Abstract
The hyperemic responses to single muscle contractions are proportional to exercise intensity, which in turn is proportional to tissue metabolic demand. Hence, we tested the hypotheses that the rapid onset vasodilatory response post single muscle contraction would be unaffected when baseline blood flow was increased via infusion of either high-dose intra-arterial infusion of adenosine (ADO) or adenosine triphosphate (ATP). Twenty-four healthy young participants (28 ± 1 years) performed a single forearm contraction (20% MVC) at minute 75 of a continuous infusion of ADO (n=6), ATP (n=8), or saline (control, n=10). Brachial artery diameter and blood velocity were measured using Doppler ultrasound. Resting forearm vascular conductance (FVC; ml·min−1·100 mmHg−1·dl FAV−1) was significantly higher during ADO (33 ± 7) and ATP (33 ± 6) compared to control (7 ± 1, p<0.05). Peak FVC post-contraction during ADO and ATP were significantly greater than control (p<0.05), but not different from one another. Peak ΔFVC from baseline was similar in all three conditions (control: 14 ± 1, ADO: 24 ± 2, ATP: 23 ± 6; p=0.15). Total FVC (area under the curve) did not significantly differ between ADO and ATP (333 ± 69 and 440 ± 125); however, ATP total FVC was significantly greater compared to control (150 ± 19, p<0.05). We conclude that the peak response to a single contraction is unaffected by augmented baseline blood flow, and thus is likely due to a feedforward vasodilatory mechanism.
Keywords: Adenosine, Exercise Hyperemia, Vascular Conductance
Introduction
At the onset of exercise, skeletal muscle blood flow rises rapidly in large part due to vasodilation of the resistance vessels (Joyner & Casey, 2015). This phenomenon has been termed “rapid onset vasodilation” as it initiates within one cardiac cycle and peaks at approximately five cardiac cycles following a single muscle contraction (Gaskell, 1877; Gorczynski, Klitzman, & Duling, 1978; Marshall & Tandon, 1984; Moore, Bearden, & Segal, 2010; VanTeeffelen & Segal, 2006). The underlying mechanisms involved in rapid onset vasodilation are thought to be due to locally derived metabolic substances including nitric oxide, prostaglandins, potassium (K+), and adenosine triphosphate (ATP) (Bergfeld & Forrester, 1992; Brock et al., 1998; Burns, Cohen, & Jackson, 2004; Casey, Walker, Ranadive, Taylor, & Joyner, 2013; Dyke, Proctor, Dietz, & Joyner, 1995; Gonzalez-Alonso, 2012; Jin et al., 2011; Mortensen, Gonzalez-Alonso, Damsgaard, Saltin, & Hellsten, 2007; Schrage, Joyner, & Dinenno, 2004; Schrage et al., 2010). These substances may participate in the matching of tissue blood flow to metabolic demand (Andersen & Saltin, 1985). Additionally, it is currently unknown if the magnitude of rapid onset vasodilation is affected by increased baseline flow. Elucidating this information would further allow us to address if there is a ceiling effect to rapid onset vasodilation.
ATP is a potent vasodilator that is released in a time frame consistent with rapid vasodilation (Hester, Guyton, & Barber, 1982; Kirby, Crecelius, Richards, & Dinenno, 2013). When released from erythrocytes, ATP binds to P2Y purinergic receptors on the endothelium, generating significant vasodilation and blunting sympathetic vasoconstriction (Kirby, Voyles, Carlson, & Dinenno, 2008; Saltin, 2012). A unique property of ATP is its capability to evoke levels of vasodilation parallel to that seen during heavy exercise when infused exogenously (Gonzalez-Alonso et al., 2008; Mortensen et al., 2009). Additionally, mechanical deformation of red blood cells by muscle contraction might stimulate ATP release from erythrocytes in a manner that could link contraction with rapid onset vasodilation (Crecelius, Kirby, Richards, & Dinenno, 2013).
Previously, our laboratory has shown that increasing resting blood flow using prolonged ATP infusions did not blunt the steady-state hyperemic response to five minutes of rhythmic handgrip exercise (Shepherd, Joyner, Dinenno, Curry, & Ranadive, 2016). However, the significance and role of individual or specific vasodilators are likely to vary during different time periods of the hyperemic response. Thus the regulatory mechanisms for initiating and maintaining exercise hyperemia may differ (Clifford & Hellsten, 2004; Sinkler & Segal, 2017). In this context, Hester et al. increased resting skeletal muscle blood flow in an isolated canine gastrocnemius muscle preparation using exogenous administration of adenosine (ADO) and superimposed an electrically stimulated single muscle contraction (Hester et al., 1982). The results showed that increasing resting blood flow with ADO did not blunt or affect rapid onset vasodilation (Hester et al., 1982). However, the effect of higher resting skeletal muscle blood flow in humans using prolonged ADO or ATP infusion on rapid onset vasodilation is unknown.
With this information as a background, the purpose of this experiment was to evaluate the effects of increasing baseline blood flow with two different vasodilators on rapid onset vasodilation following a single brief forearm muscle contraction. Based on our previous results of prolonged ATP infusion on steady state exercise blood flows (Shepherd et al., 2016), we hypothesized that the rapid onset vasodilatory response post-single muscle contraction would be unaffected when baseline blood flow was increased via infusion of either high-dose intra-arterial infusion of ADO or ATP.
Methods
Ethical Approval
Approval of the protocol was obtained by the Institutional Review Board of the Mayo Clinic (IRB: 14-005987). The study conformed to the standards set by the latest revision of the Declaration of Helsinki, except for registration in a database. Written and oral informed consent was obtained by all participants prior to any study procedures.
Human Participants
A convenience sample of 27 healthy young participants (15 men/12 women) volunteered to participate in the study. Data from two participants in the ADO group and one participant in the ATP group was excluded from data analysis because of low signal to noise ratio in the Doppler recording of blood velocity resulting in a total of 24 participants for analysis. Ten of these participants have been reported in a previously published study (Casey et al., 2013). All participants were non-obese (BMI < 30 kg/m2), non-smoking, free of any pulmonary, cardiovascular, endocrine and neurological diseases and not taking medications with known cardiovascular effects. All participants refrained from exercise, alcohol, and caffeine 24 hours prior to the study and fasted for 12 hours prior to the study. To control the effects of the reproductive hormones on cardiovascular function, all female participants were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives (Minson, Halliwill, Young, & Joyner, 2000). Women with an inter-uterine device were excluded. All women also had a negative urine pregnancy test the morning of the study day.
Subject Monitoring
A 20-gauge, 5 cm (model RA-04020; Arrow International, Reading, PA) catheter was placed in the brachial artery of the exercising non-dominant forearm using aseptic techniques under local anesthesia (2% lidocaine). Attached to the catheter was a 3-way port system connector in series and a pressure transducer (model PX600F, Edwards Lifescience, Irvine, CA). This enabled simultaneous measurement of beat-to-beat brachial arterial pressure and infusion of study drugs. Continuous heart rate (HR) was recorded via 3-lead electrocardiogram.
Experimental Protocol
Each participant completed an experimental study day in the Clinical Research and Trials Unit at the Mayo Clinic. On arrival to the laboratory, height, weight, pregnancy test (women), forearm volume (FAV) and maximal voluntary contraction (MVC) were obtained. All participants were then instrumented with a brachial arterial catheter and a 3-lead electrocardiogram followed by a 20 minute rest period. Saline Baseline: A baseline saline infusion was administered for a two minute resting period. Blood velocities were obtained continuously. Experimental (ATP, ADO or Saline) Infusion: Participants received either ATP (n= 8), ADO (n= 6), or Saline (control, n=10) as an experimental or control condition. The Saline control group was obtained from previously published data from our lab (Casey et al., 2013). Previously, the cross-sectional study method has been successfully utilized by the laboratory for pharmaco-dissection studies (Casey & Joyner, 2011a, 2011b; Casey et al., 2010). At minute 75 of continuous ADO or ATP infusion, participants performed a single, brief (approximately one second) forearm contraction at 20% of maximal voluntary contraction (MVC) via handgrip dynamometer. Although Casey et al. (2013) included 3 trials of various intensities, all trials were separated by a minimum of two minutes to allow for blood flow to return to baseline values. Therefore, there was minimal to no possibility of the first single contraction trials affecting the following trials. Blood velocities were measured continuously. The time point of minute 75 of continuous infusion was selected to ensure there was a plateau of baseline flows with drug infusion prior to initiation of tachyphylaxis during either prolonged ATP or ADO infusion; based on the animal study model where the tachyphylaxis occurred around minute 100 of ADO infusion (Hester et al., 1982). The experimental protocol is displayed in Figure 1.
Figure 1.
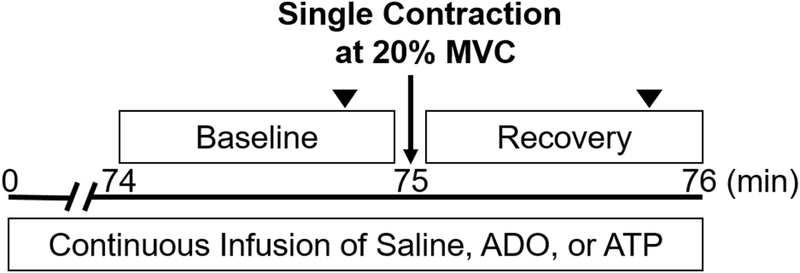
Overview of the experimental timeline. After 74 minutes of continuous intra-arterial infusion of either saline, adenosine (ADO), or adenosine triphosphate (ATP), participants performed a single forearm muscle contraction at 20% of their maximal voluntary contraction (MVC). Continuous measurements were taking for a minute following the contraction. Artery diameter measurements are indicated by the upside-down closed triangles.
Pharmacological Infusions
Drugs were administered intra-arterially via a Harvard infusion syringe pump through the brachial artery catheter. Doses were determined based on those that would evoke vasodilation at rest similar to that observed during moderate intensity exercise (Rongen, Smits, & Thien, 1994; van Ginneken, Meijer, Verkaik, Smits, & Rongen, 2004). Adenosine Triphosphate (ATP) (Sigma A7699 St. Louis, MoO, USA) was dissolved in isotonic saline (1 mg·ml−1) prepared by the Mayo Clinic Research Pharmacy to a concentration of 80 μg·ml−1. After adjusting for FAV, ATP was infused continuously at a constant infusion rate of 20 μg ·100 ml FAV−1·min−1 for 100 minutes. Adenosine (ADO) was diluted with isotonic saline to a concentration of 100 μg·ml−1. After adjusting for FAV, ADO was infused continuously at a rate of 23 μg·100 ml FAV vol−1·min−1 for 100 minutes.
Forearm Single Contraction Exercise
While supine, the participants’ non-dominant arm was abducted ~75° and rested on a table at approximately heart level. Participants performed a rapid single forearm muscle contraction with a handgrip dynamometer connected to a pulley system. Verbal instruction was given by study personnel for the timing of the contraction. Participants lifted and lowered weight a vertical distance of 4 to 5 cm over one second. Workload was set at 20% MVC, measured at the beginning of each experiment. MVC for each subject was determined as the average of three maximal squeezes of the handgrip dynamometer. This moderate intensity level was selected based upon previous studies showing it evokes significant blood flow response post single contraction in healthy participants (Casey et al., 2013). This exercise level also limits the changes in systemic hemodynamics and reflex activation of the sympathetic nervous system on exercise hyperemia (Casey & Joyner, 2012; Joyner & Casey, 2015; Richards, Crecelius, Kirby, Larson, & Dinenno, 2012).
Forearm Blood Flow (FBF)
Brachial artery diameter and brachial artery mean blood velocity (MBV, cm/s) were measured with a 10.5-MHz linear-array Doppler probe (model M12L, Vivid 7, General Electric, Milwaukee, WI). The probe was held to the skin over the brachial artery proximal to the catheter insertion site, approximately 9 cm proximal to the medial epicondyle. Measurements were collected with a probe insonation angle previously calibrated to 60°. Brachial artery diameter (cm) measurements were obtained at end diastole at rest (before contraction) and 45 seconds post contraction. This method has been previously used for several other studies in our laboratory (Casey & Joyner, 2012; Casey et al., 2013). FBF (ml·min−1) was calculated as the product of MBV and the cross-sectional area of the brachial artery: FBF = MBV·π(brachial artery diameter/2)2·60, where the brachial artery diameter is in centimeters and 60 is used for the conversion of milliliters per second into milliliters per minute (ml·min−1). Forearm Vascular Conductance (FVC, ml·min−1·100 mmHg−1) was calculated as: FVC = (FBF/mean arterial pressure)·100, where mean arterial pressure (MAP) is in mmHg. FVC was used as the primary index of vasodilation as it is linearly related to blood flow while also accounting for any changes in blood pressure.
Data Analysis
Data collection and analysis were similar between control and experimental study visits (Casey et al., 2013).
Data were collected at 250 Hz, stored on a computer and analyzed off-line with signal-processing software by a blinded team member. HR was determined from the electrocardiogram. MAP was determined from the brachial artery pressure waveform. Baseline values were determined by averaging the last 30 seconds of the resting time period prior to single contractions.
To account for baseline changes (Δ) with drug infusions, responses following the muscle contraction were adjusted (i.e., peak post contraction – baseline infusion value). The peak and total FBF and FVC responses were analyzed between conditions. Total FBF and FVC were defined as the area under the curve (AUC) via trapezoidal method after respective baseline values were subtracted for a given flow/conductance curve. To account for forearm size, all values were additionally adjusted by forearm volume: Adjusted FBF = FBF·FAV−1·100).
All values are expressed as means ± SD. All data were normally distributed and passed the Shapiro-Wilk test. One-way ANOVA was used to compare the responses between groups. When significance was detected, Bonferroni post-hoc analysis was used to identify differences between groups. The significant level was set at p<0.05. Effect size (Cohen’s d) was calculated for main effects of group by calculating the mean difference between groups divided by the standard deviation of the control group (Cohen, 2013).
Results
Participants
Control, ADO and ATP experimental groups were of similar height, weight and body mass index. However, the control group was younger than the ADO group (p<0.05). All groups showed similar systemic hemodynamic variables, MVC, and forearm volume (Table 1). Resting hemodynamic group data during control, ADO and ATP infusions are presented in Table 2.
Table 1.
Participant Characteristics.
| Control (n=10) |
ADO (n=6) |
ATP (n=8) |
|
|---|---|---|---|
| Sex (men/women) | 5/5 | 3/3 | 4/4 |
| Age (years) | 24 ± 5 | 32 ± 4* | 30 ± 5 |
| Height (cm) | 174 ± 9 | 179 ± 10 | 178 ± 9 |
| Weight (kg) | 72 ± 14 | 84 ± 17 | 79 ± 13 |
| Body Mass Index (kg/m2) | 24 ± 3 | 26 ± 3 | 25 ± 3 |
| Systolic Blood Pressure (mmHg) | 138 ± 9 | 129 ± 15 | 130 ± 13 |
| Diastolic Blood Pressure (mmHg) | 78 ± 6 | 76 ± 6 | 75 ± 7 |
| 20% Maximum Voluntary Contraction (kg) | 8 ± 3 | 10 ± 3 | 9 ± 2 |
| Forearm Volume (ml) | 879 ± 202 | 1072 ± 231 | 993 ± 181 |
Values are means ± SD for all 24 participants.
p<0.05 vs. control. cm, centimeters; kg, kilograms; m, meters; mmHg, millimeters of mercury; ml, milliliter.
Table 2:
Baseline (Resting) Infusion Hemodynamics.
| Control (n=10) |
ADO (n=6) |
ATP (n=8) |
|
|---|---|---|---|
| Mean Arterial Pressure (mmHg) | 97 ± 6 | 93 ± 8 | 95 ± 9 |
| FBF (ml·min−1) | 66 ± 33 | 306 ± 131* | 293 ± 127* |
| FVC(ml·min−1·100 mmHg−1) | 69 ± 35 | 333 ± 142* | 318 ± 156* |
| Adjusted FBF (ml·min−1· dL−1 FAV) | 7 ± 3 | 30 ± 15* | 31 ± 14* |
| Adjusted FVC (ml·min−1·100 mmHg−1· dL−1 FAV) | 8 ± 3 | 33 ± 17* | 33 ± 17* |
Values are means ± SD for all 24 participants.
p<0.05 vs. control. mmHg, millimeters of mercury; min, minute; ml, milliliter; dL, deciliter; FAV, forearm volume.
ADO vs. Control
During infusion, resting FBF and FVC was higher during ADO compared to control (p<0.01, Table 2). Peak FBF and FVC post-contraction was greater during ADO compared to control (p<0.01, Table 3). However, peak ΔFBF and peak ΔFVC in response to a single contraction was similar in control and ADO conditions (Figure 2). Total FVC over 30 cardiac cycles post contraction did not significantly differ between ADO and control (Figure 3). Time to reach peak also did not significantly differ between ADO and control (Table 3). All results remained constant after adjusting FBF and FVC for FAV.
Table 3:
Peak Post Contraction Hemodynamics.
| Control (n=10) |
ADO (n=6) |
ATP (n=8) |
|
|---|---|---|---|
| Mean Arterial Pressure (mmHg) | 98 ± 5 | 91 ± 11 | 92 ± 8 |
| FBF (ml·min−1) | 194 ± 72 | 518 ± 187* | 487 ± 188* |
| FVC (ml·min−1·100 mmHg−1) | 198 ± 70 | 575 ± 211* | 541 ± 237* |
| Adjusted FBF (ml·min−1· dL−1 FAV) | 22 ± 4 | 50± 17* | 51 ± 21* |
| Adjusted FVC (ml·min−1·100 mmHg−1· dL−1 FAV) | 22 ± 4 | 56 ± 20* | 56 ± 26* |
| Time of Peak (cardiac cycle post contraction) | 5 ± 2 | 3 ± 1 | 4 ± 2 |
| Total FVC (AUC, AU) | 22 ± 9 | 60 ± 29 | 70 ± 57* |
| Total Adjusted FVC (AUC, AU) | 3 ± 1 | 7 ± 2 | 8 ± 6* |
Values are means ± SD for all 24 participants.
p<0.05 vs. control. mmHg, millimeters of mercury; ml, milliliter; min, minute; dL, deciliter; FAV, forearm volume; AUC, area under the curve; AU, arbitrary units.
Figure 2.
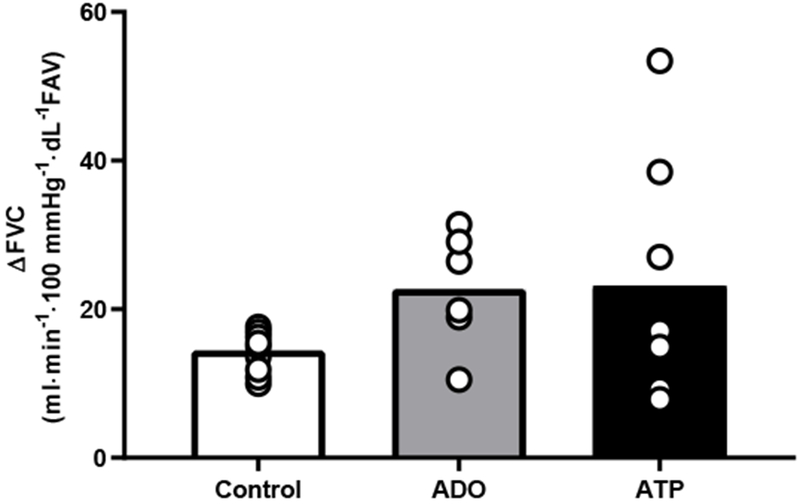
Change in FVC before and after rapid single muscle contraction did not differ between groups. Delta was calculated as peak FVC post contraction - baseline FVC, for each respective condition. Each participants is represented by an individual circle. Bar values are the mean of each group. p=0.147.
Figure 3.
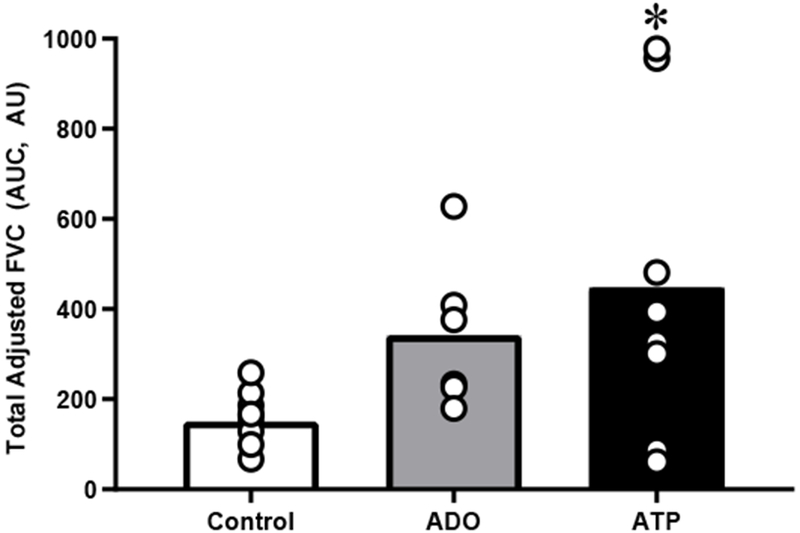
Total Adjusted FVC (AUC) over 30 cardiac cycles following a single forearm contraction at 20% maximal voluntary contraction was greater with ATP compared to control, but not ADO. Each participants is represented by an individual circle. Bar values are the mean of each group*p<0.05 ATP vs. control.
ATP vs. Control
During infusion, resting FBF and FVC was significantly higher during ATP compared to control (p<0.01, Table 2). Peak FBF and FVC post-contraction was greater during ATP compared to control (p<0.01, Table 3). However, peak ΔFBF and peak ΔFVC in response to a single contraction was similar in control and ATP conditions (Figure 2). Total FVC over 30 cardiac cycles post contraction was significantly greater during ATP compared to control (p<0.05, Figure 3). Time to reach peak did not significantly differ between ATP and control (Table 3). All results remained constant after adjusting FBF and FVC for FAV.
ADO vs ATP
Resting FBF and FVC did not significantly differ between ADO and ATP (Table 2). The change in FVC adjusted for FAV from pre- to post-drug infusion was similar between ADO and ATP (31 ± 4 and 26 ± 5 ml·min−1·100 mmHg−1· dL−1 FAV, p>0.05). Peak FBF and FVC post contraction was similar between ADO and ATP conditions (Table 3). Similarly, peak ΔFBF and peak ΔFVC from baseline was similar between ADO and ATP (Figure 2). Total FVC over 30 cardiac cycles post contraction did not significantly differ between ADO and ATP (Figure 3).
Discussion
The novel findings from the present study are: first, neither prolonged ADO nor ATP infusion blunted the hyperemic and vasodilatory responses after a single muscle contraction. Second, the peak hyperemic and vasodilator responses post-single contraction were similar between ADO, ATP and control infusions. Third, the recovery of blood flow following contraction during the ATP condition was prolonged as shown by the greater area under the recovery curve (Figure 3 and 4).
Figure 4.
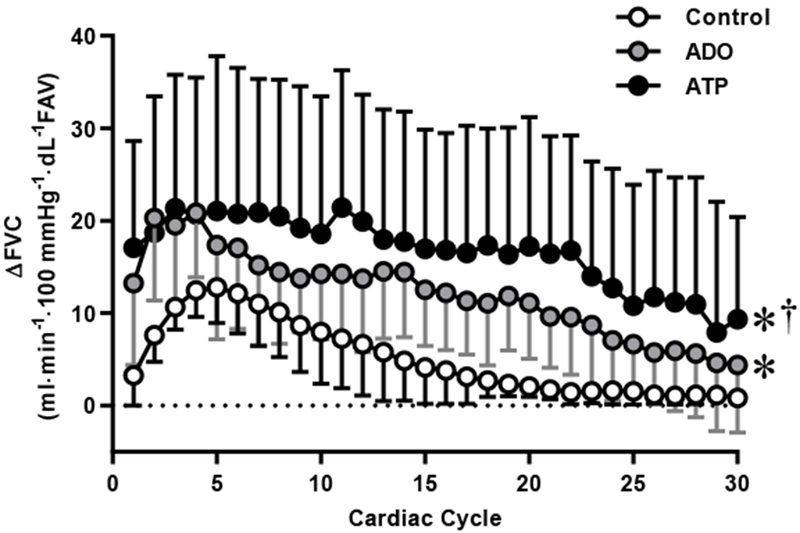
Vasodilator [change (Δ) in forearm vascular conductance (FVC)] responses over 30 cardiac cycles following a single forearm contraction at 20% maximal voluntary contraction (MVC). Values are mean ± SD. Some error bars are not visible because they are smaller than the representative symbol. *p<0.05 vs. control. †p<0.05 vs. ADO.
Our observations that peak rapid onset vasodilation following a single contraction was not blunted during ADO or ATP infusion (Figure 1) may have several explanations. First, we cannot discount the possible role of endogenous ATP released during the single contraction. As suggested by Shepherd et al., it is possible that regardless of the already high blood flow, additional ATP may have been released from deoxygenating or mechanically-deforming erythrocytes during exercise (Shepherd et al., 2016). Additionally, Crecelius et al. observed that the mechanical effects of a rhythmic muscle contractions can result in an increase in plasma ATP effluent (Crecelius, Kirby, Richards, et al., 2013). Therefore, it is possible that the release of endogenous ATP may be one of the reasons for increase in blood flow post single contraction. A second and a rather strong possibility is the contribution of other metabolites such as prostaglandins, nitric oxide, and potassium that worked synergistically after the contraction in the presence of prolonged vasodilator infusions (Crecelius, Kirby, Luckasen, Larson, & Dinenno, 2013). In this context, vasodilation via potassium-mediated hyperpolarization can account for almost half of the rapid-onset dilation seen post single contraction. When blocking potassium-mediated hyperpolarization along with nitric oxide and prostaglandins rapid onset vasodilation drops by about 60% (Crecelius, Kirby, Luckasen, et al., 2013).
Along similar lines, recent observations suggest that blockade of small and intermediate conductance calcium and potassium (sKca and iKca respectively) channels significantly attenuates for rapid onset vasodilation in mice (Sinkler & Segal, 2017). Additionally, as seen in mouse models, the signaling mechanism of endothelial dependent hyperpolarization could have been conducted along the endothelium into the proximal feed artery (brachial artery) even in the presence of local vasodilators. Taken together it suggests that hyperpolarization along the endothelium irrespective of the other metabolites may be a key mechanism (Sinkler & Segal, 2017). Currently, there is no reason to believe that the endothelial or potassium mediated hyperpolarization is compromised or disrupted due to increased baseline flow per se. This hyperpolarization mechanism serves to lower the resistance in the feed artery to increase the blood flow during the transition from rest to exercise (Sinkler & Segal, 2017).
We also observed that the ATP infusion prolonged the recovery of the vasodilator response relative to control conditions (Figure 3). One explanation for this slower recovery of blood flow during the ATP infusion could be ATP’s unique ability to mimic functional sympatholysis (DeLorey, Wang, & Shoemaker, 2002). In the current study, FVC post single contraction following ATP infusion remains at a higher level, as compared to control (Figure 4). At 30 cardiac cycles post contraction, after normalizing for baseline, ATP had a significantly higher total FVC compared to control (p<0.05). This is particularly interesting because when the baseline flow and conductance are increased by inhibiting α-adrenergic vasoconstriction, there is an increase in magnitude of flow and conductance post single contraction (Casey & Joyner, 2012).
Experimental Considerations
There are several limitations to our study. First, the change in FVC (post- to pre-contraction) was trending to be higher between both drug infusions vs control; though, statistically this was not significant (Figure 2). However, given the small study sample we have presented the effect size (Cohen’s d) to show that there was a large change during ATP (d =0.83; 95% CI: −0.14, 1.80) and ADO infusion (d =1.65; 95 %CI: 0.49, 2.81). The idea of presenting effect size in the present study helps us to reinforce our interpretation of the results because effect size provides an index of the magnitude of difference between groups. This suggests that the drug conditions may have augmented the rapid onset vasodilatory effect. Interestingly, ATP and ADO did not differ in their degree of augmentation, as peak and ΔFVC post contraction were similar between ATP and ADO.
Second, all data were collected from separate groups of participants, eliminating the ability to do inter-subject comparisons of the conditions. Although, the ADO group was statistically older than the control group, this difference likely did not influence our findings as all participants were young (i.e. under 36 years) and healthy. Based on previous studies evaluating the impact of aging on rapid onset vasodilation differences were not observed until 60 years of age (Casey & Joyner, 2012; Casey et al., 2013). Third, we did not collect blood samples, which would have allowed for the analysis of endogenous or exogenous plasma ATP concentrations throughout the study. Fourth, only one dose of ATP and ADO was used for infusion. It is possible that using a higher dose would have resulted in greater responses and differences between groups. Finally, we were unable to distinguish between any possible responders and non-responders to the drug infusions. In response to adenosine infusions, Martin et al. observed a bimodal distribution of vasodilatory responses to adenosine infusion (Martin, Nicholson, Eisenach, Charkoudian, & Joyner, 2006). It may be possible a similar response could be occurring with ATP infusion (Martin et al., 2006).
Summary
Independent prolonged exogenous ADO and ATP infusions result in a resting FBF and FVC similar to short-term moderate intensity exercise. The increased baseline flows did not result in changes to the rapid onset vasodilatory response post single handgrip exercise (20% MVC), and thus is likely due to a feedforward vasodilatory mechanism. Prolonged ATP infusion resulted in a slower recovery to a single forearm contraction.
New Findings.
What is the central question of this study?
This study investigated the effect of an elevated baseline blood flow, via either high-dose intra-arterial infusion of adenosine or adenosine triphosphate, on the rapid onset vasodilatory response to a single forearm muscle contraction.
What is the main finding and its importance?
The peak response to a single contraction is unaffected by augmented baseline blood flow, and thus is likely due to a feedforward vasodilatory mechanism.
Acknowledgements
We are grateful to the study volunteers for participation. We thank S. Baker, P. Engrav, R. Harvey, W. Holbein, C. Johnson, J. Long, J. Limberg, L. Newhouse, N. Meyer, A. Miller, W. Nicholson, H. Petersen-Jones, S. Roberts, D. Schroeder, and S. Wolhart for assistance.
Funding
National Institutes of Health Research Grants HL-119337 (M.J.J. and F.A.D.), HL-105467 (D.P. Casey), CTSA UL1 TR00013, and The Caywood Professorship via the Mayo Foundation.
Abbreviations:
- ATP
Adenosine Triphosphate
- ADO
Adenosine
- FAV
forearm volume
- FVC
forearm vascular conductance
- MVC
maximal voluntary contraction
Footnotes
Competing Interests
No conflicts of interest, financial or otherwise, are declared by the author(s). The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by ACSM.
References
- Andersen P, & Saltin B (1985). Maximal perfusion of skeletal muscle in man. J Physiol, 366, 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfeld GR, & Forrester T (1992). Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res, 26(1), 40–47. [DOI] [PubMed] [Google Scholar]
- Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, & Hughson RL (1998). Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol (1985), 85(6), 2249–2254. doi: 10.1152/jappl.1998.85.6.2249 [DOI] [PubMed] [Google Scholar]
- Burns WR, Cohen KD, & Jackson WF (2004). K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation, 11(3), 279–293. doi: 10.1080/10739680490425985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, & Joyner MJ (2011a). Contribution of adenosine to compensatory dilation in hypoperfused contracting human muscles is independent of nitric oxide. J Appl Physiol (1985), 110(5), 1181–1189. doi: 10.1152/japplphysiol.00836.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, & Joyner MJ (2011b). Prostaglandins do not contribute to the nitric oxide-mediated compensatory vasodilation in hypoperfused exercising muscle. Am J Physiol Heart Circ Physiol, 301(1), H261–268. doi: 10.1152/ajpheart.00222.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, & Joyner MJ (2012). Influence of alpha-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol (1985), 113(8), 1201–1212. doi: 10.1152/japplphysiol.00734.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, & Joyner MJ (2010). Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol, 588(Pt 2), 373–385. doi: 10.1113/jphysiol.2009.180489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DP, Walker BG, Ranadive SM, Taylor JL, & Joyner MJ (2013). Contribution of nitric oxide in the contraction-induced rapid vasodilation in young and older adults. J Appl Physiol (1985), 115(4), 446–455. doi: 10.1152/japplphysiol.00446.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS, & Hellsten Y (2004). Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol (1985), 97(1), 393–403. doi: 10.1152/japplphysiol.00179.2004 [DOI] [PubMed] [Google Scholar]
- Cohen J (2013). Statistical power analysis for the behavioral sciences: Routledge. [Google Scholar]
- Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, & Dinenno FA (2013). Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol, 305(1), H29–40. doi: 10.1152/ajpheart.00298.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Richards JC, & Dinenno FA (2013). Mechanical effects of muscle contraction increase intravascular ATP draining quiescent and active skeletal muscle in humans. J Appl Physiol (1985), 114(8), 1085–1093. doi: 10.1152/japplphysiol.01465.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey DS, Wang SS, & Shoemaker JK (2002). Evidence for sympatholysis at the onset of forearm exercise. J Appl Physiol (1985), 93(2), 555–560. doi: 10.1152/japplphysiol.00245.2002 [DOI] [PubMed] [Google Scholar]
- Dyke CK, Proctor DN, Dietz NM, & Joyner MJ (1995). Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J Physiol, 488 ( Pt 1), 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell WH (1877). The Changes of the Blood-stream in Muscles through Stimulation of their Nerves. J Anat Physiol, 11(Pt 3), 360–402.363. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J (2012). ATP as a mediator of erythrocyte-dependent regulation of skeletal muscle blood flow and oxygen delivery in humans. J Physiol, 590(20), 5001–5013. doi: 10.1113/jphysiol.2012.235002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, … Dufour SP (2008). Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol, 586(9), 2405–2417. doi: 10.1113/jphysiol.2008.152058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski RJ, Klitzman B, & Duling BR (1978). Interrelations between contracting striated muscle and precapillary microvessels. Am J Physiol, 235(5), H494–504. doi: 10.1152/ajpheart.1978.235.5.H494 [DOI] [PubMed] [Google Scholar]
- Hester RL, Guyton AC, & Barber BJ (1982). Reactive and exercise hyperemia during high levels of adenosine infusion. Am J Physiol, 243(2), H181–186. doi: 10.1152/ajpheart.1982.243.2.H181 [DOI] [PubMed] [Google Scholar]
- Jin CZ, Kim HS, Seo EY, Shin DH, Park KS, Chun YS, … Kim SJ(2011). Exercise training increases inwardly rectifying K(+) current and augments K(+)-mediated vasodilatation in deep femoral artery of rats. Cardiovasc Res, 91(1), 142–150. doi: 10.1093/cvr/cvr050 [DOI] [PubMed] [Google Scholar]
- Joyner MJ, & Casey DP (2015). Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev, 95(2), 549–601. doi: 10.1152/physrev.00035.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Richards JC, & Dinenno FA (2013). Sources of intravascular ATP during exercise in humans: critical role for skeletal muscle perfusion. Exp Physiol, 98(5), 988–998. doi: 10.1113/expphysiol.2012.071555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Carlson RE, & Dinenno FA (2008). Graded sympatholytic effect of exogenous ATP on postjunctional alpha-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol, 586(17), 4305–4316. doi: 10.1113/jphysiol.2008.154252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM, & Tandon HC (1984). Direct observations of muscle arterioles and venules following contraction of skeletal muscle fibres in the rat. J Physiol, 350, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, & Joyner MJ (2006). Bimodal distribution of vasodilator responsiveness to adenosine due to difference in nitric oxide contribution: implications for exercise hyperemia. J Appl Physiol (1985), 101(2), 492–499. doi: 10.1152/japplphysiol.00684.2005 [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, & Joyner MJ (2000). Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation, 101(8), 862–868. [DOI] [PubMed] [Google Scholar]
- Moore AW, Bearden SE, & Segal SS (2010). Regional activation of rapid onset vasodilatation in mouse skeletal muscle: regulation through alpha-adrenoreceptors. J Physiol, 588(Pt 17), 3321–3331. doi: 10.1113/jphysiol.2010.193672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, & Hellsten Y (2009). ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol, 296(4), R1140–1148. doi: 10.1152/ajpregu.90822.2008 [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, & Hellsten Y (2007). Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol, 581(Pt 2), 853–861. doi: 10.1113/jphysiol.2006.127423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JC, Crecelius AR, Kirby BS, Larson DG, & Dinenno FA (2012). Muscle contraction duration and fibre recruitment influence blood flow and oxygen consumption independent of contractile work during steady-state exercise in humans. Exp Physiol, 97(6), 750–761. doi: 10.1113/expphysiol.2011.062968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongen GA, Smits P, & Thien T (1994). Characterization of ATP-induced vasodilation in the human forearm vascular bed. Circulation, 90(4), 1891–1898. [DOI] [PubMed] [Google Scholar]
- Saltin B (2012). In search of a vasodilator: is ATP the answer? J Physiol, 590(21), 5261–5262. doi: 10.1113/jphysiol.2012.241661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Joyner MJ, & Dinenno FA (2004). Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol, 557(Pt 2), 599–611. doi: 10.1113/jphysiol.2004.061283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Wilkins BW, Johnson CP, Eisenach JH, Limberg JK, Dietz NM, … Joyner MJ (2010). Roles of nitric oxide synthase and cyclooxygenase in leg vasodilation and oxygen consumption during prolonged low-intensity exercise in untrained humans. J Appl Physiol (1985), 109(3), 768–777. doi: 10.1152/japplphysiol.00326.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JR, Joyner MJ, Dinenno FA, Curry TB, & Ranadive SM (2016). Prolonged adenosine triphosphate infusion and exercise hyperemia in humans. J Appl Physiol (1985), 121(3), 629–635. doi: 10.1152/japplphysiol.01034.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkler SY, & Segal SS (2017). Rapid versus slow ascending vasodilatation: intercellular conduction versus flow-mediated signalling with tetanic versus rhythmic muscle contractions. J Physiol, 595(23), 7149–7165. doi: 10.1113/JP275186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginneken EE, Meijer P, Verkaik N, Smits P, & Rongen GA (2004). ATP-induced vasodilation in human skeletal muscle. Br J Pharmacol, 141(5), 842–850. doi: 10.1038/sj.bjp.0705589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTeeffelen JW, & Segal SS (2006). Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol, 290(1), H119–127. doi: 10.1152/ajpheart.00197.2005 [DOI] [PubMed] [Google Scholar]


