Abstract
Objectives The study reports on the effectiveness of a ball-milled nanosized titanium dioxide composite (EB@TiO 2 ) for DH management in comparison with commercial desensitizing paste with and without saliva.
Materials and Methods Forty-nine dentine specimens were prepared from extracted bovine anterior teeth. Twenty-one of the specimens were brushed with three desensitizing toothpaste for 7 days, namely: Group 1; EB@TiO 2 , Group 2; Colgate Pro-relief; and Group 3; Sensodyne repair ( n = 7). Twenty-four specimens were brushed with the toothpaste for 7 days and stored in artificial saliva (control) after brushing. Each specimen was subsequently posttreated in citric acid solution to test its stability in acidic condition. Field scanning electron microscope was used to evaluate the effectiveness of the dentine tubules occlusion. The biocompatibility of the composite was tested using BHK21 cell line.
Statistical Analysis One-way analysis of variance was used to analyze the percentage occluded area ratio values for all specimens (α = 0.05). Independent t -test was further used to evaluate the occlusion differences with saliva and without saliva.
Results and Conclusions The number of dentine tubules decreased significantly after 7 days of brushing. Overall, the occlusion observe for EB@TiO 2 were significantly better than for Colgate Pro-relief and Sensodyne repair ( p < 0.05). BHK21 assay suggested that composite had no significant effect on the BHK21 cell line. This study demonstrated that the composite effectively occluded open dentine tubules within 7 days of brushing.
Keywords: dentine hypersensitivity, desensitizing paste, remineralization
Introduction
Globalization has created a rapid change in the diets and lifestyles of millions of people worldwide. In South Africa for example, the reintroduction of the country to the global economy postapartheid in 1994 has witnessed the proliferation of foreign goods and a rapidly changing food environment. 1 Concerning, and in the context of oral health care, this change has brought about the increase in consumption of energy drinks, and acidic beverages; which is reportedly linked to the incidence of dental diseases, such as dental caries and erosion of the enamel surface. 2 3 More worrisome is that the excessive demineralization of the tooth surface due to erosion has been reported to initiate the onset of dentine hypersensitivity (DH). 4 5 6
According to the Canadian Advisory Board on DH, 7 DH is characterized by distinctive short, sharp pain arising from exposed dentinal tubules particularly in response to external stimuli that are typically thermal, evaporative, tactile, electrical, osmotic, or chemical changes which cannot be ascribed to any other form of dental defects or pathology. As reported in the literature, DH is one of the most clinically encountered problems in dentistry affecting between 10 to 30% of people worldwide. 8 Although there have been conflicting reports on the exact prevalence of DH, nevertheless, the most common age range in which DH is frequently experienced is given as 20 to 50 years, with female patients predominantly affected. 9 10 Moreover, and as Schiff et al 11 points out, DH has a negative consequence on the quality of life for dental patient as they are less complaint with oral hygiene recommendation; thus posing a challenge for oral health care providers to manage.
Many theories have been reportedly proposed to explain the mechanism of DH. However, the hydrodynamic theory expanded upon by Brannstrom is now accepted by the dental community as the most likely mechanism for DH occurrence. 12 In an attempt to control this process, products or agents that typically aimed to reduce fluid flow, and or interfere with the nerve impulses have been reported in the literature. 11 13 At the first line of at-home therapy for DH management, the use of occluding agents is often recommended for effective treatment. 14
Several different occluding agents such as potassium oxalates, 15 sodium fluoride and sodium monofluorophosphate, 12 strontium salt, 16 amorphous calcium phosphate containing casein phosphopeptide, 17 and calcium glycerophosphate 18 have been widely utilized in desensitizing paste for their dentine tubule occluding capabilities. Still, the effectiveness of the aforementioned occluding agents will depend on the flow of saliva. Moreover, due to the chemical composition of saliva, it can play a critical role in naturally reducing DH. 13 19 Kleinberg 19 revealed that saliva could reduce DH by depositing phosphate and calcium ions in the exposed tubules which ultimately result into the ions forming a protective layer on the surface of the tubules. In some patients; however, particularly those with conditions of hyposalivation and xerostomia, the flow of saliva is limited; which could further increase the risks of caries and tooth demineralization, thereby exacerbating DH. 20
In an attempt to address the above concern, Kleinberg in 2012 at the State University of New York-Stony Brook, patented novel occluding agents based on the understanding of the role that saliva plays in naturally reducing DH. This new technology comprises arginine (an amino acid with a pH 6.5–7.5), bicarbonate, pH buffer, and calcium carbonate. 19 The said technology is marketing under the brand name Colgate Pro-Argin. 11 It is reported that Pro-Argin technology function by occluding dentinal tubules using arginine to bind to the negatively charged dentin surface, which subsequently attracts a calcium-rich layer from the saliva to infiltrate and block the dentinal tubules. 20 However, its effectiveness in a highly acidic environment has been reported to be ineffective, 21 thus leading to the reopening of the dentine tubules. Given the above drawbacks, a new occluding material consisting of an eggshell modified titanium dioxide composite recently proposed in DH management. 22
Importantly, studies 23 24 have projected that the future of tooth remineralization will be the use of eggshell owing to its high bioavailability of calcium. Likewise, the use of titanium dioxide, particularly in nano form, and their combination with other abrasive agents have been proposed in the literature to occlude open dentine tubules. 25 In a recent report, the authors demonstrated that eggshell modified with titanium dioxide (EB@TiO 2 ) significantly improved the composite acidic resistant to erosive acids. 26 This present study, therefore, aimed to evaluate EB@TiO 2 occluding characteristic against commercially available toothpaste containing Pro-Argin (Colgate Pro-relief) and NovaMin (Sensodyne repair) with and without saliva in reducing DH. The formulated hypothesis tested was: EB@TiO 2 significantly occlude the open dentine tubules with or without saliva.
Materials and Methods
Two commercially available toothpastes namely: Sensodyne repair (GlaxoSmithKline, United Kingdom) and Colgate Pro-relief (Colgate-Palmolive, Poland) were used as the test desensitizing paste. Titanium dioxide (Anatase form) and citric acid were purchased from Sigma-Aldrich (Germany), and Merck (South Africa), respectively.
Eggshell-Titanium Dioxide Composite Preparation
Eggshell and titanium dioxide composite was prepared in accordance with the method reported in literature. 22 An extensive details of the surface morphology, particle sizes, and phase of the prepared EB@TiO 2 can be found in other reported papers. 22 27 28
Preparation of Artificial Saliva
Artificial saliva was prepared following the method reported by Saporeti et al 29 with a slight modification. As specified in Table 1 , the listed chemicals were prepared in 1L of volumetric flask using deionized water. The pH of the prepared saliva was given as 6.5.
Table 1. Composition of the prepared artificial saliva (mg/L).
| Chemicals | Concentration (mg/L) | Mass (g) |
|---|---|---|
| Note: Preparation of dentine tooth specimens. | ||
| NaH 2 PO 3 H 2 O | 780 | 0.078 |
| NaCl | 500 | 0.05 |
| KCl | 500 | 0.05 |
| CaCl 2 H 2 O | 795 | 0.0795 |
| NaS 9 H 2 O | 5 | 0.0005 |
| (NH 4 ) 2 SO 4 | 300 | 0.03 |
| Citric Acid | 5 | 0.0005 |
| NaHCO 3 | 100 | 0.01 |
| Urea | 1000 | 0.1 |
Forty-nine anterior teeth extracted from bovine were collected from an abattoir, South Africa. Disinfecting and cleaning of the teeth followed by immersing in 10% chloroxylenol solution. With the aid of a diamond saw operating at a minimal speed, and cooled with water, the teeth were sectioned below the enamel-dentinal to prepare a dentine specimen having a dimension of 5 mm × 5 mm × 1 mm. A silicon carbide paper with particle size of 600 grits were further used to wet ground the specimens for 60 seconds. Thereafter, the specimens were embedded in a resin (AMT composite, South Africa). The specimens were then soaked in a solution containing 4% wt. citric acid for 2 minutes to open up the tubules. As described in Table 2 , the specimens were randomly assigned in different experimental groups.
Table 2. The distribution of specimens according to the experimental group.
| Sample groups | Treatment condition | Brushing days | Total | |
|---|---|---|---|---|
| Without saliva | With saliva | |||
| Note: Surface examination of the treated specimens. EB@TiO 2 , eggshell-titanium dioxide. | ||||
| Artificial saliva | – | 7 | Twice daily (for 7 days) | 7 |
| EB@TiO 2 | 7 | 7 | 14 | |
| Colgate Pro-relief | 7 | 7 | 14 | |
| Sensodyne repair | 7 | 7 | 14 | |
| Total | 21 | 28 | 49 | |
Each specimen from the respective groups were brushed twice daily (morning and evening) with a toothbrush powered with 1.5v alkaline battery (Oralwise, China) for 1 minute and allowed to dry for 30 seconds before rinsing with deionized water. Brushing was performed at room temperature using 100 mg of respective toothpaste. The slurry of EB@TiO 2 was prepared by mixing 100 mg of the powder/200 µL of deionized water. After each brushing protocol, the specimens were immersed in saliva or without saliva as described in Table 2 . At the end of the 7-day brushing, the treated specimens were exposed to 4% wt. citric acid solution for 2 minutes to determine the resistance to acidic challenge, and subsequently rinsed in deionized before blot drying.
Field Scanning Electron Microscope (FESEM; Carl Zeiss) was used to examine the treated specimens after each day of brushing from each respective group. The instrument was operated in controlled environment and scan at 20 kV. Prior to FESEM observation, the specimens were dehydrated, sputter coated with electric conductive gold film. Using the captured image of 1500 magnification, a software (ImageJ; National Institute of Health, United States, http://imagej.nih.gov./ij ) was used to compute the occluded tubules ratios by dividing the area of the occluded tubules by the total tubules area ( n = 7). The % occluded area ratio were counted and used for statistical evaluation.
Biocompatibility Test
A cytotoxicity assay was performed on the prepared EB@TiO 2 to evaluate its biocompatibility. Before culturing, the sample was dispersed in a solvent (Dimethyl Sulfoxide). The BHK21 hamster kidney cells were grown in the laboratory following the process of culturing normal tissues. 22 The cell viability were then evaluated using MTS assay. Auranofin was used as a negative control . All analyses were tested in duplicate and performed across two plates ( n = 6).
Statistical Analysis
One-way analysis of variance (ANOVA) was used to analyze the mean occluded area ratio within the different groups, followed by a Bonferroni test (α = 0.05). In addition, the independent t -test was used to compare the mean occluded area ratio observe for the specimens treated with saliva and without saliva (α = 0.05). All analysis was performed using statistical software (IBM SPSS Statistics v24; IBM Corp.).
Results
Dentine Specimens Treated in 7 Days (without Saliva)
Table 3 depicts the results of the dentine specimens measured in 7 days without saliva immersion. The total mean % ratio of the tubules occluded area for the dentine specimens treated with EB@TiO 2 , Colgate Pro-relief, and Sensodyne repair was statistically different ( p < 0.001).
Table 3. ANOVA test comparison of the occluded area (without saliva).
| Treatment group | N | Mean ± SD | Standard error | 95% confidence interval | p- Value | Posthoc Bonferroni’s test | |
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | p- Value | |||||
| Abbreviation: SD, standard deviation. Note: Superscript numbers indicate significant differences between the sample groups (ANOVA, p < 0.05). EB@TiO 2 , eggshell-titanium dioxide. | |||||||
| EB@TiO 2 | 7 | 64.7 ± 1.3 | 0.655 | 63.318 | 66.070 | 0.000 | 0.028 1,2 |
| Colgate Pro-relief | 7 | 62.0 ± 3.2 | 0.655 | 60.624 | 63.376 | 0.000 1,3 | |
| Sensodyne repair | 7 | 22.7 ± 4.8 | 0.655 | 21.358 | 24.111 | 0.000 2,3 | |
Notably, and after 7 days of brushing, the EB@TiO 2 group had the highest % mean occluded area (64.7 ± 1.3%), while the Sensodyne repair treated group had the lowest % mean occluded area (22.7 ± 4.8%). The Bonferroni’s correction results are given in Table 3 . The % tubules occluded for the test group (EB@TiO 2 ) were statistically higher when compared against the Colgate Pro-relief group ( p < 0.05), and the Sensodyne repair group ( p < 0.001). Equally, the % occluded area measured for the Colgate Pro-relief was significantly higher than that observed for Sensodyne repair ( p < 0.001). Fig. 1 illustrates the differences in the % tubules occluded per day with the three desensitizing paste materials (EB@TiO 2 , Colgate Pro-relief, and Sensodyne).
Fig. 1.
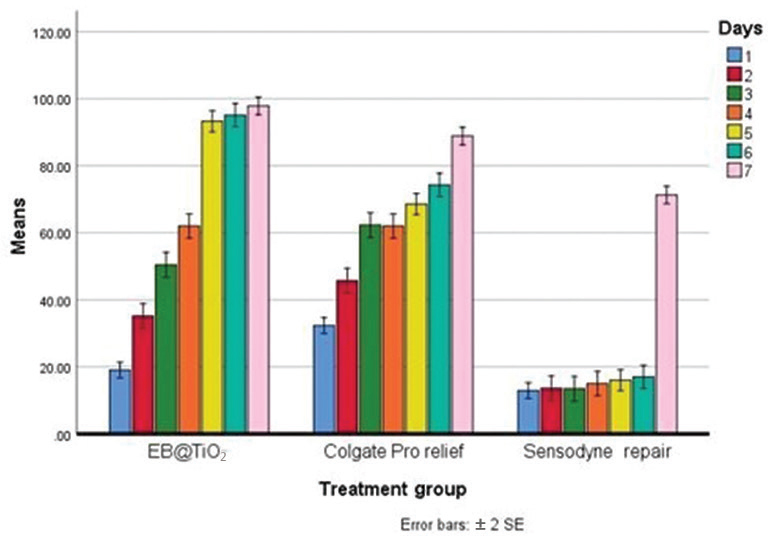
Differences in mean tubules occluded of dentine specimens treated with EB@TiO 2 Colgate Pro-relief, and Sensodyne repair desensitizing paste materials after 2 minutes of brushing without saliva immersion (7-day brushing test [ n = 7]). EB@TiO 2 , eggshell-titanium dioxide.
The Paired sample test, mean, and standard deviation results for the dentine specimen’s pre- and postacidic challenge are given in Table 4 . There was no significant different found in the EB@TiO 2 group pre- and postacidic treatment ( p > 0.05). In contrast, both the Colgate Pro-relief and Sensodyne repair treated group showed differences ( p < 0.001).
Table 4. Paired sample test comparison of occluded area ratio pre- and postacidic treatment.
| Treatment group | Occluded area (%) | p- Value | |
|---|---|---|---|
| Preacidic challenge (mean ± SD) | Postacidic challenge (mean ± SD) | ||
| Abbreviation: SD, standard deviation. | |||
| EB@TiO 2 | 97.9 ± 1.3 | 97.1 ± 1.2 | 0.318 |
| Colgate Pro-relief | 88.9 ± 3.2 | 33.9 ± 4.1 | 0.000 |
| Sensodyne repair | 71.3 ± 4.9 | 9.3 ± 2.4 | 0.000 |
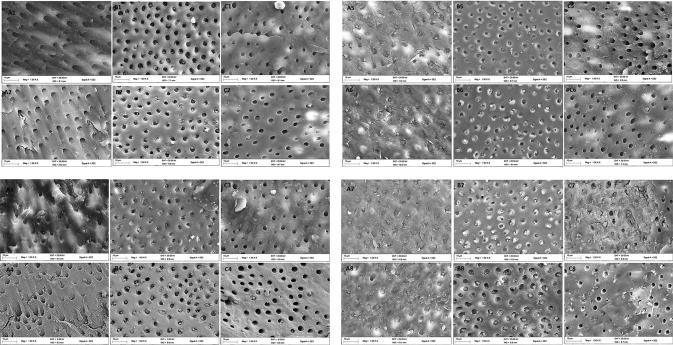
The FESEM image of the occluded dentine tubules for dentine specimens treated without storing in artificial saliva after 7 days brushing test is reflected in Fig. 2 . In day 1 ( Fig. 2B1 ), day 2 ( Fig. 2B2 ), the group treated with Colgate Pro-relief showed more evidence of tubule occlusion when compared against the group treated with EB@TiO 2 and Sensodyne repair. However, the EB@TiO 2 group showed a better evidence of tubule remineralization in day 3 ( Fig. 2A3 ) and day 4 ( Fig. 2A4 ). Similarly, there was a complete remineralization or sealing of the tubules in the EB@TiO 2 group in day 5 ( Fig. 2A5 ), day 6 ( Fig. 2A6 ), and day 7 ( Fig. 2A7 ).
Fig. 2.
Representative FESEM micrograph for the dentine surface after brushing for 7 days without saliva immersion using (A) EB@TiO 2 ; (B) Colgate Pro-relief; (C) Sensodyne repair (1–7 represents number of each day of brushing with the respective desensitizing paste, and 8 represent post acidic exposure). FESEM, field scanning electron microscope. EB@TiO 2 , eggshell-titanium dioxide.
The posttreatment in citric acid solution (4 wt.%) of the specimens are shown in Fig. 2 (A–C8) . Nonetheless, the specimen treated EB@TiO 2 ( Fig. 2A8 ) showed superior acid resistance with no visible differences pre- and postacidic challenge when compared against the specimens treated with Colgate Pro-relief ( Fig. 2B8 ) and Sensodyne repair ( Fig. 2C8 ), respectively.
Dentine Specimens Treated in 7 Days (with Saliva)
The mean, standard error, standard deviation, and ANOVA results for the dentine specimens stored in artificial saliva after brushing treatment are shown in Table 5 . The total mean % ratio of the tubules occluded area for the dentine specimens stored in artificial saliva alone, treated with EB@TiO 2 , Colgate Pro-relief, and Sensodyne repair was statistically different ( p < 0.001).
Table 5. ANOVA test Comparison of the occluded area (samples stored in artificial saliva).
| Treatment group | N | Mean ± SD | Standard error | 95% confidence interval | p- Value | Posthoc Bonferroni test | |
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | p- Value | |||||
| Abbreviation: SD, standard deviation. Note: Superscript numbers indicate significant differences between the sample groups (ANOVA, p < 0.001). EB@TiO 2 , eggshell-titanium dioxide. | |||||||
| Artificial saliva | 7.3 ± 2.3 | 0.636 | 5.953 | 8.578 | 0.000 | 0.000 1–5 | |
| EB@TiO 2 | 72.0 ± 1.0 | 0.636 | 70.708 | 73.333 | 0.000 2,3 | ||
| Colgate Pro-relief | 34.3 ± 8.6 | 0.636 | 33.034 | 35.659 | 0.000 3,4 | ||
| Sensodyne repair | 50.3 ± 3.0 | 0.636 | 49.014 | 51.639 | 0.000 2,4 | ||
It was found that the % occluded mean measured for the EB@TiO 2 group was the highest (72.0 ± 1.0%), while the specimens stored in artificial saliva alone without treatment had the lowest % mean occluded area (7.3 ± 2.3%). The Bonferroni’s correction results are shown in Table 5 . The EB@TiO 2 group mean % occluded area was statistically better when compared against the Colgate Pro-relief, and the Sensodyne repair ( p < 0.001). More so, the % occluded area measured for the Sensodyne repair was significantly higher than that observed for Colgate Pro-relief ( p < 0.001). All the treatment groups showed a significant improvement in occluding the tubules when compared against the samples stored in artificial saliva alone (< 0.001). Fig. 3 illustrates the differences in the % tubules occluded per day with the three desensitizing paste materials (EB@TiO 2 , Colgate Pro-relief, and Sensodyne) and artificial saliva.
Fig. 3.
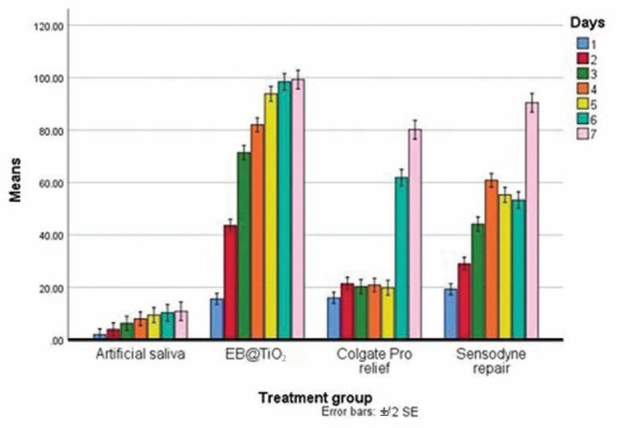
Differences in mean tubules occluded of dentine specimens treated with EB@TiO 2 Colgate Pro-relief, and Sensodyne repair desensitizing paste materials after 2 minutes of brushing and immersed in saliva (7 day brushing test [ n = 7]). EB@TiO 2 , eggshell-titanium dioxide.
Table 6 provides the paired sample test, mean, and standard deviation results for the dentine specimen’s (stored in artificial saliva) pre- and postacidic challenge. No difference was found in the Sensodyne repair group pre- and postacidic treatment ( p > 0.05). By contrast, there was a significant difference observed for the EB@TiO 2 , Colgate Pro-relief, and specimens stored in artificial saliva alone ( p < 0.001).
Table 6. Paired sample test comparison of occluded area ratio pre- and postacidic treatment (samples stored in artificial saliva).
| Treatment Group | Occluded area (%) | p- Value | |
|---|---|---|---|
| Preacidic challenge (mean ± SD) | Postacidic challenge (mean ± SD) | ||
| Abbreviation: SD, standard deviation. | |||
| Artificial saliva | 10.9 ± 2.3 | 5.7 ± 1.8 | 0.001 |
| EB@TiO 2 | 99.3 ± 1.0 | 85.0 ± 3.8 | 0.000 |
| Colgate Pro-relief | 80.1 ± 8.6 | 9.0 ± 2.2 | 0.000 |
| Sensodyne repair | 90.4 ± 3.0 | 88.1 ± 3.9 | 0.245 |
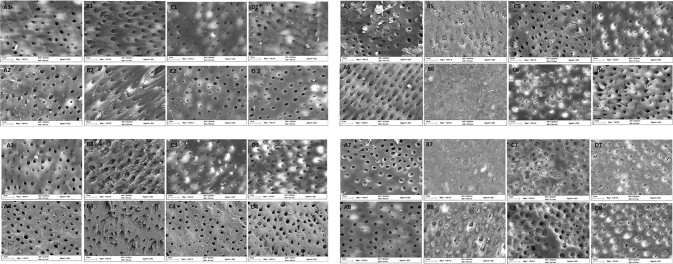
The FESEM images of the dentine specimens treated with EB-TiO 2 , Colgate Pro-relief, and Sensodyne repair for 7 days and storing in artificial saliva are shown in Fig. 4 . The observed images indicate the occlusion of EB-TiO 2 groups (A1–A7) were different from other test groups (Artificial saliva, Colgate Pro-relief, and Sensodyne repair). Fig. 4 (A–D8) revealed dissimilarity posttreatment of the specimens in citric acid solution (4 wt.%). The occluded tubules remain intact after acidic challenge for both the EB@TiO 2 and Sensodyne treated group. On the contrary, the tubules in the Colgate Pro-relief ( Fig. 4C8 ) treated specimens were visibly reopened postacidic challenge.
Fig. 4.
Representative FESEM micrograph for the dentine surface after brushing for seven days with saliva immersion using (A) Artificial saliva; (B) EB@TiO 2 ; (C) Colgate Pro-relief; (D) Sensodyne repair (1–7 represents with the respective desensitizing paste, and 8 post acidic exposure). FESEM, field scanning electron microscope. EB@TiO 2 , eggshell-titanium dioxide.
Biocompatibility Testing
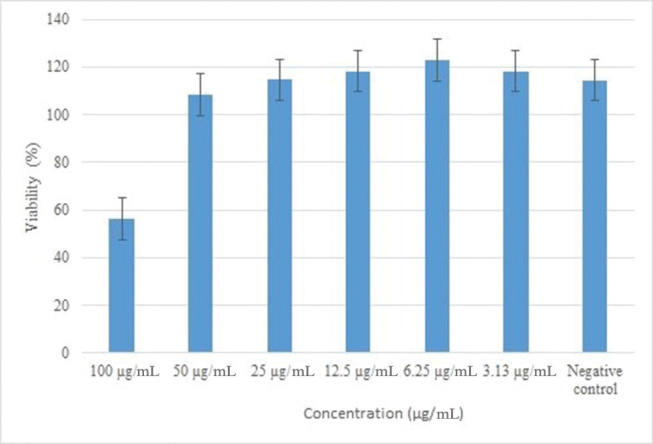
The biocompatibility of EB@TiO 2 with the BHK21 cell line is shown in Fig. 5 . In comparison to the negative control, the EB@TiO 2 appear to show little effect on the BHK21 cell lines. However, there was 56% cell viability at 100 μg/mL.
Fig. 5.
Percentage cell viability.
Discussion
Over the last decade, DH has been extensively researched owing to its widespread prevalence and noticeable painful oral health problem affecting many individuals. 30 The main aim of the paper to evaluate the effectiveness of a modified nanosized eggshell powder titanium dioxide composite (EB@TiO 2 ) in reducing DH. The composite was prepared through the mechanochemical activation method. Importantly, this method utilizes a mechanical energy to create structural changes as well as stimulate chemical reactions. 31 Consequently, it becomes possible to cause structural changes and particle size reduction in the EB@TiO 2 composite. 27 28 Conversely, the effectiveness of the prepared EB@TIO 2 in reducing DH was compared against Colgate Pro-relief and Sensodyne with or without immersion saliva. As suggested in the literature, 32 33 34 35 36 37 their occluding capabilities were evaluated using the bovine model. The morphological changes pre- and postacidic treatment of the specimens were evaluated with FESEM. The EB@TiO 2 treated specimens showed good tubule occlusion that still remain effective in acidic condition for both samples treated with and without saliva. This leads to the acceptance of the study hypothesis.
With respect to time, in the specimens treated without saliva, Colgate Pro-relief showed instant occluding of the dentine tubules. This is consistent with clinical studies 38 39 that Pro-Argin technology provides instant relief of DH. According to the mechanism proposed by Kleinberg, 19 it may be assumed that dentine with a negative charge surface attracts the positive charge arginine constituent of the Colgate Pro-relief which subsequently causes the adherence of calcium carbonate to the dentin surface. This in turn promotes the occlusion of the tubules. Despite this, the overall dentine tubule occlusion observed in the samples treated with EB@TiO 2 were significantly better than Colgate Pro-relief ( p < 0.05) and Sensodyne repair ( p < 0.001), respectively. These differences could be attributed to the modification of the carbonate structure in eggshell with titanium dioxide. 22 According to Cutler, 25 nanosized titanium dioxide, together with abrasive materials facilitate the occluding of dentine tubules, thus contributing to reducing of DH. Added to this, the nanosized calcium carbonate materials have unique high surface energy—thus facilitating the attachment of calcium-rich ions on the oral tooth surface. 40
Furthermore, the occlusion measured for Sensodyne repair was statistically lower compared against the Colgate Pro-relief ( p < 0.001). At the end of 7 days brushing, EB@TiO 2 had the highest occluded area (97.8 ± 1.3%) followed by Colgate Pro-relief (88.9 ± 3.2%), and lastly Sensodyne repair (71.3 ± 4.9%). Overall, the highest (64.7 ± 1.3) occlusion measured was in the EB@TiO 2 group, while Sensodyne repair had the lowest (22.7 ± 4.8; Table 3 ). This difference may be related to the constituent of the various test materials. Although studies 41 42 have shown that calcium sodium phosphosilicate (NovaMin) constituent of the Sensodyne repair could obstruct dentine tubules to some extent, Yu et al 43 however, argued that the Ca 2+ and PO 4 are protected by glass particles which need to be trapped for the Ca 2+ and PO 4 to be localized. The consequence of this is that there might be a delay in the action of the NovaMin to effectively promote the closing of dentine tubules. 43 Since the brushing test was performed in 7 days, without saliva, the inferior occluding characteristics observe for Sensodyne repair could be attributed to the absence of saliva to trap the Ca 2+ and PO 4 3- .
On the other hand, for the specimens treated with saliva immersion, all the tested material demonstrated a significant occlusion difference when compared with the those found in saliva alone ( p < 0.001). While saliva is reported to facilitate remineralization by the deposition of calcium and phosphate, 13 19 the finding from this study suggests that the occlusion of specimens in saliva alone without desensitizing paste treatment were highly inferior ( Table 5 ). This may; however, be attributed to the treatment duration ( Table 2 ). As reported in literature, 12 the occluding capabilities of saliva occur gradually within a long time. In support of the role saliva plays in reducing DH, the dentine tubules occlusion observed for Sensodyne repair showed an outstanding occlusion when compared against the samples treated without saliva. Similar significant occluding abilities were measured for EB@TiO 2 treated with saliva immersion ( p < 0.05).
Contrary to the above, the dentine tubules occlusion observed for the samples treated with Colgate Pro-relief with saliva treatment were consistently inferior at each day of brushing to those measured for the samples treated without saliva ( p < 0.001; Table 6 ). In contrast, other studies 11 38 claimed that the interaction of calcium carbonate and arginine encourages endogenous calcium and phosphate ions to deposit and occlude the dentin tubules. However, Yang et al 44 found that Colgate Pro-relief showed no significant changes after treatment and immersion in artificial saliva for 14 days. The above author findings corroborate with the same observation found in this study.
Moreover, the stability of occluding agents, particularly in a high acidic oral environment, is an important criterion for evaluating the efficiency of desensitizing paste in occluding dentine tubules. 45 This is more important as the oral cavity is often bombarded with citric acid that is highly common in the soft drinks and fruit juices found in our daily diets. In light of these, the effectiveness of the dentine occlusion observed with the different desensitizing paste was assessed posttreatment in a solution containing 4wt.% citric acid. The results observed for Colgate Pro-relief suggests that the product demonstrated an acid resistant to a certain extent. This can further be supported by the FESEM images that visibly showed that some of the closed dentine tubules were reopened after exposure to the citric acid solution ( Fig. 2B8 4c8 ). This; however, could be attributed to the solubility of calcium phosphates in an acidic environment. 21
In terms of the Sensodyne repair, the acid resistance effectiveness measured exhibit different behavior in the samples treated with and without saliva. In the group treated without saliva, nearly all the tubules were reopened after the exposure to citric acid ( Fig. 2C8 ). Similar findings were observed by Yu et al 43 where the deposits created by NovaMin on the dentine surface were almost completely removed by the citric acid solution. In contrast, the samples treated with saliva ( Fig. 4D8 ), the Sensodyne repair demonstrated an outstanding acidic resistance characteristic ( p > 0.05). The difference observed for both sample treatments may be associated with the role the saliva plays. It can, therefore, assume that the occlusion for the samples treated with Sensodyne and immersed in saliva had depth and penetration, thereby contributing to its acidic resistance.
As for the EB@TiO 2 group, the acidic resistant properties observed for the samples treated without saliva, pre- and postcitric acid exposure were comparable ( p > 0.05). However, slight differences were observed for the samples treated and immersed in saliva. It was found that after citric exposure, some of the obstructing tubules were reopened ( Fig. 4B8 ). This notwithstanding, the FESEM images visibly validate that the acid resistant characteristics of EB@TiO 2 were superior to that of Colgate Pro-relief and to some extent Sensodyne repair. Consistent with Tao et al, 46 the stability of EB@TiO 2 in an acidic condition may have been influenced by the modification of eggshell with titanium dioxide. Further clinical research is; however, needed to substantiate the efficiency of EB@TiO 2 as biocomposite material for the management of DH.
Conclusion
In conclusion, and within the study limitation, the study established that the EB@TiO 2 composite successfully occludes open dentine tubules with and without saliva. It was also established that EB@TiO 2 achieved effectiveness after 3 days of brushing. The composites also provide outstanding acid resistant stability. Despite this, and given the size of the sample used for the study, larger and longer duration of treatment would be required to conclusively determine the efficiency of EB@TiO 2 in reducing DH.
Funding Statement
Funding The financial support provided by the National Research Foundation of South Africa (No. 104824) is acknowledged by the authors.
Footnotes
Conflict of Interest None declared.
References
- 1.Igumbor E U, Sanders D, Puoane T R et al. “Big food,” the consumer food environment, health, and the policy response in South Africa. PLoS Med. 2012;9(07):e1001253. doi: 10.1371/journal.pmed.1001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinto S C, Bandeca M C, Silva C N, Cavassim R, Borges A H, Sampaio J E. Erosive potential of energy drinks on the dentine surface. BMC Res Notes. 2013;6:67. doi: 10.1186/1756-0500-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacaman R A, Pailahual V, Díaz-Garrido N. Cariogenicity induced by commercial carbonated beverages in an experimental biofilm-caries model. Eur J Dent. 2018;12(01):27–35. doi: 10.4103/ejd.ejd_188_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salahi S, Ghanbari M, Moosaali F. Effect of Three Common Desensitizers in Reduction of the Dentin Hypersensitivity after Periodontal Surgery. J Dent Biomater. 2016;3:169–176. [Google Scholar]
- 5.Rahardjo A, Nasia A A, Adiatman M, Maharani D. Efficacy of a toothpaste containing 5% potassium nitrate in desensitizing dentin hypersensitivity. Asian J Pharm Clin Res. 2016;9:345–347. [Google Scholar]
- 6.Mafla A C, Lopez-Moncayo L F. Dentine sensitivity risk factors: a case-control study. Eur J Dent. 2016;10(01):1–6. doi: 10.4103/1305-7456.175678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canadian Advisory Board on Dentin Hypersensitivity . Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69(04):221–226. [PubMed] [Google Scholar]
- 8.Clark D, Levin L. Non-surgical management of tooth hypersensitivity. Int Dent J. 2016;66(05):249–256. doi: 10.1111/idj.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miglani S, Aggarwal V, Ahuja B. Dentin hypersensitivity: recent trends in management. J Conserv Dent. 2010;13(04):218–224. doi: 10.4103/0972-0707.73385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colak H, Demirer S, Hamidi M, Uzgur R, Köseoğlu S. Prevalence of dentine hypersensitivity among adult patients attending a dental hospital clinic in Turkey. West Indian Med J. 2012;61(02):174–179. [PubMed] [Google Scholar]
- 11.Schiff T, Delgado E, Zhang Y P, Cummins D, DeVizio W, Mateo L R.Clinical evaluation of the efficacy of an in-office desensitizing paste containing 8% arginine and calcium carbonate in providing instant and lasting relief of dentin hypersensitivity Am J Dent 200922Spec No A:8A–15A. [PubMed] [Google Scholar]
- 12.Merh A, Singhbal K, Parikh V, Mehta S, Kulkarni G. Comparative evaluation of immediate efficacy of diode laser versus desensitizing paste containing 8% arginine and calcium carbonate in treatment of dentine hypersensitivity: an in vivo study. J Evol Med Dent Sci. 2015;4(25):4346–4355. [Google Scholar]
- 13.Panagakos F, Schiff T, Guignon A.Dentin hypersensitivity: effective treatment with an in-office desensitizing paste containing 8% arginine and calcium carbonate Am J Dent 200922Spec No A:3A–7A. [PubMed] [Google Scholar]
- 14.Yang Z Y, Wang F, Lu K, Li Y H, Zhou Z. Arginine-containing desensitizing toothpaste for the treatment of dentin hypersensitivity: a meta-analysis. Clin Cosmet Investig Dent. 2016;8:1–14. doi: 10.2147/CCIDE.S95660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Northwest PRECEDENT . Cunha-Cruz J, Stout J R, Heaton L J, Wataha J C. Dentin hypersensitivity and oxalates: a systematic review. J Dent Res. 2011;90(03):304–310. doi: 10.1177/0022034510389179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saeki K, Marshall G W, Gansky S A, Parkinson C R, Marshall S J. Strontium effects on root dentin tubule occlusion and nanomechanical properties. Dent Mater. 2016;32(02):240–251. doi: 10.1016/j.dental.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Babu K G, Subramaniam P, Teleti S. Remineralization potential of varnish containing casein phosphopeptides-amorphous calcium phosphate with fluoride and varnish containing only fluoride: a comparative study. Saudi J Oral Sci. 2018;5(01):35. [Google Scholar]
- 18.Zalite V, Locs J. Characterization and Preparation of Calcium Phosphate Model Toothpaste for Tooth Enamel Remineralization. Key Eng Mater. 2017;721:231–218. [Google Scholar]
- 19.Kleinberg I. SensiStat. A new saliva-based composition for simple and effective treatment of dentinal sensitivity pain. Dent Today. 2002;21(12):42–47. [PubMed] [Google Scholar]
- 20.Strassler H E, Serio F G.Dentinal hypersensitivity: Etiology, Diagnosis And Management. The Academy of Dental Therapeutics and Stomatology20092–7. [Google Scholar]
- 21.Arnold W H, Prange M, Naumova E A. Effectiveness of various toothpastes on dentine tubule occlusion. J Dent. 2015;43(04):440–449. doi: 10.1016/j.jdent.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Onwubu S C, Mdluli P S, Singh S, Tlapana T. A novel application of nano eggshell/titanium dioxide composite on occluding dentine tubules: an in vitro study. Braz Oral Res. 2019;33:e016. doi: 10.1590/1807-3107bor-2019.vol33.0016. [DOI] [PubMed] [Google Scholar]
- 23.Macri D V. Implementing a minimally invasive approach. Dimens Dent Hyg. 2016;14:32–37. [Google Scholar]
- 24.Haghgoo R, Mehran M, Ahmadvand M, Ahmadvand M J. Remineralization effect of eggshell versus nano-hydroxyapatite on caries-like lesions in permanent teeth (in vitro) J Int Oral Health. 2016;8:435–439. [Google Scholar]
- 25.Cutler E T.Prevention and treatment of oral diseases.In:Patent Ued.. USA: Squigle, Inc; 2014 [Google Scholar]
- 26.Onwubu S C, Mdluli P S, Singh S, Nyembe S, Thakur R. An in situ evaluation of the protective effect of nano eggshell/titanium dioxide against erosive acids. Int J Dent. 2018 doi: 10.1155/2018/4216415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onwubu S C, Mdluli P S, Singh S. Evaluating the buffering and acid-resistant properties of eggshell-titanium dioxide composite against erosive acids. J Appl Biomater Funct Mater. 2019;17(01):2.280800018809914E15. doi: 10.1177/2280800018809914. [DOI] [PubMed] [Google Scholar]
- 28.Onwubu S C, Mdluli P S, Singh S, Lawrence M, Ngombane Y. Characterization and in vitro evaluation of an acid resistant nanosized dental eggshell-titanium dioxide material. Adv Powder Technol. 2019;30(04):766–773. [Google Scholar]
- 29.Saporeti M P, Mazzieiro E T, Sales W F. In vitro corrosion of metallic orthodontic brackets: influence of artificial saliva with and without fluorides. Dental Press J Orthod. 2012;17(06):240–2.4E8. [Google Scholar]
- 30.Northwest Practice-based Research Collaborative in Evidence-based DENTistry . Cunha-Cruz J, Wataha J C, Heaton L J. The prevalence of dentin hypersensitivity in general dental practices in the northwest United States. J Am Dent Assoc. 2013;144(03):288–296. doi: 10.14219/jada.archive.2013.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hua Z, Nie M, Liu X, Wang Q. A clean strategy to prepare polylactide/hydroxyapatite bionanocomposites via solid mechanochemistry. J Macromol Sci, Part B. 2017;56(05):306–314. [Google Scholar]
- 32.Fonseca R B, Haiter-Neto F, Fernandes-Neto A J, Barbosa G AS, Soares C J. Radiodensity of enamel and dentin of human, bovine and swine teeth. Arch Oral Biol. 2004;49(11):919–922. doi: 10.1016/j.archoralbio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka J LO, Medici Filho E, Salgado J AP et al. Comparative analysis of human and bovine teeth: radiographic density. Braz Oral Res. 2008;22(04):346–351. doi: 10.1590/s1806-83242008000400011. [DOI] [PubMed] [Google Scholar]
- 34.Yassen G H, Platt J A, Hara A T. Bovine teeth as substitute for human teeth in dental research: a review of literature. J Oral Sci. 2011;53(03):273–282. doi: 10.2334/josnusd.53.273. [DOI] [PubMed] [Google Scholar]
- 35.Silva B G, Nunes Gouveia T H, Pereira da Silva M A, Bovi Ambrosano G M, Baggio Aguiar F H, Leite Lima D AN. Evaluation of home bleaching gel modified by different thickeners on the physical properties of enamel: an. in situ. study . Eur J Dent. 2018;12(04):523–527. doi: 10.4103/ejd.ejd_352_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vajrabhaya L O, Korsuwannawong S, Harnirattisai C, Teinchai C. Changes in the permeability and morphology of dentine surfaces after brushing with a Thai herbal toothpaste: a preliminary study. Eur J Dent. 2016;10(02):239–244. doi: 10.4103/1305-7456.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carvalho A O, Ayres A P, de Almeida L CAG, Briso A LF, Rueggeberg F A, Giannini M. Effect of peroxide bleaching on the biaxial flexural strength and modulus of bovine dentin. Eur J Dent. 2015;9(02):246–250. doi: 10.4103/1305-7456.156845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayad F, Ayad N, Zhang Y P, DeVizio W, Cummins D, Mateo L R. Comparing the efficacy in reducing dentin hypersensitivity of a new toothpaste containing 8.0% arginine, calcium carbonate, and 1450 ppm fluoride to a commercial sensitive toothpaste containing 2% potassium ion: an eight-week clinical study on Canadian adults. J Clin Dent. 2009;20(01):10–16. [PubMed] [Google Scholar]
- 39.Docimo R, Montesani L, Maturo P et al. Comparing the efficacy in reducing dentin hypersensitivity of a new toothpaste containing 8.0% arginine, calcium carbonate, and 1450 ppm fluoride to a commercial sensitive toothpaste containing 2% potassium ion: an eight-week clinical study in Rome, Italy. J Clin Dent. 2009;20(01):17–22. [PubMed] [Google Scholar]
- 40.Nakashima S, Yoshie M, Sano H, Bahar A. Effect of a test dentifrice containing nano-sized calcium carbonate on remineralization of enamel lesions in vitro. J Oral Sci. 2009;51(01):69–77. doi: 10.2334/josnusd.51.69. [DOI] [PubMed] [Google Scholar]
- 41.Chiang Y C, Chen H J, Liu H C et al. A novel mesoporous biomaterial for treating dentin hypersensitivity. J Dent Res. 2010;89(03):236–240. doi: 10.1177/0022034509357148. [DOI] [PubMed] [Google Scholar]
- 42.Yang H, Pei D, Chen Z, Lei J, Zhou L, Huang C. Effects of the application sequence of calcium-containing desensitising pastes during etch-and-rinse adhesive restoration. J Dent. 2014;42(09):1115–1123. doi: 10.1016/j.jdent.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 43.Yu J, Yang H, Li K, Lei J, Zhou L, Huang C. A novel application of nanohydroxyapatite/mesoporous silica biocomposite on treating dentin hypersensitivity: an in vitro study. J Dent. 2016;50:21–29. doi: 10.1016/j.jdent.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Yang J C, Hu H T, Lee S Y et al. In vitro evaluation of dentin tubule occlusion for novel calcium lactate phosphate (CLP) paste. Materials (Basel) 2017;10(03):228. doi: 10.3390/ma10030228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Sa Y, Sauro S et al. Effect of desensitising toothpastes on dentinal tubule occlusion: a dentine permeability measurement and SEM in vitro study. J Dent. 2010;38(05):400–410. doi: 10.1016/j.jdent.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Tao H, He Y, Zhao X. Preparation and characterization of calcium carbonate–titanium dioxide core–shell (CaCO 3 @ TiO 2 ) nanoparticles and application in the papermaking industry . Powder Technol. 2015;283:308–314. [Google Scholar]