Abstract
Background
Exercise training positively impacts mental health, yet remains untested in older adults with posttraumatic stress disorder (PTSD). We conducted a randomized controlled pilot trial to test the feasibility and acceptability of exercise training in older veterans with PTSD.
Methods
Fifty-four veterans ≥60 years, with a DSM-V diagnosis of PTSD, were randomized to supervised exercise (n=36) or wait-list (WL; n=18). Primary outcomes included recruitment rates, attendance, satisfaction, and retention. Secondary outcomes included changes in PTSD symptoms, depression, health-related quality of life, and sleep quality; assessed at baseline and 12 weeks.
Results
There were no adverse events. Attrition was minimal (14%), and adherence to the exercise intervention was high (82%). Clinically significant improvements in PTSD and related conditions were observed following exercise (Cohen’s d=0.36-0.81).
Conclusions
Exercise training is safe and acceptable in older adults with PTSD, may improve PTSD symptoms, and broadly impacts PTSD-related conditions. Future definitive trials are warranted.
Keywords: late life, physical activity, adjunctive, mental health, posttraumatic stress, veteran
Introduction
Posttraumatic stress disorder (PTSD) is one of the most common health conditions among U.S. military service members (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995). For many, PTSD is a chronic condition with symptoms that persist over years or even a lifetime (Chopra et al., 2014). Indeed, the estimated prevalence of PTSD is 30% among older, Vietnam-era veterans (Kulka et al., 1990). The prevalence of persistent PTSD among older Veterans is troubling in light of the negative, long-term, and often cascading, consequences of this condition. PTSD often co-occurs with depression and anxiety, and has a profound negative impact on individuals’ well-being and functioning (Dedert, Calhoun, Watkins, Sherwood, & Beckham, 2010; Farr, Sloan, Keane, & Mantzoros, 2014; Pietrzak, Goldstein, Southwick, & Grant, 2012a, 2012b). It appears that these associations are exacerbated over time, and in the context of aging; profoundly affecting one’s physical and mental health functioning into late-life (Davison et al., 2016; Durai et al., 2011; Smith, Tyzik, Neylan, & Cohen, 2015).
PTSD symptoms occur in four distinct clusters reflecting mood disturbances and emotional numbing, avoiding people or places that bring the trauma to mind, exaggerated state of alertness, and uncontrollable thoughts about the trauma. The Department of Veterans Affairs (VA) and the Department of Defense (DoD) continue to invest heavily in the development and dissemination of evidence-based psychotherapy treatments for PTSD (e.g., PE, CPT); primarily targeting younger veterans who served in Iraq and Afghanistan. The stigma attached to mental health services and the perceived unpleasantness of these therapies are barriers to engagement and adherence among older adults, with 1/3 of patients dropping out before completing a full course of treatment (Imel, Laska, Jakupcak, & Simpson, 2013; Najavits, 2015; Pless Kaiser, Seligowski, Spiro, & Chopra, 2016).
Research has also demonstrated a negative association between PTSD symptoms and health behaviors, including physical activity (van den Berk-Clark et al., 2018; Whitworth & Ciccolo, 2016). Older veterans engage in less physical activity than younger veterans, adding to their risk of functional impairment and chronic disease (Bouldin & Reiber, 2012). In a study of over 500,000 older veterans, rates of physical inactivity were highest among Veterans with PTSD (compared to Veterans with other psychiatric diagnoses), with 67.5% reporting no regular activity (Chwastiak, Rosenheck, & Kazis, 2011). Individuals with PTSD also tend to isolate at home and spend substantial amounts of time in sedentary activities, which has been shown to be a health risk factor independent of physical activity. The benefits of exercise on psychological well-being are well-established, making this a promising intervention for those suffering from PTSD in late life (American College of Sports Medicine et al., 2009; Hegberg, Hayes, & Hayes, 2019; Wetherell et al., 2013; Windle, Hughes, Linck, Russell, & Woods, 2010).
Individuals with PTSD face physical (pain, functional impairment) and psychological (depressed mood/lack of motivation, social avoidance) barriers known to hinder participation in exercise (Hall, Hoerster, & Yancy, 2015; Harada et al., 2013; Pratt et al., 2016). Although exercise among individuals with PTSD is a new area of inquiry, there is growing evidence to suggest that regular exercise may positively impact PTSD symptoms (Davidson, Babson, Bonn-Miller, Souter, & Vannoy, 2013; Hegberg et al., 2019; Rosenbaum, Sherrington, & Tiedemann, 2015; Rosenbaum, Vancampfort, et al., 2015; Whitworth, Craft, Dunsiger, & Ciccolo, 2017). Only one randomized controlled exercise trial has been reported in the literature, and showed improvements in PTSD symptoms and quality of life among patients admitted to an in-patient PTSD treatment unit (Rosenbaum, Sherrington, et al., 2015). No exercise intervention studies have been conducted in older adults with PTSD, and the impact of exercise training on late-life PTSD is not known. Warrior Wellness was a pilot randomized controlled trial of community-based exercise training for older veterans with PTSD. We examined indicators of feasibility and acceptability of the intervention including participant attendance and adherence to the program, as well as the effects of exercise training on PTSD symptoms, depression, quality of life, and sleep quality. It was hypothesized that participants in the exercise group would report reductions in PTSD and depressive symptoms, and improvements in quality of life and sleep quality.
Methods
Design
This was a 12-week, randomized controlled trial of supervised, community-based exercise compared to a usual care / wait list (WL) control group in older veterans with PTSD. A detailed description of study methods is published elsewhere (Hall et al., 2018), and CONSORT guidelines were used to guide study design and implementation. All study procedures were approved by the Durham Veterans Affairs Medical Center (VAMC) Institutional Review Board. Written informed consent was obtained for all procedures. This exercise trial for older veterans with PTSD was registered in a public registry (ClinicalTrials.gov identifier NCT02295995).
Setting and Participants
Study participants included veterans age ≥60 years who met Diagnostic and Statistical Manual of Mental Disorders (DSM-V) criteria for PTSD. Study eligibility criteria included: (1) registered for care at the Durham, NC VAMC; (2) live within 50 miles of the Durham VAMC; and (3) independently mobile (assistive devices acceptable). Exclusion criteria are described in detail elsewhere (Hall et al., 2018); in summary, these criteria included lifetime history of any psychiatric disorder with psychotic features; recent hospitalizations for cardiovascular events; evidence of lung, kidney, or congestive heart failure; uncontrolled hypertension or diabetes; significant cognitive or visual impairment; active alcohol or substance abuse; current participation in trauma-focused psychotherapies (participants were eligible for re-screening upon completion of these treatments). Participants were also excluded if they were regularly performing moderate-intensity aerobic exercise more than 150 minutes per week. Participants could be taking psychoactive medication as long as they had started it and / or had been on a stable dose for ≥6 weeks.
Participants were primarily recruited using Durham VAMC electronic health records to identify patients with PTSD. These patients were mailed introductory letters and then screened by telephone to further assess eligibility. The Primary Care PTSD Screen for DSM-V (PC-PTSD-5; Prins et al., 2016) was used to screen for PTSD, and the AUDIT-C (Bush, Kivlahan, McDonell, Fihn, & Bradley, 1998) and DAST-10 (Skinner, 1982) were used to screen for substance abuse and dependence. All participants who were eligible based on a screening telephone call were scheduled to meet a research assistant at the Durham VAMC to complete the consent process, enrollment, and baseline assessments which included the Clinician-Administered PTSD Scale for DSM-V (CAPS-5; Weathers et al., 2018) diagnostic interview and Short Portable Mental Health Status Questionnaire (SPMSQ; Pfeiffer, 1975) to determine final eligibility.
Randomization and Intervention
Randomization
Individual slips of paper indicating “PA” (physical activity) or “UC” (usual care) were placed in a sealed box at the beginning of the study. Upon completing baseline assessments, the the participant blindly selected a piece of paper from the box, and were allocated to the corresponding group. Participants were randomized to either exercise or wait list control on a 2:1 basis, respectively. Group allocation was concealed from the participant and researcher until all baseline assessments were completed. To ensure acceptance of the protocol, WL participants were told they could enroll in the exercise program after the 12-week period.
Exercise Intervention
The Warrior Wellness intervention was developed using Social Cognitive Theory (Bandura, 1986) and designed to enhance self-efficacy for exercise by integrating cognitive behavioral strategies across the duration of the exercise program. The intervention targeted the following sources of self-efficacy: 1) self-monitoring and mastery experience; 2) education of physiologic responses to exercise; 3) outcome expectations; 4) barriers to participation; and 5) modeling and social support. A full description of the theoretical constructs targeted in this intervention and the methods we implemented to address these determinants has been published previously (Hall et al., 2018).
Supervised exercise sessions were held 3 days a week (Monday, Wednesday, Friday) at a community-based fitness facility conveniently located 1 mile from the Durham VAMC. The duration of the exercise sessions ranged from 60 to 90 minutes, depending on individual pace and prescription, but sessions were only offered between 9:00-10:30 a.m. The program included an individualized exercise prescription based on the participant’s exercise history, current exercise capacity, personal preferences, and health status. Individual prescriptions and progressions were consistent with American College of Sports Medicine (ACSM) guidelines for older adults (Physical Activity Guidelines Committee, 2018). The exercise leader worked one-on-one with participants during the introduction to the program (~2 weeks), after which participants were expected to exercise independently using the exercise prescription card, with occasional feedback and guidance from the instructor. The staff-to-participant ratio for the program ranged from 1:1 up to 1:5, and typically included 2 instructors. A full description of the exercise battery and progression schedule is detailed elsewhere (Hall et al., 2018). The intervention was multi-component, emphasizing aerobic, strength, balance, and flexibility exercises. Modalities included body weight, free weight, cables, and exercise bands; treadmills and stationary bikes were used for aerobic training. The program aimed to have participants engaged in moderate to vigorous exercise for at least 150 min/week, in line with current recommendations (Physical Activity Guidelines Committee, 2018).
Wait-List Usual Care
Participants randomized to the WL group continued to receive the standard of care provided in their usual VA primary, women’s health, mental health, or geriatrics clinics. The control group did not receive exercise training. To reduce attrition, control group participants were offered the intervention after the trial; 9 of 17 WL completers chose to engage in supervised exercise at that time.
Measures
Assessments were completed in-person, at baseline and 12-week follow-up. All measures were administered by a trained research assistant on-site at the Durham VAMC, using standardized protocols. Study staff who conducted the baseline visit were blinded to participants’ randomization assignment until the end of the appointment. Due to limited resources, the same researcher delivered the intervention and administered the research measures and neither participants nor researchers were blinded to treatment allocation during intervention delivery or during outcome assessment. To maximize fidelity and reduce potential bias, both the researcher and the patient were blinded to previous results on testing, and research checklists and scripts were used to standardize testing procedures.
Screening assessments
Trauma History / PTSD symptom screen.
The PC-PTSD-5 (Prins et al., 2016) was used during the phone screen as an initial indicator of trauma history and PTSD symptom prevalence over the past month. The PC-PTSD is a 4-item screener, with one question reflecting each PTSD symptom cluster. Individuals who answered affirmatively to any of the questions were deemed eligible for further screening at the in-person baseline appointment. The PC-PTSD-5 has demonstrated excellent validity in military veterans (Prins et al., 2016).
Alcohol and Substance Abuse.
The AUDIT-C questionnaire (Bush et al., 1998) was used to assess alcohol consumption. Total scores range from 0-12. Positive results for alcohol dependence included scores ≥3 for men, and ≥2 for women (unless all points were from question 1: “how often do you have a drink containing alcohol?”). The DAST-10 (Skinner, 1982) questionnaire was used to assess recreational drug use (excluding alcohol and tobacco). Total scores range from 0-10, and positive results for substance abuse included scores ≥3. The AUDIT-C and DAST-10 have demonstrated satisfactory reliability and validity (Bush et al., 1998; Yudko, Lozhkina, & Fouts, 2007)
PTSD Diagnosis.
The Clinician-Administered PTSD Scale (CAPS; Weathers et al., 2018) is the gold-standard diagnostic measure for PTSD. The CAPS was only administered at baseline, as a diagnostic screening tool (yes/no PTSD). The CAPS-5 is a structured interview and was used to determine current (past month) diagnosis of PTSD. CAPS-5 contains 20 items, corresponding to the DSM-V diagnosis for PTSD, and assesses symptom severity based on the frequency and intensity of the symptoms. Severity ratings range from 0 (absent) to 4 (extreme/incapacitating), with a score of 2 (moderate/threshold) indicating a clinically significant problem. PTSD symptom clusters include: Re-experiencing (Criterion B), Avoidance (Criterion C), Negative alterations in cognitions or mood (Criterion D), and Hyperarousal (Criterion E). The DSM-V PTSD diagnostic rule requires: a) at least one Criterion B symptom; 2) at least one Criterion C symptom; c) at least two Criterion D symptoms; d) at least two Criterion E symptoms; d) Criterion F is met (disturbance has lasted ≥ 1 month); and Criterion G is met (disturbance causes either clinically significant distress or functional impairment). Once a patient met criteria (severity rating ≥2) for a particular symptom cluster, the interviewer skipped to the next section/cluster of the CAPS-5. This means that a total score for the CAPS-5 was not calculated. The CAPS-5 has high internal consistency and interrater reliability (Weathers et al., 2018).
Cognitive Status.
The Short Portable Mental Health Status Questionnaire (SPMSQ; Pfeiffer, 1975) is a brief, 10-item screener that is widely used to detect cognitive impairment. A positive screen for mild cognitive impairment included ≥3 errors.
Participant Characteristics
We collected patient-reported demographic and clinical characteristics including age, sex, race/ethnicity, income, education level, and comorbid health conditions.
Primary Outcomes
Program Adherence and Satisfaction.
Adherence to the prescribed protocol was calculated using program attendance logs. Acceptability of the intervention was assessed at the end of the 12-week program using a participant evaluation form.
Secondary Outcomes
PTSD Symptoms.
The PTSD checklist (PCL-5; Wortmann et al., 2016) is a 20-item self-report measure that assesses the 20 DSM-V symptoms of PTSD. All items are rated on a 5-point Likert scale ranging from 0 (not at all) to 4 (extremely), and summed to create a total score. Total scores range from 0-80, with higher scores indicating more severe PTSD symptoms. DSM-V symptom cluster severity scores were evaluated separately, and calculated by summing the scores for the items within a given category. PTSD symptom clusters include: Re-experiencing (5 items), Avoidance (2 items), Negative cognitions or mood (7 items), and Hyperarousal (6 items). The PCL-5 has excellent reliability and validity (Bovin et al., 2016).
Depressive Symptoms.
The PHQ-9 (Kroenke, Spitzer, & Williams, 2001) is a 9-item self-report questionnaire consisting of symptoms and attitudes relating to depression. The PHQ-9 includes items such as self-dislike, suicidal ideation, sadness, and sleep disturbance. The items are summed with a range of 0 to 27; higher scores indicate greater depression. The PHQ9 has been shown to be a valid and reliable measure of depression severity (Phelan et al., 2010).
Health-Related Quality of Life.
The Medical Outcomes Study 36-item Short-Form Survey (SF-36; Ware, 2000) comprises 36 items to assess health-related quality of life. The SF-36 is a widely used measure of general health that has been validated in diverse populations, is considered to be a reliable indicator of health status, and is sensitive to change (Ware Jr. & Kosinski, 2001). The SF-36 includes a Mental Health Component Score (MCS), which is the weighted sum of 4 subscales including: vitality, mental health, role-emotional, and social functioning. Higher scores indicate higher quality of life in the psychological domains.
Sleep.
The Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) is a 19-item self-report questionnaire that assesses sleep quality over the past month (e.g., “During the past month, how often have you had trouble sleeping because you wake up in the middle of the night or early morning?”). In scoring the PSQI, seven component scores were derived, each scored on a 4-point scale (0=no difficulty, 3=severe difficulty). The component scores were summed such that higher numbers indicate worse sleep quality (score range 0 to 21). The PSQI has demonstrated reliability and validity (Spira et al., 2012).
Statistical Analysis
We compared baseline characteristics of the two groups using independent-samples t tests for continuous data and Pearson’s χ2 tests for categorical data. Baseline characteristics of non-completers (i.e., individuals randomized who did not complete 12-week assessments) were compared with participants who completed all follow-up assessments. Statistical analysis was conducted on a sample size of 48. Our prespecified intention-to-treat analysis was defined as participants who were randomly assigned to treatment and for whom follow-up data were available. Data analysis was performed using PASW Statistics 18.0 (Chicago, IL) and SAS (9.3). The between-group difference at follow-up [mean difference (MD) and 95% Confidence Interval] was calculated for all outcome measures.
The primary aim of this pilot study was to determine the feasibility and acceptability of structured exercise in older adults with late-life PTSD, and was not powered to detect significant differences between the groups. As such, we estimate possible effects rather than formal hypothesis testing to infer the size and direction of treatment effect. Cohen’s d effect sizes were calculated as the difference in means between the two groups divided by the SD and is interpreted as d=0.20 (small), d=0.50 (medium), and d=0.80 (large).
Results
Recruitment and Participant Characteristics
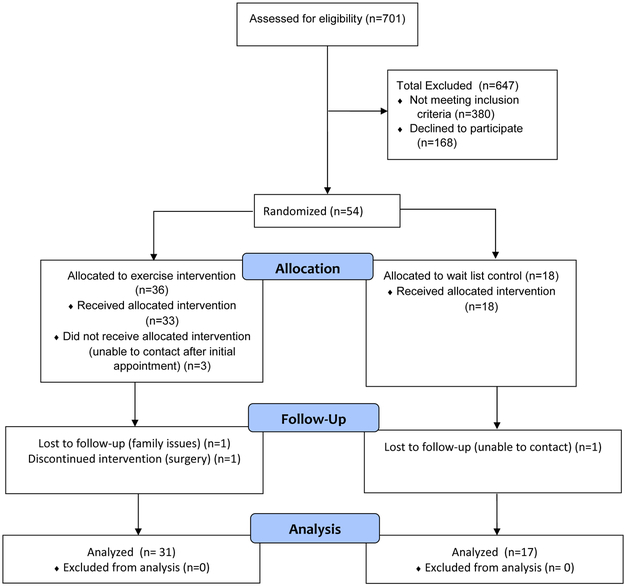
The recruitment period started November 2015 and was originally planned to last 18 months. However, this was extended to 2 years because of slow recruitment. This was not helped by recurrent personnel changes among the study team which led to disruptions in screening and recruitment activities. As shown in Figure 1, 701 patients were screened for eligibility, of which 380 did not meet inclusion criteria. The most common reasons for ineligibility were psychotic illness and active alcohol or substance abuse. Among potentially eligible participants, 166 individuals declined to participate, and 99 were unable to be contacted by our research team. The remaining 54 participants were eligible and randomized (36 to exercise, 18 to WL); 89% completed 12-week measures. Among the 6 ‘non-completers’, one (17%) had to withdraw due to a surgery, one (17%) reported family issues that prevented continued participation, and four (66%) were un-contactable after baseline measures. Overall, the dropout rate did not differ between groups. Sensitivity analyses between dropouts and those who completed the 12-week assessments revealed no significant baseline differences between groups in age, race, number of chronic conditions, or PTSD symptoms, although BMI was higher among dropouts (35.2 vs. 29.9 kg/m2, p<.05). Participant characteristics at baseline are shown in Table 1. The average age of participants was 67.4 years (range 60-78 years). The sample was mostly male (90.7%) and African American (85.2%), with significant health burden (average 3.6 comorbid health conditions). The mean baseline BMI was 30.5 kg/m2, and 83.3% of the sample were overweight or obese. Over half of the participants had served in the Army (59.3%), and reported an annual household income >$40,000 (51.9%). The severity of PTSD reported by the sample was high (mean PCL-5 = 40.9), and average duration of symptoms was 45 years. There were no significant differences between groups on any observed baseline variables at baseline.
Figure 1.
CONSORT flow diagram of patient participation
Table 1.
Baseline demographic characteristics
| Variable | Exercise (n=36) M(SD) |
WL (n=18) M(SD) |
Test statistic p value |
|---|---|---|---|
| Age (y) | 67.7(3.2) | 66.9(4.3) |
t = 0.77 p = 0.44 |
| Male gender, n(%) | 34(94%) | 15(83%) | χ2 = 1.8 p = 0.18 |
| African-American race, n(%) | 33(92%) | 13(72%) | χ2 = 1.8 p = 0.06 |
| Hispanic ethnicity, n(%) | 0 | 0 | N/A |
| ≤ High school education, n(%) | 6(17%) | 2(11%) | χ2 = 0.29 p = 0.59 |
| Number of comorbidities | 3.7(1.6) | 3.9(1.8) |
t = −.28 p = .78 |
| Body mass index (kg/m2) | 30.6(5.9) | 30.4(4.4) |
t = .12 p = .91 |
| PTSD symptoms (0-80) | 42.9(14.9) | 41.1(14.0) |
t = .43 p = .67 |
| Depressive symptoms | 10.7(5.5) | 8.5(4.7) |
t = 1.4 p = .16 |
Note. Chi-squared or t-test statistic reported depending on the data.
Adherence and Satisfaction
Six participants (11%) dropped out before completing the entire 12-week protocol. Dropout rates did not vary significantly across treatment arms (χ2(1) = .84, p= 36); 5 participants (14%) in the exercise condition and 1 (6%) in the WL condition. Three participants dropped out of the study after randomization but before exercise training was initiated, and were unable to be re-contacted by study staff. Thirty-six veterans were randomized to the exercise intervention, and 86% completed the exercise program. Individuals who completed the Warrior Wellness exercise program (n=31) reported high levels of satisfaction with the intervention (100% endorsed “Good” or “Excellent” ratings). Adherence to the intervention was evaluated by attendance logs. Among those who attended at least one session, the average attendance was 82% of all sessions (range 23% - 100%). No serious study-related adverse events were recorded, which indicates that exercise training is safe and well-tolerated in older veterans with chronic PTSD.
Changes in PTSD Symptoms
There was a small effect of differential improvement in PTSD symptoms (MD = −4.23, d=.38 [95% CI −11.7, 3.3]). Participants in the exercise group demonstrated a 7.1 point improvement (16%) in PTSD symptoms from baseline to postintervention, and patients in the WL condition showed small (<7%; 2.9 points) improvements.
When evaluating PTSD symptom clusters, small to moderate effects favoring the exercise group were observed for Negative Cognitions and Mood (MD=−2.92, d=.61 [95% CI −1.1, 1.2]), and Re-Experiencing (MD = −1.28, d=.40 [95% CI −3.4, .9]) subscales. Participants in the exercise condition achieved a 19% improvement in Negative Cognitions and Mood, and 21% improvement in Re-experiencing, whereas those in the WL group showed a 4% worsening and 11% improvement of symptoms, respectively. No between-group differences were observed in changes on Avoidance (MD=.41, d=.18 [95% CI −1.1, 1.9]) or Hyperarousal (MD=−.28, d=.07 [95% CI −2.9, 2.4]) symptom clusters between groups.
Changes in Depressive Symptoms
There was a moderate effect of differential improvement in depressive symptoms (MD=−3.28, d=.57 [95% CI −6.8, 0.3]). Exercise participants demonstrated a 17% improvement in depressive symptoms from baseline to 12 weeks, whereas the WL group demonstrated a 16% increase (worsening) of depressive symptoms. A 5-point change on the PHQ9 is clinically significant, and 8(26%) met this criteria.
Changes in Sleep and Health-Related Quality of Life
There was a small effect of differential improvement in SF-36 MCS (MD=3.19, d=.36 [95% CI −2.7, 9.1]) scores were observed for the exercise group, in comparison with the WL group. Between-group differences across the four MCS subscales ranged from small to moderate (ds=0.34 – 0.55), favoring the exercise arm. A moderate effect of differential improvements in sleep quality, favoring the exercise group, were also observed (MD=−1.47, d=.61 [95% CI −3.0, 0.5]).
Missing Data
Although the amount of missing data at 12 weeks was small (only 6 participants withdrew from the study), we conducted a post-hoc sensitivity analysis using last observation carried forward (LOCF) for these 6 cases across all outcome measures. These cases were included in analyses as “no change.” We then re-ran the analyses on this complete data set and very similar results were reported.
Discussion
The results of this pilot study provide empirical support that a multi-component, community-based exercise program is feasible in medically complex older adults with PTSD. Most participants were able to complete the exercise training protocol successfully, and retention rates (86%) and intervention adherence (82%) were high. These findings are notable given the significant psychological and emotional (i.e., lack of motivation) barriers to exercise that individuals with mental health conditions report (Crone & Guy, 2008; Soundy, Faulkner, & Taylor, 2007). Participants reported high satisfaction with the exercise program and 100% reported that they would recommend it to other veterans with PTSD. Our results also suggest that the presence of PTSD symptoms does not preclude participation in a community-based exercise program, and that exercise is both a familiar and less stigmatizing treatment option for military veterans that warrants further exploration as a health promotion tool and adjunctive therapy (Abrantes et al., 2017; Hegberg et al., 2019).
To our knowledge, this is the first published trial studying the impact of exercise training on late-life PTSD symptoms. Our results suggest that exercise training may benefit PTSD symptoms and PTSD-related conditions in older adults with PTSD. Participants in the exercise condition demonstrated greater improvement in PTSD and related symptoms than participants in the usual care wait-list condition. The sizes of these effects (Cohen’s d) ranged from small for PTSD symptoms and self-rated mental health-related quality of life, to medium for the effect of exercise on depressive symptoms and sleep. We observed an average 7-point reduction (16%) in PTSD symptoms in the exercise group. Although clinically important difference guidelines have not yet been identified for the PCL-5, evidence from the DSM-IV PCL suggests that a 5-10 point change represents reliable change, and a 10-20 point change represents clinically important difference (Monson et al., 2008; National Center for PTSD). Among those who completed exercise training, over 55% reported a ≥5 point improvement, and 42% reported a ≥10 point improvement on the PCL-5. The improvements in depressive symptoms, sleep, and mental health-related quality of life observed in the intervention group were also clinically significant.
We observed improvement across individual PTSD symptom clusters and found small to medium effects sizes, which is notable and provides some insight into possible mechanisms/pathways by which exercise impacts PTSD. The strongest effect of the intervention was seen in the Negative Cognitions and Mood symptom cluster, with veterans reporting the return of positive emotions and reductions in negative affect. The beneficial effects of exercise and enhanced physical fitness on mental health and mood states (e.g., depression, affective responses) is well-established in other populations (Penedo & Dahn, 2005; Schuch et al., 2016; Windle et al., 2010), and one study has examined these effects following a single bout of exercise in PTSD (Crombie, Brellenthin, Hillard, & Koltyn, 2018), but this is the first study to report exercise training-related changes in a population with chronic PTSD.
This is also the first trial to explore the effects of exercise in the context of long-term, chronic PTSD. Study participants reported suffering from PTSD symptoms for nearly three-quarters of their lifetime, and reported high PTSD symptom severity at the start of the study. The improvement in PTSD symptoms following exercise training reported in this trial is consistent with pilot studies in younger populations (Rosenbaum, Sherrington, et al., 2015; Rosenbaum, Vancampfort, et al., 2015). PTSD continues to have limited treatment options; exercise may be a less stigmatizing and more approachable adjunctive treatment for PTSD and related symptoms (Hegberg et al., 2019). Several study participants explicitly stated that they would not participate in the trial if they had to discuss their trauma, which points to the potential for a stepped care approach to PTSD treatment. For example, starting patients with exercise and gradually moving patients to a more health/wellness orientation may make patients more open to seeking PTSD treatment (i.e., the sugar that helps the medicine go down) (Cook, Simiola, Hamblen, Bernardy, & Schnurr, 2017). Clinical practice guidelines make no specific recommendations for the treatment of late-life PTSD, though physical activity is recommended as part of a comprehensive care plan (The Management of Posttraumatic Stress Disorder Workgroup, 2017). To our knowledge, only two trials of exercise in combination with cognitive-behavioral therapy have been published, and report beneficial effects on PTSD symptoms (Goldstein et al., 2018; Powers et al., 2015).
We also observed meaningful impact of exercise training on important clinical health indicators and patient-reported outcomes in older veterans with PTSD. Sleep problems are one of the most commonly reported symptoms by people with PTSD (Koffel, Khawaja, & Germain, 2016), and difficulty falling and/or staying asleep is considered one of the hyperarousal symptoms of PTSD. Comorbid depression is also highly prevalent in individuals with PTSD (Campbell et al., 2007). At the start of the study, 90% of participants had scores on the PSQI indicative of poor sleep quality, and 43% met criteria for moderate-to-severe depression. Mental health-related quality of life was also poor, with scores on the SF-36 falling below the 20th percentile of population norms. Following 12 weeks of exercise training, veterans reported improvements in sleep, depressive symptoms, and mental health-related quality of life compared to WL. These figures complement patient reports, where 93% of individuals reported “mental health improvements” after completing the exercise program. That exercise training alone can broadly impact PTSD and related symptoms is notable and an advantage over other traditional PTSD treatments (e.g., medications, psychotherapies), which have not demonstrated broad impact and report a less robust response in older adults compared to younger adults. Adequately powered, definitive trials to test the impact of structured exercise on PTSD symptoms and PTSD-related conditions, and that explore potential mechanisms through which these changes occur, are needed.
Strengths and Limitations
Strengths of this study include a minority sample (85% African American), an intervention targeting exercise in a vulnerable and understudied population, the randomized controlled trial design, and using validated measures of PTSD. We also observed minimal loss to follow-up in both groups, which suggests that a tailored intervention that targets key elements of behavior change is acceptable and engaging to older veterans with multiple comorbidities. Limitations include a 54% exclusion rate (due largely to comorbid psychiatric conditions and active substance abuse) reflecting limited reach of the program and generalizability of our findings, and a short exercise intervention with no long-term follow-up to assess maintenance effects. The lack of blinding of the testers to group allocation for 12-week assessments was a potential source of bias. This was countered by assessors following standardized protocols for all assessments, including giving exactly the same instructions to all participants on all occasions, and being blinded to baseline results at re-test. Challenging recruitment and a small sample size limit the generalizability of study findings and our ability to do perform formal hypothesis-testing analyses. The underrepresentation of women is this study, though expected given the demographics of the military veteran population, is another limitation. We did not have sufficient numbers to test for gender effects, but there were some indications of a gender difference in exercise adherence. We cannot draw strong conclusions from such small numbers, however, it is worth considering that women with PTSD may have unique barriers to group- and community-based exercise compared to men (Pebole & Hall, 2019; Shivakumar, Anderson, Suris, & North, 2017). It is also worth noting that while the majority of participants experienced a lessening of PTSD symptoms with exercise training, a significant minority reported a ≥5 point worsening of PTSD symptoms across both groups (16% Exercise vs. 13% WL). These results suggest there is individual variability in the responses to exercise and / or the community-based environment that warrants further exploration in future work.
Conclusions
The results of this pilot study contribute to a small, but growing, literature which suggests that exercise training is an acceptable intervention among individuals with PTSD, and improves PTSD symptoms and PTSD-related health outcomes, which is exciting. These findings are important for they show impact of exercise training on important clinical and patient-reported outcomes. As with traditional PTSD treatments, clinical symptoms of PTSD were still evident at the end of the intervention period. However, exercise training represents an empowering therapy that allows an individual to better their health and functioning in the context of a chronic mental health condition. An emphasis on physical activity is consistent with both the values cultivated during military service and geriatric and recovery models of care that prioritize quality of life in the context of chronic disease. That this exercise program was so highly rated by participants is important, for it shows that exercise is an “approachable” behavioral intervention, and it is worth considering that exercise training could be a potential bridge to initiating conversations with patients about engaging (or re-engaging) in PTSD treatment. Exercise in, and for, PTSD is a relatively new area of study. Many questions remain to be explored in future work including efficacy trials, studies that evaluate mechanistic factors (e.g., exercise capacity, sleep) as mediators of the improvement in PTSD symptoms, sub-group analyses, and implementation approaches to improve the health and well-being of individuals living with PTSD.
Table 2.
Group Means and Differences Between Group Means for All Outcomes
| Baseline | Change | Difference Between Groups | |||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | SE | Mean | SE | Mean Change | 95% CI | Cohen’s d |
| PTSD symptoms (PCL-5) | |||||||
| Exercise group | 42.97 | 2.5 | −7.10 | 2.4 | |||
| Control group | 41.06 | 3.5 | −2.87 | 2.3 | −4.23 | −11.7 to 3.3 | 0.38 |
| PTSD symptom clusters (PCL-5) | |||||||
| Re-experiencing (0-20) | |||||||
| Exercise group | 12.22 | 0.7 | −2.55 | 0.7 | |||
| Control group | 11.25 | 0.9 | −1.27 | 0.6 | −1.28 | −3.4 to 0.9 | 0.40 |
| Avoidance (0-8) | |||||||
| Exercise group | 5.25 | 0.7 | −0.52 | 0.4 | |||
| Control group | 5.07 | 0.6 | −0.93 | 0.6 | 0.41 | −1.1 to 1.9 | 0.18 |
| Negative cognitions and mood (0-28) | |||||||
| Exercise group | 12.72 | 1.0 | −2.45 | 0.9 | |||
| Control group | 12.56 | 1.2 | +0.47 | 1.1 | −2.92 | −6.1 to 0.2 | 0.61 |
| Hyperarousal (0-24) | |||||||
| Exercise group | 12.92 | 0.8 | −1.74 | 0.8 | |||
| Control group | 12.44 | 1.2 | −1.47 | 0.9 | −0.28 | −2.9 to 2.4 | 0.07 |
| Depressive symptoms (PHQ-9) | |||||||
| Exercise group | 10.67 | 0.9 | −1.87 | 1.1 | |||
| Control group | 8.50 | 1.1 | +1.41 | 1.4 | −3.28 | −6.8 to 0.3 | 0.57 |
| SF-36 Mental Health Component Score | |||||||
| Exercise group | 40.22 | 2.0 | +3.56 | 1.9 | |||
| Control group | 38.00 | 2.5 | +0.36 | 1.6 | 3.19 | −2.7 to 9.1 | 0.36 |
| Sleep (PSQI Global; 0-21) | |||||||
| Exercise group | 10.67 | 4.2 | −0.65 | 0.5 | |||
| Control group | 9.89 | 3.2 | +0.82 | 0.5 | −1.47 | −3.0 to 0.5 | 0.61 |
Note: Lower scores reflect fewer symptoms/better health with the exception of physical activity and SF-36. Cohen’s d effect sizes interpreted as d=0.20 (small), d=0.50 (medium), and d=0.80 (large).
Acknowledgements
We express our deepest gratitude to the veterans who volunteered to participate in this research study and provided valuable feedback and insights. This research was supported by a grant from the Department of Veterans Affairs Rehabilitation Research and Development Service (2RX001316 to K. Hall). Drs. Hall and Morey are supported by the Duke Claude D. Pepper Older Americans Independence Center (National Institute on Aging P30AG028716). The views expressed in this publication are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Funding: This study was funded by the Department of Veterans Affairs Rehabilitation Research and Development Service (2RX001316 to K. Hall). Drs. Hall and Morey are supported by the Duke Claude D. Pepper Older Americans Independence Center (National Institute on Aging P30AG028716).
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Abrantes AM, Reddy MK, Farris SG, Greenberg BD, Spofford C, & McLaughlin N (2017). Exercise preferences and perceived benefits and barriers of physical activity among US veterans with post-traumatic stress disorder. J Behav Health, 6(3), 111–119 [Google Scholar]
- American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, … Skinner JS (2009). American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc, 41(7), 1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c [DOI] [PubMed] [Google Scholar]
- Bandura A (1986). Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- Bouldin ED, & Reiber GE (2012). Physical Activity among Veterans and Nonveterans with Diabetes. J Aging Res, 2012. doi: 10.1155/2012/135192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, & Keane TM (2016). Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychol Assess, 28(11), 1379–1391. doi: 10.1037/pas0000254 [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, & Bradley KA (1998). The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med, 158(16), 1789–1795. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Campbell DG, Felker BL, Liu CF, Yano EM, Kirchner JE, Chan D, … Chaney EF (2007). Prevalence of depression-PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventions. J Gen Intern Med, 22(6), 711–718. doi: 10.1007/s11606-006-0101-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra MP, Zhang H, Pless Kaiser A, Moye JA, Llorente MD, Oslin DW, & Spiro A 3rd. (2014). PTSD is a chronic, fluctuating disorder affecting the mental quality of life in older adults. Am J Geriatr Psychiatry, 22(1), 86–97. doi: 10.1016/j.jagp.2013.01.064 [DOI] [PubMed] [Google Scholar]
- Chwastiak LA, Rosenheck RA, & Kazis LE (2011). Association of psychiatric illness and obesity, physical inactivity, and smoking among a national sample of veterans. Psychosomatics, 52(3), 230–236. doi: 10.1016/j.psym.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JM, Simiola V, Hamblen JL, Bernardy N, & Schnurr PP (2017). The influence of patient readiness on implementation of evidence-based PTSD treatments in Veterans Affairs residential programs. Psychol Trauma, 9(Suppl 1), 51–58. doi: 10.1037/tra0000162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie KM, Brellenthin AG, Hillard CJ, & Koltyn KF (2018). Psychobiological Responses to Aerobic Exercise in Individuals With Posttraumatic Stress Disorder. J Trauma Stress, 31(1), 134–145. doi: 10.1002/jts.22253 [DOI] [PubMed] [Google Scholar]
- Crone D, & Guy H (2008). 'I know it is only exercise, but to me it is something that keeps me going': a qualitative approach to understanding mental health service users' experiences of sports therapy. Int J Ment Health Nurs, 17(3), 197–207. doi: 10.1111/j.1447-0349.2008.00529.x [DOI] [PubMed] [Google Scholar]
- Davidson CL, Babson KA, Bonn-Miller MO, Souter T, & Vannoy S (2013). The impact of exercise on suicide risk: examining pathways through depression, PTSD, and sleep in an inpatient sample of veterans. Suicide Life Threat Behav, 43(3), 279–289. [DOI] [PubMed] [Google Scholar]
- Davison EH, Kaiser AP, Spiro A 3rd, Moye J, King LA, & King DW (2016). From Late-Onset Stress Symptomatology to Later-Adulthood Trauma Reengagement in Aging Combat Veterans: Taking a Broader View. Gerontologist, 56(1), 14–21. doi: 10.1093/geront/gnv097 [DOI] [PubMed] [Google Scholar]
- Dedert EA, Calhoun PS, Watkins LL, Sherwood A, & Beckham JC (2010). Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Annals of Behavioral Medicine, 39(1), 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durai UN, Chopra MP, Coakley E, Llorente MD, Kirchner JE, Cook JM, & Levkoff SE (2011). Exposure to trauma and posttraumatic stress disorder symptoms in older veterans attending primary care: comorbid conditions and self-rated health status. J Am Geriatr Soc, 59(6), 1087–1092. doi: 10.1111/j.1532-5415.2011.03407.x [DOI] [PubMed] [Google Scholar]
- Farr OM, Sloan DM, Keane TM, & Mantzoros CS (2014). Stress- and PTSD-associated obesity and metabolic dysfunction: a growing problem requiring further research and novel treatments. Metabolism, 63(12), 1463–1468. doi: 10.1016/j.metabol.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LA, Mehling WE, Metzler TJ, Cohen BE, Barnes DE, Choucroun GJ, … Neylan TC (2018). Veterans Group Exercise: A randomized pilot trial of an Integrative Exercise program for veterans with posttraumatic stress. J Affect Disord, 227, 345–352. doi: 10.1016/j.jad.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Hall KS, Hoerster KD, & Yancy WS Jr. (2015). Post-traumatic stress disorder, physical activity, and eating behaviors. Epidemiol Rev, 37, 103–115. doi: 10.1093/epirev/mxu011 [DOI] [PubMed] [Google Scholar]
- Hall KS, Morey MC, Beckham JC, Bosworth HB, Pebole MM, Pieper CF, & Sloane R (2018). The Warrior Wellness Study: A Randomized Controlled Exercise Trial for Older Veterans with PTSD. Transl J Am Coll Sports Med, 3(6), 43–51. doi: 10.1249/TJX.0000000000000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada ND, Wilkins SS, Schneider B, Elrod M, Hahn TJ, Kleinman L, … Dhanani S (2013). The influence of depression and PTSD on exercise adherence in older veterans. Military Behavioral Health, 1(2), 146–151. doi: 10.1080/21635781.2013.829400 [DOI] [Google Scholar]
- Hegberg NJ, Hayes JP, & Hayes SM (2019). Exercise Intervention in PTSD: A Narrative Review and Rationale for Implementation. Front Psychiatry, 10, 133. doi: 10.3389/fpsyt.2019.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imel ZE, Laska K, Jakupcak M, & Simpson TL (2013). Meta-analysis of dropout in treatments for posttraumatic stress disorder. J Consult Clin Psychol, 81(3), 394–404. doi: 10.1037/a0031474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, & Nelson CB (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry, 52(12), 1048–1060. [DOI] [PubMed] [Google Scholar]
- Koffel E, Khawaja IS, & Germain A (2016). Sleep Disturbances in Posttraumatic Stress Disorder: Updated Review and Implications for Treatment. Psychiatr Ann, 46(3), 173–176. doi: 10.3928/00485713-20160125-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, … Grady DA (1990). Trauma and the Vietnam war generation. New York: Brunner/Manzel. [Google Scholar]
- Monson CM, Gradus JL, Young-Xu Y, Schnurr PP, Price JL, & Schumm JA (2008). Change in posttraumatic stress disorder symptoms: do clinicians and patients agree? Psychol Assess, 20(2), 131–138. doi: 10.1037/1040-3590.20.2.131 [DOI] [PubMed] [Google Scholar]
- Najavits LM (2015). The problem of dropout from "gold standard" PTSD therapies. F1000Prime Rep, 7, 43. doi: 10.12703/P7-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebole MM, & Hall KS (2019). Physical activity promotion in women with PTSD: What we need for progress. Psychol Sport and Exerc, 41(March), 127–129. [PMC free article] [PubMed] [Google Scholar]
- Penedo FJ, & Dahn JR (2005). Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry, 18(2), 189–193. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E (1975). A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc, 23(10), 433–441. [DOI] [PubMed] [Google Scholar]
- Phelan E, Williams B, Meeker K, Bonn K, Frederick J, Logerfo J, & Snowden M (2010). A study of the diagnostic accuracy of the PHQ-9 in primary care elderly. BMC Fam Pract, 11, 63. doi: 10.1186/1471-2296-11-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee. (2018). 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Retrieved from Washington, D.C.: https://health.gov/paguidelines/second-edition/report/pdf/PAG_Advisory_Committee_Report.pdf [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, & Grant BF (2012a). Physical health conditions associated with posttraumatic stress disorder in U.S. older adults: Results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Am Geriatr Soc, 60, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, & Grant BF (2012b). Psychiatric comorbidity of full and partial posttraumatic stress disorder in the United States: Results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Am J Geriatr Psychiatry, 20(5), 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pless Kaiser A, Seligowski A, Spiro A III, & Chopra M (2016). Health status and treatment-seeking stigma in older adults with trauma and posttraumatic stress disorder. J Rehabil Res Dev, 53(3), 391–402. doi: 10.1682/JRRD.2015.03.0039 [DOI] [PubMed] [Google Scholar]
- Powers MB, Medina JL, Burns S, Kauffman BY, Monfils M, Asmundson GJ, … Smits JA (2015). Exercise Augmentation of Exposure Therapy for PTSD: Rationale and Pilot Efficacy Data. Cogn Behav Ther, 44(4), 314–327. doi: 10.1080/16506073.2015.1012740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt SI, Jerome GJ, Schneider KL, Craft LL, Buman MP, Stoutenberg M, … Goodrich DE (2016). Increasing US health plan coverage for exercise programming in community mental health settings for people with serious mental illness: a position statement from the Society of Behavior Medicine and the American College of Sports Medicine. Transl Behav Med, 6(3), 478–481. doi: 10.1007/s13142-016-0407-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins A, Bovin MJ, Smolenski DJ, Marx BP, Kimerling R, Jenkins-Guarnieri MA, … Tiet QQ (2016). The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): Development and Evaluation Within a Veteran Primary Care Sample. J Gen Intern Med, 31(10), 1206–1211. doi: 10.1007/s11606-016-3703-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PTSD: National Center for PTSD. Retrieved from https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
- Rosenbaum S, Sherrington C, & Tiedemann A (2015). Exercise augmentation compared with usual care for post-traumatic stress disorder: a randomized controlled trial. Acta Psychiatr Scand, 131(5), 350–359. doi: 10.1111/acps.12371 [DOI] [PubMed] [Google Scholar]
- Rosenbaum S, Vancampfort D, Steel Z, Newby J, Ward PB, & Stubbs B (2015). Physical activity in the treatment of Post-traumatic stress disorder: A systematic review and meta-analysis. Psychiatry Res, 230(2), 130–136. doi: 10.1016/j.psychres.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Schuch FB, Vancampfort D, Rosenbaum S, Richards J, Ward PB, Veronese N, …Stubbs B (2016). Exercise for depression in older adults: a meta-analysis of randomized controlled trials adjusting for publication bias. Brazilian Journal of Psychiatry, 38(3), 247–254. doi: 10.1590/1516-4446-2016-1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar G, Anderson EH, Suris AM, & North CS (2017). Exercise for PTSD in Women Veterans: A Proof-of-Concept Study. Mil Med, 182(11), e1809–e1814. doi: 10.7205/MILMED-D-16-00440 [DOI] [PubMed] [Google Scholar]
- Skinner HA (1982). The drug abuse screening test. Addict Behav, 7(4), 363–371. [DOI] [PubMed] [Google Scholar]
- Smith BN, Tyzik AL, Neylan TC, & Cohen BE (2015). PTSD and obesity in younger and older veterans: Results from the mind your heart study. Psychiatry Res, 229(3), 895–900. doi: 10.1016/j.psychres.2015.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundy A, Faulkner A, & Taylor A (2007). Exploring variability and perceptions of lifestyle physical activity among individuals with severe and enduring mental health problems; A qualitative study. J Ment Health, 16(4), 493–503. doi: 10.1080/09638230701482345 [DOI] [Google Scholar]
- Spira AP, Beaudreau SA, Stone KL, Kezirian EJ, Lui LY, Redline S, … Osteoporotic Fractures in Men, S. (2012). Reliability and validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older men. J Gerontol A Biol Sci Med Sci, 67(4), 433–439. doi: 10.1093/gerona/glr172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Management of Posttraumatic Stress Disorder Working Group. (2017). VA/DOD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder. Retrieved from https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
- van den Berk-Clark C, Secrest S, Walls J, Hallberg E, Lustman PJ, Schneider FD, & Scherrer JF (2018). Association between posttraumatic stress disorder and lack of exercise, poor diet, obesity, and co-occuring smoking: A systematic review and meta-analysis. Health Psychol, 37(5), 407–416. doi: 10.1037/hea0000593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE Jr., (2000). SF-36 health survey update. Spine (Phila Pa 1976), 25(24), 3130–3139. [DOI] [PubMed] [Google Scholar]
- Ware JE Jr., & Kosinski M (2001). SF-36 Physical and Mental Health Summary Scores: A Manual for Users of Version 1. (Second Edition ed.). Lincoln, RI: QualityMetric Incorporated. [Google Scholar]
- Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, … Marx BP (2018). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess, 30(3), 383–395. doi: 10.1037/pas0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherell JL, Petkus AJ, Thorp SR, Stein MB, Chavira DA, Campbell-Sills L, … Roy-Byrne P (2013). Age differences in treatment response to a collaborative care intervention for anxiety disorders. Br J Psychiatry, 203(1), 65–72. doi: 10.1192/bjp.bp.112.118547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth JW, & Ciccolo JT (2016). Exercise and Post-Traumatic Stress Disorder in Military Veterans: A Systematic Review. Mil Med, 181(9), 953–960. doi: 10.7205/MILMED-D-15-00488 [DOI] [PubMed] [Google Scholar]
- Whitworth JW, Craft LL, Dunsiger SI, & Ciccolo JT (2017). Direct and indirect effects of exercise on posttraumatic stress disorder symptoms: A longitudinal study. Gen Hosp Psychiatry, 49, 56–62. doi: 10.1016/j.genhosppsych.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Windle G, Hughes D, Linck P, Russell I, & Woods B (2010). Is exercise effective in promoting mental well-being in older age? A systematic review. Aging Ment Health, 14(6), 652–669. doi: 10.1080/13607861003713232 [DOI] [PubMed] [Google Scholar]
- Wortmann JH, Jordan AH, Weathers FW, Resick PA, Dondanville KA, Hall-Clark B, … Litz BT (2016). Psychometric analysis of the PTSD Checklist-5 (PCL-5) among treatment-seeking military service members. Psychol Assess, 28(11), 1392–1403. doi: 10.1037/pas0000260 [DOI] [PubMed] [Google Scholar]
- Yudko E, Lozhkina O, & Fouts A (2007). A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abuse Treat, 32(2), 189–198. doi: 10.1016/j.jsat.2006.08.002 [DOI] [PubMed] [Google Scholar]