Abstract
Elevated intraocular pressure (IOP) is a well-documented risk factor for glaucoma. Here we describe a novel, effective method for consistently inducing stable IOP elevation in mice, which mimics a post-operative complication of using silicone oil (SO) as a tamponade agent in human vitreoretinal surgery. In this protocol, SO is injected into the anterior chamber of the mouse eye to block the pupil and prevent inflow of aqueous humor. The accumulation of aqueous humor in the posterior chamber consequently increases the IOP of the posterior segment. A single SO injection produces reliable, sufficient, and stable IOP elevation, which induces significant glaucomatous neurodegeneration. This model is a true replicate of secondary glaucoma in the eye clinic. To further mimic the clinical setting, SO can be removed from the anterior chamber to reopen the drainage pathway and allow inflow of aqueous humor, which drained through the trabecular meshwork (TM) at the angle of the anterior chamber. Because IOP quickly returns to normal, the model can be used to test the effect of lowering IOP on glaucomatous retinal ganglion cells. This method is straightforward, does not require special equipment or repeat procedures, closely simulates clinical situations and may be applicable to diverse animal species with only minor modifications.
Keywords: Eye, Glaucoma, Silicone Oil, Anterior Chamber, Ocular Hypertension, Mouse Model, Intraocular Pressure, Neurodegeneration
SUMMARY:
Here, we present a protocol to induce ocular hypertension and glaucomatous neurodegeneration in mouse eyes by intracameral injection of silicone oil. We also describe the procedure for silicone oil removal from anterior chamber to return elevated intraocular pressure to normal.
INTRODUCTION:
The progressive loss of retinal ganglion cells (RGCs) and their axons is the hallmark of glaucoma, a common neurodegenerative disease in retina1. It will affect more than 100 million individuals between 40 to 80 years of age by 20402. Intraocular pressure (IOP) remains the only modifiable risk factor in the development and progression of glaucoma. In order to explore the pathogenesis, progression and potential treatments of glaucoma, a reliable, reproducible, and inducible experimental ocular hypertension/glaucoma model that replicates key features of human patients is imperative.
IOP depends on aqueous humor inflow to the anterior chamber from the ciliary body in the posterior chamber and outflow through the trabecular meshwork (TM) at the angle of the anterior chamber. When the inflow and outflow of aqueous humor reach a steady state, IOP is maintained; when the inflow surpasses or goes under outflow, IOP rises or falls respectively. By decreasing aqueous outflow either by occluding the angle of the anterior chamber or by damaging the TM, several glaucoma models have been established3–10. These models are normally associated with irreversible ocular tissue damage, and the high IOP in the anterior chamber also causes unwanted complications such as corneal edema and intraocular inflammation, which make retinal imaging and visual function assays difficult to perform and interpret.
To develop a model that overcomes these shortcomings, we turned our attention to the well documented, secondary glaucoma caused by silicone oil (SO) that occurs as a post-operative complication of using it in human vitreoretinal surgery11,12. SO is used as a tamponade in retina surgeries because of its high surface tension. However, SO can physically occlude the pupil since it is lighter than the aqueous and vitreous, which prevents aqueous flow into the anterior chamber. The obstruction causes IOP elevation in the posterior chamber due to the aqueous humor accumulation. This motivated us to develop and characterize a novel ocular hypertension mouse model based on intracameral SO injection and pupillary block13, with key features of the secondary glaucoma: effective pupillary block, significant IOP elevation that can return to normal after SO removal, and glaucomatous neurodegeneration.
Here we present a detailed protocol for SO-induced ocular hypertension in mouse eye, including SO injection and removal and IOP measurement.
PROTOCOL:
All procedures have been approved by the Institutional Animal Care and Use Committee (IACUC) of Stanford University.
1. Ocular hypertension induction by intracameral injection of SO
1.1. Prepare a glass micropipette for intracameral SO injection by pulling a glass capillary with a pipette puller to generate a micropipette. Cut an opening at the tip of the micropipette and further sharpen the tip with a microgrinder-beveling machine to make a 35° - 40° bevel.
1.2. Polish the edges of the bevel and remove all debris by washing with water. Autoclave the micropipette prior to the use.
1.3. Prepare paracentesis needle for the corneal entry. To do so, attach a 32 G needle to a 5 mL syringe on a Luer-lock, and further secure it with tape. Bend the needle bevel tip face up at 30 degree.
1.4. Prepare the SO injector by attaching and securing a blunt end 18 G needle on a 10 mL syringe first. Then attach a plastic tube with the 18 G needle on one end and fill up with SO as needed through the other end.
1.5. Attach the sterilized micropipette to the plastic tube and push the syringe plunger to fill the entire micropipette with SO.
2. Intracameral SO injection for one eye
2.1. Place 9–10-week-old male C57B6/J mouse into an induction chamber with 3% isoflurane mixed with oxygen at 2L/min for 3 min.
2.2. Intraperitoneally inject 2,2,2-Tribromoethanol at 0.3 mg/g body weight.
NOTE: Unlike ketamine/xylazine, 2,2,2-Tribromoethanol does not cause obvious pupil dilation.
2.3. Check for the lack of response to the toe pinch to determine the anesthetic depth and by the lack of movement of the whiskers or the tail.
2.4. Place the mouse in a lateral position on a surgery platform. To reduce its sensitivity during the procedure, apply one drop of 0.5% proparacaine hydrochloride to the cornea prior to injection.
2.5. Make an entry incision at the superotemporal quadrant, about 0.5 mm from the limbus with the 32G paracentesis needle.
2.6. Tunnel through the layers of the cornea for about 0.3 mm before piercing into the anterior chamber. Be careful not to touch lens or iris.
2.7. Withdraw the needle slowly to release some aqueous humor (about 1–2μl) from the anterior chamber through the tunnel (paracentesis).
2.8. Wait about 8 min to further decrease IOP (can be determined by measuring the contralateral eye).
2.9. Insert the glass micropipette pre-loaded with SO through the corneal tunnel into the anterior chamber, with the bevel facing down to the iris surface.
2.10. Push the syringe plunger slowly to inject SO into the anterior chamber until the SO droplet covers most of the iris surface, about 2.3 – 2.4 mm in diameter.
2.11. Leave the micropipette in the anterior chamber for 10 more seconds before withdrawing it slowly.
2.12. Gently push the upper eyelid to close the cornea incision to minimize SO leakage.
2.13. Apply antibiotic ointment (bacitracin-neomycin-polymyxin) to the eye surface.
2.14. Throughout the procedure, frequently moisten the cornea with artificial tears.
2.15. Keep the mouse on the heating pad until fully recovered from anesthesia.
3. SO removal
3.1. Prepare the irrigation system
3.1.1. Prepare the irrigating solution according to the manufacturer’s instructions and place it in the irrigation bottle. Elevate the irrigating solution bottle to 110–120 cm (81–88 mmHg) above the surgery platform.
3.1.2. Attach IV administration set to the irrigating solution bottle. Remove air bubbles from the IV tubing. Connect a 33 G needle bent to 20° face up to IV tubing.
3.2. To prepare the drainage system, remove the plunger from a 1 mL syringe. Attach a 33G needle to the syringe and bend the needle to 20°.
3.2 SO Removal from the anterior chamber
3.2.1. Intraperitoneally inject 2,2,2-Tribromoethanol (0.3 mg/g body weight). Check for the lack of response to the toe pinch to determine the anesthetic depth and by the lack of movement of the whiskers or the tail.
3.2.2. Place the mouse on a surgery platform and secure the lateral position with tape. Apply one drop of 0.5% proparacaine hydrochloride to the cornea to reduce its sensitivity.
3.2.3. Make two incisions in the temporal quadrant of the cornea between around 2 and 5 o’clock at the edge of the SO droplet, using the pre-made 32G paracentesis needle.
3.2.4. Insert a 33G irrigation needle connected to irrigating solution through one corneal incision, maximum speed.
3.2.5. Insert another 33G drainage needle attached to the syringe without a plunger through the other corneal incision to allow the SO droplet to exit the anterior chamber while irrigating with irrigating solution.
3.2.6. Withdraw the drainage needle, then the irrigation needle.
3.2.7. Inject an air bubble into the anterior chamber to maintain its normal depth and press to close the corneal incision.
3.2.8. Apply antibiotic ointment to both eyes.
3.2.9. Keep the mouse on the heating recovery pad until fully recovered from the anesthesia.
4. IOP measurement once a week
4.1. Place mouse into an induction chamber perfused with 3% isoflurane mixed with oxygen at 2L/minute for 3 minutes.
4.2. Intraperitoneally inject xylazine and ketamine (0.01 mg xylazine/g + 0.08 mg ketamine/g).
4.3. Keep the cornea moist by applying artificial tears throughout the procedure.
4.4. Wait about 15 min to allow the pupil to fully dilate.
4.5. Measure the IOP of both eyes using a tonometer according to product instructions. Bring the tonometer near the mouse eye. Keep the distance from the tip of the probe to the mouse cornea at about 3–4 millimeters. Press the measuring button six times to generate one reading. 3 machine-generated readings are obtained from each eye to acquire the mean IOP.
4.6. Sacrifice animals at 8 weeks after SO injection and perform immunohistochemistry of whole-mount retina, RGC counting, optic nerve semi-thin sections and quantification of surviving axons, which were described before13.
REPRESENTATIVE RESULTS:
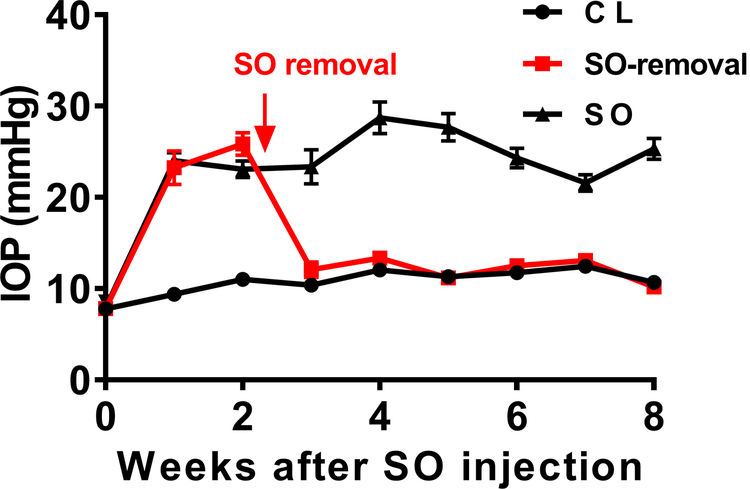
We can easily identify mice soon after injection that deemed not to produce stable ocular hypertension due to too small SO droplets (≤ 1.5mm)13, which are excluded from subsequent experiments. Following the injection procedures, we detailed before, routinely more than 80% of SO injected mice end up with droplets larger than 1.6 mm. We measured the IOP of these mouse eyes once weekly for 8 weeks after a single SO injection; the IOP of the eye receiving SO remained high, normally doubled the IOP of contralateral control eye, indicating the effective pupil blocking (Figure 1). The mouse cornea can have edema which can be checked under light dissection microscopy after intracameral injection that normally takes 2–3 days to recover. We try not to measure IOP too soon after injection. For the same reason, we do not recommend measuring IOP too often, which is also not very practical as the IOP measurement needs to wait for the pupil dilation and thus it is labor extensive. With another group of mice, we flushed out the SO from the anterior chamber at 2 weeks after SO injection, and we waited for another week to allow the cornea to recovery before measuring IOP, which stably returned the IOP to normal (Figure 1).
Figure 1. IOP measurements in SO eyes and contralateral control eyes, with or without SO removal.
SO: SO injected eyes; CL: contralateral control eyes. Data are presented as means ± S.E.M, n=12.
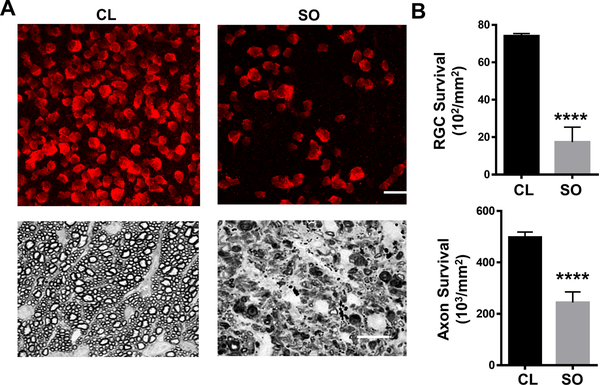
To determine the effects of ocular hypertension induced by SO injection on RGCs, we quantified surviving RGC somata in the peripheral regions of the retinal wholemounts by RBPMS staining14,15 and surviving axons in ON semithin cross-sections by PPD staining16 at 8 weeks after SO injection; glaucomatous RGC death and axon degeneration were dramatic in SOHU eyes (Figure 2).
Figure 2. Glaucomatous RGC soma and axon degeneration in SOHU eyes.
(A) Upper panel, the peripheral region of whole-mounted retinas showing RBPMS+ RGCs (red) at 8 weeks after SO injection. Scale bar, 20 μm. Lower panel, semi-thin images of cross sections of optic nerve stained with PPD at 8 weeks after SO injection. Scale bar, 10 μm. (B) Quantification data of surviving RGCs in the peripheral retina (n=12) and axons in optic nerve (n=10) at 8 weeks after SO injection compared to contralateral control (CL) eyes. Data are presented as means ± S.E.M. ****: P<0.0001; Student’s paired t test.
DISCUSSION:
Here we demonstrate a simple but effective procedure for inducing sustained IOP elevation in the mouse eye by intracameral injection of SO. This procedure can be learned pretty quickly by anyone who has experience in micro-dissection under a microscope. The primary potential risk of the failure is leakage of SO from the corneal incision. However, one of the advantages of using SO is that, because the oil droplet is visible and measurable, we can easily identify mice soon after injection that received droplets too small to induce stable ocular hypertension and exclude them from subsequent experiments. We have routinely achieved an 80% success rate and excluded about 20% of mice due to a small SO droplet (≤ 1.5mm)13. However, an experienced surgeon who can make a relatively long tunnel (0.3 mm) within the layers of the cornea before penetrating the cornea into the anterior chamber with the beveled tip, can almost prevent any SO leakage by making the inner opening of the corneal tunnel much smaller than the outer opening. Therefore, almost all the injected mice with a SO droplet larger than 1.8 mm. In addition to the length of the tunnel, some other critical points are worth emphasizing. First, it is important to keep IOP low in the injected eye to avoid pushing the SO out of the anterior chamber. One common mistake is to inject too much SO, which makes leakage easier. We limit the volume of SO in the anterior chamber to that it almost, but not entirely covers the iris surface; the diameter of the SO droplet is about 2.3–2.4 mm. Second, the corneal tunnel incision is made as close as possible to the limbus to allow the incision get close to iris but not hurt iris, so that iris can easily wedge the incision. Third, the injection speed should be as slow as possible to avoid excessive overflow of SO into the anterior chamber. Fourth, the upper eyelid massage after injection helps the corneal incision to close and sometimes assists anterior synechiae of the peripheral iris to wedge the corneal incision shut, and therefore to avoid oil leakage.
A unique feature of this model is that the IOP is elevated in the posterior part of the eye, but not in the anterior chamber. Pupil blocking prevents aqueous humor inflow into the anterior chamber and, therefore, increases IOP in the posterior part. The physical barrier formed by SO together with iris and huge lens may disconnect anterior chamber from posterior segment, which may limit IOP elevation only in the posterior segment, where the aqueous accumulates. When the mouse pupil is larger than SO droplet after dilation, the anterior and posterior chambers are re-connected, allowing quickly increase IOP in the anterior chamber by aqueous flooding into it. Therefore, tonometer can only detect the increased IOP after removing pupillary block, whereas the true IOP in the posterior segment is undoubtedly under-estimated. Therefore, we named this model as SO-induced ocular hypertension under-detected (SOHU), which more accurately and usefully reflects this key feature of the model. It would be best to be able to measure the IOP in the posterior segment directly but so far it is not possible. This unique pathogenesis of the SOHU model has two advantageous characteristics: First, the experimental eyes have clear ocular elements that allow in vivo assessment of visual function and morphology and second, the severe glaucomatous neurodegeneration allows any benefit of testing neuroprotectants to be detected.
SO injection can cause cornea edema temporarily and we recommend not performing IOP measurement too early or too often. We did not detect any inflammation in anterior chamber or cornea in SOHU eyes, although we encountered two instances of cornea neovascularization in the more than 100 mice receiving SO injections.
Since SO causes ocular hypertension in both human patients and mice, it is reasonable to postulate that this conceptually novel and practically significant glaucoma model can be adapted for larger animals that are more suitable for pre-clinical applications. The characterization of the deficits in neural function and morphology of this model will certainly encourage other investigators to take advantage of it to pursue important questions regarding glaucoma and, even more broadly, diseases that induce RGC and ON degeneration.
In summary, this is a straightforward animal glaucoma model that does not require special equipment or repeat injuries and may be applicable to other animal species. Intriguingly, the IOP elevation of SOHU model is reversible by removing SO from the anterior chamber, thus it is particularly useful for screening neuroprotective treatment combined with IOP lowering therapy
Supplementary Material
ACKNOWLEDGMENTS:
This work is supported by NIH grants EY024932, EY023295 and EY028106 to YH.
Footnotes
DISCLOSURES:
The authors have nothing to disclose.
REFERENCES:
- 1.Chang EE & Goldberg JL Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 119 (5), 979–986, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham YC et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 121 (11), 2081–2090, (2014). [DOI] [PubMed] [Google Scholar]
- 3.Pang IH & Clark AF Rodent models for glaucoma retinopathy and optic neuropathy. J Glaucoma. 16 (5), 483–505, (2007). [DOI] [PubMed] [Google Scholar]
- 4.Morrison JC, Johnson E & Cepurna WO Rat models for glaucoma research. Prog Brain Res. 173 285–301, (2008). [DOI] [PubMed] [Google Scholar]
- 5.McKinnon SJ, Schlamp CL & Nickells RW Mouse models of retinal ganglion cell death and glaucoma. Exp Eye Res. 88 (4), 816–824, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S & Zhang X The Rodent Model of Glaucoma and Its Implications. Asia Pac J Ophthalmol (Phila). 4 (4), 236–241, (2015). [DOI] [PubMed] [Google Scholar]
- 7.Sappington RM, Carlson BJ, Crish SD & Calkins DJ The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci. 51 (1), 207–216, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H et al. Optic neuropathy due to microbead-induced elevated intraocular pressure in the mouse. Invest Ophthalmol Vis Sci. 52 (1), 36–44, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cone FE, Gelman SE, Son JL, Pease ME & Quigley HA Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp Eye Res. 91 (3), 415–424, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samsel PA, Kisiswa L, Erichsen JT, Cross SD & Morgan JE A novel method for the induction of experimental glaucoma using magnetic microspheres. Invest Ophthalmol Vis Sci. 52 (3), 1671–1675, (2011). [DOI] [PubMed] [Google Scholar]
- 11.Ichhpujani P, Jindal A & Jay Katz L Silicone oil induced glaucoma: a review. Graefes Arch Clin Exp Ophthalmol. 247 (12), 1585–1593, (2009). [DOI] [PubMed] [Google Scholar]
- 12.Kornmann HL & Gedde SJ Glaucoma management after vitreoretinal surgeries. Curr Opin Ophthalmol. 27 (2), 125–131, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J et al. Silicone oil-induced ocular hypertension and glaucomatous neurodegeneration in mouse. Elife. 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwong JM, Caprioli J & Piri N RNA binding protein with multiple splicing: a new marker for retinal ganglion cells. Invest Ophthalmol Vis Sci. 51 (2), 1052–1058, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez AR, de Sevilla Muller LP & Brecha NC The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J Comp Neurol. 522 (6), 1411–1443, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith RS Systematic evaluation of the mouse eye : anatomy, pathology, and biomethods. (CRC Press, 2002). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.