Abstract
Skeletal muscle injuries activate a complex program of myogenesis that can restore normal muscle structure. We tested whether modulating the expression of klotho influenced the response of mouse muscles to acute injury. Our findings show that klotho expression in muscle declines at 3-days post-injury. That reduction in klotho expression coincided with elevated expression of targets of Wnt signaling (Ccnd1; Myc) and increased MyoD+ muscle cell numbers, reflecting the onset of myogenic cell differentiation. Klotho expression subsequently increased at 7-days post-injury with elevated expression occurring primarily in inflammatory lesions, which was accompanied by reduced expression of Wnt target genes (Ccnd1: 91%; Myc: 96%). Introduction of a klotho transgene maintained high levels of klotho expression over the course of muscle repair and attenuated the increases in Ccnd1 and Myc expression that occurred at 3-days post-injury. Correspondingly, transgene expression reduced Wnt signaling in Pax7+ cells, reflected by reductions in Pax7+ cells expressing active β-catenin, and reduced the numbers of MyoD+ cells at 3-days post-injury. At 21-days post-injury, muscles in klotho transgenic mice showed increased Pax7+ and decreased myogenin+ cell densities and large increases in myofiber size. Likewise, treating myogenic cells in vitro with Klotho reduced Myod expression but did not affect Pax7 expression. Muscle inflammation was only slightly modulated by increased klotho expression, initially reducing the expression of M2-biased macrophage markers Cd163 and Cd206 at 3-days post-injury and later increasing the expression of pan-macrophage marker F480 and Cd68 at 21-days post-injury. Collectively, our study shows that Klotho modulates myogenesis and that increased expression accelerates muscle growth after injury.
Keywords: Klotho, skeletal muscle, inflammation, acute muscle injury
1. INTRODUCTION
The long-term ability of skeletal muscle to grow and respond to injury or disease largely depends on the numbers and functions of a population of myogenic stem cells, called satellite cells. In healthy, mature muscle, satellite cells reside in a quiescent state on the surface of fully-differentiated muscle fibers (Moss & Leblond, 1970). However, in response to muscle damage, they become rapidly activated, their populations expand and some daughter cells then undergo a complex program of differentiation that is necessary to establish and maintain normal muscle health and function (Dhawan & Rando, 2005; Lepper, Partridge, & Fan, 2011; Sambasivan et al., 2011; Seale et al., 2000). Perturbations of mechanisms that regulate satellite cell function can lead to loss of normal muscle function and, in some cases, reduce lifespan.
Although numerous mechanisms interact to influence the response of satellite cells to muscle injury or disease and to determine their contributions to muscle growth and regeneration, recent discoveries have shown that the protein Klotho can play important roles in regulating the response of satellite cells to muscle disease (Wehling-Henricks et al., 2016, 2018). Klotho was originally identified as an anti-aging protein because mutations in the klotho gene cause changes in tissues that resemble accelerated aging (Kurosu et al., 2005). During our investigations into the pathophysiology of the progressive muscle wasting disease called Duchenne muscular dystrophy (DMD), we learned that klotho is epigenetically silenced in DMD muscle by increased methylation of lysine 9 on histone 3 (H3K9) in the promoter region of the klotho gene and by increased DNA methylation at CpG islands near the transcriptional start site of the klotho gene (Wehling-Henricks et al., 2016). We also demonstrated that genetic restoration of klotho to the mdx mouse model of DMD produced substantial reductions in muscle pathology and improved muscle regeneration (Wehling-Henricks et al., 2016). Similarly, a subsequent study showed that the reduced expression of klotho in aging muscle may also contribute to defects in muscle regeneration that can be reversed by delivery of exogenous Klotho to old mice (Sahu et al., 2018). Thus, loss of normal levels of klotho expression in diseased or aged muscle is associated with impaired muscle regeneration that can be improved by interventions that increase Klotho delivery. However, whether increased delivery of Klotho can improve regeneration of muscle in which klotho expression has not been silenced by disease or age is unknown.
Many of the beneficial effects of Klotho on dystrophic muscle are attributable to its influence on satellite cells. Restoring klotho expression in dystrophic mice not only prevented the loss of satellite cells from dystrophic muscle at advanced stages of the mdx pathology, the transgene increased the number of satellite cells per muscle fiber as the disease progressed and the mice aged (Wehling-Henricks et al., 2016). Those increases were also associated with increased muscle fiber size and reduced fibrosis (Wehling-Henricks et al., 2016). At least some of these effects were attributable to direct actions on proliferative satellite cells and on post-mitotic, myogenic cells called myotubes that are formed by fusion of activated satellite cells into multinucleated precursors of muscle fibers. When satellite cells in vitro were stimulated with exogenous Klotho in the presence of co-factors, satellite cell proliferation increased and when myotubes were similarly stimulated, they shifted to a more positive protein balance (Wehling-Henricks et al., 2016). In addition, age-related reductions in satellite cell mitochondrial function were reduced by treatment with Klotho in vitro, which may also contribute to Klotho-mediated improvements in function (Sahu et al., 2018).
Klotho can be expressed as a transmembrane protein (m-Klotho) or as an alternatively spliced isoform that can be secreted (s-Klotho) (Kuro-o et al., 2005; Li et al., 2004; Matsumura et al., 1998). The extracellular domain of m-Klotho can also be cleaved and released, adding to extracellular s-Klotho (Chen et al., 2007; Imura et al., 2004) which functions as a hormone (Saito et al., 2000). S-Klotho’s actions on target cells can be influenced by the presence of FGF23 (Wehling-Henricks et al., 2016) or it can act independent of FGF23 signaling (Xie et al., 2015) through mechanisms that are not yet well understood (Dalton et al., 2017). However, the pro-proliferative and anabolic effects of Klotho on myogenic cells are enhanced by the presence of FGF23, at least in vitro (Wehling-Henricks et al., 2016).
Several pathways that are important in regulating myogenesis are influenced by s-Klotho in non-muscle tissues (Doi et al., 2011; Kuro-o et al., 1997; Liu et al., 2007), which suggests they are potential targets for s-Klotho in skeletal muscle, as well. For example, Klotho can bind to Wnt1, Wnt3a, Wnt4, Wnt5a and Wnt7a (Liu et al., 2007; Zhou et al., 2013) and that binding is sufficient to inhibit activity of at least Wnt3a in a cell free system (Leung et al., 2002). Furthermore, Klotho treatment of muscle cells in vitro diminished Wnt signaling, which was attributed to Klotho binding to extracellular Wnt (Ahrens et al., 2018). Those in vitro observations suggest that if Klotho also modifies Wnt signaling in growing or regenerating muscle in vivo, myogenesis could be affected because signaling through the canonical, β-catenin-dependent Wnt pathway increases satellite cell differentiation (Brack et al., 2008; Han et al., 2011; Polesskaya, Seale, & Rudnicki, 2003; Rochat et al., 2004). Thus, the expansion of satellite cell numbers in the presence of elevated levels of Klotho in vivo or in vitro (Wehling-Henricks et al., 2016) may reflect a slowing of muscle differentiation caused by Klotho inhibition of Wnt because terminal differentiation of satellite cells and their progeny entails permanent withdrawal from the cell cycle.
Although Klotho can modulate myogenesis in diseased or injured tissue by direct actions on satellite cells, it may also play indirect roles by influencing inflammatory cell populations that regulate muscle growth. In particular, macrophages play a central role in regulating muscle growth and regeneration following injury (Tidball, 2017) and the expression of cytokines that determine macrophage function is influenced by Klotho. For example, increases in Klotho can reduce expression of tumor necrosis factor (TNF) (Hui et al., 2017) and TNF-stimulation of macrophages in the presence of interferon-gamma (IFNγ) induces a pro-inflammatory phenotype (M1-biased phenotype) that can increase muscle damage or increase myoblast proliferation (Bencze et al., 2012; Villalta et al., 2009). Klotho treatments can also increase expression of interleukin-10 (IL10) by macrophages (Wehling-Henricks et al., 2018), which deactivates the M1 biased phenotype and induces an anti-inflammatory M2 macrophage phenotype that can increase muscle growth and regeneration following injury (Deng et al., 2012).
In this investigation, we test whether increasing Klotho delivery to non-diseased, non-senescent muscles by expressing a klotho transgene improves muscle growth and regeneration following acute injury. We also assay whether elevations in Klotho influence the numbers or differentiation of satellite cells in regenerative muscle and whether the changes in myogenesis are reflected by changes in signaling through the canonical Wnt pathway or by modifications in the inflammatory response to muscle injury. Collectively, the findings indicate that modulation of Klotho expression in injured muscle can significantly affect growth following acute muscle injury, providing a potential mechanism for influencing the rate of muscle repair in vivo.
2. METHODS
2.1. Ethical approval
All animals were handled according to guidelines provided by the Chancellor’s Animal Research Committee at the University of California, Los Angeles (Animal Welfare Assurance number A-3196). The investigation complied with the principles and standards for reporting animal experiments (Grundy, 2015).
2.2. Animals
C57BL/6 (wild-type) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in specific pathogen-free vivaria at the University of California, Los Angeles. The production of transgenic mice that over-express klotho (EFmKL46) has been described previously (Kuro-o et al., 1997). The klotho transgene is under control of the elongation factor 1α promoter that causes systemic expression of the transcript and approximately doubles circulating Klotho levels (Kurosu et al., 2005). Mice carrying the klotho transgene were crossed onto the C57BL/6 background for a minimum of six generations. Mice were housed on a 12/12 hour light/dark cycle and were provided food and water ad libitum. Following euthanasia by inhalation of isoflurane, muscles were collected, weighed and flash-frozen for subsequent sectioning and histological evaluation or used for RNA isolation. Experimental group size ranged from 5–8 mice per group (48 total mice).
2.3. Acute muscle injury
Mice were briefly anesthetized with isoflurane inhalation in a chamber (4–5% isoflurane with pure oxygen for induction) then moved to a nose cone (1–2% isoflurane). Anesthesia was checked by testing mice for a positive reflex response to a hind foot pinch and by monitoring respiration. The lower limb was wiped with 70% ethanol before intramuscular injection. Sterile muscle injury was induced by the intramuscular injection of 50 μl of a 1.2% barium chloride (BaCl2) solution into the midbelly of tibialis anterior (TA) muscles of healthy, 5-month old adult male wild-type and klotho transgenic mice. Animals were monitored daily until they recovered.
2.4. RNA isolation and QPCR
RNA was isolated from muscle homogenates and electrophoresed on agarose gels and its quality assessed by 28S and 18S ribosomal RNA integrity (Wehling-Henricks et al., 2016). RNA samples (2 μg) were reverse transcribed with Super Script Reverse Transcriptase II using oligo dTs to prime extension (Invitrogen) to produce cDNA. Expression of selected transcripts was assayed using SYBR green qPCR Supermix according to the manufacturer’s protocol (BioRad) and an iCycler thermocycler system equipped with iQ5 optical system software (BioRad). Established guidelines for experimental design, data normalization and data analysis for QPCR were used to maximize the rigor of quantifying the relative levels of mRNA (Bustin et al., 2009; Nolan, Hands, & Bustin, 2006). We empirically tested up to 20 reference genes and identified those with the least variability between experimental groups. Based on that analysis, the relative expression of transcripts of interest were normalized to the reference genes Srp14 and Hagh for muscle regeneration QPCR assays. The normalization factor for each sample was calculated by geometric averaging of the Ct values of reference genes. Expression for each gene in control samples was set to 1 and the other values were scaled to the control. Primers used for QPCR are listed in Table 1. Primers for klotho were designed to a cDNA sequence that is present in both m-klotho and s-klotho. Primers for s-klotho were designed so that the downstream primer binds a region of the cDNA sequence that is absent from full-length m-klotho (Shiraki-Iida et al., 1998).
Table 1:
Primer sequences.
| Gene | Forward | Reverse |
|---|---|---|
| Axin2 | GACGCACTGACCGACGATTC | CTGCGATGCATCTCTCTCTGG |
| Ccnd1 | CGAGGAGCTGCTGCAAATG | GGGTTGGAAATGAACTTCACATC |
| Cd68 | CAAAGCTTCTGCTGTGGAAAT | GACTGGTCACGGTTGCAAG |
| Cd163 | GCAAAAACTGGCAGTGGG | GTCAAAATCACAGACGGAG |
| Cd206 | GGATTGTGGAGCAGATGGAAG | CTTGAATGGAAATGCACAGAC |
| F480 | GATACAGCAATGCCAAGCAG | CAGCACGAGGGAGACACTT |
| Hagh | CACCACTCACCACCACTGG | ACACTGAGAGACCCCACCTG |
| Il10 | CAAGGAGCATTTGAATTCCC | GGCCTTGTAGACACCTTGGTC |
| Klotho | GTCTCGGGAACCACCAAAAG | CTATGCCACTCGAAACCGTC |
| Myc | CCCTATTTCATCTGCGACGAG | GACGTAGCGACCGCAACATAG |
| Myod | GAGCGCATCTCCACAGACAG | AAATCGCATTGGGGTTTGAG |
| Myogenin | CCAGTACATTGAGCGCCTAC | ACCGAACTCCAGTGCATTGC |
| Pax7 | CTCAGTGAGTTCGATTAGCCG | AGACGGTTCCCTTTGTCGC |
| Rnsp1 | AGGCTCACCAGGAATGTGAC | CTTGGCCATCAATTTGTCCT |
| S-Klotho | CAATGGCTTTCCTCCTTTACC | GAGGCCGACACTGGGTTTTG |
| Srp14 | AGAGCGAGCAGTTCCTGAC | CGGTGCTGATCTTCCTTTTC |
| Tnf | CTTCTGTCTACTGAACTTCGGG | CACTTGGTGGTTTGCTACGAC |
2.5. Muscle fiber cross-sectional area
Frozen TA muscles were cross-sectioned at the midbelly and used for fiber cross-sectional area measurements. Sections were stained with hematoxylin and the cross-sectional areas of fibers were measured using a digital imaging system (Bioquant). In muscle that had undergone acute injury, only centrally-nucleated, regenerating muscle fibers (Chargé & Rudnicki, 2004) were measured from the area of the central lesion, identified as the region that was least regenerated, as previously described (Miller et al., 2000). The classification of small and large fibers was determined by setting three standard deviations from the mean cross-sectional area for the wild-type group at both the 7- and 21-days post-injury time points and quantifying the proportion of myofibers measured that fell within these ranges, adapted from (White et al., 2009). At 7-days post-injury, fibers were considered small or large if less than 623 μm2 or greater than 1603 μm2, respectively. The threshold for small and large fibers at 21-days post-injury was determined to be less than 458 μm2 or more than 3094 μm2, respectively.
2.6. Production of Pax7 antibody
Pax7 hybridoma cells were purchased from Developmental Studies Hybridoma Bank (Iowa City, Iowa). Cells were cultured and antibody was isolated from the supernatant as previously described (Wehling-Henricks et al., 2016).
2.7. Immunohistochemistry
We assayed for relationships between the distribution of Pax7+ cells, macrophages and Klotho by performing immunohistochemistry on serial sections obtained from injured muscles. Double- or triple-immunolabeling was not performed because each of the antigens that we probed required different fixation protocols to retain their ability to bind the primary antibodies used in the investigation. Muscles were dissected from euthanized mice and then rapidly frozen in liquid nitrogen-cooled isopentane. For identification of Pax7+ cells, 10-µm-thick cross-sections were taken from the mid-belly of muscles, air-dried for 30 min and then fixed in 4% paraformaldehyde for 10 minutes. Sections were then immersed in antigen retrieval buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) at 95–100°C for 40 minutes. Endogenous peroxidase activity in the tissue was quenched by immersion in 0.3% H2O2. Sections were then treated with blocking buffer from a mouse-on-mouse immunohistochemistry kit (M.O.M kit; Vector) for 1 hour and immunolabeled with affinity purified mouse anti-Pax7 (1:500) overnight at 4°C. Sections were washed with phosphate buffered saline solution (PBS) and then incubated with biotin-conjugated anti-mouse IgG (1:250) for 30 minutes. Sections were subsequently washed with PBS and then incubated for 30 minutes with ABC reagents from the M.O.M kit. Staining was visualized with the peroxidase substrate 3-amino-9-ethylcarbazole (AEC; Vector), yielding a red reaction product.
Macrophages were identified in adjacent sections that were fixed in ice-cold acetone and endogenous peroxidase activity was quenched, followed by incubation for 1 hour with blocking buffer (3% bovine serum albumin (BSA), 2% gelatin and 0.05% Tween-20 in 50 mM Tris-HCl pH 7.6 containing 150 mM NaCl). Sections were then incubated with rat anti-mouse CD206 (1:50, 3 hours at RT, AbD Serotec, clone MR5D3; RRID:AB_324622). The sections were washed with PBS and probed with biotin-conjugated secondary antibodies (1:200; 30 minutes at RT, Vector Laboratories). Sections were then washed with PBS and incubated with avidin D-conjugated HRP (1:1000; 30 minutes at RT, Vector) and staining was visualized with AEC. Immunohistochemistry for Klotho was performed similarly on serial sections, except the sections were fixed in 4% paraformaldehyde for 10 minutes, no antigen retrieval was performed and the primary antibody (rat anti-mouse Klotho; R&D Systems #MAB1819, clone 236214; RRID:AB_2131926) was applied overnight at 4°C.
In other immunohistochemical assays to assess numbers of myogenic cells at later stages of myogenesis, sections were prepared identically to the Pax7 labeling protocol, except mouse anti-MyoD (1:50) (BD Pharmingen #554130; RRID:AB_395255) or mouse anti-myogenin (1:50) (BD Pharmingen #556358; RRID:AB_396383) were used as primary antibodies.
2.8. Immunofluorescence
The double-staining protocol for Pax7 and activated β-catenin was similar to the anti-Pax7 method described above, although the anti-Pax7 antibody was applied to sections together with rabbit anti-non-phosphorylated (active) β-catenin (serine 45) (1:1600) (Cell Signaling Technology #19807T; RRID:AB2650576) for overnight incubation at 4°C. Sections were subsequently incubated with a combination of Dylight 488 anti-rabbit IgG and Dylight 594 anti-mouse IgG (Vector). Wnt signaling in satellite cells was determined by quantifying the percentage of satellite cells (Pax7+) that expressed active β-catenin (β-catenin+/ Pax7+ cells).
The double-staining protocol for Pax7 and Ki67 was identical to the Pax7/ β-catenin protocol except that anti-β-catenin was replaced by goat anti-Ki67 (1:200) (Santa Cruz Biotech. #sc7846) and anti-rabbit IgG was replaced by biotinylated anti-goat IgG (1:200; Vector) followed by streptavidin 488 (1:500; Vector).
2.9. Quantification of cell numbers
Numbers of Pax7+, MyoD+ and myogenin+ cells per unit volume of muscle were determined by counting the numbers of antibody-labeled cells per unit area in entire cross-sections of muscles. Muscle cross-sectional area in each section was determined by using a sterological point-counting technique and muscle volume in each section was determined by multiplying the area of the sectioned muscle by the section thickness (10 μm).
2.10. Klotho stimulation of myoblasts in vitro
Myoblasts (C2C12; ATCC; Manassas, VA, USA; RRID:CVCL_0188) were seeded in 6 cm dishes at 1.2 × 105 cells per dish (5.6 × 103 cells / cm2) and maintained in complete medium (Dulbecco’s modified Eagle media (DMEM) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin) at 37°C and 5% CO2. Attached cells were approximately 30% confluent 24 hours after plating. The myoblasts were stimulated with either heparin only (Hep; 10 µg/ml), Klotho + heparin (KL/Hep; 1 µg/ml KL; (R&D Systems, Minneapolis, MN)), recombinant fibroblast growth factor-23 (FGF/Hep; 0.5 µg/ml FGF-23; (R&D Systems)), or Klotho, FGF-23 and heparin (KL/FGF/Hep) in fresh medium at 24 and 48-hours post-plating. Five technical-replicate plates were stimulated per treatment condition. Myoblasts were approximately 90% confluent and not fused when they were collected 24 hours following the second stimulation. The cells were then washed twice with PBS and collected in Trizol. RNA was extracted and isolated with chloroform extraction and isopropyl alcohol precipitation followed by clean up using RNAeasy spin columns (Qiagen) and concentrator kit (Zymo Research). Total RNA was reverse transcribed and used for qPCR. Eff1a1 and Srp14 were used as reference genes.
2.11. Klotho stimulation of myotubes in vitro
C2C12 myoblasts were cultured as described above. When the cells reached confluence, they were transferred to differentiation medium (DMEM with 100 U/ml penicillin, 100 µg/ml streptomycin) overnight to induce differentiation and fusion to form myotubes. The cells were then stimulated with either heparin only (10 µg/ml), Klotho (1 µg/ml) + heparin, FGF-23 (0.5 µg/ml) + heparin, or Klotho, FGF-23 and heparin in complete medium at 24 and 48 hours post-differentiation. Five technical-replicate wells were stimulated per treatment condition. After treatment, the myotubes were used for RNA isolation and QPCR, as described above.
2.12. Statistical methods
All data are presented as mean ± standard deviation. Prior to analysis, statistical outliers were identified using Grubbs’ outlier test (P < 0.05). Statistical significance was calculated using unpaired Student’s t-tests or ordinary one-way ANOVA with Tukey’s multiple comparison test to determine differences among multiple groups. Differences with a p value < 0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism.
3. RESULTS
3.1. Klotho expression is influenced by acute muscle injury
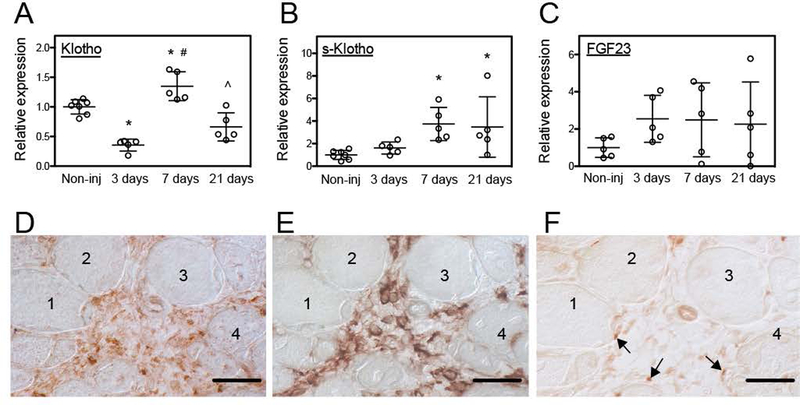
Previous investigations have shown that the onset of muscle damage and inflammation in mdx muscular dystrophy produces a drastic down-regulation of klotho expression that persists for the lifetime of the affected mice and exacerbates muscle pathology (Wehling-Henricks et al., 2016; 2018). We assayed whether muscle damage and inflammation caused by acute injury were also associated with persistent down-regulation of muscle klotho expression that could have long-term influences on muscle health. Similar to mdx muscles at the onset of pathology, we observed that klotho gene expression was reduced by ~69% at 3-days following muscle injury by BaCl2 injection (Figure 1A). In contrast to mdx muscle, the diminished expression of klotho was only transient in acutely injured muscle. At 7-days post-injury, klotho expression in muscle increased by 385% compared to 3-days post-injury and exceeded expression levels that occurred in non-injured controls. Klotho expression subsequently returned to control levels at 21-days post-injury (Figure 1A). However, expression of s-klotho did not decline at 3-days post-injury, but increased by 374% at 7-days post-injury, compared to uninjured controls (Figure 1B). Furthermore, immunohistochemistry using antibodies that recognized both m-Klotho and s-Klotho showed that inflammatory lesions enriched in CD206+ macrophages contained the highest Klotho concentration where it was detectible both within cells in the inflammatory lesions and in the extracellular space (Figures 1D, E). Few Pax7+ cells were identified in the inflammatory lesions (Figure 1F). We also observed no detectible Klotho in muscle fibers in injured muscle (Figure 1D) which is consistent with previous findings that klotho expression is silenced in inflamed, dystrophic muscle although expression in CD206+ macrophages in inflammatory lesions in muscle persists (Wehling-Henricks et al., 2016; 2018).
Figure 1.
Acute injury affected klotho expression in skeletal muscle. (A) QPCR data showing reduced klotho expression 3-days after sterile muscle injury and increased expression 7-days after injury of tibialis anterior (TA) muscle of 5-months old wild-type (Wt) mice. (B) QPCR data showing that s-klotho expression in muscle increased at 7- and 21-days following injury. (C) QPCR data showing that Fgf23 expression was not significantly affected by muscle injury. N = 5 per data set. * indicates significantly different from non-injured muscle, # indicates significantly different from muscle 3-days post-injury and ^ indicates significantly different from muscle 7-days post-injury at P < 0.05 analyzed by one-way ANOVA. Bars = standard deviation. (D-F) Serial sections of TA muscles of 5-months old mice at 7-days post-injury that were immunolabeled for Klotho (D), CD206 (E) or Pax7 (F). An inflammatory lesion surrounded by four muscle fibers (1–4) shows highest levels of Klotho (D; reddish-brown), elevated numbers of CD206+ macrophages (E; reddish brown) and Pax7+ cells associated with the neighboring muscle fibers (arrows) (F). Bars = 50 μm.
3.2. Klotho promotes muscle growth after acute injury
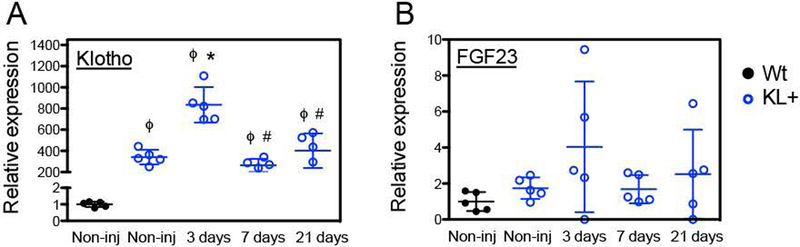
The recent finding that defects in repair and regeneration of mdx muscles can be corrected by increased delivery of Klotho to the diseased muscle by expression of a klotho transgene (Wehling-Henricks et al., 2016; 2018) suggested that increased Klotho in acutely injured muscle could also improve growth and regeneration. We tested that possibility by assaying muscle fiber growth during repair following BaCl2-mediated injury of wild-type and klotho transgenic mice. First, we established that expression of the klotho transgene was elevated over the entire course of muscle regeneration under investigation (Figure 2A). Because our previous studies showed that Klotho-induced increases in myogenic cell proliferation and myotube growth were enhanced by FGF23, we also confirmed that FGF23 expression occurred in non-injured muscles and that its expression was not significantly influenced by injury or by expression of the klotho transgene (Figures 1C and 2B).
Figure 2.
Klotho and Fgf23 expression in skeletal muscle of klotho transgenic mice after acute injury. (A) QPCR analysis of klotho expression shows a large increase in KL mRNA levels in KL+ non-injured and injured TA muscles relative to Wt non-injured TA muscle. (B) QPCR analysis reveals that the expression of a KL transgene does not significantly affect Fgf23 expression in healthy or injured muscle. N = 5 per data set. * indicates significantly different from non-injured Wt muscle. ϕ indicates significantly different from non-injured KL+ muscle, # indicates significantly different from KL+ muscle 3-days post-injury at P < 0.05
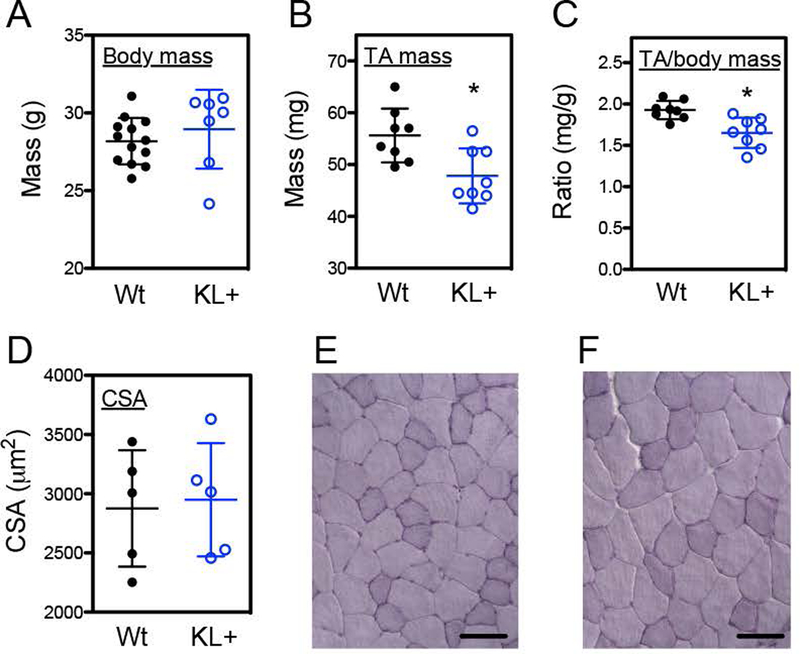
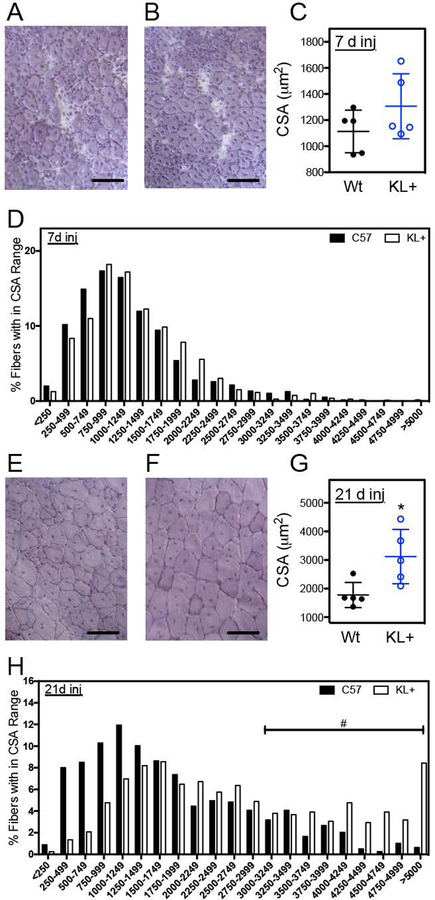
Measurements of muscle fiber cross-sectional areas were used to assay for effects of the transgene on muscle growth and regeneration. All fibers in areas of the central lesion were assayed and the number of injured fibers measured per muscle ranged from 101 to 280. Although transgene expression caused reductions in mass of non-injured TA muscles and reductions in TA mass normalized to body mass (Figures 3A–C), the transgene did not affect TA muscle fiber size in non-injured muscles (p = 0.82; two-tailed T-test) (Figures 3D–F). Muscle fiber cross-sectional areas of klotho transgenic and wild-type TA muscles also did not differ at 7-days post-injury (p = 0.19; two-tailed T-test) (Figures 4A–D). However, at 21-days post-injury, fiber cross-sectional areas of klotho transgenic mice returned to the size of non-injured muscles and were significantly larger than fiber cross-sectional areas of wild-type mice (Figures 4E–H). Furthermore, although klotho transgenic muscle fibers experienced a significant 139% increase in fiber cross-sectional area between 7- and 21-days post-injury, wild-type mice showed only a 60% increase in fiber size during the same period of muscle repair (Figures 4C, G).
Figure 3.
Expression of a klotho transgene reduced muscle mass but not muscle fiber size in non-injured adult TA muscle. Body mass (A), TA muscle mass (B) and the ratio of TA muscle mass normalized to body mass (C) was analyzed in 5-months old Wt and KL+ mice. N = 8 per data set. * indicates significantly different from Wt muscle at P < 0.05 analyzed by t test. TA muscle fiber cross-sectional area (D) was not affected by klotho transgene expression in 5-months old mice. N = 5 per data set. Representative images of cross sections of TA muscles from Wt (E) and KL+ (F) stained with hematoxylin. Bars = 100 μm.
Figure 4.
Expression of a klotho transgene improved muscle regeneration after acute injury. (A, B) Representative images of cross sections of TA muscles from Wt (A) and KL+ (B) mice 7-days post-injury stained with hematoxylin. The average cross-sectional area of fibers (C) and frequency distribution of fiber cross-sectional areas (D) for TA muscles from 5-months old Wt and KL+ mice 7-days post-injury. (E, F) Hematoxylin stained representative images of cross sections of TA muscles from Wt (E) and KL+ (F) mice 21-days post-injury. (G) The average cross-sectional area of fibers of KL+ muscle 21-days post-injury were larger than Wt. (H) Frequency distribution of fiber cross-sectional areas for TA muscles from 5-months old Wt and KL+ mice 21-days post-injury. N = 5 for each data set. * indicates significantly different from Wt muscle 21-days post-injury and # indicates a significant increase in the proportion of large muscle fibers of KL+ mice at P < 0.05 analyzed by t test. Large fibers (> 3094 μm2) are 3 standard deviations above the average cross-sectional area of Wt mice 21-days post-injury. Bars = 100 μm.
3.3. Klotho modulates myogenesis following acute muscle injury
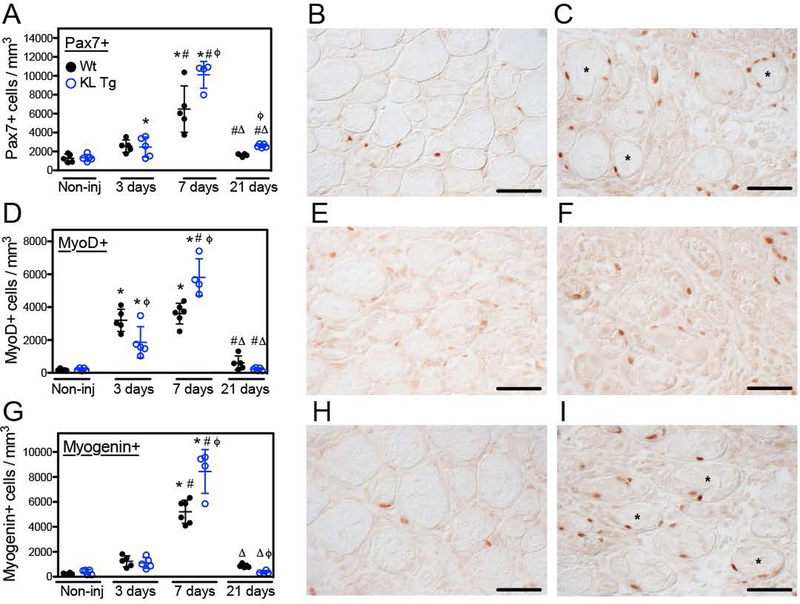
The activation, proliferation and differentiation of satellite cells and myogenic progenitor cells (MPCs) that are derived from satellite cells are essential for normal, successful repair of muscle following injury (Fry et al., 2015). We assayed for the numbers of quiescent or recently activated satellite cells (Pax7+ cells), the numbers of activated satellite cells at early stages of differentiation (MyoD+ cells) and the numbers of satellite cell progeny that were committed to terminal differentiation (myogenin+ cells). Numbers of myogenic cells expressing any of the three developmental markers did not differ between wild-type and klotho transgenic mice in non-injured muscles (Pax7: p = 0.89; MyoD: p = 0.56; myogenin: p = 0.30) (Figures 5A, D, G). Three days after injury, the number of Pax7+ cells per unit volume was similar in wild-type and klotho transgenic muscle (p = 0.87; two-tailed T-test). However, at 7- and 21-days post-injury, the number of Pax7+ cells per unit volume was 55% greater in TA muscles of klotho transgenic mice compared to wild-type mice (Figures 5A–C). In addition, individual muscle fibers in transgenic mice were frequently identified with multiple Pax7+ cells in a single plane of section, suggesting multiple rounds of division of individual satellite cells undergoing clonal expansion on the surface of injured, transgenic muscle fibers (Figure 5C). However, the proportion of Pax7+ cells that were Ki67+ did not differ significantly between wild-type vs. transgenic muscles at 7-days post-injury (wild-type: 2.25%, standard deviation = 0.55, n = 5; klotho transgenic: 1.83%, standard deviation = 0.98, n = 4; p = 0.42; two-tailed T-test). That observation suggests that there are more cycling satellite cells in the transgenic muscles, reflecting the increased number of total Pax7+ satellite cells in those muscles, although the proportion of the total population in the cell cycle did not differ.
Figure 5.
Expression of a klotho transgene affected myogenesis after acute injury. (A) Non-injured, 3-, 7- and 21-days post-injury TA muscles from 5-months old Wt and KL+ mice were immunolabeled for Pax7. Klotho transgene expression increased the number of Pax7+ cells per volume at 7- and 21-days post-injury. Representative images of TA muscle cross sections from Wt (B) and KL+ (C) mice 7-days post-injury immunolabeled with anti-Pax7 (reddish-brown nuclei). Fibers labelled with * are associated with multiple Pax7+ cells in a single plane of section. (D) TA muscle cross sections were also labeled with anti-MyoD. Injured muscle of KL+ mice showed an initial reduction in the number of MyoD+ cells per volume 3-days post-injury, which was followed by a subsequent increase in the density of MyoD+ cells 7-days post-injury. Representative images of Wt (E) and KL+ (F) TA muscle cross sections 7-days post-injury immunolabeled for MyoD (reddish-brown nuclei). (G) The expression of a klotho transgene increased the density of myogenin+ cells during the regenerative period 7-days post-injury and subsequently reduced the number of myogenin+ cells per volume 21-days post-injury relative to Wt. Photomicrographs of Wt (H) and KL+ (I) TA muscle cross sections labeled for myogenin (reddish-brown nuclei) 7-days post-injury. Data are expressed as the number of Pax7+, MyoD+ and myogenin+ cells per mm3. * in A, D and G indicates significantly different from non-injured muscle, # indicates significantly different from muscle 3-days post-injury and Δ indicates significantly different from muscle 7-days post-injury within the same genotype at P < 0.05 analyzed by one-way ANOVA. ϕ indicates significantly different from Wt in the same treatment group at P < 0.05 analyzed by t test. N = 4–6 for each data set. Bars = 100 μm.
At 3-days post-injury, MyoD+ cells in injured muscle increased tremendously in both klotho transgenic and wild-type mice, although the population was nearly 70% greater in wild-type muscles (Figure 5D). However, in the interval between 3- and 7-days post-injury, MyoD+ cell numbers did not change in wild-type muscles (p = 0.33; two-tailed T-test), but MyoD+ cell numbers in klotho transgenic muscles increased by ~212% during the same interval (Figure 5D). By 21-days post-injury, MyoD+ cell numbers nearly returned to levels that occur in uninjured muscles, and did not differ significantly between wild-type and klotho transgenic mice (Figures 5D–F).
Numbers of myogenin+ cells in muscles did not differ between wild-type and klotho transgenic mice at 3-days post-injury (p = 0.65; two-tailed T-test) (Figure 5G). At 7-days post-injury, the numbers of myogenin+ cells increased greatly in both wild-type mice and klotho transgenic mice, with the increase in klotho transgenic mice exceeding numbers in wild-type mice by ~80% (Figure 5G). At 21-days post-injury, numbers of myogenin+ cells nearly returned to levels that occurred in non-injured TA muscles for both wild-type and klotho transgenic mice, although there were significantly fewer myogenin+ cells in klotho transgenic muscles at 21-days post-injury than occurred in wild-type muscles (Figures 5G–I).
3.4. Klotho can affect MPC populations by affecting expression of myogenic transcription factors
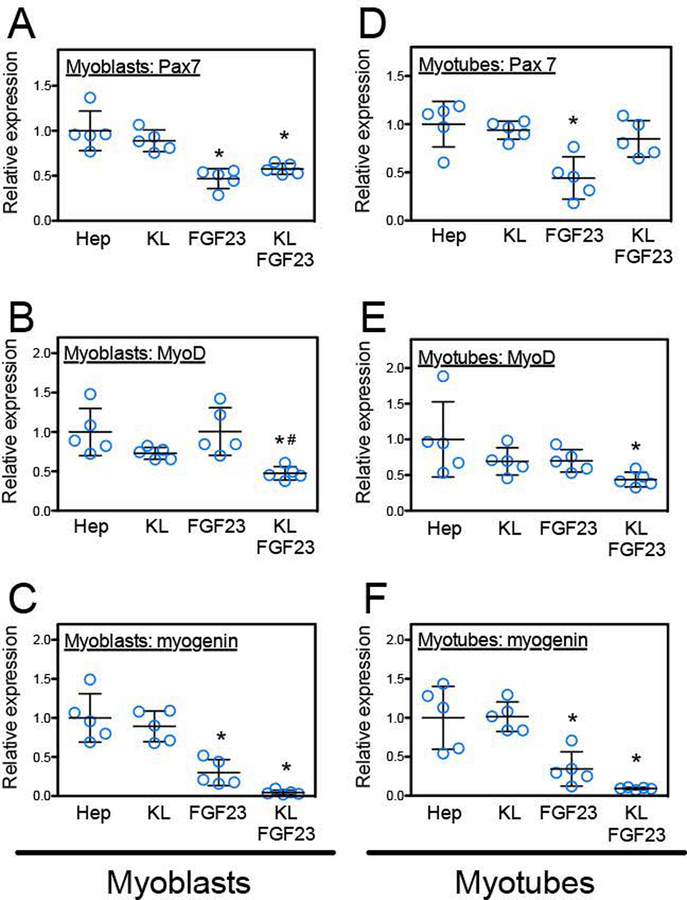
Because changes in numbers of Pax7+, MyoD+ and myogenin+ cells in injured muscle in vivo could reflect direct or indirect influences of Klotho on muscle cells, we assayed whether direct stimulation of myoblasts or myotubes with Klotho and its co-factors FGF-23 and heparin in vitro influenced expression of those myogenic transcription factors. We found that stimulation of either myoblasts or myotubes with Klotho alone had no influence on Pax7 expression (myoblasts: p = 0.23; myotubes: p = 0.78) or myogenin expression (myoblasts: p = 0.55; myotubes: p = 0.94), although FGF23 without Klotho reduced expression of both transcription factors (Figures 6A, C, D, F). However, expression of MyoD in myoblasts or myotubes was diminished by Klotho stimulation, if FGF23 were also present, although FGF23 alone had no effect on MyoD expression (myoblasts: p = 1.00; myotubes: p = 0.06), (Figures 6B, E). Because previous findings showed that stimulation of myoblasts with Klotho and co-factors significantly increases cell proliferation (Wehling-Henricks et al., 2016), these observations suggest that the expansion of Pax7+ cells in muscle following injury reflects an increase in cell proliferation and not a direct influence on the expression of Pax7 by myogenic cells. However, the reduction in MyoD+ cells in injured muscles of klotho transgenic mice at 3-days post-injury could reflect a direct, negative influence of Klotho on MyoD expression.
Figure 6.
Klotho treatment in the presence of FGF23 and heparin decreased the expression of myogenic transcription factors by MPCs in vitro. Myoblasts (A-C) or myotubes (D-F) were treated with heparin alone (Hep), Klotho and heparin (KL), FGF-23 and heparin (FGF23) or Klotho with FGF-23 and heparin (KL, FGF23). Stimulation with Klotho and its co-factors directly induced significant reductions in the expression of MyoD and myogenin in both myoblasts and myotubes. N = 5 replicates for each treatment condition. * indicates significant difference from heparin and # indicates significantly different from FGF23 by one-way ANOVA, P < 0.05.
3.5. Klotho modulates Wnt signaling in satellite cells after injury
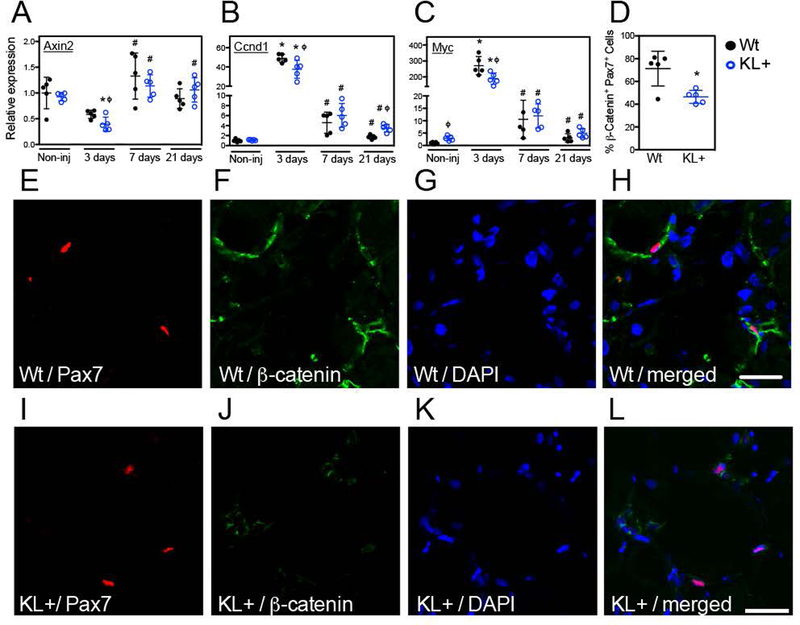
Our findings concerning the effects of klotho transgene expression on the course of myogenesis during muscle repair following injury suggest that muscle is influenced by changes in Klotho levels at early stages of MPC differentiation, which can influence the rate of muscle fiber growth during later stages of regeneration. Because the onset of MPC differentiation in postnatal myogenesis after injury is driven, at least in part, by Wnt-signaling (Brack et al., 2008) and Klotho is a Wnt antagonist (Liu et al., 2007), we tested whether klotho transgene expression affects Wnt-signaling in muscle. We assayed for changes in the expression of Wnt target genes Axin2 (Figure 7A), Ccnd1 (Figure 7B) and Myc (Figure 7C) as indices of Wnt-signaling. At 3-days post-injury Axin2, Ccnd1 and Myc were significantly reduced in klotho transgenic mice compared to wild-type. Surprisingly, klotho transgenic mice showed increased Myc gene expression in uninjured muscle (Figure 7C) and increased Ccnd1 gene expression in muscle at 21-days post-injury, compared to wild-type (Figure 7B). Because our findings showed a consensus reduction in the expression of Wnt-target genes at 3-days post-injury and because most MPCs signal through the canonical Wnt pathway at day 2 through day 5 after injury (Brack et al., 2008), we focused on the 3-day post-injury time point. We assessed Wnt activation in satellite cells using a non-phosphorylated β-catenin antibody that detects functionally active β-catenin that mediates canonical Wnt-signaling (Cadigan & Nusse, 1997; Sakanaka, 2002) and found that the proportion of Pax7+ cells expressing active β-catenin was reduced in klotho transgenic muscle 3-days post-injury relative to wild-type (Figures 7D–L). These data indicate that Klotho modulates Wnt-signaling in muscle after injury.
Figure 7.
Klotho transiently inhibited Wnt signaling in injured skeletal muscle. QPCR data showing relative expression for transcripts of Wnt signaling target genes axin2 (A), ccnd1(B) and myc (C) in TA muscle lysates of Wt and KL+ mice. * indicates significantly different from non-injured muscle and # indicates significantly different from muscle 3-days post-injury within the same genotype at P < 0.05 analyzed by one-way ANOVA. ϕ indicates significantly different from Wt in same treatment group at P < 0.05 analyzed by t test. N = 5 for each data set. (D) Immunofluorescent double labeling for Pax7 and active β-catenin in TA muscle cross sections 3-days post-injury demonstrated fewer Pax7+ cells expressing active β-catenin indicating reduced Wnt signaling in satellite cells of KL+ muscle. * indicates significantly different from Wt muscle at P < 0.05 analyzed by t test. N = 5 for each data set. Sections of Wt (E-H) and KL+ (I-L) muscle 3-days after injury labeled with anti-Pax7 (red; E, I), anti-β-catenin (green; F, J), nuclei labeled with DAPI (blue; G, K) and the merged images (from left to right; H, L). Bars = 25 μm.
3.6. Klotho modulates the inflammatory response after acute muscle injury
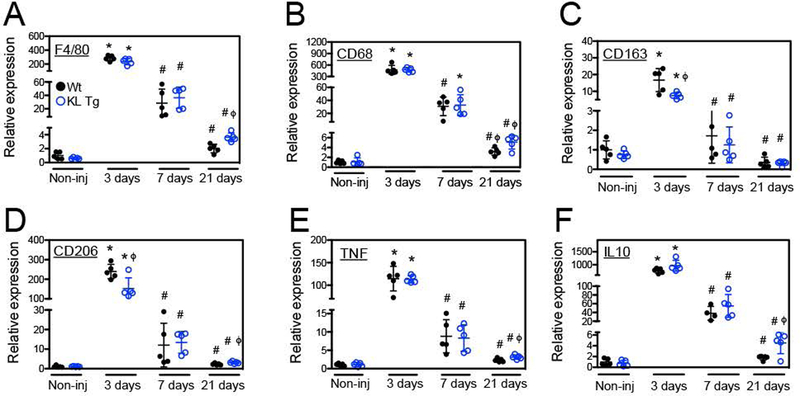
The inflammatory response to muscle injury can have major effects on determining the success or failure of muscle regeneration (Tidball, 2017) and our previous work has demonstrated that Klotho can influence cytokine production by macrophages (Wehling-Henricks et al., 2018). In particular, we found that Klotho and FGF23 stimulation can act directly on macrophages to affect TNFα and IL-10 secretion, important regulators of macrophage phenotype in muscle (Wehling-Henricks et al., 2018). Furthermore, the expression of a klotho transgene in mdx mice modifies macrophage phenotype in dystrophic muscle (Wehling-Henricks et al., 2016; 2018). Our QPCR analysis of wild-type and klotho-transgenic, uninjured muscle showed that Klotho does not affect expression of transcripts for the pan-macrophage marker F4/80 (p = 0.12; two-tailed T-test), the M1-biased macrophage marker CD68 (p = 0.84) or the M2-biased macrophage markers CD163 (p = 0.30) and CD206 (p = 0.75) (Figures 8A–D). However, transcripts for macrophage markers were affected by Klotho following muscle injury. Three days post-injury, Klotho caused small, but significant, reductions in the expression of Cd163 (Figure 8C) and Cd206 (Figure 8D). The expression of F4/80 (Figure 8A), Cd68 (Figure 8B) and Cd206 (Figure 8D) experienced small increases in expression in muscle 21-days post-injury in klotho transgenic mice. Increased F4/80 expression suggests that Klotho may prolong macrophage accumulation in muscle after acute injury, while increased Cd68 and Cd206 expression indicates increases in both M1 and M2-biased macrophages. Our findings of prolonged or increased inflammation are further supported by QPCR showing increased Tnf (Fig. 8E) and Il10 (Fig. 8F) gene expression 21-days post-injury in muscles of klotho transgenic mice relative to wild-type mice.
Figure 8.
Klotho modulated the inflammatory response in muscle after injury. QPCR data showing relative expression of transcripts for the pan-macrophage marker F4/80 (A), M1-biased macrophage marker Cd68 (B) or M2-biased macrophage markers Cd163 (C) and Cd206 (D), as well as the pro-inflammatory cytokine Tnf (E) and anti-inflammatory cytokine Il10 (F). * indicates significantly different from non-injured muscle at same genotype and # indicates significantly different from muscle 3-days post-injury within the same genotype at P < 0.05 analyzed by one-way ANOVA. ϕ indicates significantly different from Wt under same treatment conditions at P < 0.05 analyzed by t test. N = 5 for each data set.
4. DISCUSSION
The current investigation shows that modulating klotho expression influences muscle growth that follows acute injuries in healthy, adult mice and suggests that systemic elevation of klotho expression can accelerate muscle repair. Several observations indicate that at least some of these effects of Klotho on muscle repair are mediated during early stages of muscle regeneration by Klotho antagonism of Wnt signaling. As shown by other investigators, Klotho binds to several members of the Wnt family (Liu et al., 2007; Zhou et al., 2013) and that binding to Klotho can inhibit activity of at least Wnt3a (Liu et al., 2007). In addition, Klotho treatment of single, isolated muscle fibers in vitro diminished Wnt signaling, which was attributed to Klotho binding to extracellular Wnt (Ahrens et al., 2018). In the present study, we found that at 3-days post-injury in wild-type muscle, a significant reduction of klotho expression in muscle was accompanied by elevations in the expression of downstream targets of Wnt signaling (Ccnd1 and Myc), and those events coincided with large increases in numbers of MyoD+ MPCs, reflecting the initiation of satellite cell differentiation. At 7-days post-injury, klotho expression in wild-type muscle increased 3.9-fold over levels occurring in muscle at 3-days post-injury, which was accompanied by large reductions in expression of Wnt target genes (Ccnd1: 91%; Myc: 96%), and abrogation of further increases in MyoD+ cell numbers. However, expression of a klotho transgene which prevented the reduction in klotho expression that normally occurred in muscles at 3-days post-injury attenuated the increase in Ccnd1 and Myc expression that occurred at that stage in wild-type mice, and diminished numbers of MyoD+ cells by more than half. These observations show that early stages of muscle regeneration following acute injury are affected by changes in klotho expression.
Our finding that expression of the klotho transgene reduced the proportion of Pax7+ cells that express β-catenin at 3-days post-injury shows that satellite cells may be targets for Klotho inhibition of Wnt signaling in injured muscle in vivo. That observation also suggests a potential mechanism for the Klotho-induced reduction of MyoD expression at 3-days post-injury. β-catenin is an obligatory, downstream effector in the canonical Wnt signaling pathway that can bind and activate MyoD, a basic helix-loop-helix myogenic transcription factor that must be expressed for successful muscle regeneration to occur (Cadigan & Nusse, 1997; Kim et al., 2008; Megeney et al., 1996). Normally after injury, MPCs undergo a period of proliferation followed by activation of MyoD expression and the onset of muscle differentiation that is induced by Wnt signaling (Brack et al., 2008). Thus, our in vivo data suggest that the transient reduction of klotho expression that occurred in wild-type muscles at 3-days post-injury could contribute to a transient increase in Wnt signaling in satellite cells. In turn, that elevation of Wnt signaling could lead to increases in MyoD expressing MPCs, thereby promoting muscle differentiation. However, Klotho-mediated reductions in numbers of MyoD+ cells could also occur through pathways independent of Wnt signaling. Our in vitro data show that direct stimulation of myoblasts and myotubes with Klotho reduces MyoD expression, in the absence of exogenous Wnt stimulation.
Our finding that expression of endogenous klotho is significantly reduced in muscle at 3-days following injury with BaCl2, after which its expression increases above baseline levels at 7-days post-injury, supports our interpretation that endogenous modulation of klotho expression following injury can influence the course of muscle growth and regeneration. However, the time course of changes in expression that we describe differs from another report which stated that klotho expression in muscle is elevated at 3-days post-injury and then returns to baseline levels at 7-days post-injury. We cannot definitively explain the discrepancy, but we show that although klotho expression is reduced at 3-days, transcripts that specifically encode s-klotho are not reduced at that time. Because the primers used in the previous report (Sahu et al., 2018) were not identified, we cannot address whether isoform specificity of the primers contributes to the discrepancy. However, biological reasons may also contribute to the different time courses of klotho expression in the investigations. Although both the previous (Sahu et al., 2018) and present investigations use acute injury models, the previous investigation induced muscle damage by cardiotoxin (CTX) injection and our study used BaCl2 injection. An earlier investigation showed that the peak increase of satellite cell numbers was earlier and returned to levels similar to non-injured controls more rapidly in BaCl2 injured muscles than in CTX injuries (Hardy et al. 2016), indicating differences in the course and magnitude of muscle injury and repair in the two models. The injury models also produce different inflammatory responses that are potentially important because we have found that klotho is primarily expressed in inflammatory lesions in acutely injured muscle. For example, CTX-induced muscle injuries cause extensive, prolonged neutrophilia while BaCl2-induced injuries cause prolonged elevations of macrophage numbers in injured muscle (Hardy et al., 2016). In addition, CTX can contain phospholipase A2 and can interact with endogenous phospholipase A2 to affect the activation of numerous leukocyte populations, including neutrophils, mast cells and macrophages (Basanov, Dagda, & Rael, 2014; Gutierrez & Lomonte, 2004; Teixeira et al., 2003; Zuliani et al., 2005a, 2005b). Furthermore, in the previous investigation of klotho expression in CTX-injured muscle, mice were treated with non-steroidal anti-inflammatory drugs (NSAIDS) for 3 days following muscle injury, but NSAIDS were not administered in the present investigation. NSAIDS can increase numbers of M2-biased macrophages in injured muscle (Cheung and Tidball, 2003), which could affect magnitude and time course of changes in klotho expression.
Our data show that elevations in klotho expression greatly accelerate muscle fiber growth at later stages of regeneration. In contrast to wild-type muscle fibers at 21-days post-injury that grew to obtain cross-sectional areas that were only 62% of uninjured controls (~ 1800 μm2), klotho transgenic muscles returned to the size of uninjured fibers by 21-days post-injury (~ 3000 μm2). This accelerated growth in klotho transgenic muscles may be partly attributable to direct actions of Klotho on myotubes and nascent muscle fibers, because previous findings show that direct stimulation of myotubes in vitro with Klotho shifts them to a positive protein balance (Wehling-Henricks et al., 2016). However, the positive influence on muscle fiber growth may also reflect an increase in MPC proliferation, as has been demonstrated in in vitro studies in which recombinant Klotho in the presence of its co-factors more than doubled the proportion of myoblasts that were Ki67+ (Wehling-Henricks et al., 2016). Previous investigators showed that the growth of muscle fibers may be influenced by the number of satellite cells present, at least in young animals (White et al., 2010), with larger numbers of satellite cells associated with greater muscle fiber growth. Thus, the large increase in Pax7+ cells in klotho transgenic muscles at 7-days post-injury may contribute to the accelerated growth of muscle fibers in transgenic mice between 7- and 21-days post-injury.
Indirect influences of the klotho transgene may also play a role in promoting muscle fiber growth. Our findings show that klotho transgene expression increased the levels of transcripts associated with both M1-biased and M2-biased macrophage phenotypic markers at 21-days post-injury, and previous findings showed that macrophages play a significant role in increasing muscle fiber growth following injuries caused by increased muscle loading (Tidball & Wehling-Henricks, 2007). Similarly, depleting CD11b+ cells from mice (Arnold et al., 2007) or disrupting normal patterns of macrophage activation slowed muscle growth following CTX-mediated muscle injury (Perdiguero et al., 2011). Although macrophages can contribute to muscle growth following injury or disease through multiple mechanisms, including by removal of cellular debris to facilitate repair (Collins & Grounds, 2001; Deng et al., 2012; Mitchell, McGeachie, & Grounds, 1992) and participating in revascularization of the injured tissue (Shireman et al., 2007), macrophages can also act directly on muscle fibers to increase growth. For example, insulin-like growth factor-1 (IGF1) is a potent anabolic agent that can act directly on muscle fibers (Barton-Davis, Shoturma & Sweeney, 1999; Musarò et al., 1999) and the targeted deletion of IGF1 in macrophages greatly reduced growth of muscle fibers for at least 5 days following acute injury (Tonkin et al., 2015). In addition, CD206+ macrophages are also rich sources of Klotho in dystrophic muscle in which they accumulate at sites of muscle fiber regeneration and growth, and increased expression of Klotho by macrophages increases muscle fiber growth (Wehling-Henricks et al., 2018).
Other reports have shown relationships between perturbations in Klotho expression and satellite cell numbers and muscle size, although the observations collectively show that the effects on myogenesis caused by increased delivery of Klotho by a klotho transgene and by reduction of Klotho in hypomorphic mutant mice in non-injured muscles are not symmetrical. For example, data in the present investigation show that expression of the klotho transgene did not affect fiber size or numbers of satellite cells in mature, non-injured muscles. However, klotho mutation caused reductions of muscle fiber size (Ahrens et al., 2018; Iida et al., 2011), reductions in satellite cell numbers (Ahrens et al., 2018) and smaller muscle mass, at least in female mice (Phelps et al., 2013). Thus, the asymmetrical outcomes in non-injured muscles obtained by klotho overexpression and klotho mutation indicate that Klotho is essential for normal myogenesis, but supraphysiological levels do not further enhance myogenesis in healthy muscles. However, following muscle injury or disease, the effects of reduced Klotho production and increased Klotho production on muscle regeneration and growth are inverse. Elevation in klotho expression during muscle growth following acute injury or disease increases muscle fiber growth (Wehling-Henricks et al., 2016; present investigation), and klotho hypomorphic mutations reduce muscle regeneration following CTX injection (Ahrens et al., 2018).
An intriguing and unexplained observation in our investigation is that elevation of klotho expression at 3-days post-injury is associated with reductions in Wnt-signaling, but Wnt signaling is not reduced by the transgene at later stages of muscle growth and regeneration, although klotho expression levels are 263- to 836-fold higher than wild-type controls over the entire course of muscle repair that was assessed. A potential explanation for this restricted period of inhibited Wnt signaling in klotho transgenic muscles would be that Wnt signaling is more-strongly, negatively regulated through Klotho-independent pathways during later stages of myogenesis that follow acute injury. That speculation is supported by the large reductions in expression of the Wnt target genes Ccnd1 and Myc that occur between 3- and 7-days post-injury in both wild-type and klotho transgenic muscles. Our data also suggest that the Klotho-independent down regulation of Wnt signaling may be mediated by Axin2. Although Axin2 is a Wnt target, it is also a negative regulator of Wnt signaling by promoting β-catenin degradation (Liu et al., 2007; Lustig et al., 2002). Thus, the elevation in Axin2 expression in both wild-type and klotho transgenic mice between 3- and 7-days post-injury may be sufficient to drive the large reductions in Ccnd1 and Myc that obscure a less potent inhibitory affect that is mediated by Klotho.
5. CONCLUSIONS
Our study shows that elevated expression of klotho can increase muscle growth and regeneration following acute muscle injuries in healthy, adult mice, and supports the possibility that increased delivery of Klotho could have therapeutic value for promoting repair of damaged muscle. This beneficial effect is not limited to acute muscle injuries because we previously demonstrated that elevated klotho expression improved regeneration, reduced fibrosis and improved function in muscular dystrophy (Wehling-Henricks et al., 2016). However, these growth-promoting effects appear to be limited to injured or diseased muscles, because muscle fiber size and satellite cell numbers are not influenced by expression of the klotho transgene in mature, healthy muscle. As we pursue a better understanding of the regulatory influences of Klotho on skeletal muscle growth and adaptation, continuing studies are directed toward elucidating the Wnt-dependent and Wnt-independent pathways through which Klotho promotes repair of injured muscle.
NEW FINDINGS.
-
What is the central question of this study?
Does modulating the expression of Klotho affect myogenesis following acute injury of healthy, non-senescent muscle.
-
What is the main finding and its importance?
Klotho can accelerate muscle growth following acute injury of healthy, adult mice, which supports the possibility that increased delivery of Klotho could have therapeutic value for improving repair of damaged muscle.
ACKNOWLEDGEMENTS
Confocal laser scanning microscopy was performed at the California NanoSystems Institute Advanced Light Microscopy/Spectroscopy Shared Resource Facility at UCLA. The Pax7 hybridoma developed by T.M. Jessell, Columbia University, was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
FUNDING
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers F32AR065845 (to SSW), and RO1AR066036 and RO1AR062579 (to JGT).
Footnotes
COMPETING INTERESTS
The authors declare that they have no conflicts of interest. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- Ahrens HE, Huettemeister J, Schmidt M, Kaether C, & von Maltzahn J. (2018). Klotho expression is a prerequisite for proper muscle stem cell function and regeneration of skeletal muscle. Skeletal Muscle 8, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, … Chazaud B. (2007). Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. Journal of Experimental Medicine 204, 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, & Sweeney HL. (1999). Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand 167, 301–305. [DOI] [PubMed] [Google Scholar]
- Bencze M, Negroni E, Vallese D, Yacoub-Youssef H, Chaouch S, Wolff A, … Riederer I. (2012). Proinflammatory macrophages enhance the regenerative capacity of human myoblasts by modifying their kinetics of proliferation and differentiation. Molecular Therapy 20, 2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Conboy IM, Conboy MJ, Shen J, & Rando TA. (2008). A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2, 50–59. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, … Wittwer CT. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry 55, 611–622. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, & Nusse R. (1997). Wnt signaling: a common theme in animal development. Genes and Development 11, 3286–3305. [DOI] [PubMed] [Google Scholar]
- Chargé SBP, & Rudnicki MA. (2004). Cellular and molecular regulation of muscle regeneration. Physiological Reviews 84, 209–238. [DOI] [PubMed] [Google Scholar]
- Chen C-D, Podvin S, Gillespie E, Leeman SE & Abraham CR. (2007). Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proceedings of the National Academy of Sciences USA 104, 19796–19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EV & Tidball JG. (2003). Administraiton of the non-steroidal anti-inflammatory drug ibuprofen increases macrophage concentrations but reduces necrosis during modified muscle use. Inflamm Res 52, 170–176. [DOI] [PubMed] [Google Scholar]
- Collins RA, & Grounds MD. (2001). The role of tumor necrosis factor-alpha (TNF-alpha) in skeletal muscle regeneration. Studies in TNF-alpha(−/−) and TNF-alpha(−/−)/LT-alpha(−/−) mice. J Histochem Cytochem 49, 989–1001. [DOI] [PubMed] [Google Scholar]
- Dalton GD, Xie J, An S-W & Huang C-L. (2017). New insights into the mechanism of action of soluble Klotho. Frontiers in Endocrinology (Lausanne) 8, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng B, Wehling-Henricks M, Villalta SA, Wang Y & Tidball JG. (2012). IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. Journal of Immunology 189, 3669–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan J & Rando TA. (2005). Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends in Cell Biology 15, 666–673. [DOI] [PubMed] [Google Scholar]
- Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, … Kuro-o M. (2011). Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. Journal of Biological Chemistry 286, 8655–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, … Peterson CA. (2015). Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nature Medicine 21, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasanov SE, Dagda RK, & Rael ED. (2014). Snake venom cytotoxins, phospholipase A2s, and Zn2+-dependent metalloproteinases: mechanisms of action and pharmacological relevance. J. Clin Toxicol 4, 1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D. (2015). Principles and standards for reporting animal experiments. Experimental Physiology 100, 755–758. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, & Lomonte B. (2004). Phospholipase A2 myotoxins from Bothrops snake venoms. Current Organic Chemistry 8, 1677–1690. [DOI] [PubMed] [Google Scholar]
- Han XH, Jin Y-R, Seto M, & Yoon JK. (2011). A WNT/β-catenin signaling activator, R-spondin, plays positive regulatory roles during skeletal myogenesis. Journal of Biological Chemistry 286, 10649–10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thépenier C, … Chrétien F. (2016). Comparative study of injury models for studying muscle regeneration in mice. PLoS ONE 11, e0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui H, Zhai Y, Ao L, Cleveland JC, Liu H, Fullerton DA, & Meng X. (2017). Klotho suppresses the inflammatory responses and ameliorates cardiac dysfunction in aging endotoxemic mice. Oncotarget 8, 15663–15676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida R, Kanko S, Suga T, Morito M, & Yamane A. (2011). Autophagic-lysosomal pathway functions in the masseter and tongue muscles in the klotho mouse, a mouse model for aging. Molecular and Cellular Biochemistry 348, 89–98. [DOI] [PubMed] [Google Scholar]
- Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, … Nabeshima Y-I. (2004). Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Letters 565, 143–147. [DOI] [PubMed] [Google Scholar]
- Kim C-H, Neiswender H, Baik EJ, Xiong WC, & Mei L. (2008). β-Catenin interacts with MyoD and regulates its transcription activity. Molecular and Cellular Biochemistry 28, 2941–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, … Nabeshima YI. (1997). Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, … Kuro-o M. (2005). Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Partridge TA & Fan C-M. (2011). An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, … Fearon ER. (2002). Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. Journal of Biological Chemistry 277, 21657–21665. [DOI] [PubMed] [Google Scholar]
- Li S-A, Watanabe M, Yamada H, Nagai A, Kinuta M, & Takei K. (2004). Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Structure and Function 29, 91–99. [DOI] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, … Finkel T (2007). Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317, 803–806. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, … Behrens J (2002). Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Molecular and Cellular Biology 22, 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, & Nabeshima Y. (1998). Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochemical and Biophysical Research Communications 242, 626–630. [DOI] [PubMed] [Google Scholar]
- Megeney LA, Kablar B, Garrett K, Anderson JE & Rudnicki MA. (1996). MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes and Development 10, 1173–1183. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Thaloor D, Matteson S & Pavlath GK. (2000). Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. American Journal of Physiology, Cell Physiology 278, C174–181. [DOI] [PubMed] [Google Scholar]
- Mitchell CA, McGeachie JK & Grounds MD. (1992). Cellular differences in the regeneration of murine skeletal muscle: a quantitative histological study in SJL/J and BALB/c mice. Cell and Tissue Research 269, 159–166. [DOI] [PubMed] [Google Scholar]
- Moss FP & Leblond CP. (1970). Nature of dividing nuclei in skeletal muscle of growing rats. Journal of Cell Biology 44, 459–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musarò A, McCullagh KJ, Naya FJ, Olson EN, & Rosenthal N. (1999). IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 400, 581–585. [DOI] [PubMed] [Google Scholar]
- Nolan T, Hands RE, & Bustin SA. (2006). Quantification of mRNA using real-time RT-PCR. Nature Protocols 1, 1559–1582. [DOI] [PubMed] [Google Scholar]
- Phelps M, Pettan-Brewer C, Ladiges W, & Yablonka-Reuveni Z. (2013). Decline in muscle strength and running endurance in klotho deficient C57BL/6 mice. Biogerontology 14, 729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero E, Sousa-Victor P, Ruiz-Bonilla V, Jardí M, Caelles C, Serrano AL & Muñoz-Cánoves P. (2011). p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. Journal of Cell Biology 195, 307–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesskaya A, Seale P, & Rudnicki MA. (2003). Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 113, 841–852. [DOI] [PubMed] [Google Scholar]
- Rochat A, Fernandez A, Vandromme M, Molès J-P, Bouschet T, Carnac G, & Lamb NJC. (2004). Insulin and wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Molecular Biology of the Cell 15, 4544–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A, Mamiya H, Shinde SN, Cheikhi A, Winter LL, Vo NV, … Ambrosio F. (2018). Age-related declines in α-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nature Communications 9, 4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, … Nagai R (2000). In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochemical and Biophysical Research Communications 276, 767–772. [DOI] [PubMed] [Google Scholar]
- Sakanaka C. (2002). Phosphorylation and regulation of beta-catenin by casein kinase I epsilon. Journal of Biochemistry 132, 697–703. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, … & Galy A. (2011). Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647–3656. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, & Rudnicki MA. (2000). Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786. [DOI] [PubMed] [Google Scholar]
- Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, … Nabeshima Y. (1998). Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Letters 424, 6–10. [DOI] [PubMed] [Google Scholar]
- Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JE, & McManus LM. (2007). MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. Journal of Leukocyte Biology 81, 775–785. [DOI] [PubMed] [Google Scholar]
- Teixeira CF, Landucci EC, Antunes E, Chacur M, & Cury Y. (2003) Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon 42, 947–962. [DOI] [PubMed] [Google Scholar]
- Tidball JG. (2017). Regulation of muscle growth and regeneration by the immune system. Nature Reviews Immunology 17, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG, & Wehling-Henricks M. (2007). Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. Journal of Physiology 578, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, … Rosenthal N. (2015). Monocyte/macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Molecular Therapy 23, 1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta SA, Nguyen HX, Deng B, Gotoh T, & Tidball JG. (2009). Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Human Molecular Genetics 18, 482–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling-Henricks M, Li Z, Lindsey C, Wang Y, Welc SS, Ramos JN, … Tidball JG. (2016). Klotho gene silencing promotes pathology in the mdx mouse model of Duchenne muscular dystrophy. Human Molecular Genetics 25, 2465–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling-Henricks M, Welc SS, Samengo G, Rinaldi C, Lindsey C, Wang Y, … Tidball JG. (2018). Macrophages escape Klotho gene silencing in the mdx mouse model of Duchenne muscular dystrophy and promote muscle growth and increase satellite cell numbers through a Klotho-mediated pathway. Human Molecular Genetics 27, 14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JP, Baltgalvis KA, Sato S, Wilson LB & Carson JA. (2009). Effect of nandrolone decanoate administration on recovery from bupivacaine-induced muscle injury. Journal of Applied Physiology 107, 1420–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RB, Biérinx A-S, Gnocchi VF, & Zammit PS. (2010). Dynamics of muscle fibre growth during postnatal mouse development. BMC Developmental Biology 10, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Yoon J, An S-W, Kuro-o M, & Huang C-L. (2015). Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. Journal of the American Society of Nephrology 26, 1150–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li Y, Zhou D, Tan RJ, & Liu Y. (2013). Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. Journal of the American Society of Nephrology 24, 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani JP, Fernandes CM, Zamuner SR, Gutiérrez JM, & Teixeira CF. (2005a) Inflammatory events induced by Lys-49 and Asp-49 phospholipases A2 isolated from Bothrops asper snake venom: role of catalytic activity. Toxicon 45, 335–346. [DOI] [PubMed] [Google Scholar]
- Zuliani JP, Gutiérrez JM, Casais e Silva LL, Coccuzzo Sampaio S, Lomonte B, & Teixeira F. (2005b) Activation of cellular functions in macrophages by venom secretory Asp-49 and Lys-49 phospholipases A2. Toxicon 46, 523–532. [DOI] [PubMed] [Google Scholar]