Abstract
Hypertriglyceridaemia is a common clinical problem. Epidemiologic and genetic studies have established that triglyceride-rich lipoproteins (TRL) and their remnants as important contributors to ASCVD while severe hypertriglyceridaemia raises risk of pancreatitis. While low-density lipoprotein is the primary treatment target for lipid lowering therapy, secondary targets that reflect the contribution of TRL such as apoB and non-HDL-C are recommended in the current guidelines. Reduction of severely elevated triglycerides is important to avert or reduce the risk of pancreatitis. Here we discuss interventions for hypertriglyceridaemia, including diet and lifestyle, established treatments such as fibrates and omega-3 fatty acid preparations and emerging therapies, including various biological agents.
Keywords: Triglycerides, Review, Lipoproteins, Treatment, Hypertriglyceridaemia
Recent epidemiologic and genetic studies establish triglyceride (TG)-rich lipoproteins (TRL) and their remnants as important contributors to atherosclerotic cardiovascular disease (ASCVD). In addition, hypertriglyceridaemia (HTG) is a frequent cause of pancreatitis. This review article summarizes the current understanding of the TG metabolism from a clinical perspective, the current data on TRL for risk stratification and as treatment targets and discusses established and emerging treatment strategies.
Epidemiology
Hypertriglyceridaemia is a very common problem in clinical practice. Its prevalence is ∼10% in the adult population with considerable interregional variation.1–5 The prevalence of mild-to-moderate HTG parallels that of obesity and Type 2 diabetes; its increase is, therefore, unsurprising over the last few decades.5 Severe HTG, defined as plasma TG concentration >10 mmol/L (>885 mg/dL) is less common, with prevalence ranging from 0.10 to 0.20%, while very severe HTG, defined at TG >20 mmol/L (>1770 mg/dL) is rarer still (prevalence 0.014%).1,6 An upper value for ‘normal’ fasting TG of 1.7 mmol/L (150 mg/dL) is sometimes defined; when considering non-fasting TG concentration, determining HTG prevalence is more difficult since there is no accepted cut-point. However, in normolipidaemic subjects, post-prandial TG values rarely exceed 4.6 mmol/L (400 mg/dL) even post-fat challenge.7
Primary vs. secondary hypertriglyceridaemia
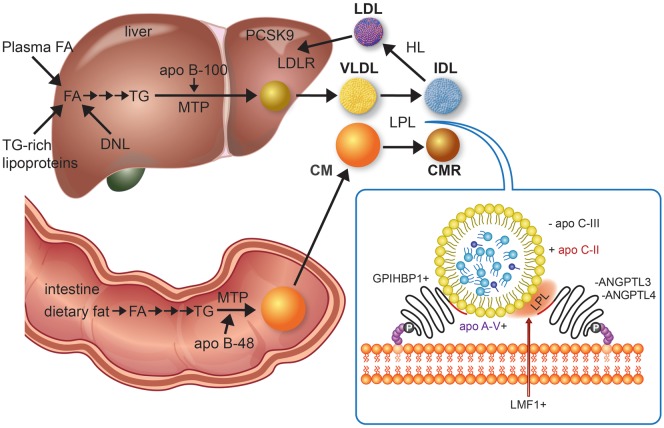
Quantifying the abnormal lipoprotein species in patients with elevated TG, as seen in the Fredrickson or WHO classification of hyperlipidaemias, is technically complicated. For this reason, the general term ‘HTG’ is usually sufficient for clinical purposes. The causes of primary HTG are listed in Table 1. Primary severe HTG has both monogenic and polygenic determinants. A tiny subset of these patients (perhaps 2%) has monogenic chylomicronaemia or familial chylomicronaemia syndrome (FCS, former Type 1), a rare form of monogenic HTG with estimated prevalence of 1–10 in a million. Definitive diagnosis of this autosomal recessive disorder requires molecular detection of rare, biallelic (homozygous or compound heterozygous) variants in one of five genes: LPL (accounting for 80% of cases), APOC2, APOA5, LMF1, and GPIHBP1. The roles of these gene products in lipolysis are shown in Figure 1. Genetic assessment has superseded biochemical assays of plasma lipolytic activity as the gold standard for diagnosis.
Table 1.
Primary causes of hypertriglyceridaemia
| A. Severe HTG (TG >10 mmol/L) | |
| Monogenic chylomicronaemia (formerly HLP Type 1 or familial chylomicronaemia syndrome) | |
| Lipoprotein lipase deficiency (Bi-allelic LPL gene mutations) | |
| Apo C-II deficiency (Bi-allelic APOC2 gene mutations) | |
| Apo A-V deficiency (Bi-allelic APOA5 gene mutations) | |
| Lipase maturation factor 1 deficiency (Bi-allelic LMF1 gene mutations) | |
| GPIHBP1 deficiency (Bi-allelic GPIHBP1 gene mutations) | |
| Multifactorial or polygenic chylomicronaemia (formerly HLP Type 5 or mixed hyperlipidaemia) | |
| Complex genetic susceptibility, including | |
| Heterozygous rare large-effect gene variants for monogenic chylomicronaemia (see above); and/or | |
| Accumulated common small-effect TG-raising polymorphisms (e.g. numerous GWAS loci including APOA1-C3-A4-A5; TRIB1, LPL, MLXIPL, GCKR, FADS1-2-3, NCAN, APOB, PLTP, ANGPTL3) | |
| Other | |
| Transient infantile HTG (glycerol-3-phosphate dehydrogenase 1 deficiency) from bi-allelic GPD1 gene mutations | |
| B. Mild-to-moderate HTG (TG 2.0–9.9 mmol/L) | |
| Multifactorial or polygenic HTG (formerly HLP Type 4 or familial HTG) | |
| Complex genetic susceptibility (see above) | |
| Dysbetalipoproteinaemia (formerly HLP Type 3 or dysbetalipoproteinaemia) | |
| Complex genetic susceptibility (see above), plus | |
| APOE E2/E2 homozygosity or | |
| APOE dominant rare variant heterozygosity | |
| Combined hyperlipoproteinaemia (formerly HLP Type 2B or familial combined hyperlipidaemia) | |
| Complex genetic susceptibility (see above), plus | |
| Accumulation of common small effect LDL-C-raising polymorphisms | |
Figure 1.
Overview of triglyceride-rich lipoprotein metabolism focused on disease genes and drug targets. Triglyceride-rich lipoprotein assembly begins with triglyceride synthesis, which derive from fatty acids in the intestine from the diet or in the liver taken up from plasma, fatty acids released from lysosomes after breakdown of endocytosed triglyceride-rich lipoproteins, and fatty acids generated from glucose by de novo lipogenesis. A series of enzymes, culminating in tissue-specific isoforms of diacylglycerol acyltransferase in intestine and liver, produce triglyceride. Microsomal triglyceride transfer protein unites triglyceride, cholesterol, and phospholipids, with tissue-specific isoforms of apolipoprotein (apo) B, i.e. small B-48, shortened as a result of RNA editing in enterocytes and full-length B-100 in hepatocytes, forming chylomicrons and very-low-density lipoprotein, respectively. Chylomicrons formation also requires Sar1 homolog B GTPase (SAR1B gene product, not shown). Chylomicrons enter plasma indirectly through lymphatics while very-low-density lipoprotein is secreted directly into the circulation. Hydrolysis of circulating chylomicrons and very-low-density lipoprotein by lipoprotein lipase releases free fatty acids and produce chylomicron remnant clearance and intermediate-density lipoprotein particles, respectively. Chylomicrons remnant clearance by the liver (not shown) requires apo E, as apo B-48 does not have the low-density lipoprotein receptor binding domain. Intermediate-density lipoprotein can also be removed by the liver (not shown) with apo B-100 and apo E both acting as ligands for the low-density lipoprotein receptor. Intermediate-density lipoprotein can be further lipolyzed by lipoprotein lipase and also remodelled by hepatic lipase to generate low-density lipoprotein, which is cleared by the low-density lipoprotein receptor, whose activity is reduced by proprotein convertase subtilisin kexin 9. The inset depicts lipoprotein lipase activity on a triglyceride-rich lipoprotein particle as well as several interacting proteins at the endothelial surface that affect lipoprotein lipase activity. A plus sign indicates enhancement or stimulation of lipolysis, whereas a minus sign indicates inhibition. Lipase maturation factor 1 (LMF1) is a chaperone protein that ensures that lipoprotein lipase attains functionality and is properly secreted from adipose cells or myocytes. Glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein-1 (GPIHBP1) is necessary for transcytosis of lipoprotein lipase across the endothelium of capillaries in adipose and muscle tissues as well as tethering lipoprotein lipase to the endothelium, thereby stabilizing it. Apo C-II activates lipoprotein lipase, while apo A-V is a stabilizing cofactor. Lipolysis is reduced by apo C-III, which is a component of triglyceride-rich lipoproteins, and by angiopoietin-like proteins 3 and 4 (ANGPTL3 and ANGPTL4), which both operate near the endothelium. Volanesorsen and AKCEA-APOCIII-LRx reduce triglyceride by targeting apo C-III, while evinacumab and IONIS-ANGPTL3-LRx lower triglyceride by targeting ANGPTL3. Peroxisome proliferator-activated receptors (not shown), particularly alpha and delta types, form a regulatory network that influences several of the above target molecules. Adapted from Ref.13
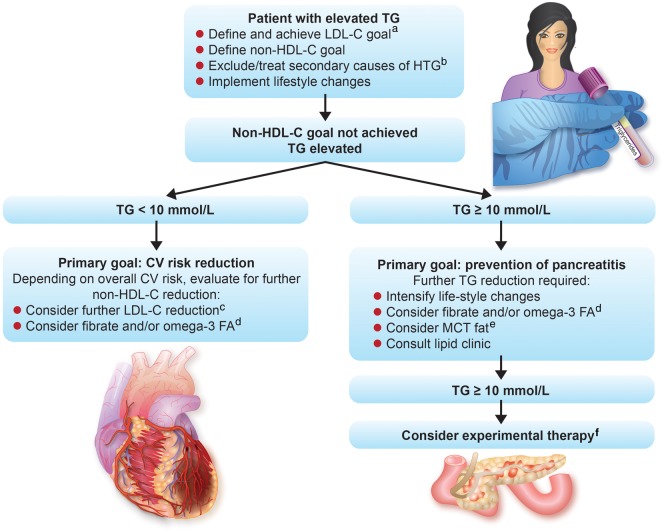
Take home figure.
Treatment algorithm for patients with elevated fasting triglycerides. aLow-density lipoprotein cholesterol goal depends on absolute cardiovascular risk. bPotential secondary causes for hypertriglyceridaemia are listed in Table 2. cFurther low-density lipoprotein cholesterol reduction will also help in achieving non-high-density lipoprotein cholesterol goals. dOmega-3-FA, omega-3 fatty acids. eMCT, fats containing medium-chain fatty acids. fExperimental therapies are listed in Table 6.
Most remaining cases of severe HTG are strongly polygenic in nature,1 which includes contributions from rare heterozygous variants in the above five canonical FCS genes and/or accumulated common variants associated with elevated TG levels identified in genome-wide association studies. Sometimes referred to as multifactorial HTG (former Type 5), certain secondary factors (Table 2) can further force the expression of severely elevated TG levels.
Table 2.
Secondary causes of hypertriglyceridaemia
| Diet with high positive energy-intake balance and high fat or high glycaemic index |
| Increased alcohol consumption (HTG risk increases with > 2 and > 1 drink(s) per day in men and women, respectively) |
| Obesity |
| Metabolic syndrome |
| Insulin resistance |
| Diabetes mellitus (predominantly Type 2) |
| Hypothyroidism |
| Renal disease (proteinuria, uraemia, or glomerulonephritis) |
| Pregnancy (particularly in the third trimester) |
| Paraproteinaemia |
| Systemic lupus erythematosis |
| Medications, including corticosteroids, oral oestrogen, tamoxifen, thiazides, non-cardioselective beta-blockers and bile acid sequestrants, cyclophosphamide, L-asparaginase, protease inhibitors, and second-generation antipsychotic agents (such as clozapine and olanzapine) |
Mild-to-moderate HTG is similarly highly polygenic in nature (Table 1). Simple HTG (former Type 4) resembles severe HTG but with a lower burden of genetic determinants. Dysbetalipoproteinaemia (former Type 3) also has a polygenic foundation, but with the additional genetic ‘hit’ of homozygosity for the binding defective apo E2/E2 isoform or a rare binding defective dominant mutation in APOE. Combined hyperlipidaemia (former Type 2B) similarly has polygenic susceptibility to HTG, but some patients, in addition, have polygenic susceptibility to elevated low-density lipoprotein (LDL) cholesterol (C). Finally, there are numerous secondary factors of HTG (Table 2), which may in some patients be sufficient to cause expression of HTG, but frequently interact with polygenic susceptibility: the resulting phenotype is related to the cumulative burden of underlying genetic risk plus intensity of the secondary factor.
Genetic hypotriglyceridaemia
Some rare heterozygous loss-of-function (LOF) variants are associated with reduced TG levels. These patients show protection from ASCVD providing important evidence for a causal role of TG for ASCVD. For instance, rare heterozygous LOF variants in APOC3 encoding apo C-III are associated with ∼50% reduced TG levels and significantly increased clearance of very-low-density lipoprotein (VLDL).8,9 Furthermore, individuals who are homozygous for APOC3 LOF variants have very-low-fasting TG levels, with no rise after a fat load.9 Such LOF variants of APOC3 are also associated with reduced ASCVD risk.10 Similarly, heterozygous LOF variants in ANGPTL311 and ANGPTL412 genes are associated with reductions both in plasma TG and ASCVD risk. These hypotriglyceridaemic states contrast with mild HTG and increased ASCVD risk seen with heterozygous LOF mutations in APOA5.11
Normal triglyceride-rich lipoprotein metabolism
Triglyceride-rich lipoproteins—i.e. chylomicrons and VLDL—are spherical complexes comprised of core lipids [TG and cholesterol esters (CE)] with surface apolipoproteins, phospholipids (PL), and free cholesterol (FC). Exogenous (dietary) TG is transported in intestinally derived chylomicrons, while TG of endogenous origin circulates in hepatically derived VLDL. An overview of the normal production and catabolism of TRL is shown in Figure 1.13
Triglyceride-rich lipoproteins metabolism in disease states
At any level of chylomicron and VLDL secretion, the efficiency of both lipoprotein lipase (LPL)-mediated lipolysis and hepatic uptake of remnant particles will determine the circulating levels of fasting and postprandial TG. Lipoprotein lipase-mediated lipolysis of newly secreted TRLs is a saturable process, and as secretion rates and plasma TG levels rise, lipolysis falls.14 In general, increased VLDL production is the commonest initiating factor for HTG. For any rate of TRL secretion, the inherited capacity of the LPL pathway will also modulate the steady-state level.
Many individuals with HTG also have insulin resistance, obesity, and Type 2 diabetes mellitus, encompassed by the term ‘metabolic syndrome’. This milieu drives increased VLDL secretion, which is particularly enhanced when FA and insulin are in excess.15 Insulin resistance with increased circulating FAs and decreased insulin signalling can also lead to increased chylomicron secretion.15 Furthermore, hyperglycaemia stimulates, while glucagon-like peptide 1 inhibits chylomicron secretion. Also, apo C-III inhibits removal of remnants; thus in states of increased VLDL secretion, when apo C-III is elevated, uptake of remnant particles will be reduced, compounding the dyslipidaemia.16
Triglyceride-rich lipoproteins and risk of atherosclerotic cardiovascular disease
Serum TRL are consistently associated with ASCVD risk, probably independent of other metabolic disturbances.17 For example, in large cohort studies from Copenhagen, non-fasting TG of 6.6 vs. 0.8 mmol/L was associated with a five-, three-, and two-fold increased adjusted risk for myocardial infarction, ischaemic stroke, and all-cause mortality, respectively.18,19 The Copenhagen General Population Study showed that TG adds important information for primary ASCVD prevention. Individuals aged 40–65 years and free of ASCVD and diabetes with TG >3.0 mmol/L (264 mg/dL) showed a similar risk of ASCVD compared to statin eligible individuals (defined according to the 2016 ESC/EAS guidelines) These data suggest an important opportunity for clinical studies in this population because 80% of ASCVD events occur in individuals who are not eligible for prophylactic statin therapy according to the 2016 ESC/EAS guidelines.20 Triglyceride levels also independently predict long- and short-term ASCVD risk in patients post an acute coronary syndrome, who are treated with a statin and therefore represent a potential target in secondary prevention.21
A causal relationship between TRL and ASCVD is supported by Mendelian randomization studies and in conditions as dysbetalipoproteinaemia, which is typically seen in some patients homozygous for the E2 allele (<1% of Europeans)18,21,22; lipoproteins with this form of apo E bind defectively cell surface receptors. However, the extent of the association of serum TG with ASCVD differs between studies and was sometimes lost in multivariate analyses.22 Indeed, not all TG particles are atherogenic. Large TRL particles such as nascent chylomicrons are unable to penetrate the vessel wall.23 Although circulating levels of TRLs predict increased ASCVD risk, it is less clear whether TG themselves contribute to atherogenesis. In contrast to cholesterol that accumulates in intimal foam cells and atherosclerotic plaques, TG is degraded by most cells. However, CE-enriched smaller TRLs (‘remnants’) promote atherogenesis via infiltration into the vessel wall and pro-inflammatory and pro-thrombotic pathways. In addition, elevated TG is frequently associated with pathological high-density lipoprotein (HDL) particles that may contribute to ASCVD risk.2
The correlation of plasma TG with ASCVD risk in epidemiological studies is attenuated or lost after adjusting for non-high-density lipoprotein cholesterol (non-HDL-C) or apo B. Most circulating TG is carried by VLDL particles and their remnants, which contain apo B. All TRL particles, as well as LDL particles, contain a single apo B molecule. An estimate for all apo B-containing lipoproteins is non-HDL-C (calculated as TC − HDL-C). A recent Mendelian randomization study found that all apoB-containing lipoproteins have a similar effect on ASCVD risk.24 Atherosclerotic cardiovascular disease risk mediated by TRLs appears to be determined by the circulating concentration of apoB-containing particles rather than their TG content and that the clinical benefit of lowering TG correlates with the reduction in apoB, rather than the change in plasma TG concentration.
Triglyceride and risk of pancreatitis
Severe HTG may lead to acute pancreatitis and HTG is believed to be causative in 1–10% of episodes of acute pancreatitis.25,26 It is, however, unclear whether all TRLs carry the same risk or whether chylomicrons and chylomicron remnants carry a greater risk than VLDL and VLDL-remnants. From a clinical perspective, this may not be very relevant, since a mixture of TRLs is present in most cases of severe HTG.
Mild-to-moderate HTG is associated with low-grade inflammation and higher risk of acute pancreatitis. While for many years it was thought that only severe HTG is associated with acute pancreatitis, newer data indicate that pancreatitis risk increases at moderately elevated TG concentrations in a dose-dependent manner.27 Thus, the risk is elevated [hazard ratio (HR) 2.4] at TG levels between 2.0 and 3.0 mmol/L (177–265 mg/dL) but is still very low in absolute terms (5.5 events per 10 000 patient-years). Even so, the majority of subjects with TG >10 mmol/L (885 mg/dL) will never experience pancreatitis. Cohort studies indicate that acute pancreatitis may occur in 3% of those with TG concentrations between 10 and 20 mmol/L (885–1770 mg/dL) and 15% of those with levels >20 mmol/L (1770 mg/dL), although higher rates were reported in some selected groups. For example, in a German cohort, 19% of those with TG >1000 mg/dL (>11.3 mmol/L) had pancreatitis.26,28 However, pancreatitis risk is highest in rare patients with monogenic chylomicronaemia, in which chylomicrons are indeed the predominant species, with low to undetectable levels of remnants and downstream lipoproteins due to severely impaired lipolytic machinery.6,14,29,30
While TG >10 mmol/L (885 mg/dL) may be sufficient to precipitate acute pancreatitis without additional factors, in many patients, especially those experiencing pancreatitis at relatively low TG levels, additional factors such as alcohol consumption, gallstone disease or certain medications are present. These factors either contribute to HTG or may directly cause acute pancreatitis. The mechanism behind HTG-associated pancreatitis is incompletely understood. Changes in the micro-environment especially changes in local pH due to very high concentrations of free fatty acids have long been postulated as the main factor.31 Genetic factors such as polymorphisms in the cystic fibrosis transmembrane conductance regulator or the presence of the APOE E4 allele may also play a role.32,33 A recent analysis from Denmark in >115 000 individuals indicates that the inflammation associated with HTG may contribute to the development of acute pancreatitis.34
Diagnosis of hypertriglyceridaemia
One reason for variability and heterogeneity of TG measurements is the post-prandial regulation of TG-rich lipoproteins.35 Atherosclerosis has been suggested to be a ‘post-prandial disease’, with non-fasting TG contributing to atherogenesis.36,37 However, an oral fat tolerance test to assess post-prandial TG kinetics did not improve risk prediction in patients with coronary artery disease compared with fasting TG; indeed post-prandial TG increase is highly correlated with fasting TG concentrations.37 Thus, a fat tolerance test provides no additional clinical information.
In general, non-HDL-C and apoB concentrations are highly correlated with each other and somewhat less with LDL-C. However, in individuals with HTG, the calculated or directly measured LDL-C level may underestimate ASCVD risk. For general risk screening, non-fasting blood samples have similar prognostic value as fasting and are recommended in order to improve patient compliance and because of practical reasons. However, non-fasting samples contain higher TG levels. When assessing patients with elevated TG, e.g. in metabolic syndrome or diabetes, calculated LDL-C should be interpreted with caution; fasting samples remain the method of choice. In a joint statement, the European Atherosclerosis Society and the European Federation of Clinical Chemistry and Laboratory Medicine recommend repeating a fasting sample when non-fasting TG >5 mmol/L (440 mg/dL).38 For non-fasting samples, TG ≥2 mmol/L (175 mg/dL) should be flagged as abnormal, for fasting samples, abnormal concentrations correspond to TG ≥1.7 mmol/L (150 mg/dL).38
Therapy—lifestyle
The most important principle for treating individuals with HTG is to manage lifestyle factors associated with elevated TG.39,40 These include obesity and metabolic syndrome resulting from physical inactivity and diets with high positive energy-intake balance due to high-fat or high-glycaemic index and, importantly, alcohol consumption. Medications that raise TG include corticosteroids, thiazides, non-selective beta-blockers, oestrogen, tamoxifen, bile acid sequestrants, cyclophosphamide, antiretroviral drugs, and second-generation antipsychotic agents. Additional contributors to secondary HTG are renal disease (uraemia or glomerulonephritis), pregnancy (particularly in the third trimester), paraproteinaemia, and systemic lupus erythematosus.40
The first practical step of lifestyle modification relates to alcohol consumption, which should be avoided in any form and quantity by individuals with high TG. Also, because of many additional beneficial metabolic and health effects, an increase of physical activity is another cornerstone of lifestyle recommendations. With regard to diet, the most important principle is to reduce net caloric intake. With regard to specific dietary recommendations, refined carbohydrate-rich foods as well as sucrose and fructose increase TG much more compared to fibre-rich, low glycaemic index foods.41 As much as possible, saturated fat should be replaced by mono- or polyunsaturated fats that improve insulin sensitivity. Trans-fatty acids should be avoided. Diets rich in saturated fatty acids (tropical oils, fatty or processed meat, sweets, cream, and butter) should be replaced (e.g. with vegetables, wholegrain foods, and fish) and with monounsaturated fat (extra virgin olive oil) and polyunsaturated fat (non-tropical vegetable oils). However, randomized control trial evidence for these specific dietary recommendations is lacking.39 The approximate effect size of lifestyle changes on serum TG is depicted in Table 3.
Table 3.
Approximate effect size of lifestyle changes on serum triglycerides
| Intervention | Lowering of triglycerides | PMID |
|---|---|---|
| Alcohol abstinence | Variable response | 6736783 |
| Up to 80% in subjects with high TG and excess intake | 4359737 | |
| Weight loss | Approximately 8 mg/dL (0.1 mM/L) per kg weight loss | 27324830 |
| 1386186 | ||
| Aerobic exercise | 10–20% | 17533202 |
| n3-PUFA (e.g. fish, flaxseed) | 10–15% | 16287956 |
PMID is the PubMed identifier of the respective literature.
For patients with severe HTG and fasting chylomicronaemia, total dietary fat should be reduced as much as possible, i.e. <30 g/day. Medium-chain TGs (6- to 12-carbon backbone), which are directly transported and metabolized in the liver following portal vein transit, thereby bypassing chylomicrons formation, can also be considered for selected cases.
Therapy—approved pharmacologic treatments
All commonly available lipid-lowering drugs such as statins, ezetimibe, PCSK9 inhibitors, fibrates, omega-3-fatty acids, and niacin affect TG levels. The effect of LDL-lowering drugs such as statins, ezetimibe and PCSK9 inhibitors on TG levels is usually modest (5–15%), while fibrates, omega-3-fatty acids, and niacin have more profound effects (25–45%). Seminal randomized cardiovascular outcome studies with TG-lowering drugs are summarized in Table 4.
Table 4.
Randomized cardiovascular outcome studies with triglyceride-lowering drugs
| Trial | Treatment | Population | n | Endpoint | Statin | P < 0.05 | PMID |
|---|---|---|---|---|---|---|---|
| Fibrates | |||||||
| WHO | Clofibrate | High cholesterol, no CHD | 5331 | Non-fatal MI + CHD death | No | Yes | 361054 |
| CDP | Clofibrate | CHD | 3892 | Non-fatal MI + CHD death | No | No | 1088963 |
| HHS | Gemfibrozil | High cholesterol, no CHD | 4081 | MI + CHD death | No | Yes | 3313041 |
| VA-HIT | Gemfibrozil | Low HDL, CHD | 2531 | Non-fatal MI + CHD death | No | Yes | 10438259 |
| BIP | Bezafibrate | Previous MI or angina | 3090 | MI + sudden death | No | No | 10880410 |
| FIELD | Fenofibrate | T2DM/CVD | 9795 | Non-fatal MI + CHD death | No | No | 16310551 |
| ACCORD | Fenofibrate | T2DM/CVD | 5518 | MI + stroke + CV death | Yes | No | 20228404 |
| Niacin | |||||||
| CDP | IR-Niacin | CHD | 3980 | Non-fatal MI + CHD death | No | No | 1088963 |
| AIM-HIGH | ER-Niacin | Dyslipidaemia + CHD | 3414 | MI + stroke + CAD death + revascularization | Yes | No | 22085343 |
| HPS2-THRIVE | ER-Niacin + Laropiprant | CHD, PAD, or DM | 25 673 | MI + stroke + CAD death + revascularization | Yes | No | 25014686 |
| High-dose omega-3-fatty acids | |||||||
| JELIS (open label in Japan) | Icosapent ethyl 1.8 g | High cholesterol | 18 645 | MI + stroke + sudden cardiac death + angina+ revascularization + PCI + CABG | Yes | Yes | 17398308 |
| REDUCE-IT | Icosapent ethyl 4 g | High TG + ASCVD or high-risk DM | 8179 | MI + stroke + CVD death + angina + revascularization | Yes | Yes | 30415628 |
The main inclusion criteria are listed under ‘Population’. ‘Endpoint’ lists the primary endpoint. ‘Statin’ indicates whether the triglyceride-lowering drug was tested on a statin background medication. ‘PMID’ is the PubMed identifier of the primary publication of the trial.
ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; CHD, coronary heart disease; CVD, cardiovascular disease; ER, extended release; HTG, hypertriglyceridaemia; IR, immediate release; MI, myocardial infarction; PCI, percutaneous coronary angioplasty; TG, triglyceride.
Clinically, there are two distinct aspects to TG reduction: (i) modifying lipid levels to decrease ASCVD risk; and (ii) reducing TG to decrease the risk for acute pancreatitis. Although LDL-lowering drugs have only moderate effects on TG levels, they reduce ASCVD risk in patients with and without HTG.42 Therefore, depending on the absolute risk, specific LDL-C and non-HDL-C goals should be achieved.40
Once the LDL-C goal is achieved, it must be decided, whether residual TG-associated risk for acute pancreatitis and/or ASCVD is sufficiently high to justify initiation of specific TG-lowering drugs, such as fibrates, omega-3-fatty-acids, or niacin. There is a lack of convincing outcomes evidence for ASCVD reduction when these medications are added to statin therapy. There are also no high-quality trial data demonstrating that acute pancreatitis risk decreases with specific TG-lowering therapy.
Fibrates can reduce TG by up to 70%, albeit with marked interindividual variation. As monotherapy, fibrates likely reduce ASCVD risk.43–45 But when used in combination with statins no further risk reduction was observed,46 although subgroup analyses indicate that patients with HTG and low HDL-C may benefit from such combination therapy.47 It is currently unclear whether the failure to show benefit relates to true failure or methodological issues with study design and enrolment criteria. An ongoing ASCVD outcome trial using the new agent, pemafibrate, will probably report in 2021.48,49 Many lipid clinics in the USA and Canada consider fibrate therapy in patients with elevated ASCVD risk and persistent TG concentrations >200 mg/dL despite reaching LDL-C goal and despite lifestyle modifications.
High doses of omega-3-fatty acids (>2 g/day) can variably decrease TG levels; at lower doses (1 g/day) these agents have generally failed to show reduced ASCVD.50,51 A recently published study, REDUCE-IT, which evaluated the effect of 4 g icosapent ethyl (Vascepa) in high-risk patients with elevated TG levels on background statin therapy, is interesting and informative in this regard. In >9000 high-risk patients, a dramatic risk reduction (HR 0.75) for ASCVD events was observed over a period of 4.9 years.52 It is currently unclear whether the observed benefit relates to the particular omega-3 fatty acid formulation used (icosapent ethyl), the high daily dose of 4 g, the carefully selected study population, or a possible deleterious effect of the mineral oil comparator used as placebo. In addition, the strongly positive outcomes seemed to be mediated by factors other than TG reduction, since benefit was seen independent of baseline TG level. Another study using high-dose omega-3 fatty acids is ongoing53; once results are reported—likely in 2021—the role of omega-3 fatty acids in treating patients with elevated TG will become clearer.
Therapy of acute pancreatitis associated with high triglyceride
Initial diagnostic and therapeutic steps should be the same as in other causes of acute pancreatitis. A TG level should be determined in all cases of acute pancreatitis as severe HTG may contribute even when the primary cause is obvious (e.g. alcohol). Initially, IV glucose should be avoided as this may further increase TG levels.
Heparin and insulin have been used in TG-associated pancreatitis without clear evidence of benefit and mostly based on case reports or small case series. Heparin releases endothelial bound LPL and may reduce TG levels.54 Thus, if there is no contraindication some lipid clinics recommend the use of heparin in this situation. Insulin would be appropriate and indeed recommended in patients with uncontrolled Type 2 diabetes or in patients with Type 1 diabetes with diabetic ketoacidosis, in whom severe HTG is not uncommon.55 However, in non-diabetics, the use of insulin, with glucose, has not been proven to be efficacious.
Plasmapheresis acutely decreases TG levels by 50–80% and may thus theoretically eliminate the causal factor in HTG-induced pancreatitis.26,56 However, a recent case series of 22 cases of acute pancreatitis with mean TG levels of 45 mmol/L showed comparable TG reductions—about 70% within 48 h—using conservative management alone, without plasmapheresis.57 Randomized, controlled studies evaluating the value of plasmapheresis vs. conservative management are desirable. Case series show great variability in triggers for the use plasmapheresis, e.g. severity of both HTG and pancreatitis, as well as in timing and frequency of plasmapheresis, and the anti-coagulant used.26 Currently, very-low-grade evidence suggests plasmapheresis might rarely be considered in certain situations, such as pregnancy, when TG >11.3 mmol/L (1000 mg/dL) and acute pancreatitis risk is imminent. TG levels rebound rapidly after plasmapheresis if the root cause is not properly managed. Treatment options for HTG-associated acute pancreatitis are summarized in Table 5.
Table 5.
Treatment options for hypertriglyceridaemia associated acute pancreatitis
| Option | Theoretical basis | Comment |
|---|---|---|
| Replete fluids with saline; avoid IV glucose | Glucose may stimulate VLDL production in the liver and thus worsen hypertriglyceridaemia. | Patients with acute pancreatitis are often hypovolemic (third spacing) and may require large amounts of fluids; this may make complete avoidance of iv glucose difficult. |
| Insulin | Insulin blocks the release of free fatty acids, which may lead to a decreased production of VLDL from the liver. | No randomized, controlled trials available; insulin should only be administered in uncontrolled Type 2 diabetes or in patients with Type 1 diabetes with diabetic ketoacidosis (in which severe hypertriglyceridaemia is not uncommon). |
| Heparin | Heparin releases LPL from the endothelium and may thus help catabolize TRL lipoproteins. | No randomized, controlled trials available, but if no contraindications are present, then iv-heparin should be considered; in the non-acute setting heparin is used to evaluate LPL activity and results in triglyceride reduction; effect may also depend on the underlying cause of hypertriglyceridaemia (no effect if LPL is deficient or malfunctioning). |
| Plasmapheresis | The procedure acutely decreases the concentration of TRL by 50–70%, but rebound is rapid unless the underlying cause of HTG is managed. | Reserved for severe HTG in pregnancy; not recommended in most cases of HTG-associated pancreatitis. |
| Fibrates, omega-3 fatty acids, niacin | May decrease TG levels by up to 70% in chronic HTG. | No role in the acute setting; fibrate and/or high dose omega-3 fatty acids should be considered after the acute episode to prevent recurrence (besides lifestyle modification). |
| Statins, Ezetimibe, and PCSK9-inhibitors | May decrease TG levels by 5–15% in chronic HTG. | No role in the acute setting; depending on the overall cardiovascular risk LDL-C (non-HDL-C) lowering should be considered after the acute episode. |
HDL-C, high-density lipoprotein cholesterol; HTG, hypertriglyceridaemia; LDL-C, low-density lipoprotein cholesterol; LPL, lipoprotein lipase; TG, triglyceride.
Novel and emerging treatments for triglycerides
New fibrate and omega-3 preparation
Pemafibrate (K-877, Kowa), a selective PPAR modulator that reduces TG levels by 35–45%, is being evaluated in PROMINENT (Pemafibrate to Reduce Cardiovascular OutcoMes by Reducing Triglycerides IN patiENts With diabeTes), a Phase 3 ASCVD outcomes study of ∼10 000 patients with Type 2 diabetes and HTG (NCT03071692)48Table 6. STRENGTH (Statin Residual Risk Reduction with EpaNova in High Cardiovascular Risk Patients with Hypertriglyceridaemia; clinicaltrials.gov: NCT02104817) is a current Phase 3 trial of >13 000 patients53 evaluating ASCVD outcomes of 4 g daily of omega-3 carboxylic acids containing eicosapentaenoic acid and docosahexaenoic acid (Epanova, AstraZeneca).
Table 6.
Emerging treatments for hypertriglyceridaemia
| Name | Company | Target | Mechanism of action | Indication | Stage | Biochemical effect | Current or possible use in 5 years |
|---|---|---|---|---|---|---|---|
| Icosapent ethyl | Amarin | Unclear | Not fully defined | Elevated TG | Phase 3 CVOT completed (REDUCE-IT) | Reduces TG | Add-on therapy to statin for ASCVD risk reduction |
| Epanova | AstraZeneca | Unclear | Not fully defined; omega-3 carboxylic acids containing EPA and DHA | Elevated TG | CVOT in progress (STRENGTH) | Reduces TG | Possible add-on therapy to statin for ASCVD risk reduction |
| Pemafibrate | Kowa | PPAR | Selective PPAR modulator | Elevated TG | CVOT in progress (PROMINENT) | Reduces TG, increases HDL-C | Possible add-on therapy to statin for ASCVD risk reduction |
| Volanesorsen (Waylivra) | Akcea | APOC3 | First-generation anti-APOC3 ASO | FCS | Approved in Europe but not North America | Reduces TG, increases HDL-C | Possible special access for high-risk patients |
| AKCEA-APOCIII-LRx | Akcea/Novartis | APOC3 | N-acetylgalactosamine (GalNac)-conjugated anti-APOC3 ASO | ASCVD | Phase 3 CVOT planned | Reduces TG, increases HDL-C | Possible add-on therapy to statin for ASCVD risk reduction; potential off label use for FCS |
| Evinacumab | Regeneron | ANGPTL3 | Anti-ANGPTL3 antibody | FH; severe HTG; FCS | Phase 2–3 | Reduces TG, LDL-C, and HDL-C | FCS; HoFH; refractory severe hyperlipidaemia |
| IONIS-ANGPTL3-LRx | Akcea-Ionis | ANGPTL3 | N-acetylgalactosamine (GalNac)-conjugated anti-ANGPTL3 ASO | FH; severe HTG; FCS | Phase 2–3 | Reduces TG, LDL-C, and HDL-C | FCS; HoFH; refractory severe hyperlipidaemia |
| Alipogene tiparvovec(Glybera) | uniQure | LPL | LPL gene therapy | FCS | Approved; but no longer marketed | Reduces TG | No obvious path forward; indication limited to FCS due to bi-allelic LPL gene mutations |
ANGPTL3, angiopoietin like 3; APOC3, apolipoprotein C-III; ASCVD, atherosclerotic cardiovascular disease; ASO, antisense oligonucleotide; CVOT, cardiovascular outcomes trial; DGAT, diacylglycerol acyltransferase; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FCS, familial chylomicronaemia syndrome; FH, familial hypercholesterolaemia; HDL-C, high-density lipoprotein cholesterol, HoFH, homozygous FH; HTG, hypertriglyceridemia; LCAT, lecithin cholesterol acyl transferase; LDL-C, low-density lipoprotein cholesterol; LDLR, LDL receptor; LIPA, lysosomal acid lipase; Lp(a), lipoprotein(a); LPL, lipoprotein lipase; PPAR, peroxisome proliferator-activated receptor; PROMINENT, Pemafibrate to Reduce Cardiovascular OutcoMes by Reducing Triglycerides IN patiENts With diabeTes; STRENGTH, Statin Residual Risk Reduction with EpaNova in High Cardiovascular Risk Patients with Hypertriglyceridaemia; TG, triglyceride.
Treatments to reduce apolipoprotein C-III
Apo C-III is expressed both intestinally and hepatically, circulates with TG-rich lipoproteins, has net deleterious effects on lipoprotein metabolism58–63 and is a genetically validated target to reduce ASCVD risk.8–10,64–66 Volanesorsen (Waylivra; ISIS 304801 or IONIS-APOCIIIRx; Ionis/Akcea), a subcutaneously injected second-generation 2'-O-methoxyethyl chimeric antisense inhibitor, impairs translation of apolipoprotein (apo) C-III mRNA.58,67 In a Phase 2 trial of volanesorsen, 300 mg given weekly to HTG patients (NCT01529424), TG levels were reduced by 80%.68 This correlated with >80% reductions in apo C-III, favourable changes across multiple lipoprotein species, unchanged LDL-C and improved carbohydrate metabolism.69–71 In three patients with FCS, volanesorsen-reduced TG levels by up to 86%,72 through a mechanism independent of LPL activity.73
APPROACH (NCT02211209) was a 52-week Phase 3 trial of 66 FCS patients with fasting TG ≥8.4 mmol/L randomized to receive weekly subcutaneous volanesorsen 300 mg or placebo.29 In volanesorsen- and placebo-treated patients, TG decreased by 77% and increased by 18%, respectively (P < 0.0001).74 Two-thirds of patients on volanesorsen had injection-site reactions. Reduced platelet counts below 100 000 per microliter was a common adverse event in the volanesorsen group (15 of 33 patients, two patients with levels < 25 000 per microliter).74 The drug-induced thrombocytopenia, possibly related to the antisense formulation, led to five early terminations of volanesorsen.75 COMPASS (NCT02300233) was a 26-week Phase 3 trial of 113 patients with fasting TG ≥5.7 mmol/L who were randomized in a 2:1 ratio to receive volanesorsen 300 mg weekly or placebo. In volanesorsen- and placebo-treated patients, TG decreased by 72% and increased by 1%, respectively (P < 0.0001). Site reactions were seen with one-quarter of volanesorsen injections. There was one treatment-related case of serum sickness, but no thrombocytopenia occurrences. In COMPASS and APPROACH studies combined, nine and one pancreatitis events occurred in placebo and volanesorsen groups, respectively (P = 0.0185). Quality of life analysis showed reduction of pancreatitis-related symptoms.76
The US Food and Drug Administration did not approve volanesorsen for FCS, while the European Medicines Agency recommended conditional marketing authorization. While the mechanism underlying the thrombocytopenia remains unclear, a next-generation N-acetylgalactosamine (GalNac)-conjugated allele-specific oligonucleotide (ASO) targeting apo C-III (AKCEA-APOCIII-LRx) may mitigate this risk; this is being developed by Akcea and Novartis. In a double-blind, placebo-controlled, dose-escalation phase 1/2a trial in healthy volunteers with mild HTG, multiple doses of AKCEA-APOCIII-LRx resulted in mean TG reductions of 65%, with other favourable changes in the lipid profile, and no safety signals. Drug development is focused on patients with ASCVD risk rather than FCS.
Treatments to reduce angiopoietin-like protein 3
ANGPTL3 is a genetically validated target for HTG to reduce ASCVD risk.11,77–79 The monoclonal antibody evinacumab and ASO IONIS-ANGPTL3-LRx reduce angiopoietin-like protein 3 (ANGPTL3), a liver-derived protein that regulates lipid metabolism through incompletely defined mechanisms. Anti-ANGPTL3 therapies reduce both severely elevated TG and severely elevated LDL-C (familial hypercholesterolaemia). In dose-finding studies, evinacumab reduced both TG and LDL-C by up to 75% and 23%, respectively; the latter effect appears to be independent of the LDL receptor pathway.80 Among homozygous familial hypercholesterolaemia patients, evinacumab reduced LDL-C, TG and HDL-C by 49%, 47%, and 36%, respectively.81
IONIS-ANGPTL3-LRx is a GalNac-modified ASO that targets ANGPTL3.82 In a dose-finding study in HTG patients, IONIS-ANGPTL3-LRx reduced TG and LDL-C by up to 63.1% and 32.9%, respectively.82 In contrast to evinacumab, which acts mainly in the plasma space to bind mature formed ANGPTL3, IONIS-ANGPTL3-LRx ASO acts primarily within hepatocytes to block production of hepatic ANGPTL3. In murine models, it reduced hepatic TG content, which differentiates it from anti-ANGPTL3 antibodies.83 A Phase 2 trial of IONIS-ANGPTL3-LRx in patients with Type 2 diabetes, hepatosteatosis, and TG >2.3 mmol/L is underway (NCT03371355).
Lipoprotein lipase gene therapy
Alipogene tiparvovec (Glybera; uniQure, Lexington, MA, USA) gene therapy for LPL deficiency was available in Europe until 2017, when the sponsor declined to renew market authorization. Spinal anaesthesia was needed for intramuscular injections of the adeno-associated virus subtype 1 vector carrying hyperfunctional LPL p.S447X; TG normalized within 12 weeks.84–86 Unfortunately, TG drifted back to baseline levels within 6 months, although a few exceptional cases experienced sustained benefits.84–87
Diacylglycerol acyltransferase inhibition
Pradigastat (Novartis) and AZD7687 (AstraZeneca) are oral inhibitors of intestinal DGAT1, which governs dietary fat absorption and TG synthesis.58 DGAT1 is not a genetically validated target for either severe HTG or ASCVD risk. Gastrointestinal adverse effects were common with AZD7687.88 In six FCS patients, pradigastat reduced fasting TG by 70%.89 In obese patients with milder dyslipidaemia, pradigastat improved TG and other metabolic traits.90 Dosage reduction and fat restriction mitigated diarrhoea and nausea, but frequent side effects have halted development.90
Treatments targeting apolipoproteins C-II and A-V and angiopoietin-like protein 4
Apo C-II is a cofactor for LPL91,92 and complete apo C-II deficiency accounts for ∼2% of all FCS cases.93 These ultra-rare patients, who are at high risk for acute pancreatitis, might benefit from an infused apo C-II peptide.94 However, apo C-II has not been genetically validated as a target for ASCVD.91 In contrast, apo A-V, which promotes LPL activity through partially defined mechanisms, is a strong genetically validated target for ASCVD.95,96 Boosting apo A-V levels might be beneficial for ASCVD prevention and also for the subset of FCS patients with complete apo A-V deficiency.93,95 Finally, angiopoietin-like protein 4 (ANGPTL4) regulates LPL activity, analogous to ANGPTL3, but its mechanism is incompletely defined.91,97,98 Genetically attenuated ANGPTL4 is associated with favourable lipid and glycaemia profiles, and with reduced ASCVD risk.12,99 Rodents given an anti-ANGPTL4 antibody developed mesenteric adenitis, providing a cautionary note about pursuing this target in humans.99
Open questions
Importantly, the efficacy and the safety of the emerging treatments for HTG depicted in Table 6 need to be assessed in outcome trials. Such trials will help to better understand whether the clinical effect of TG-lowering correlates with ApoB reduction and the importance of TG lowering in patients with well-controlled (very low) LDL-C. We need better understanding of the potentially heterogeneous pathology of different TG-rich particles and their metabolism, e.g. the importance of increased TG production vs. reduced catabolism. Since lifestyle measures are of outmost importance for the management of HTG patients, novel approaches are wanted to improve implementation, monitoring, and adherence. To this end, point of care analytical devices such as a recently reported miniaturized electrochemical TG analyzer that can be used in a smartphone may provide important options for future research and patient care.100
Summary
Epidemiologic and genetic studies establish TRL and their remnants as important contributors to ASCVD. In addition to LDL-C, the primary treatment target for lipid-lowering therapy, secondary targets that reflect the contribution of TRL such as apoB and non-HDL-C are recommended in the current guidelines.
The first step of treatment is the implementation of lifestyle interventions. Secondly, LDL-C lowering with statins is recommended to reduce vascular risk; this is independent of statin-associated lowering of TRL themselves. Previous trials with fibrates, niacin, and CETP inhibitors did not provide conclusive evidence of ASCVD reduction in patients with optimal cholesterol-lowering. However, recent and ongoing studies show the importance of TRL for the residual risk in ASCVD patients on statin. Novel and emerging data, e.g. on omega-3 fatty acids (high-dose icosapent ethyl) and the selective PPAR modulator pemafibrate may identify patients who will benefit from TRL lowering. Based on enhanced understanding of the molecular pathways of TRL, novel treatment targets have emerged that are in clinical testing. These include apo C-III antisense inhibitor (volanesorsen), inhibitors of angiopoietin-like protein 3 (evinacumab; ASO IONIS-ANGPTL3-LRx), inhibitors of intestinal diacylglycerol acyltransferase (pradigastat) and treatments targeting apo C-II and A-V, and angiopoietin-like protein 4.
In summary, TRL have emerged as important markers of residual risk and as treatment targets. Several ongoing trials will report the efficacy of novel pharmacologic strategies to lower serum TGs. We speculate that treating of TRL in specific patient populations will become an essential part of lipid-directed treatment in addition to the lowering of LDL-C in the future.
Conflict of interest: U.L.: lectures/consulting for Amgen, Boehringer-Ingelheim, Novartis, Pfizer, Regeneron, Sanofi, and Servier. K.G.P.: lectures/consulting for Aegerion, Akcea, Amgen, Amryt, Berlin-Chemie, Boehringer-Ingelheim, MSD, Novartis, Pfizer, Regeneron, and Sanofi. H.G.: grant from Medimmune/Axtrazeneca; consulting for Sanofi, Regeneron, Amgen, Resverlogix, Janssen, Pfizer, Silence Therapeutics, Merck, Kowa, and Akcea. R.A.H.: consulting for Acasti, Akcea/Ionis, Amgen, HLS Therapeutics, and Sanofi.
References
- 1. Dron JS, Wang J, Cao H, McIntyre AD, Iacocca MA, Menard JR, Movsesyan I, Malloy MJ, Pullinger CR, Kane JP, Hegele RA.. Severe hypertriglyceridemia is primarily polygenic. J Clin Lipidol 2019;13:80–88. [DOI] [PubMed] [Google Scholar]
- 2. Hegele RA, Ginsberg HN, Chapman MJ, Nordestgaard BG, Kuivenhoven JA, Averna M, Borén J, Bruckert E, Catapano AL, Descamps OS, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Raal FJ, Ray KK, Santos RD, Stalenhoef AFH, Stroes E, Taskinen M-R, Tybjærg-Hansen A, Watts GF, Wiklund O; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014;2:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jaross W, Assmann G, Bergmann S, Schulte H.. Comparison of risk factors for coronary heart disease in Dresden and Munster. Results of the DRECAN (Dresden Cardiovascular Risk and Nutrition) study and the PROCAM (Prospective Cardiovascular Munster) Study. Eur J Epidemiol 1994;10:307–315. [DOI] [PubMed] [Google Scholar]
- 4. Retterstol K, Narverud I, Selmer R, Berge KE, Osnes IV, Ulven SM, Halvorsen B, Aukrust P, Holven KB, Iversen PO.. Severe hypertriglyceridemia in Norway: prevalence, clinical and genetic characteristics. Lipids Health Dis 2017;16:115.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Truthmann J, Schienkiewitz A, Busch MA, Mensink GB, Du Y, Bosy-Westphal A, Knopf H, Scheidt-Nave C.. Changes in mean serum lipids among adults in Germany: results from National Health Surveys 1997-99 and 2008-11. BMC Public Health 2016;16:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chyzhyk V, Kozmic S, Brown AS, Hudgins LC, Starc TJ, Davila AD, Blevins TC, Diffenderfer MR, He L, Geller AS, Rush C, Hegele RA, Schaefer EJ.. Extreme hypertriglyceridemia: genetic diversity, pancreatitis, pregnancy, and prevalence. J Clin Lipidol 2019;13:89–99. [DOI] [PubMed] [Google Scholar]
- 7. Parhofer KG, Barrett PH, Schwandt P.. Atorvastatin improves postprandial lipoprotein metabolism in normolipidemlic subjects. J Clin Endocrinol Metab 2000;85:4224–4230. [DOI] [PubMed] [Google Scholar]
- 8. Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O'Connell JR, Shuldiner AR.. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 2008;322:1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saleheen D, Natarajan P, Armean IM, Zhao W, Rasheed A, Khetarpal SA, Won H-H, Karczewski KJ, O’Donnell-Luria AH, Samocha KE, Weisburd B, Gupta N, Zaidi M, Samuel M, Imran A, Abbas S, Majeed F, Ishaq M, Akhtar S, Trindade K, Mucksavage M, Qamar N, Zaman KS, Yaqoob Z, Saghir T, Rizvi SNH, Memon A, Hayyat Mallick N, Ishaq M, Rasheed SZ, Memon F-U-R, Mahmood K, Ahmed N, Do R, Krauss RM, MacArthur DG, Gabriel S, Lander ES, Daly MJ, Frossard P, Danesh J, Rader DJ, Kathiresan S.. Human knockouts and phenotypic analysis in a cohort with a high rate of consanguinity. Nature 2017;544:235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A.. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014;371:32–41. [DOI] [PubMed] [Google Scholar]
- 11. Stitziel NO, Khera AV, Wang X, Bierhals AJ, Vourakis AC, Sperry AE, Natarajan P, Klarin D, Emdin CA, Zekavat SM, Nomura A, Erdmann J, Schunkert H, Samani NJ, Kraus WE, Shah SH, Yu B, Boerwinkle E, Rader DJ, Gupta N, Frossard PM, Rasheed A, Danesh J, Lander ES, Gabriel S, Saleheen D, Musunuru K, Kathiresan S; PROMIS and Myocardial Infarction Genetics Consortium Investigators. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol 2017;69:2054–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Myocardial Infarction G, Investigators C, Stitziel NO, Stirrups KE, Masca NG, Erdmann J, Ferrario PG, Konig IR, Weeke PE, Webb TR, Auer PL, Schick UM, Lu Y, Zhang H, Dube MP, Goel A, Farrall M, Peloso GM, Won HH, Do R, van Iperen E, Kanoni S, Kruppa J, Mahajan A, Scott RA, Willenberg C, Braund PS, van Capelleveen JC, Doney AS, Donnelly LA, Asselta R, Merlini PA, Duga S, Marziliano N, Denny JC, Shaffer CM, El-Mokhtari NE, Franke A, Gottesman O, Heilmann S, Hengstenberg C, Hoffman P, Holmen OL, Hveem K, Jansson JH, Jockel KH, Kessler T, Kriebel J, Laugwitz KL, Marouli E, Martinelli N, McCarthy MI, Van Zuydam NR, Meisinger C, Esko T, Mihailov E, Escher SA, Alver M, Moebus S, Morris AD, Muller-Nurasyid M, Nikpay M, Olivieri O,L, Perreault LP, AlQarawi A, Robertson NR, Akinsanya KO, Reilly DF, Vogt TF, Yin W, Asselbergs FW, Kooperberg C, Jackson RD, Stahl E, Strauch K, Varga TV, Waldenberger M, Zeng L, Kraja AT, Liu C, Ehret GB, Newton-Cheh C, Chasman DI, Chowdhury R, Ferrario M, Ford I, Jukema JW, Kee F, Kuulasmaa K, Nordestgaard BG, Perola M, Saleheen D, Sattar N, Surendran P, Tregouet D, Young R, Howson JM, Butterworth AS, Danesh J, Ardissino D, Bottinger EP, Erbel R, Franks PW, Girelli D, Hall AS, Hovingh GK, Kastrati A, Lieb W, Meitinger T, Kraus WE, Shah SH, McPherson R, Orho-Melander M, Melander O, Metspalu A, Palmer CN, Peters A, Rader D, Reilly MP, Loos RJ, Reiner AP, Roden DM, Tardif JC, Thompson JR, Wareham NJ, Watkins H, Willer CJ, Kathiresan S, Deloukas P, Samani NJ, Schunkert H.. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med 2016;374:1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis GF, Xiao C, Hegele RA.. Hypertriglyceridemia in the genomic era: a new paradigm. Endocr Rev 2015;36:131–147. [DOI] [PubMed] [Google Scholar]
- 14. Chait A, Eckel RH.. The chylomicronemia syndrome is most often multifactorial: a narrative review of causes and treatment. Ann Intern Med 2019;170:626. [DOI] [PubMed] [Google Scholar]
- 15. Xiao C, Dash S, Morgantini C, Hegele RA, Lewis GF.. Pharmacological targeting of the atherogenic dyslipidemia complex: the next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes 2016;65:1767–1778. [DOI] [PubMed] [Google Scholar]
- 16. Stahel P, Xiao C, Hegele RA, Lewis GF.. The atherogenic dyslipidemia complex and novel approaches to cardiovascular disease prevention in diabetes. Can J Cardiol 2018;34:595–604. [DOI] [PubMed] [Google Scholar]
- 17. Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE.. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol 2018;72:330–343. [DOI] [PubMed] [Google Scholar]
- 18. Nordestgaard BG, Varbo A.. Triglycerides and cardiovascular disease. Lancet 2014;384:626–635. [DOI] [PubMed] [Google Scholar]
- 19. Madsen CM, Varbo A, Nordestgaard BG.. Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: a contemporary population-based study. Eur Heart J 2018;39:610–619. [DOI] [PubMed] [Google Scholar]
- 20. Mortensen MB, Nordestgaard BG, Afzal S, Falk E.. ACC/AHA guidelines superior to ESC/EAS guidelines for primary prevention with statins in non-diabetic Europeans: the Copenhagen General Population Study. Eur Heart J 2017;38:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, Mundl H, Olsson AG.. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol 2015;65:2267–2275. [DOI] [PubMed] [Google Scholar]
- 22. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V.. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007;115:450–458. [DOI] [PubMed] [Google Scholar]
- 23. Nordestgaard BG, Stender S, Kjeldsen K.. Reduced atherogenesis in cholesterol-fed diabetic rabbits. Giant lipoproteins do not enter the arterial wall. Arteriosclerosis 1988;8:421–428. [DOI] [PubMed] [Google Scholar]
- 24. Ference BA, Kastelein JJP, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, Laufs U, Oliver-Williams C, Wood AM, Butterworth AS, Di Angelantonio E, Danesh J, Nicholls SJ, Bhatt DL, Sabatine MS, Catapano AL.. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA 2019;321:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Searles GE, Ooi TC.. Underrecognition of chylomicronemia as a cause of acute pancreatitis. CMAJ 1992;147:1806–1808. [PMC free article] [PubMed] [Google Scholar]
- 26. Valdivielso P, Ramirez-Bueno A, Ewald N.. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med 2014;25:689–694. [DOI] [PubMed] [Google Scholar]
- 27. Pedersen SB, Langsted A, Nordestgaard BG.. Nonfasting mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis. JAMA Intern Med 2016;176:1834–1842. [DOI] [PubMed] [Google Scholar]
- 28. Sandhu S, Al-Sarraf A, Taraboanta C, Frohlich J, Francis GA.. Incidence of pancreatitis, secondary causes, and treatment of patients referred to a specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis 2011;10:157.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blom DJ, O'Dea L, Digenio A, Alexander VJ, Karwatowska-Prokopczuk E, Williams KR, Hemphill L, Muniz-Grijalvo O, Santos RD, Baum S, Witztum JL.. Characterizing familial chylomicronemia syndrome: baseline data of the APPROACH study. J Clin Lipidol 2018;12:1234–1243.e5. [DOI] [PubMed] [Google Scholar]
- 30. Hegele RA, Berberich AJ, Ban MR, Wang J, Digenio A, Alexander VJ, D'Erasmo L, Arca M, Jones A, Bruckert E, Stroes ES, Bergeron J, Civeira F, Witztum JL, Gaudet D.. Clinical and biochemical features of different molecular etiologies of familial chylomicronemia. J Clin Lipidol 2018;12:920–927.e4. [DOI] [PubMed] [Google Scholar]
- 31. Havel RJ. Pathogenesis, differentiation and management of hypertriglyceridemia. Adv Intern Med 1969;15:117–154. [PubMed] [Google Scholar]
- 32. Chang YT, Chang MC, Su TC, Liang PC, Su YN, Kuo CH, Wei SC, Wong JM.. Association of cystic fibrosis transmembrane conductance regulator (CFTR) mutation/variant/haplotype and tumor necrosis factor (TNF) promoter polymorphism in hyperlipidemic pancreatitis. Clin Chem 2007;54:131–138. [DOI] [PubMed] [Google Scholar]
- 33. Ivanova R, Puerta S, Garrido A, Cueto I, Ferro A, Ariza MJ, Cobos A, Gonzalez-Santos P, Valdivielso P.. Triglyceride levels and apolipoprotein E polymorphism in patients with acute pancreatitis. Hepatobiliary Pancreat Dis Int 2012;11:96–101. [DOI] [PubMed] [Google Scholar]
- 34. Hansen SEJ, Madsen CM, Varbo A, Nordestgaard BG.. Low-grade inflammation in the association between mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis: a study of more than 115000 individuals from the general population. Clin Chem 2019;65:321–332. [DOI] [PubMed] [Google Scholar]
- 35. Jackson KG, Poppitt SD, Minihane AM.. Postprandial lipemia and cardiovascular disease risk: interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 2012;220:22–33. [DOI] [PubMed] [Google Scholar]
- 36. Mora S, Rifai N, Buring JE, Ridker PM.. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation 2008;118:993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Werner C, Filmer A, Fritsch M, Groenewold S, Graber S, Bohm M, Laufs U.. Risk prediction with triglycerides in patients with stable coronary disease on statin treatment. Clin Res Cardiol 2014;103:984–997. [DOI] [PubMed] [Google Scholar]
- 38. Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, Watts GF, Sypniewska G, Wiklund O, Boren J, Chapman MJ, Cobbaert C, Descamps OS, von Eckardstein A, Kamstrup PR, Pulkki K, Kronenberg F, Remaley AT, Rifai N, Ros E, Langlois M; European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) joint consensus initiative. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points—a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J 2016;37:1944–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J.. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019:73:e285–e350. [DOI] [PubMed] [Google Scholar]
- 40. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT; ESC Scientific Document Group. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 41. De Natale C, Annuzzi G, Bozzetto L, Mazzarella R, Costabile G, Ciano O, Riccardi G, Rivellese AA.. Effects of a plant-based high-carbohydrate/high-fiber diet versus high-monounsaturated fat/low-carbohydrate diet on postprandial lipids in type 2 diabetic patients. Diabetes Care 2009;32:2168–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R.. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–2561. [DOI] [PubMed] [Google Scholar]
- 43. Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V, Mäenpää H, Mälkönen M, Mänttäri M, Norola S, Pasternack A, Pikkarainen J, Romo M, Sjöblom T, Nikkilä EA.. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 1987;317:1237–1245. [DOI] [PubMed] [Google Scholar]
- 44. Katsiki N, Nikolic D, Montalto G, Banach M, Mikhailidis DP, Rizzo M.. The role of fibrate treatment in dyslipidemia: an overview. Curr Pharm Des 2013;19:3124–3131. [DOI] [PubMed] [Google Scholar]
- 45. Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J.. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341:410–418. [DOI] [PubMed] [Google Scholar]
- 46. Group AS, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC Jr, Cushman WC, Simons-Morton DG, Byington RP.. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Grobbee DE, Cass A, Chalmers J, Perkovic V.. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010;375:1875–1884. [DOI] [PubMed] [Google Scholar]
- 48. Araki E, Yamashita S, Arai H, Yokote K, Satoh J, Inoguchi T, Nakamura J, Maegawa H, Yoshioka N, Tanizawa Y, Watada H, Suganami H, Ishibashi S.. Effects of pemafibrate, a novel selective PPARalpha modulator, on lipid and glucose metabolism in patients with type 2 diabetes and hypertriglyceridemia: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2018;41:538–546. [DOI] [PubMed] [Google Scholar]
- 49. Pradhan AD, Paynter NP, Everett BM, Glynn RJ, Amarenco P, Elam M, Ginsberg H, Hiatt WR, Ishibashi S, Koenig W, Nordestgaard BG, Fruchart JC, Libby P, Ridker PM.. Rationale and design of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study. Am Heart J 2018;206:80–93. [DOI] [PubMed] [Google Scholar]
- 50. Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, Chew EY, Bosch J, Collins R, Lewington S, Armitage J, Clarke R; Omega-3 Treatment Trialists’ Collaboration. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol 2018;3:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D'Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE; VITAL Research Group. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 53. Nicholls SJ, Lincoff AM, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Pedersen TR, Ridker PM, Ray K, Karlson BW, Lundstrom T, Wolski K, Nissen SE.. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: rationale and design of the STRENGTH trial. Clin Cardiol 2018;41:1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tornvall P, Olivecrona G, Karpe F, Hamsten A, Olivecrona T.. Lipoprotein lipase mass and activity in plasma and their increase after heparin are separate parameters with different relations to plasma lipoproteins. Arterioscler Thromb Vasc Biol 1995;15:1086–1093. [DOI] [PubMed] [Google Scholar]
- 55. Wolfgram PM, Macdonald MJ.. Severe hypertriglyceridemia causing acute pancreatitis in a child with new onset type I diabetes mellitus presenting in ketoacidosis. J Pediatr Intensive Care 2013;2:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ramirez-Bueno A, Salazar-Ramirez C, Cota-Delgado F, de la Torre-Prados MV, Valdivielso P.. Plasmapheresis as treatment for hyperlipidemic pancreatitis. Eur J Intern Med 2014;25:160–163. [DOI] [PubMed] [Google Scholar]
- 57. Berberich AJ, Ziada A, Zou GY, Hegele RA.. Conservative management in hypertriglyceridemia-associated pancreatitis. J Intern Med 2019;doi: 10.1111/joim.12925 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 58. Gryn SE, Hegele RA.. Novel therapeutics in hypertriglyceridemia. Curr Opin Lipidol 2015;26:484–491. [DOI] [PubMed] [Google Scholar]
- 59. Huff MW, Hegele RA.. Apolipoprotein C-III: going back to the future for a lipid drug target. Circ Res 2013;112:1405–1408. [DOI] [PubMed] [Google Scholar]
- 60. Yao Z, Wang Y.. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr Opin Lipidol 2012;23:206–212. [DOI] [PubMed] [Google Scholar]
- 61. Norata GD, Tsimikas S, Pirillo A, Catapano AL.. Apolipoprotein C-III: from pathophysiology to pharmacology. Trends Pharmacol Sci 2015;36:675–687. [DOI] [PubMed] [Google Scholar]
- 62. Dallinga-Thie GM, Kroon J, Borén J, Chapman MJ.. Triglyceride-rich lipoproteins and remnants: targets for therapy? Curr Cardiol Rep 2016;18:67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wyler von Ballmoos MC, Haring B, Sacks FM.. The risk of cardiovascular events with increased apolipoprotein CIII: a systematic review and meta-analysis. J Clin Lipidol 2015;9:498–510. [DOI] [PubMed] [Google Scholar]
- 64.TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute, Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, Hindy G, Masca N, Stirrups K, Kanoni S, Do R, Jun G, Hu Y, Kang HM, Xue C, Goel A, Farrall M, Duga S, Merlini PA, Asselta R, Girelli D, Olivieri O, Martinelli N, Yin W, Reilly D, Speliotes E, Fox CS, Hveem K, Holmen OL, Nikpay M, Farlow DN, Assimes TL, Franceschini N, Robinson J, North KE, Martin LW, DePristo M, Gupta N, Escher SA, Jansson JH, Van Zuydam N, Palmer CN, Wareham N, Koch W, Meitinger T, Peters A, Lieb W, Erbel R, Konig IR, Kruppa J, Degenhardt F, Gottesman O, Bottinger EP, O'Donnell CJ, Psaty BM, Ballantyne CM, Abecasis G, Ordovas JM, Melander O, Watkins H, Orho-Melander M, Ardissino D, Loos RJ, McPherson R, Willer CJ, Erdmann J, Hall AS, Samani NJ, Deloukas P, Schunkert H, Wilson JG, Kooperberg C, Rich SS, Tracy RP, Lin DY, Altshuler D, Gabriel S, Nickerson DA, Jarvik GP, Cupples LA, Reiner AP, Boerwinkle E, Kathiresan S.. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 2014;371:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hegele RA, Breslow JL, Cloninger CR.. Apolipoprotein genetic variation in the assessment of atherosclerosis susceptibility. Genet Epidemiol 1987;4:163–184. [DOI] [PubMed] [Google Scholar]
- 66. Jowett NI, Rees A, Williams LG, Stocks J, Vella MA, Hitman GA, Katz J, Galton DJ.. Insulin and apolipoprotein A-1/C-III gene polymorphisms relating to hypertriglyceridaemia and diabetes mellitus. Diabetologia 1984;27:180–183. [DOI] [PubMed] [Google Scholar]
- 67. Qamar A, Libby P, Bhatt DL.. Targeting RNA to lower triglycerides: long strides from short molecules. Eur Heart J 2019;40:2797–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gaudet D, Alexander VJ, Baker BF, Brisson D, Tremblay K, Singleton W, Geary RS, Hughes SG, Viney NJ, Graham MJ, Crooke RM, Witztum JL, Brunzell JD, Kastelein JJ.. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med 2015;373:438–447. [DOI] [PubMed] [Google Scholar]
- 69. Yang X, Lee SR, Choi YS, Alexander VJ, Digenio A, Yang Q, Miller YI, Witztum JL, Tsimikas S.. Reduction in lipoprotein-associated apoC-III levels following volanesorsen therapy: phase 2 randomized trial results. J Lipid Res 2016;57:706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Digenio A, Dunbar RL, Alexander VJ, Hompesch M, Morrow L, Lee RG, Graham MJ, Hughes SG, Yu R, Singleton W, Baker BF, Bhanot S, Crooke RM.. Antisense-mediated lowering of plasma apolipoprotein C-III by volanesorsen improves dyslipidemia and insulin sensitivity in type 2 diabetes. Diabetes Care 2016;39:1408–1415. [DOI] [PubMed] [Google Scholar]
- 71. Pechlaner R, Tsimikas S, Yin X, Willeit P, Baig F, Santer P, Oberhollenzer F, Egger G, Witztum JL, Alexander VJ, Willeit J, Kiechl S, Mayr M.. Very-low-density lipoprotein-associated apolipoproteins predict cardiovascular events and are lowered by inhibition of APOC-III. J Am Coll Cardiol 2017;69:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, Geary RS, Baker BF, Graham MJ, Crooke RM, Witztum JL.. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med 2014;371:2200–2206. [DOI] [PubMed] [Google Scholar]
- 73. Gordts PL, Nock R, Son NH, Ramms B, Lew I, Gonzales JC, Thacker BE, Basu D, Lee RG, Mullick AE, Graham MJ, Goldberg IJ, Crooke RM, Witztum JL, Esko JD.. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest 2016;126:2855–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, Yang Q, Hughes SG, Geary RS, Arca M, Stroes ESG, Bergeron J, Soran H, Civeira F, Hemphill L, Tsimikas S, Blom DJ, O’Dea L, Bruckert E.. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med 2019;381:531–542. [DOI] [PubMed] [Google Scholar]
- 75. Crooke ST, Baker BF, Witztum JL, Kwoh TJ, Pham NC, Salgado N, McEvoy BW, Cheng W, Hughes SG, Bhanot S, Geary RS.. The effects of 2'-O-methoxyethyl containing antisense oligonucleotides on platelets in human clinical trials. Nucleic Acid Ther 2017;27:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Arca M, Hsieh A, Soran H, Rosenblit P, O’Dea L, Stevenson M.. The effect of volanesorsen treatment on the burden associated with familial chylomicronemia syndrome: the results of the ReFOCUS study. Expert Rev Cardiovasc Ther 2018;16:537–546. [DOI] [PubMed] [Google Scholar]
- 77. Minicocci I, Tikka A, Poggiogalle E, Metso J, Montali A, Ceci F, Labbadia G, Fontana M, Di Costanzo A, Maranghi M, Rosano A, Ehnholm C, Donini LM, Jauhiainen M, Arca M.. Effects of angiopoietin-like protein 3 deficiency on postprandial lipid and lipoprotein metabolism. J Lipid Res 2016;57:1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Musunuru K, Pirruccello JP, Do R, Peloso GM, Guiducci C, Sougnez C, Garimella KV, Fisher S, Abreu J, Barry AJ, Fennell T, Banks E, Ambrogio L, Cibulskis K, Kernytsky A, Gonzalez E, Rudzicz N, Engert JC, DePristo MA, Daly MJ, Cohen JC, Hobbs HH, Altshuler D, Schonfeld G, Gabriel SB, Yue P, Kathiresan S.. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med 2010;363:2220–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, McCarthy S, Van Hout CV, Bruse S, Dansky HM, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Habegger L, Lopez A, Penn J, Zhao A, Shao W, Stahl N, Murphy AJ, Hamon S, Bouzelmat A, Zhang R, Shumel B, Pordy R, Gipe D, Herman GA, Sheu WHH, Lee I-T, Liang K-W, Guo X, Rotter JI, Chen Y-DI, Kraus WE, Shah SH, Damrauer S, Small A, Rader DJ, Wulff AB, Nordestgaard BG, Tybjærg-Hansen A, van den Hoek AM, Princen HMG, Ledbetter DH, Carey DJ, Overton JD, Reid JG, Sasiela WJ, Banerjee P, Shuldiner AR, Borecki IB, Teslovich TM, Yancopoulos GD, Mellis SJ, Gromada J, Baras A.. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med 2017;377:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang Y, Gusarova V, Banfi S, Gromada J, Cohen JC, Hobbs HH.. Inactivation of ANGPTL3 reduces hepatic VLDL-triglyceride secretion. J Lipid Res 2015;56:1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gaudet D, Gipe DA, Pordy R, Ahmad Z, Cuchel M, Shah PK, Chyu KY, Sasiela WJ, Chan KC, Brisson D, Khoury E, Banerjee P, Gusarova V, Gromada J, Stahl N, Yancopoulos GD, Hovingh GK.. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N Engl J Med 2017;377:296–297. [DOI] [PubMed] [Google Scholar]
- 82. Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, Yu R, Hurh E, Paz E, McEvoy BW, Baker BF, Pham NC, Digenio A, Hughes SG, Geary RS, Witztum JL, Crooke RM, Tsimikas S.. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med 2017;377:222–232. [DOI] [PubMed] [Google Scholar]
- 83. Gusarova V, Alexa CA, Wang Y, Rafique A, Kim JH, Buckler D, Mintah IJ, Shihanian LM, Cohen JC, Hobbs HH, Xin Y, Valenzuela DM, Murphy AJ, Yancopoulos GD, Gromada J.. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J Lipid Res 2015;56:1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gaudet D, Methot J, Dery S, Brisson D, Essiembre C, Tremblay G, Tremblay K, de Wal J, Twisk J, van den Bulk N, Sier-Ferreira V, van Deventer S.. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther 2013;20:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Carpentier AC, Frisch F, Labbe SM, Gagnon R, de Wal J, Greentree S, Petry H, Twisk J, Brisson D, Gaudet D.. Effect of alipogene tiparvovec (AAV1-LPL(S447X)) on postprandial chylomicron metabolism in lipoprotein lipase-deficient patients. J Clin Endocrinol Metab 2012;97:1635–1644. [DOI] [PubMed] [Google Scholar]
- 86. Rip J, van Dijk KW, Sierts JA, Kastelein JJ, Twisk J, Kuivenhoven JA.. AAV1-LPL(S447X) gene therapy reduces hypertriglyceridemia in apoE2 knock in mice. Biochim Biophys Acta 2006;1761:1163–1168. [DOI] [PubMed] [Google Scholar]
- 87. Gaudet D, Methot J, Kastelein J.. Gene therapy for lipoprotein lipase deficiency. Curr Opin Lipidol 2012;23:310–320. [DOI] [PubMed] [Google Scholar]
- 88. Denison H, Nilsson C, Lofgren L, Himmelmann A, Martensson G, Knutsson M, Al-Shurbaji A, Tornqvist H, Eriksson JW.. Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes Obes Metab 2014;16:334–343. [DOI] [PubMed] [Google Scholar]
- 89. Meyers CD, Tremblay K, Amer A, Chen J, Jiang L, Gaudet D.. Effect of the DGAT1 inhibitor pradigastat on triglyceride and apoB48 levels in patients with familial chylomicronemia syndrome. Lipids Health Dis 2015;14:8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Meyers CD, Amer A, Majumdar T, Chen J.. Pharmacokinetics, pharmacodynamics, safety, and tolerability of pradigastat, a novel diacylglycerol acyltransferase 1 inhibitor in overweight or obese, but otherwise healthy human subjects. J Clin Pharmacol 2015;55:1031–1041. [DOI] [PubMed] [Google Scholar]
- 91. Hegele RA. Multidimensional regulation of lipoprotein lipase: impact on biochemical and cardiovascular phenotypes. J Lipid Res 2016;57:1601–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wolska A, Dunbar RL, Freeman LA, Ueda M, Amar MJ, Sviridov DO, Remaley AT.. Apolipoprotein C-II: new findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 2017;267:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Brahm AJ, Hegele RA.. Chylomicronaemia–current diagnosis and future therapies. Nat Rev Endocrinol 2015;11:352–362. [DOI] [PubMed] [Google Scholar]
- 94. Sakurai T, Sakurai A, Vaisman BL, Amar MJ, Liu C, Gordon SM, Drake SK, Pryor M, Sampson ML, Yang L, Freeman LA, Remaley AT.. Creation of apolipoprotein C-II (ApoC-II) mutant mice and correction of their hypertriglyceridemia with an ApoC-II mimetic peptide. J Pharmacol Exp Ther 2016;356:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Forte TM, Sharma V, Ryan RO.. Apolipoprotein A-V gene therapy for disease prevention/treatment: a critical analysis. J Biomed Res 2016;30:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Do R, Stitziel NO, Won HH, Jorgensen AB, Duga S, Angelica Merlini P, Kiezun A, Farrall M, Goel A, Zuk O, Guella I, Asselta R, Lange LA, Peloso GM, Auer PL, Project NES, Girelli D, Martinelli N, Farlow DN, DePristo MA, Roberts R, Stewart AF, Saleheen D, Danesh J, Epstein SE, Sivapalaratnam S, Hovingh GK, Kastelein JJ, Samani NJ, Schunkert H, Erdmann J, Shah SH, Kraus WE, Davies R, Nikpay M, Johansen CT, Wang J, Hegele RA, Hechter E, Marz W, Kleber ME, Huang J, Johnson AD, Li M, Burke GL, Gross M, Liu Y, Assimes TL, Heiss G, Lange EM, Folsom AR, Taylor HA, Olivieri O, Hamsten A, Clarke R, Reilly DF, Yin W, Rivas MA, Donnelly P, Rossouw JE, Psaty BM, Herrington DM, Wilson JG, Rich SS, Bamshad MJ, Tracy RP, Cupples LA, Rader DJ, Reilly MP, Spertus JA, Cresci S, Hartiala J, Tang WH, Hazen SL, Allayee H, Reiner AP, Carlson CS, Kooperberg C, Jackson RD, Boerwinkle E, Lander ES, Schwartz SM, Siscovick DS, McPherson R, Tybjaerg-Hansen A, Abecasis GR, Watkins H, Nickerson DA, Ardissino D, Sunyaev SR, O'Donnell CJ, Altshuler D, Gabriel S, Kathiresan S.. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature 2015;518:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, Cohen JC.. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet 2007;39:513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Olshan DS, Rader DJ.. Angiopoietin-like protein 4: a therapeutic target for triglycerides and coronary disease? J Clin Lipidol 2018;12:583–587. [DOI] [PubMed] [Google Scholar]
- 99. Dewey FE, Gusarova V, O’Dushlaine C, Gottesman O, Trejos J, Hunt C, Van Hout CV, Habegger L, Buckler D, Lai K-MV, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Ledbetter DH, Penn J, Lopez A, Borecki IB, Overton JD, Reid JG, Carey DJ, Murphy AJ, Yancopoulos GD, Baras A, Gromada J, Shuldiner AR.. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med 2016;374:1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang J, Huang X, Tang SY, Shi GM, Ma X, Guo J.. Blood triglyceride monitoring with smartphone as electrochemical analyzer for cardiovascular disease prevention. IEEE J Biomed Health Inform 2019;23:66–71. [DOI] [PubMed] [Google Scholar]