Abstract
Anaemia is highly prevalent in cancer patients, adversely affects quality of life and impacts survival. The pathogenesis is multifactorial, with iron deficiency being a major and potentially treatable contributor. This study aimed to assess the effectiveness and economic impact of ferric carboxymaltose in chemotherapy-induced anaemia. This prospective cohort study between 2015–2016 of chemotherapy-treated patients for solid tumours, grade ≥2 anaemia and iron deficiency evaluated hematopoietic response four weeks after ferric carboxymaltose treatment. Transfusion rate of all cancer patients treated at our ambulatory unit during the two-year study period (2015–2016) was compared to a retrospective cohort (2013–2014) who received blood transfusion only. Between 2015–2016, 99 patients were included and treated with ferric carboxymaltose, the majority of whom (n = 81) had relative iron deficiency. Mean haemoglobin concentrations improved from 9.2 [6.7–10.8] g/dL to 10.6 [7.8–14.2] g/dL four weeks after treatment. A 26% reduction in the transfusion rate was observed from control retrospective to the prospective study group including ferric carboxymaltose treated patients [relative risk 0.74 (95% CI:0.66–0.83)]. The cost analysis showed a benefit for the use of ferric carboxymaltose in chemotherapy-induced anaemia. This study shows that ferric carboxymaltose is an effective, cost-saving support treatment, reducing the need for allogeneic transfusions saving blood units which are a limited resource.
Subject terms: Health care, Oncology
Introduction
Quality of life should be a priority in the management of cancer patients. As one of the most common problems reported at diagnosis and during cancer treatment, anaemia is a major concern in any Oncologist’s daily practice. The incidence of anaemia in cancer patients reported in literature varies significantly, ranging from 20% to 60% at the time of cancer diagnosis and reaching 60–90% during cancer treatments1–4. Anaemia has a negative impact on the quality of life of cancer patients. It is associated with poor performance status, fatigue, and may also jeopardize adherence to treatment, affecting therapeutic results, hospital stay and even survival4–6. According to the World Health Organization (WHO), anaemia is defined as haemoglobin (Hb) levels <12.0 g/dL in women and <13.0 g/dL in men7. The National Cancer Institute subdivides anaemia into different grades: mild – grade 1 (10 g/dl—normal), moderate – grade 2 (8–<10 g/dl), severe – grade 3 (6.5–<8 g/dl) and life threatening (<6.5 g/dl) anaemia8.
In cancer, anaemia has a multifactorial etiology. Iron deficiency (ID) is one of the underlying causes as its prevalence varies from 32 to 60%6,9,10. Anaemia may be attributed to absolute ID that can result from chronic blood loss due to gastrointestinal and gynaecological malignancies or surgery. Although less frequent, it can also derive from nutritional deficiencies due to cancer-induced anorexia, as well as reduced iron absorption due to gastrectomy, or the use of proton pump inhibitors, taken approximately by 20% of cancer patients11,12. The production of cytokines that leads to chronic inflammation and iron sequestration, and the myelosuppressive effects of chemotherapy or metastatic infiltration of the bone marrow limiting erythropoiesis are also contributing factors4,10,13,14. A variety of factors have been reported to be predictive of cancer and chemotherapy-associated anaemia: recent anticancer therapy, old age, poor performance status, advanced stage of the disease and particular tumour location (pancreatic, colorectal and lung cancer)6.
ID can be classified as absolute when iron stores are depleted, mainly due to bleeding, which corresponds to between 7 and 42% of all cases10,15, or functional (29–46% of all cases), when iron reserves are normal or increased but sequestered inside macrophages and enterocytes10,15,16. In the latter case reduction of iron availability for erythropoiesis is observed leading to anaemia.
Considering absolute ID, while in normal individuals a serum ferritin level of <30 ng/mL is virtually diagnostic, in cancer patients a higher ferritin cut-off (<100 ng/mL) appears more reliable, due to the chronic inflammation status. In functional ID, the guidelines available recommend testing both serum ferritin and transferrin saturation (TSAT). European Society for Medical Oncology (ESMO) guidelines considers functional ID when TSAT is <20%17, whereas The National Comprehensive Cancer Network (NCCN) guidelines targets ferritin level between 30 ng/mL and 500 ng/mL and a TSAT level <50%8.
Once other causes of anaemia are excluded, most patients with cancer and chemotherapy-induced anaemia (CIA) are treated with red blood cell (RBC) transfusion and/or iron supplement as monotherapy or in association to erythropoiesis-stimulating agents (ESA)8,17,18. RBC transfusions increase the risk of thrombotic events, may decrease survival19 and must be reserved for patients with severe anaemia symptoms in need of rapid Hb improvement. Safety issues regarding ESA’s link to decreased survival or increased disease progression were raised in the past. However recent data from a meta-analysis and prospective trials did not reveal links to tumour progression or reduced survival prospects20,21. Venous thromboembolic events are a known risk of ESA use in cancer patients22. Therefore, anaemia treatment guidelines do not recommend transfusions and suggest minimization of ESA dosage8,17. Some guidelines go as far as limiting their use to patients whose cancer treatment is not curative in intent18. Studies showing that intravenous (IV) iron (with or without concomitant ESA therapy) can improve Hb levels and reduce transfusion requirements in cancer patients support these goals23–25.
There are a number of IV iron formulations in the market26. In Portugal, four are available: iron sucrose, low molecular weight iron dextran (LMW-ID), iron isomaltoside and ferric carboxymaltose (FCM), all studied in CIA (reviewed in27). Because of its stable complex which allows for a slow and prolonged iron release, FCM enables administration of 1000 mg of iron in a 15-minute infusion28,29. Moreover, as reported by several authors in a variety of anaemia backgrounds, FCM is safe and effective providing a rapid correction of Hb and serum ferritin levels in iron-deficient patients30.
Therefore, the main objective of this study was to assess the effectiveness of FCM in the treatment of CIA.
Results
Between 2015 and 2016, 99 patients with at least grade 2 anaemia (Hb < 10 g/dl) and iron deficiency (defined as ferritin <800 ng/mL and TSAT <50%)8 were included in the study and treated with FCM infusions according to body weight as described in the methods section (a total of 1500 mg if 35 Kg to <70 Kg or 2000 mg if ≥70 Kg). Median age was 66 years [31–84 years] and 49.5% (n = 49) were male (Table 1). The most frequent type of tumours were gastrointestinal (44.4%, n = 44) and breast (21.2%, n = 21), and 39.4% (n = 39) had an advanced stage disease (stage IV) at diagnosis (Table 1).
Table 1.
Baseline patient characteristics (demographics and disease characteristics).
| Variables | Ferric Carboxymaltose group |
|---|---|
| Age (years), median, IQRa | 66 ± 16 |
| Gender, n (%) | |
| Male | 49 (49.5) |
| Female | 50 (50.5) |
| Cancer type, n (%) | |
| Colorectal | 22 (22.2) |
| Gastric | 22 (22.2) |
| Breast | 21 (21.2) |
| Pancreas | 11 (11.1) |
| Gynaecological | 7 (7.1) |
| Lung | 4 (4.1) |
| Othersb | 12 (12.1) |
| Disease stage at diagnosis, n (%) | |
| I-III | 60 (60.6) |
| IV | 39 (39.4) |
| Intention treatment at inclusion, n (%) | |
| Curative | 45 (45.5) |
| Palliative | 54 (54.5) |
| Ongoing chemotherapy at inclusion, n (%) | 94 (94.9%) |
| Iron Deficiency, n (%) | |
| Absolute | 18 (18.2) |
| Relative | 81 (81.8) |
aAbbreviations: IQR – interquartile range.
bOthers: head and neck, cholangiocarcinoma, oesophagus, occult primary tumour, urothelial.
The majority of patients received cytotoxic chemotherapy (94.9%, n = 94) and 5 (5.1%) participants were not receiving anti-cancer treatment at the start of the study. Treatment intent was curative in 45 patients. During the treatment phase, the median [Interquartile Range (IQR)] total dose of iron received was 1500 (±500) mg. The majority of participants (74.7%) received two infusions.
Iron status was assessed before study enrolment, and the majority (81.8%, n = 81) had functional ID (Table 1). Baseline haematological parameters are described in Table 2 and were typical of a cancer population. The baseline mean Hb concentration was 9.2 [6.7–10.8] g/dL. Four weeks after the last FCM course of treatment Hb increased in treated patients (versus baseline) on average to 10.6 [7.8–14.2] g/dL (Table 2). These results showed a statistically significant increase in Hb concentrations after FCM administration (p < 0.0001). An increment in Hb concentration from baseline was observed in 84 patients (84.8%) ranging from 0.1 to 5.3 g/dL (Fig. 1). In 23.2% of participants those increments were higher than 2 g/dL (Fig. 1).
Table 2.
Haematological parameters at study inclusion and increase in Hb from baseline until the end of study period.
| Variables | Ferric Carboxymaltose group |
|---|---|
| Baseline Hb (g/dL) | |
| Mean, SDa | 9.2 ± 0.8 |
| Min-Maxa | 6.7–10.8 |
| Post-treatment Hbb (g/dL) | |
| Mean, SDa | 10.6 ± 1.3 |
| Min-Maxa | 7.8–14.2 |
| Δ Hb (g/dL) | |
| Mean, SD | 1.37 ± 1.44 |
| Max-Min | (−1.5)–5.3 |
| p value (Baseline vs post-treatment Hb) | <0.0001 |
| Baseline ferritin (ng/mL) | |
| Mean, SDa | 255 ± 222 |
| Min-Maxa | 4.0–790 |
| Baseline TSAT (%) | |
| Mean, SDa | 15.5 ± 9.4 |
| Min-Maxa | 3.0–38.4 |
aAbbreviations: SD – standard deviation; Min – Minimum; Max – Maximum; TSAT – Transferrin saturation.
bHb value at follow-up visit during week 4.
cData were analysed by paired t-test. The significance level was considered p < 0.05.
Figure 1.
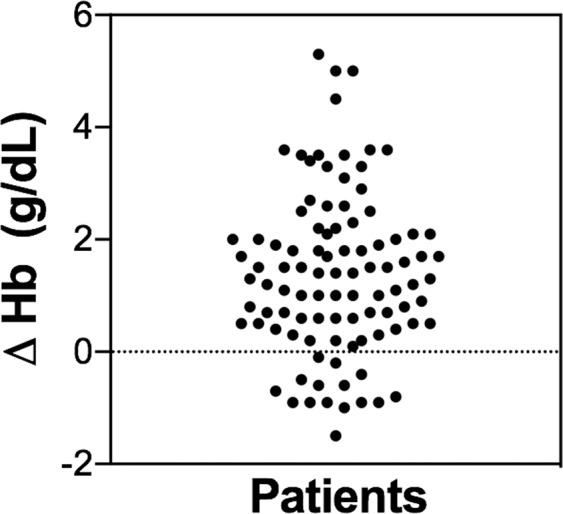
Haemoglobin level variation between baseline (pre-treatment) levels and four weeks after ferric carboxymaltose administration. Each dot represents a value relative to a patient.
Eleven patients (11.1%) received RBC transfusion within four weeks after the first FCM administration, and 26 (26.3%) after week four. RBC transfusions did not influence mean Hb concentrations achieved, as the mean post treatment Hb in the 88 patients treated with FCM-only was similar: Hb 10.7 [8.2–14.2] g/dL vs post-treatment Hb 10.6 [7.8–14.2] g/dL in all 99 FCM-treated patients.
A total of 319 vials of 500 mg of FCM were used in the study. Treatments were well tolerated, and neither hypersensitivity reactions nor other severe drug-related adverse events (immediate or delayed) occurred.
With regard to the secondary endpoint transfusion rate, we compared a cohort that comprises 99 patients treated with FCM and all patients subjected to RBC transfusion in the years 2015–2016 (prospective cohort), to a historical control group from two years before (2013–2014). The control group had received RBC transfusion as the only treatment for anaemia.
During the study period (2015–2016), a total of 13221 chemotherapy sessions were performed in 1811 patients, and a total of 517 blood units were used, representing a transfusion rate (number of RBC units per chemotherapy session) of 3.9% (Table 3). In the previous two years (control group: 2013–2014), 657 blood units were used in 12322 chemotherapy sessions, totalling a higher transfusion rate (5.3%) (Table 3). These results show a 26% reduction in the transfusion rate between the control and the study group including FCM-treated patients, with a relative risk of 0.74 (95% CI: 0.66–0.83, p < 0.0001).
Table 3.
Transfusions and FCM infusions.
| Retrospective control group (2013–2014) |
Prospective group (2015–2016) |
|
|---|---|---|
| Patients, n | 1732 | 1811 |
| ChTa cycles, n | 12322 | 13221 |
| Number of transfusions, n | 194 | 189 |
| RBCa units, n | 657 | 517 |
| Patients treated with FCM, n | 0 | 99 |
| Total FCM vials, n | 0 | 319 |
| % of patients with transfusion | 11.2 | 10.4 |
| Transfusion rateb (%) | 5.3 | 3.9 |
aAbbreviations: ChT – Chemotherapy; RBC – red blood cell; FCM – ferric carboxymaltose.
bTransfusion rate was calculated as the following: number of RBC units per chemotherapy treatment.
The unitary costs elements used for the analysis of FCM economic impact are detailed in Table 4. The results show a benefit per patient (−2.00 €) and chemotherapy cycle (−0.56 €), of using FCM treatment in CIA relative to RBC transfusion (Tables 5 and 6). Even though the direct total cost savings might appear low, the indirect cost savings are important, allowing a reduction in RBC units which are a crucial and limited resource.
Table 4.
Cost elements.
| Ferric carboxymaltose infusion (500–1000 mg/session) | |
|---|---|
| Intravenous iron | |
| FCM acquisition costa | € 95.38/500 mg |
| FCM administration costb | € 12.14/infusion |
| Red Blood Cell Transfusion | |
| RBC acquisition costc | € 164/unit |
| Pre-transfusion tests costsd | € 28 |
| RBC administration costb | € 20.90 |
Abbreviations: RBC – red blood cell; FCM – ferric carboxymaltose.aManufacturer’s selling price; bOfficial price for Portuguese Health System; cPortuguese Health System tariff for acquisition of a red blood cell unit; dIncludes two patients’ blood group assessments, one screening for irregular antibodies, one direct coombs test and compatibility tests.
Table 5.
Global treatment costs (red blood cell transfusions + Ferric carboxymaltose treatment).
| Retrospective Control group | Prospective group | |
|---|---|---|
| Transfusionsa | 139 875.30 € | 110 069.30 € |
| Ferric Carboxymaltoseb | — | 32 562.40 € |
| Total costs | 139 875.30 € | 142 631.70 € |
aTransfusion costs were calculated multiplying RBC units for the unitary cost (RBC acquisition cost + pre-transfusion tests costs + administration costs) – see Tables 3 and 4.
bFCM costs were calculated multiplying the number of vials (see Table 3) for the FCM acquisition cost, adding the cost of FCM administration taking into consideration that the infusion price is for 1000 mg/session.
Table 6.
Cost-effectiveness of Ferric Carboxymaltose treatment (per patient and chemotherapy cycle).
| Retrospective Control group | Prospective group | |
|---|---|---|
| Total cost per patient | 80.76 € | 78.76 € |
| Total cost per ChT cycle | 11.35 € | 10.79 € |
| Incremental cost per patient | — | − 2.00 € |
| Incremental cost per ChT cycle | ---- | − 0.56 € |
Costs were calculated considering the total number of patients and chemotherapy cycles described in Table 3.
Discussion
IV iron supplementation is widely used for the treatment of chronic iron deficiency anaemia. FCM was shown to be an effective treatment for anaemic patients with chronic kidney disease undergoing haemodialysis, chronic heart failure, post-partum anaemia or inflammatory bowel disease30. However, its usage in cancer patients is limited23,24. In this study, we showed that the treatment with FCM in cancer patients undergoing chemotherapy (with a grade ≥2 anaemia and iron deficiency) was effective in 85% of cases, improving the mean Hb levels of anaemic cancer patients. These observations are in line with the results published by Steinmetz et al. and Toledano et al., where the supplementation with FCM only, in anaemic cancer patients with absolute iron deficiency under chemotherapy, led to a substantial and sustained increase in Hb levels, suggesting a role for IV iron as first-line treatment for CIA23,24.
Currently, the treatment of anaemia in cancer patients consists of RBC transfusions and use of ESAs with or without iron supplementation8,17. Although ESAs are useful, ASCO guidelines limits their use to palliative intent18, and NCCN recommends its use only in patients receiving myelosuppressive chemotherapy or undergoing palliative treatment8.
At recommended doses, IV iron is well tolerated, particularly when compared with oral iron. FCM was a safe treatment as no severe drug-related adverse events were reported in this study.
Compared to other IV iron formulations, the incidence of transient hypophosphatemia with FCM is fairly high as reported in two retrospective studies31,32, and a recent prospective trial33, although no serious adverse events due to low phosphate values were seen28,31,32. Therefore, serum phosphate levels should be determined in all FCM treated subjects.
FCM has been prospectively compared to other IV iron formulations and showed similar safety and efficacy levels. As showed in a systematic review and meta-analysis of 21 randomized controlled trials by Rognoni et al., FCM resulted in a higher increase of serum ferritin levels in comparison to ferric gluconate and showed a high safety profile30, consistent with the other formulations able to be administered as a total iron dose infusion (LMW-ID, iron isomaltoside and ferumoxytol)26.
The main advantage is the administration of a single total dose, which decreases the risk of reactions to multiple courses of treatment. In addition to being a convenient option for the patient, it also reduces the number of interventions from medical staff and reduces ambulatory clinic booking hours, which contributes to a reduction in costs.
Additionally, our results showed a significant reduction in the percentage of cancer treatments that needed transfusion support in the group that included patients treated with FCM, reducing the need of allogeneic transfusion, and therefore saving RBC units.
For all these reasons, supplementation with IV iron, stands as an attractive therapeutic option for treatment of anaemic cancer patients with iron deficiency.
The cost reduction analysis was also favourable, as a direct cost saving was achieved in the FCM treatment group, due to the reduced need for RBC transfusions. More importantly, a number of indirect cost savings were achieved, namely in terms of time spent at ambulatory clinic and number of RBC units saved. From the patient’s perspective, those savings translate into fewer hospital visits and work absences, reduced transportation costs to name a few.
FCM was already shown to have reduced direct and indirect costs of hospitalization compared with iron sucrose or oral iron34. Cost reduction analysis also favours FCM over iron sucrose for the ambulatory treatment of iron deficiency35. Two studies, from Italy and Brazil, showed that FCM might be a cost-saving option for their health care systems when compared to ferric gluconate or iron sucrose in the treatment of iron-deficient patients36,37. With regards to the treatment of CIA, no data is available.
This topic is of major importance in clinical practice as anaemia is highly prevalent among cancer patients and its treatment might have a significant impact on survival and quality of life5,6. Iron deficiency is associated with impaired physical function, weakness and fatigue even in the absence of anaemia9.
One potential limitation arising from the design of our study is the lack of long term follow-up data on Hb values or patient-reported outcomes, which would have been helpful in assessing the clinical significance of our findings. Cost issues needs to be considered on a country level, as significant price differences for FCM between Europe and the United States are present.
Future studies may assess the benefit of this treatment in patients without anaemia, despite the documented iron deficiency, as it may not only prevent the occurrence of anaemia, but also improve symptoms of iron deficiency.
Methods
Patients and study design
A prospective cohort study was performed in a central Portuguese hospital (Centro Hospitalar Vila Nova de Gaia/Espinho) from 1st January 2015 to 31st December 2016. The study was reviewed and approved by the ethics committee of this hospital. All data was collected from electronic medical records. Data regarding the group which includes patients treated with FCM was collected prospectively. Data regarding the control group was collected retrospectively at baseline, for the period between the 1st January 2013 and 31st December 2014. The eligible population consisted of adults (≥18 years old) with a malignant solid tumour and diagnosed with at least grade 2 anaemia (defined by Common Terminology for Adverse Events v.5.0 as haemoglobin inferior to 10 g/dL8) and iron deficiency (defined as ferritin level <800 ng/mL and TSAT <50%)8. All were undergoing anti-cancer treatment and signed an informed consent. Subjects who received more than 50 units of blood or patients treated with ESAs were excluded from the study. Cancer staging was set in accordance to the TNM Classification (AJCC 7th edition).
At the start of the study, all patients were analysed for ferritin levels, transferrin saturation, vitamin B12 and folic acid status. The group treated with FCM consisted of subjects who initiated treatment with FCM (Ferinject, Vifor Pharma, Glattbrugg, Switzerland) between the 1st of January 2015 and 31st of December 2016. Treatment was prescribed when ferritin was lower than 800 ng/mL and TSAT was inferior to 50%. If patient body weight was 35 Kg to <70 Kg the dosage recommended was 1000 mg+ 500 mg (with at least one week interval between administrations). If patient weight was greater than 70 Kg the dosage recommended was 1000 mg + 1000 mg (with at least one week interval between administrations). Patients were examined during the first half hour post-administration for any possible side effects such as pain at the site of injection, flushing, allergic reactions, headache, dizziness, nausea, vomiting and a feeling of heaviness in the head. Haemoglobin levels were assessed four weeks after the last treatment dosage. The main aim of this study was to assess the effectiveness of FCM in the treatment of CIA in patients diagnosed with solid tumours. In order to do so, we analysed the hematopoietic response, defined as a positive haemoglobin variation 4 weeks after the post-carboxymaltose treatment.
This study also aimed to compare the transfusion rate, defined as the number of RBC units per chemotherapy treatment, between the cohort that included patients treated with FCM and those of a historical control group. The control group fulfilled the same inclusion criteria but had received RBC transfusions as the only form of treatment for anaemia.
A safety endpoint was to assess the number of adverse events during FCM treatment. The authors also analysed the economic impact of FCM protocol. Informed consent was obtained from all individual participants prior to the start of the study inclusion.
Cost and statistical analysis
Categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations, or medians and interquartile range (IQR) for variables with skewed distributions. Normal distribution was checked using skewness and kurtosis. A dependent t-test for paired samples was used for analysis of Hb variation over time (Hb at baseline vs Hb 4 weeks after treatment). Analysis of the results was performed using SPSS statistical software, version 22.With regards to the cost analysis (Table 4), for the control group transfusion costs were calculated multiplying RBC units for the unitary cost (RBC acquisition cost + pre-transfusion tests costs + administration costs). Costs for the FCM group were calculated taking into account the costs of FCM vials, costs of administration (taking into consideration that the infusion price is for 1000 mg/session) and the cost of RBC transfusions as calculated for control group. Additionally, the economic impact per patient and chemotherapy cycle was calculated. The costs were provided by the hospital and Portuguese health system tariffs. We did not include the costs of laboratory tests and investigations (to diagnose the presence and cause of anaemia); follow-up appointments (assumed to be the same in both study arms); surveillance costs, in the post-transfusion and post-FCM treatment; societal costs (e.g., loss of working hours).
Compliance with ethical standards
The study was reviewed and approved by the ethics committee of Centro Hospitalar Vila Nova de Gaia/Espinho, where the study was conducted and was approved by unanimity on the 29th December 2014. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author contributions
J.M. and I.L. collect participants data and wrote the main manuscript text; S.C., E.D., A.M.P., T.C., An. C. and M.D. contribute by reviewing the protocol and recruiting participants; H.C. was our Haematology consultant; Ang. C. was our Imunohemotherapy consultant; A.A. was responsible for collecting participants data; A.M. was responsible for the statistical analysis; A.J. is the corresponding author, was the mentor of the project and contributed by elaborating the protocol, recruiting participants and reviewing the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xu H, et al. Incidence of anemia in patients diagnosed with solid tumors receiving chemotherapy, 2010-2013. Clin. Epidemiol. 2016;8:61–71. doi: 10.2147/CLEP.S89480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludwig H, et al. The European Cancer Anaemia Survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. European Journal of Cancer. 2004;40:2293–2306. doi: 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Aravind S, Ranganathan G, Martin A, Nalysnyk L. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: a descriptive study of a large outpatient oncology practice database, 2000–2007. Clin. Ther. 2009;31 Pt 2:2416–2432. doi: 10.1016/j.clinthera.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Maccio A, et al. The role of inflammation, iron, and nutritional status in cancer-related anemia: results of a large, prospective, observational study. Haematologica. 2015;100:124–132. doi: 10.3324/haematol.2014.112813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cella D. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: results from five randomized clinical trials. Annals of Oncology. 2004;15:979–986. doi: 10.1093/annonc/mdh235. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig H, Muldur E, Endler G, Hubl W. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Annals of Oncology. 2013;24:1886–1892. doi: 10.1093/annonc/mdt118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System, http://www.who.int/vmnis/indicators/haemoglobin.pdf (2011).
- 8.National Comprehensive Cancer Network. Hematopoietic Growth Factors (Version 2.2019), https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf (2019).
- 9.de Castro J, et al. Iron deficiency in patients with solid tumours: prevalence and management in clinical practice. Clinical and Translational Oncology. 2014;16:823–828. doi: 10.1007/s12094-013-1155-5. [DOI] [PubMed] [Google Scholar]
- 10.Naoum FA. Iron deficiency in cancer patients. Revista Brasileira de Hematologia e Hemoterapia. 2016;38:325–330. doi: 10.1016/j.bjhh.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran-Duy A, et al. Use of proton pump inhibitors and risk of iron deficiency: a population-based case-control study. J. Intern. Med. 2019;285:205–214. doi: 10.1111/joim.12826. [DOI] [PubMed] [Google Scholar]
- 12.Smelick GS, et al. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Mol. Pharm. 2013;10:4055–4062. doi: 10.1021/mp400403s. [DOI] [PubMed] [Google Scholar]
- 13.Bryer E, Henry D. Chemotherapy-induced anemia: etiology, pathophysiology, and implications for contemporary practice. International Journal of Clinical Transfusion Medicine. 2018;ume 6:21–31. doi: 10.2147/ijctm.S187569. [DOI] [Google Scholar]
- 14.Dicato M, Plawny L, Diederich M. Anemia in cancer. Annals of Oncology. 2010;21:vii167–vii172. doi: 10.1093/annonc/mdq284. [DOI] [PubMed] [Google Scholar]
- 15.Lebrun F., Klastersky J., Levacq D., Wissam Y., Paesmans M. Intravenous iron therapy for anemic cancer patients: a review of recently published clinical studies. Supportive Care in Cancer. 2017;25(7):2313–2319. doi: 10.1007/s00520-017-3672-1. [DOI] [PubMed] [Google Scholar]
- 16.Busti Fabiana, Marchi Giacomo, Ugolini Sara, Castagna Annalisa, Girelli Domenico. Anemia and Iron Deficiency in Cancer Patients: Role of Iron Replacement Therapy. Pharmaceuticals. 2018;11(4):94. doi: 10.3390/ph11040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aapro M, et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018;29:iv96–iv110. doi: 10.1093/annonc/mdx758. [DOI] [PubMed] [Google Scholar]
- 18.Julia, B. et al. Management of Cancer-Associated Anemia With Erythropoiesis-Stimulating Agents: ASCO/ASH Clinical Practice Guideline Update. Journal of Clinical Oncology0, JCO.18.02142, https://doi.org/10.1200/JCO.18.02142%M30969847%U, https://ascopubs.org/doi/abs/10.1200/JCO.18.02142.
- 19.Sanon S, Lenihan DJ, Mouhayar E. Peripheral arterial ischemic events in cancer patients. Vasc. Med. 2011;16:119–130. doi: 10.1177/1358863X10388346. [DOI] [PubMed] [Google Scholar]
- 20.Glaspy J, et al. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br. J. Cancer. 2010;102:301–315. doi: 10.1038/sj.bjc.6605498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry D, et al. Randomized, double-blind, placebo (P)-controlled phase III non- inferiority study of darbepoetin alfa (D) for anemia in patients (pts) with advanced NSCLC: An ad hoc subgroup analysis of pts with baseline hemoglobin (Hb) <10.0 g/dL. Annals of Oncology. 2018;29:VIII499–VIII500. doi: 10.1093/annonc/mdy292. [DOI] [Google Scholar]
- 22.Tonia T, et al. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst. Rev. 2012;12:CD003407. doi: 10.1002/14651858.CD003407.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmetz T, et al. Clinical experience with ferric carboxymaltose in the treatment of cancer- and chemotherapy-associated anaemia. Ann. Oncol. 2013;24:475–482. doi: 10.1093/annonc/mds338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toledano A, et al. Clinical use of ferric carboxymaltose in patients with solid tumours or haematological malignancies in France. Support Care. Cancer. 2016;24:67–75. doi: 10.1007/s00520-015-2728-3. [DOI] [PubMed] [Google Scholar]
- 25.Hedenus M, et al. Intravenous iron alone resolves anemia in patients with functional iron deficiency and lymphoid malignancies undergoing chemotherapy. Med Oncol. 2014;31:302. doi: 10.1007/s12032-014-0302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auerbach M, Macdougall I. The available intravenous iron formulations: History, efficacy, and toxicology. Hemodial Int. 2017;21(Suppl 1):S83–S92. doi: 10.1111/hdi.12560. [DOI] [PubMed] [Google Scholar]
- 27.Rodgers GM, Gilreath JA. The Role of Intravenous Iron in the Treatment of Anemia Associated with Cancer and Chemotherapy. Acta Haematol. 2019;142:13–20. doi: 10.1159/000496967. [DOI] [PubMed] [Google Scholar]
- 28.Bregman DB, Goodnough LT. Experience with intravenous ferric carboxymaltose in patients with iron deficiency anemia. Therapeutic advances in hematology. 2014;5:48–60. doi: 10.1177/2040620714521127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pharma, V. Ferinject (ferric carboxymaltose). Summary Product Characteristics, https://www.medicines.org.uk/emc/product/5910 (2018).
- 30.Rognoni C, Venturini S, Meregaglia M, Marmifero M, Tarricone R. Efficacy and Safety of Ferric Carboxymaltose and Other Formulations in Iron-Deficient Patients: A Systematic Review and Network Meta-analysis of Randomised Controlled Trials. Clin. Drug. Investig. 2016;36:177–194. doi: 10.1007/s40261-015-0361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onken JE, et al. Ferric carboxymaltose in patients with iron-deficiency anemia and impaired renal function: the REPAIR-IDA trial. Nephrol. Dial. Transplant. 2014;29:833–842. doi: 10.1093/ndt/gft251. [DOI] [PubMed] [Google Scholar]
- 32.Onken JE, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2014;54:306–315. doi: 10.1111/trf.12289. [DOI] [PubMed] [Google Scholar]
- 33.Wolf, M. et al. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight3, 10.1172/jci.insight.124486 (2018). [DOI] [PMC free article] [PubMed]
- 34.Calvet X, et al. Cost-minimization analysis favours intravenous ferric carboxymaltose over ferric sucrose or oral iron as preoperative treatment in patients with colon cancer and iron deficiency anaemia. Technology and Health Care. 2016;24:111–120. doi: 10.3233/THC-151074. [DOI] [PubMed] [Google Scholar]
- 35.Calvet X, et al. Cost-minimization analysis favours intravenous ferric carboxymaltose over ferric sucrose for the ambulatory treatment of severe iron deficiency. PLoS One. 2012;7:e45604. doi: 10.1371/journal.pone.0045604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vicente AB, Decimoni TC, Quero AA. Cost-minimization analysis of the carboxymaltose ferric (i.v.) Compared with sacarato ferric (i.v.) in the treatment of anemia under suplementary health care perspective. Value in Health. 2015;18:A843. doi: 10.1016/j.jval.2015.09.386. [DOI] [Google Scholar]
- 37.Rognoni C, Tarricone R, Meregaglia M. Impatto economico dell’utilizzo di carbossimaltosio ferrico in pazienti con anemia da carenza di ferro nelle regioni italiane. MECOSAN. Manag. Econ. Sanit. 2015;93:99–114. doi: 10.3280/MESA2015-023006. [DOI] [Google Scholar]


