Abstract
There were no cases of tuberculosis in a cohort of 2531 patients who underwent hematopoietic cell transplantation from 2010 to 2015 after 7323 person-years of follow up (95% confidence interval [CI], 0.0–0.05 cases/100 person-years), including 29 (1.15%) patients with untreated latent tuberculosis after 89 person-years of follow-up (95% CI, 0.0–4.06 cases/100 person-years).
Keywords: Mycobacterium tuberculosis, TB, LTBI, allogeneic, hematopoietic cell transplantation
The identification and treatment of individuals with latent tuberculosis infection (LTBI) is crucial to preventing clinical disease [1]. Higher-risk features for LTBI reactivation include malnutrition, human immunodeficiency virus infection, diabetes mellitus, silicosis, end-stage renal disease, recent tuberculosis infection, treatment with glucocorticoids or biological agents, solid organ transplantation, and certain malignancies [2, 3]. Current North American guidelines recommend that individuals at higher risk of tuberculosis reactivation receive preventive therapy [1, 3].
The risk of developing active tuberculosis in persons with a hematological malignancy is increased compared to the general population [4]. However, the magnitude and timing of this risk has not been determined among adult hematopoietic cell transplant (HCT) recipients in low endemic settings. These patients are profoundly immunosuppressed in the peri-transplant period and allogeneic HCT recipients remain therapeutically immunosuppressed for months to prevent and treat graft-versus-host disease (GVHD).
Although rifampin may be preferred to treat LTBI in certain instances [5], isoniazid has been commonly used to prevent LTBI reactivation after HCT, as it is not associated with significant drug-drug interactions. Patients may be at greatest risk for tuberculosis reactivation early post-transplant, yet starting LTBI therapy at this time is challenging because isoniazid is associated with hepatotoxicity and HCT patients may develop liver injury due to conditioning chemotherapy and other complications. We sought to determine LTBI therapy prescription practices and tuberculosis rates among HCT recipients at our center.
METHODS
We performed a retrospective cohort study involving all adult patients who underwent HCT at the Dana-Farber Cancer Institute/Brigham and Women’s Cancer Center between 1 January 2010 and 1 January 2015. Data were censored on 1 April 2018 for the development of tuberculosis. The study was approved by the institutional review board of the Dana-Farber/Harvard Cancer Center Office for Human Research Studies. Patient characteristics and laboratory parameters were collected. Covariates of interest included patient age, sex, country of birth, comorbidities, HCT date, conditioning regimen, HCT source, human leukocyte antigen matching, GVHD prophylaxis regimens, and occurrence of GVHD.
Standard operating procedures at our center mandate that all potential allogeneic HCT recipients undergo LTBI evaluation. Pretransplant tuberculosis screening was generally performed using a 1-step tuberculin skin test (TST) with purified protein derivative without the use of a control antigen [1, 3]. However, 1 of 2 interferon-gamma release assays (IGRA), the QuantiFERON-TB Gold or T-SPOT.TB, could be ordered at the physician’s discretion when patients could have a false-positive TST due to previous BCG vaccination or for reasons of convenience. The QuantiFERON-TB Gold was used during the first 2 years of the study before the T-SPOT.TB was adopted. Per institutional standard operating procedures, patients with LTBI should begin treatment with isoniazid upon discharge from hospital or by day 28 after HCT, whichever occurs first. The infectious diseases service was consulted in cases where isoniazid could not be administered.
To assess LTBI prescription practices, data regarding antimycobacterial therapy were extracted from patient charts. All cases with a positive LTBI screening test identified in the study were independently reviewed by 2 infectious diseases physicians (M. P. C. and T. D. B.) to assess the need for LTBI therapy [1, 3]. Chest imaging, history of Bacillus Calmette–Guérin (BCG) vaccination, LTBI screening tests, and previous LTBI therapy were reviewed. To assess whether patients developed active tuberculosis, microbiological and pathological data, including autopsy records, were also reviewed. Statistical analyses were conducted using SAS® 9.4 (Cary, North Carolina).
RESULTS
In total, 2531 patients underwent HCT during the study period: 1252 underwent autologous HCT and 1279 underwent allogeneic HCT, 65 of whom had a previous autologous HCT. All patients were screened for latent tuberculosis prior to HCT. Twenty-six patients had both a TST and IGRA, 2 only had an IGRA, and all other patients were screened using a TST. Among the entire cohort, 91 (3.6%) had positive LTBI screening tests prior to HCT, of which 48 (52.7%) were foreign-born. Patient characteristics are presented in Table 1.
Table 1.
Patient Characteristics
| Patient Characteristics | LTBI (n = 91) | Non-LTBI (n = 2440) | ||
|---|---|---|---|---|
| N | (%) | N | (%) | |
| Median age, years (range) | 55 | 19–74 | 57 | 18–77 |
| Male sex | 53 | 58.2 | 1424 | 58.4 |
| Race | ||||
| White | 53 | 58.2 | 2237 | 91.7 |
| Black | 6 | 6.6 | 49 | 2.0 |
| Asian | 11 | 12.1 | 34 | 1.3 |
| Mixed | 5 | 5.5 | 32 | 1.3 |
| Unknown | 16 | 17.6 | 88 | 3.6 |
| Underlying malignancy for auto-HCT | N = 47 | N = 1205 | ||
| Multiple myeloma | 35 | 74.5 | 717 | 59.5 |
| Non-Hodgkin lymphoma | 10 | 21.3 | 360 | 29.9 |
| Hodgkin’s disease | 2 | 4.3 | 106 | 8.8 |
| Underlying malignancy for allo-HCT | N = 44 | N = 1235 | ||
| Acute myelogenous leukemia | 17 | 38.6 | 445 | 36.1 |
| Non-Hodgkin lymphoma | 3 | 7.8 | 203 | 16.4 |
| Myelodysplastic syndrome | 10 | 22.7 | 192 | 15.6 |
| Acute lymphocytic leukemia | 5 | 11.4 | 110 | 8.9 |
| Allogeneic HCT-specific Parameters | N = 44 | N = 1235 | ||
| Myeloablative conditioning | 21 | 47.7 | 402 | 32.6 |
| Reduced-intensity conditioning | 23 | 52.3 | 833 | 67.4 |
| Source of hematopoietic cells | ||||
| Peripheral blood | 33 | 75.0 | 1070 | 86.6 |
| Bone marrow | 9 | 20.5 | 108 | 8.7 |
| Cord blood | 2 | 4.5 | 57 | 4.6 |
| Allogeneic donor characteristics | ||||
| Matched-related donor | 13 | 29.5 | 375 | 30.4 |
| Matched-unrelated donor | 22 | 50.0 | 656 | 53.1 |
| Mismatched donor | 9 | 20.5 | 204 | 16.5 |
| GVHD Prophylactic regimens | ||||
| MTX and tacrolimus | 15 | 34.1 | 451 | 36.7 |
| MTX, tacrolimus, and sirolimus | 12 | 27.3 | 365 | 29.7 |
| Sirolimus and tacrolimus | 10 | 22.7 | 235 | 19.1 |
| Other | 7 | 15.9 | 184 | 14.9 |
| Acute GVHD | 22 | 50.0 | 528 | 42.8 |
Abbreviations: GVHD, graft-versus-host disease; HCT, hematopoietic cell transplant; LTBI, latent tuberculosis infection; MTX, methotrexate.
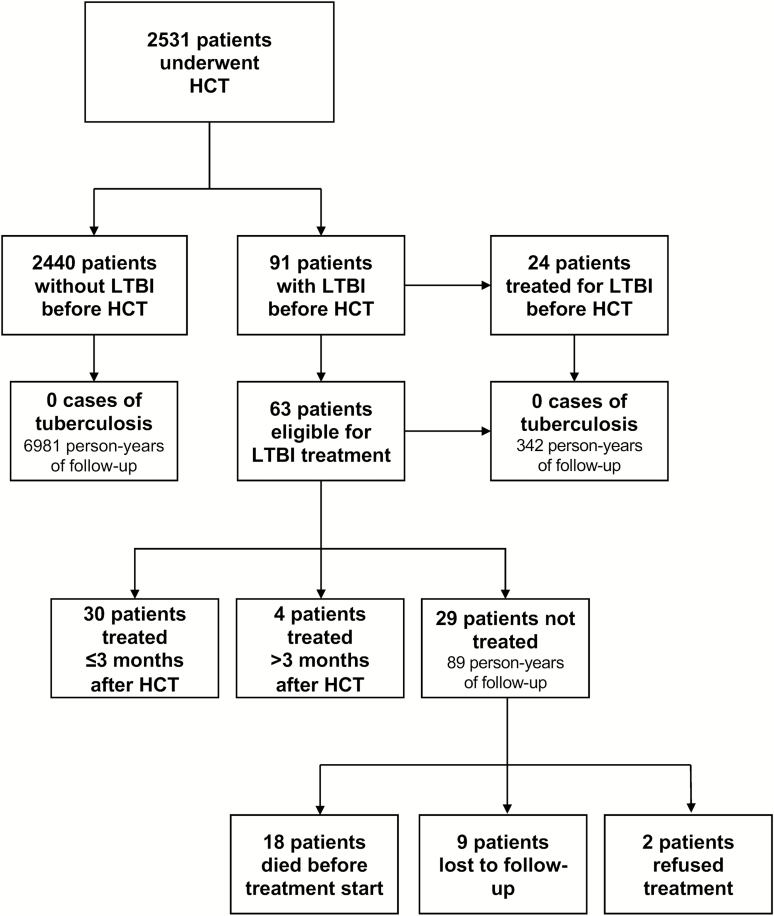
Among the 91 patients with positive screening tests, 48 (52.7%) were known to be positive before their pre-HCT evaluation. Of these, 24 had been previously treated, 20 were planned for treatment post-HCT, and 4 patients were not considered to have LTBI given previous BCG vaccination and negative IGRA results. The remaining 43 patients with positive LTBI screening tests were identified as part of their pre-HCT evaluation. Of these, 35 patients had a positive TST, 6 had a positive TST and IGRA, and 2 patients were diagnosed only with a positive IGRA. One patient had an indeterminate IGRA result but was diagnosed with LTBI on the basis of a positive TST result and immigration from a tuberculosis-endemic country. In total, 63 patients diagnosed with LTBI were identified for preventive therapy post-HCT (Figure 1), without disagreements between reviewers. No patient had chest imaging prior to HCT with findings associated with increased risk of LTBI reactivation.
Figure 1.
Summary of latent tuberculosis infection outcomes of all patients who received hematopoietic cell transplants at Dana-Farber Cancer Center from January 2010 through January 2015. See text for details. Abbreviations: HCT, hematopoietic cell transplant; LTBI, latent tuberculosis infection.
Among these 63 patients, 30 (47.6%) were treated within 3 months of HCT, including 9 patients (30.0%) who started within 28 days of transplant. Of the other patients, 4 (6.3%) initiated treatment later than 3 months post-HCT, and 29 (46.0%) did not receive treatment for reasons including physician deferral (n = 24), death within 90 days from HCT (n = 3), and patient refusal (n = 2). Treatment regimens included isoniazid (n = 29), levofloxacin (n = 4), or rifampin (n = 1). The median duration of treatment was 197 days (range, 7–326). Of the 24 patients with deferred treatment, 15 died of other causes before LTBI treatment could be initiated.
There were no cases of active tuberculosis in the cohort of patients with LTBI throughout the duration of the study, resulting in an incidence rate of 0% (95% confidence interval [CI], 0.0–1.07 cases/100 person-years) from a combined 342 person-years of follow-up. Specifically, no cases were identified in 63 patients who required LTBI therapy post-HCT (95% CI, 0.00–1.66 cases/100 person-years), including in 29 patients who did not receive preventative treatment (95% CI, 0.00–4.06/100 person-years). There were no cases of tuberculosis in the rest of the cohort either, resulting in an incidence rate of 0% (95% CI, 0.0–0.05 cases/100 person-years) from a total follow-up period of 6981 person-years.
DISCUSSION
The cumulative incidence of tuberculosis among HCT recipients in areas of high endemicity who do not use preventative therapy is between 1% and 4% [6–8]. However, the risk of reactivation is highest within the first 2 years of exposure. Furthermore, these rates likely reflect both LTBI reactivation and de novo infection. Our study suggests that the risk of developing active tuberculosis after HCT is lower in nonendemic settings. We found no cases of clinical disease among 2531 HCT recipients at our institution, including among patients who remained immunosuppressed due to post-transplant events such as GVHD and glucocorticoid treatment. Furthermore, there were no cases of tuberculosis in the peri-transplant period among patients with untreated LTBI who waited several weeks before initiating preventative therapy.
There are several potential explanations for our results, including that some of these patients received, for other indications, antibacterial therapies that are also active against M. tuberculosis. However, we do not expect this to have occurred in most patients, as empiric treatment for febrile neutropenia at our institution consists of either ceftazidime or cefepime.
Reactivation of untreated LTBI occurs at an estimated rate of 0.1% per year in the general population [9], but it is expected to be more frequent in our study population. However, relative to the low rate of LTBI reactivation, the duration of severe immunosuppression peri-HCT can be short. It is also notable that, prior to undergoing HCT, most patients had previously received immunosuppressive therapy for a hematologic malignancy and did not reactivate at that time. Stability of LTBI during a prior period of severe immunosuppression suggests either the absence of residual infection [10] or the presence of durable antituberculous immunity that may persist after HCT.
Our results must be interpreted in the context of the study characteristics. Although the majority of patients who tested positive on LTBI screening were born in countries with higher tuberculosis prevalence, supporting that these screening tests represent true-positives, there is also a risk of false-positive tests when routine screening is applied to a large population. Although our data cannot be extrapolated to areas of higher tuberculosis endemicity, our results are informative on the risk of LTBI reactivation in a low endemic setting. Finally, as data collection was obtained from medical record review, we could not determine if cases of tuberculosis were diagnosed outside of our center, although we regularly follow our post-HCT population long-term.
Although LTBI therapy remains an important consideration in this patient population, our data suggest that M. tuberculosis reactivation does not necessarily occur immediately post-HCT. These data suggest that LTBI therapy could be deferred in the immediate post-HCT setting and initiated once patients have a lower risk of hepatotoxicity. Our data also highlight the opportunity for quality improvement with regards to the management of HCT recipients with LTBI, as several eligible patients did not receive timely preventive therapy. However, the optimal timing to start LTBI therapy and the minimum duration of treatment in this population remain undefined. Newly validated methods of interpreting currently available test results could identify those at highest risk of reactivation [11] and help determine the urgency in administering preventative therapy.
Notes
Potential conflicts of interest. This article was prepared as part of routine work. S. P. H. reports research funding from Merck. All other authors report no potential conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Financial support. M. P. C. receives salary support from a postdoctoral training grant, provided by the Fonds de recherche Santé Québec.
References
- 1.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America. Controlling tuberculosis in the United States. Am J Respir Crit Care Med 2005; 172:1169–227. [DOI] [PubMed] [Google Scholar]
- 2. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5:1091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pai M, Kunimoto D, Jamieson F, Menzies D. Diagnosis of latent tuberculosis infection. Canadian tuberculosis standards 7th ed. 2014. Available at: https://www.canada.ca/content/dam/phac-aspc/migration/phac-aspc/tbpc-latb/pubs/tb-canada-7/assets/pdf/tb-standards-tb-normes-ch4-eng.pdf. [Google Scholar]
- 4. Cheng MP, Abou Chakra CN, Yansouni CP, et al. Risk of active tuberculosis in patients with cancer: a systematic review and meta-analysis. Clin Infect Dis 2016; 64:ciw838. [DOI] [PubMed] [Google Scholar]
- 5. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med 2018; 379:440–53. [DOI] [PubMed] [Google Scholar]
- 6. Agrawal N, Aggarwal M, Kapoor J, et al. Incidence and clinical profile of tuberculosis after allogeneic stem cell transplantation. Transpl Infect Dis 2018; 20:e12794. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y, Wu C, Ko P, et al. Mycobacterial infections in adult recipients of allogeneic hematopoietic stem cell transplantation: a cohort study in a high endemic area. J Microbiol Immunol Infect 2018. doi: 10.1016/j.jmii.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 8. Fan WC, Liu CJ, Hong YC, et al. Long-term risk of tuberculosis in haematopoietic stem cell transplant recipients: a 10-year nationwide study. Int J Tuberc Lung Dis 2015; 19:58–64. [DOI] [PubMed] [Google Scholar]
- 9. Comstock GW, Edwards LB, Livesay VT. Tuberculosis morbidity in the US Navy: its distribution and decline. Am Rev Respir Dis 1974; 110:572–80. [DOI] [PubMed] [Google Scholar]
- 10. Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ 2018; 362:k2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abubakar I, Drobniewski F, Southern J, et al. ; PREDICT Study Team. Prognostic value of interferon-γ release assays and tuberculin skin test in predicting the development of active tuberculosis (UK PREDICT TB): a prospective cohort study. Lancet Infect Dis 2018; 18:1077–87. [DOI] [PMC free article] [PubMed] [Google Scholar]