Abstract
Introduction:
Antiretroviral and anti-tuberculosis (TB) drugs are often co-administered in people living with HIV (PLWH). Early initiation of antiretroviral therapy (ART) during TB treatment improves survival in patients with advanced HIV disease. However, safety concerns related to clinically significant changes in drug exposure resulting from drug-drug interactions; development of overlapping toxicities and specific challenges related to co-administration during pregnancy represent barriers to successful combined treatment for HIV and TB.
Areas covered:
Pharmacokinetic interactions of different classes of ART when combined with anti-TB drugs used for sensitive-, drug-resistant (DR) and latent TB are discussed. Overlapping drug toxicities, implications of immune reconstitution inflammatory syndrome (IRIS), safety in pregnancy and research gaps are also explored.
Expert opinion:
New antiretroviral and anti-tuberculosis drugs have been recently introduced and international guidelines updated. A number of effective molecules and clinical data are now available to build treatment regimens for PLWH with latent or active TB. Adopting a systematic approach that also takes into account the need for individualized variations based on the available evidence is the key to successfully integrate ART and TB treatment and improve treatment outcomes.
Keywords: Tuberculosis, HIV, Antiretroviral, toxicity, safety, drug interactions
1. Introduction
Tuberculosis (TB) is the leading cause of death in people living with HIV (PLWH). It is estimated that PLWH have 11 to 26 times greater life time risk of developing active TB than people without HIV, with the incidence being especially high in low and middle-income countries [1]. Antiretroviral treatment (ART) decreases mortality among PLWH with active TB [2–4]. In the last few years new ART formulations with improved safety profiles have become available (e.g. the superior bone and kidney safety of tenofovir alafenamide, over tenofovir disoproxil fumarate)[5], lower doses of certain existing drugs have been evaluated and shown to be as effective and better tolerated (efavirenz 400 mg compared to 600 mg) [6,7] and combinations of long-acting injectables have recently been shown to have non-inferior efficacy relative to oral combinations [8].
However, despite important advances, co-administration of drugs that have to be taken for long periods (currently a minimum 6 months for the treatment of TB) or for life (ART) still poses safety challenges. Key issues in treatment are drug-drug interactions, which can result in subtherapeutic concentrations of ART and/or anti-TB drugs resulting in impaired efficacy, development of overlapping toxicities, immune reconstitution inflammatory syndrome (IRIS) and concerns regarding teratogenicity and safety during pregnancy.
After decades of little change, advances in the treatment of TB have been recently achieved. Whilst the recommended first-line treatment for drug sensitive TB remains the four agents RIF, isoniazid, pyrazinamide and ethambutol, data on the efficacy and safety of the newly approved agents bedaquiline and delamanid for the treatment of drug resistant (DR)-TB have become available. DR-TB is a global public health threat causing enormous morbidity and socio-economic burden in affected communities. The WHO estimated over 560,000 new cases in 2017, with at least 90,000 incident cases in the WHO African region, where the proportion of HIV-associated TB patients is up to 60% [9]. This overlapping epidemiology means that concomitant therapy for HIV and DR-TB will be frequently needed in TB treatment programs, particularly with provision of universal ART.
While awaiting more drugs to be developed, several agents that had been licenced for infections other than TB, such as clofazimine, fluoroquinolones, carbapenems and oxazolidinones have been recently repurposed for the treatment of DR-TB. The evidence of their efficacy and safety is based on observational studies [10,11].
Regardless of the recent introduction of new ART and anti-TB drugs, co-managing TB with HIV simultaneously is a complex clinical challenge that also demands a systematic approach in order to minimize treatment failure and reduce morbidity and mortality [12]. Adverse drug reactions due to overlapping toxicities and drug-drug interactions may be factors that result in the worse TB treatment outcomes observed in HIV [13,14], and are therefore important to anticipate and actively manage [15].
The objective of this review is to discuss the safety of co-administration of ART and anti-TB drugs and to propose a practical approach to help improve patients’ safety during co-administration. Gengiah et al reviewed this topic in 2011, predominately focusing on drug safety of agents used in drug-sensitive TB when administered with ART [16]. In this review, PK and safety data of drugs recommended for the treatment of DR- and latent TB (latent TB) in adults will be also discussed. An additional focus on the management of HIV-associated TB during pregnancy is added. Considerations of the impact of ART and TB treatment co-administration in infants and children are also of great importance, but are outside the scope of this review.
2. Drug-drug interactions between anti-TB drugs and ART
2.1. First-line anti–TB drugs
Amongst first-line anti-TB drugs, rifamycins (rifampicin (RIF), rifabutin and rifapentine) are key agents due to their effective sterilising action. They are also responsible for most of the relevant drug-drug interactions with antiretrovirals. RIF is a potent inducer of hepatic phase I and phase II enzymes [17–19] [cytochrome P450 (CYP450) and uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1)] and of drug transporter P-glycoprotein (P-gp) [20]. Co-administration of antiretroviral drugs which are substrates of these enzymes and transporter leads to significant reduction in their bioavailability. Here we discuss the PK interactions between rifamycins and anti-HIV agents and recommended drug dosing (Table 1).
Table 1:
Drug-drug interactions of antiretrovirals with rifampicin and rifabutin
| Rifampicin | Rifabutin | |||
|---|---|---|---|---|
| Antiretroviral | Effect | Dose adjustment and comments | Effect | Dose adjustment and comments |
| NNRTI | ||||
| NVP | ↓↓NVP | Avoid concomitant use | ↓RBT | Can be co-administered with RBT 300 mg once daily |
| EFV | ↓/= EFV | EFV 600 mg can be co-administered without dose adjustments Non-significant changes in EFV 400mg exposure in a small clinical PK study[35] |
↓RBT | Can be co-administered with RBT 450 mg once daily |
| RPV | ↓↓RPV | Avoid concomitant use | ↓RPV | Avoid concomitant use When necessary, can be co-administered with RPV 50 mg once daily with caution (ECG monitoring due to risk of QT prolongation) |
| ETR | ↓↓ETR | Avoid concomitant use | ↓ETR | Can be co-administered without dose adjustments Limited data available |
| DOR | ↓↓DOR | Avoid concomitant use | ↓DOR | Can be co-administered with DOR 100 mg twice daily Limited data available derived only from PK studies |
| PI | ||||
| LPV/r | ↓↓LPV/r | Avoid concomitant use | ↑RBT | Can be co-administered with RBT 150 mg once daily Monitor closely due to potential increased RBT toxicity |
| ATV/r | ↓↓ATV/r | ↑RBT | ||
| DRV/r | ↓↓DRV/r | ↑RBT | ||
| Cobi | ↓↓Cobi | Avoid concomitant use | ↓↓Cobi | Avoid concomitant use |
| INSTI | ||||
| RAL | ↓RAL | Can be co-administered with RAL 400 or 800 mg twice daily Avoid RAL 1200 mg once-daily dose |
= RAL | Can be co-administered with RBT 300 mg once daily, RAL standard dose |
| EVG/c | ↓↓EVG/c | Avoid concomitant use | ↓EVG/c ↑RBT |
Avoid concomitant use |
| DTG | ↓DTG | Can be co-administered with DTG 50 mg twice daily | = DTG | Can be co-administered with RBT 300 mg once daily |
| BIC | ↓↓BIC | Avoid concomitant use | ↓BIC | Avoid concomitant use No data available |
| NRTI | ||||
| TDF, ABC, 3TC, FTC | =TDF =ABC =3TC =FTC |
Can be co-administered without dose adjustments | =TDF =ABC =3TC =FTC |
Can be co-administered without dose adjustments |
| TAF | ↓↓TAF | Co-administration with TAF 25 mg once daily resulted in non-significant changes in intracellular tenofovir diphosphate exposure in an healthy volunteer PK study [21] | ↓TAF | Avoid concomitant use until further data available |
| CCR5 receptor antagonists | ||||
| MVC | ↓↓MVC | Can be co-administered with MVC 600 mg twice daily | ↓MVC | Can be co-administered without dose adjustments |
NNRTIs, non-nucleoside reverse transcriptase inhibitors; NVP, nevirapine; EFV, efavirenz; RPV, rilpivirine; ETR, etravirine; DOR, doravirine; PIs, protease inhibitors; LPV, lopinavir; ATV, atazanavir, DRV, darunavir;/r, ritonavir; cobi, cobicistat; INSTIs, integrase inhibitors; RAL, raltegravir; EVG, elvitegravir; DTG, dolutegravir; BIC, bictegravir; NRTIs, nucleoside reverse transcriptase inhibitors; TDF, tenofovir disoproxil fumarate; ABC, abacavir; 3TC, lamivudine; FTC, emtricitabine; TAF, tenofovir alafenamide; MVC, maraviroc; PK, pharmacokinetic; RBT, rifabutin
2.1.1. Nucleos(t)ide reverse transcriptase inhibitors (NRTI)
Most NRTI are not subject to drug-drug interactions with RIF. One exception is tenofovir alafenamide, which is a substrate of P-gp. A PK study of RIF and tenofovir alafenamide in healthy volunteers [21] showed a reduction in plasma tenofovir alafenamide AUC by 55%, and decreased intracellular (IC) tenofovir-diphosphate concentrations (the active molecule) by 36%. The authors noted that the IC concentrations were still 76% higher than those with standard dose tenofovir disoproxil fumarate, meaning that tenofovir alafenamide efficacy should not be compromised with concomitant RIF co-administration. However, clinical efficacy data is warranted before tenofovir alafenamide is used in routine clinical practice with RIF [22].
2.1.2. Non-nucleoside reverse transcriptase inhibitors (NNRTI)
The strongest clinical evidence of safe and effective co-administration with RIF is with efavirenz [23–27]. Efavirenz is a substrate of the CYP450 family [28], mainly CYP2B6, with minor contributions from CYP3A4/5 [29]. There is a high variability in efavirenz concentrations When given with or without RIF [30,31], mainly due to CYP2B6 polymorphisms [32]. Various studies have shown good virologic efficacy and clinical outcomes with standard efavirenz dosing (600 mg) and RIF. When efavirenz is given at reduced doses (of 400 mg once daily) with RIF the plasma exposures are within efficacy ranges (Ctrough between 1–4 mcg/mL)[33], irrespective of CYP2B6 genotype [34]. These results were confirmed in a subsequent pharmacokinetic trial conducted in Uganda enrolling ten patients with HIV-associated TB who were switched to efavirenz 400 mg during treatment with RIF and isoniazid [35]. Nevertheless, validation in a larger cohort is needed.
Nevirapine is extensively metabolized via CYP3A4. Numerous studies have reported sub-therapeutic concentrations of nevirapine with RIF, and increased virological failures in patients starting antiretroviral treatment [27].
Rilpivirine [36], etravirine [37], and doravirine are also primarily metabolized via CYP3A4 and their concentrations fall significantly with RIF[38], such that they should not be co-administered.
Doravirine exposure decreased by 50% with rifabutin, although twice daily dosing of doravirine appears to effectively overcome the induction [39].
2.1.3. Integrase inhibitors (INSTI)
Of the INSTI, raltegravir and dolutegravir are both substrates of UGT1A1. RIF co-administration reduces raltegravir exposure by 40% and dolutegravir AUC by 54% [19,40]. Both drugs are commonly and effectively used in clinical practice at a standard dose of 400 mg twice daily or double dose (800 mg twice daily) for raltegravir as investigated in the Reflate TB study [41], and at a double dose of 50 mg twice daily for dolutegravir [42]. There is lack of clinical and PK data for raltegravir 1200 mg (once daily dose) with RIF which should not be used. Dolutegravir 50 mg once daily allows for trough concentrations still above the protein binding-adjusted IC90 of 64 ng/mL in a PK study on healthy volunteers[43].
Both elvitegravir/cobicistat and bictegravir are CYP3A4 and UGT1A1 substrates: RIF greatly reduces their concentrations and their concurrent use is contraindicated [44–46]. Use of elvitegravir/cobicistat with rifabutin is also contraindicated due to reduced elvitegravir exposure and there are little data with concomitant use of rifabutin and bictegravir whose AUC is reduced by 38% [47].
2.1.4. Protease inhibitors (PI)
Protease inhibitors (PI) should not be used with RIF as the latter has a profound impact on their concentrations. However, if no other options are available, a dose adjustment of lopinavir/ritonavir 800/200 mg twice daily has demonstrated been effective in overcoming the RIF induction under close safety and therapeutic drug monitoring [48,49]. Rifabutin is the rifamycin of choice when use of PI is necessary, as it induces CYP3A4 to a lesser degree. Nevertheless, concentrations of rifabutin and its active metabolite increase significantly as it is a substrate of CYP 3A4 when administered with a boosted-PI and rifabutin dose needs to be reduced in order to avoid excessive exposure and subsequent adverse effects. There is a lack of consensus on the optimal dose of boosted PI to adopt [50], although rifabutin at 150 mg once daily is recommended by most international guidelines [51,52]. Cobicistat should not be used with rifabutin as its exposure is reduced by 66% and there is a more than five-fold increase in 25 OH desacetyl rifabutin [52,53].
2.1.5. Other drugs
The use of long-acting injectables (cabotegravir plus rilpivirine) for the treatment of HIV has recently demonstrated great efficacy and safety in clinical trials [8], although once again cabotegravir is subject to RIF-induction and therefore their co-administration contraindicated [54]. Novel classes of antiretrovirals in the HIV pipeline present improved options for the treatment of multidrug resistant-HIV. Among those recently approved, Ibalizumab is a post-attachment inhibitor with no significant pharmacological interactions expected with RIF, however its role and safety in patients infected with TB is not known. [55]. Likewise, albuvirtide, a fusion inhibitor currently approved in China, lacks data regarding its administration with other antivirals and during TB treatment [56]. Fostemsavir is an attachment inhibitor tested in Phase III studies. The active component of fostemsavir, temsvir, is a P-gp and CYP3A4 substrate. A PK study in healthy volunteers RIF resulted in significantly reduced temsvir exposure when co-administered with fostemsavir [57], whilst no major decrease was seen when fostemsavir was administered with rifabutin.[58]. Fostemsavir is a promising salvage therapy for multidrug-resistant HIV, however data are needed before its use can be considered during TB treatment with rifabutin or other anti-TB drugs.
2.2. Anti-TB drugs used for latent TB
Drugs recommended for latent TB treatment include INH, RIF and rifapentine. For regimens including RIF, all the drug-drug interactions described above and summarised in Table 1 apply.
INH has also potential for drug-drug interactions. Efavirenz is indeed also metabolized by an accessory pathway involving CYP2A6, that is inhibited by INH thus slow metabolizers can result in increased efavirenz concentrations [59].
Rifapentine induces CYP450 and glucuronosyltransferases [60], and when given daily is a more potent inducer than daily RIF therapy [61]. Rifapentine can be given as a once weekly regimen with INH for 12 weeks [62], or daily with INH, for 4 weeks [63]. Rifapentine can be safely administered with efavirenz both daily or weekly and daily or weekly when combined with raltegravir [64] [65][66]. Although a previous clinical trial in healthy volunteers was stopped prematurely because unexpected adverse reactions[67], a recent study with dolutegravir given with weekly rifapentine/isoniazid in PLWH showed that dolutegravir could be administered safely and without dose adjustment [68].
2.3. Second-line anti-TB drugs
Most agents used in conventional DR-TB treatment regimens have no major PK drug-drug interactions with ART[69]. An exception is moxifloxacin, which was found in a population PK study to have higher clearance and lower concentrations in patients with HIV-associated TB on efavirenz, possibly due to induction by the latter of moxifloxacin metabolism via UDP-glucuronosyltransferase (UGT)[70]. The clinical relevance of this is unknown, and no dose adjustments are currently recommended.
Introduction of highly effective new and repurposed drugs have changed the treatment landscape for DR-TB, introducing new challenges for co-administration with ART.
2.3.1. Bedaquiline
Perhaps the most important advance in DR-TB treatment has been the introduction of bedaquiline into treatment programs. A diarylquinoline, bedaquiline has a unique mechanism of action by inhibiting mycobacterial ATP synthesis. Due to potent antimycobacterial activity, bedaquiline enables DR-TB treatment shortening resulting in improved outcomes, including survival in a large retrospective cohort analysis [71]. Although well tolerated, it is associated with prolongation of the QT interval. Another drawback is that bedaquiline is primarily metabolised by cytochrome P450 3A4 (CYP3A4)[72], and is thus subject to a drug-drug interactions with ART drugs that modulate activity of this isoenzyme. Drugs that inhibit CYP3A4, such as ritonavir-boosted PIs,[73,74] may lead to increased bedaquiline concentrations, potentially increasing the risk for toxicity, especially QT prolongation. CYP3A4 inducers, especially RIF, may affect the PK of bedaquiline in the opposite direction, potentially leading to reduced efficacy and resistance selection.
The NNRTIs efavirenz and nevirapine are CYP3A4 inducers [75], and widely used in high HIV-TB burden settings, posing potential problems for co-administration with bedaquiline. A population PK model based on phase 1 trial data predicted a 50% reduction in steady-state bedaquiline exposure when co-administered with efavirenz over time [76][77] This combination is likely to compromise treatment efficacy as bedaquiline’s PD effect is driven by average concentration (area under the concentration-time curve, AUC), and should be avoided. In contrast, non-linear mixed effects modelling of healthy volunteer data suggested no effect of nevirapine on bedaquiline PK, and that the drugs could be co-administered without dose adjustment [78]. This finding was confirmed by non-compartmental analysis of clinical data from a small PK study involving patients with HIV-associated TB (n = 17) on nevirapine -based ART, who had similar bedaquiline exposures to no-ART controls [79]. Rilpivirine, at standard dose of 25 mg daily, are unlikely to have an important impact on bedaquiline exposure. There may be additive effect on QT prolongation, especially at supratherapeutic doses of rilpivirine [80].
In that study, bedaquiline PK was also evaluated in patients on lopinavir/ritonavir-based ART. As predicted by a PK model [78,81], which showed substantially reduced clearance of bedaquiline and its N-monodesmethyl metabolite, M2, during chronic co-administration, patients taking concomitant lopinavir/ritonavir had significantly increased bedaquiline exposure (62% increase in AUC) compared to the no-ART group [79]. The clinical significance of this is unknown, but there are concerns that elevated concentrations of bedaquiline, and particularly M2 (which is thought to be more toxic than the parent molecule [82,83]), may enhance QT-prolonging effects. Consequently, PIs should be used with caution and with closer electrocardiogram (ECG) monitoring in combination with bedaquiline, and avoided if other ART options are available. This is especially relevant when used in combination with other QT-prolonging agents. Other drugs which result in QT prolongation, such as fluoroquinolones,(in particular moxifloxacin) [84,85], clofazimine [86], and delamanid [87], are frequently co-administered with bedaquiline in DR-TB regimens, with the possibility for additive toxicity [88]. However, emerging clinical data suggest that the combined use of these drug classes is safe [89,90].
Co-administration of bedaquiline with other antiretroviral agents has not been studied. Based on known metabolism of integrase inhibitors, which do not influence CYP3A4, a clinically significant interaction with bedaquiline is not expected although the use of cobicistat-boosted INSTIs may increase bedaquiline exposure. Table 2 summarizes the DDI between bedaquiline and ART.
Table 2:
Drug-drug interactions of antiretrovirals with bedaquiline
| Antiretroviral | Effect on bedaquiline | Dose adjustment and comments | |
|---|---|---|---|
| NNRTI | |||
| EFV | ↓↓ BDQ | Average steady-state concentrations reduced by 52% (model-based prediction)54 | Risk of subtherapeutic BDQ concentrations and treatment failure: do not co-administer |
| NVP | = BDQ | Non-significant increase in average steady-state concentrations of BDQ (9%) and decrease (5%) in M2 metabolite (PK model)55 Non-significant changes in BDQ AUC in clinical PK study[79] |
Can be co-administered without dose adjustments |
| RPV | = BDQ | Not studied, but clinically relevant DDIs not expected | Can be co-administered without dose adjustment |
| PI | |||
| LPV/r | ↑BDQ | 3- and 2-fold increases in average steady state concentrations of BDQ and M2 metabolite, respectively (model-based) 55 62% increased BDQ AUC in clinical DDI study[79] |
Avoid concomitant use when possible. Implement more frequent ECG monitoring for QT prolongation if used together |
| Other PIs (ATV/r, DRV/r) | ↑BDQ | Not studied; increased BDQ exposures expected | Avoid concomitant use when possible. Implement more frequent ECG monitoring for QT prolongation if used together |
| INSTI | |||
|
DTG RAL |
= BDQ | Not studied, but clinically relevant DDI not expected | Can be co-administered without dose adjustment |
| EVG/cobi | ↑BDQ | Not studied; may result in increased BDQ concentrations | Avoid due to potential risk of cardiac toxicity. Implement more frequent ECG monitoring for QT prolongation if used together |
| NRTI | |||
| TDF, ABC, 3TC, FTC | = BDQ | clinically significant DDIs not expected | Can be co-administered without dose adjustment |
| CCR5 receptor antagonists | |||
| MVC | = BDQ | Not studied; clinically significant DDIs not expected | Can be co-administered without dose adjustment |
NNRTIs, non-nucleoside reverse transcriptase inhibitors; NVP, nevirapine; EFV, efavirenz; RPV, rilpivirine; PIs, protease inhibitors; LPV, lopinavir; ATV, atazanavir, DRV, darunavir; INSTIs, integrase inhibitors; RAL, raltegravir; EVG, elvitegravir; cobi, cobicistat;/r, ritonavir-boosted; DTG, dolutegravir; NRTI, nucleoside reverse transcriptase inhibitors; TDF, tenofovir disoproxil fumarate; ABC, abacavir; 3TC, lamivudine; FTC, emtricitabine; TAF, tenofovir alafenamide; DDIs, drug-drug interactions;
2.3.2. Nitroimidazoles
The nitroimidazoles, delamanid and pretomanid (formerly PA-824), are the second new class of anti-TB drugs that have entered clinical practice. Of these, delamanid has been most extensively evaluated and is now registered for clinical use. Delamanid is primarily metabolized by albumin, with a smaller contribution by CYP. [91,92]. Like bedaquiline [72], delamanid does not affect the activities of drug transporters or CYP enzymes [93,94], and there is no impact on NNRTI or PI exposures with concomitant use [95,96]. A healthy volunteer study demonstrated a moderate impact of lopinavir/ritonavir on delamanid exposures, with 18% and 22% increase in delamanid Cmax and AUC, respectively. This is unlikely to result in clinically-important QT effects, but more frequent ECG monitoring is advised. Reassuringly, use of efavirenz had no impact on delamanid after multiple doses in healthy subjects, and may be used concurrently without dose adjustment [96].
CYP3A is a minor metabolic pathway for the investigational nitroimidazole, pretomanid. A phase 1 study found substantial reductions in pretomanid exposures with efavirenz co-administration, with 28%, 35% and 46% reduction in pretomanid Cmax, AUC and trough concentration respectively, but minimal effect from lopinavir/ritonavir, supporting combination therapy with PI [97].
2.3.3. Linezolid
The oxazolidinone, linezolid, has been repurposed as an anti-TB agent, and now has a central role in DR-TB treatment [98]. Linezolid is thought to undergo mainly non-oxidative enzymatic metabolism and significant PK drug-drug interactions with ART are considered unlikely [99]. However, CYP may play a minor role in linezolid disposition and indirect evidence suggest that linezolid may be a substrate of P-gp [100] [101]. Thus, powerful inhibition of CYP and drug efflux pumps by PIs could theoretically result in increased linezolid concentrations and associated toxicity. Reassuringly, a PK study involving patients with DR-TB in South Africa found no association between lopinavir/ritonavir use and linezolid exposure, although numbers of patients studied were small [102]. There are important PD interactions between linezolid and ART that may partially explain the signal of increased linezolid toxicity observed amongst HIV-infected patients [103]. Both linezolid and zidovudine cause anaemia, which may be more severe when co-prescribed; and the use of other NRTI, which inhibit mitochondrial gamma polymerase [104], may theoretically potentiate the mitochondrial toxicity of linezolid [105] (mediated through a different mechanism) [106].
2.3.4. Clofazimine
Clofazimine, another repurposed drug recently introduced into DR-TB treatment guidelines, is now recommended for inclusion in all DR-TB regimens [98]. The PK of clofazimine is poorly understood. It is thought to be eliminated largely unchanged in faeces both as unabsorbed drug and via biliary excretion [107], and is thus unlikely to be an major CYP substrate or subject to significant PK drug-drug interactions with ART. There are, however, in vitro data suggesting clofazimine may potentially have PK interactions via inhibition of drug transporters (P-gp, BRCP, MRP1) and CYP3A4 [108][109], but this has been inconsistent between different cell lines [110]. Secondary data analysis from a clinical PK study found that clofazimine had no effect on bedaquiline clearance, a CYP3A4 substrate [111]. No drug-drug interactions studies have been performed for clofazimine and ART; and currently no dose adjustments are recommended.
3. Overlapping toxicities of ART and anti-TB drugs
The recommended composition of treatment regimens for PLWH with both sensitive- and DR-TB do not differ from HIV uninfected individuals.
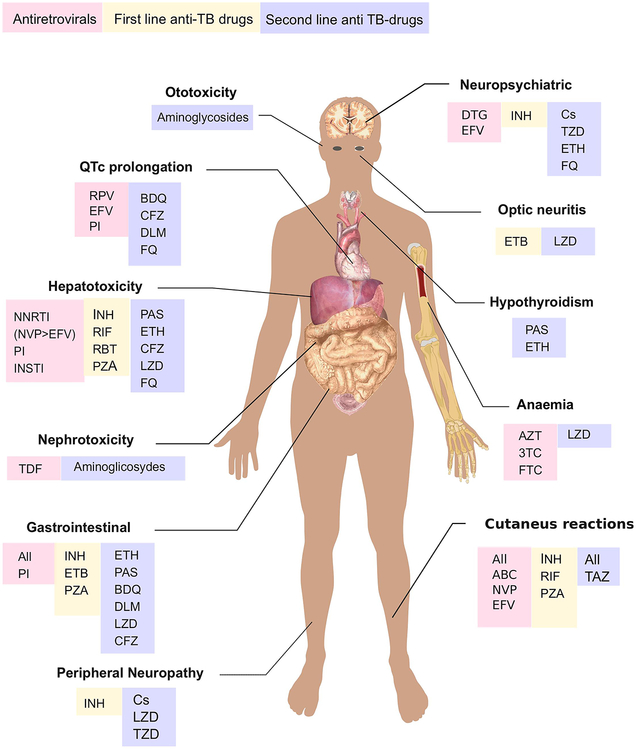
Shared toxicity is the major challenge with concomitant use of ART and anti-TB drugs [15,112]. It is unclear whether risk of adverse reactions to TB treatment are increased with concurrent ART administration [113], but identification of the culprit drug is often difficult and complicates management of both infections when toxicity occurs [15,114]. Hepatotoxicity, peripheral neuropathy, central nervous system side effects, QT prolongation and cutaneous adverse drug reactions are the most important toxicities that can arise during co-administration of ART and anti-TB drugs and will be discussed in detail below. Other important adverse reactions include nephrotoxicity and electrolyte abnormalities with injectable agents (aminoglycosides and capreomycin) and tenofovir disoproxil fumarate [115]. Gastrointestinal (GI) disturbances, such as diarrhoea, nausea, vomiting and abdominal pain are events associated with all antiretrovirals (ARV), especially the PIs and in particular lopinavir/ritonavir. Notably, GI side effects are an important cause of interruption of lopinavir/ritonavir-based ART [116]. Among anti-TB drugs, ethionamide and para-aminosalicylic acid are most frequently associated with GI intolerance. Hypothyroidism can occur in over a half of patients treated for DR-TB, and is associated with exposure to para-aminosalicylic acid and ethionamide [117,118]. Ototoxicity is another important side effect of aminoglycosides (streptomycin, kanamycin, amikacin). Therefore audiometry monitoring is recommended at baseline and during treatment with aminoglycosides [119]
3.1. Hepatotoxicity
Drug-induced liver injury (DILI) is the most serious adverse reaction to anti-TB drugs, occurring in 2–28% of patients treated for TB [120], with estimates varying widely between different cohorts in part due to different definitions of DILI used in studies [121–125]. Given that the mortality in patients with TB treatment-associated DILI is high, up to 27% in one study [126], it is imperative to promptly diagnose this complication in order to discontinue treatment with potentially causative drugs, and switch to appropriate alternative therapy [123,127].
Three of the anti-TB drugs used in standard treatment for drug sensitive-TB (RIF, INH and pyrazinamide) can cause liver injury [15]. Following treatment initiation, an asymptomatic, low-grade and transient elevation of transaminases is relatively common, and may reflect physiological hepatic adaptation to drugs exposure which does not require drug cessation [128]. INH, for instance, causes a transient asymptomatic and mild increase in liver enzymes in up to 20% of patients, which resolves spontaneously despite continuous administration of the drug [129]. Distinguishing adaptation from liver injury is important, as unnecessary treatment discontinuation can lead to worsening of TB disease and emergence of drug resistance, consequently increasing morbidity and mortality.
Causality assessment in patients with liver injury on TB treatment is challenging due to multiple possible disease and drug causes of the liver injury, including ARVs and cotrimoxazole in addition to anti-TB drugs. Although some studies have found that the incidence of hepatoxicity is similar in PLWH and HIV uninfected individuals [130–132], several others identified HIV co-infection itself as an independent risk factor for anti-TB drug-induced hepatotoxicity [121,123,124]. A large observational study conducted in Ethiopia enrolling 1060 patients examined the patterns of liver toxicity in patients with HIV and/or TB and found that amongst a total of 159 patients who developed DILI, being HIV positive was associated with increased risk of DILI (OR=4.11, 95%CI: 1.45 to 11.96; p<0.01) [124].
In addition, ARVs may cause liver injury by a variety of mechanisms including hypersensitivity reactions (nevirapine, abacavir), mitochondrial toxicity (NRTI as a class) or a combination of mitochondrial and endoplasmic reticulum stress (efavirenz) [133]. This may explain why concomitant ART treatment is an independent risk factor for TB-DILI in some studies. In a retrospective analysis of a South Africa cohort, which included 868 PLWH on ART, concomitant TB treatment increased risk of hepatotoxicity by 8.5 fold (HR 8.5, 95%CI 2.7–27; p<0.001) [134]. Araujo-Mariz et al monitored liver injury in a cohort of 173 PLWH during the first 60 days of TB treatment and found that hepatotoxicity (defined as aminotransferase levels three times higher than they were prior to TB treatment and associated with symptoms of hepatitis) occurred in 30.6%(53/173) and the majority of the individuals enrolled were on ART (78.6%).
DILI may also occur during treatment for DR-TB, with an observed prevalence between 3.9–16.5% in different studies [135–138]. Among the drugs used in second-line treatment for TB, prothionamide [139], rifabutin [140], para-aminosalicylic acid, linezolid [141] and fluoroquinolones have all been associated with increased risk of liver injury [142]. Bedaquiline can also cause hepatotoxicity[143].
Many guidelines are available advising the management of DILI in HIV-associated TB [52,144,145], however the definition of DILI is not standardized and criteria for TB treatment discontinuation and re-challenge vary [144]. In general, is preferred to re-introduce first-line TB drugs over the switch to second-line drugs; importantly, rechallenge is not recommended in patients who had fulminant hepatitis (involving coagulopathy and hepatic encephalopathy). Knowing which ART drugs possess higher hepatotoxic profile (e.g. nevirapine over efavirenz and integrase inhibitors) is important, as a switch to a less liver toxic ART agent before starting on TB treatment is a strategy to adopt in case of DILI in HIV-associated TB patients also possibly associated to ART.
The risk of hepatotoxicity may be increased by other acquired and genetic factors. As an example, N-acetyltransferase 2 (NAT2) acetylator status associates with INH-related DILI; those individuals classified as slow acetylators are at higher risk of hepatotoxicity [146]. Among studies investigating potential risk factors for development of liver injury during TB treatment, female sex, older age, low weight and alcohol consumption are associated with increased risk [123,128,147]. Hepatitis B and/or C chronic infections increase risk of liver injury and are associated with increased mortality during treatment of both sensitive and DR-TB [137,148]. Hence, screening for viral hepatitis is important at the start of TB treatment or when patients present with abnormal liver function during treatment. In addition, other concomitant medications known to be potentially hepatotoxic and frequently prescribed to PLWH with low CD4 count as treatment or prophylaxis for opportunistic infections (e.g. fluconazole and cotrimoxazole) should be stopped when liver injury develops on anti-TB drugs.
3.2. Peripheral neuropathy
HIV infection has been shown since early studies to affect both the central and peripheral nervous system [149]. In a systematic review including 25 studies, the prevalence of peripheral neuropathy (PN) in PLWH was 69%, and distal sensory polyneuropathy was the most common neurologic disorder in PLWH [150].
Up to 10% of patients treated for drug-sensitive TB develop PN [151]. In a South African cohort of 400 individuals treated for active TB, a greater proportion of PLWH developed PN than non-HIV infected individuals (8.3% vs 1.9%, p=0.009)[132]. INH is known to interfere with pyridoxine metabolism and depletes its levels leading to neurotoxicity in up to 10% of all patients [152]. In particular, PN may especially develop during treatment for DR-TB when higher doses of INH are used [153]. In addition, PLWH, especially with advanced disease, frequently suffer of nutritional deficiencies, thus HIV infection may predispose to pyridoxine depletion [154]. Pyridoxine supplementation dose of 10–50 mg daily is therefore advised to prevent PN in all PLWH receiving INH [52,155], including pregnant women and those treated for latent TB, whilst higher doses of pyridoxine are recommended in the treatment of INH-related PN.
Optic neuropathy is another manifestation of drug neurotoxicity and a well-recognised complication of ethambutol administration [156]. The exact mechanism is unknown, but the ability of ethambutol to chelate metals and to affect different mitochondrial metal-containing enzymes has been hypothesized. Although data are missing, an additive negative effect played by ethambutol and NRTI on mitochondrial function has been postulated, leading to an increased risk of toxic neuropathy in PLWH on NRTI-based treatment [157]. Monitoring visual function to detect the development of optic nerve damage during therapy assessing visual acuity (Snellen test) and applying colour discrimination tests should therefore be considered. Among the drugs used in the treatment of DR-TB, PN and optic neuritis are the most common side effect during linezolid administration, with reported cases of reversible or irreversible damage likely as a results of mitochondrial dysfunction [153,158]. An Indian cohort of 86 patients treated with linezolid for DR-TB reported an incidence of 28% of ocular complaints (mainly blurred vision) and 5.8% optic neuropathy [159]. Ethionamide has been frequently associated with PN causing pyridoxine deficiency similar to INH whilst cycloserine and aminoglycosides also have been associated with PN, although to a lesser extent [152,160]. Additionally, exposure to terizidone has also been linked to symptomatic PN during treatment for DR-TB [161]. Finally, clinicians should look for co-morbidities causing PN that may coexist in patients with HIV-associated TB that can be treated, such as diabetes mellitus and substance abuse, particularly of alcohol [152].
3.3. Central nervous system toxicities
Dolutegravir and efavirenz, both commonly used in first-line ART, are both associated with neuropsychiatric adverse events [162,163]. In a cohort of 197 patients prospectively enrolled in Uganda, higher efavirenz concentrations and CYP2B6 genotype, were the main predictors for efavirenz -related neuropsychiatric symptoms, underscoring the potential role for pharmacogenomic testing and therapeutic drug monitoring (TDM) to prevent drug toxicity [164]. Of the first-line anti-TB drugs, INH can cause a range of symptoms from mild insomnia to severe psychosis that generally reverses after treatment interruption or completion and are often associated with high doses of INH. As previously mentioned, INH neurotoxicity associates with pyridoxine depletion and therefore higher doses of pyridoxine, INH substitution and psychiatric management, are warranted in the treatment of INH-related neuropsychiatric symptoms [151,165]. Among second-line anti-TB drugs, a meta-analysis showed that cycloserine and terizidone associate with a higher rate of a CNS-related adverse reactions than other second-line drugs. Attention should be given to patients’ mental health during the administration of anti-TB drugs, especially those taking cycloserine, which may cause psychosis and/or suicidal ideation [151,166]. Switching from efavirenz to an antiviral agent less likely to cause neurotoxicity (e.g. frequently dolutegravir, as proved less frequently causing neuropsychiatric events in a large randomised trial [167], or alternatively dose-adjusted lopinavir/ritonavir or raltegravir) should be considered in patients who develop neuropsychiatric side effects during TB treatment in order to avoid TB treatment interruption or switch to second-line anti-TB drugs that can be less effective and still cause adverse effects.
3.4. QT prolongation
Prolongation of the corrected QT interval is a risk factor for torsades de pointes, a ventricular arrhythmia with potentially fatal consequences [168]. QT prolongation is more common in PLWH and this appear to be influenced by the duration of HIV infection itself, the induced systemic inflammation, CD4 count below 200 cell/mm3 and different ART agents, although reports vary [169–174]. Among ARVs, NNRTI (especially efavirenz and rilpivirine) and PI have been reported as potential causes of QT interval prolongation; however clinical complications are uncommon and therefore regular ECG monitoring is not recommended during ART [80,175–178]. Several drugs used in TB treatment are known to prolong the QT interval, including fluoroquinolones (especially moxifloxacin), bedaquiline and clofazimine. The safety of rilpivirine administered with moxifloxacin was studied in a controlled crossover study enrolling 60 healthy volunteers that did not find any clinically relevant effect on QT [179]. However, levofloxacin should be the fluoroquinolone of choice when combined with other potential QT prolonging drugs due to a lower potential to prolong QT. The recent introduction of bedaquiline and delamanid, both linked with QT prolongation as discussed in the pharmacokinetics section, demands ECG monitoring as a safety requirement during the treatment of DR-TB [180].
3.5. Cutaneous adverse drug reactions
In clinical practice, recognizing which drug is responsible for a cutaneous adverse drug reactions can be challenging as virtually all ART and anti-TB drugs and some agents used for treatment or prophylaxis of opportunistic infections can cause skin reactions. Importantly, clinicians should be aware that the onset of cutaneous adverse drug reactions is often delayed and differ between ART and anti-TB drugs, varying between few days up to 3 months after the introduction of a new agent.
Most mild-moderate cutaneous adverse drug reactions do not require drug cessation. However, the presence of systemic symptoms or organ involvement or severe cutaneous adverse drug reactions warrant drug interruption, eventually followed by a careful re-challenge or desensitization under close supervision if a specific agent is required in the treatment regimen. Importantly, life-threatening conditions such like Stevens-Johnson syndrome and hypersensitivity to abacavir are contraindications to drug re-challenge [181]. However, in selected cases when alternative treatment options are limited, patients with Steven-Johnson syndrome may be cautiously rechallenged with anti-tuberculosis treatment under close clinical supervision.
PLWH are at higher risk to develop cutaneous adverse drug reactions to anti-TB drugs. The pathogenesis remains unclear but growing evidence supports the role of multiple factors, including genetic and immunological, that predispose PLWH to increased incidence of drug hypersensitivity reactions [132,182–186]. Cutaneous adverse drug reactions severity can range from transient erythematous rash without consequences, to life-threatening complications such as Steven-Johnson syndrome and toxic epidermal necrolysis (TEN), which have been described frequently in PLWH treated with thioacetazone and therefore its use in HIV is not recommended. The role of ART in the pathogenesis of cutaneous adverse drug reactions during TB-treatment has still not been fully elucidated. A study conducted in South Africa of 141 patients with HIV-associated TB (only 16% on ART) failed to find any association between the use of ART and adverse events [132]. In a cohort of 307 patients with HIV-associated TB in Thailand where the incidence of cutaneous adverse drug reactions was 16% (48/307), ART administration was associated with a lower risk of cutaneous adverse drug reactions (HR 0.23, 95%CI 0.09–0.61, P=0.003) [187]. However, several anti-HIV agents are well-known causes of skin reactions. Among ART agents, NNRTI have been associated with rash in up to 17% of patients. In particular, nevirapine can cause cutaneous adverse drug reactions in over a third of the patients during administration, often associated with hepatotoxicity [186]. The role of genetic factors involved in the pathogenesis of cutaneous adverse drug reactions in PLWH is recognised in the case of abacavir: hypersensitivity reactions to abacavir associate with genetic polymorphisms of the class I major histocompatibility complex (MHC). As a result of this observation being confirmed in several studies, HLA-B*5701 testing prior to starting abacavir is now recommended by many international guidelines [186]. Finally, to complicate even further the diagnosis and management of cutaneous adverse drug reactions in HIV-associated TB, concomitant drugs used in the prophylaxis of opportunistic infections, in particular cotrimoxazole, that may be initiated around the same time as ART and TB treatment, can cause severe cutaneous adverse drug reactions. In a multivariate analysis performed by Boonyagars et al in their Thai cohort on individuals with HIV-associated TB, it was observed that concomitant use of cotrimoxazole was an independent risk factor for cutaneous adverse drug reactions (HR 2.36, 95% CI 0.09–0.61) [187].
4. Immune reconstitution inflammatory syndrome
PLWH initiating ART are at risk of TB-IRIS. IRIS is an inflammatory reaction resulting from recovery of the immune system once ART has started, and its presentation can follow two main patterns: unmasking TB-IRIS refers to the presentation of TB in association with signs of acute inflammation after starting ART; paradoxical TB-IRIS occurs in patients already appropriately treated for TB who, following the introduction of ART, present paradoxical worsening or recurrence of TB symptoms and signs [188]. Paradoxical TB-IRIS is the most frequent and studied presentation, with an estimated incidence of 18% (95%CI 16–21 %) but occasionally affecting more that 50% of individuals in cohorts with a high prevalence of TB-IRIS risk factors [188]. In a systemic review and metanalysis, the morbidity resulting from paradoxical TB-IRIS was relatively high, with 25% of affected patients requiring hospitalization; all-cause mortality among patients was 7% (95% CI:4–11%), although only 2% (95% CI: 1–3%) of deaths attributable to TB-IRIS [189]. Clinical presentation often includes worsening of dyspnoea and cough, constitutional symptoms (fever, night sweats), enlarging lymphadenopathy and new or enlarging infiltrates on chest X-ray [190]. However, TB-IRIS can affect any organ system, with TB-IRIS of the CNS having particularly high mortality (30%) [191]. Extrapulmonary, short interval between starting TB treatment and ART, high HIV viral load and low CD4 T-cell count at baseline have been shown to be major risks factors for TB-IRIS in a systematic review and metanalysis [189].
Although patients with CD4 T-cell count below 50/mm3 encounter a relatively higher incidence of TB-IRIS, early ART initiation within 2 weeks of TB treatment initiation improves overall survival [192,193]. The diagnosis of TB-IRIS is challenging due to the lack of specific diagnostic tests and the non-specific symptoms, hence clinicians should carefully evaluate all patients presenting with unexpected deterioration of symptoms who start ART while on anti-TB drugs, excluding drug toxicities or the development of other opportunistic infections or DR-TB that could explain the aggravation of symptoms.
The management of paradoxical TB-IRIS involves supportive therapy in conjunction with corticosteroids [194]; importantly, ART should be continued. A recent randomised placebo-controlled controlled trial (PredART trial) also showed that the administration of prednisone during the first four weeks of ART to prevent TB-IRIS resulted in a 30% lower incidence of TB-IRIS in individuals at high risk compared to the placebo arm [195]. Prophylaxis with prednisone should be therefore considered in PWLH infected with sensitive-TB who have a CD4 nadir less than 100/mm3 and no evidence of Kaposi’s sarcoma [51,188].
The more rapid decrease in HIV viral load that has been observed during treatment with integrase inhibitors (INSTI), raised concern that the sudden decline of HIV viremia could increase the risk of paradoxical TB-IRIS in patients started on dolutegravir. However, a recent metanalysis failed to demonstrate association between the use of dolutegravir and IRIS [196]. Notably, the INSPIRING study evaluated the incidence of IRIS after initiation of twice-daily dolutegravir in PLWH ART-naïve on treatment for TB reported and found a low rate of IRIS [42].
6. ART and anti-TB drugs during pregnancy
Changes in the PK, tolerability and side effects of HIV and anti-TB drugs can occur during pregnancy, along with the potential for foetal toxicities. Data to inform practice and their association with treatment outcome of both TB and HIV are scarce, thus the management of TB in HIV-infected pregnant women represents a significant challenge. The regimen of choice for drug sensitive-TB during pregnancy does not differ from the standard four drug regimen and does not carry major toxic effects. A PK study conducted in HIV/TB pregnant women found that RIF exposure during pregnancy was only slightly increased in a South African cohort and therefore dose adjustment is not necessary. The options for DR-TB, by contrary, are limited as many drugs have been associated with risk of maternal and infant toxicities [197], and therefore their use during pregnancy and breastfeeding may be considered when an effective treatment regimen cannot otherwise be provided and benefits outweigh the risks. The use of aminoglycosides is not advised because associated with congenital deafness. A summary of evidences of the available data in human and animal studies in pregnancy and breastfeeding are summarised in table 3. Pregnant women are often underrepresented in clinical trials and although progress have been made in the treatment of DR-TB, data informing on the administration of drugs for DR-TB and ART in this population are missing. For example, the STREAM trial did not enrol pregnant women as the shorter regimen for DR-TB investigated included two component (injectables and ethionamide/prothionamide) usually not recommended during pregnancy [198].
Table 3.
Summary of evidences for the use of second-line anti-tuberculosis drugs during pregnancy and breastfeeding (see separate word document)
| WHO Group | Drug | FDA Category | Animal and human evidences | Breastfeeding |
|---|---|---|---|---|
| A | Levofloxacin [1] | C | There are no or limited amount of data in pregnant women. No effect of foetuses in rats except for delayed maturation as a result of maternal toxicity. However, levofloxacin showed effects on cartilage (blistering and cavities) in rats and dogs. | No data in lactating or nursing women. However other fluoroquinolones are excreted in breast milk. |
| Moxifloxacin [2] | C | There are no or limited amount of data in pregnant women. Observed teratogenic potential in rabbits and rats at higher doses than those recommended in humans. | No data in lactating or nursing women. Preclinical data indicate that small amounts of moxifloxacin are secreted in milk | |
| Bedaquiline [3] | B | There are no or limited amount of data in pregnant women. No adverse effects observed in a pre- and post- natal development study in rats. | Unknown whether bedaquiline or its metabolites are excreted in human milk. In rats, bedaquiline concentrations in milk were 6- to 12-fold higher than the maximum concentration observed in maternal plasma | |
| Linezolid [4] | C | There are no or limited amount of data in pregnant women. Not teratogenic effect in mice and rats at exposures 4 times or equivalent to those in humans; however, the same concentrations caused maternal toxicity in mice and were related to increased embryo death. Minor maternal toxicity was observed at exposures lower than clinical exposures in rats. | Linezolid and its metabolites may be excreted in human milk | |
| B | Clofazimine [5] | C | There are no or limited amount of data in pregnant women. Cases of skin pigmentation at birth from infants born to pregnant mothers who had received clofazimine during pregnancy. In mice studies clofazimine induced embryotoxicity and fetotoxicity at doses equivalent to the 0.6 times maximum recommended human daily dose. No developmental effects observed in rats or rabbits. | Excreted in human milk. Skin discoloration observed in breast fed neonates of mothers receiving clofazimine. |
| Cycloserine [6] | C | There are no or limited amount of data in pregnant women. No teratogenic effect observed in rats | Excreted in human milk in concentrations that can be similar to maternal serum levels | |
| Terizidone [7] | C | There are no or limited amount of data in pregnant women. Animal data do not indicate potential for teratogenicity | May be excreted in human milk but no adverse effects observed | |
| C | Amikacin, streptomycin [8] | D | Aminoglycosides can cause foetal harm. Reports of total and irreversible bilateral congenital deafness in children born from mothers exposed to streptomycin during pregnancy. Foetal adverse effects have not been reported in pregnant women exposed to other aminoglycosides. | Streptomycin is excreted in human milk, no data for amikacin |
| Ethionamide [9] | C | There are no or limited amount of data in pregnant women. Reported teratogenic effects in mice, rabbits and rats at doses considerably higher than those recommended in humans. | No data in lactating or nursing women. | |
| Prothionamide [10] | C | There are no or limited amount of data in pregnant women. Embryotoxic and teratogenic effects in mice, rabbits and rats | Excreted in human milk. | |
| Delamanid [11] | Not assigned | There are no or limited amount of data in pregnant women. Embryo-fetal toxicity observed at maternally toxic dosages in rabbits | No data in lactating or nursing women. In lactating rats, the maximum concentration for delamanid in breast milk was 4-fold higher than plasma | |
| Para-aminosalicylic acid [12] | C | There are no or limited amount of data in pregnant women. In an embryofoetal development study in rats observed potential for malformations including bone defects at doses below those proposed clinically. In the rabbit, no effects on embryofoetal development; however, the doses evaluated were below those proposed clinically. | Para-aminosalicylic acid is excreted in human milk. There is insufficient information on the effects of para-aminosalicylic acid in newborns/infants.. | |
| Imipenem-cilastatin [13] | C | There are no or limited amount of data in pregnant women. In cynomolgus monkeys, at doses 3 times the usual recommended daily human intravenous dose, there was minimal maternal intolerance, no teratogenicity, but an increase in embryonic loss relative to control groups | Imipenem and cilastatin are excreted into the mother’s milk in small quantities. | |
| Meropenem [14] | B | There are no or limited amount of data in pregnant women. No teratogenic potential in studies in rats up to 750 mg/kg and in monkeys up to 360 mg/kg. | Small amounts of meropenem have been reported to be excreted in human milk. |
References
Accord-UK Ltd. Levofloxacin, Summary of Product Characteristics. 2019.
Bayer plc. Avelox - Summary of Product characteristics [Internet]. 2019 [cited 2019 Jul 7]. Available from: https://www.medicines.org.uk/emc/product/6771/smpc.
Janssen-Cilag Ltd. Sirturo 100 mg - Summary of Product characteristics [Internet]. 2019 [cited 2019 Jul 7]. Available from: https://www.medicines.org.uk/emc/product/3560/smpc.
Pfizer. Zyvox 600 mg - Summary of Product characteristics [Internet]. 2018 [cited 2019 Jul 7]. Available from: https://www.medicines.org.uk/emc/product/1688/smpc.
Novartis. Lamprene - Highlights of prescribing information [Internet]. 2016 [cited 2019 Jul 7]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019500s013lbl.pdf.
King Pharmaceuticals Ltd. Cycloserine - Summary of Product characteristics [Internet]. 2018 [cited 2019 Jul 7]. Available from: https://www.medicines.org.uk/emc/product/2252/smpc.
Macleods Pharmaceuticals Limited. Terizidone - Summary of Product characteristics [Internet]. 2018 [cited 2019 Jul 7]. Available from: https://extranet.who.int/prequal/sites/default/files/TB303part4v1.pdf.
Hospira UK Ltd. Amikacin [Internet]. 2015 [cited 2019 Jul 7]. Available from: https://www.medicines.org.uk/emc/product/3784/smpc.
Wyeth Pharmaceuticals Inc. Trecator [Internet]. 2018. Available from: http://labeling.pfizer.com/showlabeling.aspx?id=473.
Micro Labs Ltd. Protionamide [Internet]. 2016 [cited 2019 Jul 7]. Available from: https://extranet.who.int/prequal/sites/default/files/TB239part4v02.pdf.
Otsuka Novel Products GmbH. Deltyba [Internet]. 2019 [cited 2019 Jul 7]. Available from: https://www.medicines.org.uk/emc/product/5413/smpc.
Lucane Pharma. Granupas [Internet]. [cited 2019 Jul 7]. Available from: http://ec.europa.eu/health/documents/community-register/2018/20181218143055/anx_143055_en.pdf.
Merck Sharp & Dohme Limited. Primaxin [Internet]. 2018 [cited 2019 Jul 7]. Available from: https://www.medicines.org.uk/emc/product/1515/smpc.
Pfizer Limited. Meronem [Internet]. 2019 [cited 2019 Jul 7]. Available from: https://www.medicines.org.uk/emc/product/6731/smpc.
Other important considerations concerning the treatment of latent TB are the immunological changes that occur during pregnancy put women with HIV at high risk of progression from latent TB to active TB [199]. INH preventive therapy (IPT) is strongly recommended in HIV-infected individuals with latent TB, but the evidence of efficacy and safety of this regimen in pregnant women on ART is still unclear [200]. The TB APPRISE, a placebo-controlled randomised study comparing the efficacy and safety of immediate versus deferred IPT in pregnant women with HIV living in a high TB burden area, found that IPT during pregnancy associated with higher risk of adverse pregnancy outcomes [201]. However, more recently data showed no association between IPT and poor maternal and infant outcome [202] and therefore further studies are needed to elucidate the safety implications of IPT during pregnancy.
Among ART, recent concerns on dolutegravir exposure during conception and first trimester and increased risk of neural tube defects has arisen, thus its use in nonpregnant women of childbearing age is not recommended [203][204].
7. Conclusions
Several new ART agents with high efficacy and better tolerability have become available in the last few years. RIF-containing TB treatment remains the standard of care for first-line treatment of rifamycin-sensitive TB, and there are increasing number of ART treatment options that can be administered concomitantly. There is growing evidence for bedaquiline and delamanid efficacy and safety in management of DR-TB from both observational and interventional studies. Drugs originally approved for use in other infections, like linezolid and clofazimine, are increasingly being used in DR-TB management. Thus, finally patients with DR-TB have a wider range of choices available to build a more efficacious regimen and to individualize their therapy in case of contraindication or drug toxicity.
However, concomitant treatment for HIV and TB still poses challenges. Metabolism of many of the new antiretrovirals (e.g. bictegravir, doravirine) is induced by RIF and these drugs cannot be co-administered with standard first-line anti-TB drugs. For some drugs, dose adjustment (e.g. raltegravir, dolutegravir) or the alternative use of rifabutin in place of rifampicin (e.g. in combination with PI) is recommended, (Table 1). However, access to rifabutin is still limited in many low and middle-income countries and it is currently not available in fixed dose combinations with other anti-tuberculosis drugs and consequently rifabutin may not represent a practicable option and a switch to a different antiretroviral agent that can be administered with RIF is advised in this case. There is extensive data on use of efavirenz in combination with RIF, and it remains the drug with the most data to support its use during RIF-based TB treatment as well as being safe during pregnancy.
Pharmacokinetic considerations of bedaquiline and ART coadministration and recommendations for their use are reviewed in Table 2. Overlapping toxicities between ART and other anti-TB drugs for DR-TB are the main concern and limiting factor for their combined administration. Drug-related hepatotoxicity, neuropathy, neuro-psychiatric side effects, skin reactions, QT prolongation and other toxicities arising from the concomitant treatment of HIV and TB are not uncommon and can lead to drug discontinuation, subsequent poor treatment outcome and occasionally to fatal events. Clinicians should avoid, whenever possible, the combination of drugs with overlapping toxicities based on the most common reported adverse events shown in Figure 1.
Figure 1: Most common antiretrovirals and anti-tuberculosis drug toxicities.
Figure 1 has been adapted from ‘Adult male diagram template.svg’ available https://commons.wikimedia.org/wiki/File:Adult_male_diagram_template.svg using Inkscape software.
RPV, rilpivirine; EFV, efavirenz; 3TC, lamivudine; FTC, emtricitabine; PI, protease inhibitors; BDQ, bedaquiline; CFZ, clofazimine; DLM, delamanid; FQ, fluoroquinolones; NNRTI, non-nucleoside reverse transcriptase inhibitors; INSTI, integrase inhibitors; NVP, nevirapine; INH, isoniazid; RIF, rifampicin; PZA, pyrazinamide; PAS, para-aminosalicylic acid; ETB, ethambutol; LZD, linezolid; TDF, tenofovir disoproxil fumarate; Cs, cycloserine; TZD, terizidone; DTG, dolutegravir; AZT, zidovudine; ABC, abacavir; TAZ, thioacetazone;
Patient education and clinician awareness on the recognition of signs and symptoms of adverse drug reactions is crucial to prevent and promptly manage drug toxicities.
Diagnosing and managing TB IRIS is essential to successfully integrate ART and TB treatment and healthcare practitioners should be able to recognise early signs of IRIS to adequately start appropriate treatment. Risk of TB-IRIS is not a reason to defer ART initiation in those with low CD4 counts.
Finally, data on safe therapeutic options for the treatment of DR-TB in pregnant women are scarce. Supporting the use of condoms and performing counselling to promote the importance of adopting contraceptive methods during TB treatment to delay the pregnancy after the completion of TB treatment are key measures to protect future mothers and infants from the exposure to potential mutagenic drugs.
8. Expert opinion
The pharmacokinetic properties and toxicities of many antiretroviral and anti-TB drugs are well-recognised. Combined with the growing experience in the use of different regimens for both HIV and TB derived from recent observational and randomized trials, policymakers and healthcare professionals now have better data to facilitate early concomitant initiation of ART and TB treatment to improve the overall survival of PLWH with TB.
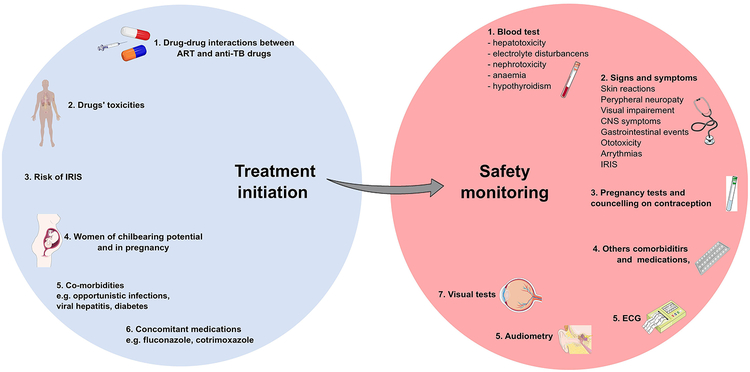
The development of toxicities is unpredictable, and, in several cases, the full range of antimicrobial agents cannot be used, for instance due to drug-resistance, economic resources limiting access to more expensive agents or contraindications during pregnancy. Clinicians should adopt a systematic approach that takes into consideration all the possible barriers that can interfere with an optimal integration of ART and TB treatment. An individualized assessment of each patient with HIV-associated TB and the application of a different range of tools beside laboratory tests (e.g. ECG, visual tests, audiometry, pregnancy test) as proposed in Figure 2, as a strategy that can be adopted to prevent and detect early adverse events. This approach is critical both at the start and also during the course of therapy to improve the overall treatment outcome.
Figure 2: Proposed approach to adopt to safely integrate antiretrovirals and anti-tuberculosis (TB) treatment.
The following considerations are crucial when approaching a patient with HIV-associated TB to combine antiretroviral therapy (ART) and TB treatment: 1. Consider drug-drug interactions between ART and anti-TB drugs, essential to avoid subtherapeutic or excessive drug exposure; 2. Avoid and minimise potential overlapping toxicities, for example favouring the use of levofloxacin over moxifloxacin when combined with other drugs with potential to prolong QT, like bedaquiline; 3. Consider risks factors for immune reconstitution inflammatory syndrome (IRIS) (e.g. CD4 count, HIV viral load) and consider prophylaxis with prednisone if appropriate; 4. Perform pregnancy test at baseline in all women of childbearing potential and discuss importance of contraception and methods during TB treatment (especially for drug resistant-TB); 5. Consider concomitant comorbidities (e.g. Hepatitis B/C, diabetes mellitus, alcohol) and opportunistic infection treatments and prophylaxis that could exacerbate drug toxicities; 6. Evaluate concomitant medications with potential overlapping toxicities and/or drug interactions and consider alternatives.
During the course of ART and TB treatment safety monitoring is essential and should include: 1. Blood tests to detect early signs of drug toxicities; 2. Monitoring signs and symptoms of drug toxicities, educating patients to recognise and report them to the healthcare team; 3. Routine pregnancy tests and reinforced counselling to promote contraception and the use of condoms; 4. Regular evaluation of concomitant comorbidities and medications that could increase risk of toxicities. 5. Regular ECG monitoring during the course of drugs associated with QT prolongation, especially when used in combination (e.g. bedaquiline, delamanid, fluoroquinolones): 6. Audiometry follow up should be considered during the treatment with aminoglycosides to detect early signs of ototoxicity; 7. Visual function tests should be used to identify early signs of optic neuropathy during treatment with ethambutol and linezolid.
This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com
Limitations of current knowledge and future research challenges
Due to its high potency and genetic barrier to resistance, dolutegravir is the drug of choice for the treatment of HIV recommended by the WHO. Nevertheless, its administration with RIF is currently recommended at the double dose of 50 mg twice daily. Adding an extra dose can represent a challenge to its implementation in low and middle-income countries, due to potential for decreased patient adherence and increased cost. Preliminary data on using the standard dolutegravir 50mg daily dose together with RIF in healthy volunteers are promising [43], hence assessing the safety and efficacy of the standard dose of dolutegravir of 50 mg once daily in PLWH infected with TB as a next step is a research priority to accelerate dolutegravir global implementation. More data informing on the most appropriate dose of raltegravir to adopt in patients with HIV-associated TB are also needed in order to expand its use in this population. Tenofovir alafenamide is gradually replacing tenofovir disoproxil fumarate in many of the newly approved ART combinations and also as pre-exposure prophylaxis option for HIV [205] in light of its safer profile in terms of nephrotoxicity and bone health. Drug interaction and efficacy studies in patients with HIV-associated TB are necessary to expand the access to patients on TB treatment, especially when concomitant renal impairment is present.
Rifamycins still represent the core agents for the treatment of drug sensitive-TB and no valid substitutive options have so far proven equivalent efficacy. While effort should be addressed to develop new TB regimens that do not include rifamycins, future work will also need to be directed to generate antiviral agents compatible with TB drugs.
Rifabutin represents a valuable option combined with many of ART agents that are subject to RIF induction, however its use is limited due to the higher cost compared to RIF and the lack of co-formulations with other anti-TB drugs that would reduce the pill burden and improve adherence. In addition, pharmacokinetic data of rifabutin administered with tenofovir alafenamide are missing; efficacy and safety data of rifabutin administered with doravirine, dolutegravir or bictegravir come from individual PK studies enrolling few subjects and therefore a need for larger trials including PLWH with TB informing on the use of rifabutin are warranted.
Amongst newly introduced molecules and shortened regimens for the treatment of DR-TB, efficacy and safety data in PLWH are still scarce and investigators should ensure the inclusion of larger numbers of HIV-infected individuals in future clinical trials for DR-TB. An exceptionally difficult challenge is represented by the management of DR-TB in HIV infected pregnant women, as many HIV and anti-TB drugs are not recommended particularly during early gestation or data informing on their use during pregnancy are not available. Pregnant women have been underrepresented or excluded from most pharmacokinetic and efficacy trials investigating the treatment of HIV-infected individuals with TB. As a consequence, building an efficacious and safe regimen in this context, especially when some of the remaining drugs cannot be reintroduced due to the appearance of drug reactions, requires extensive expertise as most of the current knowledge is derived from case reports or clinicians’ personal experience. Capturing and ultimately publishing data of pregnancy-associated outcomes and perinatal follow-up appear fundamental to inform guidelines and physicians when managing this vulnerable population.
The length of treatment required for both latent TB and TB can often lead to premature discontinuation and subsequent failure. Long-acting injectable (LAI) formulations of antipsychotics and contraceptives have been successfully used to improve adherence and treatment outcome. Developing LAI for TB prevention and treatment, to be administered for instance monthly, could enhance adherence and could represent a future successful strategy [206]. Not all anti-TB drugs have, however, the appropriate physicochemical composition that allow them to be suitable for long-acting formulations, nevertheless preliminary data on bedaquiline show promising results and further studies should further investigate this [207].
Article highlights.
Overview of the recent advances in HIV and TB treatment.
Pharmacokinetic data available on the co-administration of ART, and anti-TB drugs used in drug-sensitive, drug-resistant and latent TB.
Current evidence on overlapping toxicities between ART and first- and second-line anti-TB drugs.
Treatment issues during pregnancy.
Update on risk factors, management and prevention of tuberculosis-immune reconstitution inflammatory syndrome (TB-IRIS).
Adopting a systematic approach to successfully integrate treatment for HIV and TB.
Expert opinion and future research challenges.
Funding
This paper was not funded.
Footnotes
Declaration of interest
M Cerrone has received a travel grant from Gilead over 2 years ago.
RJ Wilkinson has performed consultancy on vaccines against tuberculosis for GSK. RJ Wilkinson receives support from Wellcome (104803, 203135) and the Francis Crick institute which is funded by Wellcome (FC0010218), UKRI (FC0010218) and CRUK (FC0010218); NIH (U01 AI 5940); EDCTP (SRIA 2015–1065); FNIH (WILKI16PTB).
A Pozniak joins advisory boards and symposia for ViiV, Gilead, Janssen and Merck. A Pozniak chairs BHIVA TB guidelines, is a panel member on the EACS OI guidelines committee, and a member of the WHO ARV guidelines committee.
G Meintjes was supported by the Wellcome Trust (098316 and 203135/Z/16/Z), the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa (Grant No 64787), NRF incentive funding (UID: 85858) and the South African Medical Research Council through its TB and HIV Collaborating Centres Programme with funds received from the National Department of Health (RFA# SAMRC-RFA-CC).
S Wasserman is supported by the European & Developing Countries Clinical Trials Partnership (CDF1018) and Wellcome Trust (203135/Z/16/Z).
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- [1].WHO Organization. Fact sheets - Tuberculosis [Internet] 2018. [cited 2019 May 30]. Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
- [2].Zhou J, Elliott J, Li PC, et al. Risk and prognostic significance of tuberculosis in patients from The TREAT Asia HIV Observational Database. BMC Infect. Dis 2009;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Manosuthi W, Chottanapand S, Thongyen S, et al. Survival Rate and Risk Factors of Mortality Among HIV/Tuberculosis-Coinfected Patients With and Without Antiretroviral Therapy. JAIDS J. Acquir. Immune Defic. Syndr 2006;43:42–6. [DOI] [PubMed] [Google Scholar]
- [4].Sungkanuparph S, Manosuthi W, Kiertiburanakul S, et al. Initiation of antiretroviral therapy in advanced AIDS with active tuberculosis: Clinical experiences from Thailand. J. Infect [Internet] 2006;52:188–194. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0163445305001398. [DOI] [PubMed] [Google Scholar]
- [5].Wang H, Lu X, Yang X, et al. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy. Medicine (Baltimore) [Internet] 2016;95:e5146 Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00005792-201610110-00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Amin J, Becker S, Belloso W, et al. Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): A randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet 2014;383:1474–82. [DOI] [PubMed] [Google Scholar]
- [7].Dickinson L, Amin J, Else L, et al. Pharmacokinetic and Pharmacodynamic Comparison of Once-Daily Efavirenz (400 mg vs. 600 mg) in Treatment-Naive HIV-Infected Patients: Results of the ENCORE1 Study. Clin. Pharmacol. Ther 2015;98:406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Orkin Chloe, Arastéh Keikawus H-M MG et al. LONG-ACTING CABOTEGRAVIR + RILPIVIRINE FOR HIV MAINTENANCE: FLAIR WEEK 48 RESULTS. Conf. Retrovir. Opportunistic Infect. Seattle, March 4–7, 2019 [Internet] 2019. Available from: http://www.croiconference.org/sessions/long-acting-cabotegravir-rilpivirine-hiv-maintenance-flair-week-48-results. [Google Scholar]
- [9].World Health Organization. WHO Global Tuberculosis Report 2018. Pharmacol. Reports 2018.
- [10].Tiberi S, Muñoz-Torrico M, Duarte R, et al. New drugs and perspectives for new anti-tuberculosis regimens. Rev. Port. Pneumol (English Ed. 2018;24:86–98. [DOI] [PubMed] [Google Scholar]
- [11].Van Deun A, Maug AKJ, Salim MAH, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am. J. Respir. Crit. Care Med 2010;182:684–92. [DOI] [PubMed] [Google Scholar]; *’The Bangladesh study’ is a crucial study on the efficacy of shorter regimens for DR-TB
- [12].Hurtado RM, Meressa D, Goldfeld AE. Treatment of drug-resistant tuberculosis among people living with HIV. Curr. Opin. HIV AIDS 2018;13:478–85. [DOI] [PubMed] [Google Scholar]; *Recent comprehensive review on this topic
- [13].Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am. J. Respir. Crit. Care Med 2010;181:80–6. [DOI] [PubMed] [Google Scholar]
- [14].Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006;368:1575–80. [DOI] [PubMed] [Google Scholar]
- [15].McIlleron H, Meintjes G, Burman WJ, et al. Complications of Antiretroviral Therapy in Patients with Tuberculosis: Drug Interactions, Toxicity, and Immune Reconstitution Inflammatory Syndrome. J. Infect. Dis [Internet] 2007;196:S63–S75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17624828. [DOI] [PubMed] [Google Scholar]; *Detailed review on this topic
- [16].Gengiah TN, Holford NHG, Botha JH, et al. The influence of tuberculosis treatment on efavirenz clearance in patients co-infected with HIV and tuberculosis. Eur. J. Clin. Pharmacol 2012;68:689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Niemi M, Backman JT, Fromm MF, et al. Pharmacokinetic interactions with rifampicin: Clinical relevance. Clin. Pharmacokinet 2003. p. 819–50. [DOI] [PubMed] [Google Scholar]
- [18].Baciewicz AM, Chrisman CR, Finch CK ST. Update on rifampin and rifabutin drug interactions. Am J Med Sci 2008. February;335(2)126–36. doi 10.1097/MAJ.0b013e31814a586a. [DOI] [PubMed] [Google Scholar]
- [19].Wenning LA, Hanley WD, Brainard DM, et al. Effect of rifampin, a potent inducer of drug-metabolizing enzymes, on the pharmacokinetics of raltegravir. Antimicrob. Agents Chemother 2009;53:2852–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Greiner B, Eichelbaum M, Fritz P, et al. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J. Clin. Invest 1999;104:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cerrone M, Alfarisi O, Neary M, et al. Rifampicin effect on intracellular and plasma pharmacokinetics of tenofovir alafenamide. J. Antimicrob. Chemother 2019;74:1670–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gilead sciences. Descovy - Summary of Product Characteristics [Internet] [cited 2019 May 30]. Available from: https://www.medicines.org.uk/emc/product/2108/smpc.
- [23].Gengiah T, Baxter C, Gray AL, et al. Integration of Antiretroviral Therapy with Tuberculosis Treatment. N. Engl. J. Med 2011;365:1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Blanc F-X, Sok T, Laureillard D, et al. Earlier versus Later Start of Antiretroviral Therapy in HIV-Infected Adults with Tuberculosis. N. Engl. J. Med [Internet] 2011;365:1471–81. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Havlir DV, Kendall MA, Ive P, et al. Timing of Antiretroviral Therapy for HIV-1 Infection and Tuberculosis. N. Engl. J. Med 2011;365:1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mfinanga SG, Kirenga BJ, Chanda DM, et al. Early versus delayed initiation of highly active antiretroviral therapy for HIV-positive adults with newly diagnosed pulmonary tuberculosis (TB-HAART): A prospective, international, randomised, placebo-controlled trial. Lancet Infect. Dis 2014;14:563–571. [DOI] [PubMed] [Google Scholar]; *Important study informing on antiretroviral initiation during TB treatment
- [27].Boulle A, Van Cutsem G, Cohen K, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA - J. Am. Med. Assoc [Internet] 2008;300:530–39. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- [28].Ward BA. The Cytochrome P450 2B6 (CYP2B6) Is the Main Catalyst of Efavirenz Primary and Secondary Metabolism: Implication for HIV/AIDS Therapy and Utility of Efavirenz as a Substrate Marker of CYP2B6 Catalytic Activity. J. Pharmacol. Exp. Ther [Internet] 2003;306:287–300. Available from: http://jpet.aspetjournals.org/cgi/doi/10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- [29].Itoh M, Nakajima M, Higashi E, et al. Induction of Human CYP2A6 Is Mediated by the Pregnane X Receptor with Peroxisome Proliferator-Activated Receptor- Coactivator 1. J. Pharmacol. Exp. Ther 2006;319:693–702. [DOI] [PubMed] [Google Scholar]
- [30].Lopez-Cortes LF, Ruiz-Valderas R, Viciana P, et al. Pharmacokinetic Interactions Between Efavirenz and Rifampicin in HIV-Infected Patients with Tuberculosis. Clin. Pharmacokinet 2002;41:681–90. [DOI] [PubMed] [Google Scholar]
- [31].Luetkemeyer AF, Rosenkranz SL, Lu D, et al. Relationship between weight, efavirenz exposure, and virologic suppression in HIV-infected patients on rifampin-based tuberculosis treatment in the AIDS clinical trials group A5221 STRIDE study. Clin. Infect. Dis 2013;57:586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Manosuthi W, Sukasem C, Lueangniyomkul A, et al. Impact of pharmacogenetic markers of CYP2B6, clinical factors, and drug-drug interaction on efavirenz concentrations in HIV/tuberculosis-coinfected patients. Antimicrob. Agents Chemother 2013;57:1019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marzolini C, Telenti A, Decosterd LA, et al. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 2001;15:71–75. [DOI] [PubMed] [Google Scholar]
- [34].Cerrone M, Wang X, Neary M, et al. Pharmacokinetics of Efavirenz 400 mg Once Daily Coadministered With Isoniazid and Rifampicin in Human Immunodeficiency Virus-Infected Individuals. Clin. Infect. Dis 2019;68:446–52. [DOI] [PubMed] [Google Scholar]
- [35].Kaboggoza JP, Wang X, Neary M, et al. A Lower Dose of Efavirenz Can Be Coadministered With Rifampicin and Isoniazid in Tuberculosis Patients. Open Forum Infect. Dis 2019;6(2):ofz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Crauwels H, Van Heeswijk RPG, Stevens M, et al. Clinical perspective on drug-drug interactions with the non-nucleoside reverse transcriptase inhibitor rilpivirine. AIDS Rev 2013. p. 87–101. [PubMed] [Google Scholar]
- [37].Kakuda TN, Schöller-Gyüre M, Hoetelmans RMW. Pharmacokinetic interactions between etravirine and non-antiretroviral drugs. Clin. Pharmacokinet 2011;50:25–39. [DOI] [PubMed] [Google Scholar]
- [38].Yee KL, Khalilieh SG, Sanchez RI, et al. The Effect of Single and Multiple Doses of Rifampin on the Pharmacokinetics of Doravirine in Healthy Subjects. Clin. Drug Investig 2017;37:659–67. [DOI] [PubMed] [Google Scholar]
- [39].Khalilieh SG, Yee KL, Sanchez RI, et al. Multiple Doses of Rifabutin Reduce Exposure of Doravirine in Healthy Subjects. J. Clin. Pharmacol 2018;58:1044–52. [DOI] [PubMed] [Google Scholar]
- [40].ViiV Healthcare UK Ltd. Tivicay, Summary of Product Characteristics [Internet] 2019. [cited 2019 May 30]. Available from: https://www.medicines.org.uk/emc/product/5248/smpc.
- [41].Grinsztejn B, De Castro N, Arnold V, et al. Raltegravir for the treatment of patients co-infected with HIV and tuberculosis (ANRS 12 180 Reflate TB): A multicentre, phase 2, non-comparative, open-label, randomised trial. Lancet Infect. Dis 2014;14:459–467. [DOI] [PubMed] [Google Scholar]
- [42].Dooley KE, Kaplan R, Mwelase N, et al. Dolutegravir-Based Antiretroviral Therapy for Patients Co-Infected with Tuberculosis and Hiv: A Multicenter, Noncomparative, Open-Label, Randomized Trial. Clin. Infect. Dis [Internet] 2019;[Epub ahead of print]. Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciz256/5421214. [DOI] [PubMed] [Google Scholar]; *Important clinical trial on the use of dolutegravir twice daily with rifampicin
- [43].Wang X, Cerrone M, Ferretti F, et al. Pharmacokinetics of dolutegravir 100 mg once daily with rifampicin. Int. J. Antimicrob. Agents [Internet] 2019;[Epub ahead of print]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0924857919300950. [DOI] [PubMed] [Google Scholar]
- [44].Gilead Sciences Ltd. Stribild - Summary of Product Characteristics [Internet] 2019. [cited 2019 May 30]. Available from: https://www.medicines.org.uk/emc/product/3154/smpc.
- [45].Custodio Joseph M., Steve K. West SC et al. PHARMACOKINETICS OF BICTEGRAVIR ADMINISTERED TWICE DAILY IN COMBINATION WITH RIFAMPIN Conf. Retrovir. Opportunistic Infect. Boston, March 4–7, 2018 2019. [Google Scholar]
- [46].Gilead Sciences Ltd. Biktarvy - Summary of Product Characteristics [Internet] [cited 2019 May 27]. Available from: https://www.medicines.org.uk/emc/product/9313/smpc.
- [47].Zhang H, Custodio JM, Wei X et al. Clinical pharmacology of the HIV integrase strand transfer inhibitor bictegravir Conf. Retrovir. Opportunistic Infect. Seattle, Febr. 13–16, 2017 p. Abstract 40. [Google Scholar]
- [48].Decloedt EH, McIlleron H, Smith P, et al. Pharmacokinetics of Lopinavir in HIV-Infected Adults Receiving Rifampin with Adjusted Doses of Lopinavir-Ritonavir Tablets. Antimicrob. Agents Chemother 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].La Porte CJL, Colbers EPH, Bertz R, et al. Pharmacokinetics of Adjusted-Dose Lopinavir-Ritonavir Combined with Rifampin in Healthy Volunteers. Antimicrob. Agents Chemother 2004; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yapa HM, Boffito M, Pozniak A. What dose of rifabutin is recommended with antiretroviral therapy? J. Acquir. Immune Defic. Syndr 2016;72:138–52. [DOI] [PubMed] [Google Scholar]
- [51].European AIDS Clinical Society. European AIDS Clinical Society (EACS) Guidelines Version 9 [Internet] 2017;72 Available from: http://www.eacsociety.org/files/guidelines_9.0-english.pdf%0Ahttp://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. [Google Scholar]
- [52].BHIVA. British HIVAssociation guidelines for the management of tuberculosis in adults living with HIV 2019 [Internet] HIV Med 2019. p. s2–s83. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/hiv.12748. [DOI] [PubMed] [Google Scholar]
- [53].Gilead Sciences Ltd. Tybost - Summary of Product Characteristics [Internet] 2019. [cited 2019 May 30]. Available from: https://www.medicines.org.uk/emc/product/1277/smpc.
- [54].Ford SL, Sutton K, Lou Y, et al. Effect of Rifampin on the Single-Dose Pharmacokinetics of Oral Cabotegravir in Healthy Subjects. Antimicrob. Agents Chemother [Internet] 2017;61 Available from: http://aac.asm.org/lookup/doi/10.1128/AAC.00487-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Emu B, Fessel J, Schrader S, et al. Phase 3 Study of Ibalizumab for Multidrug-Resistant HIV-1. N. Engl. J. Med 2018;379:645–654. [DOI] [PubMed] [Google Scholar]
- [56].Yang W, Xiao Q, Wang D, et al. Evaluation of pharmacokinetic interactions between long-acting HIV-1 fusion inhibitor albuvirtide and lopinavir/ritonavir, in HIV-infected subjects, combined with clinical study and simulation results. Xenobiotica 2017;47:133–43. [DOI] [PubMed] [Google Scholar]
- [57].Hruska M, Anderson J, Bedford B et al. The effect of rifampin on the pharmacokinetics of the HIV-1 attachment inhibitor prodrug BMS-663068 in healthy subjects 14th Int. Work. Clin. Pharmacol. HIV Ther. April 22–24, 2013; Amsterdam, Netherlands. [Google Scholar]
- [58].Adamczyk R, Griffies A, Ravindran P, Smith C, Anderson J et al. HIV-1 Attachment Inhibitor Prodrug BMS-663068: Interactions with Rifabutin, with or without Ritonavir, in Healthy Subjects 8th IAS Conf. HIV Pathog. Treat. Prev. 2015. [Google Scholar]
- [59].McIlleron HM, Schomaker M, Ren Y, et al. Effects of rifampin-based antituberculosis therapy on plasma efavirenz concentrations in children vary by CYP2B6 genotype. AIDS 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].COHN D Treatment of latent tuberculosis infection. Semin. Respir. Infect 2003;18:249–262. [DOI] [PubMed] [Google Scholar]
- [61].Dooley KE, Bliven-Sizemore EE, Weiner M, et al. Safety and Pharmacokinetics of Escalating Daily Doses of the Antituberculosis Drug Rifapentine in Healthy Volunteers. Clin. Pharmacol. Ther 2012;91:881–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Centers for Disease Control and Prevention (CDC). Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR. Morb. Mortal. Wkly. Rep [Internet] 2011;60:1650–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22157884. [PubMed] [Google Scholar]
- [63].Swindells S, Ramchandani R, Gupta A, et al. ONE MONTH OF RIFAPENTINE/ISONIAZID TO PREVENT TB IN PEOPLE WITH HIV: BRIEF-TB/A5279 Conf. Retrovir. Opportunistic Infect. Boston, March 4–7, 2018 2018. [Google Scholar]
- [64].Podany AT, Bao Y, Swindells S, et al. Efavirenz Pharmacokinetics and Pharmacodynamics in HIV-Infected Persons Receiving Rifapentine and Isoniazid for Tuberculosis Prevention. Clin. Infect. Dis 2015;61:1322–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Farenc C, Doroumian S, Cantalloube C, et al. Rifapentine Once-Weekly Dosing Effect On Efavirenz, Emtricitabine and Tenofovir PKs. Top. Antivir. Med 2014;Conference:233–234. [Google Scholar]
- [66].Weiner M, Egelund EF, Engle M, et al. Pharmacokinetic interaction of rifapentine and raltegravir in healthy volunteers. J. Antimicrob. Chemother 2014;69:1079–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Brooks KM, George JM, Pau AK, et al. Cytokine-mediated systemic adverse drug reactions in a drug-drug interaction study of dolutegravir with once-weekly isoniazid and rifapentine. Clin. Infect. Dis 2018;67:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dooley KE, Churchyard G, Savic RM, et al. SAFETY & PK OF WEEKLY RIFAPENTINE/ISONIAZID (3HP) IN ADULTS WITH HIV ON DOLUTEGRAVIR Conf. Retrovir. Opportunistic Infect. Seattle, March 4–7, 2019 [Internet]. 2019. p. Abstract number 80. Available from: http://www.croiconference.org/sessions/safety-pk-weekly-rifapentineisoniazid-3hp-adults-hiv-dolutegravir. [Google Scholar]; *Important study on safety of rifapentine/isoniazid and dolutegravir
- [69].Coyne KM, Pozniak AL, Lamorde M, et al. Pharmacology of second-line antituberculosis drugs and potential for interactions with antiretroviral agents. AIDS 2009;23:437–46. [DOI] [PubMed] [Google Scholar]
- [70].Naidoo A, Chirehwa M, McIlleron H, et al. Effect of rifampicin and efavirenz on moxifloxacin concentrations when co-administered in patients with drug-susceptible TB. J. Antimicrob. Chemother 2017;72:1441–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Schnippel K, Ndjeka N, Maartens G, et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir. Med 2018; [DOI] [PubMed] [Google Scholar]
- [72].van Heeswijk RPG, Dannemann B, Hoetelmans RMW. Bedaquiline: A review of human pharmacokinetics and drug-drug interactions. J. Antimicrob. Chemother 2014;69:2310–2318. [DOI] [PubMed] [Google Scholar]
- [73].Hsu A, Granneman GR, Bertz RJ. Ritonavir: Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin. Pharmacokinet 1998;35:275–91. [DOI] [PubMed] [Google Scholar]
- [74].Kirby BJ, Collier AC KE. Complex drug interactions of HIV protease inhibitors 1: Inactivation, induction, and inhibition of cytochrome P450 3A by ritonavir or nelfinavir. Drug Metab. Dispos 2011. p. 1070–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Usach I, Melis V, Peris J-E. Non-nucleoside reverse transcriptase inhibitors: a review on pharmacokinetics, pharmacodynamics, safety and tolerability. J. Int. AIDS Soc 2013;16:18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Dooley KE, Park JG, Swindells S, et al. Safety, tolerability, and pharmacokinetic interactions of the antituberculous agent TMC207 (Bedaquiline) with efavirenz in healthy volunteers: AIDS clinical trials group study A5267. J. Acquir. Immune Defic. Syndr 2012;59:455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Svensson EM, Aweeka F, Park J-G, et al. Model-Based Estimates of the Effects of Efavirenz on Bedaquiline Pharmacokinetics and Suggested Dose Adjustments for Patients Coinfected with HIV and Tuberculosis. Antimicrob. Agents Chemother 2013;57:2780–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Svensson EM, Dooley KE, Karlsson MO. Impact of Lopinavir-Ritonavir or Nevirapine on Bedaquiline Exposures and Potential Implications for Patients with Tuberculosis-HIV Coinfection. Antimicrob. Agents Chemother 2014;58:6406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Pandie M, Wiesner L, McIlleron H, et al. Drug-drug interactions between bedaquiline and the antiretrovirals lopinavir/ritonavir and nevirapine in HIV-infected patients with drug-resistant TB. J. Antimicrob. Chemother 2016;71:1037–40. [DOI] [PubMed] [Google Scholar]
- [80].Sharma M, Saravolatz LD. Rilpivirine: A new non-nucleoside reverse transcriptase inhibitor. J. Antimicrob. Chemother 2013;68:250–56. [DOI] [PubMed] [Google Scholar]
- [81].Brill MJE, Svensson EM, Pandie M, et al. Confirming model-predicted pharmacokinetic interactions between bedaquiline and lopinavir/ritonavir or nevirapine in patients with HIV and drug-resistant tuberculosis. Int. J. Antimicrob. Agents 2017;49:212–17. [DOI] [PubMed] [Google Scholar]
- [82].Mesens N, Verbeeck J, Rouan M, De Beule KVP. Elucidating the role of M2 in the preclinical safety profile of TMC207 38th World Conf. Lung Heal. Int. Union Against Tuberc. Lung Dis. 2007. 2007. p. 2007 p. 8–12. [Google Scholar]
- [83].Tanneau L, Svensson EM, Rossenu S, et al. Bedaquiline appears to antagonize its main metabolite ‘s QTcF interval prolonging effect. Tuberc. PK 2018, Netherlands, 23 Oct. 2018 [Internet] 2016. p. 2016 Available from: http://regist2.virology-education.com/presentations/2018/11TBPK/03_Tanneau.pdf.
- [84].Ball P Quinolone-induced QT interval prolongation: a not-so-unexpected class effect. J. Antimicrob. Chemother 2000;45:557–59. [DOI] [PubMed] [Google Scholar]
- [85].Démolis JL, Kubitza D, Tennezé L, et al. Effect of a single oral dose of moxifloxacin (400 mg and 800 mg) on ventricular repolarization in healthy subjects. Clin. Pharmacol. Ther 2000;68:658–66. [DOI] [PubMed] [Google Scholar]
- [86].Diacon AH, Dawson R, Von Groote-Bidlingmaier F, et al. Bactericidal activity of pyrazinamide and clofazimine alone and in combinations with pretomanid and bedaquiline. Am. J. Respir. Crit. Care Med 2015;191:943–53. [DOI] [PubMed] [Google Scholar]
- [87].Lynch J, Szumowski J. Profile of delamanid for the treatment of multidrug-resistant tuberculosis. Drug Des. Devel. Ther [Internet] 2015;677 Available from: http://www.dovepress.com/profile-of-delamanid-for-the-treatment-of-multidrug-resistant-tubercul-peer-reviewed-article-DDDT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dannemann B, Bakare NMT. QTcF prolongation in a Phase II trial of TMC207 plus background regimen as treatment for multidrug-resistant tuberculosis: effect of co-administration with clofazimine 52nd Intersci. Conf. Antimicrob. Agents Chemother 2012 9–12 Sept. 2012; San Fr. CA, USA; 2012. [Google Scholar]
- [89].Ferlazzo G, Mohr E, Laxmeshwar C, et al. Early safety and efficacy of the combination of bedaquiline and delamanid for the treatment of patients with drug-resistant tuberculosis in Armenia, India, and South Africa: a retrospective cohort study. Lancet Infect. Dis 2018;18:536–44. [DOI] [PubMed] [Google Scholar]
- [90].Dooley KE, Rosenkranz SL, Conradie F, et al. Qt Effects of Bedaquiline, Delamanid or Both in MDR-TB Patients : the Deliberate Trial Conf. Retrovir. Opportunistic Infect. Seattle, March 4–7, 2019 2019 p. 1–4. [Google Scholar]
- [91].Sasahara K, Shimokawa Y, Hirao Y, et al. Pharmacokinetics and metabolism of delamanid, a novel anti-tuberculosis drug, in animals and humans: Importance of albumin metabolism in vivo. Drug Metab. Dispos 2015;43:1267–76. [DOI] [PubMed] [Google Scholar]
- [92].Shimokawa Y, Sasahara K, Koyama N, et al. Metabolic mechanism of delamanid, a new anti-tuberculosis drug, in human plasma. Drug Metab. Dispos 2015;43:1277–83. [DOI] [PubMed] [Google Scholar]
- [93].Sasabe H, Shimokawa Y, Shibata M, et al. Erratum for Sasabe et al., Antitubercular Agent Delamanid and Metabolites as Substrates and Inhibitors of ABC and Solute Carrier Transporters. Antimicrob. Agents Chemother [Internet] 2016;60:4431–31. Available from: http://aac.asm.org/lookup/doi/10.1128/AAC.01094-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shimokawa Y, Sasahara K, Yoda N, et al. Delamanid does not inhibit or induce cytochrome p450 enzymes in vitro. Biol. Pharm. Bull [Internet] 2014;37:1727–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25366478. [DOI] [PubMed] [Google Scholar]
- [95].Paccaly A, Petersen C, Patil S, Bricmont P, Kim J, Harlin M et al. Absence of clinically relevant drug interaction between delamanid, a new drug for multidrug-resistant tuberculosis (MDR-TB) and tenofovir or lopinavir/ritonavir in healthy subjects 19th Int. AIDS Conf. 2012. 2012. p. 22–7. [Google Scholar]
- [96].Mallikaarjun S, Wells C, Petersen C, et al. Delamanid Coadministered with Antiretroviral Drugs or Antituberculosis Drugs Shows No Clinically Relevant Drug-Drug Interactions in Healthy Subjects. Antimicrob. Agents Chemother 2016;60:5976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Dooley KE, Luetkemeyer AF, Park J-G, et al. Phase I Safety, Pharmacokinetics, and Pharmacogenetics Study of the Antituberculosis Drug PA-824 with Concomitant Lopinavir-Ritonavir, Efavirenz, or Rifampin. Antimicrob. Agents Chemother 2014;58:5245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].WHO Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment [Internet] 2019. Available from: http://apps.who.int/bookorders.%0Ahttp://www.ncbi.nlm.nih.gov/pubmed/30946559. [PubMed]; *WHO guidelines on DR-TB treatment
- [99].Slatter JG, Stalker DJ, Feenstra KL, et al. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [14C]linezolid to healthy human subjects. Drug Metab. Dispos 2001;29:1136–45. [PubMed] [Google Scholar]
- [100].Gandelman K, Zhu T, Fahmi OA, et al. Unexpected effect of rifampin on the pharmacokinetics of linezolid: In silico and in vitro approaches to explain its mechanism. J. Clin. Pharmacol 2011;51:229–36. [DOI] [PubMed] [Google Scholar]
- [101].Bolhuis MS, Van Altena R, Van Soolingen D, et al. Clarithromycin increases linezolid exposure in multidrug-resistant tuberculosis patients. Eur. Respir. J 2013;42:1614–21. [DOI] [PubMed] [Google Scholar]
- [102].Wasserman S, Denti P, Brust JCM, et al. Linezolid Pharmacokinetics in South African Patients with Drug-Resistant Tuberculosis and a High Prevalence of HIV Coinfection. Antimicrob. Agents Chemother [Internet] 2019;63 Available from: http://aac.asm.org/lookup/doi/10.1128/AAC.02164-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Hughes J, Isaakidis P, Andries A, et al. Linezolid for multidrug-resistant tuberculosis in HIV-infected and -uninfected patients. Eur. Respir. J 2015;46:271–4. [DOI] [PubMed] [Google Scholar]
- [104].Brinkman K, Ter Hofstede HJM, Burger DM, et al. Adverse effects of reverse transcriptase inhibitors: Mitochondrial toxicity as common pathway. AIDS 1998. p. 1735–44. [DOI] [PubMed] [Google Scholar]
- [105].Wasserman S, Meintjes G, Maartens G. Linezolid in the treatment of drug-resistant tuberculosis: the challenge of its narrow therapeutic index. Expert Rev. Anti. Infect. Ther 2016. p. 901–15. [DOI] [PubMed] [Google Scholar]
- [106].De Vriese AS, Van Coster R, Smet J, et al. Linezolid-Induced Inhibition of Mitochondrial Protein Synthesis. Clin. Infect. Dis 2006;42:1111–17. [DOI] [PubMed] [Google Scholar]
- [107].Holdiness MR. Clinical Pharmacokinetics of Clofazimine: A Review. Clin. Pharmacokinet 1989. p. 74–85. [DOI] [PubMed] [Google Scholar]
- [108].Te Brake LHM, Russel FGM, Van Den Heuvel JJMW, et al. Inhibitory potential of tuberculosis drugs on ATP-binding cassette drug transporters. Tuberculosis 2016;96:150–7. [DOI] [PubMed] [Google Scholar]
- [109].Shimokawa Y, Yoda N, Kondo S, et al. Inhibitory Potential of Twenty Five Anti-tuberculosis Drugs on CYP Activities in Human Liver Microsomes. Biol. Pharm. Bull 2015;38:1425–29. [DOI] [PubMed] [Google Scholar]
- [110].Horita Y, Doi N. Comparative Study of the Effects of Antituberculosis Drugs and Antiretroviral Drugs on Cytochrome P450 3A4 and P-Glycoprotein. Antimicrob. Agents Chemother 2014;58:3168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Maartens G, Brill MJE, Pandie M, et al. Pharmacokinetic interaction between bedaquiline and clofazimine in patients with drug-resistant tuberculosis. Int. J. Tuberc. Lung Dis 2017;22:26–9. [DOI] [PubMed] [Google Scholar]
- [112].Schnippel K, Berhanu RH, Black A, et al. Severe adverse events during second-line tuberculosis treatment in the context of high HIV Co-infection in South Africa: a retrospective cohort study. BMC Infect. Dis [Internet] 2016;16:593 Available from: http://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-016-1933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Isaakidis P, Varghese B, Mansoor H, et al. Adverse Events among HIV/MDR-TB Co-Infected Patients Receiving Antiretroviral and Second Line Anti-TB Treatment in Mumbai, India. PLoS One 2012;7:e40781. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Informative cohort study on this topic
- [114].Ramachandran G, Swaminathan S. Safety and Tolerability Profile of Second-Line Anti-Tuberculosis Medications. Drug Saf 2015;38:253–69. [DOI] [PubMed] [Google Scholar]
- [115].Kenyon C, Wearne N, Burton R, et al. The risks of concurrent treatment with tenofovir and aminoglycosides in patients with HIV-associated tuberculosis. South. Afr. J. HIV Med 2017;12:43–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Neuman MG, Schneider M, Nanau RM, et al. HIV-Antiretroviral Therapy Induced Liver, Gastrointestinal, and Pancreatic Injury. Int. J. Hepatol 2012;2012:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Munivenkatappa S, Anil S, Naik B, et al. Drug-Induced Hypothyroidism during Anti-Tuberculosis Treatment of Multidrug-Resistant Tuberculosis: Notes from the Field. J. Tuberc. Res 2016;04:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Silva GAR da, Andrade MCT, Sugui D de AS, et al. Association between antiretrovirals and thyroid diseases: a cross-sectional study. Arch. Endocrinol. Metab 2015;59:116–22. [DOI] [PubMed] [Google Scholar]
- [119].Hong H, Dooley KE, Starbird LE, et al. Adverse outcome pathway for aminoglycoside ototoxicity in drug-resistant tuberculosis treatment. Arch. Toxicol 2019;93:1385–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Tostmann A, Boeree MJ, Aarnoutse RE, et al. Antituberculosis drug-induced hepatotoxicity: Concise up-to-date review. J. Gastroenterol. Hepatol [Internet] 2008;23:192–202. Available from: http://doi.wiley.com/10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- [121].Tostmann A, Boeree MJ, Aarnoutse RE, et al. Antituberculosis drug-induced hepatotoxicity: Concise up-to-date review. J. Gastroenterol. Hepatol 2008;23:192–202. [DOI] [PubMed] [Google Scholar]
- [122].Singanayagam A, Sridhar S, Dhariwal J, et al. A comparison between two strategies for monitoring hepatic function during antituberculous therapy. Am. J. Respir. Crit. Care Med 2012;185:653–9. [DOI] [PubMed] [Google Scholar]
- [123].Abbara A, Chitty S, Roe JK, et al. Drug-induced liver injury from antituberculous treatment: a retrospective study from a large TB centre in the UK. BMC Infect. Dis 2017;17:231. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Informative cohort on this topic
- [124].Yimer G, Gry M, Amogne W, et al. Evaluation of patterns of liver toxicity in patients on antiretroviral and anti-tuberculosis drugs: A prospective four arm observational study in Ethiopian patients. PLoS One 2014;9:e94271. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Informative study on liver toxicity during antiretroviral and TB treatment
- [125].Saha A, Margaret Shanthi FX, Blessed Winston A, et al. Prevalence of hepatotoxicity from antituberculosis therapy: A five-year experience from South India. J. Prim. Care Community Heal 2016;7:171–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].S C, M S, B R, et al. Burden of antituberculosis and antiretroviral drug-induced liver injury at a secondary hospital in South Africa. South African Med. J [Internet] 2012;102:506–11. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22668951%0Ahttp://www.samj.org.za/index.php/samj/article/download/5650/4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Devarbhavi H, Singh R, Patil M, et al. Outcome and determinants of mortality in 269 patients with combination anti-tuberculosis drug-induced liver injury. J. Gastroenterol. Hepatol 2013;28:161–7. [DOI] [PubMed] [Google Scholar]
- [128].Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am. J. Respir. Crit. Care Med [Internet] 2006;174:935–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17021358. [DOI] [PubMed] [Google Scholar]
- [129].Ramappa V, Aithal GP. Hepatotoxicity Related to Anti-tuberculosis Drugs: Mechanisms and Management. J. Clin. Exp. Hepatol 2013. p. 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].El-Sadr W, Tsiouris S. HIV-Associated Tuberculosis: Diagnostic and Treatment Challenges. Semin. Respir. Crit. Care Med 2008;29:525–31. [DOI] [PubMed] [Google Scholar]
- [131].Breen RAM, Miller RF, Gorsuch T, et al. Adverse events and treatment interruption in tuberculosis patients with and without HIV co-infection. Thorax 2006;61:791–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Marks DJB, Dheda K, Dawson R, et al. Adverse events to antituberculosis therapy: influence of HIV and antiretroviral drugs. Int. J. STD AIDS 2009;20:339–45. [DOI] [PubMed] [Google Scholar]
- [133].Polo M, Alegre F, Funes HA, et al. Mitochondrial (dys)function - A factor underlying the variability of efavirenz-induced hepatotoxicity? Br. J. Pharmacol 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: The effect of tuberculosis and hepatitis B. AIDS 2007;21:1301–8. [DOI] [PubMed] [Google Scholar]
- [135].Keshavjee S, Gelmanova IY, Shin SS, et al. Hepatotoxicity during treatment for multidrug-resistant tuberculosis: Occurrence, management and outcome. Int. J. Tuberc. Lung Dis 2012;16:596–603. [DOI] [PubMed] [Google Scholar]
- [136].Törün T, Güngor G, Özmen J, et al. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis 2005. p. 1373–7. [PubMed] [Google Scholar]
- [137].Lee SS, Lee CM, Kim TH, et al. Frequency and risk factors of drug-induced liver injury during treatment of multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis 2016;20:800–5. [DOI] [PubMed] [Google Scholar]
- [138].Yang TW, Park HO, Jang HN, et al. Side effects associated with the treatment of multidrug-resistant tuberculosis at a tuberculosis referral hospital in South Korea. Med. (United States) [Internet] 2017;96:e7482 Available from: http://journals.lww.com/md-journal%0Ahttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed18&NEWS=N&AN=617549636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hsu HL, Bai KJ, Chiang YC, et al. Hepatitis associated with prothionamide for treatment of multidrug-resistant tuberculosis. J. Formos. Med. Assoc 2010;109:923–7. [DOI] [PubMed] [Google Scholar]
- [140].Chien JY, Chien ST, Huang SY, et al. Safety of rifabutin replacing rifampicin in the treatment of tuberculosis: A single-centre retrospective cohort study. J. Antimicrob. Chemother 2014;69:790–6. [DOI] [PubMed] [Google Scholar]
- [141].de Bus L, Depuydt P, Libbrecht L, et al. Severe Drug-induced Liver Injury Associated with Prolonged Use of Linezolid. J. Med. Toxicol 2010;6:322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Alshammari TM, Larrat EP, Morrill HJ, et al. Risk of hepatotoxicity associated with fluoroquinolones: A national case-control safety study. Am. J. Heal. Pharm 2014;71:37–43. [DOI] [PubMed] [Google Scholar]
- [143].Guglielmetti L, Le Dû D, Jachym M, et al. Compassionate use of bedaquiline for the treatment of multidrug-resistant and extensively drug-resistant tuberculosis: Interim analysis of a french cohort. Clin. Infect. Dis 2015;60:188–194. [DOI] [PubMed] [Google Scholar]
- [144].Jong E, Conradie F, Berhanu R, et al. Consensus statement: Management of drug-induced liver injury in HIV-positive patients treated for TB. South. Afr. J. HIV Med 2013;14:113–9. [Google Scholar]; *Detailed statement informing on the management of DILI during antiretriviral and TB treatment
- [145].Nahid P, Dorman SE, Alipanah N, et al. Executive Summary: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin. Infect. Dis 2016. p. 853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Shi J, Xie M, Wang J, et al. Susceptibility of N-acetyltransferase 2 slow acetylators to antituberculosis drug-induced liver injury: A meta-analysis. Pharmacogenomics 2015. p. 2083–97. [DOI] [PubMed] [Google Scholar]
- [147].Makhlouf HA, Helmy A, Fawzy E, et al. A prospective study of antituberculous drug-induced hepatotoxicity in an area endemic for liver diseases. Hepatol. Int 2008;2:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Mo P, Zhu Q, Teter C, et al. Prevalence, drug-induced hepatotoxicity, and mortality among patients multi-infected with HIV, tuberculosis, and hepatitis virus. Int. J. Infect. Dis 2014;28:95–100. [DOI] [PubMed] [Google Scholar]
- [149].Robertson K, Kumwenda J, Supparatpinyo K, et al. A multinational study of neurological performance in antiretroviral therapy-naïve HIV-1-infected persons in diverse resource-constrained settings. J. Neurovirol 2011;17:438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Vivithanaporn P, Heo G, Gamble J, et al. Neurologic disease burden in treated HIV/AIDS predicts survival: A population-based study. Neurology 2010;75:1150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Kass JS, Shandera WX. Nervous system effects of antituberculosis therapy. CNS Drugs 2010; 2:655–667. [DOI] [PubMed] [Google Scholar]; *Comprehensive review on CNS symptoms during TB treatment
- [152].Mafukidze AT, Calnan M, Furin J. Peripheral neuropathy in persons with tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis 2016. p. 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Tiwari M, Patel M, Shamaliya K. Peripheral neuropathy in XDR-TB patients on second line anti-tubercular therapy. 10.2 Tuberc. European Respiratory Society; 2015. p. PA2710. [Google Scholar]
- [154].Baum MK, Mantero-Atienza E, Shor-Posner G, et al. Association of vitamin B6 status with parameters of immune function in early HIV-1 infection. J. Acquir. Immune Defic. Syndr 1991;4:1122–32. [PubMed] [Google Scholar]
- [155].Daley CL, Brozek JL, Grzemska M, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin. Infect. Dis 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Chan RYC. Ocular toxicity of ethambutol. Chest 2006. p. 56–60. [PubMed] [Google Scholar]
- [157].Mustak H, Rogers G, Cook C. Ethambutol induced toxic optic neuropathy in HIV positive patients. Int. J. Ophthalmol [Internet] 2013;6:542–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23991394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Javaheri M, Khurana RN, O’Hearn TM, et al. Linezolid-induced optic neuropathy: A mitochondrial disorder? Br. J. Ophthalmol 2007;91:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Mehta S, Das M, Laxmeshwar C, et al. Linezolid-Associated Optic Neuropathy in Drug-Resistant Tuberculosis Patients in Mumbai, India. Bhattacharya S, editor. PLoS One 2016;11:e0162138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Shin SS, Hyson AM, Castañeda C, et al. Peripheral neuropathy associated with treatment for multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis 2003;7:347–53. [PubMed] [Google Scholar]
- [161].Conradie F, Mabiletsa T, Sefoka M, et al. Prevalence and incidence of symmetrical symptomatic peripheral neuropathy in patients with multidrug-resistant TB. South African Med. J 2014;104:24–6. [DOI] [PubMed] [Google Scholar]
- [162].Hoffmann C, Llibre JM. Neuropsychiatric adverse events with dolutegravir and other integrase strand transfer inhibitors. AIDS Rev 2019;21:4–10. [DOI] [PubMed] [Google Scholar]
- [163].Gaida R, Truter I, Grobler C, et al. A review of trials investigating efavirenz-induced neuropsychiatric side effects and the implications. Expert Rev. Anti. Infect. Ther 2016. p. 377–88. [DOI] [PubMed] [Google Scholar]
- [164].Mukonzo JK, Okwera A, Nakasujja N, et al. Influence of efavirenz pharmacokinetics and pharmacogenetics on neuropsychological disorders in Ugandan HIV-positive patients with or without tuberculosis: a prospective cohort study. BMC Infect. Dis 2013;13:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Prasad R, Garg R, Verma S. Isoniazid- and ethambutol-induced psychosis. Ann. Thorac. Med 2009;3:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Hwang TJ, Wares DF, Jafarov A, et al. Safety of cycloserine and terizidone for the treatment of drug-resistant tuberculosis: A meta-analysis. Int. J. Tuberc. Lung Dis 2013. p. 1257–66. [DOI] [PubMed] [Google Scholar]
- [167].Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus Abacavir–Lamivudine for the Treatment of HIV-1 Infection. N. Engl. J. Med 2013; [DOI] [PubMed] [Google Scholar]
- [168].Schwartz PJ, Woosley RL, Woosley RL. Predicting the Unpredictable: Drug-Induced QT Prolongation and Torsades de Pointes. J. Am. Coll. Cardiol 2016. 67(13):1639–50. [DOI] [PubMed] [Google Scholar]
- [169].Moreno T, Pérez I, Isasti G, et al. Prevalence and Factors Associated with a Prolonged QTc Interval in a Cohort of Asymptomatic HIV-Infected Patients. AIDS Res. Hum. Retroviruses 2013;29:1195–98. [DOI] [PubMed] [Google Scholar]
- [170].McIntosh RC, Lobo JD, Hurwitz BE. Current assessment of heart rate variability and QTc interval length in HIV/AIDS. Curr. Opin. HIV AIDS 2017;12:528–33. [DOI] [PubMed] [Google Scholar]
- [171].Wu KC, Zhang L, Haberlen SA, et al. Predictors of electrocardiographic QT interval prolongation in men with HIV. Heart 2019;105:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Reinsch N, Arendt M, Geisel MH, et al. Prolongation of the QTc interval in HIV-infected individuals compared to the general population. Infection 2017;45:659–67. [DOI] [PubMed] [Google Scholar]
- [173].Gili S, Mancone M, Ballocca F, et al. Prevalence and predictors of long corrected QT interval in HIV-positive patients: A multicenter study. J. Cardiovasc. Med 2017;18:539–44. [DOI] [PubMed] [Google Scholar]
- [174].Charbit B, Rosier A, Bollens D, et al. Relationship between HIV protease inhibitors and QTc interval duration in HIV-infected patients: A cross-sectional study. Br. J. Clin. Pharmacol 2009;67:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Castillo R, Pedalino RP, El-Sherif N, et al. Efavirenz-associated QT prolongation and torsade de pointes arrhythmia. Ann. Pharmacother 2002;36:1006–8. [DOI] [PubMed] [Google Scholar]
- [176].Chinello P, Petrosillo N. QT Interval Prolongation and Antiretroviral Treatment: Another Point of Interest. Clin. Infect. Dis 2007;44:1388–9. [DOI] [PubMed] [Google Scholar]
- [177].Abdelhady AM, Shugg T, Thong N, et al. Efavirenz Inhibits the Human Ether-A-Go-Go Related Current (hERG) and Induces QT Interval Prolongation in CYP2B6*6*6 Allele Carriers. J. Cardiovasc. Electrophysiol 2016;27:1206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Ly T, Ruiz ME. Prolonged QT Interval and Torsades de Pointes Associated with Atazanavir Therapy. Clin. Infect. Dis 2007;44:e67–e68. [DOI] [PubMed] [Google Scholar]
- [179].Janssen-Cilag Ltd. Edurant - Summary of Product Characteristics [Internet] 2019. Available from: https://www.medicines.org.uk/emc/product/4968/smpc.
- [180].World Health Organization. Companion handbook to the WHO guidelines for the porgrammatic managment of drug-resistance tuberculosis [Internet] 2014. Available from: https://www.who.int/tb/publications/pmdt_companionhandbook/en/. [PubMed]
- [181].Yunihastuti E, Widhani A, Karjadi TH. Drug hypersensitivity in human immunodeficiency virus-infected patient: challenging diagnosis and management. Asia Pac. Allergy 2014;4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Coopman SA, Johnson RA, Platt R, et al. Cutaneous Disease and Drug Reactions in HIV Infection. N. Engl. J. Med 2002;328:1670–4. [DOI] [PubMed] [Google Scholar]
- [183].Lehloenya RJ, Dheda K. Cutaneous adverse drug reactions to anti-tuberculosis drugs: State of the art and into the future. Expert Rev. Anti. Infect. Ther 2012. p. 475–86. [DOI] [PubMed] [Google Scholar]
- [184].Nunn P, Brindle R, Wasunna K, et al. Cutaneous hypersensitivity reactions due to thiacetazone in HIV-1 seropositive patients treated for tuberculosis. Lancet 1991;337:927–30. [DOI] [PubMed] [Google Scholar]
- [185].Chintu C, Luo C, Bhat G, et al. Cutaneous hypersensitivity reactions due to thiacetazone in the treatment of tuberculosis in Zambian children infected with HIV-1. Arch. Dis. Child 1993;68:665–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Chaponda M, Pirmohamed M. Hypersensitivity reactions to HIV therapy. Br. J. Clin. Pharmacol 2011;71:659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Boonyagars L, Hirunwiwatkul P, Hurst CP. CD4 count and risk of anti-tuberculosis drug-associated cutaneous reactions in HIV-infected Thai patients. Int. J. Tuberc. Lung Dis 2017;21:338–44. [DOI] [PubMed] [Google Scholar]
- [188].Walker NF, Stek C, Wasserman S, et al. The tuberculosis-associated immune reconstitution inflammatory syndrome: recent advances in clinical and pathogenesis research. Curr. Opin. HIV AIDS 2018;13:512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Namale PE, Abdullahi LH, Fine S, et al. Paradoxical TB-IRIS in HIV-infected adults: A systematic review and meta-analysis. Future Microbiol 2015. p. 1077–99. [DOI] [PubMed] [Google Scholar]
- [190].Luetkemeyer AF, Kendall MA, Nyirenda M, et al. Tuberculosis Immune Reconstitution Inflammatory Syndrome in A5221 STRIDE. JAIDS J. Acquir. Immune Defic. Syndr 2014. p. 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Bahr N, Boulware DR, Marais S, et al. Central nervous system immune reconstitution inflammatory syndrome. Curr. Infect. Dis. Rep 2013;15:583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Abay SM, Deribe K, Reda AA, et al. The effect of early initiation of antiretroviral therapy in TB/HIV-coinfected patients: A systematic review and meta-analysis. J. Int. Assoc. Provid. AIDS Care 2015;14:560–70. [DOI] [PubMed] [Google Scholar]
- [193].Uthman OA, Okwundu C, Gbenga K, et al. Optimal timing of antiretroviral therapy initiation for HIV-infected adults with newly diagnosed pulmonary tuberculosis: A systematic review and meta-analysis. Ann. Intern. Med 2015;163:32–9. [DOI] [PubMed] [Google Scholar]
- [194].Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010;24:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Meintjes G, Stek C, Blumenthal L, et al. Prednisone for the Prevention of Paradoxical Tuberculosis-Associated IRIS. N. Engl. J. Med [Internet] 2018;379:1915–25. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1800762. [DOI] [PubMed] [Google Scholar]
- [196].Hill AM, Mitchell N, Hughes S, et al. Risks of cardiovascular or central nervous system adverse events and immune reconstitution inflammatory syndrome, for dolutegravir versus other antiretrovirals: Meta-analysis of randomized trials. Curr. Opin. HIV AIDS 2018. p. 102–11. [DOI] [PubMed] [Google Scholar]
- [197].Bates M, Ahmed Y, Kapata N, et al. Perspectives on tuberculosis in pregnancy. Int. J. Infect. Dis 2015;32:124–7. [DOI] [PubMed] [Google Scholar]
- [198].Nunn AJ, Phillips PPJ, Meredith SK, et al. A Trial of a Shorter Regimen for Rifampin-Resistant Tuberculosis. N. Engl. J. Med 2019;380:1201–13. [DOI] [PubMed] [Google Scholar]
- [199].Mayer KH, Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: Epidemiology, management, and research gaps. Clin. Infect. Dis 2012;55:1532–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management 2018; Available from: https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. [PubMed]
- [201].Gupta A, Montepiedra G, Aaron L, et al. RANDOMIZED TRIAL OF SAFETY OF ISONIAZID PREVENTIVE THERAPY DURING OR AFTER PREGNANCY Conf. Retrovir. Opportunistic Infect. Boston, March 4–7, 2018 2018. [Google Scholar]
- [202].Salazar-Austin N, Lala S, Waja Z, et al. IPT AND PREGNANCY OUTCOMES IN HIV-POSITIVE WOMEN: THE TSHEPISO COHORT Conf. Retrovir. Opportunistic Infect. Seattle, March 4–7, 2018 2019. [Google Scholar]
- [203].WHO Organization. Statement on DTG [Internet] 2018. [cited 2019 May 30]. Available from: https://www.who.int/hiv/WHO_q-and-a-DTG-21may2018.pdf?ua=1.
- [204].Zash R, Makhema J, Shapiro RL. Neural-Tube Defects with Dolutegravir Treatment from the Time of Conception. N. Engl. J. Med [Internet] 2018;379:979–981. Available from: http://www.nejm.org/doi/10.1056/NEJMc1807653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Hare CB, Coll J, Ruane P, et al. THE PHASE 3 DISCOVER STUDY: DAILY F/TAF OR F/TDF FOR HIV PREEXPOSURE PROPHYLAXIS Conf. Retrovir. Opportunistic Infect. Seattle, March 4–7, 2019 [Internet] 2019. Available from: http://www.croiconference.org/sessions/phase-3-discover-study-daily-ftaf-or-ftdf-hiv-preexposure-prophylaxis. [Google Scholar]
- [206].Swindells S, Siccardi M, Barrett SE, et al. Long-acting formulations for the treatment of latent tuberculous infection: opportunities and challenges. Int. J. Tuberc. Lung Dis 2018;22:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Nicole C Ammerman EN. Long-acting injectables for TB prevention in people living with HIV: prospects and challenges 2019.