Abstract
Mitochondria have emerged as important pharmacological targets due to their key role in cellular proliferation and death. In tumor tissues, mitochondria can switch metabolic phenotypes to meet the challenges of high energy demand and macromolecular synthesis. Furthermore, mitochondria can engage in crosstalk with the tumor microenvironment, and signals from cancer-associated fibroblasts can impinge on mitochondria. Cancer cells can also acquire a hybrid phenotype in which both glycolysis and OXPHOS can be utilized. This hybrid phenotype can facilitate metabolic plasticity of cancer cells more specifically in metastasis and therapy-resistance. In light of the metabolic heterogeneity and plasticity of cancer cells that had until recently remained unappreciated, strategies targeting cancer metabolic dependency appear promising to develop novel and effective cancer therapeutics.
Mitochondria and Cancer
Abnormal mitochondrial morphology, functions, and dynamics have long been associated with malignant transformation. Otto Warburg in the 1920s was one of the first to observe this phenomenon where cancer cells utilize glycolysis, even under oxygen rich conditions, and produce excess lactate, a process defined as “aerobic glycolysis”, which is often referred to as the “Warburg effect” [1, 2]. This finding suggested that mitochondrial dysfunction is the hallmark of cancer tumorigenesis and became the foundation for the visual detection of cancer via the development of positron emission tomography (PET) imaging, both as a staging tool and to evaluate response to therapy. Another observation that supported this viewpoint was the production of high levels of mitochondrial ROS. Enhanced ROS leads to the activation of cellular signaling cascades and oncogene activation that result in cell proliferation and genomic instability [3, 4]. However, this view was challenged when it was demonstrated that mitochondria are not wholly dysfunctional in cancer and in fact, produce similar levels of intermediates of the tricarboxylic acid (TCA) cycle and fatty acid oxidation as non-cancerous cells [5-7]. Even while aerobic glycolysis is increased in an oxygen-rich setting, oxidative phosphorylation (OXPHOS, see Glossary) still continues. Rather, in response to oncogenic signals, their metabolic signaling is upregulated, thus producing increased TCA cycle intermediates that promote and meet the metabolic demands of cancer growth and proliferation. Additionally, studies have demonstrated that ATP produced by tumor cells via glycolysis comprises only a small proportion of the total ATP, and tumor cells are vulnerable to the inhibition of OXPHOS [8, 9]. Thus, these observations suggested that mitochondrial dysfunction is not necessary for tumorigenesis, and mitochondria perhaps even fluctuate between differing metabolic phenotypes [10].
Indeed, it is now well documented that in highly aggressive tumors, mitochondrial energy pathways are reprogrammed to meet the challenges of high energy demand, with better utilization of available fuels and macromolecular synthesis for rapid cell division and migration [11-13]. Furthermore, recent evidence indicates that mitochondrial energy reprogramming also regulates oncogenic pathways via mitochondria-to-nucleus retrograde signaling and post-translational modification of the encoded oncoproteins. This signaling facilitates communication between mitochondria and the nucleus [14-16], and can be triggered by alterations in mitochondrial DNA copy numbers, mutations, defects in respiratory chain complexes, as well as changes in mitochondrial ROS levels [17]. In fact, it has been demonstrated that retrograde signaling can even alter nuclear gene expressions for metabolic reprogramming in response to these altered mitochondrial activities [13], Consistent with these observations, a recent report showed that, in breast cancer cells, the small GTPase Arf6-based pathway promotes the localization of ILK to focal adhesions to block RhoT1-TRAK2 association, which controls mitochondrial retrograde trafficking [18]. Furthermore, blocking the RhoT1-TRAK1 machinery impaired cell invasion, but not cell migration. Interestingly, in non-invasive cells or those that are weakly invasive, the TRAK proteins are undetectable. Taken together, these observations have uncovered a novel association between cell movement and mitochondrial dynamics, which is specific to invasion and is necessary for avoiding detrimental ROS production [18].
Mitochondria can also engage in crosstalk with the tumor microenvironment. For example, signals from cancer-associated fibroblasts can impinge on mitochondria to utilize OXPHOS [19], a process referred to as the ‘reverse Warburg effect’ [12]. Indeed, emerging evidence shows that cancer cells can also acquire a hybrid glycolysis/OXPHOS phenotype in which both glycolysis and OXPHOS can be utilized for energy production and biosynthesis of macromolecules [20]. Furthermore, the hybrid glycolysis/OXPHOS phenotype is thought to facilitate metabolic plasticity of cancer cells more specifically in metastasis and therapy-resistance. Moreover, cancer cells can switch their metabolic phenotypes in response to external stimuli for better survival [13]. Thus, in light of the metabolic heterogeneity and plasticity of cancer cells that had until recently remained unappreciated, strategies targeting cancer metabolic dependency appear promising to develop novel and effective cancer therapeutics. Here, we review recent trends in targeting mitochondria in cancer. More specifically, we provide a clinical perspective and focus on new drugs that target the mitochondria and are currently under investigation for different types of cancers (Table 1 and Table 2). As described in Figure 1, they include drugs that target the metabolic functions of mitochondria, modulate mitochondrial dynamics, morphogenesis (e.g. fission/fusion dynamics), mitogenesis (e.g. cell death), and evasion of apoptosis.
Table 1.
Selected completed clinical trials in adult patients.
| Agent | Target or Mechanism |
Trial phase/Tumor type | Regimen (single agent unless specified) |
Outcome | NCT Number | Reference | |
|---|---|---|---|---|---|---|---|
| Targeting mitochondrial metabolism | CPI-613 | Inhibits KGDH and PDH | 1/relapse/refractory AML | CPI-613 + cytarabine + mitoxantrone | ORR 48% (n=26), pts >60 years of age CR/CRi 46% (12/26), poor-risk cytogenetics CR/CRi 48% (11/23) |
[] | [129] |
| 1/newly diagnosed metastatic pancreatic adenocarcinoma | CPI-613 + mFOLFIRINOX | 16.6% CR, 50% PR, 11% SD, 22% PD | [] | [105] | |||
| 1/locally advanced or metastatic pancreatic cancer | Unreported | [] | |||||
| 1/recurrent small cell lung cancer | Unreported | [] | |||||
| 1/2/advanced or metastatic cholangiocarcinoma | Unreported | [] | |||||
| 2/relapsed/refractory SCLC | No treatment responses, median OS 4.3 months | [] | [104] | ||||
| Dichloroacetate (DCA) |
Inhibits PDK and activates PDH | 1/refractory or metastatic solid tumors | Well tolerated, 35% SD | [] | [130] | ||
| 1/recurrent head and neck cancers | Unreported | [] | |||||
| 2/WHO grade III-IV gliomas | Well tolerated | [] | [131] | ||||
| AG-22 (enasidenib) |
IDH inhibitor | 1/2 relapsed/refractory IDH2 mutated AML | ORR 38.5%, with 20.2% CR | [] | [132] | ||
| AG-120 | 1/glioma | 73% SD in patients in dose escalation cohort and 88% in expansion cohort | [] | [133] | |||
| 1/cholangiocarcinoma | 6% of patients with PR, and 56% SD; 6-month PFS 40% | [] | [134] | ||||
| 1/IDH1 mutated AML | ORR 41.6% | [] | [135] | ||||
| AG-881 | 1/IDH mutant solid malignancies | 22% of patients remained on therapy after about 2.5 years | [] | [45] | |||
| Targeting apoptotic pathways | ABT-199 (venetoclax) |
BCL-2 inhibitor | II/mantle cell lymphoma | Venetoclax + ibrutinib | 42% response rate | [] | [136] |
| III/relapsed/refractory CLL | Venetoclax + rituximab | PFS 84.9% at 2 years | [] | [137] | |||
| Birinapant | Mimics SMAC | 1/relapsed epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer | Birinapant + conatumumab | 18 pts enrolled; well-tolerated; 1 pt with PR and 4 pts with SD; median time on study 1.9 months | [] | [138] | |
| 2/relapsed/refractory epithelial ovarian cancer | SD in 2/11 pts; closed after 11 patients enrolled as clinical benefit unlikely | [] | [139] | ||||
| Debio 1143 | 1/advanced or metastatic solid tumors and lymphoma | 5/31 patients with SD for a median duration of 93 days | [] | [140] | |||
| 1/poor-risk AML | Debio 1143 + cytarabine + daunorubicin | 38% CR | [] | [141] | |||
| LCL 161 | 2/relapsed/refractory multiple myeloma | LCL-161 +/− cyclophosphamide | No responses with single-agent; with combination, median PFS 10 months in 5 of 25 patients (1 pt with CR, 3 pts with PR) | [] | [142] | ||
| ME-344 | Decreases mitochondrial ATP production and ROS | 1/refractory solid tumors | 1 of 30 pts with PR for >1 year, 4 pts with SD | [] | [143] | ||
| 1/previously treated, locally advanced or metastatic small cell lung, ovarian, and cervical cancers | ME-344 + topotecan | ORR 2.4%, clinical benefit rate 53.7%, 21 of 41 pts with SD; Terminated due to lack of efficacy | [] | [144] | |||
| Minnelide | Regulation of Sirt3, increase ROS, active caspase-3 | 1/refractory GI malignancies | Well tolerated, disease control observed with progression after dose reduction or treatment breaks | [] | [145] | ||
| ONC201 | Induces transcription of TNF-related apoptosis by inducing TRAIL ligand | 1/advanced solid tumors | 8/10 pts with stable disease; Suspended due to lack of funding | [] | [146] | ||
| 1/advanced solid tumors | Recruiting; preliminary data with 1 pt with prostate ca with stable disease at 6 months and 2 pts with prostate cancer after 4 cycles | [] | [147] |
Abbreviations: ATP adenosine triphosphate, CR complete response, CRi Complete response with incomplete hematologic recovery, KDGH alpha-ketoglutarate dehydrogenase, PDH pyruvate dehydrogenase, PDK pyruvate dehydrogenase kinase, PFS progression-free survival, PR partial response, Pt(s) patient(s), ROS reactive oxygen species, SD stable disease, TNF tumor necrosis factor, TRAIL TNF-related apoptosis inducing ligand, ORR overall response rate
Table 2.
Selected ongoing clinical trials in adult patients.
| Agent | Trial phase/Tumor type | Regimen (single agent unless specified) | NCT Number | |
|---|---|---|---|---|
| Targeting mitochondrial metabolism | CPI-613 | 1/metastatic, unresectable colorectal cancer | CPI-613 + fluorouracil | [] |
| 1/untreated locally advanced or metastatic pancreatic cancer | CPI-613 + gemcitabine + nab-paclitaxel | [] | ||
| 1/relapsed/refractory T-cell non-Hodgkin lymphoma or Hodgkin lymphoma | CPI-613 + bendamustine hydrochloride | [] | ||
| 2/locally advanced pancreatic cancer | CPI-613 + FOLFIRINOX | [] | ||
| 2/relapsed/refractory AML or granulocytic sarcoma | CPI-613 + high dose cytarabine + mitoxantrone | [] | ||
| 2/relapsed/refractory Burkitt Lymphoma/Leukemia or High-grade B-cell lymphoma with high-risk translocations | [] | |||
| 3/ patients ≥ years with relapsed/refractory AML | CPI-613 + high dose cytarabine + mitoxantrone | [] | ||
| 3/metastatic pancreatic adenocarcinoma | CPI-613 + mFOLIRINOX vs FOLFIRINOX | [] | ||
| Targeting apoptotic pathways | BI 891065 | 1/advanced or metastatic malignancies | BI 891065 + BI 754091 | [] |
| Birinapant | 1/locally recurrent head and neck squamous cell carcinoma | Birinapant + IMMRT | [] | |
| 1/2 relapsed or refractory solid tumors | Birinapant + Pembrolizumab | [] | ||
| Debio 1143 | 1/with advanced solid tumor malignancies, including metastatic non-small cell lung cancer refractory to platinum-containing doublet therapy | Debio 1143 + avelumab | [] | |
| IACS 01759 | 1/relapsed/refractory AML | [] | ||
| 1/advanced, metastatic, or unresectable solid tumor malignancies | [] | |||
| IDH inhibitors | 1/IDH1 mutated myeloid neoplasms after SCT | Ivosidenib | [] | |
| 1/brain neoplasms | PEPIDH1M vaccine + temozolomide | [] | ||
| 1/2 advanced hematologic malignancies, AML | AG-120 (Ivosidenib) + venetoclax | [] | ||
| 1/IDH mutant glioma, chondrosarcoma, glioma | AG-120 (Ivosidenib) | [] | ||
| 1/AML and CML after SCT | Enasidenib | [] | ||
| 1/glioma | AG-881 | [] | ||
| 2/relapsed/refractory AML with IDH2 mutation | Enasidenib | [] | ||
| 2/IDH2 recurrent AML | Enasidenib + CPX-351 | [] | ||
| 2/AML and CML | Enasidenib + azacitidine | [] | ||
| 3/newly diagnosed IDH1 mutated AML | AG-120 (Ivosidenib) + Azacitidine | [] | ||
| 3/IDH2 mutated AML | AG-221 + azacitidine + cytarabine | [] | ||
| LCL 161 | 1/relapsed/refractory multiple myeloma | CJM 112 vs PDR001 + CJM122 vs CJM112 + LCL 161 | [] | |
| 1/advanced or metastatic solid tumors | PDR001 + LCL 161 vs PDR001 + everolimus vs PDR001 + panobinostat + PDR001 + QBM076 vs PDR001 + HDM201 | [] | ||
| 1/2 relapsed/refractory SCLC and ovarian cancers | LCL 161 + topotecan | [] | ||
| ME-344 | 0/treatment-naïve HER2+ breast ca | Me-344 + bevacizumab | [] | |
| Minnelide | 1/relapsed/refractory AML | [] | ||
| 1/advanced solid tumors | [] | |||
| 2/refractory pancreatic cancer | [] | |||
| ONC201 | 1/advanced solid tumors | Continuation trial for patients who previously received benefit from ONC201 | [] | |
| 1/advanced solid tumors and multiple myeloma | [] | |||
| 1/2 metastatic colorectal cancer, MSS | ONC201 + nivolumab | [] | ||
| 1/2 relapsed/refractory multiple myeloma | ONC201 + ixazomib + dexamethasone | [] | ||
| 1/2 relapsed/refractory multiple myeloma | [] | |||
| 1/2 relapsed/refractory Non-Hodgkin’s lymphoma | [] | |||
| 1/2 relapsed/refractory acute leukemias and HR-MDS | ONC201 + cytarabine | [] | ||
| 2/recurrent or metastatic type II endometrial cancer | [] | |||
| 2/recurrent or metastatic breast cancer and advanced endometrial carcinoma | [] | |||
| 2/recurrent or metastatic endometrial cancer | [] | |||
| 2/recurrent or metastatic neuroendocrine tumors | [] | |||
| 2/metastatic triple negative breast cancer | ONC201 + methionine restricted diet | [] | ||
| 2/recurrent GBM, H3 K27M glioma, and midline glioma | [] |
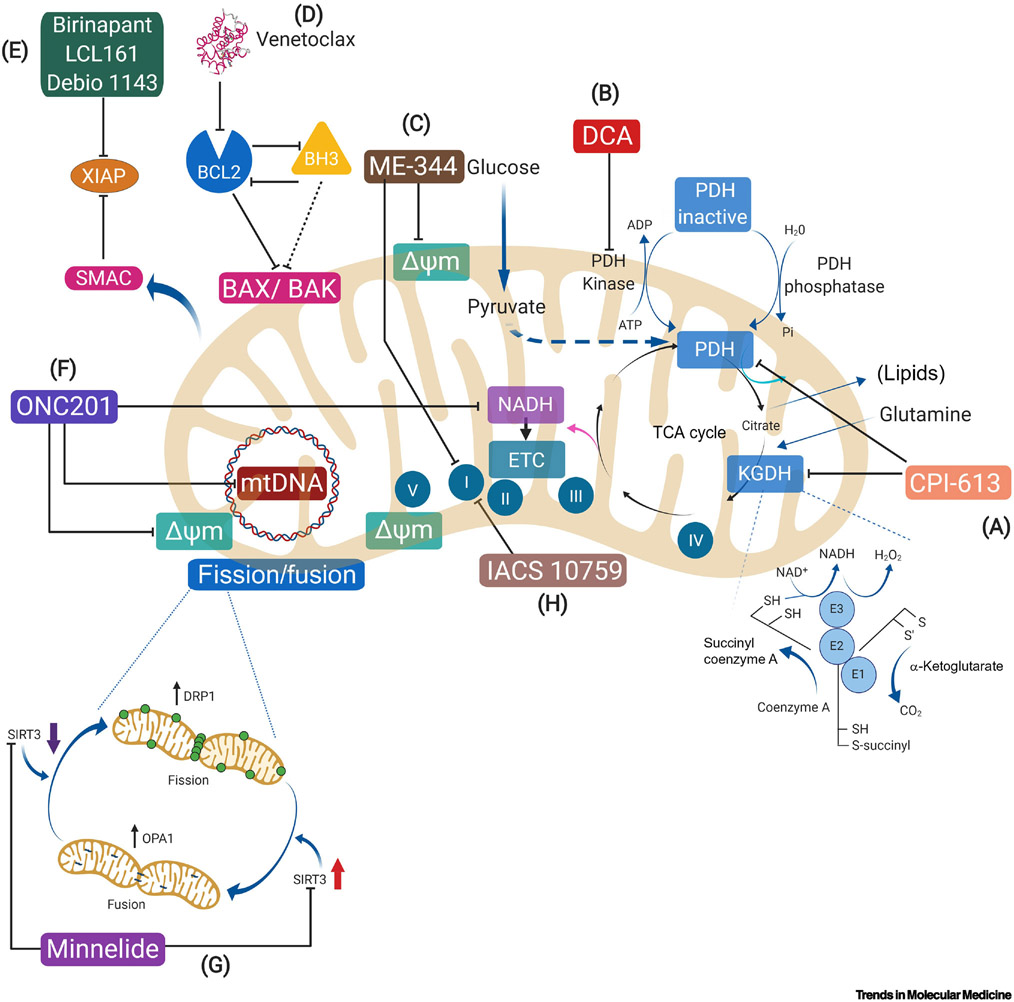
Figure 1. Mitochondrial Therapeutic Targets Undergoing Evaluation in Trials and Their Proposed Mechanism of Action.
This figure is a schematic of a mitochondrion, including a generalized representation of the highlighted therapeutic targets and each of their proposed mechanism of action. The mechanisms of action and selected clinical outcomes are more fully discussed in this review, and selected clinical trials evaluating these therapies are summarized in Table 1 and Table 2. A) CPI-613 inhibits two major mitochondrial enzyme complexes in the TCA cycle, (KGDH) and pyruvate dehydrogenase (PDH).B) DCA (Dichloroacetate) inhibits pyruvate dehydrogenase kinase (PDK), thus activating pyruvate dehydrogenase (PDH) leading to glucose oxidation. C)ME-344 directly targets complex I of OXPHOS. D) Venetoclax is a BH3 mimetic that inhibits BCL2, an anti-apoptotic protein. E) Birinapant, LCL 161, and Debio 1143 are SMAC mimetics which bind to inhibitors of apoptosis which, in turn, enable tumor cell apoptosis. F) ONC201’s mechanism of action is not fully understood, but it has been shown to deplete mitochondrial DNA and disrupt ATP production. G) Minnelide is a water-soluble prodrug of triptolide, a potent heatshock protein (HSP) 70 inhibitor, which releases triptolide into the blood stream to slow tumor growth. H) IACS 10759 is a selective inhibitor of OXPHOS.
Abbreviations: BAK BCL2-antagonist/killer, BAX BCL2-associated X protein, BCL2 B-cell lymphoma 2, BH3 Bcl-2 Homology 3, DCA Dichloroacetate, Delta psi m mitochondrial membrane potential, DRP1 Dynamin-related protein 1, KDGH α-ketoglutarate dehydrogenase, mtDNA Mitochondrial DNA, OPA1 Optic atrophy 1, PDH Pyruvate dehydrogenase, SIRT3 Sirtuin-3 mitochondrial NAD-dependent deacetylase, SMAC Second mitochondria-derived activator of caspase, XIAP X-linked inhibitor of apoptosis.
Mitochondrial Metabolism as Cancer Therapeutic Target
Mitochondrial dysfunction varies depending on the initial conditions of tumor origin and often involves the inhibition of mitochondrial respiration [21]. Tumors are able to compensate for this by utilizing alternate methods of bio-energetics to meet metabolic demands of the cell, and thereby fuel proliferation [22, 23]. This is achieved by deregulated signaling pathways, activation of oncogenes as well as loss of tumor suppressors. Growing evidence has characterized the activity of oxidative phosphorylation in multiple tumor types [24]. For example, melanoma has been shown to have two subpopulations of cells with high and low levels of PGC1α. Melanoma cells with high PGC1α expression are OXPHOS-dependent and resistant to oxidative stress, through high mitochondrial oxidative metabolism and efficient ROS detoxification. Meanwhile, melanoma cells with low levels of PGC1α have lower survival and proliferation potential, but are more capable of forming metastases [25]. Other High-OXPHOS tumors are characterized by upregulation of genes encoding respiratory chain components, together with increased mitochondrial respiration and enhanced antioxidant defense [26].
Aerobic glycolysis circumvents mitochondrial oxidative phosphorylation (OxPhos) facilitating an increased rate of glucose hydrolysis that enables cancer cells to successfully compete with normal cells for glucose uptake in order to maintain uninterrupted growth [27]. Cancer cells use glucose and other glycolytic intermediates as precursors for the synthesis of amino acids, nucleotides, lipids and other building blocks and molecules for new cells. The study of cancer cell glycolysis continues to expand, revealing further associations between a metabolic switch in cancer cells, mutations in mitochondrial metabolic enzymes, and altered mitochondrial function [28, 29]. The upregulation of glycolysis is mostly due to the increased expression of enzymes and transporters involved in glucose uptake, lactate production, and lactate secretion. The growing list of mitochondrial enzymes includes glucose transporters (GLUT1-4), hexokinase 2 (HK2), glyceraldehyde-3- phosphate dehydrogenase (GAPDH), 6-phosphofructo-1-kinase (PFK1), aldolase (ALDO), triose-phosphate isomerase (TPI), phosphoglycerate kinase 1 (PGK1), phosphoglycerate mutase (PGM), enolase 1 (ENO1), pyruvate kinase (PKM2), lactate dehydrogenase (LDHA), and monocarboxylate transporters (MCTs).
The TCA cycle intermediates are utilized as biosynthetic precursors for macromolecules necessary for DNA synthesis and proliferation, including lipids, nucleotides, and proteins. Both glucose, with the production of pyruvate via glycolysis in the cytosol, and glutamine, in its deamination to alpha-ketoglutarate, provide continued replenishment of TCA cycle intermediates in what are termed “anaplerotic reactions” [30]. Pyruvate is transported to the mitochondrial matrix and irreversibly converted to acetyl-CoA by pyruvate dehydrogenase (PDH). Alpha-ketoglutarate is decarboxylated by alpha-ketoglutarate dehydrogenase (KDGH) into succinate and NADH, both of which serve to inhibit this enzyme. Citrate, one of the TCA cycle intermediates, is shuttled and converted to acetyl-CoA in the cytosol by ATP citrate lyase. Acetyl-CoA is irreversibly converted by acetyl-CoA carboxylase to malonyl-CoA, which is involved in fatty acid synthesis. Activation of the PI3K pathway, which has been well-characterized in its role in activating tumorigenesis [31], leads to downstream activation of AKT and mTOR pathways, both of which promote lipid synthesis as well through the activation of sterol regulatory element-binding protein transcription factors (SREBP), which stimulate the transcription of the gene for acetyl-CoA carboxylase. In addition to its role in lipid synthesis, in animal models, AKT has been shown to be activated by insulin-like growth factor (IGF-1) and translocate to the mitochondria where it binds to mitochondrial hexokinase II enzyme, which catalyzes the first step in glycolysis [32]. Thus, developing therapeutics that disrupt these mitochondrial metabolic pathways may be an effective approach for cancer therapy, and in Box 1 we have identified a few of these drugs that are currently under investigation in clinical trials.
Box 1. Mitochondrial metabolism targets in clinical trials.
CPI-613
CPI-613 is a first-in-class lipoate analog that has been shown to inhibit two major mitochondrial enzyme complexes in the TCA cycle, α-ketoglutarate dehydrogenase (KGDH) and pyruvate dehydrogenase (PDH). Lipoate is a co-factor for PDH and other mitochondrial enzymes. CPI-613 selectively inhibits KGDH in tumor cells resulting in ROS generation [100]. CPI-613 has also been demonstrated to inactivate PDH selectively in tumor cells by inducing phosphorylation [101]. CPI-613 is well tolerated overall [102], and has demonstrated promising response rates when used in combination with chemotherapy in patients with solid tumor malignancies [] [103-105].
Dichloroacetate
Dichloroacetate (DCA) is a novel metabolic modulator that has shown in pre-clinical studies to trigger apoptosis of human lung, breast, and brain cancer cells [106]. DCA modulates mitochondrial metabolism by inhibiting pyruvate dehydrogenase kinase (PDK) and activating pyruvate dehydrogenase (PDH), which results in increased delivery of pyruvate to the mitochondria allowing for normalized glucose oxidation to occur [106]. Initial findings identified DCA’s anti-cancer activity in several cancer types to be through inhibition of aerobic glycolysis and activation of mitochondrial potassium ion channels [106]. However, single-agent clinical studies found that DCA, while well-tolerated, was cytostatic possibly because in-vitro studies utilized higher concentrations of DCA than could be tolerated in humans [, ] [107].
Mitochondria and Genomic Instability
Although most cancer cells have functional mitochondria, some cancers, as Warburg postulated, have been shown to have dysfunctional mitochondria, particularly those cancers associated with mutations of mitochondrial enzymes, such as isocitrate dehydrogenase (IDH) and succinate dehydrogenase. IDH enzymes catalyze the conversion of isocitrate to alpha ketoglutarate (α-KG) in the TCA cycle, reducing NAD/NADP+ to NADH/NADPH and CO2 [33]. There are three enzymes that catalyze this same reaction but differ in their cell localization and substrate: IDH1 is located in the cytoplasm and peroxisomes, IDH2 and IDH3 are located in the mitochondria. IDH1 and IDH2 use NADP+ while IDH3 uses NAD+ as its substrate [34]. This energy production is critical for exchanging electrons and metabolites between the mitochondria and cytosol. In cancer, mutations are frequently found in IDH1 and IDH2, most commonly in low-grade gliomas, cartilaginous tumors, angioimmunoblastic T-cell lymphoma, and cholangiocarcinoma [35]. Mutations in these metabolic genes not only lead to a loss of their normal function described above, but also result in the production of 2-hydroxyglutarate (2-HG), an oncometabolite, which acts as a competitor of α-KG and has been shown to alter histone methylation and gene expression [36-38]. This hypermethylation results in chromosome instability and gene silencing, promoting tumorigenesis [39, 40]. Elevated levels of 2-HG have been detected in patients with IDH mutated acute myeloid leukemia (AML) and gliomas, and 2-HG is currently being explored as a potential biomarker [41]. IDH1 mutated AML cells demonstrated increased oxidative phosphorylation activity, which is associated with chemoresistance, and thus has been used as a predictor of treatment response. In vitro and in vivo studies have shown that IDH1 mutated AML cells are more sensitive to Bcl-2 inhibitors which can induce hypoxia versus cytarabine, an anti-metabolite [42].
Preclinical studies showed efficacy of IDH-targeted therapy in patients with relapsed or refractory IDH2 mutated AML, and subsequently AG-22 (enasidenib), an IDH2 inhibitor, was developed and is now FDA approved for these patients harboring an IDH2 mutation [43]. In an evaluation of non-responders, the investigators found that mutations in the RAS pathway were correlated with a decreased response, and response did not necessarily correlate with a decrease in 2-HG levels as described above. Patients who did respond had repaired hematopoietic differentiation of their leukemia cells into mature neutrophils, with studies showing about 12% of patients having the potentially lethal manifestation of this response, differentiation syndrome [44]. Another approved IDH inhibitor is AG-120, ivosidenib, which targets IDH1 mutations. In animal studies, AG-120 demonstrated brain penetration, and is currently being evaluated in clinical trials for patients with AML and solid tumors, thus far with favorable results [, , ]. AG-881 inhibits both IDH1 and IDH2 and has been shown to penetrate the brain, and a phase 1 study was conducted for patients with IDH-mutant solid malignancies, including gliomas. Approximately two and a half years after the start of the trial, about 22% of patients remained on therapy [] [45].
Mitochondrial Dynamics in Cancer
Mitochondrial morphology is mitigated by fission and fusion dynamics, which are most commonly regulated by Dynamin-related protein 1 (DRP1) [fission] and Mitofusin-2 (MFN2) [fusion] proteins. The disruption of these dynamics has a direct effect on the morphology of the mitochondrial network within the cell and can be associated with dysregulated cellular functions [46], as observed in cancer cells [47]. DRP1 is a cytoplasmic protein but translocates to the mitochondria and links to fission 1 (FIS1) and mitochondrial fission factor (MFF) proteins, thereby constricting the outer mitochondria membrane and completing mitochondrial fission [48, 49]. DRP1 functions as the dysregulated protector of dysfunctional mitochondria by preserving an elongated mitochondrial network which prevents mitophagy, autophagy of defective or damaged mitochondria, after nutrient deprivation [50]. While fission dynamics occur even in healthy cells to remove damaged mitochondria from the network through mitophagy, the excess of this dynamic observed in cancer cells has been shown to be associated with OXPHOS defects, nutrient excess, cellular dysfunction, and mitochondrial dysfunction, which are all thought to contribute to the high proliferation and invasiveness in some cancer cells [51]. The nuclear encoded mitochondrial gene MFN2 is a GTPase that is a component of the outer mitochondrial membrane and regulates the fusion of one mitochondrion to another [52, 53]. MFN2 and mitofusin 1 (MFN1) promote mitochondrial elongation to create an elongated network of mitochondria within cells [54]. Fusion of individual mitochondrion to the elongated network of interconnected organelles is a crucial step to meet the bioenergetic demands of the cell. Increased fission and decreased fusion have been shown to attenuate cell mitosis and increase cell proliferation, which have been linked with the fragmented mitochondrial network phenotype and thus can be attributed to high levels of dysfunctional mitochondria in cancer cells [55]. In multiple evaluations of lung cancer cell lines, it was determined that mitochondria in cancer cells, as compared to primary lung cells, were highly fragmented with increased levels of DRP1 and reduced levels of MFN2, wherein DRP1 upregulation and increased expression have been linked to response to hypoxic conditions, less metabolically active mitochondria, and increased mitochondrial biogenesis [55-57].
Several preclinical studies have attempted to restore the functional mitochondrial network observed in healthy cells by targeting fission regulating proteins and exploit this unique mitochondrial morphology as a biomarker of therapeutic efficacy [58, 59]. Minnelide induces mitochondrial impairment through the regulation of SIRT3 [60], a major mitochondrial deacetylase that regulates respiration and ATP production [61]. Sirtuins are a family of nicotinamide adenine dinucleotide (NAD)-dependent deacetylases comprised of seven family members (SIRT1-SIRT7) in humans, and among these three of the members (SIRT3, SIRT4, and SIRT5) are exclusively localized to the mitochondria [62]. These deacetylases have been emerging as important factors in age-related diseases, genomic stability, angiogenesis, metabolism and anoikis. SIRT3 is an active mitochondrial deacetylase that deacetylates and activates complex I of the mitochondrial electron transport chain [61]. It has also been implicated in energy metabolism, nutrient metabolism, glycolysis, oxidative stress, and importantly mitochondrial biogenesis and dynamics. SIRT3 expression and proteins levels have been shown to be regulated by PGC1α alongside ERRα under oxidative stress conditions [63, 64]. Peroxisome proliferator-activated receptor-gamma coactivator (PCG1α) is the master regulator of mitogenesis and actively modulates mitochondrial homeostasis through autophagy and mitophagy [65].
Cancer cells utilize PGC1α as an enhancer of oxidative phosphorylation, mitochondrial biogenesis, and the oxygen consumption rate [66]. The self-sustaining signal transduction mechanisms associated with mitochondrial biogenesis are precipitated by metabolic reprogramming that relies on oxidative phosphorylation to supply the energy required for cancer biosynthesis [66]. SIRT3 is further interconnected with PGC1α through the activation of the AMPK signaling pathway, which increases PGC1α gene-expression [67]. Minnelide is a water-soluble prodrug of triptolide, a potent heatshock protein (HSP) 70 inhibitor, which releases triptolide into the blood stream to slow tumor growth. It has also been suggested that triptolide inhibits transcription in A549 cancer cells, resulting in the disruption of mitochondrial functions including increasing ROS, decreasing the mitochondrial membrane potential, and activating caspase-3 [68]. In preclinical studies, Minnelide was shown to be a promising cancer therapeutic in lung cancer [69], pancreatic cancer [70], liver cancer [71], and breast cancer [72] models. It is currently being evaluated in phase I and II studies with relapsed or refractory AML, refractory pancreatic cancer, and other advanced solid tumors [, , ]. To further enhance the preclinical models of mitochondrial therapeutics it will be vital to understand the relationship between mitochondrial morphology and dynamics using novel fractal mathematical measures as described in Box 2.
Box 2. Mathematic modeling of mitochondrial dynamics in cancer.
Mitochondrial networks can be visually characterized by their tubular connections that retain fractal, or self-similar, patterns. Recent evidence suggests that the morphological structure of the mitochondria network is closely related to the fission/fusion dynamics occurring within the cell as well as changes in response to chemotherapeutic treatment [108, 109]. These networks are often elongated, fragmented, and reticulated fractal constructs that show self-similarity at different scales. Fractals are characterized by three properties: self-similarity, scaling, and a fractional (non-integer) dimension [110]. Fractal measurements were pioneered by Benoit Mandelbrot, who coined the term fractal, and was able to measure the complex irregular curved shapes of the coast of Great Britain using fractal dimension (FD) [111]. FD is a measure of the complexity of the shape, where higher values correspond to more complex patterns. After this discovery lacunarity (LC) has also been introduced to measure how fractal objects fill that space, where higher values correspond with spatial complexity [110]. The complex and fractal geometry of mitochondria and their dynamics requires novel methods of quantification. There are many quantification methods coming to fruition [112, 113] and one such method is the use of these fractal measurements, FD and LC, to help understand therapeutic efficacy of novel drugs and their effect on mitochondrial dynamics in cancer [110, 114]. Fractal measures have already been implemented in various diseases including COPD [115], colorectal adenocarcinoma [116], breast cancer [117], and others [118, 119]. While these studies focused on tissue and radiological scans, more recent progress has been made in evaluating the fractal network of mitochondria [120]. One such study was able to correlate sensitivity to metformin with low mitochondrial FD and high LC in malignant mesothelioma [121]. The use of these and other mitochondrial quantification methods, such as MitoGraph [112], alongside the metabolic profile of tumors may offer tools to understand the hyperplasticity, morphology, dynamics, and sensitivity to mitochondrial therapeutics.
Targeting Mitochondrial Evasion of Apoptosis
Apoptosis is a distinct form of programmed cell death that is characterized by specific morphological and biochemical changes and is essential for homeostasis of vital processes including development, cell turnover, and the functioning of the immune system. Mitochondria are known to have an important role in tumor priming and chemotherapy effectiveness [73, 74] and there are several apoptotic pathways: the extrinsic death receptor pathway, the perforin/granzyme pathway, and the intrinsic BCL-2 regulated mitochondrial caspase dependent pathway [75]. In the mitochondrial apoptotic pathway, various stimuli initiate changes in the mitochondrial inner membrane ultimately leading to the activation of BH3-only containing proteins and the creation of a mitochondrial permeability transition (MPT) pore. A hallmark of apoptosis is the breakdown of the mitochondrial network, involving both fission and fusion dynamics as previously described. During apoptosis, the pro-apoptotic BCL-2 family proteins, BAX and BAK, translocate to the outer membrane and cluster in foci with DRP1 and MFN2 fission and fusion proteins [76]. Subsequently, BAX and BAK interact with the pro-apoptotic BH3-only proteins, leading to the triggering apoptotic event: mitochondrial outer membrane permeabilization (MOMP), after which mainly cytochrome c and other proteins, including SMAC (second mitochondria-derived activator of caspase), are released into the cytosol and activate caspases. MOMP can also result in cell death independently of caspase activation [77]. Evidence suggests that the BH3-only proteins interrupt the anti-apoptotic BLC-2 protein’s inhibition of the BAX and BAK pro-apoptotic proteins, allowing for fission and the release of cytochrome c [78]. Additionally, it has also been suggested that the BH3-only proteins directly activate the pro-apoptotic BAX and BAK proteins [79]. The anti-apoptotic BCL-2 proteins can also bind to the BH3 proteins, preventing the interaction of BH3 proteins with BAX and BAK, or by binding BAX and BAK directly, thus inhibiting MOMP and apoptosis. To overcome this potential inhibition of apoptosis, BH3 mimetics have been developed to bind directly to and inhibit the anti-apoptotic BCL-2 proteins [74].
ME-344 is a synthetic, active metabolite of NV-128, which is a flavonoid that inhibits mTOR by downregulating the AKT/mTOR pathway, and functions independently of caspases. NV-128 was shown to activate the AMPK-mTOR pathway as well as the extracellular signal-regulated kinase-Bax pathway to induce death in both epithelial ovarian cells and subsequently in ovarian cancer stem cells [80]. In pre-clinical studies, ME-344 was shown to have more activity than NV-128 [80], and also demonstrated to reduce the activity of mitochondrial complexes I and III in 143B osteosarcoma, HeLa and HEK293T human embryonic kidney cells [81], thus resulting in a decrease in mitochondrial ATP production and ROS production [80].One current area of interest is the combination of ME-344 and bevacizumab. Preclinical data in breast and lung cancer xenograft models demonstrate that anti-angiogenic therapies result in increased tumor hypoxia and a decrease in aerobic glycolysis, and that these therapies synergistically inhibit tumor growth when combined with ME-344 [82]. ONC201, originally identified as inducer of TNF-related apoptosis inducing ligand (TRAIL) [83], was more recently shown to have effects on mitochondrial respiration [84], similar to oligomycin, a known inhibitor of oxidative phosphorylation [85]. This effect may possibly occur through AMP-dependent kinase (AMPK) and the indirect disruption of mitochondrial ATP production and depletion of mtDNA [84]. Phase I studies in patients with advanced solid tumors have thus far shown promising results [, ].
ABT-737 is a BH3 mimetic that has been developed for clinical use as a cancer therapy. However, it has limited bioavailability and, since it does not inhibit several of the pro-survival proteins, specifically Mcl-1, tumors overexpressing these proteins are resistant to the drug. Navitoclax (ABT-263) has also been tested but has a dose limiting toxicity of thrombocytopenia [86]. Thus, venetoclax (ABT-199) was developed as a BH3 mimetic that spares Bcl-XL, described above, to avoid this toxicity [87]. Venetoclax is currently FDA approved as a single agent and in combination with rituximab for patients with chronic lymphocytic leukemia or small lymphocytic leukemia with or without a 17p deletion who have received at least one prior therapy as well as for use in combination with low-dose cytarabine, azacitidine, or decitabine for treatment in newly-diagnosed acute myeloid leukemia in patients who are 75 years of age or older or are not candidates for standard induction chemotherapy.
SMAC, one of the proteins released from MOMP, promotes apoptosis by activating caspases [88, 89] and has been shown to sensitize tumor cells by enabling cytokines, such as TNFα, thereby activating the extrinsic apoptosis pathway while simultaneously turning off the canonical NF-kB survival pathway that leads to cancer cell death [90]. Preclinical data showed sensitivity of some lung cancer cell lines to single-agent SMAC mimetics [91], and several studies showed synergistic effects when used in combination [92, 93]. Birinapant and LCL 161 are two SMAC mimetic therapies that have been evaluated in trials, with results suggesting that therapeutic efficacy is improved when used in combination with chemotherapy [, , ]. Other apoptotic targets such as inhibitor of apoptosis proteins (IAPs) that are currently under consideration in clinical trials are described in Box 3.
Box 3. Evasion of apoptosis and mitochondrial targets.
Inhibitor of apoptosis proteins (IAPs) are endogenous proteins that regulate both the extrinsic death receptor pathway and the intrinsic mitochondrial pathway through diverse mechanisms, including the inhibition of caspase activation [122], and are regulated by SMAC. Overexpression of IAPs is one of the mechanisms by which cancer is thought to evade apoptosis. Debio 1143 (formerly AT-406 and SM-406) is another SMAC mimetic that targets cIAP1, cIAP2, and XIAP [123]. In ovarian cancer, the overexpression of IAPs, specifically cIAP1 and XIAP, has been identified as a mechanism of resistance to platinum chemotherapy, and targeting these proteins enhances apoptosis in platinum-resistant cell lines and inhibits tumor growth in mice xenografts [124]. While single agent Debio 1143 was not cytotoxic, it increased sensitivity to carboplatin in carboplatin-resistance cell lines. Like Birinapant and LCL 161, it has also shown synergy with chemotherapies in other solid tumor and hematologic malignancies and additionally has demonstrated that it enhances the activity of anti-PD-L1 therapy [125]. Additionally, in preclinical studies, Debio 1143 showed a synergistic effect with radiation in head and neck squamous cell carcinoma cell lines in a dose-dependent manner [126].
Mitochondria are being studied to explore their role in activating the innate immune system, which is partly triggered in response to metabolic dysregulation including ROS production [51, 127]. SMAC mimetics also stimulate a pro-inflammatory state, as mentioned above, through promoting cytokine release and, by inducing necrosis, triggering an immune response [128]. A preclinical study of human peripheral blood mononuclear cells showed that Debio 1143, when used with anti-PD-L1 therapy, enhanced the immune response in a concentration-dependent manner [125]. Clinical trials evaluating this combination are ongoing, including a phase I study of Debio 1143 in combination with avelumab, a PD-L1 antibody, for patients with advanced solid tumor malignancies [].
Concluding Remarks
Altered mitochondrial function, which originates primarily due to defects from within the organelle or in response to extraneous factors such as stress, plays a critical role in human disease. In the past couple of decades there has been an explosion in interest in targeting this subcellular organelle for various indications, particularly cancer [94, 95]. Furthermore, recent progress in metabolic research has unequivocally demonstrated that altered metabolism in cancer is not merely a secondary effect due to the signaling regulation for growth and proliferation but that it can also be a primary cause for tumor growth, metastasis, and stem-like properties [13, 17, 22, 96]. Nonetheless, to date, there are only a few drugs approved by the FDA that specifically target mitochondria. However, given the vast and rapid progress, and promising results from clinical testing, we can be hopeful of seeing breakthroughs in mitochondrial medicine in the near future. Mitochondria-targeting ligand-conjugated anticancer agents, and mitochondria-targeting nanocarrier system for anticancer drug delivery [97], among other recent developments such as the ‘omics’ technologies [98], herald novel and exciting opportunities for new therapeutics (see Clinician’s Corner). As we enter this new era of mitochondrial medicine, these discoveries will continue to shed light on unresolved mitochondrial questions (see Outstanding Questions), paving the way for improved outcomes for cancer patients.
Box 4. Clinician’s Corner.
In highly aggressive tumors, mitochondrial energy pathways are reprogrammed to meet the challenges of high energy demand through a streamlined utilization of fuels and macromolecular synthesis that drives rapid cell division and migration.
Mitochondria exhibit high levels of metabolic plasticity where cancer cells can even acquire a hybrid glycolysis and OXPHOS phenotype to produce energy and create biomass, which may be associated with metastasis and therapy-resistance.
Mitochondrial inhibitors in preclinical studies are often dosed at concentrations that are too lethal for patients and monotherapy for many of the inhibitors has not shown dramatic benefit in clinical trials. Therefore, it will be vital to utilize mitochondrial inhibitors in combination with other therapies to accentuate response.
Recent discoveries in mitochondrial metabolism have demonstrated that altered metabolism in cancer is not only a secondary effect of signaling regulation, but can also be a primary cause for tumor initiation and proliferation.
IDH inhibitors have been approved in relapsed or refractory IDH2 mutated AML and preliminary results in solid tumors especially in gliomas show promise in clinical trials.
Mitochondrial metabolism and mitochondrial inhibitors such as SMAC mimetics and IAPs have been shown to have a role in the immune microenvironment and early preclinical data show that when they are used in combination with immunotherapy they enhance immune response.
What are the mechanisms of mitochondrial hyperplasticity that allow cancer cells to switch metabolic phenotypes?
What regulates mitochondrial crosstalk within the tumor microenvironment?
How does mitochondrial motility influence cancer progression?
What is the role of the mitochondria in driving tumor heterogeneity and resistance?
Can mitochondrial inhibitors be utilized synergistically with other inhibitors to improve efficacy?
Highlights.
Mitochondria display a dynamic and heterogenous phenotype, facilitating metabolic heterogeneity and plasticity of cancer cells. The altered metabolic functions, dynamics of mitochondria, and evasion of apoptosis in cancer cells provide a target for novel cancer therapeutics.
The mitochondrial network has distinct morphological features that appear to be interrelated with mitochondrial dynamics. Fractal measurements to evaluate the mitochondrial network alongside the metabolic profile of tumors may provide insight into the sensitivity to mitochondrial therapeutics.
Although there are a few FDA-approved cancer-directed therapies that specifically target mitochondria, numerous therapies have been evaluated in clinical trials that target the mitochondrial metabolism and evasion of apoptosis with promising results in a variety of cancer types.
Acknowledgments
Funding: This publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers P30CA033572, U54CA209978 and R01CA218545. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary:
- Adenosine triphosphate (ATP)
A nucleotide that stores and transfers energy for cellular metabolism
- Anaplerotic Reactions
Reactions that form intermediates of a metabolic pathway
- Anoikis
Apoptosis induced by the detachment of cells from the extracellular matrix
- B-cell lymphoma-2 (BCL-2) protein family
The BCL-2 gene translocation is found frequently in B-cell lymphomas, and its family of proteins has now been well-characterized as a major regulator of mitochondrial permeability and apoptosis [99]. The BCL-2 family of proteins have both pro- and anti-apoptotic activities and play a crucial role in committing a cell to apoptosis. There are four characterized protein domains, with the BCL-2 protein itself containing all four (BH1, BH2, BH3, and BH4), which distinctively includes the BH4 domain and has been characterized as anti-apoptotic. There are three characterized classes of BCL-2 family proteins, the anti-apoptotic group including the BCL-2 protein itself, the pro-apoptotic group including the BAX and BAK proteins, and a third pro-apoptotic group that uniquely only contains the BH3 domain.
- Oncometabolite
Small-molecule components of normal cell metabolism whose accumulation leads to signaling and metabolic dysregulation resulting in carcinogenesis.
- Oxidative phosphorylation (OXPHOS)
The final pathway in cellular respiration in which protein complexes located in the inner mitochondrial membrane perform redox reactions, during which electrons are transferred across the membrane ultimately leading to the production of ATP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: All authors in our study have no conflicts of interest.
References
- 1.Warburg O (1956) On the origin of cancer cells. Science 123 (3191), 309–14. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O (1956) On respiratory impairment in cancer cells. Science 124 (3215), 269–70. [PubMed] [Google Scholar]
- 3.Cross CE et al. (1987) Oxygen radicals and human disease. Ann Intern Med 107 (4), 526–45. [DOI] [PubMed] [Google Scholar]
- 4.Liou GY and Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44 (5), 479–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenner CE et al. (1952) Metabolism of neoplastic tissue. II. A survey of enzymes of the citric acid cycle in transplanted tumors. Cancer Res 12 (1), 44–9. [PubMed] [Google Scholar]
- 6.Fantin VR et al. (2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9 (6), 425–34. [DOI] [PubMed] [Google Scholar]
- 7.Weinhouse S et al. (1951) Metabolism of neoplastic tissue. I. The oxidation of carbohydrate and fatty acids in transplanted tumors. Cancer Res 11 (11), 845–50. [PubMed] [Google Scholar]
- 8.Muller M et al. (1986) Quantification of ATP-producing and consuming processes of Ehrlich ascites tumour cells. Eur J Biochem 161 (3), 701–5. [DOI] [PubMed] [Google Scholar]
- 9.Griguer CE et al. (2005) Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J Neurooncol 74 (2), 123–33. [DOI] [PubMed] [Google Scholar]
- 10.Sonveaux P et al. (2008) Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 118 (12), 3930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward PS and Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21 (3), 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida GJ (2015) Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res 34, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia D et al. (2018) Elucidating the Metabolic Plasticity of Cancer: Mitochondrial Reprogramming and Hybrid Metabolic States. Cells 7 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Q et al. (2002) A mitochondrial specific stress response in mammalian cells. EMBO J 21 (17), 4411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold I et al. (2006) Evidence for a novel mitochondria-to-nucleus signalling pathway in respiring cells lacking i-AAA protease and the ABC-transporter Mdl1. Gene 367, 74–88. [DOI] [PubMed] [Google Scholar]
- 16.Houtkooper RH et al. (2013) Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497 (7450), 451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guha M and Avadhani NG (2013) Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion 13 (6), 577–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onodera Y et al. (2018) Arf6-driven cell invasion is intrinsically linked to TRAK1-mediated mitochondrial anterograde trafficking to avoid oxidative catastrophe. Nat Commun 9 (1), 2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arcucci A et al. (2016) Cancer: An Oxidative Crosstalk between Solid Tumor Cells and Cancer Associated Fibroblasts. Biomed Res Int 2016, 4502846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu L et al. (2017) Modeling the Genetic Regulation of Cancer Metabolism: Interplay between Glycolysis and Oxidative Phosphorylation. Cancer Res 77 (7), 1564–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trachootham D et al. (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8 (7), 579–91. [DOI] [PubMed] [Google Scholar]
- 22.Pavlova NN and Thompson CB (2016) The Emerging Hallmarks of Cancer Metabolism. Cell Metab 23 (1), 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim JH et al. (2014) Targeting mitochondrial oxidative metabolism in melanoma causes metabolic compensation through glucose and glutamine utilization. Cancer Res 74 (13), 3535–45. [DOI] [PubMed] [Google Scholar]
- 24.Ashton TM et al. (2018) Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin Cancer Res 24 (11), 2482–2490. [DOI] [PubMed] [Google Scholar]
- 25.Bost F and Kaminski L (2019) The metabolic modulator PGC-α in cancer. American journal of cancer research 9 (2), 198–211. [PMC free article] [PubMed] [Google Scholar]
- 26.Gentric G et al. (2019) PML-Regulated Mitochondrial Metabolism Enhances Chemosensitivity in Human Ovarian Cancers. Cell Metab 29 (1), 156–173.el0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganapathy-Kanniappan S (2018) Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit Rev Biochem Mol Biol 53 (6), 667–682. [DOI] [PubMed] [Google Scholar]
- 28.Zheng L et al. (2015) Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat Commun 6, 6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orang AV et al. (2019) Micromanaging aerobic respiration and glycolysis in cancer cells. Mol Metab 23, 98–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen OE et al. (2002) The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277 (34), 30409–12. [DOI] [PubMed] [Google Scholar]
- 31.Fruman DA et al. (2017) The PI3K Pathway in Human Disease. Cell 170 (4), 605–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts DJ et al. (2013) Akt phosphorylates HK-II at Thr-473 and increases mitochondrial HK-II association to protect cardiomyocytes. J Biol Chem 288 (33), 23798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krell D et al. (2013) IDH mutations in tumorigenesis and their potential role as novel therapeutic targets. Future Oncol 9 (12), 1923–35. [DOI] [PubMed] [Google Scholar]
- 34.Reitman ZJ and Yan H (2010) Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst 102 (13), 932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye D et al. (2018) Metabolism, Activity, and Targeting of D- and L-2-Hydroxyglutarates. Trends Cancer 4 (2), 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang H et al. (2012) IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin Cancer Res 18 (20), 5562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dang L et al. (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462 (7274), 739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figueroa ME et al. (2010) Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18 (6), 553–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu W et al. (2011) Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 19 (1), 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Deimling A et al. (2011) The next generation of glioma biomarkers: MGMT methylation, BRAF fusions and IDH1 mutations. Brain Pathol 21 (1), 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waitkus MS et al. (2018) Biological Role and Therapeutic Potential of IDH Mutations in Cancer. Cancer Cell 34 (2), 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stuani L et al. (2017) Utilization of a-Ketoglutarate for Synthesis of 2-Hydroxyglutarate Oncometabolite Promotes Catabolic Flexibility, Redox Perturbation and Mitochondrial Activity That Supports Chemoresistance in IDH1 Mutant Acute Myeloid Leukemia. Blood 130 (Suppl 1), 5080–5080. [Google Scholar]
- 43.Wang F et al. (2013) Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science 340 (6132), 622–6. [DOI] [PubMed] [Google Scholar]
- 44.Fathi AT et al. (2018) Differentiation Syndrome Associated With Enasidenib, a Selective Inhibitor of Mutant Isocitrate Dehydrogenase 2: Analysis of a Phase 1/2 StudyDifferentiation Syndrome Associated With EnasidenibDifferentiation Syndrome Associated With Enasidenib. JAMA Oncology 4 (8), 1106–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mellinghoff IK et al. (2018) Phase 1 study of AG-881, an inhibitor of mutant IDH1/IDH2, in patients with advanced IDH-mutant solid tumors, including glioma. Journal of Clinical Oncology 36 (15_suppl), 2002–2002.29746224 [Google Scholar]
- 46.Seo BJ et al. (2018) Mitochondrial Dynamics in Stem Cells and Differentiation. Int J Mol Sci 19 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campello S et al. (2014) Mitochondrial dismissal in mammals, from protein degradation to mitophagy. Biochim Biophys Acta 1837 (4), 451–60. [DOI] [PubMed] [Google Scholar]
- 48.Kamerkar SC et al. (2018) Dynamin-related protein 1 has membrane constricting and severing abilities sufficient for mitochondrial and peroxisomal fission. Nat Commun 9 (1), 5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serasinghe MN et al. (2015) Mitochondrial division is requisite to RAS-induced transformation and targeted by oncogenic MAPK pathway inhibitors. Mol Cell 57 (3), 521–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rambold AS et al. (2011) Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A 108 (25), 10190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H and Chan DC (2017) Mitochondrial Dynamics in Regulating the Unique Phenotypes of Cancer and Stem Cells. Cell Metab 26 (1), 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feely SM et al. (2011) MFN2 mutations cause severe phenotypes in most patients with CMT2A. Neurology 76 (20), 1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rojo M et al. (2002) Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 115 (Pt 8), 1663–74. [DOI] [PubMed] [Google Scholar]
- 54.Miret-Casals L et al. (2018) Identification of New Activators of Mitochondrial Fusion Reveals a Link between Mitochondrial Morphology and Pyrimidine Metabolism. Cell Chem Biol 25 (3), 268–278 e4. [DOI] [PubMed] [Google Scholar]
- 55.Rehman J et al. (2012) Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J 26 (5), 2175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuo CW et al. (2017) mtDNA as a Mediator for Expression of Hypoxia-Inducible Factor 1alpha and ROS in Hypoxic Neuroblastoma Cells. Int J Mol Sci 18 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou P et al. (2016) Coordinated Upregulation of Mitochondrial Biogenesis and Autophagy in Breast Cancer Cells: The Role of Dynamin Related Protein-1 and Implication for Breast Cancer Treatment. Oxid Med Cell Longev 2016, 4085727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bukoreshtliev NV et al. (2009) Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett 583 (9), 1481–8. [DOI] [PubMed] [Google Scholar]
- 59.Wang X and Gerdes HH (2015) Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ 22 (7), 1181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar A et al. (2016) Minnelide/Triptolide Impairs Mitochondrial Function by Regulating SIRT3 in P53-Dependent Manner in Non-Small Cell Lung Cancer. PLoS One 11 (8), e0160783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahn BH et al. (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105 (38), 14447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hallows WC et al. (2009) Ure(k)a! Sirtuins Regulate Mitochondria. Cell 137 (3), 404–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kong X et al. (2010) Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5 (7), e11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giralt A et al. (2011) Peroxisome proliferator-activated receptor-gamma coactivator-1alpha controls transcription of the Sirt3 gene, an essential component of the thermogenic brown adipocyte phenotype. J Biol Chem 286 (19), 16958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ploumi C et al. (2017) Mitochondrial biogenesis and clearance: a balancing act. FEBS J 284 (2), 183–195. [DOI] [PubMed] [Google Scholar]
- 66.LeBleu VS et al. (2014) PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 16 (10), 992–1003, 1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi T et al. (2005) SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 280 (14), 13560–7. [DOI] [PubMed] [Google Scholar]
- 68.Vispe S et al. (2009) Triptolide is an inhibitor of RNA polymerase I and II-dependent transcription leading predominantly to down-regulation of short-lived mRNA. Mol Cancer Ther 8 (10), 2780–90. [DOI] [PubMed] [Google Scholar]
- 69.Rousalova I et al. (2013) Minnelide: a novel therapeutic that promotes apoptosis in non-small cell lung carcinoma in vivo. PLoS One 8 (10), e77411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chugh R et al. (2012) A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med 4 (156), 156ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Banerjee S and Saluja A (2015) Minnelide, a novel drug for pancreatic and liver cancer. Pancreatology 15 (4 Suppl), S39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh MK et al. (2017) Abstract 5119: Evaluation of Minnelide as potential targeted therapy for triple negative breast cancer. Cancer Research 77 (13 Supplement), 5119–5119. [Google Scholar]
- 73.Sarosiek KA et al. (2013) Mitochondria: gatekeepers of response to chemotherapy. Trends Cell Biol 23 (12), 612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sarosiek KA and Letai A (2016) Directly targeting the mitochondrial pathway of apoptosis for cancer therapy using BH3 mimetics - recent successes, current challenges and future promise. FEBS J 283 (19), 3523–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tait SW and Green DR (2010) Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11 (9), 621–32. [DOI] [PubMed] [Google Scholar]
- 76.Karbowski M et al. (2002) Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol 159 (6), 931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tait SW et al. (2014) Die another way--non-apoptotic mechanisms of cell death. J Cell Sci 127 (Pt 10), 2135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Willis SN et al. (2007) Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315 (5813), 856–9. [DOI] [PubMed] [Google Scholar]
- 79.Youle RJ (2007) Cell biology. Cellular demolition and the rules of engagement. Science 315 (5813), 776–7. [DOI] [PubMed] [Google Scholar]
- 80.Alvero AB et al. (2011) Targeting the mitochondria activates two independent cell death pathways in ovarian cancer stem cells. Mol Cancer Ther 10 (8), 1385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lim SC et al. (2015) Anti-cancer analogues ME-143 and ME-344 exert toxicity by directly inhibiting mitochondrial NADH: ubiquinone oxidoreductase (Complex I). Am J Cancer Res 5 (2), 689–701. [PMC free article] [PubMed] [Google Scholar]
- 82.Navarro P et al. (2016) Targeting Tumor Mitochondrial Metabolism Overcomes Resistance to Antiangiogenics. Cell Rep 15 (12), 2705–18. [DOI] [PubMed] [Google Scholar]
- 83.Allen JE et al. (2013) Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med 5 (171), 171ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Greer YE et al. (2018) ONC201 kills breast cancer cells in vitro by targeting mitochondria. Oncotarget 9 (26), 18454–18479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joshi S and Huang YG (1991) ATP synthase complex from bovine heart mitochondria: the oligomycin sensitivity conferring protein is essential for dicyclohexyl carbodiimide-sensitive ATPase. Biochim Biophys Acta 1067 (2), 255–8. [DOI] [PubMed] [Google Scholar]
- 86.Wilson WH et al. (2010) Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol 11 (12), 1149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Souers AJ et al. (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19 (2), 202–8. [DOI] [PubMed] [Google Scholar]
- 88.Du C et al. (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102 (1), 33–42. [DOI] [PubMed] [Google Scholar]
- 89.Benetatos CA et al. (2014) Birinapant (TL32711), a bivalent SMAC mimetic, targets TRAF2-associated cIAPs, abrogates TNF-induced NF-kappaB activation, and is active in patient-derived xenograft models. Mol Cancer Ther 13 (4), 867–79. [DOI] [PubMed] [Google Scholar]
- 90.Smith MA et al. (2012) Birinapant (TL32711), a Small Molecule Smac Mimetic, Induces Regressions in Childhood Acute Lymphoblastic Leukemia (ALL) Xenografts That Express TNFα and Synergizes with TNFα in Vitro – A Report From the Pediatric Preclinical Testing Program (PPTP). Blood 120 (21), 3565–3565. [Google Scholar]
- 91.Petersen SL et al. (2007) Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 12 (5), 445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chauhan D et al. (2007) Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM). Blood 109 (3), 1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bockbrader KM et al. (2005) A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene 24 (49), 7381–8. [DOI] [PubMed] [Google Scholar]
- 94.Picard M et al. (2016) The rise of mitochondria in medicine. Mitochondrion 30, 105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sotgia F et al. (2018) A mitochondrial based oncology platform for targeting cancer stem cells (CSCs): MITO-ONC-RX. Cell Cycle 17 (17), 2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hanahan D and Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144 (5), 646–74. [DOI] [PubMed] [Google Scholar]
- 97.Battogtokh G et al. (2018) Mitochondrial-Targeting Anticancer Agent Conjugates and Nanocarrier Systems for Cancer Treatment. Front Pharmacol 9, 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rahman J and Rahman S (2018) Mitochondrial medicine in the omics era. Lancet 391 (10139), 2560–2574. [DOI] [PubMed] [Google Scholar]
- 99.McClintock DS et al. (2002) Bcl-2 family members and functional electron transport chain regulate oxygen deprivation-induced cell death. Mol Cell Biol 22 (1), 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stuart SD et al. (2014) A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process. Cancer Metab 2 (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zachar Z et al. (2011) Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J Mol Med (Berl) 89 (11), 1137–48. [DOI] [PubMed] [Google Scholar]
- 102.Pardee TS et al. (2014) A phase I study of the first-in-class antimitochondrial metabolism agent, CPI-613, in patients with advanced hematologic malignancies. Clin Cancer Res 20 (20), 5255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.O'Brien ME et al. (2006) Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 24 (34), 5441–7. [DOI] [PubMed] [Google Scholar]
- 104.Lycan TW et al. (2016) A Phase II Clinical Trial of CPI-613 in Patients with Relapsed or Refractory Small Cell Lung Carcinoma. PLoS One 11 (10), e0164244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alistar AT et al. (2016) CPI-613 enhances FOLFIRINOX response rate in stage IV pancreatic cancer. Annals of Oncology 27 (suppl_6). [Google Scholar]
- 106.Bonnet S et al. (2007) A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11 (1), 37–51. [DOI] [PubMed] [Google Scholar]
- 107.Kankotia S and Stacpoole PW (2014) Dichloroacetate and cancer: new home for an orphan drug? Biochim Biophys Acta 1846 (2), 617–29. [DOI] [PubMed] [Google Scholar]
- 108.Giedt RJ et al. (2016) Computational imaging reveals mitochondrial morphology as a biomarker of cancer phenotype and drug response. Sci Rep 6, 32985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Von Stockum S et al. (2016) Mitochondrial dynamics and mitophagy in Parkinson's disease: A fly point of view. Neurobiol Dis 90, 58–67. [DOI] [PubMed] [Google Scholar]
- 110.Lennon FE et al. (2015) Lung cancer-a fractal viewpoint. Nat Rev Clin Oncol 12 (11), 664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mandelbrot B (1967) How long is the coast of britain? Statistical self-similarity and fractional dimension. Science 156 (3775), 636–8. [DOI] [PubMed] [Google Scholar]
- 112.Viana MP et al. (2015) Quantifying mitochondrial content in living cells. Methods Cell Biol 125, 77–93. [DOI] [PubMed] [Google Scholar]
- 113.Harwig MC et al. (2018) Methods for imaging mammalian mitochondrial morphology: A prospective on MitoGraph. Anal Biochem 552, 81–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahmed E (1993) Fractals and chaos in cancer models. International Journal of Theoretical Physics 32 (2), 353–355. [Google Scholar]
- 115.Bodduluri S et al. (2018) Airway fractal dimension predicts respiratory morbidity and mortality in COPD. J Clin Invest 128 (12), 5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stefanescu D et al. (2016) Computer Aided Diagnosis for Confocal Laser Endomicroscopy in Advanced Colorectal Adenocarcinoma. PLoS One 11 (5), e0154863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pribic J et al. (2017) Fractal Parameters of Tumour Microscopic Images as Prognostic Indicators of Clinical Outcome in Early Breast Cancer. [DOI] [PubMed] [Google Scholar]
- 118.Hernandez Velazquez JD et al. (2018) Fractal properties of biophysical models of pericellular brushes can be used to differentiate between cancerous and normal cervical epithelial cells. Colloids Surf B Biointerfaces 170, 572–577. [DOI] [PubMed] [Google Scholar]
- 119.Bikou O et al. (2016) Fractal Dimension as a Diagnostic Tool of Complex Endometrial Hyperplasia and Well-differentiated Endometrioid Carcinoma. In Vivo 30 (5), 681–90. [PubMed] [Google Scholar]
- 120.Vargas I et al. (2018) Rapid quantification of mitochondrial fractal dimension in individual cells. Biomed Opt Express 9 (11), 5269–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lennon FE et al. (2016) Unique fractal evaluation and therapeutic implications of mitochondrial morphology in malignant mesothelioma. Sci Rep 6, 24578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deveraux QL and Reed JC (1999) IAP family proteins--suppressors of apoptosis. Genes Dev 13 (3), 239–52. [DOI] [PubMed] [Google Scholar]
- 123.Cai Q et al. (2011) A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment. J Med Chem 54 (8), 2714–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen X et al. (2016) Sequential combination therapy of ovarian cancer with cisplatin and gamma-secretase inhibitor MK-0752. Gynecol Oncol 140 (3), 537–44. [DOI] [PubMed] [Google Scholar]
- 125.Attinger A et al. (2018) Abstract 4703: The inhibitor of apoptosis protein (IAP) antagonist Debio 1143 enhances the immune response to anti-PD1/L1 inhibitors in vitro and in vivo. Cancer Research 78 (13 Supplement), 4703–4703. [Google Scholar]
- 126.Matzinger O et al. (2015) The radiosensitizing activity of the SMAC-mimetic, Debio 1143, is TNFalpha-mediated in head and neck squamous cell carcinoma. Radiother Oncol 116 (3), 495–503. [DOI] [PubMed] [Google Scholar]
- 127.Dougan SK and Dougan M (2018) Regulation of innate and adaptive antitumor immunity by IAP antagonists. Immunotherapy 10 (9), 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lecis D et al. (2013) Smac mimetics induce inflammation and necrotic tumour cell death by modulating macrophage activity. Cell Death Dis 4, e920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pardee TS et al. (2015) Activity of the mitochondrial metabolism inhibitor cpi-613 in combination with high dose Ara-C (HDAC) and mitoxantrone in high risk relapsed or refractory acute myeloid leukemia (AML). Journal of Clinical Oncology 33 (15_suppl), 7015–7015. [Google Scholar]
- 130.Chu QS et al. (2015) A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Invest New Drugs 33 (3), 603–10. [DOI] [PubMed] [Google Scholar]
- 131.Dunbar EM et al. (2014) Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs 32 (3), 452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stein EM et al. (2017) Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130 (6), 722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mellinghoff IK et al. (2017) ACTR-46. AG-120, A FIRST-IN-class MUTANT IDH1 INHIBITOR IN PATIENTS WITH RECURRENT OR PROGRESSIVE IDH1 MUTANT GLIOMA: UPDATED RESULTS FROM THE PHASE 1 NON-ENHANCING GLIOMA POPULATION. Neuro-Oncology 19 (suppl_6), vilO–vill. [Google Scholar]
- 134.Lowery MA et al. (2017) Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: Results from the cholangiocarcinoma dose escalation and expansion cohorts. Journal of Clinical Oncology 35 (15_suppl), 4015–4015. [Google Scholar]
- 135.DiNardo CD et al. (2018) Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med 378 (25), 2386–2398. [DOI] [PubMed] [Google Scholar]
- 136.Tam CS et al. (2018) Ibrutinib plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. New England Journal of Medicine 378 (13), 1211–1223. [DOI] [PubMed] [Google Scholar]
- 137.Seymour JF et al. (2018) Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. New England Journal of Medicine 378 (12), 1107–1120. [DOI] [PubMed] [Google Scholar]
- 138.Hamilton EP et al. (2015) A phase lb, open-label, non-randomized multicenter study of birinapant in combination with conatumumab in subjects with relapsed epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer. Journal of Clinical Oncology 33 (15_suppl), 5571–5571. [Google Scholar]
- 139.Noonan AM et al. (2016) Pharmacodynamic markers and clinical results from the phase 2 study of the SMAC mimetic birinapant in women with relapsed platinum-resistant or -refractory epithelial ovarian cancer. Cancer 122 (4), 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hurwitz HI et al. (2015) Safety, pharmacokinetics, and pharmacodynamic properties of oral DEBIO 1143 (AT-406) in patients with advanced cancer: results of a first-in-man study. Cancer Chemother Pharmacol 75 (4), 851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.DiPersio JF et al. (2015) Oral Debio1143 (AT406), an Antagonist of Inhibitor of Apoptosis Proteins, Combined With Daunorubicin and Cytarabine in Patients With Poor-Risk Acute Myeloid Leukemia—Results of a Phase I Dose-Escalation Study. Clinical Lymphoma Myeloma and Leukemia 15 (7), 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chesi M et al. (2016) IAP antagonists induce anti-tumor immunity in multiple myeloma. Nat Med 22 (12), 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bendell JC et al. (2015) Phase 1, open-label, dose escalation, safety, and pharmacokinetics study of ME-344 as a single agent in patients with refractory solid tumors. Cancer 121 (7), 1056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Diamond JR et al. (2017) Phase Ib study of the mitochondrial inhibitor ME-344 plus topotecan in patients with previously treated, locally advanced or metastatic small cell lung, ovarian and cervical cancers. Invest New Drugs 35 (5), 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Greeno E et al. (2016) Phase I dose escalation and pharmokinetic study of a modified schedule of 14-o-phosphonooxymethyltriptolide. Journal of Clinical Oncology 34 (4_suppl), TPS472–TPS472. [Google Scholar]
- 146.Stein MN et al. (2017) First-in-Human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors. Clin Cancer Res 23 (15), 4163–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stein MN et al. (2016) Clinical activity of ONC201 in metastatic castrate resistant prostate cancer (mCRPC). Journal of Clinical Oncology 34 (15_suppl), e16514–e16514. [Google Scholar]