 AI068634, UM1 AI068636, and UM1 AI106701.
Potential conflicts of interest.K. M. E. has received grant support from Gilead Sciences and serves on an advisory panel for ViiV. S. L. K. has received grant support from Gilead Sciences. F. J. P. is a consultant and/or on the speakers bureau for Gilead Sciences, Janssen Pharmaceuticals, Merck and Co., and ViiV. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 2. Palella FJ Jr, Baker RK, Moorman AC, et al. ; HIV Outpatient Study Investigators Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43:27–34. [DOI] [PubMed] [Google Scholar]
- 3. Bergersen BM, Sandvik L, Bruun JN, Tonstad S. Elevated Framingham risk score in HIV-positive patients on highly active antiretroviral therapy: results from a Norwegian study of 721 subjects. Eur J Clin Microbiol Infect Dis 2004; 23:625–30. [DOI] [PubMed] [Google Scholar]
- 4. Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 5. Rodger AJ, Lodwick R, Schechter M, et al. ; INSIGHT SMART, ESPRIT Study Groups Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS 2013; 27:973–9. [DOI] [PubMed] [Google Scholar]
- 6. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–56. [DOI] [PubMed] [Google Scholar]
- 7. Desquilbet L, Jacobson LP, Fried LP, et al. ; Multicenter AIDS Cohort Study HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 2007; 62:1279–86. [DOI] [PubMed] [Google Scholar]
- 8. Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology 2009; 55:539–49. [DOI] [PubMed] [Google Scholar]
- 9. Erlandson KM, Perez J, Abdo M, et al. Frailty, neurocognitive impairment, or both in predicting poor health outcomes among adults living with human immunodeficiency virus. Clin Infect Dis 2019; 68:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tassiopoulos K, Abdo M, Wu K, et al. Frailty is strongly associated with increased risk of recurrent falls among older HIV-infected adults. AIDS 2017; 31:2287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erlandson KM, Allshouse AA, Jankowski CM, et al. Risk factors for falls in HIV-infected persons. J Acquir Immune Defic Syndr 2012; 61:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smurzynski M, Collier AC, Koletar SL, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials 2008; 9:269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erlandson KM, Wu K, Koletar SL, et al. Association between frailty and components of the frailty phenotype with modifiable risk factors and antiretroviral therapy. J Infect Dis 2017; 215:933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White DK, Neogi T, Nevitt MC, et al. Trajectories of gait speed predict mortality in well-functioning older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2013; 68:456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akgün KM, Tate JP, Crothers K, et al. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr 2014; 67:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guaraldi G, Brothers TD, Zona S, et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS 2015; 29:1633–41. [DOI] [PubMed] [Google Scholar]
- 17. Piggott DA, Muzaale AD, Mehta SH, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One 2013; 8:e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desquilbet L, Jacobson LP, Fried LP, et al. A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A Biol Sci Med Sci 2011; 66:1030–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cacciatore F, Abete P, Mazzella F, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest 2005; 35:723–30. [DOI] [PubMed] [Google Scholar]
- 20. Ensrud KE, Ewing SK, Taylor BC, et al. ; Study of Osteoporotic Fractures Research Group Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 2007; 62:744–51. [DOI] [PubMed] [Google Scholar]
- 21. Kojima G, Kendrick D, Skelton DA, Morris RW, Gawler S, Iliffe S. Frailty predicts short-term incidence of future falls among British community-dwelling older people: a prospective cohort study nested within a randomised controlled trial. BMC Geriatr 2015; 15:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vermeiren S, Vella-Azzopardi R, Beckwée D, et al. ; Gerontopole Brussels Study Group Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc 2016; 17:1163.e1–1163.e17. [DOI] [PubMed] [Google Scholar]
- 23. Buchman AS, Wilson RS, Bienias JL, Bennett DA. Change in frailty and risk of death in older persons. Exp Aging Res 2009; 35:61–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Althoff KN, Smit M, Reiss P, Justice AC. HIV and ageing: improving quantity and quality of life. Curr Opin HIV AIDS 2016; 11:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Newman AB, Gottdiener JS, Mcburnie MA, et al. ; Cardiovascular Health Study Research Group Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001; 56:M158–66. [DOI] [PubMed] [Google Scholar]
- 26. Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–83. [DOI] [PubMed] [Google Scholar]
- 27. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 2006; 295:2018–26. [DOI] [PubMed] [Google Scholar]
- 28. Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med 2007; 167:635–41. [DOI] [PubMed] [Google Scholar]
- 29. Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab 2001; 86:3574–8. [DOI] [PubMed] [Google Scholar]
- 30. Althoff KN, Jacobson LP, Cranston RD, et al. ; Multicenter AIDS Cohort Study (MACS) Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014; 69:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kenny AM, Waynik IY, Smith J, Fortinsky R, Kleppinger A, McGee D. Association between level of frailty and bone mineral density in community-dwelling men. J Clin Densitom 2006; 9:309–14. [DOI] [PubMed] [Google Scholar]
- 32. Bregigeon S, Galinier A, Zaegel-Faucher O, et al. Frailty in HIV infected people: a new risk factor for bone mineral density loss. AIDS 2017; 31:1573–7. [DOI] [PubMed] [Google Scholar]
- 33. Erlandson KM, Allshouse AA, Jankowski CM, et al. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis 2013; 208:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Piggott DA, Varadhan R, Mehta SH, et al. Frailty, inflammation, and mortality among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci 2015; 70:1542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Erlandson KM, Ng DK, Jacobson LP, et al. Inflammation, immune activation, immunosenescence, and hormonal biomarkers in the frailty-related phenotype of men with or at risk for HIV infection. J Infect Dis 2017; 215:228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Margolick JB, Bream JH, Martínez-Maza O, et al. Frailty and circulating markers of inflammation in HIV+ and HIV– men in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr 2017; 74:407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeoh HL, Cheng AC, Cherry CL, et al. Immunometabolic and lipidomic markers associated with the frailty index and quality of life in aging HIV+ men on antiretroviral therapy. EBioMedicine 2017; 22:112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duprez DA, Neuhaus J, Kuller LH, et al. ; INSIGHT SMART Study Group Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fitch KV, Srinivasa S, Abbara S, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis 2013; 208:1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012; 206:1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr 2013; 63:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thompson-Paul AM, Lichtenstein KA, Armon C, et al. Cardiovascular disease risk prediction in the HIV outpatient study. Clin Infect Dis 2016; 63:1508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stephens KI, Rubinsztain L, Payan J, Rentsch C, Rimland D, Tangpricha V. Dual-energy x-ray absorptiometry and calculated FRAX risk scores may underestimate osteoporotic fracture risk in vitamin D-deficient veterans with HIV infection. Endocr Pract 2016; 22:440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 1990; 263:3029–34. [PubMed] [Google Scholar]
- 45. Pahor M, Guralnik JM, Ambrosius WT, et al. ; LIFE Study Investigators Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014; 311:2387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cesari M, Vellas B, Hsu FC, et al. ; LIFE Study Group A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci 2015; 70:216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O’Brien KK, Tynan AM, Nixon SA, Glazier RH. Effectiveness of progressive resistive exercise (PRE) in the context of HIV: systematic review and meta-analysis using the Cochrane Collaboration Protocol. BMC Infect Dis 2017; 17:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Erlandson KM, MaWhinney S, Wilson M, et al. Physical function improvements with moderate or high-intensity exercise among older adults with or without HIV infection. AIDS 2018; 32:2317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]