Abstract
Background:
Electronic cigarette (e-cigarette) conventions regularly bring together thousands of users around the world. In these environments, secondhand exposures to high concentrations of e-cigarette emissions are prevalent. Some biomarkers for tobacco smoke exposure may be used to characterize secondhand e-cigarette exposures in such an environment.
Methods:
Participants who did not use any tobacco product attended four separate e-cigarette events for approximately six hours. Urine and saliva samples were collected from participants prior to the event, immediately after the event, 4-h after the event, and the next morning (first void). Urine samples from 34 participants were analyzed for cotinine, trans-3′-hydroxycotinine, S-(3-hydroxypropyl)-N-acetylcysteine (3-HPMA), S-carboxyethyl-N-acetylcysteine (CEMA), select tobacco-specific nitrosamines (TSNAs), and 8-isoprostane. Saliva samples were analyzed for cotinine and trans-3′-hydroxycotinine.
Results:
Data from 28 of 34 participants were used in the data analysis. Creatinine-adjusted urinary cotinine concentrations increased up to 13-fold and peaked 4-h after completed exposure (range of adjusted geometric means [AGMs] = 0.352–2.31 μg/g creatinine). Salivary cotinine concentrations were also the highest 4-h after completed exposure (range of AGMs = 0.0373–0.167 ng/mL). Salivary cotinine and creatinine-corrected concentrations of urinary cotinine, trans-3′-hydroxycotinine, CEMA, and 3-HPMA varied significantly across sampling times. Urinary and salivary cotinine, urinary trans-3′-hydroxycotinine, and urinary 3-HPMA concentrations also varied significantly across events.
Conclusion:
Secondhand e-cigarette exposures lasting six hours resulted in significant changes in exposure biomarker concentrations of both nicotine and acrolein but did not change exposure to tobacco-specific nitrosamines. Additional research is needed to understand the relationship between biomarker concentrations and environmental concentrations of toxicants in e-cigarette emissions.
Keywords: Electronic cigarettes, Biological monitoring, Secondhand exposures, Nicotine, Cotinine
1. Introduction
Tobacco products contribute to the death of nearly a half a million Americans every year (CDC, 2017a). Tobacco is used primarily because of nicotine addiction (U.S. Department of Health Human Services, 1988). To provide a less toxic smoking experience, devices have emerged that deliver nicotine without the high concentrations of many harmful chemicals in tobacco smoke. One such device is the electronic cigarette. Electronic cigarettes (e-cigarettes) aerosolize a liquid containing nicotine without producing tobacco combustion products (AIHA, 2014). E-cigarettes have rapidly grown in popularity and are now the most commonly used nicotine delivery products among youth (U.S. Department of Health and Human Services, 2016).
Because e-cigarettes are often excluded from indoor smoke-free laws (Tobacco Control Legal Consortium, 2015; U.S. Department of Health and Human Services, 2016) many users begin using them in places where smoking is banned (Marynak et al., 2014). As of June 30, 2018, approximately 20% of US states, the District of Columbia, and Puerto Rico banned e-cigarette use in bars, restaurants, and private worksites (CDC, 2017c). In comparison, nearly 60% of states and Puerto Rico and the District of Columbia ban traditional cigarettes in bars, restaurants, and private worksites (CDC, 2017b).
Often studies characterize passive e-cigarette emission exposures in a controlled environment, but few characterize exposures in a real-use or public setting. Studies in controlled environments are often short in duration and cannot account for the variety of e-cigarette devices, liquids, and user behaviors that influence exposure (Melstrom et al., 2017; U.S. Department of Health and Human Services, 2016; Wang et al., 2017). Some studies use a regulatory commercial smoking machine to mimic the first-hand exposure of an e-cigarette device. These studies fail to account for the lung absorption of e-cigarette emissions that occurs when a human participant operates the device (Schripp et al., 2013). Understanding the secondhand exposures to toxicants in e-cigarette emissions under real-use conditions in natural settings is an important public health priority.
Because validated biomarkers specific to e-cigarette exposures have yet to be identified (Schick et al., 2017), we used conventional tobacco smoke exposure biomarkers to characterize e-cigarette emissions exposures. One of the most sensitive and specific tobacco exposure biomarkers is cotinine, the primary proximate metabolite of nicotine (Benowitz, 1999). Approximately 75% of absorbed nicotine is converted to cotinine, and approximately 60% of cotinine is further metabolized to trans-3′-hydroxycotinine (Hukkanen et al., 2005). The sum of these metabolites accounts for 60–80% of absorbed nicotine. Because nicotine is typically present in e-cigarette liquids, cotinine and trans-3′-hydroxycotinine are useful biomarkers for characterizing e-cigarette exposure (Schick et al., 2017).
Tobacco-specific nitrosamines (TSNAs) are a class of compounds only found in tobacco products (Schick et al., 2017). Several TSNAs have been detected in e-cigarette emissions (Goniewicz et al., 2014). A metabolite of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), 4-(methylnitrosamino)-1-(3-pyridyl) butanol (NNAL) is often used as a tobacco exposure biomarker because it is stable, abundant in urine of smokers and tobacco users, and indicative of cancer risk (Schick et al., 2017). Acrolein is a potent irritant formed when glycerin and propylene glycol in e-cigarette liquids are heated inside an e-cigarette and oxidized to a variety of carbonyl compounds (IARC, 1993; Ohta et al., 2011; Sleiman et al., 2016). Although acrolein exposures are not specific to tobacco products, acrolein metabolites (i.e. S-(3-hydroxypropyl)-N-acetylcysteine [3-HPMA], S-carboxyethyl-N-acetylcysteine [CEMA]), may help assess the extent of tobacco or e-cigarette exposure.
Environmental toxicants, such as tobacco smoke, are known to generate reactive oxygen species in humans (CDC, 2010). A non-enzymatic peroxidation product of arachidonic acid, 8-isoprostane is a known biomarker for estimating oxidative stress (Kadiiska et al., 2005). Like acrolein, 8-isoprostane is not specific to tobacco products but can also aid in understanding exposures from tobacco or e-cigarette products.
The purpose of this study was to conduct a secondhand exposure assessment using biomonitoring to characterize passive e-cigarette exposures in a real-use setting with a high concentration of e-cigarette emissions. E-cigarette conventions are large social e-cigarette events described previously (Johnson et al., 2018a; Williams, 2015). E-cigarette conventions attract hundreds to thousands of e-cigarette users who gather in a relatively small space (i.e. convention hall). This environment provides a unique opportunity to conduct a secondhand exposure assessment representative of high exposures in public settings and exposures that last for approximately the length of a work shift.
2. METHODS
2.1. Study locations
This study was conducted at four e-cigarette events in the Southeastern United States between April 2016 and March 2017 described previously (Johnson et al., 2018b). Event 1 was held in a large convention center in Daytona Beach, Florida in April 2016. Event 2 was held in a small concert venue in Athens, GA in September 2016. Event 3 was held in a large convention center in Chattanooga, Tennessee in October 2016. Event 4 was held in a tradeshow venue in Atlanta, Georgia in March 2017. Events 1 and 4 attracted ≥1000 attendees. Events 2 and 3 attracted smaller crowds. Event and venue summaries are presented in Table 1.
Table 1.
E-Cigarette event characteristics.
| VARIABLES | EVENT 1 | EVENT 2 | EVENT 3 | EVENT 4 |
|---|---|---|---|---|
| Location | Daytona Beach, Florida | Athens, Georgia | Chattanooga, Tennessee | Atlanta, Georgia |
| Venue type | Convention Center | Concert Venue | Convention Center | Exhibition/Tradeshow |
| Date | April 2016 | September 2016 | October 2016 | March 2017 |
| Estimate number of attendees | 1000 | 300 | 150 | 1500 |
| Number of Study Participants | 10 | 9 | 11 | 4 |
| Exposure Duration (mins) | (341–351) | (350) | (340) | (360–363) |
2.2. Study participants
Study participants were recruited from University of Georgia (UGA) students and staff or friends and family members of the researchers. All participants gave written informed consent and completed a screening questionnaire to determine their eligibility. Participants received a $25 gift card, lodging (if necessary), and per diem for each event they completed. In order to participate, participants had to be healthy and at least 18 years old. Females could not be pregnant or breastfeeding. Additionally, participants could not be current e-cigarette, tobacco, nicotine replacement therapy, or smokeless tobacco users or live with anyone who uses these products. Thirty-four volunteers participated in this study. This total participant count includes 26 unique participants and 5 participants who attended two or more events. Participants ranged from 19 to 30 years old (Females = 19–28 years old; Males = 19–30 years old). Most participants were female (n = 23, 68%). The UGA Institution Review Board reviewed and approved this study.
2.3. Event visits
Prior to entering the venue, participants completed an entry survey that asked about confounding exposures they may have received in the past 6 days (i.e. exposure to secondhand smoke or e-cigarette emissions, wood smoke, and charcoal). The survey also asked the participant to list the food and drinks they had consumed in the past 24 h. Inside the venue, participants participated in the event as members of the public. Participants attended the events with a researcher for approximately six hours (Table 1). All participants remained inside the venue for the duration of sampling. A researcher had to exit the venue for less than 30 min during Event 1. No confounding exposures were noted during this time. Participants were instructed not to use an e-cigarette, nicotine replacement-therapy, or other tobacco product while attending the event. An exit survey verified the participants had not used any nicotine product and asked about any adverse health effects experienced. The exit survey also asked participants what food and drink they consumed during the event inside the venue.
2.4. Biological sample collection
Urine and saliva samples were collected from each participant before entering the venue (“pre-exposure”), immediately prior to or just after they exited the venue (“immediate post-exposure”), 4-h after exiting the venue (”4-h post-exposure”), and first thing in the morning the day after the event (“first-void”).
All urine and saliva samples were collected in urine collection cups and Salivettes®, respectively. Participants were instructed not to touch the inside of the collection cups or the Salivette to prevent contamination. Each urine cup and Salivette was labeled with a unique barcode to identify the participant, event, biological medium, and sampling time. Sampling supplies were provided to the participants to take home for samples not collected when the researchers were present (i.e. select 4-h post-exposure samples and first-void samples).
Sample collection locations and storage methods are described in the supplementary material (Appendix A, Table 1). When participants collected samples after the events on their own (i.e. 4-hours post exposure and first void samples), they were instructed to place the samples in their freezer immediately or place them on wet ice until they could place them in their freezer. Most participants delivered these samples to a researcher the following morning. For the few remaining samples, the researcher traveled to an agreed upon location or drove to the participant’s residence to collect the remaining samples the day after the event. One Event 3 participant lived a significant distance from UGA, and this participant’s sample was kept in a freezer and delivered to a researcher on wet ice one week later. All samples were transported on wet ice to the U.S. Centers for Disease Control and Prevention (CDC) within a few weeks of collection.
The Division of Laboratory Sciences, National Center for Environmental Health, U.S. CDC analyzed urine samples for cotinine, trans-3′-hydroxycotinine, NNAL, N′-nitrosonornicotine (NNN), N′-nitrosoanabasine (NAB) and N′-nitrosoanatabine (NAT), 8-isoprostane, 3- HPMA, and CEMA. Saliva samples were analyzed for cotinine and trans-3′-hydroxycotinine.
2.5. Biological sample analysis
2.5.1. Cotinine and trans-3′-Hydroxycotinine analyses
2.5.1.1. Salivary measurements.
Salivary cotinine and trans-3′-hydroxycotinine were measured by isotope dilution high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry (HPLC-APCI-MS/MS) using a modified version of a published procedure (Bernert et al., 2000). The limits of detection were 0.015 ng/mL for both analytes.
2.5.1.2. Urinary measurements.
Urinary “total” (free plus conjugated glucuronide forms) cotinine and trans-3′-hydroxycotinine were measured by isotope dilution high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry (HPLC-APCI-MS/MS) using a modified version of a published procedure (Bernert et al., 2005). The limits of detection were 0.030 ng/mL for both analytes.
2.5.2. Volatile organic compound metabolites in urine (VOCM)
Urinary VOC metabolite (VOCM) concentrations were measured using ultrahigh performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI-MS/MS) according to a published procedure (Alwis et al., 2012). The limits of detection for CEMA and 3-HPMA were 6.96 ng/mL and 1.3 ng/mL, respectively.
2.5.3. Urinary tobacco specific nitrosamines (TSNAs)
Urinary “total” (free plus conjugated glucuronide forms) NNAL, NNN, NAB, and NAT were measured by isotope dilution high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry (HPLC-MS/MS) using a modified version of a published procedure (Xia et al., 2014). The limit of detection for urinary TSNAs ranged from 0.0006 to 0.0042 ng/mL, depending on the analyte.
2.5.4. 8-Isoprostane
Urinary “total” (free plus conjugated glucuronide forms) 8-isoprostane (iPF2α-III) (8-iso-15(S)-Prostaglandin F2α) (8-epi PGF2α) (15-F2t-isoprostane) (9α,11α,15S-trihydroxy-(8β)-prosta-5Z,13E-dien-1-oic acid) was measured by isotope dilution ultrahigh-performance liquid chromatography/electrospray ionization tandem mass spectrometry (UHPLC–MS/MS) following urine digestion using β-glucuronidase. The limit of detection for urinary 8-isoprostane was 8.8 pg/mL.
2.5.5. Creatinine
Creatinine in urine was measured by a commercial automated, colorimetric enzymatic (creatinase) method implemented on a Roche/Hitachi Cobas 6000 Analyzer.
2.6. Data analysis
Concentrations below the limit of detection (LOD) were substituted with an imputed value () (Hornung and Reed, 1990). All urinary endpoints were corrected for creatinine. Data were not normally distributed and so were log-transformed for analysis. Adjusted geometric means (AGM) and 95% confidence intervals (95% CI) of biomarker concentrations were calculated for the four sampling times and sampling events (Table 2). The median and range of select biomarker concentrations across events and sampling times are presented in Figs. 1–4.
Table 2.
Adjusted geometric means and confidence intervals of biomarker concentrationsa.
| Sample Time | Event | Value | Urinary Cotinine (μg/g) |
Salivary Cotinine (ng/ mL) |
Urinary Trans-3′- Hydroxycotinine (μg/g) |
3-HPMA (μg/ g) |
CEMA (μg/g) | 8-Isoprostane (ng/ g) |
|---|---|---|---|---|---|---|---|---|
| Pre-Exposure | 1 | Geometric Mean (GM) | 0.106 | 0.0204 | 0.134 | 275 | 71.8 | 341 |
| 95% CI of GM | 0.0751–0.150 | 0.0150–0.0277 | 0.0775–0.231 | 183–413 | 52.2–98.8 | 261–446 | ||
| 2 | Geometric Mean | 0.159 | 0.0172 | 0.168 | 186 | 61.5 | 346 | |
| 95% CI of GM | 0.113–0.225 | 0.0127–0.0233 | 0.0974–0.290 | 124–279 | 44.8–84.5 | 265–452 | ||
| 3 | Geometric Mean | 0.120 | 0.0185 | 0.147 | 196 | 64.7 | 346 | |
| 95% CI of GM | 0.0805–0.179 | 0.0129–0.0266 | 0.0821–0.264 | 123–311 | 44.9–93.1 | 252–473 | ||
| 4 | Geometric Mean | 0.191 | 0.0139 | 0.223 | 324 | 100 | 302 | |
| 95% CI of GM | 0.118–0.307 | 0.00895–0.0216 | 0.116–0.429 | 187–560 | 64.8–155 | 207–442 | ||
| Immediate Post-Exposure | 1 | Geometric Mean | 0.379 | 0.0838 | 0.354 | 625 | 95.6 | 446 |
| 95% CI of GM | 0.268–0.536 | 0.0617–0.114 | 0.205–0.612 | 415–940 | 69.6–131 | 336–590 | ||
| 2 | Geometric Mean | 0.738 | 0.113 | 0.434 | 199 | 97.0 | 260 | |
| 95% CI of GM | 0.523–1.04 | 0.083–0.154 | 0.251–0.748 | 132–299 | 70.6–133 | 199–340 | ||
| 3 | Geometric Mean | 0.282 | 0.0325 | 0.249 | 252 | 82.7 | 300 | |
| 95% CI of GM | 0.189–0.420 | 0.0226–0.0467 | 0.139–0.447 | 159–401 | 57.4–119 | 219–411 | ||
| 4 | Geometric Mean | 1.08 | 0.169 | 0.848 | 424 | 113 | 381 | |
| 95% CI of GM | 0.673–1.75 | 0.109–0.262 | 0.441–1.63 | 245–735 | 73.1–175 | 260–557 | ||
| 4-Hours Post-Exposure | 1 | Geometric Mean | 0.814 | 0.0836 | 0.731 | 734 | 116 | 373 |
| 95% CI of GM | 0.575–1.15 | 0.0594–0.1178 | 0.423–1.26 | 488–1100 | 84.1–159 | 286–487 | ||
| 2 | Geometric Mean | N.A.b | N.A. | N.A. | N.A. | N.A. | N.A. | |
| 95% CI of GM | … | … | … | … | … | … | ||
| 3 | Geometric Mean | 0.352 | 0.0373 | 0.349 | 455 | 110 | 314 | |
| 95% CI of GM | 0.236–0.524 | 0.0259–0.0537 | 0.194–0.626 | 286–723 | 76.5–159 | 229–430 | ||
| 4 | Geometric Mean | 2.31 | 0.167 | 2.22 | 808 | 107 | 414 | |
| 95% CI of GM | 1.43–3.72 | 0.107–0.259 | 1.15–4.26 | 466–1400 | 69.4–166 | 283–605 | ||
| First Void | 1 | Geometric Mean | 0.801 | 0.0649 | 0.884 | 839 | 169 | 297 |
| 95% CI of GM | 0.567–1.13 | 0.0478–0.0882 | 0.512–1.53 | 558–1260 | 123–233 | 225–392 | ||
| 2 | Geometric Mean | 1.09 | 0.111 | 0.957 | 163 | 90.0 | 323 | |
| 95% CI of GM | 0.768–1.53 | 0.0821–0.151 | 0.555–1.65 | 109–245 | 66.2–125 | 248–422 | ||
| 3 | Geometric Mean | 0.312 | 0.0280 | 0.406 | 262 | 96.3 | 369 | |
| 95% CI of GM | 0.209–0.465 | 0.0194–0.0402 | 0.226–0.728 | 165–417 | 66.9–139 | 269–505 | ||
| 4 | Geometric Mean | 2.21 | 0.148 | 2.67 | 407 | 97.8 | 377 | |
| 95% CI of GM | 1.37–3.56 | 0.0951–0.230 | 1.39–5.13 | 235–706 | 63.3–151 | 258–551 | ||
All urinary endpoints were corrected for creatinine.
4h post-exposure samples were not collected after Event 2.
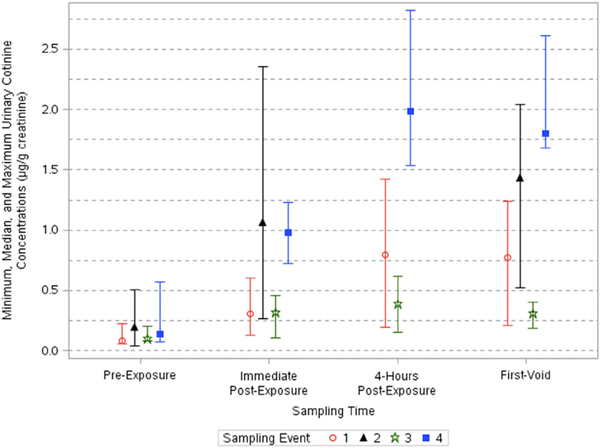
Fig. 1.
Minimum, median, and maximum creatinine-corrected urinary cotinine concentrations across sampling times and events.
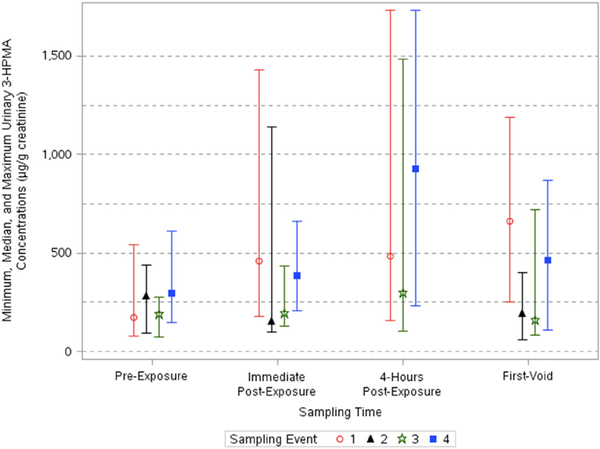
Fig. 4.
Minimum, median, and maximum creatinine-corrected urinary 3-HPMA concentrations across sampling times and events.
A linear mixed effects model was used to analyze the log-transformed data across the four sampling times and four events. Sample time, event, and time by event interactions were treated as fixed effects. Participants were treated as random effects. A p-value ≤0.05 was considered statistically significant. Results are presented in Table 3. The adjusted mean ratio (i.e. the difference) between participants’ log-transformed maximum biomarker concentrations and their log-transformed baseline biomarker concentrations for each endpoint and location are presented in Table 4.
Table 3.
| Biological Endpoint | Effect | F-Value | p-Value |
|---|---|---|---|
| Urinary Cotinine | Sampling Time | 116.91 | < 0.0001*** |
| Event | 28.88 | < 0.0001*** | |
| Sampling | 3.60 | 0.0015* | |
| Time*Event | |||
| Salivary Cotinine | Sampling Time | 95.12 | < 0.0001*** |
| Event | 23.01 | < 0.0001*** | |
| Sampling | 6.66 | < 0.0001*** | |
| Time*Event | |||
| Urinary Trans-3′-Hydroxycotinine | Sampling Time | 87.31 | < 0.0001*** |
| Event | 17.28 | < 0.0001*** | |
| Sampling | 2.61 | 0.0149* | |
| Time*Event | |||
| Urinary 3-HPMA | Sampling Time | 11.45 | < 0.0001*** |
| Event | 11.99 | < 0.0001*** | |
| Sampling | 2.75 | 0.0106* | |
| Time*Event | |||
| Urinary CEMA | Sampling Time | 6.47 | 0.0006** |
| Event | 1.35 | 0.2647 | |
| Sampling | 1.88 | 0.0780 | |
| Time*Event | |||
| Urinary 8-isoprostane | Sampling Time | 0.10 | 0.9594 |
| Event | 0.62 | 0.6019 | |
| Sampling | 1.64 | 0.1300 | |
| Time*Event | |||
Significant at p ≤ 0.05.
Significant at p ≤ 0.001.
Significant at p ≤0.0001.
All urinary endpoints were adjusted for creatinine for this analysis.
Sampling Events included Events 1, 2, 3, and 4. Sampling times included pre-exposure, immediate post-exposure, 4-h post-exposure (Events 1, 3, and 4 only), and the morning after the events.
Table 4.
Adjusted mean ratio of participants’ maximum over baseline biomarker concentrations by endpoint and locationa.
| Biological Endpoint | Event | Adjusted Mean Ratio |
|---|---|---|
| Urinary Cotinine | 1 | 8.14 |
| 2 | 6.77 | |
| 3 | 2.67 | |
| 4 | 13.16 | |
| Salivary Cotinine | 1 | 4.58 |
| 2 | 7.07 | |
| 3 | 2.02 | |
| 4 | 12.68 | |
| Urinary Trans-3′-Hydroxycotinine | 1 | 6.84 |
| 2 | 5.68 | |
| 3 | 2.24 | |
| 4 | 8.79 | |
| Urinary 3-HPMA | 1 | 3.82 |
| 2 | 1.28 | |
| 3 | 2.18 | |
| 4 | 1.83 | |
| Urinary CEMA | 1 | 2.40 |
| 2 | 1.82 | |
| 3 | 1.92 | |
| 4 | 1.16 | |
| Urinary 8-Isoprostane | 1 | 1.37 |
| 2 | 0.95 | |
| 3 | 1.07 | |
| 4 | 1.48 | |
All urinary endpoint were corrected for creatinine.
3. Results
To ensure only participants with minimal to no recent secondhand tobacco exposures were included, participants with a salivary cotinine concentration > 0.1 ng/mL at the pre-exposure sampling time were excluded from the analysis (n = 6 total [Event 1 = 1; Event 3 = 5]). Samples from 28 of the 34 participants were analyzed. Two participants did not collect 4-h post exposure saliva samples for Event 1. A substance interfered with 8-isoprostane analysis in two Event 1 samples. Insufficient quantities of urine prevented the analysis of TSNAs in one Event 1 and one Event 2 sample. These data were treated as missing data in the analysis. Approximately 3 people smoking cigarettes passed by participants as they walked towards the Event 2 venue. One smoker was present as they exited the venue. Participants walked quickly past the smokers and avoided inhaling any secondhand smoke as much as possible.
A total of 103 urine and 101 saliva samples were collected across the four events and used in this data analysis. A total of 34 saliva and 36 urine samples were analyzed and used in this data analysis for Event 1, 27 saliva and urine samples for Event 2, 24 saliva and urine samples for Event 3, and 16 saliva and urine samples for Event 4.
Samples were collected prior to exposure, immediately after exposure, 4-h after exposure, and first thing the following morning. Three participants in Event 1 forgot to collect first void samples, but did collect early morning samples. These are considered as first-void samples in this analysis. Samples were not collected 4-h after exposure for Event 2 because the sampling event ended at midnight.
Among the 28 participants used in the statistical analysis, most participants (n = 19, 68%) reporting sitting in a designated eating area inside the event venue 75% of their time. The remaining participants reported spending at least 75% of their time walking around and visiting vendors (n = 2, 7%), standing in e-cigarette use sections (n = 4, 14%), or split their time equally between sitting in the designated area and visiting vendors (n = 3, 11%). All participants verified they had not used a tobacco or e-cigarette product or touched e-cigarette liquid during the e-cigarette event.
3.1. Nicotine metabolites
3.1.1. Cotinine
Urinary cotinine concentrations corrected for creatinine varied significantly across sampling times (p < 0.0001) and events (p < 0.0001) (Table 3). A significant interaction between location and sampling time was also found (p < 0.05). Urinary cotinine concentrations corrected for creatinine increased up to 13-fold after the events (Table 4). The adjusted geometric means of urinary cotinine concentrations across all events ranged from 0.106 to 0.191, 0.282–1.08, 0.352–2.31, and 0.312–2.21 μg/g creatinine for pre-exposure, immediate post-exposure, 4-h post-exposure, and first-void samples, respectively (Table 2). Urinary cotinine concentrations were all below 0.58 μg/g creatinine in the pre-exposure samples. After exposure, concentrations increased through post-exposure sampling times and then decreased slightly in the first-void samples (Fig. 1). The largest increases in creatinine-corrected cotinine were observed in Event 4 and Event 2, while Event 3 showed little variation in cotinine exposure across time. The highest concentrations were detected in samples collected 4-h post-exposure for Event 4 (AGM: 2.31 μg/g creatinine [95% CI: 1.43,3.72]).
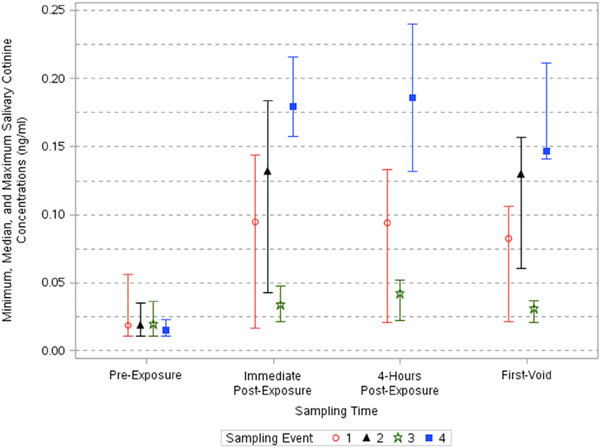
Salivary cotinine concentrations varied significantly across sampling times (p < 0.0001) and events (p < 0.0001) (Table 3). A significant interaction between sampling time and event was also found (p < 0.0001). Salivary cotinine concentrations increased up to 12-fold after the events (Table 4). Concentrations were elevated through post-exposure sampling times and decreased slightly in first-void samples (Fig. 2). The highest concentrations were measured in Event 4 samples collected 4-h after exposure (AGM: 0.17 ng/mL [95% CI: 0.11, 0.26]). The geometric mean and 95% CIs for this sample time were the same as those calculated for immediate post-exposure samples, but concentrations collected 4-h post-exposure had a wider range of concentrations. The adjusted geometric means of salivary cotinine across all events ranged from 0.0139 to 0.0204, 0.0325–0.169, 0.0373–0.167, and 0.0280–0.148 ng/mL for pre-exposure, immediate post-exposure, 4-h post-exposure, and first-void samples (Table 2).
Fig. 2.
Minimum, median, and maximum salivary cotinine concentrations across sampling times and events.
3.1.2. Trans-3′-Hydroxycotinine
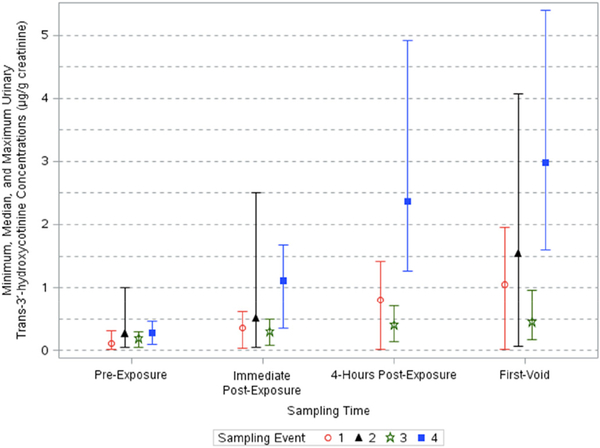
Creatinine-corrected urinary trans-3′-hydroxycotinine concentrations varied significantly across sampling times (p < 0.0001) and sampling events (p < 0.0001) (Table 3). A significant interaction between sampling time and location was detected (p < 0.05). Urinary trans-3′-hydroxycotinine concentrations increased up to 8.8-fold after the events (Table 4). The adjusted geometric means of concentrations ranged from 0.134 to 0.223, 0.249–0.848, 0.349–2.22, and 0.406–2.67 μg/g creatinine pre-exposure, immediate post-exposure, 4-h post-exposure, and first void samples, respectively (Table 2). Creatinine-corrected concentrations increased at each successive post-exposure sampling time and peaked in first-void samples (Fig. 3). Event 4 first-void concentrations were the highest among events and sampling times (AGM: 2.67 μg/g creatinine [95% CI: 1.39, 5.13]).
Fig. 3.
Minimum, median, and maximum creatinine-corrected urinary trans-3′-hydroxycotinine concentrations across sampling times and events.
Salivary trans-3′-hydroxycotinine concentrations were below the limit of detection for 66% of samples (n = 67). This was most pronounced in Event 3 samples where concentrations were < LOD for 92% of samples (n = 22). Salivary trans-3′-hydroxycotinine was not included in statistical analyses because of the low detection rates.
3.2. Urinary acrolein metabolites
3.2.1. 3-HPMA
Creatinine-corrected 3-HPMA urinary concentrations varied significantly across sampling times (p < 0.0001) and events (p < 0.0001) (Table 3). A significant interaction between sampling time and event existed (p < 0.05). Urinary 3-PHMA concentrations corrected for creatinine increased up to 3.8-fold after the events (Table 4). The adjusted geometric means of pre-exposure, immediate post-exposure, 4-h post exposure, and first-void adjusted concentrations ranged from 186 to 324, 199–625, 455–808, and 163–839 μg/g creatinine, respectively (Table 2). Event 1, 3, and 4 concentrations of creatinine-corrected 3-HPMA peaked at different times after events but they all increased after exposure, unlike Event 2 concentrations which did not increase appreciably (Table 2, Fig. 4).
3.2.2. CEMA
Creatinine-corrected CEMA concentrations varied significantly across sampling times (p < 0.01) but not across sampling events. Urinary CEMA concentrations increased up to 2.4-fold after the events (Table 4). The adjusted geometric means of concentrations ranged from 61.5 to 100, 82.7–113, 107–116, and 91.0–169 μg/g creatinine in pre-exposure, immediate post-exposure, 4-h post-exposure, and first void samples, respectively (Table 2).
3.3. Urinary tobacco-specific nitrosamines
Total NAB, NAT, and NNN concentrations were below the limit of detection in all samples for all sampling times and sampling events. NNAL was < LOD in 84% of samples (n = 85). Interestingly, 38% of detected NNAL concentrations were in pre-exposure samples (n = 6/16) collected from participants prior to Events 1 (n = 1), 2 (n = 3), and 3 (n = 2). TSNAs were not included in the statistical analyses because of the low detection rates.
3.4. Urinary 8-isoprostane
Creatinine-corrected 8-isoprostane concentrations did not vary significantly across sampling times or events (Table 3). The adjusted geometric mean of concentrations ranged from 302 to 346, 260–446, 314–414, 297–377 ng/g creatinine in pre-exposure, immediate post-exposure, 4-h post-exposure, and first void samples, respectively (Table 2).
3.5. Health effects
Participants completed an exit survey that asked about adverse health effects they experienced during the event. No adverse health effects were reported during Event 2. Among Event 1, 3, and 4 participants, 15% (n = 5) reported experiencing some type of adverse health effect (i.e. headache, dry mouth, cough, dry/burning eyes) during the e-cigarette convention that they attributed to secondhand exposures to e-cigarette emissions. One participant reported experiencing a headache but thought that it could be due to factors other than the event.
4. Discussion
This study characterized secondhand exposures to e-cigarettes by analyzing tobacco exposure biomarkers in urine and saliva of 28 non-users who attended at least one large e-cigarette event. Secondhand exposures to e-cigarette emissions lasting approximately six hours resulted in significant increases in salivary and urinary cotinine and urinary trans-3′-hydroxycotinine, 3-HPMA, and CEMA concentrations. Urinary and salivary cotinine and urinary trans-3′-hydroxycotinine and 3-HPMA concentrations varied significantly across sampling events. Significant interaction effects between sampling event and sampling time were found for urinary and salivary cotinine, urinary trans-3′-hydroxycotinine, and urinary 3-HPMA.
Significant interaction effects indicate the effect of sampling time is dependent on the sampling event. This can likely be explained by the differences in exposures inside each event venue determined by variables such as venue size, the number of active e-cigarette users, venue ventilation rates, mixing of outdoor air from opened doors, etc. Biomarker concentrations post-exposure are largely dependent on the extent of exposure inside the sampling event.
The highest urinary cotinine concentrations were observed after Event 4. Urinary cotinine concentrations measured 4-h after this event were (AGM [95% CI]) 2.31 μg/g creatinine (1.43, 3.72). Ballbe et al. (2014) measured cotinine concentrations in urine from five participants passively exposed to e-cigarette emissions at least two hours a day by living in the homes of e-cigarette users. Though there are differences between the Ballbe et al. (2014) and the present study such as e-cigarette user density, length of exposure, etc., the Ballbe et al. (2014) study offers valuable comparison data. Reported urinary cotinine concentrations (GM ± Geometric SD [GSD]: 1.75 ± 2.67 μg/g creatinine) were slightly lower than those found in this current study. For comparison, the reported urinary concentrations for twenty-five non-users living in homes with cigarette smokers were (GM ± GSD) 2.46 ± 2.67 μg/g creatinine in the Ballbe et al. study. It is not clear whether Ballbe et al. measured total cotinine or only the free form. The latter may account for the lower concentrations they found.
Salivary cotinine concentrations in this study also reached the highest values at 4-h after Event 4 (AGM: 0.167 ng/mL [95% CI: 0.107, 0.259]). These concentrations are slightly lower than salivary cotinine concentrations reported for non-users living with e-cigarette users in the Ballbe et al. (2014) study (GM ± GSD: 0.19 ± 2.17 ng/mL). Ballbe et al. (2014) reported salivary cotinine concentrations of non-users who lived with tobacco cigarette smokers were twice as high (GM ± GSD: 0.38 ± 2.34 ng/mL). Salivary cotinine concentrations in the current study were similar to those reported in a study of second- hand tobacco exposures in a bar in Athens, GA (St Helen et al., 2012). In that study, participants stood or sat near tobacco smokers in a bar for three hours. After the three hours, mean salivary cotinine concentrations were (GM [95% CI]) 0.161 ng/mL (0.14, 0.18]). Results indicate that six hours of e-cigarette secondhand exposures can result in salivary cotinine concentrations similar to those reported for people living in homes with e-cigarette users or those exposed for a few hours to secondhand tobacco smoke in a bar. These concentrations are approximately twenty times lower than those reported for participants exposed to sidestream smoke from approximately three tobacco cigarettes in a chamber study (Avila-Tang et al., 2013).
Urinary acrolein metabolites increased after secondhand exposure to e-cigarette emissions. The CDC reports the average 3-HPMA and CEMA urinary concentrations among a representative sample of nonsmokers in the U.S. population from 2005 to 2006 were (Median [25th, 75th]) 219 μg/g creatinine (140, 353) and 78.8 μg/g creatinine (51.8, 121), respectively (Alwis et al., 2015). Adjusted average concentrations of 3-HPMA in this study exceeded these estimates by up to four-fold. Average concentrations of CEMA in this study were similar to or slightly higher than the median reported by CDC. Similarly, Schober et al. (2014) reported the 3-HPMA was elevated among e-cigarette users, but they found no elevation in CEMA (Schober et al., 2014). 3-HPMA is the major metabolite and CEMA is a minor metabolite of acrolein (Alwis et al., 2015). This could explain the discrepancy in patterns of change observed. There are many sources of acrolein exposures both in the environment and endogenously, thus the acrolein concentrations measured in this study are only partially attributable to passive e-cigarette exposure.
E-cigarette use has been shown to result in inflammation in the user, but inflammation from secondhand e-cigarette exposures has not been reported. For example, two studies used the concentrations of fractional exhaled nitric oxide (FeNO) to measured bronchial inflammation in e-cigarette users (Schober et al., 2014; Vardavas et al., 2012). In both studies, the concentration of FeNO changed after primary e-cigarette use, though the responses were in opposite directions. Propylene glycol exposures have resulted in ocular and airway irritation, though the concentrations used to cause these health effects were much higher than those likely present at an e-cigarette event (GM = 309 mg/m3) (Wieslander et al., 2001). 8-Isoprostane is recognized as the most specific and sensitive biomarker for oxidative stress (Czerska et al., 2016; Montuschi et al., 2007). Cigarette smoking is associated with increased urinary 8-isoprostane (Morrow and Roberts, 1997). However it was the only biomarker in this study that did not significantly change across sampling events or sampling times. Secondhand e-cigarette exposures in this study did not result in oxidative stress in those passively exposed, suggesting that an increase in oxidative stress may be a chronic effect biomarker of exposure or that the exposure levels in this study did not have an effect on oxidative stress.
Limitations of this study include a small sample size and a subset of only four e-cigarette events. This study only measured acute exposures. Chronic exposures may result in different outcomes. Future research should analyze the relationship between environmental components of e-cigarette emissions and biomarkers of e-cigarette exposure. Participants’ consumption of food and drink was recorded but not incorporated into biological analysis. All participants consumed food or drinks inside the venue. Participants could have received third-hand e- cigarette exposures from e-cigarette emissions present on food or drink products, serving containers, or the participants’ hands during consumption. Future research should consider the contribution of food and drink to biomarker concentrations, because chemicals of interest (i.e. acrolein) are inherently in many foods and drinks and third-hand exposures can contribute to overall e-cigarette exposures.
Ventilation rates inside e-cigarette event venues should also be considered in future research. Researchers did not observe any additional tobacco products being used inside the venues, but it is possible that attendees used other tobacco products during the events that could contribute to the concentrations of contaminants reported here.
5. Conclusion
This study is the first to characterize secondhand exposures to chemicals present in e-cigarette emissions in public settings. While the exposure duration was relatively short (∼6 h), participants’ salivary and urinary cotinine concentrations were comparable to those reported for non-users living with e-cigarette users or sitting near tobacco smokers in a bar (Ballbe et al., 2014). Secondhand e-cigarette emissions may be a source of acrolein exposures but are not a strong source of tobacco-specific nitrosamines. Secondhand e-cigarette exposures occurring for a short period of time do not result in measurable increases in an oxidative stress biomarker.
Supplementary Material
Acknowledgments
Funding source
This publication was supported by Grant # 2T420H008436 from the National Institute for Occupational Safety and Health (NIOSH). Technical assistance was provided by the Division of Laboratory Sciences in the National Center for Environmental Health (NCEH). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not constitute endorsement by the Public Health Service or by the U.S. Department of Health and Human Service.
Footnotes
Declarations of interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijheh.2019.04.013.
References
- AIHA, 2014. White Paper: Electronic Cigarettes in the Indoor Environment. American Industrial Hygiene Association. [Google Scholar]
- Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL, 2012. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal. Chim. Acta 750, 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwis KU, deCastro BR, Morrow JC, Blount BC, 2015. Acrolein exposure in U.S. Tobacco smokers and non-tobacco users: NHANES 2005–2006. Environ. Health Perspect. 123, 1302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Tang E, Al-Delaimy WK, Ashley DL, Benowitz N, Bernert JT, Kim S, Samet JM, Hecht SS, 2013. Assessing secondhand smoke using biological markers. Tobac. Contr. 22, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballbe M, Martinez-Sanchez JM, Sureda X, Fu M, Perez-Ortuno R, Pascual JA, Salto E, Fernandez E, 2014. Cigarettes vs. e-cigarettes: passive exposure at home measured by means of airborne marker and biomarkers. Environ. Res. 135, 76–80. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, 1999. Biomarkers of environmental tobacco smoke exposure. Environ. Health Perspect. 107, 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert JT, Harmon TL, Sosnoff CS, McGuffey JE, 2005. Use of continine immunoassay test strips for preclassifying urine samples from smokers and nonsmokers prior to analysis by LC-MS-MS. J. Anal. Toxicol. 29, 814–818. [DOI] [PubMed] [Google Scholar]
- Bernert JT Jr., McGuffey JE, Morrison MA, Pirkle JL, 2000. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J. Anal. Toxicol. 24, 333–339. [DOI] [PubMed] [Google Scholar]
- CDC, 2010. How Tobacco Smoke Causes Disease: the Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General, Publications and Reports of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA. [PubMed] [Google Scholar]
- CDC, 2017a. Burden of Tobacco Use in the U.S. Office on Smoking and Health. National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. [Google Scholar]
- CDC, 2017b. Maps of Smokefree Indoor Air–Private Worksites. Restaurants, and Bars, Atlanta, Georgia. [Google Scholar]
- CDC, 2017c. STATE System E-Cigarette Fact Sheet. [Google Scholar]
- Czerska M, Zielinski M, Gromadzinska J, 2016. Isoprostanes - a novel major group of oxidative stress markers. Int. J. Occup. Med. Environ. Health 29, 179–190. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P 3rd, Benowitz N, 2014. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobac. Contr. 23, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg 5, 46–51. [Google Scholar]
- Hukkanen J, Jacob P 3rd, Benowitz NL, 2005. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 57, 79–115. [DOI] [PubMed] [Google Scholar]
- IARC, 1993. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Acrolein. World Health Organization, Lyon, France. [Google Scholar]
- Johnson JM, Muilenburg JL, Rathbun SL, Yu X, Naeher LP, Wang JS, 2018. Feba Elevated nicotine dependence scores among electronic cigarette users at an electronic cigarette convention. J. Community Health 43 (1), 164–174. 10.1007/s10900-017-0399-3. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Naeher LP, Yu X, Rathbun SL, Muilenburg JL, Wang JS, 2018b. Air monitoring at large public electronic cigarette events. Int. J. Hyg Environ. Health 221, 541–547. [DOI] [PubMed] [Google Scholar]
- Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ 2nd, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC, 2005. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 38, 698–710. [DOI] [PubMed] [Google Scholar]
- Marynak K, Holmes CB, King BA, Promoff G, Bunnell R, McAfee T, 2014. State laws prohibiting sales to minors and indoor use of electronic nicotine delivery systems–United States, November 2014. MMWR (Morb. Mortal. Wkly. Rep.) 63, 1145–1150. [PMC free article] [PubMed] [Google Scholar]
- Melstrom P, Koszowski B, Thanner MH, Hoh E, King B, Bunnell R, McAfee T, 2017. September 1 Measuring PM2.5, ultrafine particles, air nicotine and wipe samples following the use of electronic cigarettes. Nicotine Tob. Res. : Off. J. Soc. Res. Nicotine Tob. 19 (9), 1055–1061. 10.1093/ntr/ntx058. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Barnes P, Jackson Roberts L, 2007. Insights into oxidative stress: the isoprostanes. Curr. Med. Chem. 14, 703–717. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ, 1997. The isoprostanes: unique bioactive products of lipid peroxidation. Prog. Lipid Res. 36, 1–21. [DOI] [PubMed] [Google Scholar]
- Ohta K, Uchiyama S, Inaba Y, Nakagome H, Kunugita N, 2011. Determination of carbonyl compounds generated from the electronic cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine. Bunseki Kagaku 60. [DOI] [PubMed] [Google Scholar]
- Schick SF, Blount BC, Jacob P 3rd, Saliba NA, Bernert JT, El Hellani A, Jatlow P, Pappas RS, Wang L, Foulds J, Ghosh A, Hecht SS, Gomez JC, Martin JR, Mesaros C, Srivastava S, St Helen G, Tarran R, Lorkiewicz PK, Blair IA, Kimmel HL, Doerschuk CM, Benowitz NL, Bhatnagar A, 2017. September 1 Biomarkers of exposure to new and emerging tobacco and nicotine delivery products. Am. J. Physiol. Lung Cell. Mol. Physiol. 313 (3), L425–L452. 10.1152/ajplung.00343.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, Jorres RA, Fromme H, 2014. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int. J. Hyg Environ. Health 217, 628–637. [DOI] [PubMed] [Google Scholar]
- Schripp T, Markewitz D, Uhde E, Salthammer T, 2013. Does e-cigarette consumption cause passive vaping? Indoor Air 23. [DOI] [PubMed] [Google Scholar]
- Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, Destaillats H, 2016. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ. Sci. Technol. 50, 9644–9651. [DOI] [PubMed] [Google Scholar]
- St Helen G, Bernert JT, Hall DB, Sosnoff CS, Xia Y, Balmes JR, Vena JE, Wang JS, Holland NT, Naeher LP, 2012. Exposure to secondhand smoke outside of a bar and a restaurant and tobacco exposure biomarkers in nonsmokers. Environ. Health Perspect. 120, 1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacco Control Legal Consortium, 2015. Regulating Electronic Cigarettes and Similar Devices. Public Health Law Center, Sain Paul, Minnestoa. [Google Scholar]
- U.S. Department of Health and Human Services, 2016. E-cigarette Use Among Youth and Young Adults A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA. [Google Scholar]
- U.S. Department of Health Human Services, 1988. The Health Consequences of Smoking: Nicotine Addiction: A Report of the Surgeon General (DHHS Publication No. CDC 88–8406). U.S. Department of Health and Human Services, Washington, DC. [Google Scholar]
- Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK, 2012. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 141, 1400–1406. [DOI] [PubMed] [Google Scholar]
- Wang P, Chen W, Liao J, Matsuo T, Ito K, Fowles J, Shusterman D, Mendell M, Kumagai K, 2017. A device-independent evaluation of carbonyl emissions from heated electronic cigarette solvents. PLoS One 12, e0169811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander G, Norback D, Lindgren T, 2001. Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup. Environ. Med. 58, 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, 2015. VapeCons: E-cigarette user conventions. J. Public Health Policy 36, 440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia B, Xia Y, Wong J, Nicodemus KJ, Xu M, Lee J, Guillot T, Li J, 2014. Quantitative analysis of five tobacco-specific N-nitrosamines in urine by liquid chromatography-atmospheric pressure ionization tandem mass spectrometry. Biomed. Chromatogr. : BMC (Biomed. Chromatogr.) 28, 375–384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.