Abstract
Temporal lobe epilepsy (TLE) is the most common form of epilepsy in adults, but current treatment options provide limited efficacy, leaving as many as one third of patients with uncontrolled seizures. Recently, attention has shifted towards more closed-loop therapies for seizure control, and on-demand optogenetic modulation of the cerebellar cortex was shown to be highly effective at attenuating hippocampal seizures. Intriguingly, both optogenetic excitation and inhibition of cerebellar cortical output neurons, Purkinje cells, attenuated seizures. The mechanisms by which the cerebellum impacts seizures, however, are unknown. In the present study, we targeted the immediate downstream projection of vermal Purkinje cells -- the fastigial nucleus -- in order to determine whether increases and/or decreases in fastigial output can underlie seizure cessation. Though Purkinje cell input to fastigial neurons is inhibitory, direct optogenetic inhibition of the fastigial nucleus had no effect on seizure duration. Conversely, however, fastigial excitation robustly attenuated hippocampal seizures. Seizure cessation was achieved at multiple stimulation frequencies, regardless of laterality relative to seizure focus, and even with single light pulses. Seizure inhibition was greater when selectively targeting glutamatergic fastigial neurons than when an approach that lacked cell-type specificity was used. Together, these results suggest that stimulating excitatory neurons in the fastigial nucleus may be a promising approach for therapeutic intervention in TLE.
INTRODUCTION
Temporal lobe epilepsy (TLE), a disorder characterized by spontaneous, recurrent seizures typically emerging from the hippocampus, is the most common form of epilepsy in adults, with 150,000 new diagnoses per year in the US alone (England et al., 2012). Total healthcare costs associated with epilepsy in the US overall exceed $2.7 billion (Vivas et al., 2012). Despite this high prevalence, current treatment options have limited efficacy and carry the potential for problematic side effects, leaving 30-40% of epilepsy patients with uncontrolled seizures (England et al., 2012). Clearly, there is an immense need to improve both current treatment options and our understanding of the seizure network in temporal lobe epilepsy.
Recent attention has shifted towards more targeted interventions such as deep brain and on-demand stimulation, which has been shown to reduce seizure duration in some patients (Fisher et al., 2010; Valentin et al., 2013; Bergey et al., 2015; Pakdaman et al., 2016). Targeting areas outside the seizure focus has shown some promise (Fisher et al., 2010; Salanova et al., 2015; Elder et al., 2019), and recent optogenetic work has renewed interest in the cerebellum as a potential therapeutic target (Krook-Magnuson et al., 2014; Kros et al., 2015a; Miterko et al., 2019). For example, on-demand optogenetic modulation of the midline cerebellar cortex, the vermis, was recently found to robustly terminate hippocampal seizures (Krook-Magnuson et al., 2014). The mechanisms by which cerebellar stimulation inhibits seizures, however, is unknown, and complications associated with targeting the cerebellar cortex may limit translational opportunities.
Vermal Purkinje cells project to and inhibit neurons in the fastigial nucleus (Itō, 1984). Glutamatergic fastigial neurons in turn project to further downstream targets, including the superior colliculus, thalamus, and reticular formation (Angaut & Bowsher, 1970). An immediate and important question is whether fastigial neurons can be leveraged, similar to Purkinje cells, to attenuate hippocampal seizures. Intriguingly, both on-demand excitation and inhibition of Purkinje cells was highly effective in reducing seizure duration (Krook-Magnuson et al., 2014). As both excitation or inhibition of Purkinje cells can attenuate seizures, it is possible that simply disrupting on-going activity in the cerebellum is sufficient to stop seizures. If true, then excitation or inhibition of the fastigial nucleus would also inhibit seizures. However, alternative explanations are possible. During optogenetic excitation, Purkinje cell firing increases overall during light stimulation, including robust increases during each pulse of light (Tsubota et al., 2011; Chaumont et al., 2013; Witter et al., 2013; Krook-Magnuson et al., 2014; Kruse et al., 2014; Lee et al., 2015). However, after each individual pulse of light there is a decrease in Purking cell firing (Krook-Magnuson et al., 2014; Lee et al., 2015; El-Shamayleh et al., 2017; Brown & Raman, 2018). Similarly, with optogenetic inhibition, Purkinje cell firing decreases overall during the period of pulsed light delivery, but there are brief increases in firing between pulses (Krook-Magnuson et al., 2014; Lee et al., 2015). Previous work has shown that even brief pauses in Purkinje cell firing results in increased firing of downstream deep cerebellar nuclear neurons (Gauck & Jaeger, 2000; Lee et al., 2015; Brown & Raman, 2018). Therefore, interpreting previous optogenetic targeting of the cerebellar cortex, in the context of the cerebellar nuclei, is not straightforward. Additionally, Purkinje cells project to multiple types of neurons in the nuclei, including both excitatory and inhibitory neurons (Bagnall et al., 2009; Uusisaari & Knopfel, 2012).
A major unanswered question then is what ultimately can lead to the cessation of seizures, such that we could mimic the effects through direct manipulation of the fastigial nucleus. Is seizure suppression mediated through i) merely the disruption of ongoing cerebellar activity, such that either excitation or inhibition of fastigial neurons would be sufficient to terminate seizures, ii) inhibiton of fasitigial neurons, or iii) excitatation of fastigial neurons? To test these possibilities, we implemented direct inhibition and excitation of the fastigial nucleus during hippocampal seizures in a mouse model of TLE.
We find that on-demand excitation of the fastigial nucleus is required for hippocampal seizure attenuation. Direct on-demand inhibition of the fastigial nucleus, tested across multiple different stimulation frequencies, has no effect on the duration of hippocampal seizures. In contrast, robust attenuation of hippocampal seizures is observed with on-demand excitation of the fastigial nucleus. Stimulation is highly effective regardless of the side targeted relative to the seizure focus or the stimulation frequency tested. Even a single pulse of light is able to truncate seizures. We additionally find that excitation of glutamatergic nuclear neurons selectively provides greater seizure inhibition than non-cell-type-selective excitation of the fastigial nucleus. While additional circuit elements may be able to also provide some degree of seizure control, these results clearly indicate that increases in excitatory fastigial output, rather than decreases, provide strong inhibition of seizures. These results further suggest that the fastigial nucleus is a potential target for therapeutic neuromodulation in TLE.
MATERIALS AND METHODS
Ethical approval
The present study conforms to the ethical principles and regulations of the Journal of Physiology. All experimental protocols were approved by the University of Minnesota’s Institutional Animal Care and Use Committee (IACUC protocol 1801-35497A).
Animals
For all experiments, mice were bred in-house and had ad libitum access to food and water in all housing conditions. Mice with opsin expression were generated by crossing mice expressing Cre selectively in VGluT2-expressing neurons (Vong et al., 2011)(B6J.129S6(FVB)-Slc17a6tm2(cre)Lowl/MwarJ; Jackson Laboratory stock 028863), referred to as VGluT2-cre in the text, with either floxed-STOP channelrhodopsin (ChR2) mice (Madisen et al., 2012)(B6;129S-Gt(ROSA)26Sortm32.1(CAG-COP4*H134R/EYFP)Hze/J; Jackson Laboratory stock 012569) or floxed-STOP halorhodopsin (HR) mice (Madisen et al., 2012)(B6;129S-Gt(ROSA)26Sortm39(CAG-HOP/EYFP)Hze/J; Jackson Laboratory stock 014539). These crosses generated mice with opsin expression restricted to VGluT2 expressing neurons, referred to as VGluT2-ChR and VGluT2-HR in the text. For experiments with virally induced opsin expression, Black-6 (C57BL/6J; Jackson Laboratory stock 000664) mice were used for cre-independent viruses and VGluT2-cre mice for cre-dependent viruses.
Opsin-negative littermates were used as light only controls for experiments with VGluT2-ChR and -HR animals. For virally induced opsin expression, mice injected with virus inducing expression of fluorescent protein only were used as controls.
Both male and female mice were used for experiments. Until optical fiber and electrode implantation, animals were housed in standard housing conditions in the animal facility at the University of Minnesota. Following implantation, animals were singly housed in investigator managed housing. In all conditions, animals were allowed ad libitum access to food and water, and were on a 12 hour light; 12 hour dark (/low red light) cycle.
Stereotactic surgeries
Epilepsy induction
The mouse unilateral intra-hippocampal kainic acid model of epilepsy was utilized (Bouilleret et al., 1999; Bragin et al., 1999), as described previously with minor modifications (Armstrong et al., 2013). Briefly, adult mice (postnatal day 45 or later) under isoflurane anesthesia were injected with 100nL of kainic acid (KA) unilaterally into the right hippocampus (2.0 mm posterior, 1.25 mm right, 1.6 mm ventral from bregma). Animals were subsequently removed from isoflurane less than five minutes post injection. In this model, spontaneous recurrent electrographic seizures emerge after a period of weeks (Bouilleret et al., 1999).
Electrode and fiber implantation
A minimum of 1 week post kainic acid injection, mice were implanted with a twisted wire bipolar (local reference, differential) electrode (PlasticsOne) ipsilateral to the site of kainate (2.6 mm posterior, 1.75 mm right, 1.6 mm ventral from bregma). Optical fibers (ThorLabs) targeting the left and right fastigial nucleus were implanted 6.48 mm posterior, 0.75 left/right, and 3.5 mm ventral from bregma. Implants were secured to the skull using surgical screws and dental cement, as described previously (Armstrong et al., 2013). Animals were allowed to recover a minimum of five days prior to video and local field potential (LFP) monitoring for seizures and closed-loop interventions.
Viral targeting
For all viral experiments, AAV serotype 9 was selected from other options due to its optimal expression in the fastigial nucleus with no apparent retrograde expression. Mice were injected with 120nL of virus via a Hamilton Neuros syringe into the left cerebellar fastigial nucleus (6.48 posterior, 0.75 left, 3.7 mm ventral from bregma) under isoflurane anesthesia. Black-6 mice were injected with virus encoding Channelrhodopsin fused to enhanced yellow fluorescent protein (AAV9-CAG-ChR2-GFP, titer of 2.1×10e12, UNC vector core lot #AV5406D, provided by Edward Boyden), or AAV9-CAG-GFP (titer 2×1012, UNC vector core lot #AV5221, provided by Edward Boyden). VGluT2-cre mice were injected with virus encoding Channelrhodopsin in a cre-dependent manner (pAAV-EF1a-double floxed-hChR2(H134R)-EYFP-WPRE-HGHpA, titer of 2.2×1013 Addgene viral prep #202198-AAV9, lot #V22125, gift from Karl Deisseroth)(Gradinaru et al., 2007), virus encoding Halorhodopsin in a cre-dependent manner (pAAV-Ef1a-DIO eNpHR 3.0-EYFP, titer of 2.2 × 1013, Addgene viral prep #26966-AAV9, lot #V44904, gift from Karl Deisseroth)(Gradinaru et al., 2010), or GFP alone in a cre-dependent manner (AAV9-CAG-Flex-GFP, titer 3.7×1012, UNC vector core lot #AV5220C, provided by Edward Boyden). After injection, the syringe was held in place for a minimum of 10 minutes before being withdrawn.
Subsequent KA injections and implantations were performed as for experiments with transgenic animals, with the KA injections performed a minimum of two weeks post virus injection. On-demand interventions were conducted a minimum of 6 weeks post viral injection.
Post-operative care
For all surgical procedures, post-operative care consisted of recovery from anesthesia on a heating pad with regular visual inspection, followed by daily post-operative monitoring for a minimum of three days to inspect comfort level and healing of the surgical site. Neopredef powder was applied to the closed incisions as a topical antibiotic and analgesic. In the case of KA injection, no additional post-op analgesics were given. For viral injections and implantations, carprofen was administered subcutaneously (5mg/kg) during surgery. For implantations, post-operative ibuprofen was also administered orally (50-80mg/kg/day in water) for three days post-surgery as an additional analgesic.
Closed-loop seizure detection and interventions
After recovery from implantation, animals were placed in investigator managed housing for 24 hour video and LFP recordings. Electrographic hippocampal seizures were detected and on-demand optogenetic interventions were triggered as described previously (Armstrong et al., 2013; Krook-Magnuson et al., 2014). Briefly, animals were connected via electrical patch cords through an electrical commutator (PlasticsOne). Hippocampal LFP was amplified (Brownlee), digitized (National instruments), and analyzed in real time by custom MATLAB seizure detection software. Light interventions were delivered via optical patch cords (ThorLabs, Doric lenses) through an optical commutator connected to either LEDs (Plexon) or lasers (Shanghai Laser & Optics Century) of the appropriate wavelength (473 nm for ChR2, 589nm for HR). Power, measured post-hoc from the tip of implanted fibers, ranged between 0.2-2.4mW for ChR2 and 1.5-9.8mW for HR. There was no correlation between light power and effect on seizure duration for either blue light or amber light (p > 0.05, Spearman’s correlations). Optogenetic interventions were triggered for 50% of events in a random fashion using custom closed-loop MATLAB software similar to that described previously and available for download (Armstrong et al., 2013). Three seconds of pulsed light was delivered unilaterally to the fastigial nucleus either contralateral or ipsilateral to the site of kainic acid injection. A variety of pulse lengths were tested, as discussed in the text.
Tissue harvesting and imaging of opsin expression
In order to confirm appropriate optical fiber targeting and viral expression, after on-demand interventions, mice were deeply anesthetized with 5% isoflurane and decapitated. Their brains were subsequently harvested and drop fixed in 4% paraformeldahyde. Sagittal brain sections of 50μm were collected in 0.1M phosphate buffer using a vibratome (Leica VT1000S). After sectioning, every third section was mounted with Vectashield mounting media with DAPI and covered with glass coverslips. Sections were visualized with epifluorescence microscopy (Leica DM2500). Confocal imaging of representative images was performed on an Olympus FluoView FV1000 BX2 upright confocal microscope (University Imaging Center, University of Minnesota).
Immunohistochemistry
In order to confirm specificity of the VGluT2-cre mouse line in targeting VGluT2-expressing neurons, VGluT2 immunohistochemistry was performed on brains of VGluT2-cre mice injected with cre-dependent virus for GFP expression. Tissue was harvested and fixed as described above. After sectioning, every third sagittal section containing the virally injected fastigial nucleus was transferred into 0.1M phosphate buffer and rinsed three times for 10 min. Slices were incubated for 1 hour at room temperature in a blocking solution containing 10% bovine serum and 0.5% triton diluted in TBS. This was followed by overnight incubation with primary antibody for VGluT2 (Millipore Sigma, 1:1000 diluted in TBS containing 2% bovine serum and 0.4% triton). Slices were subsequently rinsed 3 times for 10 minutes in TBS and incubated for 2 hours with Alexa fluor 594 anti-guinea pig (Jackson, 1:500 diluted in TBS containing 2% bovine serum and 0.4% triton). Sections were rinsed 3 times for 15 minutes in PB, mounted with Vectashield and visualized with epifluorescence microscopy (Leica DM2500) for cell counting. A total of three 50μm sagittal slices containing the fastigial nucleus were included for each animal, resulting in 352 total fastigial neurons counted between two mice. Confocal imaging of representative images was performed on an Olympus FluoView FV1000 BX2 upright confocal microscope (University Imaging Center, University of Minnesota).
Statistical analyses
Seizure duration after the time of trigger was analyzed off-line using a combination of manual and automated methods. Seizure events are processed automatically based on user-identified characteristics of spikes including amplitude, peak width, spike frequency and deflection (positive, negative, or both), and the resulting post detection seizure duration of all events were confirmed via manual inspection (Zeidler et al., 2018). No consistent effects on time to next seizure were noted. Reviewers were blinded to the light condition of triggers, and at least 100 events were analyzed per animal per condition. Seizure durations are normalized to percent duration relative to no-light condition. Comparisons of post-trigger seizure duration between light and no light conditions were compared in each animal using two-sample Kolmogorov-Smirnov and two-tailed Mann-Whitney tests. Effects on post-detection seizure duration were examined at the group level using Wilcoxon signed rank tests. A p value < 0.05 was considered statistically significant. Statistical analyses were conducted using MATLAB. Values are presented as mean ± SEM.
RESULTS
On-demand inhibition of the fastigial nucleus fails to attenuate hippocampal seizures
On-demand modulation of cerebellar Purkinje cells is highly effective at inhibiting hippocampal seizures (Krook-Magnuson et al., 2014). Intriguingly, either excitation or inhibition of Purkinje cells robustly terminated seizures, with Purkinje cell firing entraining to pulsed light delivery. Purkinje cells in the vermis project to, and inhibit, neurons in the fastigial nucleus. An important question is if similar on-demand inhibition of seizures can be achieved through direct modulation of neurons in the fastigial nucleus. Optogenetics also allows us to further ask whether inhibition, excitation, or simply a disruption of ongoing activity of fastigial neurons can provide seizure cessation similar to that previously seen with on-demand optogenetic modulation of Purkinje cells in the cerebellar cortex. We thus first sought to directly inhibit the fastigial nucleus in a similar on-demand fashion to determine whether this could attenuate seizures.
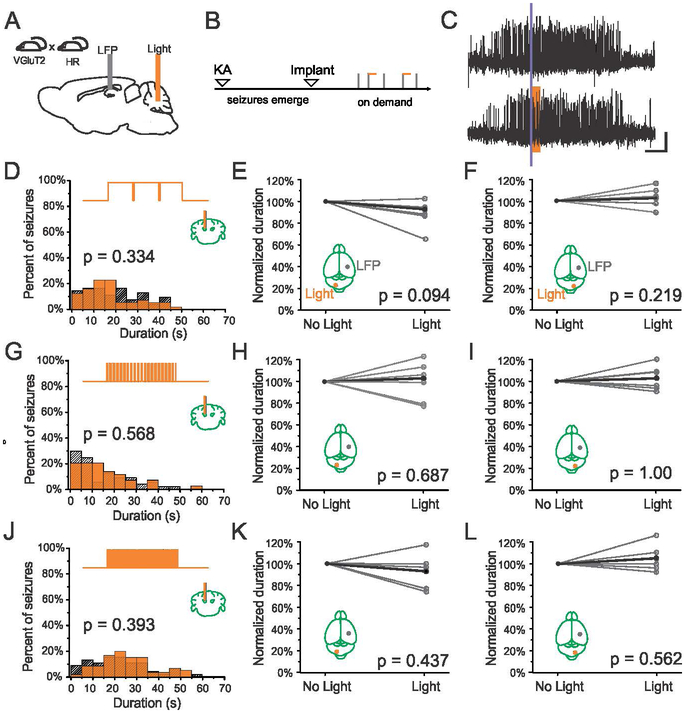
In order to implement optogenetic inhibition of excitatory neurons in the fastigial nucleus, mice expressing Cre in VGluT2-expressing neurons were crossed with mice expressing the inhibitory opsin, Halorhodopsin (HR) in a Cre-dependent fashion as detailed in Materials and Methods (Fig. 1A). On-demand optogenetic inhibition was achieved in these animals via amber light (589nm) delivered through an optical fiber targeting the fastigial nucleus. Chronic seizure induction, monitoring, and on-demand interventions were implemented as described previously (Fig. 1B) (Armstrong et al., 2013; Krook-Magnuson et al., 2014). Using these methods, electrographic seizure events are detected in real time, with 50% of detected events randomly selected to receive light delivery (Fig. 1C).
Figure 1.
Fastigial intervention in VGluT2-HR mice. A-B) Schematics of experimental design. Note that seizures are recorded from the hippocampus and light is delivered in an on-demand fashion to the fastigial nucleus during the chronic phase of the disorder. C) Example seizure events detected on-line (denoted by purple bar) that were either randomly selected not to receive light (top trace) or receive light (bottom trace, 3 seconds of light delivery denoted by amber box). Scale bar: 5s, 0.05mV. D-F) 3 seconds of long light pulses (1000ms on, 50ms off) to inhibit the fastigial nucleus produces no significant change in seizure duration (D, example animal; amber bars: events receiving light intervention; hashed bars: no-light internal controls; top trace illustrates pulsed light delivery paradigm) when light (589nm) is delivered to the contralateral (E) or ipsilateral (F) fastigial nucleus (each gray point represents data from one animal, black points represent mean). Similarly, 3 seconds of shorter light pulses (G-I) at 7Hz (50ms on, 100ms off), or (J-L) 10 Hz (50ms on, 50ms off), produce no significant change in seizure duration.
Given the inhibitory input of Purkinje cells to neurons in the deep cerebellar nuclei, we hypothesized that direct inhibition of neurons in the fastigial nucleus would mimic the effects of cerebellar cortical stimulation. Previous work targeting Purkinje cells has shown that 3 seconds of long light pulses (1000ms on, 50ms off) as well as shorter pulses (50ms on, 100ms off) is highly effective at attenuating hippocampal seizures (Krook-Magnuson et al., 2014). We therefore tested these pulse paradigms targeting the fastigial nucleus.
Surprisingly, we found that 3 seconds of long pulses of light (589nm) delivered to the nucleus to inhibit fastigial neurons fails to attenuate hippocampal seizures (Fig 1C), with no significant difference between the durations of seizure events receiving light versus no light (Fig. 1D). Across the population, zero of six animals showed a significant effect of light delivery on post-detection seizure duration. There is no effect of fastigial inhibition on seizure duration regardless of whether the contralateral (Fig. 1E, p = 0.094, Wilcoxon signed rank test, n = 6 animals) or ipsilateral (Fig. 1F, p = 0.219, Wilcoxon signed rank test, n = 6 animals) fastigial nucleus is targeted.
We next examined whether shorter inhibitory pulses of amber light (589nm) at higher frequencies delivered to the fastigial nucleus could affect the duration of hippocampal seizures. As with longer light pulses, shorter 50ms pulses of light delivered at ~7Hz (50ms on, 100ms off; (Krook-Magnuson et. al, 2014)) had no significant effect on hippocampal seizure duration (Fig. 1G-I). Across the population, zero of six animals showed a significant reduction in seizure duration for either contralateral (Fig. 1H, p = 0.687, Wilcoxon signed rank test, n = 6 animals) or ipsilateral (Fig. 1I, p > 0.99, Wilcoxon signed rank test, n = 6 animals) fastigial inhibition. To further explore possible light parameters, we additionally examined 50ms on, 50ms off pulsed light delivery (10Hz) and found similar results (Fig. 1J-L). Zero of six animals showed a significant reduction in seizure duration for either contralateral (Fig. 1K, p = 0.437, Wilcoxon signed rank test, n = 6 animals) or ipsilateral (Fig. 1L, p = 0.562, Wilcoxon signed rank test, n = 6 animals) fastigial inhibition with this intervention approach. Together, these results suggest that on-demand direct inhibition of the fastigial nucleus is insufficient to disrupt hippocampal seizures.
On-demand excitation of the fastigial nucleus robustly attenuates hippocampal seizures
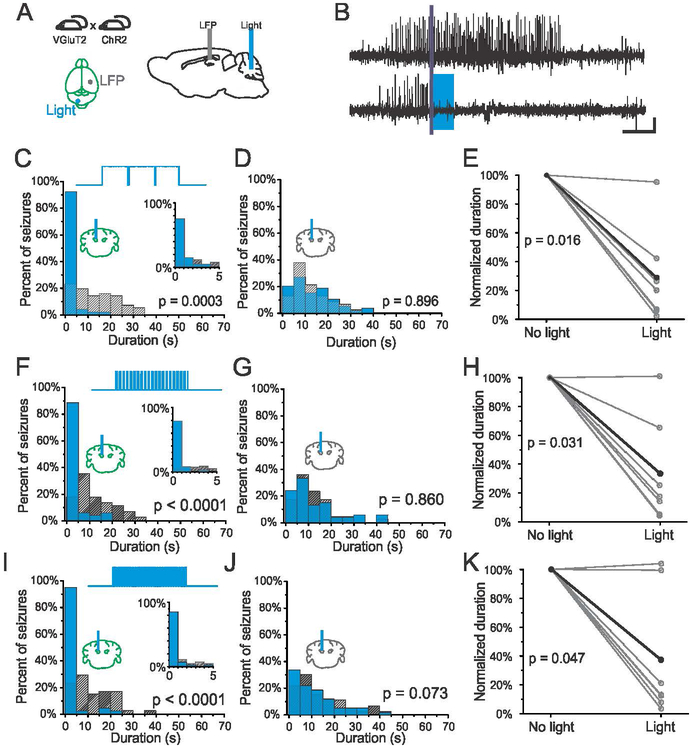
Given the inefficacy of direct inhibition of fastigial neurons, we next asked whether direct excitation of the fastigial nucleus could reduce the duration of hippocampal seizures. A similar transgenic approach was utilized for targeted opsin expression, with mice expressing Cre in VGluT2-expressing neurons crossed with mice expressing the excitatory opsin, Channelrhodopsin (ChR2) in a Cre-dependent fashion. Excitation was achieved via blue (473nm) light delivered to the fastigial nucleus (Fig. 2A), using the same light pulse paradigms as used in the previously described experiments.
Figure 2.
On-demand optogenetic excitation of the fastigial nucleus contralateral to KA injection. A) Spontaneous seizures are recorded from the KA-injected hippocampus and blue (473nm) light is delivered in an on-demand fashion to the contralateral fastigial nucleus in VGluT2-ChR animals. B) Example seizure events detected on-line (denoted by purple bar) that were either randomly selected not to receive light (top trace) or receive light (bottom trace, 3 seconds of light delivery denoted by blue box). Scale bar: 5s, 0.05mV. Three seconds of pulsed light delivery (1000ms on, 50ms off) significantly reduces seizure duration. C) Post-detection seizure duration distributions for an example animal (93% reduction, p < 0.001, two sample Kolmogorov-Smirnov test). Blue bars: events receiving light intervention; hashed bars: no-light internal controls. Top trace illustrates pulsed light delivery paradigm. Inset: first 5s bin expanded, 1s bin size. D) No effect of light delivery on seizure duration in an opsin negative animal (p = 0.896, two sample Kolmogorov-Smirnov test). E) Light delivery produces a significant reduction of seizure duration in opsin positive VGluT2-ChR mice (each gray data point represents one animal, black data points represent mean). Similarly, 3 seconds of shorter light pulses (F-H) at 7Hz (50ms on, 100ms off), or (I-K) 10 Hz (50ms on, 50ms off), produce a significant reduction in seizure duration.
Three seconds of pulsed blue light delivery (1000ms on, 50ms off) to the contralateral fastigial nucleus successfully terminated hippocampal seizure events (Fig. 2B-C), with a majority of events terminating within 1 second of stimulation onset (Fig. 2C, inset). Given that we saw no effect in VGluT2-HR expressing animals, the termination of seizures with light delivery in VGluT2-ChR2 animals is unlikely to be due to off-target effects of light delivery. Further supporting this, there was no effect of blue light delivery in an opsin negative control littermate (Fig. 2D), illustrating that excitation via opsin activation underlies anti-seizure effects. Across the population in opsin-positive VGluT2-ChR2 animals, on-demand excitation of the contralateral fastigial nucleus significantly reduced the duration of hippocampal seizures (Fig. 2E, 71 ± 13% reduction, p = 0.016, Wilcoxon signed rank test, n = 7 animals), with 6/7 animals showing a significant effect of light delivery. These results demonstrate that on-demand direct activation of the fastigial nucleus is able to attenuate hippocampal seizures.
Fastigial attenuation of hippocampal seizures occurs at multiple stimulation frequencies
We next tested whether longer pulses of blue light (1000ms on, 50ms off, for 3s) were necessary to disrupt seizures. The first additional stimulation parameter tested was 7Hz (50ms on, 100ms off), which produced robust seizure attenuation when targeting the cerebellar cortex (Krook-Magnuson 2014). As seen with longer light pulses, ~7Hz stimulation robustly attenuated hippocampal seizures, with a majority of seizure events terminating within one second of light delivery (Fig. 2F). Seizure disruption was not seen in an opsin negative control (Fig. 2G), again confirming that effects in opsin-positive animals were due to opsin activation rather than light delivery per se. Across the population of opsin-positive VGluT2-ChR2 animals, the duration of hippocampal seizures was significantly reduced with ~7Hz blue (473nm) light delivery (Fig. 2H, 67 ± 15 % reduction, p = 0.031, Wilcoxon signed rank test, n = 7 animals), with 6/7 animals showing a significant effect of light delivery.
To further assess the efficacy of fastigial stimulation in terminating hippocampal seizures, we also tested whether higher frequency (10Hz; 50ms on, 50ms off) blue light delivery to excite the fastigial nucleus could also be an effective intervention. We found that higher frequency 10Hz stimulation also robustly attenuated hippocampal seizures, with a majority of seizure events terminating within 1 second of blue light delivery (Fig. 2I). As seen with previous experiments, blue light delivery had no effect on seizure duration in an opsin negative control (Fig. 2J). Across the population, 10Hz stimulation produced a significant reduction in seizure duration in VGluT2-ChR2 opsin-positive animals (Fig. 2K, 63 ± 18% reduction, p = 0.047, Wilcoxon signed rank test, n = 7 animals), with 5/7 animals showing a significant effect of light delivery.
Fastigial interventions are effective irrespective of the side relative to seizure focus
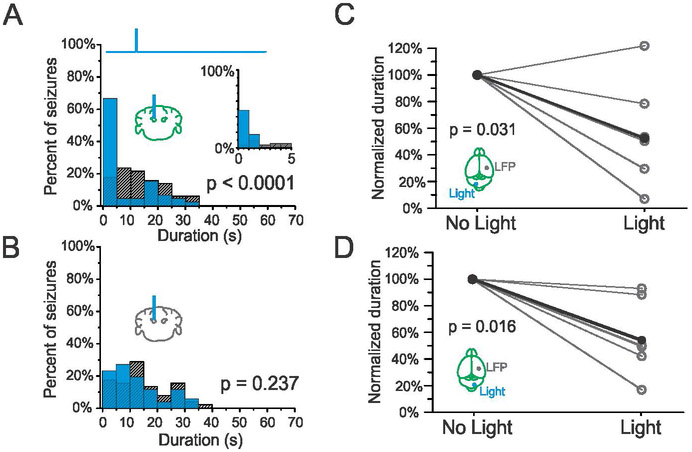
Ascending fastigial outputs cross the midline via the superior cerebellar peduncle, resulting in a largely contralateral organization relative to forebrain structures (Angaut & Bowsher, 1970). However, previous results indicate that inhibition of hippocampal seizures through modulation of the cerebellar cortex is not strongly lateralized (Krook-Magnuson et al., 2014). We thus tested whether excitation of the fastigial nucleus ipsilateral to the seizure focus could also disrupt hippocampal seizures. As with contralateral excitation, blue (473nm) light delivered to the ipsilateral fastigial nucleus successfully terminated seizures in VGlut2-ChR2 animals (Fig. 3A), across all stimulation paradigms tested. Seizure cessation induced by on-demand fastigial excitation is robust and rapid, with a majority of seizure events terminating within 1 second of light delivery (Fig. 3B). As with contralateral stimulation, no change in seizure duration was seen in an opsin negative control (Fig. 3C). Across the population in opsin-positive VGluT2-ChR2 animals, on-demand stimulation of the ipsilateral fastigial nucleus significantly reduced the duration of hippocampal seizures with long blue light pulses (Fig. 3D, 57 ± 12 % reduction, p = 0.016, Wilcoxon signed rank test, n = 7 animals), with 6/7 animals showing a significant effect of light delivery, shorter blue light pulses at ~7Hz (Fig. 3E, 54 ± 15% reduction, p = 0.047, Wilcoxon signed rank test, n = 7 animals), and shorter blue light pulses at 10Hz (Fig. 3F, 57 ± 12% reduction, p = 0.016, Wilcoxon signed rank test, n = 7 animals). These results indicate that on-demand excitation of fastigial neurons can disrupt hippocampal seizures regardless of the side targeted relative to the seizure focus.
Figure 3.
On-demand optogenetic excitation of the fastigial nucleus ipsilateral to KA injection. A) Example seizure events detected on-line (denoted by purple bar) that were either randomly selected not to receive light (top trace) or receive light (bottom trace, 3 seconds of light delivery denoted by blue box). Scale bar: 5s, 0.05mV. Three seconds of pulsed blue light delivery (1000ms on, 50ms off) significantly reduces seizure duration. B) Post-detection seizure duration distribution for an example VGluT2-ChR animal; blue bars: events receiving light intervention; hashed bars: no-light internal controls; top trace illustrates pulsed light delivery paradigm (57% reduction, p < 0.001, two sample Kolmogorov-Smirnov test). Inset: first 5s bin expanded, 1s bin size. C) No effect of light delivery on seizure duration in an opsin negative animal (p = 0.423, two sample Kolmogorov-Smirnov test). D) Light delivery produces a significant reduction of seizure duration in opsin positive VGluT2-ChR mice (each gray data point represents one animal, black data points represent mean). Similar results are seen for 7Hz (E) and 10Hz (F) stimulation.
Together, these results suggest that fastigial disruption of hippocampal seizures is not highly lateralized, and is not restricted to specific pulse durations or stimulation frequencies, but is dependent on excitation, rather than inhibition, of fastigial neurons.
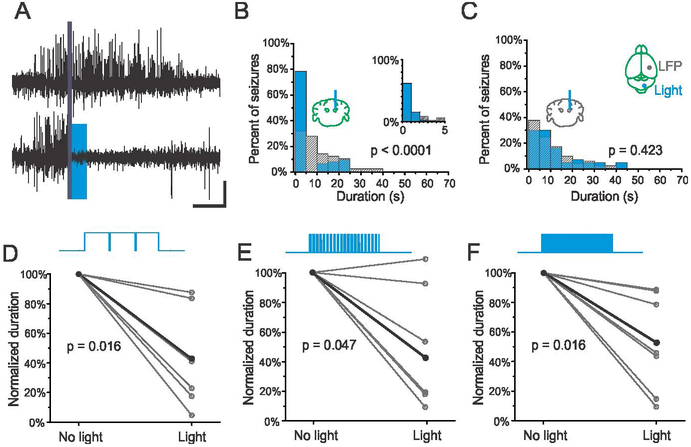
Single pulse stimulation of the fastigial nucleus is an effective intervention
Given the diversity of stimulation frequencies and pulse durations that effectively terminate hippocampal seizures, and that the majority of seizure events stopped within 1 second of fastigial intervention, we next asked whether a single excitatory pulse of light delivered to the fastigial nucleus could also be effective. To test this, on-demand stimulation of either the contralateral and ipsilateral fastigial nucleus was implemented, with a single 50ms pulse of blue (473nm) light delivered at the time of seizure detection. We found that single blue light pulses to excite the fastigial nucleus are highly effective at reducing the duration of hippocampal seizures (Fig. 4A). As seen with previous interventions, there was no effect of light delivery in an opsin negative control (Fig. 4B). Across the VGluT2-ChR2 opsin-positive population, single pulses significantly reduced seizure duration when targeting either the contralateral (Fig. 4C, 47 ± 15% reduction, p = 0.031, Wilcoxon signed rank test, n = 7 animals) or ipsilateral (Fig. 4D, 44 ± 10% reduction, p = 0.016, Wilcoxon signed rank test, n = 7 animals) fastigial nucleus, with 6/7 animals and 5/7 animals showing a significant effect of light delivery for contralateral and ipsilateral stimulation, respectively. These results illustrate the powerful influence that even brief activation of the fastigial nucleus can exert over hippocampal seizure activity.
Figure 4.
A single 50ms pulse of light significantly reduces seizure duration in VGluT-ChR animals. A) Data from an example opsin-positive animal; blue bars: events receiving blue (473nm) light intervention; hashed bars: no-light internal controls; top trace illustrates pulsed light delivery paradigm (50% reduction, p < 0.001, two sample Kolmogorov-Smirnov test). Inset: first 5s bin expanded, 1s bin size. B) No effect of blue (473nm) light delivery in an opsin negative animal (p = 0.237, two sample Kolmogorov-Smirnov test). Single pulses of light in opsin positive VGluT2-ChR animals significantly reduce seizure duration when targeting either contralateral (C) or ipsilateral (D) fastigial nucleus (each gray data point represents one animal, black data points represent mean).
Specific targeting of fastigial neurons using a viral approach
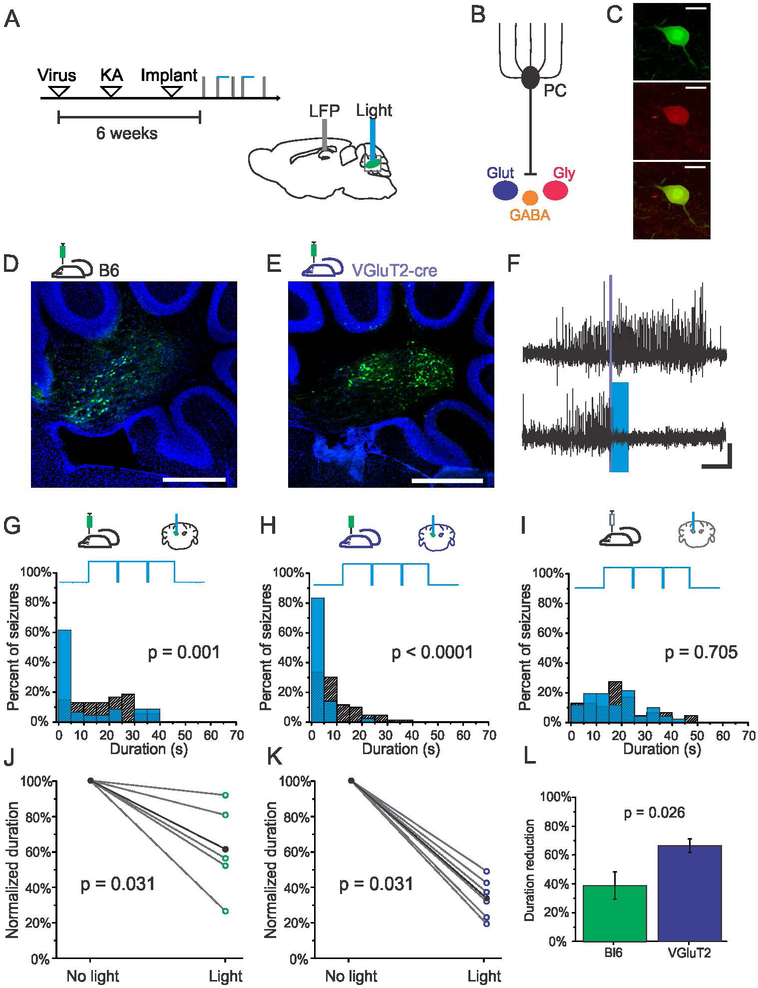
While VGluT2 is expressed by glutamatergic neurons of the fastigial nucleus (Itō, 1984), it is also found in the collaterals of both mossy and climbing fibers terminating in the deep cerebellar nuclei (Hioki et al., 2003). We thus used a viral approach to further increase the specificity of on-demand interventions to neurons of the fastigial nucleus. The contralateral fastigial nucleus was injected with viruses inducing the expression of ChR2. Subsequently, KA injections and implantations were performed as in previous experiments, with on-demand interventions implemented a minimum of 6 weeks post viral injection (Fig. 5A). Two separate viral targeting techniques were used to target fastigial neurons, as the fastigial nucleus contains glutamatergic, glycinergic and GABAergic neurons, all of which can be inhibited by Purkinje cells (Uusisaari & Knopfel, 2012)(Fig. 5B). In the first set of experiments, global targeting of fastigial neurons was achieved via injecting Black-6 mice with the virus AAV9-CAG-ChR2-GFP for the expression of channelrhodopsin (Fig. 5D), or, as a control, AAV9-CAG-GFP. We also selectively targeted glutamatergic neurons of the fastigial nucleus by injecting VGluT2-cre mice with a cre-dependent virus encoding channelrhodopsin (pAAV9-EF1a-double floxed-hChR2(H134R)-EYFP-WPRE-HGHpA), or, as a control, AAV9-CAG-Flex-GFP (Fig. 5E). The selectivity of expression of virally-targeted glutamatergic neurons in VGluT2-cre mice was confirmed by performing immunohistochemistry against VGluT2 (Fig. 5C); greater than 99% of GFP expressing neurons (349 out of 352 neurons, n = 2 mice) were immunopositive for VGluT2.
Figure 5. Selective excitation of fastigial glutamatergic neurons provides robust seizure control.
A) Schematic of experimental design and timeline. Viral approaches allowed for the targeting of nuclear neurons broadly, including nuclear glutamatergic, GABAergic, and glycinergic neurons (B), or selective targeting of glutamatergic nuclear neurons (shown in dark blue in the figure). (C) Selective expression in glutamategic neurons is achieved following injection of cre-dependent viruses in VGluT2-cre transgenic mice (Top- Green: GFP, Middle- Red: VGluT2 immunohistochemistry, Bottom- overlay, scale bar 70μm). D) GFP expression in nuclear neurons following injection in a Black-6 mouse. E) GFP expression in nuclear neurons following injection of cre-dependent virus in a VGluT2-cre mouse. Scale bars for D and E: 500μm. Green: GFP. Blue: DAPI. F) Example seizure events detected on-line (denoted by purple bar) that were either randomly selected not to receive light (top trace) or receive light (bottom trace, 3 seconds of blue (473nm) light delivery denoted by blue box). Scale bar: 5s, 0.05mV. G-H) Light delivery significantly reduces seizure duration in virally injected Black-6 (G, 44% reduction, p = 0.001, two sample Kolmogorov-Smirnov test) and VGluT2-cre (H, 81% reduction, p < 0.001, two sample Kolmogorov-Smirnov test) mice expressing channelrhodopsin. Blue bars: events receiving light intervention; hashed bars: no-light internal controls; top trace illustrates pulsed light delivery paradigm. I) No effect of light delivery in a mouse injected with AAV9-CAG-GFP control vector (p = 0.705, two sample Kolmogorov-Smirnov test). J-K) Stimulation significantly reduces the duration of hippocampal seizures across the population of channelrhodospin-expressing virally injected Black-6 (J) and VGluT2 (K) mice. Each open circle represents one animal. Black data points represent mean. L) Selective targeting of glutamatergic neurons in the fastigial nucleus produces significantly greater seizure attenuation than targeting fastigial neurons more broadly (p = 0.026, Mann-Whitney test).
A significant reduction in seizure duration was observed for both virally injected Black-6 (Fig. 5G) and VGluT2-cre (Fig. 5H) mice expressing channelrhodopsin with on-demand light delivery. Across the population of opsin-expressing virally injected Black-6 animals, on-demand excitation of the fastigial nucleus significantly reduced seizure duration (Fig. 5J, 39 ± 9% reduction, p = 0.031, Wilcoxon signed rank test, n = 6 animals), with 4/6 animals showing a significant effect of light delivery. On-demand selective excitation of glutamatergic fastigial neurons was also highly effective at reducing seizure duration (Fig. 5K, 66 ± 5% reduction, p = 0.031, Wilcoxon signed rank test, n = 6 animals), with 6/6 animals showing a significant effect of light delivery. Selective excitation of fastigial glutamatergic neurons produced a significantly greater reduction in seizure duration compared to exciting the fastigial nucleus more broadly (Fig. 5L, p = 0.026, Mann-Whitney test), suggesting that the improved cell-type targeting can provide greater seizure suppression benefits.
Single pulse stimulation also significantly reduced seizure duration in virally injected animals (39 ± 9% reduction, p = 0.016, Wilcoxon signed rank test, n = 3 Black-6 and n = 4 VGluT2 animals), illustrating that even brief activation of excitatory fastigial neurons is able to disrupt seizures.
As seen in our other experiments, opsin expression was required, as mice injected with control virus inducing the expression of only fluorescent protein showed no effect of light delivery on seizure duration (Fig. 5I, not significant in 4/4 animals). As an additional control, we also injected VGluT2-cre mice with a virus allowing cre-dependent expression of halorhodopsin (pAAV9-Ef1a-DIO-eNpHR 3.0-EYFP), to inhibit glutamatergic fastigial neurons. In support of earlier experiments, on-demand inhibition using a viral approach also had no effect on seizure duration, with 0/2 animals showing a significant effect of light delivery.
Together, these results show that excitation of fastigial glutamatergic neurons can robustly inhibit hippocampal seizures.
DISCUSSION
Using a combination of transgenic and viral approaches, this study reveals a powerful influence of fastigial neurons on hippocampal seizures. Seizures are robustly inhibited by multiple stimulation parameters, regardless of laterality of the fastigial nucleus targeted for stimulation. However, excitation, rather than inhibition, is required. Even a single excitatory light pulse delivered to the fastigial nucleus is sufficient to terminate seizures, emphasizing the profound influence of fastigial activation on hippocampal seizures. Viral targeting experiments reveal that selective activation of glutamatergic fastigial neurons provides attenuation of hippocampal seizures. Together, these results indicate that the fastigial nucleus could represent a promising target for therapeutic stimulation in TLE. Additionally, they highlight the importance of excitation in the deep cerebellar nuclei for cerebellar attenuation of seizures.
The finding that direct inhibition of the fastigial nucleus fails to affect hippocampal seizure duration was initially surprising. Given that Purkinje cells are inhibitory and that direct excitation of Purkinje cells robustly attenuates hippocampal seizures, a reasonable expectation is that direct inhibition of the fastigial nucleus should mimic the effects of Purkinje cell excitation. How then, can optogenetic excitation of Purkinje cells inhibit seizures, but not optogenetic inhibition of nuclear neurons? One possible explanation is the complex response to optogenetic manipulation of Purkinje cells themselves. During optogenetic excitation of Purkinje cells, cerebellar nuclei neurons show appropriately decreased firing rates (Chaumont et al., 2013; Witter et al., 2013; Brown & Raman, 2018). However, optogenetic excitation of Purkinje cells is subsequently followed by a pause in Purkinje cell activity (Krook-Magnuson et al., 2014; Lee et al., 2015; Brown & Raman, 2018) and a corresponding increase in firing in the cerebellar nuclei (Lee et al., 2015; Brown & Raman, 2018). Therefore, both optogenetic excitation or inhibition of Purkinje cells could result in excitation of nuclear neurons, and thereby inhibition of seizures. Our finding that on-demand excitation, but not on-demand inhibition, of fastigial neurons successfully terminates seizures strongly suggest that an increase in firing of nuclear neurons may be key for successful seizure intervention. Minimally, excitation of excitatory fastigial neurons is sufficient for seizure inhibition.
While our results provide strong support for a role of excitatory fastigial neurons in the suppression of seizures, our results do not rule out other potential contributors. For example, Purkinje cells can have direct projections to areas beyond the cerebellar nuclei (Schwarz et al., 2015; Hashimoto et al., 2018). The ability of these projections to inhibit seizures was not tested in this study. Additionally, the deep cerebellar nuclei have a diversity of cell types, including local interneurons and excitatory and inhibitory projection neurons (Uusisaari & Knopfel, 2012). While our results demonstrate that selectively targeting excitatory cells provides greater seizure control than broadly targeting fastigial neurons, selectively targeting other cell populations may also provide additional effects and interesting insights. For example, the fastigial nucleus contains a unique subpopulation of glycinergic projection neurons (Bagnall et al., 2009). The impact of selectively activating or inhibiting this population of neurons on seizure activity remains unknown. Similarly, while we have focused on the fastigial nucleus, previous work targeting the cerebellar cortex (Krook-Magnuson et al., 2014) suggests that other cerebellar nuclei may also be capable of producing seizure suppressive effects in temporal lobe epilepsy.
While the cerebellum is not typically associated with epilepsy, cerebellar impairments are often observed with epilepsy (Marcian et al., 2016; Boscolo Galazzo et al., 2018; Lee et al., 2018; Allen et al., 2019; Li et al., 2019), changes in cerebellar actvity are associated with seizure events (Niedermeyer & Uematsu, 1974; Mitra & Snider, 1975; Kandel & Buzsaki, 1993; Blumenfeld et al., 2009; Krook-Magnuson et al., 2014; Kros et al., 2017), and there have been noted cases of seizures originating in the cerebellum (Harvey et al., 1996; Mesiwala et al., 2002; Norden & Blumenfeld, 2002; Boop et al., 2013; Lascano et al., 2013; Martins et al., 2016). Indeed, the cerebellum was previously a target for seizure control of considerable interest and investigation (Cooke & Snider, 1955; Babb et al., 1974; Maiti & Snider, 1975). However, while early studies suggested that seizure inhibition with electrical stimulation of the cerebellum is possible, small clinical trials (and further animal studies) ultimately produced mixed results (for reviews, see (Miller, 1992; Fountas et al., 2010; Zhong et al., 2011)). As a result, the cerebellum fell out of favor. One reason for the discrepancy between these initial results and optogenetic stimulation of the cerebellum shown here and in previous work (Krook-Magnuson et al., 2014; Kros et al., 2015a)may be the closed-loop nature of the interventions. In on-demand interventions, stimulation is only applied on an ‘as-needed’ basis, limiting potential mal-adaptive plasticity or other negative effects of constant intervention; on-demand interventions, with their improved temporal alignment of intervention, may be inherently more effective (Good et al., 2009; Krook-Magnuson et al., 2015; Thomas & Jobst, 2015). Another benefit of optogenetic approaches is the cell type specificity, which may also aid in successful intervention (Krook-Magnuson & Soltesz, 2015). Here we find that cell-type specificity when targeting the fastigial nucleus for seizure control can indeed provide improved outcomes. Finally, as also demonstrated here, the direction of modulation can be crucial, raising the possibility that previous electrical stimulation efforts did not consistently produce excitation of the cerebellar nuclei.
While the cerebellum has long been considered a purely motor structure, increasing evidence suggests a role for the cerebellum in more cognitive functions (for reviews, see (Popa et al., 2014; Sokolov et al., 2017)). Mice with impaired cerebellar plasticity show disruptions in hippocampal place cells (Rochefort et al., 2011; Lefort et al., 2019), cerebellar activity can be synchronized with hippocampal oscillations (Wikgren et al., 2010; McAfee et al., 2019), and inhibition of Purkinje cells leads to functional activation of the hippocampus (Choe et al., 2018). However, while previous studies examining degenerating axons suggested a direct fastigial to hippocampal connection (Heath & Harper, 1974), evidence for a direct connection has not been replicated in later studies (Strick et al., 2009; Rochefort et al., 2013; Bohne et al., 2019), and we see no fibers from fastigial neurons in the hippocampus in our virally injected animals (data not shown). This suggests that other downstream pathways engaged by cerebellar stimulation must underlie hippocampal seizure attenuation (for a review, see (Yu & Krook-Magnuson, 2015)). Potential fastigial downstream targets of interest could include the reticular formation (Browning, 1985), superior colliculus (Soper et al., 2016), and thalamus (Fisher et al., 2010; Kros et al., 2015b; Salanova et al., 2015), but future experiments will be necessary to determine which fastigial targets are responsible. Notably, the vermis projects not only to the fastigial nucleus, but also the vestibular nuclei. Given our findings that direct stimulation of fastigial neurons is sufficient to inhibit seizures, vermal projections to the vestibular nuclei are unlikely to mediate seizure termination observed with optogenetic modulation of the vermis, or, minimally, are not required for it.
Our findings provide key insight into the mechanism by which modulation of the cerebellar cortex controls temporal lobe seizures. Specifically, we find that excitation of nuclear neurons is required, as inhibition of fastigial neurons had no effect. Excitation of fastigial neurons, in contrast, provided robust seizure control across a range of stimulation frequencies. In particular, excitation of fastigial excitatory neurons (which project to a number of downstream regions) was sufficient to terminate temporal lobe seizures. The deep cerebellar nuclei may be counterintuitively more accessible for intervention (Fountas et al., 2010; Wathen et al., 2018), as surgical complications including electrode displacement have been noted when targeting the cerebellar cortex (Wright et al., 1984; Fountas et al., 2010). The range of effective stimulation parameters and ability to successfully target either the fastigial nucleus contralateral or ipsilateral to the site of seizure focus add to its promise as a target for stimulation. Additionally, the ability to terminate hippocampal seizures with only a single light pulse is of therapeutic interest, as it provides seizure control while minimizing disruptions to ongoing cerebellar function. Therefore, targeting the fastigial nucleus for seizure control clinically should be revisited, as our data suggest it may be a powerful target for therapeutic intervention in temporal lobe epilepsy.
KEY POINTS.
On-demand optogenetic inhibition of glutamatergic neurons in the fastigial nucleus of the cerebellum does not alter hippocampal seizures in a mouse model of temporal lobe epilepsy.
In contrast, on-demand optogenetic excitation of glutamatergic neurons in the fastigial nucleus successfully inhibits hippocampal seizures. With this approach, even a single 50ms pulse of light is able to significantly inhibit seizures.
On-demand optogenetic excitation of glutamatergic fastigial neurons either ipsilateral or contralateral to the seizure focus is able to inhibit seizures.
Selective excitation of glutamatergic nuclear neurons provides greater seizure inhibition than broadly exciting nuclear neurons without cell-type specificity.
Acknowledgments
The authors thank Chris Krook-Magnuson, Brandon McCollam, and Jane Yap for technical assistance, Dr. Michael Benneyworth and the University of Minnesota Mouse Behavioral Core, the University of Minnesota University Imaging Centers, and Dr. Erin Larson and the University of Minnesota Optogenetics Core for technical assistance and equipment.
Funding
This work was supported in part by The Winston and Maxine Wallin Neuroscience Discovery Fund Award, an American Epilepsy Society Postdoctoral Fellowship (MLS), NIH R01-NS104071-01, a University of Minnesota McKnight Land-Grant Professorship award, and the University of Minnesota’s MnDRIVE (Minnesota’s Discovery, Research and Innovation Economy) initiative.
Biography

Martha Streng received a B.A. in Neuroscience and Behavior from Mount Holyoke College and a Ph.D. in Neuroscience from the University of Minnesota, Twin Cities, where she studied the encoding of motor predictive and feedback information by Purkinje cells during online motor control tasks. Her current research focuses on the characterization of cerebellar circuitry and output channels in health and disease.
Footnotes
Competing interests
The authors declare no competing financial interests
All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Reference list
- Allen LA, Vos SB, Kumar R, Ogren JA, Harper RK, Winston GP, Balestrini S, Wandschneider B, Scott CA, Ourselin S, Duncan JS, Lhatoo SD, Harper RM & Diehl B. (2019). Cerebellar, limbic, and midbrain volume alterations in sudden unexpected death in epilepsy. Epilepsia 60, 718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angaut P & Bowsher D. (1970). Ascending projections of the medial cerebellar (fastigial) nucleus: an experimental study in the cat. Brain Res 24, 49–68. [DOI] [PubMed] [Google Scholar]
- Armstrong C, Krook-Magnuson E, Oijala M & Soltesz I. (2013). Closed-loop optogenetic intervention in mice. Nature protocols 8, 1475–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TL, Mitchell AG Jr. & LCrandall PH. (1974). Fastigiobulbar and dentatothalamic influences on hippocampal cobalt epilepsy in the cat. Electroencephalogr Clin Neurophysiol 36, 141–154. [DOI] [PubMed] [Google Scholar]
- Bagnall MW, Zingg B, Sakatos A, Moghadam SH, Zeilhofer HU & du Lac S. (2009). Glycinergic projection neurons of the cerebellum. J Neurosci 29, 10104–10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, Srinivasan S, Jobst B, Gross RE, Shields DC, Barkley G, Salanova V, Olejniczak P, Cole A, Cash SS, Noe K, Wharen R, Worrell G, Murro AM, Edwards J, Duchowny M, Spencer D, Smith M, Geller E, Gwinn R, Skidmore C, Eisenschenk S, Berg M, Heck C, Van Ness P, Fountain N, Rutecki P, Massey A, O’Donovan C, Labar D, Duckrow RB, Hirsch LJ, Courtney T, Sun FT & Seale CG. (2015). Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 84, 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, Levin AR, Hirsch LJ, Tikofsky R, Zubal IG, Paige AL & Spencer SS. (2009). Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain 132, 999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne P, Schwarz MK, Herlitze S & Mark MD. (2019). A New Projection From the Deep Cerebellar Nuclei to the Hippocampus via the Ventrolateral and Laterodorsal Thalamus in Mice. Front Neural Circuits 13, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boop S, Wheless J, Van Poppel K, McGregor A & Boop FA. (2013). Cerebellar seizures. J Neurosurg Pediatr 12, 288–292. [DOI] [PubMed] [Google Scholar]
- Boscolo Galazzo I, Storti SF, Barnes A, De Blasi B, De Vita E, Koepp M, Duncan JS, Groves A, Pizzini FB, Menegaz G & Fraioli F. (2018). Arterial Spin Labeling Reveals Disrupted Brain Networks and Functional Connectivity in Drug-Resistant Temporal Epilepsy. Front Neuroinform 12, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouilleret V, Ridoux V, Depaulis A, Marescaux C, Nehlig A & Le Gal La Salle G (1999). Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience 89, 717–729. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J Jr., Wilson CL, Vizentin E & Mathern GW (1999). Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia 40, 1210–1221. [DOI] [PubMed] [Google Scholar]
- Brown ST & Raman IM. (2018). Sensorimotor Integration and Amplification of Reflexive Whisking by Well-Timed Spiking in the Cerebellar Corticonuclear Circuit. Neuron 99, 564–575 e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning RA. (1985). Role of the brain-stem reticular formation in tonic-clonic seizures: lesion and pharmacological studies. Fed Proc 44, 2425–2431. [PubMed] [Google Scholar]
- Chaumont J, Guyon N, Valera AM, Dugue GP, Popa D, Marcaggi P, Gautheron V, Reibel-Foisset S, Dieudonne S, Stephan A, Barrot M, Cassel JC, Dupont JL, Doussau F, Poulain B, Selimi F, Lena C & Isope P. (2013). Clusters of cerebellar Purkinje cells control their afferent climbing fiber discharge. Proc Natl Acad Sci U S A 110, 16223–16228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KY, Sanchez CF, Harris NG, Otis TS & Mathews PJ. (2018). Optogenetic fMRI and electrophysiological identification of region-specific connectivity between the cerebellar cortex and forebrain. Neuroimage 173, 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PM & Snider RS. (1955). Some cerebellar influences on electrically-induced cerebral seizures. Epilepsia 4, 19–28. [DOI] [PubMed] [Google Scholar]
- El-Shamayleh Y, Kojima Y, Soetedjo R & Horwitz GD. (2017). Selective Optogenetic Control of Purkinje Cells in Monkey Cerebellum. Neuron 95, 51–62 e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder C, Friedman D, Devinsky O, Doyle W & Dugan P. (2019). Responsive neurostimulation targeting the anterior nucleus of the thalamus in 3 patients with treatment-resistant multifocal epilepsy. Epilepsia Open 4, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England MJ, Liverman CT, Schultz AM & Strawbridge LM. (2012). Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy & behavior : E&B 25, 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N & Group SS. (2010). Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51, 899–908. [DOI] [PubMed] [Google Scholar]
- Fountas KN, Kapsalaki E & Hadjigeorgiou G. (2010). Cerebellar stimulation in the management of medically intractable epilepsy: a systematic and critical review. Neurosurgical focus 29, E8. [DOI] [PubMed] [Google Scholar]
- Gauck V & Jaeger D. (2000). The control of rate and timing of spikes in the deep cerebellar nuclei by inhibition. J Neurosci 20, 3006–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good LB, Sabesan S, Marsh ST, Tsakalis K, Treiman D & Iasemidis L. (2009). Control of synchronization of brain dynamics leads to control of epileptic seizures in rodents. International journal of neural systems 19, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB & Deisseroth K. (2007). Targeting and readout strategies for fast optical neural control in vitro and in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 14231–14238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR & Deisseroth K. (2010). Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AS, Jayakar P, Duchowny M, Resnick T, Prats A, Altman N & Renfroe JB. (1996). Hemifacial seizures and cerebellar ganglioglioma: an epilepsy syndrome of infancy with seizures of cerebellar origin. Annals of neurology 40, 91–98. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Yamanaka A, Kato S, Tanifuji M, Kobayashi K & Yaginuma H. (2018). Anatomical Evidence for a Direct Projection from Purkinje Cells in the Mouse Cerebellar Vermis to Medial Parabrachial Nucleus. Front Neural Circuits 12, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RG & Harper JW. (1974). Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Experimental neurology 45, 268–287. [DOI] [PubMed] [Google Scholar]
- Hioki H, Fujiyama F, Taki K, Tomioka R, Furuta T, Tamamaki N & Kaneko T. (2003). Differential distribution of vesicular glutamate transporters in the rat cerebellar cortex. Neuroscience 117, 1–6. [DOI] [PubMed] [Google Scholar]
- Itō M (1984). The cerebellum and neural control. Raven Press, New York. [Google Scholar]
- Kandel A & Buzsaki G. (1993). Cerebellar neuronal activity correlates with spike and wave EEG patterns in the rat. Epilepsy research 16, 1–9. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E, Gelinas JN, Soltesz I & Buzsaki G. (2015). Neuroelectronics and Biooptics: Closed-Loop Technologies in Neurological Disorders. JAMA Neurol 72, 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E & Soltesz I. (2015). Beyond the hammer and the scalpel: selective circuit control for the epilepsies. Nature neuroscience 18, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M & Soltesz I. (2014). Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. eNeuro 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros L, Eelkman Rooda OH, Spanke JK, Alva P, van Dongen MN, Karapatis A, Tolner EA, Strydis C, Davey N, Winkelman BH, Negrello M, Serdijn WA, Steuber V, van den Maagdenberg AM, De Zeeuw CI & Hoebeek FE. (2015a). Cerebellar output controls generalized spike-and-wave discharge occurrence. Annals of neurology 77, 1027–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros L, Eelkman Rooda OHJ, De Zeeuw CI & Hoebeek FE. (2015b). Controlling Cerebellar Output to Treat Refractory Epilepsy. Trends Neurosci 38, 787–799. [DOI] [PubMed] [Google Scholar]
- Kros L, Lindeman S, Eelkman Rooda OHJ, Murugesan P, Bina L, Bosman LWJ, De Zeeuw CI & Hoebeek FE. (2017). Synchronicity and Rhythmicity of Purkinje Cell Firing during Generalized Spike-and-Wave Discharges in a Natural Mouse Model of Absence Epilepsy. Front Cell Neurosci 11, 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse W, Krause M, Aarse J, Mark MD, Manahan-Vaughan D & Herlitze S. (2014). Optogenetic modulation and multi-electrode analysis of cerebellar networks in vivo. PLoS One 9, e105589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascano AM, Lemkaddem A, Granziera C, Korff CM, Boex C, Jenny B, Schmitt-Mechelke T, Thiran JP, Garibotto V, Vargas MI, Schaller K, Seeck M & Vulliemoz S. (2013). Tracking the source of cerebellar epilepsy: hemifacial seizures associated with cerebellar cortical dysplasia. Epilepsy Res 105, 245–249. [DOI] [PubMed] [Google Scholar]
- Lee KH, Mathews PJ, Reeves AM, Choe KY, Jami SA, Serrano RE & Otis TS. (2015). Circuit mechanisms underlying motor memory formation in the cerebellum. Neuron 86, 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Rodriguez OC, Albanese C, Santos VR, Cortes de Oliveira JA, Donatti ALF, Fernandes A, Garcia-Cairasco N, N’Gouemo P & Forcelli PA. (2018). Divergent brain changes in two audiogenic rat strains: A voxel-based morphometry and diffusion tensor imaging comparison of the genetically epilepsy prone rat (GEPR-3) and the Wistar Audiogenic Rat (WAR). Neurobiology of disease 111, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort JM, Vincent J, Tallot L, Jarlier F, De Zeeuw CI, Rondi-Reig L & Rochefort C. (2019). Impaired cerebellar Purkinje cell potentiation generates unstable spatial map orientation and inaccurate navigation. Nature communications 10, 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Hu C, Wang L, Liu D, Liu D, Liao W, Xiao B, Chen H & Feng L. (2019). Disruption of functional connectivity among subcortical arousal system and cortical networks in temporal lobe epilepsy. Brain Imaging Behav. [DOI] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ 3rd, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsaki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE & Zeng H (2012). A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature neuroscience 15, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti A & Snider RS. (1975). Cerebellar control of basal forebrain seizures: amygdala and hippocampus. Epilepsia 16, 521–533. [DOI] [PubMed] [Google Scholar]
- Marcian V, Filip P, Bares M & Brazdil M. (2016). Cerebellar Dysfunction and Ataxia in Patients with Epilepsy: Coincidence, Consequence, or Cause? Tremor Other Hyperkinet Mov (N Y) 6, 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins WA, Paglioli E, Hemb M & Palmini A. (2016). Dysplastic Cerebellar Epilepsy: Complete Seizure Control Following Resection of a Ganglioglioma. Cerebellum 15, 535–541. [DOI] [PubMed] [Google Scholar]
- McAfee SS, Liu Y, Sillitoe RV & Heck DH. (2019). Cerebellar Lobulus Simplex and Crus I Differentially Represent Phase and Phase Difference of Prefrontal Cortical and Hippocampal Oscillations. Cell Rep 27, 2328–2334 e2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesiwala AH, Kuratani JD, Avellino AM, Roberts TS, Sotero MA & Ellenbogen RG. (2002). Focal motor seizures with secondary generalization arising in the cerebellum. Case report and review of the literature. Journal of neurosurgery 97, 190–196. [DOI] [PubMed] [Google Scholar]
- Miller JW. (1992). The role of mesencephalic and thalamic arousal systems in experimental seizures. Prog Neurobiol 39, 155–178. [DOI] [PubMed] [Google Scholar]
- Miterko LN, Baker KB, Beckinghausen J, Bradnam LV, Cheng MY, Cooperrider J, DeLong MR, Gornati SV, Hallett M, Heck DH, Hoebeek FE, Kouzani AZ, Kuo SH, Louis ED, Machado A, Manto M, McCambridge AB, Nitsche MA, Taib NOB, Popa T, Tanaka M, Timmann D, Steinberg GK, Wang EH, Wichmann T, Xie T & Sillitoe RV. (2019). Consensus Paper: Experimental Neurostimulation of the Cerebellum. Cerebellum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra J & Snider RS. (1975). Effects of hippocampal afterdischarges on Purkinje cell activity. Epilepsia 16, 235–243. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E & Uematsu S. (1974). Electroencephalographic recordings from deep cerebellar structures in patients with uncontrolled epileptic seizures. Electroencephalography and clinical neurophysiology 37, 355–365. [DOI] [PubMed] [Google Scholar]
- Norden AD & Blumenfeld H. (2002). The role of subcortical structures in human epilepsy. Epilepsy Behav 3, 219–231. [DOI] [PubMed] [Google Scholar]
- Pakdaman H, Amini Harandi A, Abbasi M, Karimi M, Arami MA, Mosavi SA, Haddadian K, Rezaei O, Sadeghi S, Sharifi G, Gharagozli K, Bahrami P, Ashrafi F, Kasmae HD, Ghassemi A, Arabahmadi M & Behnam B. (2016). Vagus nerve stimulation in drug-resistant epilepsy: the efficacy and adverse effects in a 5-year follow-up study in Iran. Neurol Sci 37, 1773–1778. [DOI] [PubMed] [Google Scholar]
- Popa LS, Hewitt AL & Ebner TJ. (2014). The cerebellum for jocks and nerds alike. Front Syst Neurosci 8, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Arabo A, Andre M, Poucet B, Save E & Rondi-Reig L. (2011). Cerebellum shapes hippocampal spatial code. Science 334, 385–389. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Lefort JM & Rondi-Reig L. (2013). The cerebellum: a new key structure in the navigation system. Frontiers in neural circuits 7, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, Labar D, Sperling MR, Sharan A, Sandok E, Handforth A, Stern JM, Chung S, Henderson JM, French J, Baltuch G, Rosenfeld WE, Garcia P, Barbaro NM, Fountain NB, Elias WJ, Goodman RR, Pollard JR, Troster AI, Irwin CP, Lambrecht K, Graves N, Fisher R & Group SS. (2015). Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 84, 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ & Luo L. (2015). Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov AA, Miall RC & Ivry RB. (2017). The Cerebellum: Adaptive Prediction for Movement and Cognition. Trends Cogn Sci 21, 313–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper C, Wicker E, Kulick CV, N’Gouemo P & Forcelli PA. (2016). Optogenetic activation of superior colliculus neurons suppresses seizures originating in diverse brain networks. Neurobiology of disease 87, 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP & Fiez JA. (2009). Cerebellum and nonmotor function. Annual review of neuroscience 32, 413–434. [DOI] [PubMed] [Google Scholar]
- Thomas GP & Jobst BC. (2015). Critical review of the responsive neurostimulator system for epilepsy. Med Devices (Auckl) 8, 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota T, Ohashi Y, Tamura K, Sato A & Miyashita Y. (2011). Optogenetic manipulation of cerebellar Purkinje cell activity in vivo. PLoS One 6, e22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusisaari MY & Knopfel T. (2012). Diversity of neuronal elements and circuitry in the cerebellar nuclei. Cerebellum 11, 420–421. [DOI] [PubMed] [Google Scholar]
- Valentin A, Garcia Navarrete E, Chelvarajah R, Torres C, Navas M, Vico L, Torres N, Pastor J, Selway R, Sola RG & Alarcon G. (2013). Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia 54, 1823–1833. [DOI] [PubMed] [Google Scholar]
- Vivas AC, Baaj AA, Benbadis SR & Vale FL. (2012). The health care burden of patients with epilepsy in the United States: an analysis of a nationwide database over 15 years. Neurosurg Focus 32, E1. [DOI] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S Jr. & Lowell BB (2011). Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen CA, Frizon LA, Maiti TK, Baker KB & Machado AG. (2018). Deep brain stimulation of the cerebellum for poststroke motor rehabilitation: from laboratory to clinical trial. Neurosurg Focus 45, E13. [DOI] [PubMed] [Google Scholar]
- Wikgren J, Nokia MS & Penttonen M. (2010). Hippocampo-cerebellar theta band phase synchrony in rabbits. Neuroscience 165, 1538–1545. [DOI] [PubMed] [Google Scholar]
- Witter L, Canto CB, Hoogland TM, de Gruijl JR & De Zeeuw CI. (2013). Strength and timing of motor responses mediated by rebound firing in the cerebellar nuclei after Purkinje cell activation. Front Neural Circuits 7, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GD, McLellan DL & Brice JG. (1984). A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. Journal of neurology, neurosurgery, and psychiatry 47, 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W & Krook-Magnuson E. (2015). Cognitive Collaborations: Bidirectional Functional Connectivity Between the Cerebellum and the Hippocampus. Frontiers in systems neuroscience 9, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler Z, Brandt-Fontaine M, Leintz C, Krook-Magnuson C, Netoff T & Krook-Magnuson E. (2018). Targeting the Mouse Ventral Hippocampus in the Intrahippocampal Kainic Acid Model of Temporal Lobe Epilepsy. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XL, Yu JT, Zhang Q, Wang ND & Tan L. (2011). Deep brain stimulation for epilepsy in clinical practice and in animal models. Brain research bulletin 85, 81–88. [DOI] [PubMed] [Google Scholar]