Abstract
Background
In holoendemic areas, children suffer the most from Plasmodium falciparum malaria, yet newborns and young infants express a relative resistance to both infection and severe malarial disease (SM). This relative resistance has been ascribed to maternally-derived anti-parasite immunoglobulin G; however, the targets of these protective antibodies remain elusive.
Methods
We enrolled 647 newborns at birth from a malaria-holoendemic region of Tanzania. We collected cord blood, measured antibodies to Plasmodium falciparum Schizont Egress Antigen-1 (PfSEA-1), and related these antibodies to the risk of severe malaria in the first year of life. In addition, we vaccinated female mice with PbSEA-1, mated them, and challenged their pups with P. berghei ANKA parasites to assess the impact of maternal PbSEA-1 vaccination on newborns’ resistance to malaria.
Results
Children with high cord-blood anti–PfSEA-1 antibody levels had 51.4% fewer cases of SM compared to individuals with lower anti–PfSEA-1 levels over 12 months of follow-up (P = .03). In 3 trials, pups born to PbSEA-1–vaccinated dams had significantly lower parasitemia and longer survival following a P. berghei challenge compared to pups born to control dams.
Conclusions
We demonstrate that maternally-derived, cord-blood anti–PfSEA-1 antibodies predict decreased risk of SM in infants and vaccination of mice with PbSEA-1 prior to pregnancy protects their offspring from lethal P. berghei challenge. These results identify, for the first time, a parasite-specific target of maternal antibodies that protect infants from SM and suggest that vaccination of pregnant women with PfSEA-1 may afford a survival advantage to their offspring.
Keywords: malaria, vaccine, cord blood, maternal antibodies
Maternally-derived, cord-blood anti–Plasmodium falciparum Schizont Egress Antigen-1 antibodies significantly decrease infants’ risks of severe Plasmodium falciparum malaria. The vaccination of mice with P. berghei Schizont Egress Antigen-1 prior to pregnancy protects offspring from lethal P. berghei parasite challenges.
Plasmodium falciparum malaria is a leading cause of morbidity and mortality in developing countries, infecting hundreds of millions of individuals and killing up to 300000 children in sub-Saharan Africa each year [1]. In holoendemic areas, children suffer the most from malaria, particularly after 6 months of age. Both the relative resistance to infection and severe malarial disease (SM) expressed by neonates and young infants, as well as the hypothesis that this resistance is mediated by maternally-derived IgG, have been recognized for decades [2–4]. Despite these early observations, the antigenic targets of protective, maternally-derived cord-blood antibodies remain elusive.
Recently, we demonstrated that antibodies to Plasmodium falciparum Schizont Egress Antigen-1 (PfSEA-1) predict a decreased risk of SM in 1.5–4-year-olds living in a holoendemic area of Tanzania [5]. Here we demonstrate, in the same cohort, that maternally-derived anti–PfSEA-1 antibodies cross the placenta, and higher cord-blood levels of these antibodies predict a significantly decreased risk of SM in infants. Further, in maternal vaccination studies in mice, pups born to dams that were immunized with PbSEA-1 prior to pregnancy had significantly lower parasitemia and longer survival times following a lethal P. berghei ANKA challenge compared to pups born to dams treated with adjuvant alone. Together, these results identify, for the first time, a specific parasite-antigen target of maternal antibodies that transfer to the offspring and are associated with protection from SM, and suggest that vaccination of pregnant women with PfSEA-1 may afford a survival advantage to their offspring.
METHODS
Detailed methods are provided in the Supplementary Materials.
Tanzanian Birth Cohort
Subjects participated in the Mother Offspring Malaria Studies (MOMS) project, which is based at Muheza Designated District Hospital in northeastern Tanzania [5–7].
Inclusion Criteria and Clinical Monitoring
We monitored children for P. falciparum infection at well-child visits every 2 weeks from birth to 1 year of age, including via blood smear analyses. We obtained cord-blood samples from N = 647 infants and 12 months of follow-up data on N = 583 infants who were included in the current analysis.
Sample Collection and Processing
Maternal peripheral, placental [8], and cord blood were collected in heparinized tubes and stored at 4°C until processing.
Case Definitions
Severe malaria was defined as a positive bloodsmear and 1 or more of the following: (1) respiratory distress, defined by a respiratory rate of >40 breaths/min for children older than 2 months of age or a respiratory rate of >50 breaths/min for children less than 2 months of age; (2) a history of 1 or more convulsions in the 24 hours prior to or during hospitalization; (3) prostration, defined as the inability to move or feed; (4) hypoglycemia, defined by a glucose level < 2.2 mmol/L; (5) severe anemia, defined by hemoglobin <6 g/dL; or (6) oral temperature >40°C.
Maternal Murine Vaccination Studies
In each of 3 trials, we immunized 12-week-old female BALB/cJ mice with 50 ug of rPbSEA-1A emulsified in 100 ul of TiterMax Gold adjuvant or adjuvant alone. Mice were immunized subcutaneously on days 0, 14, 27, and 41. On day 45, mice were bred with male BALB/cJ mice and the resultant pups were challenged intraperitoneally on day-of-life 9 +/-1 (first trial) or 12 +/-1 (second and third trials) with 104P. berghei ANKA-infected red blood cells. Pups were monitored from day 5 post-challenge with blood films.
Statistics in Tanzanian Birth Cohort
We used generalized estimating equation (GEE) models to determine if anti–PfSEA-1 antibodies modified the risk of SM during the follow-up period.
Statistics in Murine Protection Experiments
Parasitemia was analyzed by Mann Whitney U-test. Survival time was analyzed with the Kaplan-Meier log-rank test.
RESULTS
Human Studies
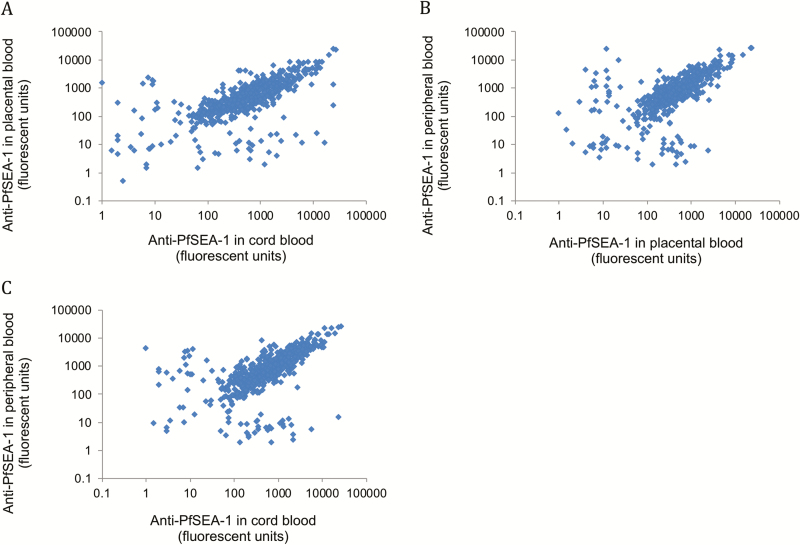
We enrolled and collected cord blood on N = 647 maternal-child dyads at delivery in a P. falciparum–holoendemic region of Tanzania. We obtained maternal peripheral, placental, and cord-blood plasma and measured anti–PfSEA-1 IgG antibody levels using a multiplexed, bead-based assay in each compartment. As expected [9], anti–PfSEA-1 antibodies were highly correlated across all 3 compartments (all Spearman Rho > 0.69, all P < .001; Figure 1), consistent with the known transplacental transport of IgG via neonatal Fc receptor (FcRn) [10].
Figure 1.
Anti–PfSEA-1 antibody levels are highly correlated in maternal peripheral, placental, and cord-blood compartments. Anti–PfSEA-1 IgG levels were measured by bead-based assays in paired samples from maternal peripheral (n = 581), placental (n = 615), and cord-blood (n = 647) plasma. Abbreviation: PfSEA-1, Plasmodium falciparum Schizont Egress Antigen-1.
To assess the impact of cord-blood anti–PfSEA-1 on subsequent experience of malaria parasitemia and disease, children were evaluated at routine, well-child visits by a clinician every 2 weeks from birth to 12 months of age and a blood smear was obtained at each visit. We selected 12 months as the endpoint for evaluating the impact of cord-blood antibody levels because maternally-derived, functional anti-parasite antibodies are still detectable at 6 months of age [11] and maternally-derived antibodies remain detectible in some 9- to 12-month-olds [12]. We also conducted analyses using clinical follow-up data through 9 months of life.
To answer the question, do cord-blood anti–PfSEA-1 antibodies predict a reduced risk of SM, we constructed GEE models to evaluate the impact of cord-blood PfSEA-1 antibodies on malaria outcomes after adjusting for confounders and repeated measures within individuals.
We observed n = 7 cases of SM in the first 3 months of life, n = 52 cases of SM in the first 6 months of life, n = 112 cases of SM in the first 9 months of life, and n = 166 cases of SM in the first 12 months of life. Of the n = 166 cases of SM in our cohort, there were n = 75 cases of respiratory distress, n = 62 cases of severe anemia, n = 20 cases of prostration, and n = 20 cases of convulsion. The majority of SM cases presented with a single clinical syndrome.
We fit GEE models to determine whether cord-blood antibodies to PfSEA-1 predicted a decreased risk of SM over 9 and 12 months of follow-up in n = 583 children with complete follow-up data. When analyzed as a continuous variable, anti–PfSEA-1 antibody levels measured in cord blood were associated with significantly reduced incidence of SM over the 12 months of follow-up in univariate analyses (odds ratio [OR] = 0.38, 95% confidence interval [CI] 0.16–0.90, P = .028; Supplementary Table S1). This relationship remained significant (OR = 0.42, 95% CI 0.18–0.95, P = .038; Table 1) after adjusting for parity, average prior parasitemia, age, and, to allow for a potential non-linear relationship, age2. Using this same GEE model, anti–PfSEA-1 antibody levels measured in cord blood were associated with significantly reduced incidence of SM when children were followed for only 9 months of follow-up (OR = 0.31, 95% CI 0.12–0.84, P = .02; Supplementary Table S2).
Table 1.
Multivariable Generalized Estimating Equation Model Estimating Risk of Severe Malaria During First 12 Months of Life
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Age in years | 1.23 (1.16–1.30) | <.001 |
| Age2 in years | 0.997 (0.996–0.998) | <.001 |
| Average prior parasite density | 1.002 (1.001–1.003) | <.001 |
| Parasites/200 WBCs | ||
| Parity | ||
| Primiparous | Reference | .044 |
| Secundiparous | 1.80 (1.10–2.94) | |
| Multiparous | 1.19 (0.76–1.85) | |
| Anti–PfSEA-1 in cord blood (fluorescence units/10 000) | 0.42 (0.18–0.95) | .038 |
Abbreviations: CI, confidence interval; OR, odds ratio; PfSEA-1, Plasmodium falciparum Schizont Egress Antigen-1; WBC, white blood cell.
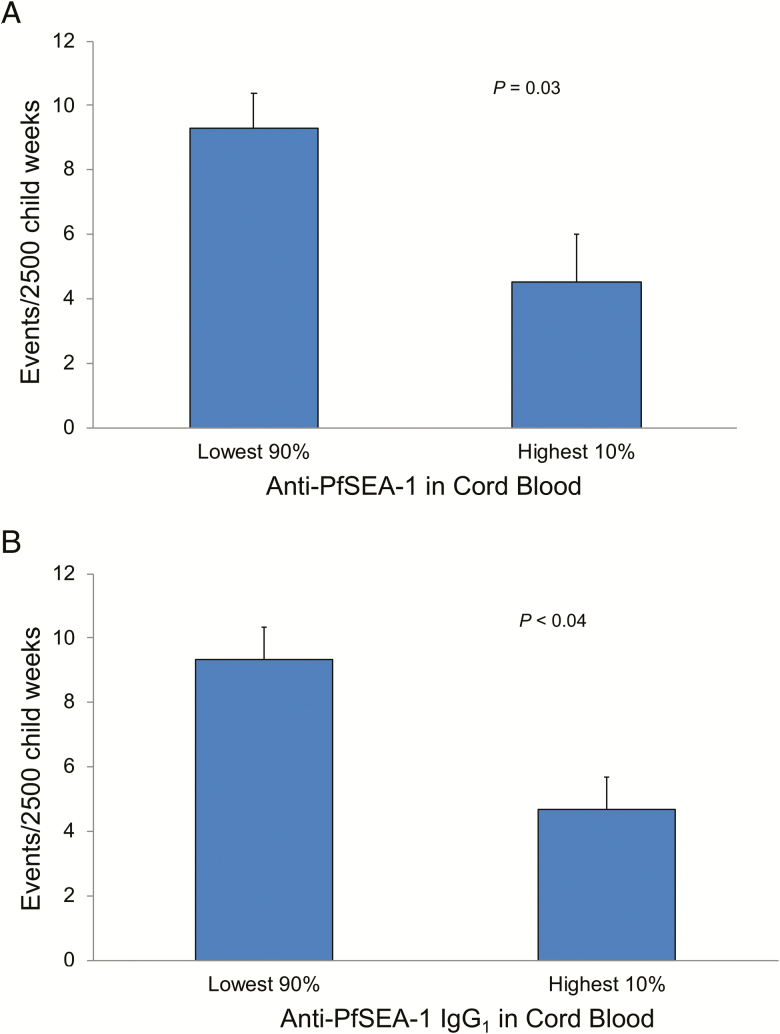
After demonstrating a significant relationship between anti–PfSEA-1 antibody levels (analyzed as a continuous variable) and the risk of SM, we explored cutoff levels of anti–PfSEA-1 antibodies to identify levels necessary for protection from SM. Children with anti–PfSEA-1 levels in the top 10% had 51.4% fewer cases of SM compared to individuals with lower anti–PfSEA-1 levels over 12 months of follow-up (9.3 +/- 1.11 cases/2500 observation weeks vs 4.5 +/- 1.47 cases/2500 observation weeks, respectively, P = .03; Figure 2A). The high levels of cord blood anti–PfSEA-1 antibodies necessary for protection are consistent with the expected decay of these maternally-derived antibodies from their cord-blood maxima, even in exclusively breast-fed infants [13, 14].
Figure 2.
Incidence of severe malaria in Tanzanian children from birth to 12 months of age, dichotomized into those with the highest 10% (n = 64) and lowest 90% (n = 583) levels of anti–PfSEA-1 IgG in cord blood. Bars represent the incidence of severe malaria after adjustment for age, age2, parity, average prior parasitemia, and repeated measures within individuals in GEE models. Error bars represent SEM. (A) Total IgG anti–PfSEA-1. (B) IgG1 anti–PfSEA-1. Abbreviations: GEE, generalized estimating equation; IgG; PfSEA-1, Plasmodium falciparum Schizont Egress Antigen-1; SEM, standard error of the mean.
Because of the differential transport of IgG subclasses across the human placenta by FcRn [15], as well as their unique interactions with immune effector functions, we performed isotype-specific anti–PfSEA-1 antibody assays on all 583 available cord-blood sera. Using the same GEE-based statistical analyses employed for dichotomous total IgG anti–PfSEA-1 analyses, cord-blood anti–PfSEA-1 antibodies of the IgG1 subclass predicted a significantly decreased risk of SM over the first 12 months of life (P < .04; Figure 2B). Anti–PfSEA-1 responses of other isotypes were not associated with protection.
To place the anti–PfSEA-1 results in context with antibodies directed against other malarial vaccine candidates/antigens and to demonstrate the antigenic specificity of these protective anti–PfSEA-1 responses, we performed multiplexed bead-based antibody analyses using the same cord-blood plasma in which we measured anti–PfSEA-1 antibody levels. Using this multiplexed platform, we measured IgG antibody levels to merozoite surface protein (MSP)-1–19 (3D7 variant), MSP-3 (99–265 amino acid [aa]), MSP-7 (117–248 aa), liver stage antigen (LSA)-N (28–150 aa), LSA-C (1630–1909 aa), rhoptry associated membrane antigen (RAMA)-E (759–840 aa), and RAMA-Pr (582–767 aa). Cord-blood IgG antibody responses to these malaria antigens were not related to the risk of severe malaria in the first 9 or 12 months of life using the same GEE-based statistical analyses employed for PfSEA-1 when analyzed as continuous variables (all P = not significant) or when analyzed as dichotomous variables (all P = not significant; Supplementary Figure S1). We note that MSP-3 responses did show a trend toward protection in the dichotomous analysis. These results support the interpretation that protection against SM was not due to higher titers of anti-malarial antibodies in general, but rather due, in part, to the specific, known anti-parasite effect of anti–PfSEA-1 antibodies.
Murine Studies
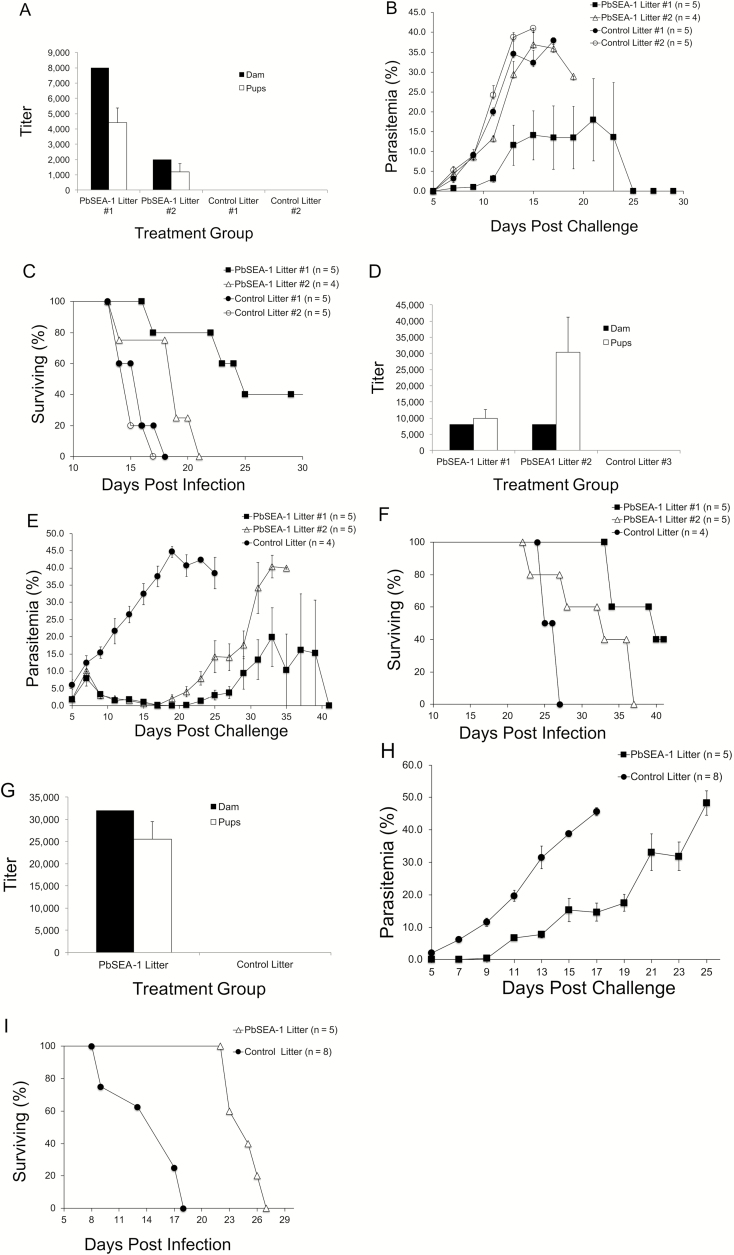
To demonstrate that anti-SEA-1 antibodies protect newborns from malaria in an experimental model, we conducted 3 independent vaccine trials in mice. In all trials, the immunization of dams with PbSEA-1A induced IgG antibodies that were detectible in their pups (Figure 3A, D and G). In the first trial, pups born to control dams (Control Litter #1 and #2, total n = 10) had 8.4-fold higher parasitemia on day 9 post challenge compared to pups born to the PbSEA-1A vaccinated dam (n = 5) who produced high titer anti-PbSEA-1 antibodies (PbSEA-1 Litter #1, P <.002, Figure 3B). In addition, pups born to the vaccinated dam who produced high titer anti-PbSEA-1A (PbSEA-1 Litter #1, n = 5) had 1.67-fold longer median survival times compared to pups born to control dams (Control Litter #1 and #2, total n = 10, P < .001, Figure 3C). Pups born to the vaccinated dam who produced low titer anti-PbSEA-1A (PbSEA-1 Litter #2, n = 4) did not have significantly lower parasite densities on day 9 post-challenge, but did have 1.27-fold longer median survival times compared to pups born to control dams (Control Litter #1 and #2, total n = 10, P <.017, Figure 3C). Pups born to the vaccinated dam who produced high titer anti-PbSEA-1A (PbSEA-1 Litter #1, n = 5) had 1.32-fold longer median survival times compared to pups born to the vaccinated dam who produced low titer anti-PbSEA-1A (PbSEA-1 Litter #2, n = 4, P =.038, Figure 3C). Of particular note, 2 out of 5 pups born to the dam with the highest anti-PbSEA-1A titer self-cured on days 15 and 17 post-infection and had no detectable parasites in their blood films for the remainder of the 41-day experiment.
Figure 3.
Vaccination of female BALB/cJ mice with rPbSEA-1A protects their pups from challenge with P. berghei ANKA. Antibody responses of rPbSEA-1A assayed by bead-based endpoint titration ELISA in vaccinated female mice and their offspring in the (A) first, (D) second, and (G) third trials. The bars represent mean fluorescence; the error bars represent SEMs. Black bars represent anti–rPbSEA-1A IgG responses in female adult mice immunized with rPbSEA-1A or adjuvant alone. The white bars represent anti–rPbSEA-1A IgG responses in their pups. (B) In the first trial, pups born to control dams (Control Litters #1 and #2, total n = 10) had 8.4-fold higher parasitemia on day 9 post-challenge compared to pups born to the PbSEA-1A vaccinated dam (n = 5), who produced high-titer anti–PbSEA-1 antibodies (PbSEA-1 Litter #1, P < .002). Error bars represent SEMs. (C) In the first trial, BALB/cJ pups born to the vaccinated dam who produced high-titer anti–PbSEA-1A (PbSEA-1 Litter #1, n = 5) had 1.67-fold longer median survival times compared to pups born to control dams (Control Litters #1 and #2, total n = 10, P < .001). (E) In the second trial, BALB/cJ pups born to the control dam (Control Litter, n = 4) had 4.8-fold higher parasitemia on day 9 post-challenge (and a 170-fold higher parasitemia on day 17, the day of maximal parasitemia difference) compared to pups born to the 2 PbSEA-1A–vaccinated dams (PbSEA-1 Litters #1 and #2, n = 10, P < .005). Error bars represent SEMs. (F) In the second trial, BALB/cJ pups born to the vaccinated dams had 1.36-fold longer median survival times compared to pups born to the control dam (P < .003). (H) In the third trial, BALB/cJ pups born to the control dam had 27-fold higher parasitemia on day 9 post-challenge compared to pups born to the PbSEA-1A–vaccinated dam (P < .0001). Error bars represent SEMs. (I) In the third trial, BALB/cJ pups born to the vaccinated dam had 1.47-fold longer median survival times compared to pups born to the control dam (P < .001). Abbreviations: IgG, immunoglobulin G; ELISA, enzyme linked immunosorbent assay; PbSEA-1, Plasmodium berghei Schizont Egress Antigen-1; PfSEA-1, Plasmodium falciparum Schizont Egress Antigen-1; SEM, standard error of the mean.
In the second trial, pups born to the control dam (Control Litter, n = 4) had 4.8-fold higher parasitemia on day 9 post challenge (and a 170-fold higher parasitemia on day 17, the day of maximal parasitemia difference) compared to pups born to the 2 PbSEA-1A vaccinated dams (PbSEA-1 Litter #1 and #2, n = 10, P <.005; Figure 3E). In addition, pups born to the two vaccinated dams (PbSEA-1 Litter #1 and #2, n = 10) had 1.36-fold longer median survival times compared to pups born to the control dam (Control Litter, n = 4, P < .003; Figure 3F). Again, 2 out of 5 pups born to one of the dams (PbSEA-1 Litter #1) self-cured on day 19 post-infection and had no detectable parasites in their blood films for the remainder of the 41-day experiment.
In the third trial, pups born to the control dam (Control Litter, n = 8) had 27-fold higher parasitemia on day 9 post challenge compared to pups born to the PbSEA-1A vaccinated dam (PbSEA-1 Litter, n = 5, P <.0001). In addition, pups born to the vaccinated dam had 1.47-fold longer median survival times compared to pups born to control dam (P <.001).
DISCUSSION
Several hypotheses have been advanced to explain the relative resistance to P. falciparum infection and SM enjoyed by neonates, infants, and young children, including transfer of protective maternal antibodies [2, 3], poor parasite growth in hemoglobin F–containing RBCs [16, 17], low levels of the parasite growth–limiting nutrient para-aminobenzoic acid in breast milk [18, 19], the presence of lactoferrin and secretory IgA in breast milk [20], and decreased mosquito exposure resulting from behavioral features such as the swaddling of infants [21].
Several reports have attempted to implicate specific maternally-derived anti-malarial antibodies with neonatal resistance [22]. Antibody levels to both crude parasite extracts [23] and recombinant P. falciparum proteins [24, 25] are highly correlated in maternal and cord-blood compartments. Surprisingly, cord-/infant-blood antibody responses to parasite extracts, as well as several specific malaria antigens, including Pf (RESA [ring-infected erythrocyte surface antigen]), Pf (circumsporozoite protein [CSP]), PfLSA-1, PfMSP-119, apical membrane antigen (AMA)-1, and PfMSP-2, and Rh2, were not associated with decreased risks of parasitemia, clinical malaria, or severe malaria in multiple studies [26–32]. Similarly, cord-blood antibody levels to surface antigens expressed on chondroitin sulfate A–selected parasites predict a shorter time to first infection and higher parasite density [33].
The lack of protection associated with these specific responses is in marked contrast to evidence that (1) the presence of anti-parasite IgG2 antibodies (but not their titers) in cord blood was associated with a decreased risk of parasite infection in the first 6 months of life [34]; (2) cord-blood sera exhibits anti-parasite activity in growth inhibition assays [11]; and (3) parasite-specific antibody-dependent respiratory burst activity measured in cord-blood sera predicts a decreased risk of severe malaria for up to 9 months in a case-control study [32].
While the majority of reports have found no association between maternally-derived, parasite-antigen–specific antibodies and protection from malaria, there have been 2 reports of protection associated with anti–PfMSP-1 responses [25, 35]. In a Liberian birth cohort [35], anti–MSP-119 IgG levels measured in 100 infants were related to a risk of malaria parasitemia with axillary temperatures greater than 37.5℃ in the first 12 months of life. Unfortunately, these antibodies were not measured in cord blood, but rather in peripheral blood collected sometime after delivery. Notably, there was a 66% loss to follow-up during the study and no assessment of bias resulting from this loss. In a Kenyan birth cohort [25], cord blood was available for only n = 21 maternal/infant dyads. Therefore, the authors measured anti–MSP-119 within the final 30 days of pregnancy in n = 60 mothers. Maternal anti–MSP-119 was associated with an increased time to the first positive blood film in offspring using monthly blood smears. Unfortunately, neither of these reports directly measured maternally-derived antibodies transferred to the neonate (ie, cord-blood antibody levels) and neither of these reports measured antibody responses to any other specific malarial antigens. These data suggest that maternally-transferred antibodies could influence neonatal outcomes; however, study limitations and the failure of mouse pups suckled on P. yoelii MSP-119–vaccinated dams to achieve similar levels of protection as those enjoyed by their mothers [36] have precluded the broad acceptance of anti–MSP-1 antibody-mediated protection of neonates.
In recent work, we identified PfSEA-1, a highly invariant protein necessary for merozoite egress from rupturing infected erythrocytes, as a target of antibodies that protect young children from severe malaria [5] and demonstrated that anti–PfSEA-1 antibodies purified from placental blood samples obtained from mothers in the current cohort significantly attenuated parasite growth in vitro (see Figure S6 in reference [5]). In the present study, we measured the level of anti–PfSEA-1 antibodies in maternal peripheral, placental, and cord-blood plasma from n = 647 mother-infant dyads residing in a P. falciparum–holoendemic region of Tanzania. We evaluated the relationship between cord-blood antibody levels and the risk of severe malaria in the first 9 and 12 months of life, the time period of greatest risk of severe malaria in our cohort. In our study, anti–PfSEA-1 antibody levels were linearly related to a decreased risk of SM and individuals with high levels of cord-blood anti–PfSEA-1 enjoyed a 51% decreased risk of SM in their first year of life. This protective relationship between antimalarial antibodies and the risk of SM was not observed for several other malaria antigens evaluated. Because the potential for residual confounding cannot be eliminated from longitudinal cohort studies, we validated our results in an experimental murine model.
We evaluated the impact of maternal vaccination with PbSEA-1 on resistance to a P. berghei ANKA challenge in their pups. Previously, we demonstrated that vaccination of mice with PbSEA-1 confers protection against a lethal P. berghei ANKA challenge and that this protection is transferable to naive mice with anti–PbSEA-1 antisera (see Figure 5 of reference [5]). While immunization of female mice with irradiated P. berghei sporozoites confers resistance to a sporozoite challenge in their pups [37], protection in pups born to dams vaccinated with specific malaria antigens has not been reported in P. berghei.
In 3 independent trials, vaccination of female mice prior to pregnancy generated significant protection against parasite challenge in their offspring. Strikingly, we observed that 40% of PbSEA-1 vaccinated mice in the first 2 trials were able to self-cure, a phenomenon that has not been reported for any blood-stage sub-unit vaccine in this model. Together with the marked prolongation in survival times (32–67%) in our 3 trials, these data suggest that similar results in humans could carry significant clinical benefit.
We note that across the 3 trials, antibody titers in the vaccinated groups were not linearly related to protection from challenge infection. Because pups acquire a significant portion of their endowment of maternal antibodies via breast milk [38, 39] and we measured their endowment on only 1 occasion (the day of inoculation), it is likely that there is unmeasured variation in antibody levels that was not captured by our single antibody assessment.
Together, our results from human observational studies and interventional mouse models represent the first demonstration that maternally-derived, cord-blood antibodies recognizing a specific malarial protein (PfSEA-1/PbSEA-1) confer resistance to SM or death in offspring. This resistance may result from the impact of anti–PfSEA-1 antibodies alone or, more likely, involve cooperation with additional antibodies targeting as-yet-unidentified antigens. Our human cohort data indicate that only offspring with high anti–PfSEA-1 levels benefit from a reduced incidence of SM, consistent with the decay of maternally-derived antibodies over time. These data provide the first direct support for the decades-old hypothesis that antigen-specific maternally derived anti-malarial antibodies protect infants from SM, and suggest that vaccination of pregnant women with PfSEA-1 may afford a survival advantage to their infants.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contribution. J. F. F., I. C. M., M. F., P. E. D., and J. D. K. designed the study. J. F. F., J. D. K., S. P., I. C. M., M. F., and P. E. D. drafted the manuscript. M. F., E. R. K., and P. E. D. conducted the field-based sample and epidemiologic data collection. S. P.-T. and C. P. N. expressed and purified the recombinant proteins and performed the human antibody assays. J. F. F. and S. P. designed and performed the statistical analyses. D. K. R., C. E. N., E. A. M., R. J. T., and A. J. performed the murine vaccination experiments.
Acknowledgments. The authors thank Mother Offspring Malaria Studies (MOMS) Project staff for their efforts collecting clinical data, processing samples, and interpreting malaria blood smears. The authors also thank the study subjects and their families.
Financial support. This work was supported by grants from the US National Institutes of Health (grant number R01-AI52059) and the Bill & Melinda Gates Foundation (grant number 1364) to P. E. D.; the Intramural Research Program of the National Institute for Allergy and Infectious Disease, National Institutes of Health; and the US National Institutes of Health (grant numbers R01-AI076353 and R01 AI110699-01) and the March of Dimes Foundation to J. D. K. This work was further supported by seed funds from both the Department of Pathology and the Office of Research Administration, Rhode Island Hospital. The US National Institutes of Health has awarded grants to J. F. F. (grant number K24-AI112964), C. E. N. (grant number T32-DA013911), I. C. M. (grant number 1K08-AI100997-01A1), and E. A. M. (grant number 1K01-AI113068). All other authors report no potential conflicts.
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. The world malaria report. Geneva, Switzerland: World Health Organization, 2016. [Google Scholar]
- 2. Williams AI, McFarlane H. Distribution of malarial antibody in maternal and cord sera. Arch Dis Child 1969; 44:511–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruce-Chwatt LJ. Malaria in African infants and children in Southern Nigeria. Ann Trop Med Parasitol 1952; 46:173–200. [DOI] [PubMed] [Google Scholar]
- 4. Macdonald G. The analysis of malaria parasite rates in infants. Trop Dis Bull 1950; 47:915–38. [PubMed] [Google Scholar]
- 5. Raj DK, Nixon CP, Nixon CE, et al. . Antibodies to PfSEA-1 block parasite egress from RBCs and protect against malaria infection. Science 2014; 344:871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mutabingwa TK, Bolla MC, Li JL, et al. . Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med 2005; 2:e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Decreased susceptibility to Plasmodium falciparum infection in pregnant women with iron deficiency. J Infect Dis 2008; 198:163–6. [DOI] [PubMed] [Google Scholar]
- 8. Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 1996; 272:1502–4. [DOI] [PubMed] [Google Scholar]
- 9. Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012; 2012:985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leitner K, Ellinger I, Grill M, Brabec M, Fuchs R. Efficient apical IgG recycling and apical-to-basolateral transcytosis in polarized BeWo cells overexpressing hFcRn. Placenta 2006; 27:799–811. [DOI] [PubMed] [Google Scholar]
- 11. Wilson PT, Malhotra I, Mungai P, King CL, Dent AE. Transplacentally transferred functional antibodies against Plasmodium falciparum decrease with age. Acta Trop 2013; 128:149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicoara C, Zäch K, Trachsel D, Germann D, Matter L. Decay of passively acquired maternal antibodies against measles, mumps, and rubella viruses. Clin Diagn Lab Immunol 1999; 6:868–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iyengar L, Selvaraj RJ. Intestinal absorption of immunoglobulins by newborn infants. Arch Dis Child 1972; 47:411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshida M, Claypool SM, Wagner JS, et al. . Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 2004; 20:769–83. [DOI] [PubMed] [Google Scholar]
- 15. Einarsdottir HK, Stapleton NM, Scherjon S, et al. . On the perplexingly low rate of transport of IgG2 across the human placenta. PLoS One 2014; 9:e108319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pasvol G, Weatherall DJ, Wilson RJ, Smith DH, Gilles HM. Fetal haemoglobin and malaria. Lancet 1976; 1:1269–72. [DOI] [PubMed] [Google Scholar]
- 17. Amaratunga C, Lopera-Mesa TM, Brittain NJ, et al. . A role for fetal hemoglobin and maternal immune IgG in infant resistance to Plasmodium falciparum malaria. PLoS One 2011; 6:e14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maegraith BG, Deegan T, Jones ES. Suppression of malaria (P. berghei) by milk. Br Med J 1952; 2:1382–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hawking F. Milk diet, p-aminobenzoic acid, and malaria (P. berghei); preliminary communication. Br Med J 1953; 1:1201–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kassim OO, Ako-Anai KA, Torimiro SE, Hollowell GP, Okoye VC, Martin SK. Inhibitory factors in breastmilk, maternal and infant sera against in vitro growth of Plasmodium falciparum malaria parasite. J Trop Pediatr 2000; 46:92–6. [DOI] [PubMed] [Google Scholar]
- 21. Riley EM, Wagner GE, Akanmori BD, Koram KA. Do maternally acquired antibodies protect infants from malaria infection?Parasite Immunol 2001; 23:51–9. [DOI] [PubMed] [Google Scholar]
- 22. Dobbs KR, Dent AE. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology 2016; 143:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rasheed FN, Bulmer JN, De Francisco A, et al. . Relationships between maternal malaria and malarial immune responses in mothers and neonates. Parasite Immunol 1995; 17:1–10. [DOI] [PubMed] [Google Scholar]
- 24. Yang JC, Blanton RE, King CL, Fujioka H, Aikawa M, Sam-Yellowe TY. Seroprevalence and specificity of human responses to the Plasmodium falciparum rhoptry protein Rhop-3 determined by using a C-terminal recombinant protein. Infect Immun 1996; 64:3584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Branch OH, Udhayakumar V, Hightower AW, et al. . A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kiloDalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am J Trop Med Hyg 1998; 58:211–9. [DOI] [PubMed] [Google Scholar]
- 26. Achidi EA, Salimonu LS, Perlmann H, Perlmann P, Berzins K, Williams AI. Lack of association between levels of transplacentally acquired Plasmodium falciparum-specific antibodies and age of onset of clinical malaria in infants in a malaria endemic area of Nigeria. Acta Trop 1996; 61:315–26. [DOI] [PubMed] [Google Scholar]
- 27. Zhou Z, Xiao L, Branch OH, Kariuki S, Nahlen BL, Lal AA. Antibody responses to repetitive epitopes of the circumsporozoite protein, liver stage antigen-1, and merozoite surface protein-2 in infants residing in a Plasmodium falciparum-hyperendemic area of Western Kenya. XIII. Asembo Bay Cohort Project. Am J Trop Med Hyg 2002; 66:7–12. [DOI] [PubMed] [Google Scholar]
- 28. Riley EM, Wagner GE, Ofori MF, et al. . Lack of association between maternal antibody and protection of African infants from malaria infection. Infect Immun 2000; 68:5856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitua AY, Urassa H, Wechsler M, et al. . Antibodies against Plasmodium falciparum vaccine candidates in infants in an area of intense and perennial transmission: relationships with clinical malaria and with entomological inoculation rates. Parasite Immunol 1999; 21:307–17. [DOI] [PubMed] [Google Scholar]
- 30. Dechavanne C, Cottrell G, Garcia A, Migot-Nabias F. Placental malaria: decreased transfer of maternal antibodies directed to Plasmodium falciparum and impact on the incidence of febrile infections in infants. PLoS One 2015; 10:e0145464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dent AE, Malhotra I, Wang X, et al. . Contrasting patterns of serologic and functional antibody dynamics to plasmodium falciparum antigens in a Kenyan birth cohort. Clin Vaccine Immunol 2016; 23:104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murungi LM, Sondén K, Odera D, et al. . Cord blood IgG and the risk of severe Plasmodium falciparum malaria in the first year of life. Int J Parasitol 2017; 47:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cot M, Le Hesran JY, Staalsoe T, Fievet N, Hviid L, Deloron P. Maternally transmitted antibodies to pregnancy-associated variant antigens on the surface of erythrocytes infected with Plasmodium falciparum: relation to child susceptibility to malaria. Am J Epidemiol 2003; 157:203–9. [DOI] [PubMed] [Google Scholar]
- 34. Deloron P, Dubois B, Le Hesran JY, et al. . Isotypic analysis of maternally transmitted Plasmodium falciparum-specific antibodies in Cameroon, and relationship with risk of P. falciparum infection. Clin Exp Immunol 1997; 110:212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Høgh B, Marbiah NT, Burghaus PA, Andersen PK. Relationship between maternally derived anti-Plasmodium falciparum antibodies and risk of infection and disease in infants living in an area of Liberia, west Africa, in which malaria is highly endemic. Infect Immun 1995; 63:4034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stanisic DI, Martin LB, Good MF. The role of the 19-kDa region of merozoite surface protein 1 and whole-parasite-specific maternal antibodies in directing neonatal pups’ responses to rodent malaria infection. J Immunol 2003; 171:5461–9. [DOI] [PubMed] [Google Scholar]
- 37. Orjih AU, Cochrane AH, Nussenzweig RS. Active immunization and passive transfer of resistance against sporozoite-induced malaria in infant mice. Nature 1981; 291:331–2. [DOI] [PubMed] [Google Scholar]
- 38. Abrahamson DR, Powers A, Rodewald R. Intestinal absorption of immune complexes by neonatal rats: a route of antigen transfer from mother to young. Science 1979; 206:567–9. [DOI] [PubMed] [Google Scholar]
- 39. González JE, León M, Hernández I, Garrido G, Casacó A. Effect of the maternofetal and milk transfer of the anti-epidermal growth factor receptor monoclonal antibody 7A7 in mice. Placenta 2011; 32:470–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.