Abstract
Mitochondrial dysfunction is involved in the pathology of two major blinding retinal diseases, Diabetic Retinopathy (DR) and Age-related Macular Degeneration (AMD). These diseases accumulate mitochondrial defects in distinct retinal subcellular structures, the vascular/neural network in DR and the retinal pigment epithelium in AMD. These mitochondrial defects cause a metabolic crisis that drives disease. With no treatments to stop these diseases, coupled with an increasing population suffering from AMD and DR, there is an urgent need to develop new therapeutics targeting the mitochondria to prevent or reverse disease-specific pathology.
Keywords: Diabetes, retinopathy, age-related macular degeneration, mitochondria
Cell-Specific Mitochondrial Defects Cause Distinct Diseases
Diabetic Retinopathy (DR) and Age-related Macular Degeneration (AMD), covering the age spectrum from working-age adults (DR) through the elderly (AMD), are responsible for the majority of patients with vision loss in the industrialized world [1,2]. The global prevalence of DR was approximately 126.6 million in 2010, and this number is expected to escalate to 191 million by 2030 [1]. For AMD, the global estimate for the number of people with AMD in 2020 is 196 million and will increase to 288 million by 2040 [2]. These blinding diseases affect different retinal cells and manifest vision changes that are distinct from each other.
Persistent, fluctuating hyperglycemia is the major cause of morbidity and mortality in diabetes, with the deleterious effects categorized into macrovascular (cardiovascular, cerebrovascular, and peripheral artery diseases) and microvascular (retinopathy, nephropathy, and neuropathy) complications [3]. DR is the most common microvascular complication of diabetes. The earliest clinical signs of DR (nonproliferative) are mainly micro-aneurysms and intra-retinal hemorrhages, which can lead to fluid accumulation in the macula [3]. Macular swelling that leads to blurred vision and is known as diabetic macular edema (DME), a complication of DR. In late stage DR (proliferative), the number and size of hemorrhages increase and cause disruptions in the visual field (Fig. 1). Hypoxia in the capillaries leads to neovascularization that can cause permanent vision loss without prompt treatment with either laser surgery or ocular injections of vascular endothelial growth factor (VEGF, see Glossary)
Figure 1. Disease-dependent Changes in Vision and Retinal Anatomy.
Examples of vision (left panels) in patients with normal vision (A), with diabetic retinopathy (DR) (B), and with age-related macular degeneration (AMD) (C). With normal vision an individual can easily recognize faces. DR causes visual blurring and black patches throughout the visual field. Vision loss with AMD includes distortion of horizontal and vertical lines (arrow) and a black spot (scotoma) in the central vision.
Schematic of the healthy eye (A) indicates structures associated with vision. Light passes through the cornea (C) and iris, and is focused by the lens on the back of the eye at the macula, a small, central retinal region responsible for high acuity central vision. Light is sensed by photoreceptors (PR) in the retina, and the signal is sent through the secondary neurons (bipolar, horizontal, and ganglion cells) to the optic nerve.
Clinical symptoms of DR (B) include hemorrhages, aneurysms (A), neovascularization (NV), and “cotton-wool” spots (CWS). Hemorrhaging from the inner retinal vessels (IV) of the neural retina is depicted.
AMD exists in a “dry” and “wet” form (C). The dry form is characterized by the presence of drusen, deposits of lipids and proteins that form between the RPE and Bruch’s membrane. Loss of RPE and photoreceptors (PR) occurs with advance dry AMD. In wet AMD, choroidal neovascularization (CNV), which is abnormal growth of blood vessels from the choroid into the retina, results in leakage of fluid that can cause rapid vision loss.
AMD includes two forms of the disease. The less common “wet” AMD involves the abnormal growth of blood vessels into the retina from the choroid, the outer retina blood supply (Fig. 1) [4]. Leakage of vascular fluid causes rapid vision loss that can be reversed by anti-VEGF ocular injections. Approximately 80% of AMD patients have the dry form of the disease, for which there are no effective therapies and will be the focus of this review. In contrast to DR, the site of primary defect in dry AMD occurs in the retinal pigment epithelium (RPE) (Fig. 1). The RPE supports the health and function of the retina, therefore RPE dysfunction precipitates the death of photoreceptors (PR), the light sensing neurons of the retina. Vision changes associated with AMD include distortion of vertical and horizontal lines and a scotoma, or black spot, in the area of central vision. The loss of central vision is due to the death of RPE and photoreceptors primarily in the macula, which is a small, centrally located region of the retina responsible for high-resolution and color vision.
Vision loss due to either DR or AMD has a significant negative impact on activities of daily living, including reading, driving, and recognizing faces. Thus, there is an urgent need to develop new therapies that can prevent or reverse cellular changes that cause disease-specific pathology. Recent evidence points to mitochondrial dysfunction in the microvasculature and retina in DR and the RPE in AMD as the newly emerging hypotheses for their pathologies and offers potential novel targets for intervention. This review will discuss the use of model systems to study DR and AMD, as well as the recent findings that support the hypothesis that mitochondrial defects drive both diseases. Finally, current and emerging strategies to treat disease will provide insight into future direction for improved therapies.
Model Systems to Study Degenerative Retinal Diseases
In humans, DR is a slow, progressive disease with characteristics that can be partially replicated in animal models and cultured cells. Development of DR in animal models is accomplished through genetic mutation or induction via laser or chemical damage, surgery, diet, or drugs [5]. These different models in a range of species, including mice, rats, cats, dogs, pigs, and non-human primates, exhibit many retinal changes that mimic the human disease. However, replicating specific aspects of DR pathology is dependent on the model system [See table 1 in 6]. DR pathology mainly includes damage to the microvasculature, but can also effect retinal neurons, as evidenced by changes in the electroretinogram, visual acuity, color sensitivity, and damage to photoreceptors [6,7,8]. Culture models of DR include the use of primary retinal endothelial cells and pericytes, retinal pigment epithelium, Muller cells, or ganglion cells cultured in high glucose. This review will focus on findings from a variety of tissues and cells, including human and mouse models. Most studies of DR discussed herein utilize streptozotocin-induced diabetic rats or mice, or isolated retinal cells in culture. A caveat of these model systems is their applicability to human disease. However, both human donors with established DR and these model systems exhibit similar mitochondrial defects. While no model replicates all aspects of DR, the advantage of model systems is that they allow investigations into specific mechanisms of the human disease.
The unique characteristic of AMD presents several challenges in selecting a model system to study this disease. AMD affects the macula, a retinal structure found only in primates. The age-dependent onset suggests aging is an important contributing factor that is difficult to replicate in more traditional cell culture and mouse models. Animal models for AMD have been developed by manipulating both genetic and environmental factors, however no animal model recapitulates all disease phenotypes [9,10]. The use of human donor tissue circumvents these challenges and provides the most accurate insight into AMD pathobiology. Thus, this review article will focus on information provided by human retinal tissue and primary RPE cell cultures comparing donors with and without AMD.
Altered Metabolism is a Shared Mechanism
Central to both diseases is the accumulation of reactive oxygen species (ROS)-induced damage (Fig 2). While our discussion focuses on the damaging effects of ROS overproduction on mitochondrial function, it is important to note ROS make positive contributions, for example in cell signaling. The overproduction and imbalance in ROS can have serious consequences on cellular signaling and other processes as discussed in Box 1.
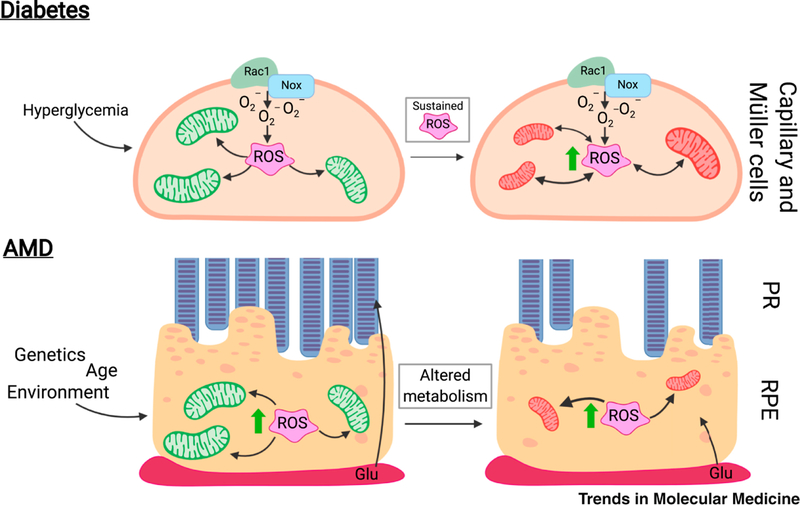
Figure 2. Metabolic changes drive DR and AMD.
A. Hyperglycemia is considered as the major causal factor in the development of diabetic retinopathy (DR). The hyperglycemic milieu activates Rac1-Nox2, generating the reactive oxygen species (ROS) superoxide (O2−). Overactive Rac1-Nox2, along with an impaired cytosolic antioxidant defense mechanism, leads to accumulation of cytosolic ROS. Right Panel: With diabetes progression, the sustained increase in cytosolic ROS damages mitochondrial membranes, proteins, and DNA, leading to an imbalance in mitochondrial dynamics. Impaired mtDNA transcription compromises the electron transport chain, decreasing mitochondrial function while stimulating production of mitochondrial ROS. Mitochondrial damage can stimulate apoptotic cell death, as observed in the vascular histopathology associated with DR.
B. The retinal pigment epithelium (RPE) supplies oxygen and glucose from the choroid (Ch) to the photoreceptors (PR). Age-related macular degeneration (AMD) disrupts this system. Advanced age, along with numerous genetic and environmental factors, is thought to contribute to the development of AMD. In early AMD, an imbalance in RPE reactive oxygen species (ROS) damages mitochondrial proteins, lipids, and DNA (mtDNA) and causes mitochondrial dysfunction. Right panel: Without fully functioning RPE mitochondria, RPE begin utilizing glucose to generate energy via glycolysis, thereby usurping this energy source away from the PR, which rely heavily on the glycolytic pathway. This alteration to retinal metabolism causes a bioenergetics crisis in the retina that ultimately leads to death of photoreceptors and RPE, a hallmark of advanced disease.
Text Box 1. Mitochondria and the “Jekyll and Hyde” of Producing Energy.
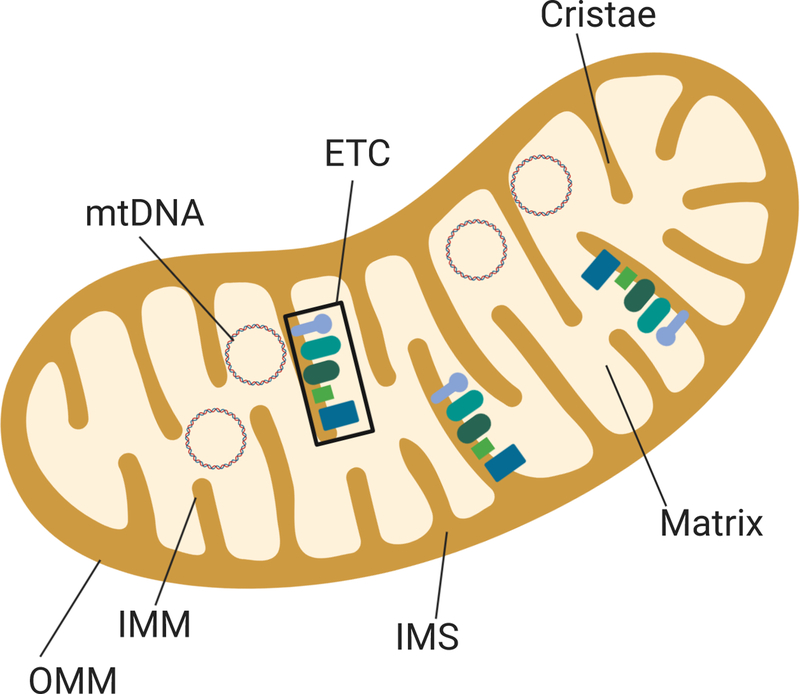
Mitochondria have two membranes (outer and inner mitochondrial membranes, OMM, IMM) that define functionally distinct regions of the inner mitochondrial space (IMS) and the matrix (M) (Fig. I, Box 1). The surface area of the IMM is increased by multiple invaginations, called cristae, where proteins of the electron transport chain (ETC) are embedded. The ETC consists of multi-subunit complexes (I to IV) that perform oxidative phosphorylation (OxPhos), the main pathway for ATP generation. OxPhos involves the reduction of molecular oxygen coupled to the generation of an electrochemical gradient across the IMM, driving the conversion of ADP to ATP by Complex V, also known as ATP Synthase. Under normal physiological conditions, approximately 2% of the total oxygen is incompletely reduced and forms superoxide radicals (O2−) [66]. These reactive oxygen species (ROS) are continuously formed in the mitochondria, with Complexes I and III identified as the major sites of ROS leakage [67]. ROS are important signaling molecules that allow for communication between the mitochondria and nucleus through numerous transcription factors regulated by ROS [67]. In addition to ROS, changes in mitochondrial membrane potential, the quantity of ATP, calcium, and unfolded proteins are other signals that initiate an adjustment in gene expression to accommodate changes in energy demands and cellular environment.
While ROS provide an important means of communication between the mitochondria and nucleus, overproduction of ROS can damage mtDNA, proteins, and lipids. mtDNA, located within the matrix, is particularly prone to ROS damage due to several factors, including its close proximity to the ETC, the site of ROS production. Unlike the nuclear genome, mtDNA are not protected from ROS-induced damage by histones. However, some protection is provided by mitochondrial transcription factor A (TFAM) and the nucleoid complexes of multiple proteins [68]. There is only limited DNA repair available in the mitochondria, so mtDNA damage can be long lasting and accumulate under conditions of increased oxidative stress, a hallmark of both diabetic retinopathy and AMD.
In summary, ATP generation produces ROS, which has both positive and negative consequences. The positive “Jekyll” effect includes its role as a critical signaling molecule. The negative “Hyde” aspect involves molecular damage due to ROS overproduction.
In diabetes, saturated glycolysis, increased flux through the polyol and hexosamine pathways, impaired protein kinase C activation, increased formation of advanced glycation end-products, and elevated oxidative stress are some of the biochemical changes contributing to DR pathology [11]. The accelerated production of the ROS superoxide is initiated by hyperglycemia -induced activation of Ras-related C3 botulinum toxin substrate 1 (Rac1) and NADPH oxidase 2 (Nox2), and by other metabolic abnormalities including activation of polyol pathway [12]. Importantly, Nox2 stimulation and a cytosolic increase in ROS precedes mitochondrial damage in the retinal capillaries [13,14] (Fig. 2a).
Another major source of ROS are mitochondria, which generate ROS as a byproduct of OxPhos. Under conditions of hyperglycemia and saturated glycolysis, the flux through OxPhos is increased. When the proton gradient reaches capacity, slower transfer of electrons through complex III of the electron transport chain (ETC) increases ROS generation [15]. (See Box 1 for in-depth discussion of mitochondrial architecture.) The increased concentration of mitochondrial ROS damages mitochondria, and combined with damage from cytosolic ROS, leads to mitochondrial dysfunction.
Hyperglycemia in many retinal cells, including microvascular cells, photoreceptors, and ganglion cells, is known to lead to ROS-induced mitochondrial dysfunction [16,17]. As reported in rodent retina and endothelial cells isolated from humans and bovine, mitochondria become swollen, their membrane potential is decreased, and membrane permeability is increased [17]. Damaged membranes allow cytochrome c to escape from the mitochondria into the cytosol, inducing apoptosis, a phenomenon that precedes the histopathologic development of DR. This process is the apparent link between mitochondrial oxidative stress, mitochondrial dysfunction, and DR pathology [18,19].
While hyperglycemia-induced metabolic changes play a major role in the development of DR, no single cause for AMD has been identified. Instead, AMD is a multi-factorial disease that is associated with at least 34 genetic loci and numerous environmental factors that place individuals at greater risk for the disease [20] (Fig. 2b). These genetic and environmental factors increase oxidative stress and inflammation, two stressors that are clearly linked to mitochondrial damage [21]. Recent studies investigating retinal metabolism have demonstrated the metabolic codependence of the RPE and retina [22,23,24,25,26]. Together, these studies have developed a new model for a “metabolic ecosystem” [24]. In the healthy eye, glucose from the choroidal circulation remains largely unused by the RPE and is transported to the photoreceptors. Photoreceptors and Mueller glia cells utilize glucose to generate ATP via glycolysis [24,25]. In contrast, RPE rely almost exclusively on the mitochondria, which generates energy via OxPhos using a number of substrates such as lipids, ketone bodies, and pyruvate to satisfy its energy requirements [22,23,26].
In AMD, mitochondrial damage and dysfunction disrupts this delicate metabolic ecosystem by causing RPE to rely on glucose and glycolysis to supply its energy requirements (Fig. 2b) [27]. With limited availability of glucose, the photoreceptors starve. RPE’s reliance on glycolysis eventually leads to death of both photoreceptors and RPE, a characteristic associated with late-stage AMD. This concept of a bioenergetic crisis provides a potential explanation for how reduced RPE mitochondrial function could have a global effect on multiple cells in the retina.
Mitochondria at the Nexus of Both Diseases
In addition to generating energy, mitochondria perform a number of other functions essential for cell survival such as calcium buffering and metabolism of cholesterol and iron. Importantly, mitochondria must maintain all of these processes while adjusting to changing cellular conditions. Mitochondria form dynamic and extensive cellular networks that maintain homeostasis through fusion, fission, mitophagy, and biogenesis (Fig. 3). Fission culls damaged fragments from the mitochondrial network, which are subsequently degraded through mitochondrial autophagy, coined mitophagy [28]. With fusion, healthy mitochondria merge and mix their lipids, proteins, and mitochondrial DNA (mtDNA) [28]. Biogenesis replenishes the mitochondrial pool by generating new mitochondrial membranes, proteins, and mtDNA through the coordinated effort of nuclear and mitochondrial DNA gene expression [29]. Epigenetic modifications also regulate nuclear and mitochondrial gene expression, influencing mitochondrial homeostasis (see Box 2). The process of mitochondrial homeostasis is a delicate balance, and disruptions to any of the pathways involved could lead to disease.
Figure 3. Overview of Mitochondrial Homeostasis.
Mitochondrial homeostasis is regulated through multiple steps that preserve a healthy network of mitochondria (green). Mitochondrial damage causes fragmentation (fission), regulated by the large GTPases dynamin-like protein 1 (Drp1) and dynamin 2 (Dnm2). Importantly, fission results in segregation of the damaged mitochondrial remnants (red). If not repaired, these remnants are isolated for degradation through mitochondrial autophagy (mitophagy). The mitochondria are encapsulated by a double membrane autophagosome (A) containing microtubule-associated proteins 1A/1B (LC3). These autophagosomes merge with a lysosome (L) where the acidic environment and lysosomal enzymes degrade and recycle the mitochondria. Healthy mitochondria continually fuse, mixing lipids, proteins, and mtDNA using mitofusin 1 and 2 (Mfn1 and Mfn2) for outer mitochondrial membrane fusion and Opa1 for inner mitochondrial membrane fusion. New components are added to expand and replenish mitochondrial networks through mitochondrial biogenesis, a process that involves both mitochondrial and nuclear transcription and translation, lipid synthesis, and replication of the mitochondrial genome.
Text Box 2. Epigenetic Changes Associated with Diabetes.
Gene expression can be regulated by external factors and disease states via epigenetic modifications, which includes DNA methylation and modifications such as acetylation and phosphorylation of histones. Epigenetic modifications can either be passed to the next generation or be erased, thus potentially making them good therapeutic targets [69].
In DR, many epigenetic modifications are implicated in dysregulation of mitochondrial homeostasis. Retinal microvasculature from diabetic rodents and human donors with established DR, and retinal endothelial cells cultured in high glucose, have hypermethylated DNA at the promoters for Mfn2, Mlh1 and POLG, affecting both structural and genomic stability of the mitochondria [17, 31, 70]. Dynamic DNA methylation is also implicated in the transcription of MMP-9, a mitochondrial damaging enzyme, and Rac1, an obligatory component of the Nox2 holoenzyme [14,17]. Increased import of the methyl transfer enzyme Dnmt1 into the mitochondria and subsequent hypermethylated mtDNA have been observed in experimental models of DR [30]. Furthermore, mtDNA is more extensively methylated at its D-Loop, the site of transcriptional regulation, compared to the other regions of the mtDNA [30]. Due to hypermethylation, transcription of mtDNA-encoded genes, including some subunits of the ETC, is impaired, and the compromised ETC feeds into the vicious cycle of ROS production and damage [15,71,72,73]. In DR, DNA methylation and base-mismatches show a positive correlation [73], suggesting a close relation between epigenetic modifications and mtDNA damage.
Histone modifications are also implicated in mitochondrial homeostasis. For example, increased methylation at H3K9 and H4K20 reduced transcription factor binding at the promoter for Sod2, a key mitochondrial antioxidant that is downregulated in DR [74]. Histone modifications are also associated with increased expression of the Nrf2 inhibitor, Kelch-like ECH-associated protein 1, thus impeding Nrf2 movement into the nucleus, and increasing oxidative stress [45]. Thus, the current evidence clearly suggests a major role of epigenetic modifications in the structural, functional and genomic stability of the mitochondria, and the processes of mitochondrial fusion and biogenesis [15,75]. Although the majority of diabetes-induced epigenetic modifications are reported in the retinal vasculature from diabetic rodents, human donors with established DR, and primary retinal endothelial cells cultured in high glucose, similar epigenetic modifications in other retinal cells and their role in the pathogenesis of DR remain to be investigated.
In summary, the diabetic environment favors epigenetic modifications in the retina and its vasculature, and these modifications affect many aspects of mitochondrial homeostasis. In contrast, no genome-wide methylation profiles or histone modifications have been published for AMD. Thus, this area of research is largely untouched and should be investigated.
Many facets involved in maintaining mitochondrial homeostasis are altered in diabetes. Direct evidence for impaired mitochondrial biogenesis in retinal endothelial cells and microvasculature from streptozotocin-induced diabetic rats and mice includes a significant decrease in mtDNA copy number [30]. Other changes include a 50% reduction in the mitochondrial DNA replication enzymes, DNA polymerase gamma and mitochondrial DNA helicase (Twinkle), which may be caused by the sustained increase in ROS associated with hyperglycemic conditions [30,31]. Additionally, the import of nuclear-encoded mtDNA replication enzyme polymerase gamma and the mitochondrial transcription factor, TFAM, inside the retinal mitochondria is significantly reduced by 35–45% [31,32].
Fission and fusion are also affected as evidenced by ~50% increase in Drp1 (fission) protein and a similar decrease in Mfn2 (fusion) content in the retina and its vasculature from streptozotocin-induced diabetic rats, and from human donors with established DR [17]. Decreased Mfn2 affects not only mitochondrial morphology, but also causes a loss in OxPhos function [33]. Mitophagy is increased under high glucose conditions in cultured RPE and Mueller cell lines, as observed by the co-localization of fragmented mitochondria with the autophagosome and lysosomal markers [33,34]. However, in cultured retinal capillary pericytes, the effect of accelerated autophagy is dependent on the stage of the disease. Autophagy induction under mild stress induced by low concentrations of heavily-oxidized low density lipoproteins is protective [35]. In contrast, conditions of severe stress induced by high concentrations of oxidized lipoproteins upregulates autophagy and promotes cell death, suggesting that autophagy could be cytoprotective for vascular cells in the early stages of DR [35]. No matter the mechanism, mitochondrial damage and dysfunctional mitochondrial homeostasis (summarized in Table 1) occupies a central role in the pathogenesis of DR.
Table 1.
Summary of Mitochondrial Changes in Diabetic Retinopathy and Age-related Macular Degeneration
| Disease | Change | Model System | Ref |
|---|---|---|---|
| Diabetic Retinopathy | Decreased Mitochondrial content | Rodents, Primary RECs | 19, 30 |
| mitochondrial morphology (Cristae and fragmentation) | Rodents, Primary RECs, Muller cells | 19, 74, 76, 78 | |
| Increased mtDNA damage | Rodents, Human donors with DR, Primary RECs | 31, 44, 45 | |
| Decreased Mitochondrial function | Rodents, Primary RECs, Muller cells | 8, 18, 76 | |
| Mitochondrial membrane damage, transporters and protein transport | Rodents, Primary RECs, | 45, 74, 77 | |
| ETC components | Rodents, Primary RECs, | 44, 79 | |
| Fusion-Fission, Autophagy, Biogenesis | Rodents, Primary RECs, Muller cells | 17, 19, 30, 3, 74 | |
| Mitochondrial superoxide scavengers | Rodents, Primary RECs | 18, 19 | |
| Epigenetic modification of mtDNA | Rodents, Human donors with DR, Primary RECs | 72, 73 | |
| Age-related Macular Degeneration | Decreased Mitochondrial Content | hRPE-T | 36 |
| Decreased Mitochondrial Surface Area | hRPE-T, Primary hRPE | 36, 41 | |
| Mitochondrial Morphology(Cristae) | hRPE-T, Primary hRPE | 36, 41 | |
| Increased mtDNA Damage | hRPE-T | 39, 47 | |
| Decreased Mitochondrial Function | Primary hRPE | 41, 42 | |
| Mitochondrial protein trafficking and refolding, apoptosis | hRPE-T | 37 | |
| ETC components, cristae morphology, protein import | hRPE-T | 38 | |
| Metabolism, chaperones, protein homeostasis | hRPE-T | 80 | |
| Regulators of metabolism, oxidative stress response | Primary hRPE | 42 | |
| Autophagy | Primary hRPE | 41 | |
| Altered response to drugs | Primary hRPE | 47 | |
Abbreviations: Primary retinal endothelial cells (Primary RECs); Primary Human RPE Cell Culture (Primary hRPE); Human RPE Tissue (hRPE-T).
RPE from AMD human donor tissue also show mitochondrial damage potentially due to disrupted mitochondrial homeostasis. Data that support diminished mitochondrial biogenesis includes a decrease in mitochondrial number in RPE from AMD compared with age-matched donors [36], and lower content of proteins in the ETC, such as a ~55% decrease in subunits of Complex IV and V [37,38]. A 60% reduction in mitochondrial heat shock protein 70, a protein involved in the import of nuclear encoded proteins that reside in the mitochondria, has also been reported [37,38]. Protein import is integral to mitochondrial biogenesis since the vast majority of mitochondrial proteins are nuclear-encoded, produced in the cytosol, and imported into the mitochondria.
Evidence for defects in the isolation and removal of damaged mitochondria include a significant increase of mtDNA damage in the macula of human donor RPE [39,40]. Additional evidence for the accumulation of damaged mitochondria includes the observed disruption and disorganization in mitochondrial cristae (see Box 1) in electron micrographs from AMD donor RPE [36], and the increased content of mitofilin at an early stage of AMD [38]. Mitofilin is involved in stabilizing cristae, so its upregulation could be a compensatory response to altered mitochondrial remodeling [38]. The removal of damaged mitochondria through mitophagy may be impaired due to the significant decrease in cellular autophagy in cultured RPE from AMD donors [41]. While mitophagy has not been directly measured, this overall decrease in autophagy would lead to decreased mitophagy as well.
Data from primary RPE culures have shown that the accumulation of damaged mitochondria have functional consequences on metabolism. Two laboratories reported decreased mitochondrial function in cultured RPE from AMD donors [41,42]. Taken together, these results show that mitochondrial damage occurs in the RPE of AMD donors (summary in Table 1) and support the notion that RPE mitochondrial dysfunction contributes to AMD pathology. Importantly, failures in any pathways of mitochondrial homeostasis could lead to the mitochondrial damage and dysfunction observed in RPE with AMD. This accumulation of dysfunctional mitochondria eventually leads to RPE death, the hallmark of dry AMD.
Mitochondrial Genomic Stability and Damage
Mitochondria contain their own 16.5 kb double-stranded, circular DNA. This genome encodes 13 proteins, including essential components of the ETC [43]. The remaining genome encodes for the machinery (16S and 12S ribosomal RNAs, 22 transfer RNAs) required to produce the 13 mitochondrial encoded proteins. Additionally, there is a non-coding region containing the Displacement loop (D-loop) that regulates mtDNA replication and translation. (For a more extensive discussion of mtDNA, see Box 1.)
With diabetes, mitochondrial DNA damage includes an increase in oxidatively modified guanine bases (8-OHdG) and sequence variants in retinal microvessels and endothelial cells in culture [44,45]. Although the entire mitochondrial genome is damaged, the non-coding D-Loop is the most extensively damaged region [15]. The effect of this damage is further amplified by impaired machinery responsible for repairing the damaged mtDNA. A reduction in both the base excision repair enzyme, 8-oxoguanine DNA glycosylase (Ogg1), and the mismatch repair enzyme MutLhomolog 1 (Mlh1), have been reported in endothelial cells and microvessels [44,45,46]. As a consequence of the increased mtDNA damage and suboptimal DNA repair systems the transcription of mtDNA-encoded genes is decreased, further compromising the already damaged ETC system [44,45]. This mitochondrial damage and decreased transcription induces a vicious cycle of free radical production that eventually leads to the capillary cell death observed in DR.
With AMD, there are numerous reports of elevated mtDNA damage in the RPE from both human donor tissue as well as in primary RPE cultures. In the RPE from donors with AMD, mtDNA damage increased ~350% compared to RPE from age-matched controls [39,47]. This damage was localized to specific regions of the genome, including the regulatory D-loop and in coding regions for subunits of Complex I, Complex IV, and Complex V [47]. Consistent with findings in donor tissue, analysis of primary cultures of RPE cells reported increased mtDNA damage and 5-fold increase in somatic mutations in RPE cultures from AMD donors compared with age-matched controls [40].
Comparison of the type and extent of mtDNA damage in each disease is limited due to the different assays used. However, both find damage localized to the D-Loop, the regulatory region of mtDNA. This damage would lead to decreased replication and transcription, thereby affecting production of new mitochondria and nascent mitochondrial encoded proteins, thereby negatively impacting mitochondrial homeostasis.
Current Treatments and Novel Strategies
Standard of Care
The complex etiology of DR has allowed only limited therapeutic advances. Therefore, tight glycemic control and laser photocoagulation remain the conventional management options for controlling its progression. Insulin not only helps regulated blood glucose levels, but may have a positive effect on mitochondrial function since it has been shown to maintain cardiac mitochondrial homeostasis in streptozotocin-induced diabetic rats [48]. Maintenance of glycemic control for long durations becomes challenging for some diabetic patients, and laser photocoagulation has the possibility of unwanted damage to the retina and loss of central vision. Although intravitreal anti-VEGF administration is routinely used for DME, its use for non-proliferative DR still requires further investigation [49]. The limitation of these treatments is that they only treat disease symptoms rather than targeting the pathological mechanism. Thus, there is an urgent need to identify novel therapies targeting the mechanism that could be used to treat patients prior to development of DR.
Currently, the only treatment available for dry AMD is a supplement of antioxidants plus zinc, identified by the Age-related Eye Disease Study (AREDS), which slowed AMD progression [50]. However, only ~20% of patients with intermediate AMD had a positive response to the AREDS formulation, so additional treatments that affect a larger patient population is needed. Several clinical trials have tested new drugs that target specific pathways or mechanisms linked to AMD, including complement, inflammation, neuroprotection, and oxidative stress [51]. For example, drugs targeting different points in the three pathways (Classical, Lectin, and Alternative) that make up the complement cascade capitalize on blocking the formation of the membrane attack complex, which can cause cell lysis and release cytokines that trigger the recruitment of inflammatory cells. One of the early successes for inhibiting the complement system in the retina was Lampalizumab, an antibody that inhibits Complement Factor D, the final step in the alternative pathway. The outcome of the phase 2 multicenter, randomized sham-controlled trial () I noted a significant 20% reduction in mean growth of geographic atrophy lesions, which are retinal areas where RPE have died. Publication of these encouraging results [52] prompted initiation of two identically designed phase 3 trials (, ) II conducted at 275 sites in 23 countries. The outcome of the double-masked, sham-controlled trials with 1733 participants found no significant benefit with lampalizumab versus sham controls over 48 weeks of treatment [53]. These trials were the most comprehensive studies of geographic atrophy to date and while their results did not support lampalizumab’s efficacy, they did provide a wealth of information about the natural history of AMD in a diverse population with geographic atrophy.
While several additional drugs targeting specific molecules in the complement cascade are currently in clinical trials (see 51 for summary), two on-going phase 3 clinical trials ( and )III are worth noting. These trials are testing the efficacy and safety of intravitreal injections of the drug APL-2, a cyclic peptide that inhibits C3. This protein is located downstream of Complement Factor D at the convergence of the three pathways within the complement system. As APL-2 affects C3, which is involved in all complement pathways, it may be more effective than lampalizumab, which inhibits one arm of the complement cascade. The multi-center phase 3 trials are randomized, double-masked, sham-controlled studies of subjects with advanced dry AMD that will use growth of geographic atrophy lesions as the primary outcome. These phase 3 trials are still recruiting to meet their target of 600 subjects, with an expected completion date of November, 2022.
There are a number of challenges associated with conducting AMD clinical trials that likely contribute to the limited success observed to date. For example, due to slow disease progression, significant changes in outcomes (i.e., size of atrophic lesion) require lengthy clinical trials that are cost-prohibitive. Additionally, most trials randomly assign patients to treatment or control groups without consideration of their genetic background or environmental factors that could influence the results. Due to constraints placed by the FDA, most clinical trials are treating patients with late stage disease when the ability to “rescue” the retina is very limited. The mode of drug delivery (oral, intravitreal, subcutaneous, or intravenous injections) could also influence the outcome due to challenges associated with the blood/retina barrier and in maintaining an optimal dose that targets the correct cell. It is also important to note that most clinical trials may not be targeting the correct molecular pathway. Therefore, a greater understanding of disease mechanism is required in order to develop better therapies that target the primary defect in the retina.
Emerging Strategies that Target Mitochondria
Based on the central role of mitochondria in DR and in AMD, strategies targeting mitochondrial homeostasis have great potential. For example, the tetrapeptide SS-31, which targets mitochondrial cardiolipin, was effective in reversing visual decline in a streptozotocin-induced mouse model of diabetes [54]. Cardiolipin is a lipid found in the mitochondrial inner membrane and is crucial for maintaining OxPhos function [55]. Since this drug promotes proper mitochondrial function, SS-31 has also been used to treat diseases involving mitochondrial pathology [56]. SS-31 (commercial name Elamipretide) is currently in a phase 2 randomized, double-masked, placebo-controlled clinical trial to evaluate safety, efficacy, and the pharmacokinetics in subjects with dry AMD ()IV. This clinical trial is still recruiting subjects, with a target of 180 patients, and will use best-corrected visual acuity under low light conditions as the primary outcome.
In addition to chemical modulators of the mitochondria, far-red to near-infrared light (590–850 nm), referred to as photobiomodulation, has also demonstrated beneficial effects in multiple retinal degenerative models and patients with DR or AMD [11,57]. Photobiomodulation targets mitochondrial cytochrome oxidase C, a protein that modulates the transfer of electrons between ETC complexes, increasing mitochondrial membrane potential and ATP synthesis [57]. In streptozotocin-diabetic mice, treatment with 670 nm light inhibited the diabetes-induced leakage of retinal capillaries and reduction in vision [58]. In aged mice and an AMD mouse model with a genetic disruption of complement factor H, photobiomodulation significantly increased retinal mitochondrial function and reduced signs of inflammation [59,60]. These studies provide insight into the molecular basis for the beneficial effects of photobiomodulation.
Clinical studies have also reported positive results after photobiomodulation for patients with DR. In a non-randomized, consecutive, case study of four type 2 diabetic patients with DME, photobiomodulation treatment caused a significant reduction in macular thickness [61]. A pilot study evaluating photobiomodulation therapy for DME, initiated in 2019, will evaluate macular thickness, mean change in retinal volume, and visual acuity from baseline to four months of treatment ()V. This randomized, sham-controlled, quadruple-masked trial anticipates 134 participants.
Multiple clinical studies treating AMD patients with photobiomodulation have also produced promising results. In a prospective intervention study with 21 dry AMD patients, photobiomodulation significantly improved vision (best-corrected visual acuity and contrast sensitivity) and reduced drusen volume [62]. Based on these positive outcomes, a pilot study with 30 participants was conducted and importantly, this study design included double-masked, randomized patient assignment to either treatment or sham control groups ()VI. Outcomes were visual acuity, contrast sensitivity, and retina structural analysis measured by Optical Coherence Tomography. Published results reported a statistically significant improvement in visual acuity, contrast sensitivity, and a reduction in drusen volume [63]. As a follow up from this first study, a second multi-center clinical trial based in Europe is currently enrolling dry AMD patients ()VII. This double-masked, randomized, sham-controlled, study has an anticipated completion date of December, 2020.
The antidiabetic drug metformin has also demonstrated promise in improving mitochondrial function. Systemic treatment of mice with metformin was effective in increasing mitochondrial biogenesis and improving mitochondrial health [64]. The mechanism may be due to metformin’s activation of adenosine monophosphate-activating protein kinase (AMPK), which in turn activates PGC-1α, the regulator of mitochondrial biogenesis. The link between improved mitochondrial health and reduced risk for AMD was recently reported in a retrospective, case-controlled study that used univariate conditional multivariate logistic regression to examine the association between metformin use and AMD diagnosis in over 7000 patients. The significant odds ratio of 0.58 shows diabetic patients taking metformin had significantly decreased risk for developing AMD [65].
These early studies and clinical trials show promising effects by targeting the mitochondria. SS-31 and photobiomodulation interact with the ETC and improve bioenergetics. Metformin has many targets, and therefore its beneficial effect on bioenergetics is less clearly defined. As early intervention strategies, these treatments should have minimal side effects and demonstrate long term benefits. Important optimization must be conducted once their efficacy is established, including timing, dose, and method of therapeutic delivery.
Concluding Remarks
Mitochondrial dysfunction drives disease pathology in two distinct retinal diseases affecting unique cell types (Fig. 2). While recent advances highlight the importance of mitochondrial homeostasis in disease mechanism, several outstanding questions and key issues remain to be investigated (see Outstanding Questions). For example, even though mitochondrial damage is observed in specific cells (e.g., vasculature in DR and RPE in AMD) the entire neural retina is ultimately affected as the disease progresses. It is unclear how metabolic defects in a single cell can affect the health of adjacent cells. One of the challenges is the complexity of the retina, which consists of eight distinct cell types, including neurons (PR, horizontal, bipolar, ganglion), glia (Mueller, microglia, astrocytes), and RPE. An additional layer of complexity comes from region specific differences in distribution and multiple subpopulations of these cell types. For example, the macula has a high density of cone photoreceptors, with the rest of the retina containing primarily rod photoreceptors. To thoroughly understand effects of the metabolic defect on each cell type within the retina, single cell analysis is required to grant insight into how specific retinal populations are affected.
OUTSTANDING QUESTIONS.
Why is retinal microvascular mitochondrial damage not an early event in the pathogenesis of DR? In the initial stages of DR, mitophagy may efficiently remove the damaged mitochondria. But, with time, the mitophagy machinery becomes exhausted, and damaged mitochondria accumulate. A systematic temporal relationship between mitochondrial mitophagy, biogenesis, mtDNA damage and DNA repair machinery could help unravel this mystery.
With DR, what cell types exhibit mitochondrial damage and when does it occur? We need new models and techniques to decipher cell-specific changes with mitochondrial damage and dysfunction. A better understanding of epigenetic regulation of mitochondrial homeostasis would shine light on this question.
Why is there preferential death of macular RPE and photoreceptors when both clinical and biochemical evidence shows that the detrimental effect of AMD impacts the entire retina? A potential mechanism involves the higher bioenergetic demand of the macula and metabolic uncoupling of the retinal ecosystem with AMD.
With AMD, why is mitochondrial damage limited to the RPE? The answer may involve cell-specific differences in metabolism. In the healthy retina, RPE rely almost exclusively on mitochondria to generate energy, whereas the photoreceptors depend on glycolysis.
What drugs will provide the greatest protection of mitochondrial function? It is important that new and current drugs are investigated for their effect on mitochondria homeostasis and function in both DR and AMD. It is important to test these drugs in the most appropriate model system and cell type.
Fortunately, with technical advances, our understanding of the molecular mechanism(s) responsible for imbalanced mitochondrial homeostasis in DR and AMD is improving. Major effort needs to be placed in developing therapies that target the mitochondria and are administered early in disease, so that irreversible cellular damage can be prevented. Currently, new mitochondria-targeted molecules are being generated, and advanced strategies for sustained drug delivery to the back of the eye are now becoming a reality. Thus, the future for preventing vision loss in patients with DR or AMD looks optimistic.
CLINICIAN’S CORNER.
Diabetes is a chronic disease that can lead to diabetic retinopathy (DR), the leading cause of blindness in working age adults. Its effective management depends heavily on early diagnosis, and maintenance of tight glycemic control, which becomes difficult or impossible for many patients.
Current options to prevent progression of diabetic retinopathy remain focal laser and pan-retinal laser photocoagulation, both carrying risks of visual field deficits and exudative retinal detachments.
Age-related macular degeneration (AMD) is the leading cause of blindness in the elderly, manifesting in loss of central vision and blurring of horizontal and vertical lines, due to cell death in the macula.
Age-related macular degeneration (AMD) includes two forms of the disease, “wet” and “dry”. While wet AMD is treated with ocular injections of anti-VEGF, there are no effective treatments for dry AMD.
As both DR and AMD develop due to mitochondrial damage and dysfunction, new therapies that target the mitochondria may be a viable option.
HIGHLIGHTS.
In diabetic retinopathy (DR), cytosolic ROS accumulation in retinal vasculature occurs before mitochondrial damage, and mitochondrial damage precedes capillary cell loss.
In DR, mitochondrial fission is increased and mitochondrial DNA has increased damage, lower copy numbers, and is hypermethylated.
The retina and retinal pigment epithelium (RPE) are co-dependent tissues that exchange energy substrate required for their survival.
RPE mitochondrial damage occurs early in age-related macular degeneration (AMD) and leads to a bioenergetic crisis due to an imbalance in available energy substrates.
Therapeutic strategies targeted towards maintaining mitochondrial homeostasis (structural, functional and genomic) have the potential to prevent the development and progression of both DR and AMD.
Acknowledgements:
We acknowledge the contributions of laboratory associates (past and present) to the work cited in this review article, Nikhil S. Sahajpal for his help in preparing the initial draft, Jorge Polanco for help with developing figures, and Parvathy Hariharan for editorial assistance.
Funding: (National Institutes of Health (T32 AG029796), VitroRetinal Surgery Foundation Fellowship to CF); (National Institutes of Health (NEI EY028554 and EY 026012), Elaine and Robert Larson Endowed Research Chair, Anonymous Benefactor for Macular Degeneration Research, Lindsay Family Foundation to DAF), (National Institutes of Health (EY014370, EY017313 and EY022230) and from The Thomas Foundation to RK), and an unrestricted grant from Research to Prevent Blindness to the WSU’s Ophthalmology Department.
Glossary
- Cristae
folds of the inner mitochondrial membrane that increase surface area, allowing for efficient packing of the ETC and isolated generation of proton gradients to fuel ATP generation.
- Displacement loop (D-Loop)
a non-coding region of the mitochondrial DNA and the initiation site of mtDNA replication.
- Electron Transport Chain (ETC)
consists of multi-subunit complexes contained within the mitochondrial inner membrane. Complexes I, II, III, and IV consume NADH and FADH2 to generate a proton gradient. This proton gradient is utilized by Complex V to produce ATP.
- Macula
a small pea-sized region adjacent to the optic nerve where light is focused. Responsible for high acuity central vision.
- Nicotinamide adenine dinucleotide (phosphate) (NAD(P)H) oxidase 2 (Nox2)
A cytosolic, membrane bound, enzyme that generates reactive oxygen species.
- Oxidative Phosphorylation (OxPhos)
process of producing ATP via the mitochondrial ETC, involving the transfer of electrons from NADH or FADH2 to oxygen.
- Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α)
regulates genes involved in energy metabolism. Importantly, this transcriptional coactivator is known as the master regulator of mitochondrial biogenesis.
- Photoreceptors (PR)
the light sensing neurons of the retina consisting of two main subpopulations. Cones are responsible for color vision while rods are responsible for vision under low light conditions.
- Ras-related C3 botulinum toxin substrate 1 (Rac1)
small G-protein, required for the assembly of Nox2 holoenzyme.
- Reactive oxygen species (ROS)
include any chemically reactive species containing oxygen, including peroxide, superoxide, and hydroxyl radicals. ROS are natural byproducts of the metabolism of oxygen and act as signaling molecules within the cell. However, ROS are able to damage cell structures, including lipids, protein, and DNA.
- Retina
The light-sensitive layer of the eye, comprised of neurons, glia, and RPE that converts light signals into electrical neural impulses.
- Retinal pigment epithelium (RPE)
perform multiple functions to maintain health of the retina. Including acting as the blood-retina barrier and transporting essential nutrients and oxygen from the choroid.
- Vascular endothelial growth factor (VEGF)
a signaling molecule that stimulates the formation of blood vessels.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: Authors have no ongoing commercial relationships. DAF has received past research funds from Stealth BioTherapeutics
Resources:
, The MAHALO phase 2 clinical trial is registered with Clinicaltrials.gov. (https://clinicaltrials.gov/ct2/show/NCT01602120)
and , The CHROMA and SPECTRI phase 3 clinical trials are registered with Clinicaltrials.gov. (https://clinicaltrials.gov/ct2/show/NCT02247531) (https://clinicaltrials.gov/ct2/show/NCT02247479)
and , The Oaks and Derby phase 3 clinical trials are registered with Clinicaltrials.gov. (https://clinicaltrials.gov/ct2/show/NCT03525613)
, The ReCLAIM-2 study is registered with Clinical Trials.gov. (https://clinicaltrials.gov/ct2/show/NCT03891875)
, This study is registered with Clinical Trials.gov (https://clinicaltrials.gov/ct2/show/NCT03866473)
, the LIGHTSITE I study is registered with Clinicaltrials.gov. (https://clinicaltrials.gov/ct2/show/NCT02725762)
, the LIGHTSITE II study is registered with Clinical Trials.gov. (https://clinicaltrials.gov/ct2/show/NCT03878420)
References
- 1.Zheng Y et al. (2012) The worldwide epidemic of diabetic retinopathy. Indian J Ophthalm 60, 428–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong WL et al. (2014) Global prevalence of age-related macular degeneration and disease burden projections for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014 2, e106–16 [DOI] [PubMed] [Google Scholar]
- 3.Kusuhara S et al. (2018) Pathophysiology of diabetic retinopathy: the old and the new. Diabetes Metab 42, 364–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Layana A et al. (2017) Early and intermediate age-related macular degeneration: update and clinical review. Clin Interv Aging 12, 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olivares AM et al. (2017) Animal models of diabetic retinopathy. Curr Diab Rep 17, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim MK et al. (2018) Dopamine deficiency mediates early rod-driven inner retinal dysfunction in diabetic mice. Invest Ophthalmol Vis Sci 59, 572–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin T et al. (2019) Loss of XBP1 leads to early-onset retinal neurodegeneration in a mouse model of type I diabetes. J Clin Med 8, pii:E906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du Y et al. (2013) Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci 110, 16586–16591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landowski M et al. (2019) Human complement factor H Y402H polymorphism causes an age-related macular degeneration phenotype and lipoprotein dysregulation in mice. PNAS 116, 3703–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujihara M et al. (2008) Chronic cigarette smoke causes oxidative damage and apoptosis to retinal pigmented epithelial cells in mice. PLoS One 3, e3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy S et al. (2017) Mechanistic insights into pathological changes in diabetic retina: implications for targeting diabetic retinopathy. Am J Pathology 187, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowluru RA et al. (2014) TIAM1-RAC1 signaling axis-mediated activation of NADPH oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy. Diabetologia 57, 1047–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar B et al. (2015) Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 56, 2985–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duraisamy AJ et al. (2018) Epigenetics and regulation of oxidative stress in diabetic retinopathy. Invest Ophthalmol Vis Sci 59, 4831–4830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowluru RA and Mishra M (2015) Oxidative stress, mitochondrial damage and diabetic retinopathy, Biochim Biophys Acta 1852, 2474–2483 [DOI] [PubMed] [Google Scholar]
- 16.Mishra M and Kowluru RA (2017) Role of PARP-1 as a novel transcriptional regulator of MMP-9 in diabetic retinopathy. Biochim Biophys Acta 1863, 1761–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duraisamy AJ et al. (2019) Mitochondrial fusion and maintenance of mitochondrial homeostasis in diabetic retinopathy. Biochim Biophys Acta 1865, 1617–1626 [DOI] [PubMed] [Google Scholar]
- 18.Kanwar M et al. (2007) Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophtahlmol Vis Sci 48, 3805–3811 [DOI] [PubMed] [Google Scholar]
- 19.Santos JM et al. (2011) Mitochondrial biogenesis and the development of diabetic retinopathy. Free Radical Biol Med 51, 1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritsche LG et al. (2016) A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet 48, 134–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grazioli S and Pugin J (2018) Mitochondrial damage-associated molecular patterns: from inflammatory signaling to human diseases. Front Immunol 9, 832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao JR et al. (2017) Human retinal pigment epithelial cells prefer proline as a nutrient and transport metabolic intermediates to the retinal side. J Biol Chem 292,12895–12905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du J et al. (2016) Reductive carboxylation is a major metabolic pathway in the retinal pigment epithelium. Proc Natl Acad Sci U.S.A 113, 14710–14715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurley JB et al. (2015) Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J Neurosci Res 93, 1079–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanow MA et al. (2017) Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. Elife 6, Pii:e28899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes-Reveles J et al. (2017) Phagocytosis-dependent ketogenesis in retinal pigment epithelium. J Biol Chem 292, 8038–8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher CR and Ferrington DA (2018) Perspective on AMD pathobiology: a bioenergetics crisis in the RPE. Invest Ophthalmol Vis Sci 59, AMD41–AMD47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra P and Chan DC (2016) Metabolic regulation of mitochondrial dynamics. J Cell Biol 212, 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ploumi C et al. (2017) Mitochondrial biogenesis and clearance: a balancing act. FEBS J 284, 183–195 [DOI] [PubMed] [Google Scholar]
- 30.Tewari S et al. (2012) Damaged mitochondrial DNA replication system and the development of diabetic retinopathy. Antiox Redox Signal 17, 492–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tewari S et al. (2012) Regulation of mitochondrial DNA damage and POLG in diabetic retinopathy. Invest Ophthalmol Vis Sci 53, 5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos JM and Kowluru RA (2013) Impaired transport of mitochondrial transcription factor A (TFAM) and the metabolic memory phenomenon associated with the progression of diabetic retinopathy. Diabetes Metab Res Rev 29, 204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devi TS et al. (2019) TXNIP mediates high glucose-induced mitophagic flux and lysosome enlargement in human retinal pigment epithelial cells. Biology open 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devi TS et al. (2017) TXNIP regulates mitophagy in retinal Muller cells under high-glucose conditions: implications for diabetic retinopathy. Cell Death Dis e2777. [DOI] [PMC free article] [PubMed]
- 35.Fu D et al. (2016) Survival or death: a dual role for autophagy in stress-induced pericyte loss in diabetic retinopathy. Diabetologia 59, 2251–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feher J et al. (2006) Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging 27, 983–993 [DOI] [PubMed] [Google Scholar]
- 37.Nordgaard CL et al. (2006) Proteomics of the retinal pigment epithelium reveals altered protein expression at progressive stages of age-related macular degeneration. Invest Ophthalmol Vis Sci 47, 815–822. [DOI] [PubMed] [Google Scholar]
- 38.Nordgaard CL et al. (2008) Mitochondrial proteomics of the retinal pigment epithelium at progressive stages of age-related macular degeneration. Invest Ophthalmol Vis Sci 49, 2848–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karunadharma PP et al. (2010) Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Invest Ophthalmol Vis Sci 51, 5470–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin H et al. (2011) Mitochondrial DNA damage and repair in RPE associated with aging and age-related macular degeneration. Invest Ophthalmol Vis Sci 52, 3521–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golestaneh N et al. (2017) Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis 8, e2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrington DA et al. (2017) Altered bioenergetics and enhanced resistance to oxidative stress in human retinal pigment epithelial cells from donors with age-related macular degeneration. Redox Biol 13, 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson S et al. (1981) Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 [DOI] [PubMed] [Google Scholar]
- 44.Madsen-Bouterse SA et al. (2010) Oxidative damage of mitochondrial DNA in diabetes and its protection by manganese superoxide dismutase. Free Rad Biol Med 44, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra M and Kowluru RA (2014) Retinal mitochondrial DNA mismatch repair in the development of diabetic retinopathy, and its continued progression after termination of hyperglycemia. Invest Ophtahlmol Vis Sci 55, 6960–6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohammad G et al. (2019) Epigenetic modifications compromise mitochondrial DNA quality control in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 60, 3943–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terluk MR et al. (2015) Investigating mitochondria as a target for treating age-related macular degeneration. J Neurosci 35, 7304–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Remor AP et al. (2011) Differential effects of insulin on peripheral diabetes-related changes in mitochondrial bioenergetics: involvement of advanced glycosylated end products. Biochim Biophys Acta 1812, 1460–1471 [DOI] [PubMed] [Google Scholar]
- 49.Karst SJ, et al. (2018) Association of Changes in Macular Perfusion with Ranibizumab Treatment for Diabetic Macular Edema: A Subanalysis of the RESTORE (Extension) Study. JAMA Ophthalmol 136, 315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Age-relaed Eye Disease Study Research Group. (2001) A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. AREDS report no 8. Arch Ophthalmol 119, 1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nebbioso M (2019) Therapeutic approaches with intravitreal injections in geographic atrophy secondary to age-related macular degeneration: current drugs and potential molecules. Int J Mol Sci 20, 1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yaspan BL et al. (2017) Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med 9 [DOI] [PubMed] [Google Scholar]
- 53.Holtz FG et al. (2018) Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri phase 3 randomized clinical trials. JAMA Ophthalmol 136, 666–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alam NM et al. (2015) A mitochondrial therapeutic reverses visual decline in mouse models of diabetes. Dis Model Mech 8, 701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson NC (1993) Functional binding of cardiolipin to cytochrome c oxidase. J Bioenerg Biomembr 2, 153–163 [DOI] [PubMed] [Google Scholar]
- 56.Birk AV et al. (2014) Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol 8, 2017–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ao J et al. (2018) Retinal pigment epithelium in the pathogenesis of age-related macular degeneration and photobiomodulation as a potential therapy? Clin Exp Ophthalmol 46, 670–686 [DOI] [PubMed] [Google Scholar]
- 58.Cheng T et al. (2018) Photobiomodulation inhibits long-term structural and function lesions of diabetic retinopathy. Diabetes 67, 291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kokkinopoulos I et al. (2013) Age-related retinal inflammation is reduced by 670nm light via increased mitochondrial membrane potential. Neurobiol Aging 34, 602–609 [DOI] [PubMed] [Google Scholar]
- 60.Begum R et al. (2013) Treatment with 670nm light upregulates cytochrome c oxidase expression and reduces inflammation in an age-related macular degeneration model. PLoS One 8, e57828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang J et al. (2014) Photobiomodulation in the treatment of patients with non-center-involving diabetic macular oedema. Br J Ophthalmol 98, 1013–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merry GF et al. (2017) Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol 95, e270–e277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Markowitz SN et al. (2019) A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina 00, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown EE et al. (2018) Mitochondria: potential targets for protection in age-related macular degeneration. Adv Exp Med Biol 1074, 11–17 [DOI] [PubMed] [Google Scholar]
- 65.Brown EE et al. (2019) The common antidiabetic drug metformin reduces odds of developing age-related macular degeneration. Invest Ophthalmol Vis Sci 60, 1470–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Q et al. (2003) Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278, 36027–36031 [DOI] [PubMed] [Google Scholar]
- 67.Sena LA and Chandel NS (2012) Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48, 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanki T et al. (2004) Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol Cell Biol 24, 9823–9834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feil R and Fraga MF (2012) Epigenetics and the environment: emerging patterns and implications, Nat Rev Genet 13, 97–109 [DOI] [PubMed] [Google Scholar]
- 70.Duraisamy AJ et al. (2017) Crosstalk between histone and DNA methylation in regulation of retinal matrix metalloproteinase-9 in diabetes. Invest Ophtahlmol Vis Sci 58, 6440–6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kowluru RA and Mishra M (2017) Epigenetic regulation of redox signaling in diabetic retinopathy: Role of Nrf2. Free Rad Biol Med 103, 155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mishra M and Kowluru RA (2015) Epigenetic Modification of Mitochondrial DNA in the Development of Diabetic Retinopathy. Invest Ophthalmol Vis Sci 56, 5133–5142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mishra M and Kowluru RA (2019) DNA methylation-a potential source of mitochondria DNA base mismatch in the development of diabetic retinopathy. Mol Neurobiol 56, 88–101 [DOI] [PubMed] [Google Scholar]
- 74.Zhong Q and Kowluru RA (2011) Diabetic retinopathy and damage to mitochondrial structure and transport machinery. Invest Ophthalmol Vis Sci 52, 8739–8746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kowluru RA (2019) Mitochondrial stability in diabetic retinopathy: Lessons learned from epigenetics. Diabetes 68, 141–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tien T et al. (2017) High Glucose Induces Mitochondrial Dysfunction in Retinal Müller Cells: Implications for Diabetic Retinopathy. Invest Ophthalmol Vis Sci 58, 2915–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haider SZ et al. (2019) Early Diabetes Induces Changes in Mitochondrial Physiology of Inner Retinal Neurons. Neuroscience 406, 140–149 [DOI] [PubMed] [Google Scholar]
- 78.Kowluru RA et al. (2011) Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes 60, 3023–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Madsen-Bouterse SA et al. (2010) Role of mitochondrial DNA damage in the development of diabetic retinopathy, and the metabolic memory phenomenon associated with its progression. Antioxid Redox Signal 13, 797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Decanini A et al. (2007) Changes in select redox proteins of the retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol 143, 607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]