Abstract
Background:
Temsirolimus has level 1 evidence for initial treatment of poor-risk patients with advanced renal cell carcinoma (mRCC), but its efficacy has not been directly compared with an antiangiogenic tyrosine kinase inhibitor (vascular endothelial growth factor receptor tyrosine kinase inhibitor [VEGFR TKi]) in this setting.
Objective:
To evaluate temsirolimus versus pazopanib as first-line therapy in patients with mRCC, predominant clear-cell features, and clinical characteristics of a poor prognosis.
Design, setting, and participants:
A randomized (1:1) phase II trial in 69 treatment-naïve mRCC patients and with three or more predictors of short survival for temsirolimus was conducted during 2012–2017 in a single academic cancer center. Crossover to the alternative treatment upon discontinuation of the first-line agent was permitted.
Intervention:
Mechanistic target of rapamycin inhibitor temsirolimus and VEGFR TKi pazopanib.
Outcome measurements and statistical analysis:
The primary endpoint was progression-free survival (PFS), and the secondary endpoints were overall survival (OS), objective response rate (ORR), safety, and patient-reported outcomes (PROs). Radiographic response was assessed by blinded radiologists. Efficacy outcomes were adjusted by prior nephrectomy status, prior interleukin-2 treatment, and the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) score.
Results and limitations:
Thirty-five patients received temsirolimus and 34 received pazopanib upfront; 72% overall had poor risk by IMDC. Median PFS in the first line was 2.7 mo with temsirolimus and 5.2 mo with pazopanib (adjusted hazard ratio [HR] 1.36, 95% confidence interval [CI] 0.84–2.22; p = 0.210). Median OS was 7.1 mo with temsirolimus and 11.9 mo with pazopanib (adjusted HR 1.16, 95% CI 0.70–1.93; p = 0.558), and ORRs were 5.9% and 21.2%, respectively (adjusted odds ratio 5.2, 95% CI 0.9–29.3; p = 0.062). PRO measures favored pazopanib. Five patients discontinued first-line therapy due to adverse events.
Conclusions:
Temsirolimus and pazopanib had modest activity in patients with poor-risk clear-cell mRCC, and therefore their use should be discouraged in this setting.
Patient summary:
We evaluated outcomes of advanced renal cell carcinoma patients presenting with aggressive features when treated with temsirolimus or pazopanib as first-line therapy. Survival was <1 yr for most, suggesting that more efficacious alternative treatments should be favored for these patients.
Keywords: Cytokines and angiogenic factors, First line, International Metastatic Renal Cell Carcinoma Database Consortium risk groups, Pazopanib, Poor-risk metastatic renal cell carcinoma, Temsirolimus
1. Introduction
The mechanistic target of rapamycin (mTOR) inhibitor temsirolimus is considered a category 1 recommendation for untreated patients with relapsed or advanced, unresectable renal cell carcinoma (mRCC) with predominant clear-cell histology and poor-risk characteristics [1–3]. This is based on a multicenter phase 3 trial (ARCC) comparing open-label temsirolimus, interferon-alpha, or both in patients who had at least three of six characteristics of an unfavorable prognosis [4]. This study demonstrated that patients who received temsirolimus alone had improved overall survival (OS) and progression-free survival (PFS) compared with patients who received interferon alone [4].
Although the combination of the immune checkpoint inhibitors nivolumab and ipilimumab or the multi–tyrosine kinase inhibitor (multi-TKi) cabozantinib has become the recommended first-line (1L) therapy in patients with mRCC and intermediate- or poor-risk disease, the vascular endothelial growth factor receptor (VEGFR) TKis sunitinib and pazopanib were considered appropriate 1L therapeutic options until recently. These two agents had demonstrated significant antitumor activity in phase 3 studies in patients with no prior treatment or who had failed cytokine therapy, thus becoming the standard of care, but the vast majority of the patients included had favorable- or intermediate-risk disease [5,6].
No molecular marker has yet been validated to predict a response in patients with mRCC who were treated with targeted therapies. One study reported circulating interleukin (IL)-6 levels (alone and as part of a 6-cytokine and angiogenic factor [CAF] signature) as predictive of PFS benefit in patients treated with pazopanib versus placebo [7]. Another study reported serum lactate dehydrogenase (LDH) as a predictor of survival in patients treated with temsirolimus versus interferon [8]. These results were derived retrospectively from phase III registration trials for both agents. More recently, an analysis of plasma samples from patients in the RECORD-3 phase II trial of sunitinib versus everolimus identified candidate biomarkers associated with a greater relative PFS benefit from the latter [9].
Here, we report results from the TemPa trial, the first head-to-head comparison of temsirolimus versus pazopanib as 1L therapy primarily in patients with mRCC and clinical characteristics of a poor prognosis. We also include analyses of patient-reported outcomes (PROs) and circulating biomarkers.
2. Patients and methods
2.1. Patients
Eligible patients were ≥18 yr of age and had pathologic confirmation of locally advanced or metastatic RCC with a clear-cell component. Measurable disease by the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, Karnofsky performance status (PS) ≥60%, and adequate organ and bone marrow function were also required. Poor-risk disease was defined, similar to the ARCC trial [4], by the presence of three or more of the following factors: Eastern Cooperative Oncology Group PS 2, anemia, elevated serum LDH, hypercalcemia, time from initial diagnosis to trial registration of <1 yr, and metastatic disease in more than one organ site. Patients having received any prior targeted therapy/chemotherapy were excluded, but those who had received prior immunotherapy (cytokines or vaccines) were eligible.
2.2. Trial design and treatments
TemPa was a randomized, open-label, phase II trial of temsirolimus versus pazopanib conducted at the University of Texas MD Anderson Cancer Center. Patients were randomized in a 1:1 ratio, and were stratified at the time of randomization by nephrectomy status and prior treatment with immunotherapy. Treatment was continued until disease progression (PD) or unacceptable toxicity. At that time, crossover to receive the alternative treatment was offered as an option.
Patients received temsirolimus 25 mg intravenously weekly or pazopanib 800 mg orally daily. Dose modifications were permitted for adverse events (AEs). Temsirolimus could be decreased to 20 mg weekly and then to 15 mg weekly. Pazopanib could be decreased to 600 mg daily and then to 400 mg daily.
2.3. Endpoints and assessments
The primary efficacy endpoint was PFS. Secondary endpoints were OS, objective response rate (ORR), and safety. The ORR was determined based on evaluation every 8 wk after randomization by computed tomography and/or magnetic resonance imaging, or every 16 wk for patients remaining progression free after 1 yr of treatment. Responses were determined based on RECIST v1.1, as assessed by radiologists blinded to the assigned treatment.
The Functional Assessment of Cancer Therapy—General (FACT-G); the Kidney Symptom Index, Disease-related Symptoms (FKSI-DRS); and the Center for Epidemiologic Studies— Depression (CES-D) questionnaires were used to assess PROs at baseline and on each evaluation on protocol.
AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0.
2.4. CAF analysis
Fourteen CAFs and four additional analytes (insulin, c-peptide, growth hormone, and C-reactive protein), previously identified as prognostic or predictive of benefit and relevant to angiogenesis and mTOR blockade, were evaluated in EDTA plasma collected before starting treatment with the SearchLight Protein Array, as described previously [7]. Supplementary Table 1 includes the biomarkers tested and their concentrations.
2.5. Statistical design and analyses
The maximum sample size to be accrued was determined to be 90 patients, 45 per treatment arm, based on the assumption that pazopanib would result in 60% improvement in the median PFS in the 1L setting, from 3.8 mo to 6.1 mo, with temsirolimus (one-sided log-rank test with type-1 error 0.10 and power 0.80). One interim analysis for futility was conducted after 42 total events. The Kaplan-Meier product-limit method was used to estimate the probabilities of PFS/OS. Multivariable Cox proportional hazard regression (PFS and OS) or logistic regression (ORR) models were fit using Stata/SE v15.1 (Stata Corp., College Station, TX, USA) while adjusting the effects of the following covariates: prior nephrectomy status, prior IL-2/vaccine treatment, and the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) score.
Treatment-by-subgroup interactions were not tested due to the small sample sizes. In addition, Cox proportional hazard regression analyses were performed to assess the association between CAF biomarkers as continuous variables and survival outcomes.
3. Results
3.1. Patients and disposition
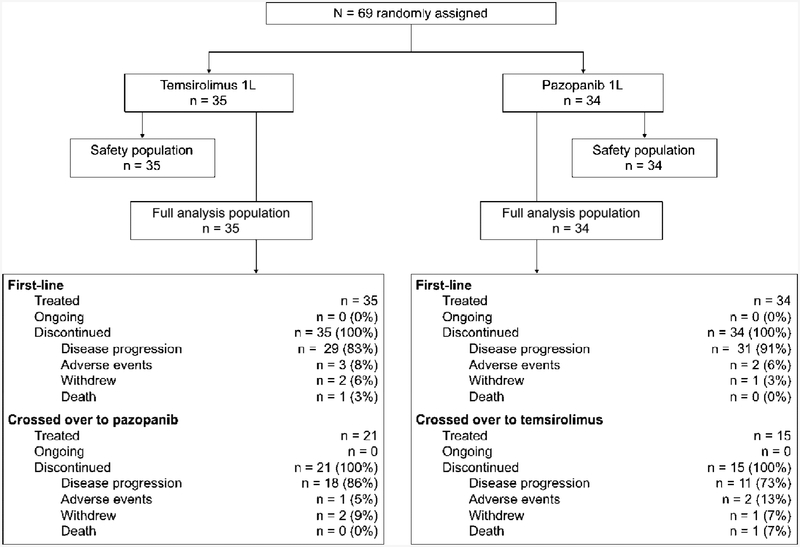
A total of 69 patients were enrolled from November 2012 to June 2017 (Fig. 1). The study was closed to new patient enrollment in September 2017 after the results of the CheckMate 214 and CABOSUN trials showed improved OS and ORR for ipilimumab with nivolumab, and improved PFS and ORR for cabozantinib, compared with sunitinib, in patients with intermediate- and poor-risk mRCC [10,11]. All consenting participants underwent randomization; 35 and 34 patients were, respectively, randomized to receive temsirolimus or pazopanib in the 1L setting. Patients’ characteristics were similar between treatment groups (Table 1). Only two patients (3%) had PS 0. By IMDC risk score [12], the majority of patients in both treatment groups had poor-risk disease (pazopanib 77% and temsirolimus 69%); none had a favorable risk. Patients’ characteristics were also similar between arms in the 2L setting (Supplementary Table 2). Thirty six patients (52%) continued 2L treatment on protocol: 21 patients crossed over from temsirolimus to pazopanib (60% of the initial 35) and 15 crossed over from pazopanib to temsirolimus (44% of the initial 34). The interim futility analysis was performed after 43 events had been observed, and the log-rank test p value was 0.016, thus allowing the trial to continue with patient enrollment.
Fig. 1–
CONSORT diagram. 1L = first line.
Table 1–
Baseline patient characteristics for first-line treatment
| Treatment arm | ||||
|---|---|---|---|---|
| Temsirolimus (n = 35) |
Pazopanib (n = 34) |
|||
| Characteristic | No. | % | No. | % |
| Age (yr) | ||||
| Median | 61 | 61 | ||
| Range | 42–80 | 37–74 | ||
| Gender | ||||
| Female | 11 | 31.4 | 6 | 17.7 |
| Male | 24 | 68.6 | 28 | 82.3 |
| Ethnicity | ||||
| White | 27 | 77.1 | 26 | 76.5 |
| Hispanic | 5 | 14.3 | 3 | 8.8 |
| Other | 3 | 8.6 | 4 | 11.7 |
| ECOG PS | ||||
| 0 | 1 | 2.9 | 1 | 2.9 |
| 1 | 14 | 40.0 | 12 | 35.3 |
| 2 | 20 | 57.1 | 21 | 61.8 |
| Previous nephrectomy |
15 | 42.9 | 15 | 44.1 |
| Previous IL-2 | 2 | 5.7 | 1 | 2.9 |
| IMDC risk | ||||
| Intermediate | 11 | 31.4 | 8 | 23.5 |
| Poor | 24 | 68.6 | 26 | 76.5 |
ECOG PS = Eastern Cooperative Group performance status; IL-2 = interleukin-2; IMDC = International Metastatic Renal Cell Carcinoma Database Consortium.
3.2. Efficacy
3.2.1. Primary endpoint
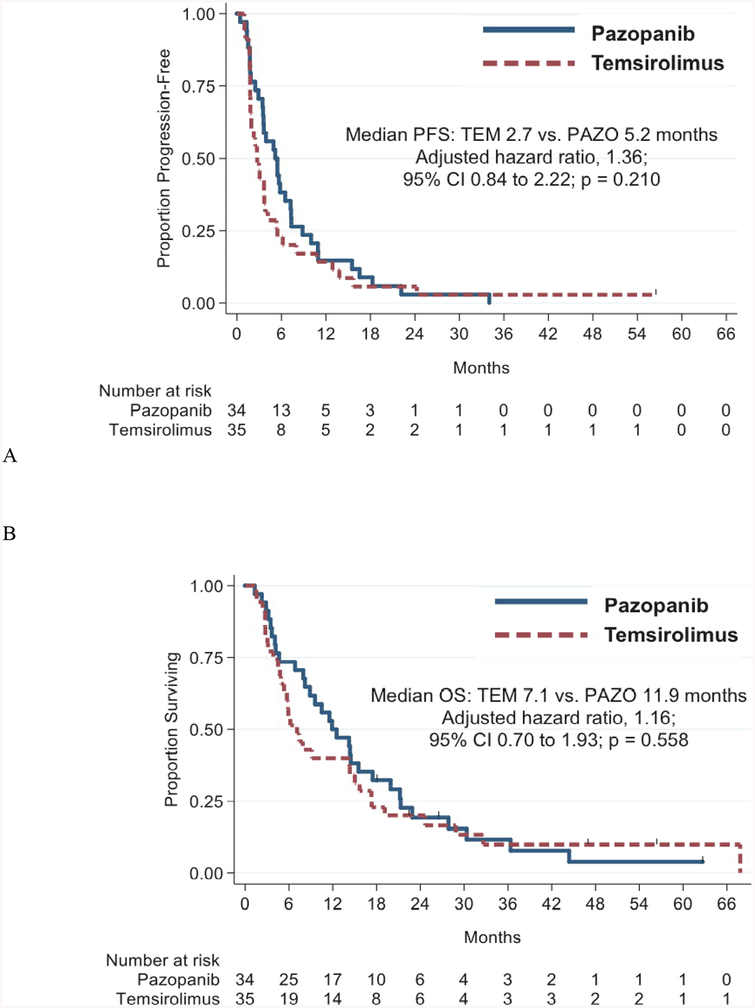
All 69 patients have discontinued 1L treatment or died. The primary reason for the discontinuation was PD, which occurred in 71% of patients. The median PFS using temsirolimus-1L versus pazopanib-1L was 2.7 versus 5.2 mo (adjusted hazard ratio [HR] 1.36, 95% confidence interval [CI] 0.84–2.22, p = 0.210; Fig. 2A).
Fig. 2–
(A) Progression-free survival and (B) overall survival for first-line temsirolimus and first-line pazopanib. Survival endpoints adjusted by prior nephrectomy status, prior IL-2 treatment, and the International Metastatic Renal Cell Carcinoma Database Consortium score. CI = confidence interval; IL-2 = interleukin-2; OS = overall survival; PAZO = pazopanib; PFS = progression-free survival; TEM = temsirolimus.
3.2.2. Secondary efficacy endpoints
Sixty-three patients (91.3%) have died (median follow-up 9.6 mo). The median OS for all patients was 9.6 mo (95% CI 6.2–14.3 mo), 7.1 mo in the temsirolimus group versus 11.9 mo in the pazopanib group (adjusted HR 1.16, 95% CI 0.70–1.93, p = 0.558; Fig. 2B).
Data on the best overall response were available for 67 patients (34 temsirolimus-1L and 33 pazopanib-1L). Of these patients, only two (5.9%) receiving temsirolimus-1L achieved a confirmed partial response (PR) to treatment, while seven (21.2%) receiving pazopanib-1L achieved a PR (adjusted odds ratio [OR] 5.2, 95% CI 0.9–29.3, p = 0.062).
Thirty-three of the 36 patients (92%) crossing over to the alternative treatment have died. Median PFS and OS, respectively, were 3.0 mo (95% CI 1.9–5.3) and 10.5 mo (95% CI 7.1–17.3) overall; 5.2 mo (95% CI 1.7–7.2) and 10.5 mo (95% CI 4.2–17.5) for the 15 patients treated with temsirolimus-2L; and 2.3 mo (95% CI 1.8–3.8) and 14.3 mo (95% CI 5.9–19.1) for the 21 patients treated with pazopanib-2L (adjusted p = 0.516 for PFS and adjusted p = 0.267 for OS). Three of the patients (20%) treated with temsirolimus-2L and two of those (10%) treated with pazopanib-2L did not reach the first evaluation of response on treatment. In those who did, the best overall response was stable disease, which was achieved in five patients on temsirolimus-2L (42%) and in nine patients on pazopanib-2L (47%; adjusted OR 1.01, 95% CI 0.21–4.87, p = 0.992). Ten patients did not cross over and were treated off protocol in the 2L setting with different agents: nivolumab (n = 4), cabozantinib (n = 3), pazopanib (n = 2), and everolimus (n = 1).
3.3. Safety
Common treatment-emergent AEs are listed in Table 2. Profiles were consistent with previously reported AEs with temsirolimus and pazopanib. No treatment-related grade 5 AEs occurred.
Table 2–
Treatment-emergent adverse events with ≥10% incidence during first-line therapy
| Adverse event | Event rate by treatment arm and grade (%) | |||||
|---|---|---|---|---|---|---|
| Pazopanib (n = 34) |
Temsirolimus (n = 35) |
|||||
| All grades |
Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | |
| Diarrhea | 71 | 3 | 0 | 17 | 0 | 0 |
| Fatigue | 65 | 21 | 0 | 46 | 17 | 0 |
| Nausea | 62 | 9 | 0 | 20 | 0 | 0 |
| Hypertension | 53 | 29 | 0 | 11 | 6 | 0 |
| Rash maculopapular | 53 | 3 | 0 | 29 | 3 | 0 |
| Hypothyroidism | 50 | 0 | 0 | 0 | 0 | 0 |
| Skin hypopigmentation | 47 | 0 | 0 | 0 | 0 | 0 |
| Dysgeusia | 44 | 0 | 0 | 20 | 0 | 0 |
| Vomiting | 44 | 6 | 0 | 6 | 0 | 0 |
| Anorexia | 38 | 0 | 0 | 14 | 3 | 0 |
| Aspartate aminotransferase increased |
35 | 9 | 0 | 6 | 0 | 0 |
| Proteinuria | 35 | 3 | 0 | 26 | 6 | 0 |
| Alanine aminotransferase increased |
32 | 12 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 32 | 0 | 0 | 63 | 6 | 3 |
| Mucositis oral | 32 | 0 | 0 | 43 | 6 | 0 |
| Alkaline phosphatase increased | 29 | 6 | 0 | 26 | 0 | 0 |
| Constipation | 26 | 3 | 0 | 17 | 0 | 0 |
| Pain | 26 | 3 | 0 | 9 | 3 | 0 |
| Weight loss | 26 | 0 | 0 | 9 | 0 | 0 |
| Anemia | 24 | 0 | 0 | 60 | 17 | 0 |
| Hyponatremia | 24 | 6 | 0 | 6 | 0 | 0 |
| Platelet count decreased | 24 | 3 | 0 | 9 | 0 | 0 |
| Creatinine increased | 21 | 0 | 3 | 31 | 0 | 0 |
| Hyperkalemia | 21 | 0 | 0 | 6 | 0 | 0 |
| Hypoalbuminemia | 21 | 0 | 0 | 9 | 0 | 0 |
| White blood cell decreased | 21 | 0 | 0 | 11 | 0 | 0 |
| Abdominal pain | 18 | 0 | 0 | 11 | 0 | 0 |
| Bilirubin increased | 18 | 3 | 0 | 0 | 0 | 0 |
| Lymphocyte count decreased | 18 | 3 | 0 | 9 | 0 | 0 |
| Alopecia | 15 | 0 | 0 | 6 | 0 | 0 |
| Dyspnea | 15 | 0 | 0 | 17 | 0 | 0 |
| Hoarseness | 15 | 0 | 0 | 0 | 0 | 0 |
| Hypomagnesemia | 15 | 0 | 0 | 0 | 0 | 0 |
| Palmar-plantar | ||||||
| erythrodysesthesia | 15 | 0 | 0 | 0 | 0 | 0 |
| syndrome | ||||||
| Paresthesia | 15 | 0 | 0 | 9 | 0 | 0 |
| Chest wall pain | 12 | 0 | 0 | 3 | 0 | 0 |
| Edema limbs | 12 | 0 | 0 | 26 | 3 | 0 |
| Hypertriglyceridemia | 9 | 0 | 0 | 57 | 3 | 0 |
| Hypokalemia | 3 | 0 | 0 | 14 | 0 | 0 |
| Cholesterol high | 0 | 0 | 0 | 31 | 0 | 0 |
| Dry skin | 0 | 0 | 0 | 14 | 0 | 0 |
Note: Incidence was ≥10% for at least one of the first-line agents.
3.4. Patient-reported outcomes
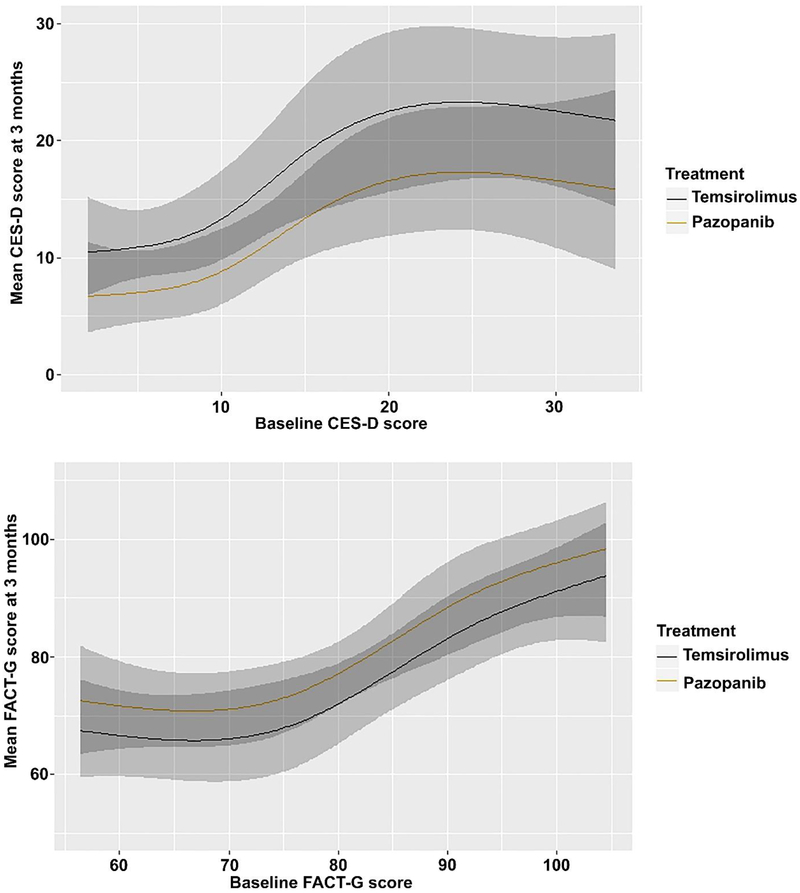
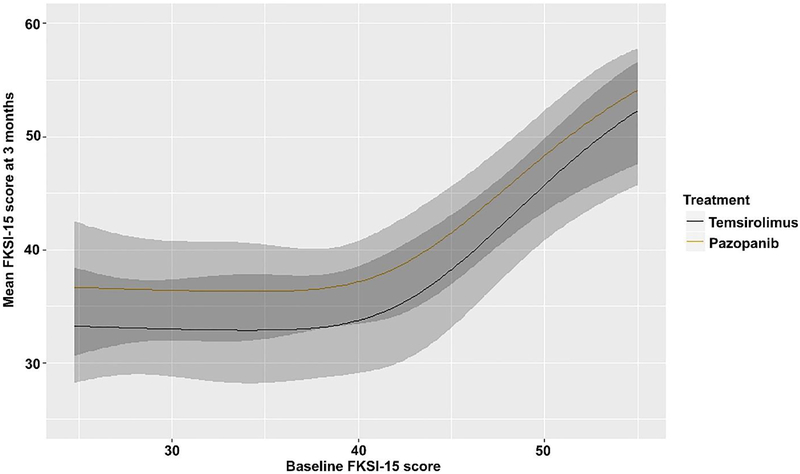
Higher FKSI-15, higher FACT-G, and lower CES-D scores are preferable. Only patients who had no missing data for CES-D (57/69), FACT-G (57/69), and FKSI-15 (58/69) were included. Pazopanib was associated (OR 0.31, 95% CI 0.11–0.89, p = 0.0296) with a lower adjusted mean CES-D score at 3 mo (9.6, 95% CI 6.8–13.1) compared with temsirolimus (14.3, 95% CI 10.6–18.6). Our analyses did not significantly contradict the supposition that pazopanib and temsirolimus yield the same FKSI-15 scores (adjusted mean FKSI-15 scores: 36.6, 95% CI 33.0–40.1 for pazopanib vs 33.1, 95% CI 28.7–37.8 for temsirolimus; OR 2.07, 95% CI 0.76–5.61, p = 0.1549) or FACT-G scores (adjusted mean FACT-G scores: 78.1, 95% CI 72.8–83.6 for pazopanib vs 73, 95% CI 66.4–79.1 for temsirolimus; OR 2.11, 95% CI 0.80–5.60, p = 0.1325) at 3 mo. Figure 3 shows a smooth association between baseline and mean PRO outcomes at 3 mo by treatment group.
Fig. 3–
Association between baseline and mean patient-reported outcomes at 3 mo by treatment group. The shaded areas represent the 95% confidence bands for each curve, with the darker shade representing the region in which the confidence intervals overlap. CES-D = Center for Epidemiologic Studies—Depression; FACT-G = Functional Assessment of Cancer Therapy— General; FKSI-15 = 15-item Kidney Symptom Index.
3.5. CAF analysis
Plasma samples collected before the initiation of treatment were available from 59 patients (31 temsirolimus-1L and 28 pazopanib-1L). By univariable analysis, we identified baseline/pretreatment osteopontin (OPN), tissue inhibitor of metalloproteinase-1 (TIMP-1), hepatocyte growth factor (HGF), IL-6, C-reactive protein, LDH, and vascular endothelial growth factor (VEGF) as prognostic for PFS, and TIMP-1, IL-6, HGF, VEGF, and C-reactive protein as prognostic for OS (Table 3; Supplementary Table 3 includes the additional biomarkers). For all, higher levels were associated with worse survival outcomes. No biomarker demonstrated distinct interaction effect with 1L treatment in fitted Cox models for PFS (Supplementary Table 4).
Table 3–
Univariable Cox proportional hazard model for circulating biomarkers in relation to survival outcomes
| Biomarker | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| VEGF | 1.38 | 1.01–1.90 | 0.045 | 1.57 | 1.08–2.28 | 0.018 |
| CRP | 1.24 | 1.02–1.51 | 0.031 | 1.27 | 1.03–1.56 | 0.023 |
| IL-6 | 1.43 | 1.11–1.83 | 0.006 | 1.39 | 1.08–1.79 | 0.011 |
| HGF | 1.88 | 1.27–2.79 | 0.002 | 1.74 | 1.13–2.67 | 0.012 |
| TIMP-1 | 3.93 | 2.12–7.28 | <0.001 | 3.43 | 1.88–6.27 | <0.001 |
| OPN | 1.57 | 1.23–2.01 | <0.001 | 1.28 | 0.98–1.66 | 0.069 |
| LDH | 2.44 | 1.08–5.53 | 0.032 | 1.77 | 0.77–4.08 | 0.177 |
CI = confidence interval; CRP = C-reactive protein; HGF = hepatocyte growth factor; HR = hazard ratio; IL-6 = interleukin-6; LDH = lactate dehydrogenase; OPN = osteopontin; OS = overall survival; PFS = progression-free survival; TIMP-1 = tissue inhibitor of metalloproteinase-1; VEGF = vascular endothelial growth factor.
4. Discussion
TemPa is the only clinical trial to directly compare temsirolimus, the treatment with the best available evidence of frontline efficacy in poor-risk mRCC until 2017, with a standard VEGFR TKi (pazopanib). Large studies of angiogenesis inhibitors in the 1L setting had mostly included patients with good- and intermediate-risk disease [5,6], but clinical experience suggested greater potential to induce antitumor responses and longer duration of disease control with these agents than with mTOR inhibitors. On this basis, we designed TemPa in 2011 as a randomized phase II trial under the proposition that pazopanib would improve PFS compared with temsirolimus.
Our results did not contradict the hypothesis that pazopanib and temsirolimus yield the same PFS and OS in mRCC patients with poor-risk disease. Pazopanib produced more objective responses than temsirolimus and showed consistently better PRO scores at 3 mo after treatment initiation, although the FACT-G and FKSI-15 comparisons did not reach statistical significance. Thus, if choosing between these two options, pazopanib should be favored over temsirolimus as frontline treatment in patients with poor-risk mRCC. However, the efficacy of both agents was modest, with median PFS and OS of 2.7 and 7.1 mo, respectively, for temsirolimus, and 5.2 and 11.9 mo, respectively, for pazopanib.
We chose to define the risk for eligibility based on the predictors of short survival used by Hudes et al [4] in the original ARCC trial, since these are the criteria used to treat poor-risk mRCC patients with frontline temsirolimus. In that trial, single-agent temsirolimus resulted in median PFS of 5.5 mo and median OS of 10.9 mo [4]. The VEGFR TKi–validated IMDC model [12], which instead of elevating LDH and metastasis to more than one organ site uses elevated neutrophil and platelet counts as risk factors, was not widely used at the time we designed our study, but we adjusted for it in our final analyses because it is an established predictor of outcomes. Fifty of our 69 participants (72%) had a poor IMDC risk with more than two factors, and almost one-third had four or more risk factors. The outcomes of the poor-IMDC-risk patients were abysmal, particularly of those treated with temsirolimus-1L (median PFS 1.9 mo and median OS 5.3 mo). These results are comparable with those reported in the contemporary phase II RECORD-3 trial of the other approved mTOR inhibitor everolimus versus sunitinib (VEGFR TKi) frontline, in which median PFS for poor-risk patients (as per the Memorial Sloan Kettering Cancer Center model) was 2.6 mo for everolimus versus 3 mo for sunitinib [13]. In addition, similar to that study, only 52% of our patients (vs 50–54% in RECORD-3) [13] crossed over to the alternative treatment, more frequently from temsirolimus-1L to pazopanib-2L than to the opposite sequence. The relatively long median PFS of 5.2 mo found in patients treated with temsirolimus-2L was driven by a few comparatively durable stable disease cases. In the INTORSECT randomized phase 3 trial in patients with clear-cell mRCC previously treated with 1L sunitinib, temsirolimus was associated with inferior OS compared with sorafenib [14].
The observed AEs were consistent with the known safety profiles of temsirolimus and pazopanib, including those most characteristic of these agents [4,6]. The rate of noninfectious pneumonitis with temsirolimus was similar to that previously reported, at 11% overall (grade 1 in three patients and grade 2 in one patient, all 1L). However, rates of grade 3 hypertension and hepatotoxicity associated with pazopanib were slightly higher than expected in 1L and 2L settings: respectively, 32% and 24% for hypertension, and 15% and 14% for hepatotoxicity.
Regarding circulating biomarkers, here we confirm in an independent and higher-risk dataset our previous results demonstrating robust prognostic significance for OPN, TIMP-1, IL-6, HGF, and VEGF concentrations in clear-cell mRCC [15]. We did not, however, identify any obvious value for either IL-6 or LDH to predict a differential benefit from a VEGFR TKi in comparison with an mTOR inhibitor [7,8]. Larger sample sets will be needed for more definitive evidence.
The present study is limited by the small sample size, as we stopped accrual before reaching our target number of patients after the results of the CheckMate 214 phase III and CABOSUN phase II trials became available. CheckMate 214 revealed significant improvement in OS and ORR for mRCC patients with intermediate and poor risk treated in the 1L setting with the combination of the immune checkpoint inhibitors nivolumab and ipilimumab compared with sunitinib (37% lower risk of death; PFS 11.6 vs 8.4 mo; ORR = 42% vs 27%, including 9% complete responses), and this combination became the standard of care treatment for these patients [2,10]. In CABOSUN, cabozantinib yielded longer PFS and a higher ORR compared with sunitinib, a TKi with similar activity to pazopanib in mRCC [16], as 1L therapy in intermediate- or poor-risk mRCC patients [11].
5. Conclusions
Our study provides direct evidence of the shortcomings of the mTOR inhibitor temsirolimus and pazopanib as single agents for mRCC patients with a poor prognosis. Unless molecular biomarkers are conclusively shown to guide treatment selection in specific patient subsets [17], the use of these agents as frontline therapy should be discouraged in mRCC patients with poor-risk disease.
Supplementary Material
Acknowledgments:
We are grateful to our patients and their families. We also thank the investigators, nurses, statistical team, and research staff for their dedication to this project.
Funding/Support and role of the sponsor: The TemPa trial was supported by Novartis Pharmaceuticals and in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Obtaining funding: Tannir.
Administrative, technical, or material support: Pruitt.
Supervision: Tannir, Zurita.
Other: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: Nizar M. Tannir certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Tannir has received grant funding and personal fees for consultancy from Pfizer and Novartis. Karam has received personal fees for consultancy from Pfizer. Wood has received grant funding from Pfizer. Zurita has received grant funding and personal fees for consultancy from Pfizer, and grant funding from Novartis.
References
- [1].Atkins MB, Tannir NM. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev 2018;70:127–37. [DOI] [PubMed] [Google Scholar]
- [2].NCCN. Clinical practice guidelines in oncology. Kidney cancer (version 3.2019). [DOI] [PubMed] [Google Scholar]
- [3].Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. In press. 10.1093/annonc/mdz056 [DOI] [PubMed] [Google Scholar]
- [4].Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–81. [DOI] [PubMed] [Google Scholar]
- [5].Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24. [DOI] [PubMed] [Google Scholar]
- [6].Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061–8. [DOI] [PubMed] [Google Scholar]
- [7].Tran HT, Liu Y, Zurita AJ, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol 2012;13:827–37. [DOI] [PubMed] [Google Scholar]
- [8].Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol 2012;30:3402–7. [DOI] [PubMed] [Google Scholar]
- [9].Voss MH, Chen D, Marker M, et al. Circulating biomarkers and outcome from a randomised phase II trial of sunitinib vs everolimus for patients with metastatic renal cell carcinoma. Br J Cancer 2016;114:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Choueiri TK, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. Eur J Cancer 2018;94:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 2013;14:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Motzer RJ, Barrios CH, Kim TM, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol 2014;32:2765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hutson TE, Escudier B, Esteban E, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 2014;32:760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zurita AJ, Gagnon RC, Liu Y, et al. Integrating cytokines and angiogenic factors and tumour bulk with selected clinical criteria improves determination of prognosis in advanced renal cell carcinoma. Br J Cancer 2017;117:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722–31. [DOI] [PubMed] [Google Scholar]
- [17].Voss MH, Chen D, Reising A, et al. PTEN expression, not mutation status in TSC1, TSC2, or mTOR, correlates with the outcome on everolimus in patients with renal cell carcinoma treated on the randomized RECORD-3 trial. Clin Cancer Res 2019;25:506–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.