Abstract
Background
Currently, there is debate over whether the daptomycin susceptibility breakpoint for enterococci (ie, minimum inhibitory concentration [MIC] ≤4 mg/L) is appropriate. In bacteremia, observational data support prescription of high doses (>8 mg/kg). However, pharmacodynamic targets associated with positive patient outcomes are undefined.
Methods
Data were pooled from observational studies that assessed outcomes in daptomycin-treated enterococcal bacteremia. Patients who received an additional antienterococcal antibiotic and/or a β-lactam antibiotic at any time during treatment were excluded. Daptomycin exposures were calculated using a published population pharmacokinetic model. The free drug area under the concentration-time curve to MIC ratio (fAUC/MIC) threshold predictive of survival at 30 days was identified by classification and regression tree analysis and confirmed with multivariable logistic regression. Monte Carlo simulations determined the probability of target attainment (PTA) at clinically relevant MICs.
Results
Of 114 patients who received daptomycin monotherapy, 67 (58.8%) were alive at 30 days. A fAUC/MIC >27.43 was associated with survival in low-acuity (n = 77) patients (68.9 vs 37.5%, P = .006), which remained significant after adjusting for infection source and immunosuppression (P = .026). The PTA for a 6-mg/kg/day (every 24 hours) dose was 1.5%–5.5% when the MIC was 4 mg/L (ie, daptomycin-susceptible) and 91.0%–97.9% when the MIC was 1 mg/L.
Conclusions
For enterococcal bacteremia, a daptomycin fAUC/MIC >27.43 was associated with 30-day survival among low-acuity patients. As pharmacodynamics for the approved dose are optimized only when MIC ≤1 mg/L, these data continue to stress the importance of reevaluation of the susceptibility breakpoint.
Keywords: pharmacodynamics, daptomycin, Enterococcus, bacteremia
Daptomycin monotherapy–treated enterococcal bacteremia with an area under the free drug concentration-time curve to minimum inhibitory concentration ratio >27.43 was associated with 30-day survival. At the current susceptibility breakpoint, target attainment was <90% (6–12 mg/kg/day), justifying a lower breakpoint.
Daptomycin was approved in the United States in 2003 for the treatment of complicated skin and skin structure infections and in 2006 for Staphylococcus aureus bacteremia [1]. Relative to enterococcal infection, the Food and Drug Administration (FDA) susceptibility breakpoint (minimum inhibitory concentration [MIC] ≤4 mg/L) pertains only to vancomycin-susceptible Enterococcus faecalis for complicated skin and skin structure infections [1]. However, daptomycin is among the few antibiotics that retain bactericidal activity against vancomycin-resistant E. faecium [2], and the susceptibility breakpoint defined by the Clinical and Laboratory Standards Institute (also ≤4 mg/L) applies to all Enterococcus spp. [3]. Recent reports of failure in daptomycin-treated enterococcal bacteremia suggest reduced efficacy against susceptible isolates with MICs of 3–4 mg/L [4–6], but contrasting findings are also described [7, 8]. At the molecular level, the potential inadequacy of the established breakpoint is evidenced by mutations in genes encoding the LiaFSR regulatory system among daptomycin-susceptible E. faecium isolates with MICs >2 mg/L [9, 10].
The FDA-approved dose for S. aureus bacteremia is 6 mg/kg (actual body weight) [1]. However, in line with higher reported daptomycin MIC distributions for Enterococcus spp. [9], doses prescribed when treating enterococcal bacteremia frequently exceed the approved dose [11]. Observational studies indicate an association between improved survival and doses ≥9 mg/kg [7] and ≥10 mg/kg [12], yet 6 mg/kg remains a commonly-prescribed regimen in clinical practice [13]. Combination therapy with one of many β-lactams is also frequently utilized, a practice supported by favorable clinical [14] and in vitro [15] observations.
Antibiotic dosage selection should be supported by pre-clinical pharmacodynamic (PD) data and confirmed in randomized, controlled trials. Against enterococcal species, data from neither methodology are available to justify daptomycin dosing for the treatment of serious enterococcal infection or the optimal susceptibility breakpoint. Nonetheless, pre-clinical data have established a free (unbound) drug area under the concentration-time curve to MIC ratio (fAUC/MIC) as the PD parameter most predictive of bacterial killing [16–18]. The precise fAUC/MIC required for successful outcomes in severe enterococcal infections, however, is unknown. Therefore, analyzing human PD exposure is a logical next step for the identification of PD thresholds associated with optimized clinical outcomes. These data may also be useful for establishing dosing regimens and clinical breakpoints. To address this knowledge gap, the primary aim of this study was to determine the daptomycin fAUC/MIC associated with improved 30-day survival rates in enterococcal bacteremia. The secondary aim was to use the identified PD threshold to support clinical breakpoints.
METHODS
Patients
Data were pooled from 7 observational studies assessing outcomes in daptomycin-treated enterococcal bacteremia [4, 7, 8, 12, 19–21]. Institutional Review Board approval and informed consent waivers were obtained at all sites, as only de-identified data were used. Patients were excluded either if the duration of daptomycin treatment was <72 hours or in the setting of concomitant administration of an antibiotic with intrinsic or synergistic activity against enterococci. Specifically, patients administered 1 or more of: (1) any β-lactam, (2) linezolid, (3) tigecycline, (4) any intravenous aminoglycoside, or (5) vancomycin (for vancomycin-susceptible isolates) at any time during daptomycin therapy were considered to have received combination therapy. In addition, patients were excluded if information required for calculation of exposure (outlined below) was unavailable, continuous renal replacement therapy occurred during treatment, or clearance of infection preceded daptomycin therapy. In recurrent bacteremia, only the first episode was included.
Data collected included age, sex, weight, daptomycin regimen (dose and frequency), daptomycin ETEST MIC, source of bacteremia, concomitant antibiotics, concomitant hemodialysis, microbiological response, and survival to 30 days. Patients were considered high acuity if any of the following conditions were met: Acute Physiology and Chronic Health Evaluation (APACHE)-II score ≥21, Charlson comorbidity index ≥5, or Pitt bacteremia score ≥4. These scores were selected because they have been previously associated with hospital mortality in critically-ill patients with sepsis [22].
Daptomycin Exposure
For each patient, a validated population pharmacokinetic model was used to estimate daptomycin clearance (CL) [23]:
| 1 |
| 2 |
Here, y was 0.8 for females and 1 for males, CLR (L/h) was renal daptomycin clearance, TEMP was patient temperature (°C), and CLCR was creatinine clearance, calculated by the Cockcroft-Gault equation [24] using actual body weight (150 mL/min maximum) [23]. In intermittent hemodialysis, CLR was 0.269 L/h [23]. As temperature data were unavailable and had only marginal contributions to calculated exposure, 37.2°C was assumed for all patients. The fAUC of daptomycin was calculated as follows [25]:
Here, 0.1 was the free daptomycin fraction in plasma [1] and the dose was in milligrams. For patients that received daptomycin doses every 48 hours, the dose was divided by 2 to reflect the average 24-hour exposure. The calculated fAUC was divided by the baseline daptomycin ETEST MIC provided by the study site to determine each fAUC/MIC.
Statistical Analysis
The primary outcome was survival at 30 days, assessed from the first day of daptomycin therapy. In a minority of bacteremias, it was not possible to conclusively discriminate survival from the time of diagnosis of enterococcal bacteremia versus from the initiation of daptomycin therapy in the previously-collected data. The former definition was allowed with the assumption that minimal discordance existed between the 2 definitions (i.e., daptomycin therapy was initiated within 1–2 days following diagnosis in all patients). The secondary outcome was microbiological response, which was variably defined by the individual studies as bacteremia clearance prior to day 4 from the first positive blood culture [4, 7, 8, 12, 21], the absence of recurrent infection after initial clearance within 7 days [19], and clearance while receiving daptomycin (Supplementary Table 1) [20].
To transform fAUC/MIC into a binary PD threshold (i.e., exposures achieving or not achieving the threshold), a classification and regression tree (CART) analysis (Salford Systems, San Diego, California) was performed to identify the fAUC/MIC thresholds most predictive of survival to 30 days and of a microbiological response.
Statistical analyses were conducted in Sigma Plot v13.0 (Systat Software Inc., San Jose, California). T-tests or Mann-Whitney Rank Sum tests and χ2 tests were performed for continuous and categorical variables, respectively. To confirm associations between the CART-derived PD threshold and outcomes, a multivariable logistic regression analysis was performed, with the PD threshold as the exposure of interest and selected covariates with bivariate P-values <.2. Covariates that were co-linear with fAUC/MIC (e.g., dose) were not included. The Hosmer-Lemeshow statistic was used to assess the model for goodness of fit.
Monte Carlo Simulations
The CART-derived PD threshold was applied in Monte Carlo simulations using Oracle Crystal Ball v11.1 (Oracle, Redwood Shores, California) to determine the probability of target attainment (PTA) of various daptomycin dosing regimens (6, 8, 10, and 12 mg/kg/day). Simulations recapitulated study cohort characteristics, including actual body weight as a log-normal distribution between 37 and 180 kg and CLCR as a uniform distribution between 30 and 150 mL/min, to include the range for once-daily dosing [1]. Assumptions for the unbound daptomycin fraction (uniform distribution, 0.07–0.10) were derived from prescribing information [1]. The fAUC/MIC forecasts were performed at doubling MIC dilutions between 0.25 and 16 mg/L, and completed separately for 5000 females and 5000 males to account for gender disparities in daptomycin CL (Equation 1). An a priori PTA >90% was defined as optimal.
RESULTS
Cohort Characteristics
A total of 460 patients with enterococcal bacteremia were identified. After applying exclusion criteria, the final cohort included 114 patients, 90 of which were also evaluated for a microbiological response. Patients were excluded primarily for receipt of additional antibiotics (n = 240). Other reasons included loss to follow up prior to the 30-day survival assessment (n = 2), missing the data required to determine daptomycin exposure (n = 48), the clearance of bacteremia prior to daptomycin initiation (n = 18), recurrent bacteremia (n = 16), a duration of daptomycin therapy <72 hours (n = 13), and the receipt of continuous renal replacement therapy (n = 9). The number of patients included from each study was 9 [4], 28 [7], 33 [8], 1 [12], 10 [19], 31 [20], and 2 [21]. Vancomycin MICs were not collected; however, an estimated 86% of bacteremias in the final cohort were caused by vancomycin-resistant enterococci (VRE), based upon the proportion of VRE infections included in each original study. Patient characteristics are summarized in Table 1 for the total population and the low-acuity cohort.
Table 1.
Cohort Characteristics
| Characteristic | All Patients (n = 114) | Low-acuity Cohort (n = 77) |
|---|---|---|
| Age in y | 55.0 (18.6), 59.0 | 53.8 (17.6), 58.5 |
| Female sex, n (%) | 48 (42.1) | 36 (46.8) |
| Weight in kg | 76.2 (21.2), 74.1 | 79.1 (22.1), 76.7 |
| Serum creatinine in mg/dL | 1.2 (0.9), 0.9 | 1.0 (0.6), 0.8 |
| Low-acuity, n (%)a | 77 (67.5) | 77 (100.0) |
| APACHE-II scorea | 14.4 (7.0), 14.0 | 11.5 (4.9), 13.0 |
| Charlson co-morbidity indexa | 3.3 (2.5), 3.0 | 1.5 (1.4), 2.0 |
| Pitt bacteremia scorea | 2.1 (2.5), 3.0 | 0.8 (1.0), 0.0 |
| Infection source | … | … |
| Catheter, n (%) | 54 (47.4) | 36 (46.8) |
| Endocarditis, n (%) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal, n (%) | 20 (17.5) | 17 (22.1) |
| Urinary tract, n (%) | 8 (7.0) | 5 (6.5) |
| Other or unknown, n (%) | 32 (28.1) | 19 (24.7) |
| Intermittent hemodialysis, n (%) | 5 (4.4) | 0 (0.0) |
| Immunosuppressed, n (%)b | 91 (79.8) | 64 (83.1) |
| Enterococcus faecium bacteremia, n (%) | 103 (90.4) | 68 (88.3) |
| ETEST daptomycin MIC, mg/L, median (range) | 2 (0.5–4) | 2 (0.5–4) |
| Daptomycin dose, mg/dose | 553 (169), 500 | 575 (176), 500 |
| Daptomycin dose, mg/kg/dose | 7.4 (1.5), 7.2 | 7.4 (1.8), 7.0 |
| Survived at 30 days, n (%) | 67 (58.8) | 43 (55.8) |
Data are presented as mean (standard deviation), median unless otherwise noted.
Abbreviations: APACHE-II, Acute Physiology and Chronic Health Evaluation II; MIC, minimum inhibitory concentration.
aLow acuity indicates that none of following conditions were met: APACHE-II score ≥21 (available for 32 patients in the total population and 24 in the low-acuity cohort), Charlson comorbidity index ≥5 (available for 41 patients in the total population and 24 in the low-acuity cohort), or Pitt bacteremia score ≥4 (available for 60 patients in the total population and 41 in the low-acuity cohort).
bImmunosuppressed patients were those with active malignancy, a history of solid organ/bone marrow transplantation, neutropenia (absolute neutrophil count <500), human immunodeficiency virus infection, or those receiving chemotherapy, mycophenolate, tacrolimus, cyclosporine, biological therapy, or corticosteroids.
Daptomycin Exposure
In the total population (N = 114), the mean daptomycin clearance was 0.7 ± 0.2 L/h, translating to a mean fAUC equal to 74.3 ± 25.8 mg*h/L and a mean fAUC/MIC equal to 35.0 ± 20.2 (range: 10.1–1235.9). In the low-acuity cohort (n = 77), the mean daptomycin clearance was 0.8 ± 0.2 L/h and the mean fAUC and fAUC/MIC were nearly identical to the values in all patients.
Survival Pharmacodynamic Threshold
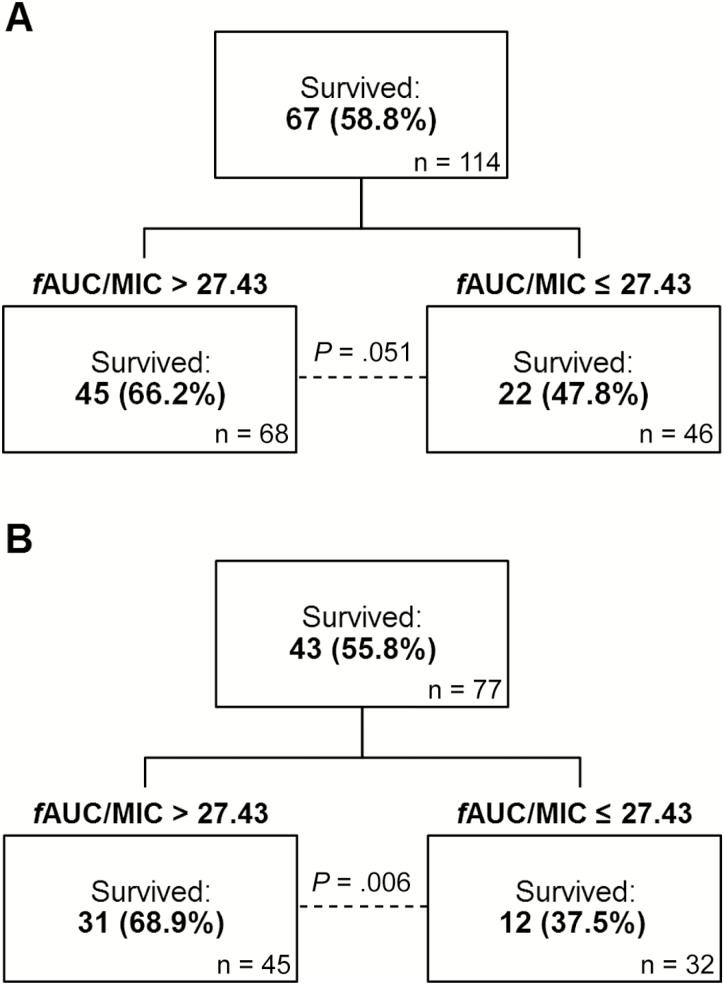
In patients treated with daptomycin monotherapy, 67 (58.8%) were alive at 30 days. The CART-derived PD threshold associated with survival to 30 days is depicted in Figure 1. Although a greater proportion of total patients survived when daptomycin fAUC/MIC was greater than 27.43, compared with patients that did not achieve the threshold, the difference was not significant (Figure 1, P = .051). However, in low-acuity patients (n = 77), the CART-derived fAUC/MIC threshold was identical and was associated with survival (Figure 1, P = .006). Controlling for catheter source and immunosuppression, the fAUC/MIC >27.43 was independently associated with survival (P = .026, Table 2). In low-acuity patients alive at 30 days, the median fAUC/MIC was higher (32.9 versus 26.0, P = .085), and significantly higher daptomycin doses were prescribed (7.7 versus 6.3 mg/kg/dose, P = .023). The median daptomycin MIC was 2 mg/L, and did not differ between those alive and deceased at 30 days (P = .607). Significant relationships with fAUC/MIC thresholds were not identified when assessed in high-acuity patients only (data not shown).
Figure 1.
CART-derived pharmacodynamic thresholds in (A) all patients and (B) the low-acuity cohort for the 30-day survival outcome. Survival thresholds were identified in low-acuity patients to control for unknown confounding variables, which may contribute to overall mortality risk. Low acuity was defined as the absence of any of the following conditions: APACHE-II score ≥21, Charlson comorbidity index ≥5, and Pitt bacteremia score ≥4. Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CART, classification and regression tree analysis; fAUC/MIC, area under the free drug concentration-time curve to minimum inhibitory concentration ratio.
Table 2.
Effect of Characteristics on 30-Day Survival in the Low-acuity Cohort (n = 77)
| Characteristic | Survived ( n = 43), n (%) | Deceased n = 34, n (%) |
Bivariate OR (95% CI) | Bivariate P Value |
Multivariable OR (95% CI) | Multivariable P Value |
|---|---|---|---|---|---|---|
| Immunosuppressed | 32 (74.4) | 32 (94.1) | 0.18 (0.04–0.89) |
.022 | 0.24 (0.05–1.21) | .084 |
| Bacteremia source | ||||||
| Gastrointestinal | 11 (25.6) | 6 (17.6) | 1.60 (0.53–4.90) | .405 | … | … |
| Urinary tracta | 4 (9.3) | 1 (2.9) | 3.38 (0.36–31.79) | .376 | … | … |
| Catheter-related | 16 (37.2) | 20 (58.8) | 0.42 (0.17–1.04) | .059 | 0.51 (0.19–1.38) | .185 |
| Other or unknown | 12 (27.9) | 7 (20.6) | 1.49 (0.51–4.33) | .459 | … | … |
| fAUC/MIC >27.43 | 31 (72.1) | 14 (41.2) | 3.69 (1.42–9.58) | .006 | 3.10 (1.14–8.38) | .026 |
Abbreviations: CI, confidence interval; fAUC/MIC, area under the free drug concentration-time curve to minimum inhibitory concentration ratio; OR, odds ratio
aBivariate statistical analysis performed with Fisher’s exact test.
Microbiologic Response Pharmacodynamic Threshold
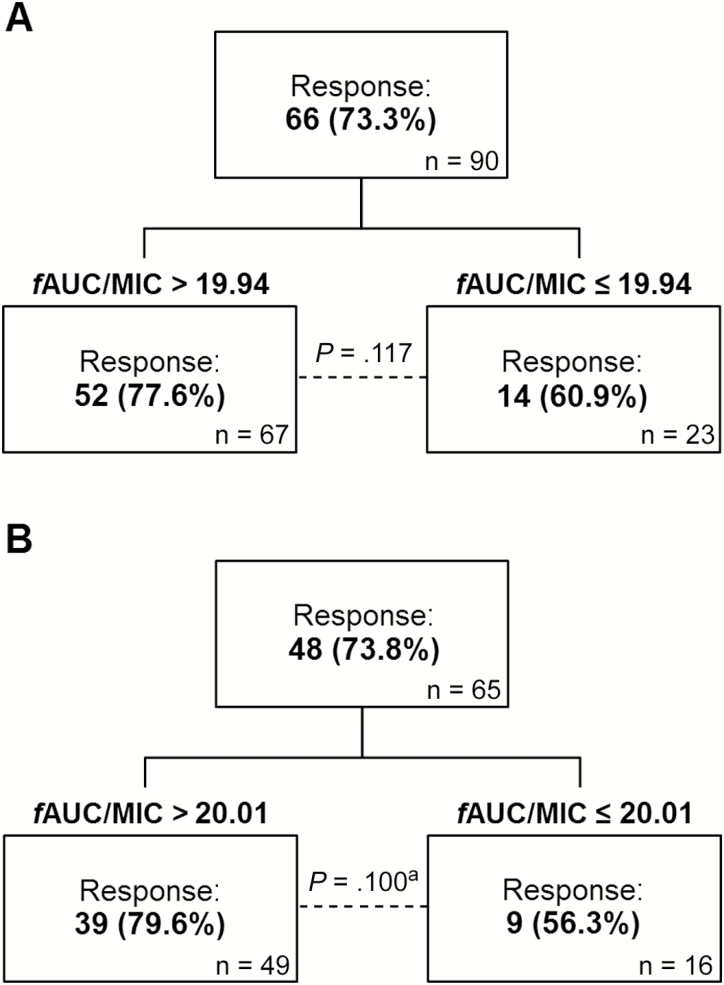
In total, 66 (73.3%) patients achieved a microbiological response. However, despite higher observed microbiological response rates in patients with exposures exceeding the CART-derived thresholds both in all patients (fAUC/MIC >19.94) and low-acuity patients (fAUC/MIC >20.01), neither threshold was statistically significant in bivariate analyses (Figure 2).
Figure 2.
CART-derived pharmacodynamic thresholds in (A) all patients and (B) the low-acuity cohort for the microbiological response outcome. Low acuity was defined as the absence of any of the following conditions: APACHE-II score ≥21, Charlson comorbidity index ≥5, and Pitt bacteremia score ≥4. Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CART, classification and regression tree analysis; fAUC/MIC, area under the free drug concentration-time curve to minimum inhibitory concentration ratio. aDifference assessed by Fisher’s exact test.
Monte Carlo Simulations
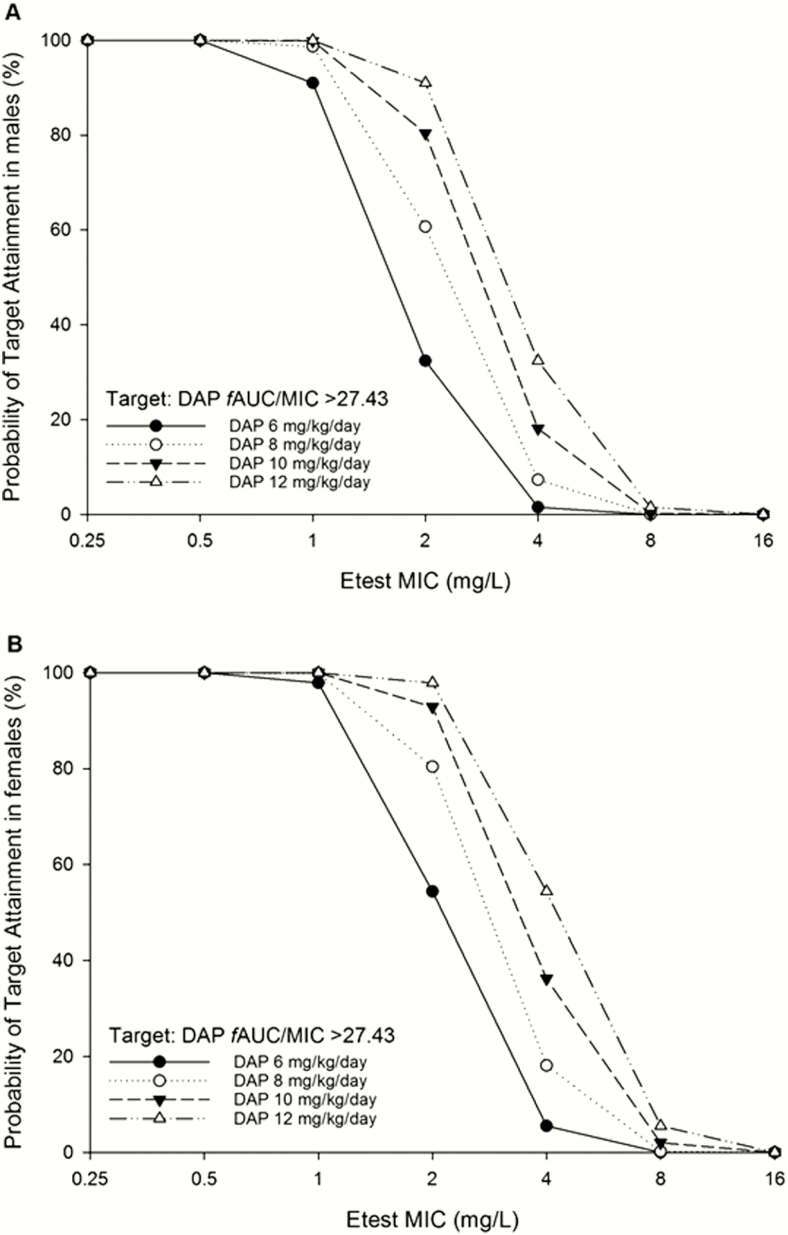
Using the approved daptomycin dose (6 mg/kg/day), the PTA of the PD threshold associated with survival (fAUC/MIC >27.43) remained >90% when exposures were simulated for susceptible isolates with MICs of 0.25, 0.5, and 1 mg/L (Figure 3). At MICs of 2 mg/L, the PTA dropped precipitously, to 32.4% for males and 54.4% for females (Supplementary Table 2). As the relationship between dose and AUC is directly proportional, an identical pronounced decline was present between MICs of 2 and 4 mg/L with 12 mg/kg/day.
Figure 3.
Monte Carlo simulation results for (A) males and (B) females. The probability of target attainment (PTA) was higher in simulations for female patients (Supplementary Table 2); however, both PTAs were either above or below 90% at each dose simulated, except for 10 mg/kg/day (males: 80.4%, females: 92.9%). Abbreviations: DAP, daptomycin; fAUC/MIC, area under the free drug concentration-time curve to minimum inhibitory concentration ratio; MIC, minimum inhibitory concentration.
DISCUSSION
The appropriateness of the daptomycin susceptibility breakpoint for enterococcal infection is uncertain [4, 9, 13, 15], and the controversy has been propagated by conflicting reports of the effect of MIC on patient outcomes [4, 5, 7, 8]. PD relationships that may explain these observations were previously unexplored. Therefore, we conducted a human PD analysis utilizing global data generated from diverse populations (Supplementary Table 1), and demonstrated an association between a daptomycin fAUC/MIC >27.43 and improved 30-day survival rates in enterococcal bacteremia. Among low-acuity patients administered monotherapy, the odds of survival for those that achieved the optimal PD threshold were 3.1-fold higher than those with sub-threshold exposures.
No other human PD studies are available for comparison. However, the PD threshold of fAUC/MIC >27.43 is higher than the target for 2-log10 kill reported in 2003 by Alder and colleagues following an assessment of daptomycin PD in a murine renal infection model [16]. Given that daptomycin is primarily excreted as an intact drug in the urine, the authors cautioned that their results may not be applicable to other infection sites [16]. Indeed, Kidd and colleagues recently reassessed the in vivo pharmacodynamics utilizing human-simulated doses in a murine thigh infection model, and reported a 1-log10 kill target of fAUC/MIC >12.9 for E. faecium isolates [26], which is 3-fold higher than the murine target reported by Alder and colleagues [16]. Thus, our human PD analysis and the new murine PD target suggest that higher exposures are associated with survival and antibacterial efficacy, respectively. Moreover, our data were also supportive of higher doses and a higher fAUC/MIC (when analyzed as a continuous variable, data not shown) being associated with survival, albeit the relationship with fAUC/MIC was not statistically significant, which was likely due to lack of power. Notably, the median MIC was the same regardless of survival, thereby suggesting that it is the PD exposure (i.e., fAUC/MIC) that is most important for daptomycin in predicting survival in patients with enterococcal bacteremia.
The exclusion of patients that received an active combination therapy for enterococcal bacteremia was imperative in order to conduct an analysis that appropriately informs daptomycin breakpoint decisions. However, many have extensively described the observed enhancement of daptomycin in the presence of β-lactams [27–31], as well as the increased activity of β-lactams after the cell envelope changes induced by daptomycin [32] in some enterococcal strains. Thus, future analyses that define the PD effect of combination therapy on daptomycin-treated enterococcal bacteremia are needed, especially in light of a recent finding of improved end-of-treatment survival rates with high-dose daptomycin (≥9 mg/kg/day) plus a β-lactam, compared with high-dose daptomycin monotherapy [14].
When the survival PD threshold was explored in Monte Carlo simulations, the FDA-approved dose produced a poor PTA when corresponding isolate MICs exceeded 1 mg/L. When exposures for 8 and 10 mg/kg/day regimens were simulated, PTAs remained <90% for MICs ≥2 mg/L. Even when the dose was maximized at 12 mg/kg/day, the PTA was suboptimal for MICs of 4 mg/L. Thus, an optimal PTA was only achievable at an MIC of 2 mg/L using a 12 mg/kg/day regimen. Overall, the PTA estimations best support a susceptibility breakpoint of an MIC ≤1 mg/L for daptomycin monotherapy with the licensed dose (6 mg/kg/day) and the susceptible–dose dependent designation at an MIC of 2 mg/L for daptomycin 12 mg/kg/day.
Although most bacteremias were caused by E. faecium in this analysis, breakpoint modifications should apply to all enterococcal species to avoid reporting errors and misinterpretation. Even if vancomycin-resistant, E. faecalis are rarely ampicillin–non-susceptible [11], and the reduction of daptomycin breakpoints is therefore unlikely to impact overall prescribing trends. Nonetheless, it should be recognized that there are important biological differences between pathogenic enterococcal species [11]. For example, the emerging data reported by Kidd and colleagues supported a bacteriostatic target for E. faecalis (fAUC/MIC >7.2), as 1-log10 kill was not achieved [26]. This observation may indicate differing responses to daptomycin between the 2 enterococcal species in this murine model, though further studies are required to ultimately determine whether separate PD targets exist in humans.
While our analysis reveals that revised breakpoints are warranted to optimize PD exposures, hypotheses regarding the safety of such changes cannot be gleaned from these data. Clinicians may fear that high-dose daptomycin translates to an increased frequency of adverse effects; however, the literature does not support dose-dependent toxicities related to creatine phosphokinase elevation [12] or eosinophilic interstitial pneumonia [33, 34]. In fact, recently-updated treatment guidelines for enterococcal endocarditis recommend doses up to 12 mg/kg [35]. Moreover, a prospective review of high-dose daptomycin in the treatment of endocarditis captured only 15 (15%) patients with creatine phosphokinase elevation, while 11 (73%) continued treatment without any alteration of muscle function. There were 3 cases of eosinophilic interstitial pneumonia, and no patient discontinued due to renal toxicity, leading the authors to conclude that high-dose daptomycin was well tolerated in patients with multiple comorbidities [36].
In line with the existing literature, we observed mortality rates >40% [37] and daptomycin cure rates <80% [9] in enterococcal bacteremia. However, we were unable to detect the PD thresholds associated with microbiological eradication. A contributory factor may be the variability in microbiological response definitions used by the original studies, as well as the wide range of response rates among data sets (47% to 94%, Supplementary Table 1). Others have failed to observe associations between daptomycin doses and microbiological outcomes [7, 38], though Britt and colleagues reported a significant dose-dependent relationship via the investigation of response as a continuous variable of time to microbiologic clearance [12]. Furthermore, Shukla and colleagues reported a clear influence of MIC in microbiological eradication [4]. Data are conflicting, but with robust in vitro evidence of enterococcal activity [39], the observed effect of optimized daptomycin exposure on survival is likely mediated by microbiological clearance.
The current body of literature includes multiple reports of daptomycin failures in susceptible enterococcal infections, as well as the presence of mutations that confer resistance in isolates with MICs ≤4 mg/L. Thus, we believe that the human PD relationships presented here indicate that the time has come to revise daptomycin breakpoints for enterococcal infections. Our identification of the thresholds predictive of survival is consistent with previous observations of improved survival with high-dose daptomycin for enterococcal bacteremia [7, 12] as patients administered higher doses achieve higher fAUC and are more likely to attain optimized fAUC/MIC exposures. However, several limitations should be noted. First, the measured daptomycin concentrations were unavailable. However, the mean calculated fAUCs for patients that received 6 mg/kg/day were within the range of measured values in small pharmacokinetic analyses conducted in other patient populations [40, 41]. Second, as patient data originated from individually-published studies, different acuity scoring systems were utilized; thus, we applied cut-offs that had been previously identified in critically-ill patients to partition the high-acuity from low-acuity patients. Third, our results are only generalizable to adult populations. In addition, as our findings were subject to the inherent limitations of a retrospective observational study design, the results signify the presence of an association between PD exposure and 30-day survival, rather than a causal relationship. It is also important to recognize that we were unable to control for time to adequate therapy or source control, which may impact mortality in cases of enterococcal bacteremia.
CONCLUSIONS
This study identified a daptomycin fAUC/MIC >27.43 to be associated with improved 30-day survival rates in low-acuity patients with enterococcal bacteremia. The probability of a 6 mg/kg/day daptomycin regimen achieving this PD threshold is negligible for isolates with MICs ≥2 mg/L; thus, clinicians may be misguided by susceptibility reports. Our results support reduced daptomycin susceptibility breakpoints to optimize patient outcomes. Furthermore, strategies that employ higher daptomycin doses to maximize fAUC/MIC should be seriously considered in deep-seated enterococcal infections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by internal funding of the Center for Anti-Infective Research and Development, Hartford Hospital, Connecticut.
Potential conflicts of interest. J. L. K. has served as a consultant for Merck & Co., Inc., and Pfizer, Inc.; a grant investigator for Merck & Co., Inc., Theravance Biopharma, and Shionogi Inc.; and a member of the Speakers Bureau/Advisory Board for Allergan. C. A. A. has served as a grant investigator for Merck & Co., Inc., MeMed, and Allergan. N. S. B. has served as a grant investigator for Merck & Co., Inc., and Gilead Sciences, Inc., and maintains a research relationship with Theravance Biopharma. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Merck & Co. Inc. Cubicin package insert-US. Kenilworth, New Jersey: Merck & Co. Inc, 2017. Available at: https://www.merck.com/product/usa/pi_circulars/c/cubicin/cubicin_pi.pdf. Accessed 1 May 2018. [Google Scholar]
- 2. Munita JM, Murray BE, Arias CA. Daptomycin for the treatment of bacteraemia due to vancomycin-resistant enterococci. Int J Antimicrob Agents 2015; 344:1173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 28th ed. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute, 2018. [Google Scholar]
- 4. Shukla BS, Shelburne S, Reyes K, et al. Influence of minimum inhibitory concentration in clinical outcomes of Enterococcus faecium bacteremia treated with daptomycin: Is it time to change the breakpoint?Clin Infect Dis 2016; 62:1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moise PA, Sakoulas G, McKinnell JA, et al. Clinical outcomes of daptomycin for vancomycin-resistant enterococcus bacteremia. Clin Ther 2015; 37:1443–53.e2. [DOI] [PubMed] [Google Scholar]
- 6. Munita JM, Mishra NN, Alvarez D, et al. Failure of high-dose daptomycin for bacteremia caused by daptomycin-susceptible Enterococcus faecium harboring LiaSR substitutions. Clin Infect Dis 2014; 59:1277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chuang YC, Lin HY, Chen PY, et al. Effect of daptomycin dose on the outcome of vancomycin-resistant, daptomycin-susceptible Enterococcus faecium bacteremia. Clin Infect Dis 2017; 64:1026–34. [DOI] [PubMed] [Google Scholar]
- 8. Chong PP, van Duin D, Bangdiwala A, et al. Vancomycin-resistant enterococcal bloodstream infections in hematopoietic stem cell transplant recipients and patients with hematologic malignancies: Impact of daptomycin MICs of 3 to 4 mg/L. Clin Ther 2016; 38:2468–76. [DOI] [PubMed] [Google Scholar]
- 9. Humphries RM, Pollett S, Sakoulas G. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev 2013; 26:759–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Munita JM, Panesso D, Diaz L, et al. Correlation between mutations in liaFSR of Enterococcus faecium and MIC of daptomycin: revisiting daptomycin breakpoints. Antimicrob Agents Chemother 2012; 56:4354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller WR, Murray BE, Rice LB, Arias CA. Vancomycin-resistant enterococci: Therapeutic challenges in the 21st century. Infect Dis Clin North Am 2016; 30:415–39. [DOI] [PubMed] [Google Scholar]
- 12. Britt NS, Potter EM, Patel N, Steed ME. Comparative effectiveness and safety of standard-, medium-, and high-dose daptomycin strategies for the treatment of vancomycin-resistant enterococcal bacteremia among Veterans Affairs patients. Clin Infect Dis 2017; 64:605–13. [DOI] [PubMed] [Google Scholar]
- 13. Patel R, Gallagher JC. Vancomycin-resistant enterococcal bacteremia pharmacotherapy. Ann Pharmacother 2015; 49:69–85. [DOI] [PubMed] [Google Scholar]
- 14. Chuang YC, Chen PY, Lin CY, Chen YC, Wang JT, Chang SC. A retrospective clinical comparison of daptomycin vs daptomycin and a beta-lactam antibiotic for treating vancomycin-resistant Enterococcus faecium bloodstream infections. Sci Rep 2018; 8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yim J, Smith JR, Rybak MJ. Role of combination antimicrobial therapy for vancomycin-resistant Enterococcus faecium infections: Review of the current evidence. Pharmacotherapy 2017; 37:579–92. [DOI] [PubMed] [Google Scholar]
- 16. Alder J, Li T, Yu D, et al. Analysis of daptomycin efficacy and breakpoint standards in a murine model of Enterococcus faecalis and Enterococcus faecium renal infection. Antimicrob Agents Chemother 2003; 47:3561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dandekar PK, Tessier PR, Williams P, Nightingale CH, Nicolau DP. Pharmacodynamic profile of daptomycin against Enterococcus species and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. J Antimicrob Chemother 2003; 52:405–11. [DOI] [PubMed] [Google Scholar]
- 18. Safdar N, Andes D, Craig WA. In vivo pharmacodynamic activity of daptomycin. Antimicrob Agents Chemother 2004; 48:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egli A, Schmid H, Kuenzli E, et al. Association of daptomycin use with resistance development in Enterococcus faecium bacteremia: A 7-year individual and population based analysis. Clin Microbiol Infect 2016; 23:118.e1–7. [DOI] [PubMed] [Google Scholar]
- 20. Casapao AM, Kullar R, Davis SL, et al. Multicenter study of high-dose daptomycin for treatment of enterococcal infections. Antimicrob Agents Chemother 2013; 57:4190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DiPippo AJ, Tverdek FP, Tarrand JJ, et al. Daptomycin non-susceptible Enterococcus faecium in leukemia patients: Role of prior daptomycin exposure. J Infect 2017; 74:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rhee JY, Kwon KT, Ki HK, et al. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: a comparison of the Pitt bacteremia score and the Acute Physiology and Chronic Health Evaluation II scoring systems. Shock 2009; 31:146–50. [DOI] [PubMed] [Google Scholar]
- 23. Dvorchik B, Arbeit RD, Chung J, Liu S, Knebel W, Kastrissios H. Population pharmacokinetics of daptomycin. Antimicrob Agents Chemother 2004; 48:2799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 25. Benet LZ, Zia-Amirhosseini P. Basic principles of pharmacokinetics. Toxicol Pathol 1995; 23:115–23. [DOI] [PubMed] [Google Scholar]
- 26. Kidd JM, Abdelraouf K, Asempa TE, Humphries RM, Nicolau DP. Pharmacodynamics of daptomycin against Enterococcus faecalis and Enterococcus faecium in the neutropenic murine thigh infection model. Antimicrob Agents Chemother 2018; 62:e00506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith JR, Barber KE, Raut A, Aboutaleb M, Sakoulas G, Rybak MJ. β-Lactam combinations with daptomycin provide synergy against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium. J Antimicrob Chemother 2015; 70:1738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakoulas G, Rose W, Nonejuie P, et al. Ceftaroline restores daptomycin activity against daptomycin-nonsusceptible vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 2014; 58:1494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sakoulas G, Bayer AS, Pogliano J, et al. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 2012; 56:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hall Snyder A, Werth BJ, Barber KE, Sakoulas G, Rybak MJ. Evaluation of the novel combination of daptomycin plus ceftriaxone against vancomycin-resistant enterococci in an in vitro pharmacokinetic/pharmacodynamic simulated endocardial vegetation model. J Antimicrob Chemother 2014; 69:2148–54. [DOI] [PubMed] [Google Scholar]
- 31. Smith JR, Barber KE, Raut A, Rybak MJ. β-Lactams enhance daptomycin activity against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium in in vitro pharmacokinetic/pharmacodynamic models. Antimicrob Agents Chemother 2015; 59:2842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diaz L, Tran TT, Munita JM, et al. Whole-genome analyses of Enterococcus faecium isolates with diverse daptomycin MICs. Antimicrob Agents Chemother 2014; 58:4527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Uppal P, LaPlante KL, Gaitanis MM, Jankowich MD, Ward KE. Daptomycin-induced eosinophilic pneumonia: A systematic review. Antimicrob Resist Infect Control 2016; 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higashi Y, Nakamura S, Tsuji Y, et al. A case of daptomycin-induced eosinophilic pneumonia and a review of the published literature. Intern Med 2018; 57:253–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 36. Durante-Mangoni E, Andini R, Parrella A, et al. Safety of treatment with high-dose daptomycin in 102 patients with infective endocarditis. Int J Antimicrob Agents 2016; 48:61–8. [DOI] [PubMed] [Google Scholar]
- 37. Zhao M, Liang L, Ji L, et al. Similar efficacy and safety of daptomycin versus linezolid for treatment of vancomycin-resistant enterococcal bloodstream infections: a meta-analysis. Int J Antimicrob Agents 2016; 48:231–8. [DOI] [PubMed] [Google Scholar]
- 38. King EA, McCoy D, Desai S, Nyirenda T, Bicking K. Vancomycin-resistant enterococcal bacteraemia and daptomycin: are higher doses necessary?J Antimicrob Chemother 2011; 66:2112–8. [DOI] [PubMed] [Google Scholar]
- 39. Foolad F, Taylor BD, Shelburne SA, Arias CA, Aitken SL. Association of daptomycin dosing regimen and mortality in patients with VRE bacteraemia: a review. J Antimicrob Chemother 2018; 73:2277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cojutti PG, Candoni A, Ramos-Martin V, et al. Population pharmacokinetics and dosing considerations for the use of daptomycin in adult patients with haematological malignancies. J Antimicrob Chemother 2017; 72:2342–50. [DOI] [PubMed] [Google Scholar]
- 41. Bubalo JS, Munar MY, Cherala G, Hayes-Lattin B, Maziarz R. Daptomycin pharmacokinetics in adult oncology patients with neutropenic fever. Antimicrob Agents Chemother 2009; 53:428–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.