Abstract
Decorin, a prototype small leucine-rich proteoglycan, regulates a vast array of cellular processes including collagen fibrillogenesis, wound repair, angiostasis, tumor growth, and autophagy. This functional versatility arises from a wide array of decorin/protein interactions also including interactions with its single glycosaminoglycan side chain. The decorin-binding partners encompass numerous categories ranging from extracellular matrix molecules to cell surface receptors to growth factors and enzymes. Despite the diversity of the decorin interacting network, two main roles emerge as prominent themes in decorin function: maintenance of cellular structure and outside-in signaling, culminating in anti-tumorigenic effects. Here we present contemporary knowledge regarding the decorin interacting network and discuss in detail the biological relevance of these pleiotropic interactions, some of which could be targeted by therapeutic interventions.
Keywords: Decorin, Proteoglycan, Extracellular matrix, Tumorigenesis, Angiogenesis, Autophagy, Binding-partners
Introduction
The extracellular matrix (ECM) is renowned for its collection of molecules that governs both structural integrity and function of the cell [1–4]. An extensively studied member of the ECM is the canonical class I small, leucine-rich proteoglycan (SLRP) [5], decorin, whose complex gene is located on chromosome 12 [6]. Though famously studied in the context of collagen fibrillogenesis [7–12], decorin is not merely a structural entity. In fact, it is one of the most versatile proteoglycans regulating a range of cellular processes [13–18] including but not limited to angiogenesis [19,20], myocardial infarct [21], innate immunity [22], inflammation [23,24], diabetic nephropathy [25], fibrosis [26], wound healing [27] and autophagy [28–32]. In order to facilitate signaling through these and other pathways, decorin must interact with a variety of different classes of ligands encompassing ECM constituents, cellular receptors, growth factors, proteases, and other signaling molecules. The interactions between decorin and its ligands involve its glycosaminoglycan (GAG) chain as well as specific binding motifs in its protein core. The diversity of this binding repertoire permits decorin to partake in a multitude of functions through its ability to activate or inhibit receptor signaling [33–37], sequester growth factors [26,38–42,42,43], and, either directly or indirectly, alter the processing of key matrix components [44]. Here we provide a representation of the current decorin interactome in order to more completely define the functional role of decorin in outside-in signaling in the cell. In addition, this compendium of decorin interactions may allow for the identification of novel decorin-binding partners, which can further illuminate the role of this SLRP in normal and pathological processes as well as initiate new therapeutic approaches to combat disease.
Structure and ligand-binding regions of decorin
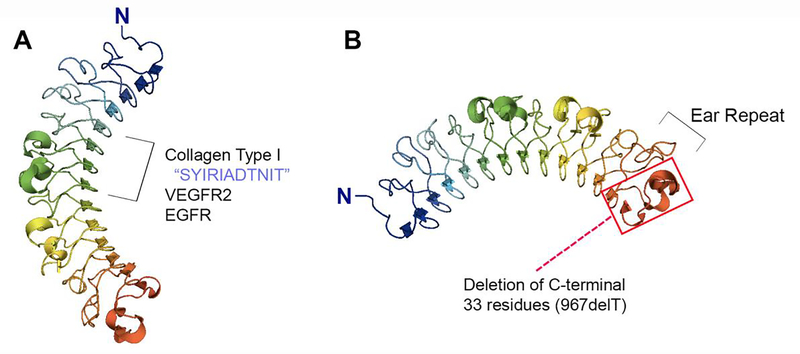
Structurally, decorin is highly conserved across multiple species where it possesses a central domain harboring 12 leucine rich repeats (LRR) and an N-terminal attachment site for a single GAG chain of chondroitin or dermatan sulfate [12] (Fig. 1). Its shape has been described as either a horseshoe or banana [45,46], where the inner concave surface comprises 14 curved β-sheets and the outer convex surface includes multiplea-helices [12]. Though the GAG chain has been shown to be important for some decorin/ligand interactions, most decorin-binding partners share an interface with decorin at its core protein. Indeed, LRR 4–6 act as high-affinity binding sites for collagen I, the most well-known binding partner of decorin [47] (Fig. 1A). In addition, these same leucine-rich regions bear the responsibility for the binding of decorin to receptor tyrosine kinases (RTKs), such as the vascular endothelial growth factor receptor 2 (VEGFR2) [48] and the epidermal growth factor receptor (EGFR) [49] (Fig. 1A).
Fig. 1.
Graphical representation of the crystal structure of monomeric bovine decorin created via Pymol v1.7 (PDB accession number 1XKU) depicting its curved appearance and leucine rich repeats. (A) LRR 4–6 are important for binding of decorin to collagen and receptor tyrosine kinases, including VEGFR2 and EGFR. (B) The penultimate ear repeat of decorin is important for proper folding of this SLRP. Mutations of decorin resulting in loss of this ear repeat cause congenital stromal corneal dystrophy, a disease characterized by corneal opacity and visual disturbances. The red box indicates the region of decorin that is lost with the most commonly observed mutation, 967delT.
Furthermore, and unsurprisingly given the aforementioned information, it appears that monomeric decorin accounts for most, if not all of its interactions, as dimerization of decorin [46,50,51] renders its core region unavailable for binding other substrates. In fact, recent binding studies demonstrate the reversibility of decorin dimerization as well as highlight the ability of decorin to alternate between forming homodimers or interacting with collagen as a monomer [50]. Additionally, in response to earlier controversy regarding the stability of decorin as a monomer in solution, these same studies illustrate that dimerization is not necessary for decorin stabilization [50]. Therefore, decorin functions as a monomer where it can act as a soluble paracrine factor to regulate a variety of downstream signaling pathways by binding to its respective partners via the concave surface of its core.
We must also emphasize the importance of the penultimate and longest LRR of decorin known as the ear repeat, a feature shared by several other SLRPs (Fig. 1B). The first mouse model developed for congenital stromal corneal dystrophy (CSCD), the only documented human disease associated with mutations in decorin [52–54], contains a C-terminal truncation of decorin protein core resulting in a shorter core lacking the C-terminal 33 amino acid residues (Fig. 1B). This deletion faithfully replicates the corneal opacification and severe visual impairment [55] seen in these patients. Mechanistically, truncated decorin (952delT) lacking the ear repeat results in a misfolded SLRP, leading to intracellular accumulation of mutated decorin and consequently activation of the ER stress response [55]. Interestingly, mice with global deletion of decorin do not exhibit this phenotype [56], suggesting that this disease is solely due to an unfolded protein response stemming from impaired secretion and, thus, intracellular accumulation of decorin. However, further study on this topic has illustrated that the 952delT mutation in the original disease model may not be consistent with human pathology as human CSCD (harboring the 967delT mutation) depends on extracellular accumulation of the truncated version of decorin [57], where this mutant decorin may act in a dominant negative fashion to obstruct normal signaling pathways. The end product is an abnormal corneal stroma where collagen fibrils lack the precise interfibrillar spacing of normal transparent cornea [58].
It is also important to note that, though the crystal structure of decorin has been elucidated and some ligand-binding sites identified at its concave surface (Fig. 1), many of the decorin/ligand interfaces remain uncharacterized.
Contemporary data regarding the decorin interacting network
Decorin embraces numerous interacting partners of different categories (Fig. 2). Here we will describe, in detail, these interactions and their physiological relevance. Though originally christened as PG40 [59], the proteoglycan we now know as decorin gained its name from the observation that it bound to and “decorated” collagen fibrils [60]. Via its core protein, decorin binds at the D period of collagen type I approximately every 67 nm [61–63]. Importantly, the N-terminal GAG chain of decorin also plays a role in arranging adjacent collagen fibrils. Specifically, the dermatan sulfate chain simultaneously binds to a separate collagen molecule in the vicinity of the collagen fiber interfacing with the decorin protein core [9]. Functionally, this interaction between decorin and collagen is crucial for proper fibril formation and fibrillar spacing as Dcn−/− mice exhibit increased fibril diameter as well as increased diameter variability, resulting in a skin fragility phenotype [64]. In addition, though the interaction between decorin and collagen type I is the best studied, decorin also binds collagens II, III, IV, V, VI, XII, and XIV [65–71] (Fig. 3), where the interaction with collagen type VI is of particularly high affinity [65,66].
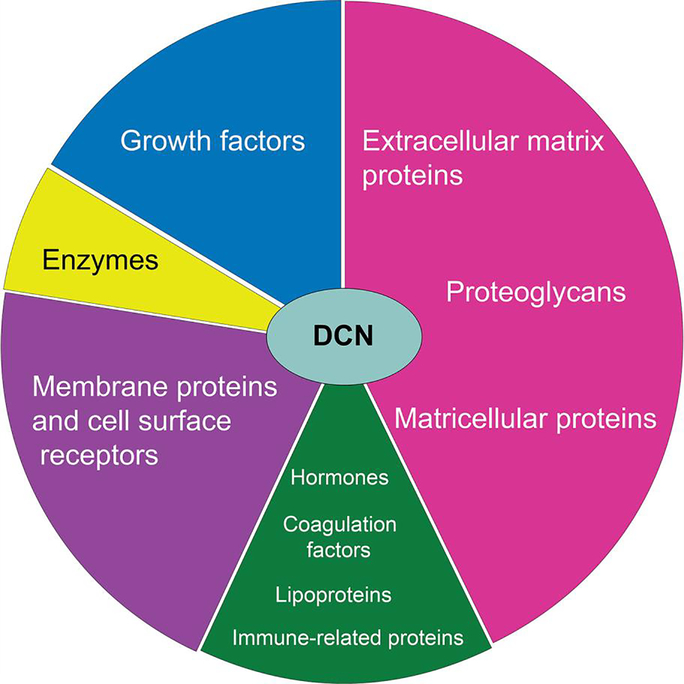
Fig. 2.
Pie chart rendition of the various categories of decorin-binding partners. Note that the most prominent types of ligands are members of the extracellular matrix, cell surface receptors, and growth factors.
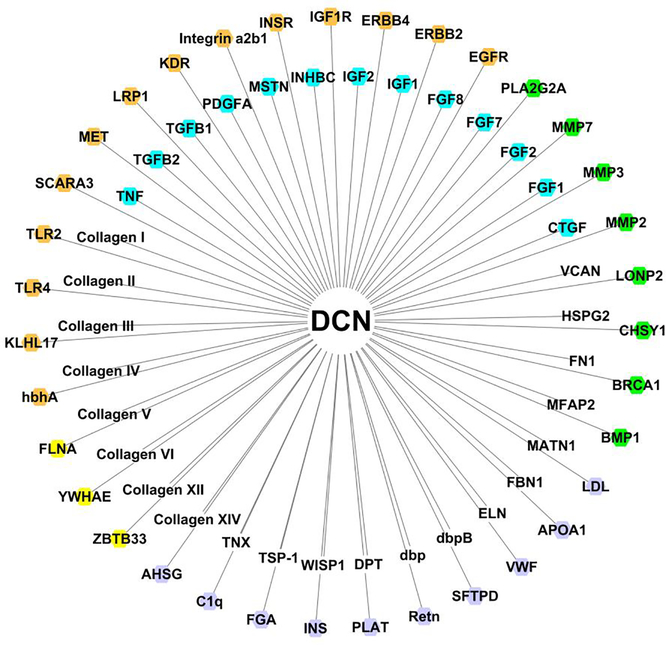
Fig. 3.
Compilation of known decorin-binding ligands depicted by their gene or the name of the multimeric protein and identified in the literature and in interaction databases of the International Molecular Exchange consortium including MatrixDB (http://matrixdb.univ-lyon1.fr) [179], No color: extracellular matrix proteins, proteoglycans, and matricellular proteins; turquoise: growth factors; orange: membrane proteins; green: enzymes; purple: hormones, (anti)-coagulation factors, immune-related and carrier proteins; yellow: intracellular proteins.
Related to collagen structure is the complex formed between decorin and matrilin-1 [72], a protein involved in matrix assembly whose relative, matrilin-3, has been associated with certain chondrodysplasias [73]. The decorin/matrilin-1 complex functions as a linkage between collagen VI and either aggrecan or collagen type II [72] (Fig. 3). Similarly, decorin interacts with dermatopontin, a low molecular weight extracellular matrix constituent involved in fibroblast adhesion and migration [74] (Fig. 3). This interaction accelerates the process of collagen fibrillogenesis as well as alters the collagen diameter [75].
Although most ligands of decorin bind to its core, an example of an unusual decorin-binding partner that does not rely on the protein core is tenascin X (Fig. 3), an extracellular glycoprotein expressed in connective tissues that modulates matrix maturation and is implicated in the pathogenesis of Ehlers-Danlos syndrome and glaucoma [76,77]. In this particular case, the GAG chain of decorin is solely responsible for its binding to tenascin X as treatment of decorin with chondroitinase ABC abrogates this interaction [78]. Interestingly, as tenascin X surrounds collagen fibrils, it is plausible that its partnership with decorin helps to preserve mechanical strength of connective tissues. Taken together, the presence of decorin in vivo is exceptionally important for proper collagen formation and matrix integrity.
Along with collagen and related matrix proteins, decorin interacts with other structural ECM components, such as microfibril-associated glycoprotein-1 (MFAP-2) [79] and fibrillin 1 [79] (Fig. 3). The microfibril-associated glycoproteins and fibrillins interact with each other and form a niche for growth factors and mechanosensation [80,81]. Notably, ADAMTS can regulate microfibril assembly [82] and latent TGFβ-binding proteins interact with microfibrils [83]. Any alterations of these fine-tuned complexes could lead to activation of TGFβ and ultimately to fibrosis [84,85].
Though these microfibril-associated proteins form a complex with decorin [79], the role of this interaction remains unknown. However, given the previously-mentioned role of decorin in the assembly and spacing of collagen fibrils, its interactions with MFAP-2 and fibrillin 1 suggest that it may be similarly involved in the organization of elastic microfibrils. Intriguingly, decorin can also interact with elastin itself via its protein core [86] (Fig. 3) where this interaction is stronger in normal ligaments vis-à-vis ossified ligaments. However, despite these interactions, Dcn−/− mice appear to have structurally and functionally normal elastic tissues [79], signifying there must be compensation by other factors for proper elastic fibril formation in the absence of decorin.
Decorin interacts with fibronectin, a protein responsible for cell adhesion, migration, and differentiation [87] (Fig. 3). As with collagen, both decorin proteoglycan and its protein core are able to interact with fibronectin [87]. In particular, amino acids 85–89 of the core protein, which harbor the NKISK sequence, seem to be especially important for this interaction [87]. However, they are not the only protein core sites responsible for this interface as competitive binding with a pentapeptide inhibitor of identical amino acid composition cannot completely prevent the interaction between decorin core and fibronectin [87]. From a functional viewpoint, as decorin treatment inhibits fibroblast adhesion to a fibronectin substrate, this interaction between decorin and fibronectin in vivo may result in impaired cell adhesion to the extracellular matrix [88].
Though its association with structural ECM members is important for matrix integrity, decorin correspondingly interacts with matrix components involved in cellular signaling. One example is thrombospondin-1 [89], a secreted protein that functions to inhibit angiogenesis and endothelial cell motility [90], as well as many other biological functions [91–97] (Fig. 3). Interestingly, in addition to acting as a ligand for this SLRP, thrombospondin-1 is also induced by decorin in MDA-231 triple-negative breast carcinoma cells via activation of the EGFR signaling axis [20]. Thus, these data suggest a complex functional role for decorin both by directly interacting with thrombospondin-1, perhaps to bring it into close proximity with its signaling receptors, as well as by regulating its expression to help thwart tumorigenesis.
Though technically a growth factor involved in WNT-signaling, we are including the decorin/WNT-inducible signaling pathway protein 1 (WISP-1) interaction in this ECM section (Fig. 3) as WISP-1 is secreted by fibroblasts where it binds to cell surface receptors to initiate signaling cascades related to proliferation and migration [98]. As it does with other growth factors, decorin binds WISP-1 via its dermatan sulfate GAG chain [99]. This interaction may contribute to the progression of tumorigenesis as the WISP-1/decorin complex likely prevents decorin from acting in an inhibitory fashion in the tumor microenvironment [99].
Also of note is the observation that, in the bovine ovarian follicle, decorin co-localizes with versican and perlecan [100] (Fig. 3), two large proteoglycans expressed in many different tissues and implicated in various signaling pathways including angiogenesis, development, and cell migration and proliferation [101–107] (Fig. 3). Hence, this information illustrates that proteoglycans, including decorin itself, comprise yet another important class of matrix constituents that are part of this wide-ranging interacting network.
We must also recognize two non-eukaryotic proteins that interact with decorin in the extracellular matrix. These spirochete-derived proteins, decorin-binding proteins A and B (Fig. 3), are vital for virulence of Borrelia burgdorferi, the pathogen responsible for Lyme disease [108–110]. Loss of both binding proteins significantly reduces the bacterial load in skin and heart [110], thereby designating an important role for decorin as a host-ligand for bacterial infection.
Overall, the class of ECM molecules as binding partners for decorin is quite extensive illustrating the propensity for decorin to support both structural integrity of the cell and cellular function. This list may not be exhaustive. Therefore, it is possible that emerging techniques with increased sensitivity will promote new discoveries that will broaden our knowledge of the decorin/ECM interactome.
A ligand for multiple receptor tyrosine kinases and membrane proteins
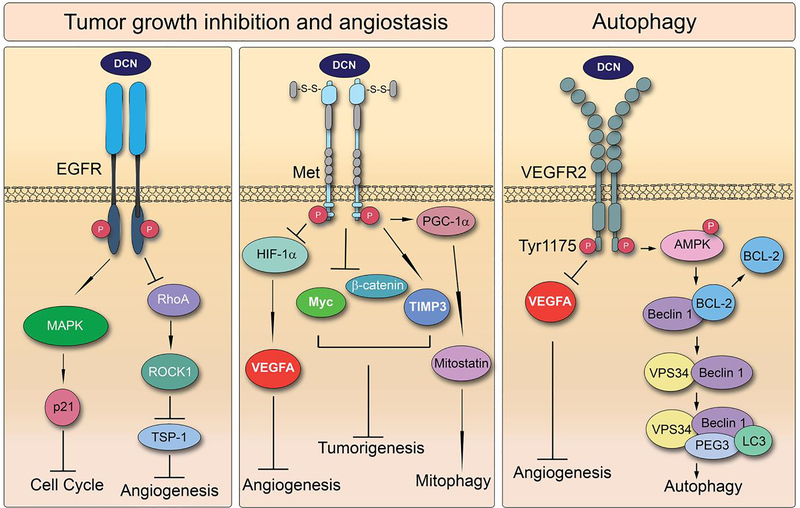
For many years, decorin has been recognized as a “guardian from the matrix” as much of its anti-tumorigenic activity results from its pan-inhibition of numerous pathways emanating from receptor tyrosine kinase signaling (Fig. 4). As such, a prominent class of decorin-binding partners resides in these cell surface receptors. The first notable decorin/RTK interaction was discovered between decorin and the epidermal growth factor receptor (EGFR) in A431 squamous carcinoma cells [37,49,111]. Indeed, decorin binds EGFR via its central leucine-rich domain leading to dimerization, phosphorylation, and ultimately caveolin-mediated degradation of the receptor, resulting in diminished activity of this signaling pathway [112] (Fig. 4). Deletion mutants of decorin demonstrate that binding to EGFR is dependent specifically upon LRR6 [49]. This interaction is particularly interesting, as decorin shares no homology with epidermal growth factor (EGF) or other related compounds despite the fact that its binding region slightly overlaps with that of the endogenous ligand [49]. Ultimately, by binding to and quelling signaling through EGFR, decorin is able to suppress the cell cycle through induction of p21WAF1, which accounts for some of decorin’s ability to curb tumorigenesis [111] (Fig. 4).
4.
Decorin-induced signaling is centralized on its ability to bind RTKs including EGFR, Met, and VEGFR2. These signaling cascades result in numerous downstream effects including cell cycle arrest, angiostasis, and autophagic induction ultimately converging on reduced tumorigenesis.
Furthermore, as EGFR is a member of the ErbB family, it comes as no surprise that decorin also interacts with other ErbB members, specifically ErbB2 and ErbB4 [113,114] (Fig. 3). By binding to ErbB4, decorin prevents dimerization with ErbB2, causing growth arrest and differentiation of breast carcinoma cells [114], again culminating in decreased tumor growth.
Another significant decorin/RTK interaction in tumor cells is that between decorin and Met, also known as hepatocyte growth factor or scatter factor receptor [36] (Fig. 3). As in the case with EGFR, decorin binding to Met results in rapid receptor phosphorylation and eventual degradation in caveolin-mediated endosomes [36]. Functionally, this interaction silences downstream Met mediators, such as β-catenin and Myc resulting in decreased tumor burden [34]. Furthermore, decorin-dependent inhibition of Met signaling also induces TIMP3, a potent anti-angiogenic protein [20]. Even more striking is that this interaction concurrently decreases HIF-1α and VEGFA, both of which are strongly pro-angiogenic [20]. Thus, the decorin/Met interaction not only decreases tumor growth due to actions of decorin directly at the tumor cell surface, but also from inhibition of blood supply to tumor cells due to alteration of the tumor secretome. Of note is the requirement of Met signaling for decorin-induced mitochondrial autophagy in cancer cells [115]. In this situation, decorin acts as a partial agonist of Met in order to induce mitostatin, an oncostatic mitochondrial protein that is required for tumor cell mitophagy [115]. Thus, a single interaction between a SLRP and an RTK leads to numerous changes in cell signaling, with the cumulative effect being diminished tumorigenesis (Fig. 4).
Unlike in cancer cells where decorin functions primarily to inhibit RTK activity, normal cells can utilize decorin as an agonist for these same signaling pathways. A classic example of this paradigm can be found in the interaction between decorin and IGF1R (Fig. 3). As a ligand for this receptor in urothelial tumor cells, decorin antagonizes IGF1R and its subsequent downstream signaling cascades resulting in decreased cell motility [116]. Conversely, in normal endothelial cells and renal fibroblasts, decorin stimulates IGF1R [117], activating the Akt pathway [117]. On a related note, decorin also binds to insulin receptor A isoform [117] resulting in inhibition of IGF-II-mediated proliferation without affecting insulin-dependent activation of mitosis [117] (Fig. 3). These findings illustrate the complexity of the decorin/receptor interactome where context-dependent interactions result in different cellular consequences contingent upon unique cellular milieux [118].
Finally, the most recent development regarding decorin/RTK interactions occurs with VEGFR2. VEGFR2 is the fundamental receptor for endothelial cell signaling where it regulates angiogenesis and other cellular processes critical for cell viability [48]. Though decorin can compete with the endogenous ligand of VEGFR2, VEGFA, to inhibit VEGFR2 signaling [119], decorin also binds VEGFR2 with high affinity and acts as a partial agonist causing an induction of autophagy in these cells [29,30] (Fig. 3). Thus, the role of the interaction between decorin and VEGFR2 is two-fold: 1. To reduce angiogenesis by inhibiting VEGFA-dependent signaling through this receptor and 2. To induce endothelial cell autophagy, which may directly inhibit angiogenesis, although the intricate details of this mechanism remain mostly uncharacterized (Fig. 4).
Though we have discussed mainly the interface between decorin and RTKs, decorin similarly interacts with the Toll-like receptors (Fig. 3). These receptors are involved in the regulation of inflammation [22], thereby adding versatility for decorin as a soluble ligand in the ECM. In particular, decorin binds TLR2/4 with high affinity [22] resulting in a pro-inflammatory response leading to the synthesis and secretion of cytokines, including TNF-α. This ability for decorin to regulate inflammation is important for understanding its potency in tumor biology, as a pro-inflammatory tumor microenvironment results in diminished tumor growth [120,121].
Related to immune responses, a receptor specifically expressed on the surface of macrophages is the Class A scavenger receptor [122]. This receptor has been implicated in the uptake and modification of lipoproteins and cholesterol [123] and its interaction with decorin results in adhesion of macrophages to the cell matrix [122] (Fig. 3). Interestingly, decorin appears to have a lower affinity for this receptor than biglycan, another class I SLRP that is structurally decorin’s closest family member [122,124]. This interaction, as well as binding of this receptor with other proteoglycans such as versican, mechanistically implicates matrix proteoglycans in the retention of macrophages in atherosclerotic lesions [122].
Moreover, decorin can interact with LDL-receptor related protein 1 (LRP-1) via LRR 5 and 6 [125–127] (Fig. 3). Specifically, LRP-1 serves as an endocytic receptor for decorin and is fundamentally involved in mediating TGF-β-dependent signaling in the setting of fibrosis, particularly in musculoskeletal diseases [128]. Given this information, decorin may be useful as a novel therapeutic intervention for dystrophic muscle disorders, many of which are characterized by excessive fibrosis, by targeting this pathway.
Furthermore, via its GAG chain, decorin interacts with α2β1 integrin (Fig. 3), though not in the canonical binding region in the A-domain of the α2 subunit [129]. This interaction may act in concert with binding of decorin to collagen type I and IGF-1R [118], resulting in crosstalk among several receptors leading to downstream consequences specifically in the context of endothelial cell signaling.
As in the previous section, there are non-eukaryotic membrane proteins that interact with decorin. A particular example is found in the heparin-binding mycobacterial surface protein (HBHA) [130] (Fig. 3). Like the previously described interface with spirochete-specific proteins, this finding demonstrates that decorin acts as a receptor for mycobacterial infection, once again implicating decorin in the facilitation of pathogen/host interactions.
Consequently, the ability to act as a ligand for a diverse array of receptors and membrane-associated proteins permits decorin to profoundly affect cell signaling. Importantly, there are likely many other membrane-associated proteins that, in the future, could be identified as members of the decorin interactome. For example, decorin is predicted to associate with Kelch-like protein 17, a membrane protein that functions as a substrate adaptor for E3 ubiquitin ligases. Thus, the current knowledge of decorin’s signaling capacity, both by its direct communication with receptors at the tumor cell surface as well as by its capability to initiate signaling cascades in crucial stromal cells, can be improved with future discovery of unique decorin ligands.
A reservoir for growth factors
Several matrix proteoglycans are important hubs for growth factors due to their ability to sequester these signaling molecules via their GAG chains. Decorin is no exception where it sequesters several different growth factors in the extracellular matrix, both by its GAG chain and also by regions in its core. Most famously, decorin harbors two binding sites of varying affinity in its core protein for TGF-β [131] (Fig. 3). Importantly, this interaction between TGF-β and decorin can occur while decorin is bound to collagen [40], suggesting a mechanism for decorin to keep TGF-β isolated in the matrix and unable to bind to its receptors to initiate growth factor-dependent signaling. Indeed, high concentrations of decorin in vitro inhibit TGF-β signaling [132,133]. In functional studies in vivo, decorin’s aptitude for binding to TGF-β decreases fibrosis [26]. Interestingly, a complex relationship between decorin and TGF-β exists in that TGF-β is able to reciprocally inhibit decorin gene expression in fibroblasts [134] suggesting an intricate feedback loop between these two factors as a potential means of maintaining cellular homeostasis.
Myostatin, another TGF-β superfamily member that negatively regulates muscle mass [135], binds the protein core of decorin in a zinc-dependent manner [136] (Fig. 3). This interaction results in its sequestration, preventing myostatin-dependent inhibition of myoblast proliferation [136]. Furthermore, connective tissue growth factor (CTGF) is a TGF-β-inducible factor involved in the onset and progression of fibrosis [137]. Interestingly, Dcn−/− myoblasts respond more acutely to CTGF than their wild-type counterparts, suggesting an inhibitory role for decorin in this growth factor-mediated pathway [41]. Indeed, LRR 10–12 of the core protein of decorin bind CTGF, which negatively regulates the activity of this important cytokine [41] (Fig. 3). Moreover, CTGF also stimulates decorin expression [41], suggesting, as in the case of TGF-β, a complex feedback mechanism for this biological pathway. In addition, decorin binds activin C, another TGF-β relative [138] (Fig. 3). This interaction prevents proliferation and migration of colorectal cancer cells by promoting caveolin-mediated degradation of this growth factor [138].
TNF-α, a cytokine known to be involved in the inflammatory response, is another factor that shares an interface with decorin [139] (Fig. 3). Like TGF-β, TNF-α is sequestered away from its receptors by decorin in order to dampen the inflammatory response under normal conditions. This hypothesis is curious as decorin itself induces a pro-inflammatory environment via its direct interaction with TLR2/4 [22]. Thus, the overall function of decorin in inflammation appears to be quite sophisticated and may depend on other inflammatory mediators as well as the setting of inflammation (i.e. whether it is due to microbial pathogenesis or cancer). Furthermore, in analogy to TGF-β, TNF-α is also capable of downregulating decorin expression in fibroblasts, further suggesting an elaborate regulatory network for these signaling molecules [134].
Though decorin typically interferes with RTK signaling by interacting with the receptor as described in the section above, its attenuation of the platelet-derived growth factor receptor (PDGFR) pathway is unique and occurs through a direct interaction between decorin and the ligand, platelet-derived growth factor (PDGF) [42] (Fig. 3). Functionally, this interaction prevents hepatic fibrosis [140] as well as intimal hyperplasia through obstruction of this signaling pathway [140]. Therefore, this interaction exemplifies the multi-faceted ability of decorin to alter RTK signaling.
As mentioned in a previous section, decorin plays an important role in the regulation of the insulin signaling pathway via its direct interaction with IGF1R and the insulin receptor A isoform [117]. Decorin also binds IGF-I, although with a much lower affinity than the typical binding partners of this growth factor [118] (Fig. 3). It is likely that this interaction does not occur much endogenously, but rather only when decorin concentrations greatly exceed normal levels, as would be utilized in a therapeutic context, since it can then compete with endogenous IGF-I binding proteins. Furthermore, decorin binds IGF-II and insulin [141] (Fig. 3), illustrating that the function of decorin in this signaling pathway is quite sophisticated.
Finally, another important group of growth factors that interacts with decorin is the fibroblast growth factor (FGF) family. Specifically, FGF 1, 2, 7, and 8 bind the dermatan sulfate chain of decorin [142] (Fig. 3). In the context of wound healing [27], these interactions suggest a potential mechanism for decorin to localize these factors near their cognate receptors.
In summary, the capacity for decorin to bind growth factors is crucial for the understanding of how decorin modifies signaling pathways in concert with its ability to directly interact with cell surface receptors. This current knowledge may prompt future studies to find novel small, molecule drugs that can be used as analogs of decorin in the treatment of diseases such as cancer or fibrosis.
At the interface with proteases and other enzymes
Though not a ligand in the traditional sense, proteases are notable players in the decorin interacting network. These proteases primarily include the matrix metalloproteinases 2, 3, and 7 [143–146] (Fig. 3), all of which are involved in turnover of the extracellular matrix and possess the capacity to digest decorin. MMP2 (gelatinase A), MMP3 (stromelysin) and MMP7 (matrilysin) cleave decorin into fragments via interactions with its protein core where MMP7 demonstrates the highest efficiency for this degradation [147]. Since the MMPs digest decorin at its core protein, it is possible that this enzymatic action may hinder the ability of decorin to bind collagen, thereby disrupting matrix stability. Furthermore, as TGF-β and TNF-α also bind to the protein core of decorin, the activity of these MMPs toward decorin can correspondingly allow for the release of these growth factors, further perturbing normal signaling in the context of fibrosis and inflammation [148]. Likewise, BMP-1, a peptidase involved in bone and cartilage development, also processes decorin in a manner similar to the MMPs [149,150] (Fig. 3).
Additionally, as decorin has been implicated in atherosclerosis, it comes as no surprise that phospholipase A(2) group IIA is a member of the decorin interactome [151] (Fig. 3). Functionally, this enzyme is involved in collagen assembly in the extracellular matrix of atherosclerotic plaques [152]. This interaction with decorin serves to activate this enzyme resulting in the pathogenesis of atherosclerosis by mediating the release of inflammatory lipids and modifying lipoproteins retained in the arterial subendothelium [152].
Lastly, other enzymes such as chondroitin synthase I, lon protease homolog 2 (peroxisomal), and breast cancer susceptibility protein have been suggested as potential members of the decorin interactome (Fig. 3). However, specific data are limited regarding the exact nature of these interactions. Overall, it is noteworthy to comment on the extensive range of different known and predicted decorin ligands in order to underscore the importance of decorin in cell signaling.
Other decorin/ligand interactions
Though the main binding partners of decorin have been discussed extensively in the previous sections, there exist other proteins that do not fit neatly into the aforementioned categories that interact with this SLRP. One prominent protein is Von Willebrand factor (VWF), a glycoprotein involved in the adhesion of platelets to the endothelial matrix [153]. Following endothelial injury, VWF binds to platelets to promote rolling and tethering of these cells at the site of injury to allow for clot formation [154]. The binding between decorin and VWF is saturable and dose-dependent and, like the case of tenascin X, involves the GAG chain of decorin [155] (Fig. 3). However, the interaction between decorin and tenascin X relies on the dermatan sulfate side chain of decorin whereas the composition of GAG chain does not seem to matter appreciably in the interaction between decorin and VWF [155].
Decorin also interacts with other members related to the coagulation cascade including fibrinogen [156] and tissue plasminogen activator [157] (Fig. 3). The decorin/fibrinogen interaction is zinc-dependent and functionally results in the ability of decorin to modulate clot formation [156]. Decorin concurrently causes increased tissue-type plasminogen activator-dependent fibrinolysis [157], validating the importance of ECM proteoglycans in the regulation of wound repair, clotting, and thrombus formation and breakdown.
Another example of an uncategorized decorin-binding partner is the apolipoprotein portion of lipoprotein(a). Lipoprotein(a) is a lipoprotein particle containing apolipoprotein(a) [apo(a)], which varies in size based on different isoforms [158]. This apo(a) protein binds via its kringle domain to decorin’s protein core, but not to its GAG chain [159] (Fig. 3). As apo(a) is involved in the inflammatory response of the arterial wall in atherosclerosis, it is possible that this connection between apo(a) and decorin may be linked to cardiovascular disease by causing retention of the pro-atherogenic lipoprotein(a) in atherosclerotic lesions [159]. Moreover, LDL itself is a known decorin ligand [160] as it binds the negatively charged GAG chains of this SLRP [160,161] (Fig. 3). This interaction serves to enhance the association between collagen and LDL subsequently resulting in increased retention of LDL in blood vessels [160].
Though decorin primarily interacts with ECM and cell surface-associated proteins, it also peculiarly binds filamin A (Fig. 3), a cytoskeletal protein [162]. Interestingly, this interaction occurs only between the de-glycosylated form of decorin suggesting that decorin potentially interacts with filamin A prior to its GAG modification and secretion. However, studies in neuroblastoma cells suggest that the C-terminus of filamin A may be accessible to the ECM, allowing for this interaction [163].
Other unclassified decorin-binding ligands include resistin [164], an adipokine implicated in hypercholesterolemia and insulin resistance; pulmonary surfactant-associated protein D [165], a surfactant protein involved in immunity, alveolar-wall remodeling, and lipid homeostasis; complement component C1q, a crucial member of the classical complement cascade [166]; and α2 HS glycoprotein, a serum protein involved in bone resorption and lymphocyte reactivity [167] (Fig. 3). These examples further emphasize the promiscuity of decorin in terms of binding partners, which allows it to possess a breadth of functions ranging from proliferation to inflammation. These functions support the importance of decorin in vivo and truly accentuate its designation as “guardian from the matrix.”
Overarching themes in decorin signaling
As its interactome suggests, decorin is unmistakably versatile. Though it is associated with other ancillary roles, the web of cellular processes linked to decorin consists of two main categories: cellular infrastructure and tumor stasis. Structurally, decorin maintains extracellular matrix form, which in turn supports matrix function. As mentioned above, an example of this tenet is seen in Dcn−/− mice, which exhibit skin fragility [64], illuminating the necessity for this small, leucine-rich proteoglycan in the maintenance of proper collagen assembly to yield a normal phenotype.
The function of decorin in tumor stasis is much more complicated as it involves a variety of processes that interconnect to yield a cumulative anti-tumorigenic effect. First, through its interaction with RTKs, decorin can directly alter the tumor parenchyma to inhibit cell growth and migration [34,115,168–173] (Fig. 4). Additionally, decorin modifies the secretome of tumor cells as evidenced by its ability to reduce the efflux of pro-angiogenic signaling factors, like VEGFA, while simultaneously promoting the secretion of anti-angiogenic proteins, like thrombospondin-1 [20,170]. Moreover, the concurrent deletion of decorin and p53 in mice results in more aggressive tumorigenesis than in mice harboring only a deletion of p53 [174], and mice deficient in decorin, when challenged with a hypercaloric diet, develop intestinal tumors [175,176]. All these studies emphasize the importance of decorin as a potent tumor repressor.
Complementing these mechanisms is the capability of decorin to interact in a straight-forward manner with the tumor microenvironment. For example, a recent microarray screen capable of detecting differences in gene expression in the tumor proper versus the stroma of xenografts derived from MDA-231 triple-negative breast carcinoma cells, illustrates that decorin-dependent genetic alterations occur predominantly in the stroma [177]. These findings and follow-up analyses point to an elaborate signaling nexus for decorin in supporting cells that, with future inquiry, may provide more detailed answers for the mechanisms by which decorin curtails tumor growth. Specifically, decorin induces autophagy in endothelial cells via a Peg3-dependent mechanism stemming from its interaction with VEGFR2 [29,30]. These findings link a soluble matrix proteoglycan to the induction of a pro-autophagic complex consisting of Beclin 1 and LC3 (Fig. 4). As several studies posit a role for decorin in diminishing angiogenesis and, as we have shown that decorin induces autophagy exclusively in endothelial cells, it is possible that the two processes are linked. Interestingly, thrombospondin-1, both a ligand and downstream effector of decorin, can induce autophagy in RAS-positive cancer cells [178]. This autophagic induction inhibits tumor growth, and thus, may be another mechanism by which decorin-mediated thrombospondin-1 secretion is necessary to combat tumorigenesis. As we continue to study the impact of decorin on endothelial cell signaling, we hope to pinpoint the exact responsibility decorin holds in the regulation of these key pathways.
The story becomes even more complex as decorin gene expression is induced itself by pro-autophagic stimuli such as starvation (in mice) and serum deprivation (in cells). Specifically, decorin is an autophagy-inducible proteoglycan where inhibition of canonical mTOR signaling is capable of stimulating decorin gene and protein expression both in fibroblasts and in vivo in cardiac tissue [28,31]. As fibroblasts are one of the few cell types that endogenously express and secrete decorin, it is possible that pro-autophagic stimuli may evoke decorin secretion from fibroblast cells, leading to increased levels of autophagy in surrounding stromal cells by soluble decorin. In turn, this may lead to decreased tumorigenesis. Taken together, the ability to stimulate endogenous decorin levels proposes an inviting non-toxic and efficacious therapeutic option for quelling tumor growth. In addition, the decorin interactome itself may change in a pro-autophagic environment. The comparison of decorin-binding partners under normal conditions versus conditions following stimulation by pro-autophagic compounds may be a worthwhile endeavor to undertake in the matrix biology field in the future.
Overall, a predominant theme that emerges from studying the decorin interacting network appears to be related to reducing tumorigenesis by several different mechanisms. These mechanisms include altering signaling pathways in tumor cells themselves as well as by reducing tumor angiogenesis, partly through modification of the tumor secretome and possibly also through the induction of autophagy in vascular cells (Fig. 4). Additionally, the secondary, but imperative, theme of maintaining extracellular matrix structure by decorin cannot be pushed to the wayside, as this subject is where the significance of decorin in vivo began. Accordingly, a greater comprehension of the decorin interactome is quite important as the knowledge gained from these and prospective studies should allow for a better understanding of how decorin functions both in standard as well as in aberrant cell signaling.
Final considerations and future directions
We have just hit the tip of the iceberg regarding understanding the vast signaling capabilities of decorin. To add to the current awareness of the many functions of this SLRP, it is essential that we parse out additional details regarding the decorin interactome as so many of its abilities rely on its interaction with different signaling molecules. For example, future work in this field may identify novel decorin-binding partners, which can provide supplementary data about the intricacies of current signaling pathways as well as link previously unrelated cellular processes. Additionally, as decorin is one of many proteoglycans in the family of SLRPs, it is feasible to assume that other homologous SLRPs may share partners in this interactome, resulting in a sophisticated signaling network that can be altered under diverse cellular conditions. Therefore, we hope that this review provides a cohesive compilation of the current understanding of the decorin interacting network as well as sparks innovative research to decipher more of the mysteries surrounding extracellular matrix signaling complexes.
Highlights.
Decorin embraces a variety of binding partners branding it as a multi-purpose small, leucine-rich proteoglycan
Structural integrity and cellular signaling are two prominent themes found in the decorin interactome
Decorin anti-tumorigenic effects result from multiple concurrent interactions with receptor tyrosine kinases
Emerging functions include angiostasis and autophagic induction
Acknowledgements
We would like to thank all current and previous members of the Iozzo laboratory as well as our long-standing collaborators and fellow scientists who have made important contributions to the knowledge surrounding decorin and its interactome. Some of the original research was supported in part by the National Institutes of Health grants RO1 CA39481, RO1 CA47282, and RO1 CA164462 (to RVI). M. A. Gubbiotti was supported in part by NIH training grant T32 AA07463.
Abbreviations used
- Akt/PKB
protein kinase B
- apo(a)
apolipoprotein a1
- BMP
bone morphogenetic protein
- CTGF
connective tissue growth factor
- Dcn
decorin
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ErbB
erythroblastic leukemia viral oncogene homolog
- FGF
fibroblast growth factor
- GAG
glycosaminoglycan
- HBHA
heparin-binding mycobacterial hemagglutinin
- HIF-1α
hypoxia-inducible factor-1α
- IGF
insulin-like growth factor
- IGF-1R
insulin-like growth factor 1 receptor
- LC3
microtubule-associated light chain protein 3
- LDL
low-density lipoprotein; LRP-1, LDL receptor-related protein 1
- LRR
leucine-rich repeat
- MAGP1
microfibril-associated glycoprotein 1
- Met
hepatocyte growth factor receptor
- MMP
matrix metalloproteinase
- mTOR
mammalian target of rapamycin
- Myc
avian myelocytomatosis virus oncogene cellular homolog
- p21Waf1
cyclin-dependent kinase inhibitor 1
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- Peg3
paternally expressed gene 3
- PG40
proteoglycan 40
- RTK
receptor tyrosine kinase
- SLRP
small, leucine-rich proteoglycan
- TGF-β
transforming growth factor β
- TIMP3
tissue inhibitor of metalloproteinase 3
- TLR2/4
toll-like receptor 2/4
- TNF-α
tumor necrosis factor alpha
- VEGFA
vascular endothelial growth factor A
- VEGFR2
vascular endothelial growth factor receptor 2
- VWF
von Willebrand factor
- WISP1
WNT1-inducible signaling pathway protein 1
- WNT
wingless-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Iozzo RV, The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth, Crit. Rev. Biochem. Mol. Biol 32 (1997) 141–174, [DOI] [PubMed] [Google Scholar]
- [2].Iozzo RV, Schaefer L, Proteoglycans in health and disease: Novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans, FEBS J. 277 (2010) 3864–3875, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Iozzo RV, Sanderson RD, Proteoglycans in cancer biology, tumour microenvironment and angiogenesis, J. Cell. Mol. Med 15 (2011) 1013–1031, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO, The extracellular matrix: Tools and insights for the “omics” era, Matrix Biol. 49 (2016) 10–24, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Iozzo RV, Schaefer L, Proteoglycan form and function: A comprehensive nomenclature of proteoglycans, Matrix Biol. 42 (2015) 11–55, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Danielson KG, Fazzio A, Cohen I, Cannizzaro LA, Eichstetter I, Iozzo RV, The human decorin gene: intron-exon organization, discovery of two alternatively spliced exons in the 5’ untranslated region, and mapping of the gene to chromosome 12q23, Genomics 15 (1993) 146–160, [DOI] [PubMed] [Google Scholar]
- [7].Keene DR, San Antonio JD, Mayne R, McQuillan DJ, Sarris G, Santoro SA et al. , Decorin binds near the C terminus of type I collagen, J. Biol. Chem 275 (2000) 21801–21804, [DOI] [PubMed] [Google Scholar]
- [8].Reed CC, Iozzo RV, The role of decorin in collagen fibrillogenesis and skin homeostasis, Glycoconj. J 19 (2002) 249–255, [DOI] [PubMed] [Google Scholar]
- [9].Rühland C, Schönherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P et al. , The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis, FEBS J. 274 (2007) 4246–4255, [DOI] [PubMed] [Google Scholar]
- [10].Robinson PS, Lin TW, Jawad AF, Iozzo RV, Soslowsky LJ, Investigating tendon fascicle structure-function relationship in a transgenic age mouse model using multiple regression models, Ann. Biomed. Eng 32 (2004) 924–931, [DOI] [PubMed] [Google Scholar]
- [11].Sanches JCT, Jones CJP, Aplin JD, Iozzo RV, Zorn TMT, Oliveira SF, Collagen fibril organization in the pregnant endometrium of decorin-deficient mice, J. Anat 216 (2010) 144–155, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Weber IT, Harrison RW, Iozzo RV, Model structure of decorin and implications for collagen fibrillogenesis, J. Biol. Chem 271 (1996) 31767–31770, [DOI] [PubMed] [Google Scholar]
- [13].Nikolovska K, Renke JK, Jungmann O, Grobe K, Iozzo RV, Zamfir AD et al. , A decorin-deficient matrix affects skin chondroitin/dermatan sulfate levels and keratinocyte function, Matrix Biol. 35 (2014) 91–102, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen S, Young MF, Chakravarti S, Birk DE, Interclass small leucine-rich repeat proteoglycan interactions regulate collagen fibrillogenesis and corneal stromal assembly, Matrix Biol. 35 (2014) 103–111, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu Z, Horgan CE, Carr O, Owens RT, Iozzo RV, Lechner BE, Biglycan and decorin differentially regulate signaling in the fetal membranes, Matrix Biol. 35 (2014) 266–275, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Kumar A et al. , The injury response of aged tendons in the absence of biglycan and decorin, Matrix Biol. 35 (2014) 232–238, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Häkkinen L, Strassburger S, Kahari VM, Scott PG, Eichstetter I, Iozzo RV et al. , A role for decorin in the structural organization of periodontal ligament, Lab. Invest 80 (2000) 1869–1880, [DOI] [PubMed] [Google Scholar]
- [18].Ichii M, Frank MB, Iozzo RV, Kincade PW, The canonical Wnt pathway shapes niches supportive of hematopoietic stem/progenitor cells, Blood 119 (2012) 1683–1692, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Järveläinen H, Sainio A, Wight TN, Pivotal role for decorin in angiogenesis, Matrix Biol. 43 (2015) 15–26, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Neill T, Painter H, Buraschi S, Owens RT, Lisanti MP, Schaefer L et al. , Decorin antagonizes the angiogenic network. Concurrent inhibition of Met, hypoxia inducible factor-1α and vascular endothelial growth factor A and induction of thrombospondin-1 and TIMP3, J. Biol. Chem 287 (2012) 5492–5506, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Weis SM, Zimmerman SD, Shah M, Covell JW, Omens JH, Ross J Jr. et al. , A role for decorin in the remodeling of myocardial infarction, Matrix Biol. 24 (2005) 313–324, [DOI] [PubMed] [Google Scholar]
- [22].Frey T, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L, Biological interplay between proteoglycans and their innate immune receptors in inflammation, FEBS J. 280 (2013) 2165–2179, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bocian C, Urbanowitz AK, Owens RT, Iozzo RV, Gotte M, Seidler DG, Decorin potentiates interferon-gamma activity in a model of allergic inflammation, J. Biol. Chem 288 (2013) 12699–12711, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Borges MC, Narayanan V, Iozzo RV, Ludwig MS, Deficiency of decorin induces expression of Foxp3 in CD4(+) CD25(+) T cells in a murine model of allergic asthma, Respirology. 20 (2015) 904–911, [DOI] [PubMed] [Google Scholar]
- [25].Williams KJ, Qiu G, Usui HK, Dunn SR, McCue P, Bottinger E et al. , Decorin deficiency enhances progressive nephropathy in diabetic mice, Am. J. Pathol 171 (2007) 1441–1450, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baghy K, Iozzo RV, Kovalszky I, Decorin-TGFβ axis in hepatic fibrosis and cirrhosis, J. Histochem. Cytochem 60 (2012) 262–268, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Höök M, Iozzo RV et al. , A role for decorin in cutaneous wound healing and angiogenesis, Wound Rep. Reg 14 (2006) 443–452, [DOI] [PubMed] [Google Scholar]
- [28].Gubbiotti MA, Neill T, Frey H, Schaefer L, Iozzo RV, Decorin is an autophagy-inducible proteoglycan and is required for proper in vivo autophagy, Matrix Biol. 48 (2015) 14–25, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Neill T, Torres AT, Buraschi S, Iozzo RV, Decorin has an appetite for endothelial cell autophagy, Autophagy 9 (2013) 1626–1628, [DOI] [PubMed] [Google Scholar]
- [30].Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT et al. , Decorin causes autophagy in endothelial cells via Peg3, Proc. Natl. Acad. Sci. U. S. A 110 (2013) E2582–E2591, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gubbiotti MA, Iozzo RV, Proteoglycans regulate autophagy via outside-in signaling: An emerging new concept, Matrix Biol. 48 (2015) 6–13, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Goyal A, Neill T, Owens RT, Schaefer L, Iozzo RV, Decorin activates AMPK, an energy sensor kinase, to induce autophagy in endothelial cells, Matrix Biol. 34 (2014) 46–54, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dellett M, Hu W, Papadaki V, Ohnuma S, Small leucine rich proteoglycan family regulates multiple signalling pathways in neural development and maintenance, Dev. Growth Differ 54 (2012) 327–340, [DOI] [PubMed] [Google Scholar]
- [34].Buraschi S, Pal N, Tyler-Rubinstein N, Owens RT, Neill T, Iozzo RV, Decorin antagonizes Met receptor activity and downregulates β-catenin and Myc levels, J. Biol. Chem 285 (2010) 42075–42085, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Csordás G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D et al. , Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo, J. Biol. Chem 275 (2000) 32879–32887, [DOI] [PubMed] [Google Scholar]
- [36].Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ et al. , Decorin is a novel antagonistic ligand of the Met receptor, J. Cell Biol 185 (2009) 743–754, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Iozzo RV, Moscatello D, McQuillan DJ, Eichstetter I, Decorin is a biological ligand for the epidermal growth factor receptor, J. Biol. Chem 274 (1999) 4489–4492, [DOI] [PubMed] [Google Scholar]
- [38].Ferdous Z, Wei VM, Iozzo RV, Höök M, Grande-Allen KJ, Decorin-transforming growth factor-β interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices, J. Biol. Chem 282 (2007) 35887–35898, [DOI] [PubMed] [Google Scholar]
- [39].Jarvinen TA, Prince S, Decorin: A Growth Factor Antagonist for Tumor Growth Inhibition, Biomed. Res Int 2015 (2015) 654765, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Markmann A, Hausser H, Schonherr E, Kresse H, Influence of decorin expression on transforming growth factor-beta-mediated collagen gel retraction and biglycan induction, Matrix Biol. 19 (2000) 631–636, [DOI] [PubMed] [Google Scholar]
- [41].Vial C, Gutierrez J, Santander C, Cabrera D, Brandan E, Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity, J. Biol. Chem 286 (2011) 24242–24252, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Baghy K, Horvath Z, Regos E, Kiss K, Schaff Z, Iozzo RV et al. , Decorin interferes with platelet-derived growth factor receptor signaling in experimental hepatocarcinogenesis, FEBS J. 280 (2013) 2150–2164, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Baghy K, Tatrai P, Regos E, Kovalszky I, Proteoglycans in liver cancer, World J. Gastroenterol 22 (2016) 379–393, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mimura T, Han KY, Onguchi T, Chang J-H, Kim T-I, Kojima T et al. , MT1-MMP-mediated cleavage of decorin in corneal angiogenesis, J. Vasc. Res 46 (2008) 541–550, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Scott JE, Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen, Biochemistry 35 (1996) 8795–8799, [DOI] [PubMed] [Google Scholar]
- [46].Scott PG, McEwan PA, Dodd CM, Bergmann EM, Bishop PN, Bella J, Crystal structure of the dimeric protein core of decorin, the archetypal small leucine-rich repeat proteoglycan, Proc. Natl. Acad. Sci. USA 101 (2004) 15633–15638, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Svensson L, Heinegård D, Oldberg Å, Decorin binding sites for collagen type I are mainly located in leucine rich repeats 4–5, J. Biol. Chem 270 (1995) 20712–20716, [DOI] [PubMed] [Google Scholar]
- [48].Khan GA, Girish GV, Lala N, DiGuglielmo GM, Lala PK, Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast, Mol. Endocrinol 25 (2011) 1431–1443, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Santra M, Reed CC, Iozzo RV, Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping with but distinct from the EGF-binding epitope, J. Biol. Chem 277 (2002) 35671–35681, [DOI] [PubMed] [Google Scholar]
- [50].Islam M, Gor J, Perkins SJ, Ishikawa Y, Bãchinger HS, Hohenester E, The concave face of decorin mediates reversible dimerization and collagen binding, J. Biol. Chem 288 (2013) 35526–35533, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE et al. , Biologically active decorin is a monomer in solution, J. Biol. Chem 279 (2004) 6606–6612, [DOI] [PubMed] [Google Scholar]
- [52].Bredrup C, Knappskog PM, Majewski J, Rødahl E, Boman H, Congenital stromal dystrophy of the cornea caused by a mutation in the decorin gene, Inv. Ophtalm. Vis. Sci 46 (2005) 420–426, [DOI] [PubMed] [Google Scholar]
- [53].Kim J-H, Ko JM, Lee I, Kim JY, Kim MJ, Tchah H, A novel mutation of the decorin gene identified in a korean family with congenial hereditary stromal dystrophy, Cornea 30 (2011) 1473–1477, [DOI] [PubMed] [Google Scholar]
- [54].Rødahl E, Van Ginderdeuren R, Knappskog PM, Bredrup C, Boman H, A second decorin frame shift mutation in a family with congenital stromal corneal dystrophy, Am. J. Opthalmol 142 (2006) 520–521, [DOI] [PubMed] [Google Scholar]
- [55].Chen S, Sun M, Meng X, Iozzo RV, Kao WWY, Birk DE, Pathophysiological mechanisms of autosomal dominant congenital stromal corneal dystrophy. C-terminal-truncated decorin results in abnormal matrix assembly and altered expression of small leucine-rich proteoglycans, Am. J. Pathol 179 (2011) 2409–2419, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Breuer B, Schmidt G, Kresse H, Non-uniform influence of transforming growth factor-β on the biosynthesis of different forms of small chondoitin sulphate/dermatan sulphate proteoglycan, Biochem. J 269 (1990) 551–554, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mellgren AE, Bruland O, Vedeler A, Saraste J, Schonheit J, Bredrup C et al. , Development of congenital stromal corneal dystrophy is dependent on export and extracellular deposition of truncated decorin, Invest. Ophthalmol. Vis. Sci 56 (2015) 2909–2915, [DOI] [PubMed] [Google Scholar]
- [58].Kamma-Lorger CS, Pinali C, Martinez JC, Harris J, Young RD, Bredrup C et al. , Role of Decorin Core Protein in Collagen Organisation in Congenital Stromal Corneal Dystrophy (CSCD), PLoS ONE 11 (2016) e0147948, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Adany R, Heimer R, Caterson B, Sorrell JM, Iozzo RV, Altered expression of chondroitin sulfate proteoglycan in the stroma of human colon carcinoma. Hypomethylation of PG-40 gene correlates with increased PG-40 content and mRNA levels, J. Biol. Chem 265 (1990) 11389–11396, [PubMed] [Google Scholar]
- [60].Ruoslahti E, Structure and biology of proteoglycans, Annu. Rev. Cell Biol 4 (1988) 229–255, [DOI] [PubMed] [Google Scholar]
- [61].Scott JE Dermatan sulfate proteoglycans, London, Portland Press, 1993. [Google Scholar]
- [62].Scott JE, Orford CR, Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region, Biochem. J 197 (1981) 213–216, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Font B, Eichenberger D, Goldschmidt D, Boutillon M-M, Hulmes DJS, Structural requirements for fibromodulin binding to collagen and the control of type I collagen fibrillogenesis. Critical roles for disulphide bonding and the C-terminal region, Eur. J. Biochem 254 (1998) 580–587, [DOI] [PubMed] [Google Scholar]
- [64].Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV, Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility, J. Cell Biol 136 (1997) 729–743, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bidanset DJ, Guidry C, Rosenberg LC, Choi HU, Timpl R, Höök M, Binding of the proteoglycan decorin to collagen type VI, J. Biol. Chem 267 (1992) 5250–5256, [PubMed] [Google Scholar]
- [66].Nareyeck G, Seidler DG, Troyer D, Rauterberg J, Krese H, Schönherr E, Differential interactions of decorin and decorin mutants with type I and type VI collagens, Eur. J. Biochem 271 (2004) 3389–3398, [DOI] [PubMed] [Google Scholar]
- [67].Font B, Aubert-Foucher E, Goldschmidt D, Eichenberger D, van der Rest M, Binding of collagen XIV with dermatan sulfate side chain of decorin, J. Biol. Chem 268 (1993) 25015–25018, [PubMed] [Google Scholar]
- [68].Font B, Eichenberger D, Rosenberg LM, van der Rest M, Characterization of the interactions of Type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin, Matrix Biol. 15 (1996) 341–348, [DOI] [PubMed] [Google Scholar]
- [69].Vogel KG, Paulsson M, Heinegard D, Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon, Biochem. J 223 (1984) 587–597, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ramamurthy P, Hocking AM, McQuillan DJ, Recombinant decorin glycoforms. Purification and structure, J. Biol. Chem 271 (1996) 19578–19584, [DOI] [PubMed] [Google Scholar]
- [71].Douglas T, Hempel U, Mietrach C, Viola M, Vigetti D, Heinemann S et al. , Influence of collagen-fibril-based coatings containing decorin and biglycan on osteoblast behavior, J. Biomed. Mater. Res 84A (2008) 805–816, [DOI] [PubMed] [Google Scholar]
- [72].Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegard D et al. , Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan, J. Biol. Chem 278 (2003) 37698–37704, [DOI] [PubMed] [Google Scholar]
- [73].Otten C, Wagener R, Paulsson M, Zaucke F, Matrilin-3 mutations that cause chondrodysplasias interfere with protein trafficking while a mutation associated with hand osteoarthritis does not, J. Med. Genet 42 (2005) 774–779, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Liu X, Meng L, Shi Q, Liu S, Cui C, Hu S et al. , Dermatopontin promotes adhesion, spreading and migration of cardiac fibroblasts in vitro, Matrix Biol 32 (2013) 23–31, [DOI] [PubMed] [Google Scholar]
- [75].Takeda U, Utani A, Wu J, Adachi E, Koseki H, Taniguchi M et al. , Targeted disruption of dermatopontin causes abnormal collagen fibrillogenesis, J. Invest Dermatol 119 (2002) 678–683, [DOI] [PubMed] [Google Scholar]
- [76].Burch GH, Gong Y, Liu W, Dettman RW, Curry CJ, Smith L et al. , Tenascin-X deficiency is associated with Ehlers-Danlos syndrome, Nat. Genet. 17 (1997) 104–108, [DOI] [PubMed] [Google Scholar]
- [77].Wallace DM, Murphy-Ullrich JE, Downs JC, O’Brien CJ, The role of matricellular proteins in glaucoma, Matrix Biol. 37 (2014) 174–182, [DOI] [PubMed] [Google Scholar]
- [78].Elefteriou F, Exposito J-Y, Garrone R, Lethias C, Binding of tenascin-X to decorin, FEBS Lett 495 (2001) 44–47, [DOI] [PubMed] [Google Scholar]
- [79].Trask BC, Trask TM, Broekelmann T, Mecham RP, The microfibrillar proteins MAGP-1 and fibrillin-1form a ternary complex with the chondroitin sulfate proteoglycan decorin, Mol. Biol. Cell 11 (2000) 1499–1507, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mecham RP, Gibson MA, The microfibril-associated glycoproteins (MAGPs) and the microfibrillar niche, Matrix Biol. 47 (2015) 13–33, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sengle G, Sakai LY, The fibrillin microfibril scaffold: A niche for growth factors and mechanosensation?, Matrix Biol. 47 (2015) 3–12, [DOI] [PubMed] [Google Scholar]
- [82].Hubmacher D, Apte SS, ADAMTS proteins as modulators of microfibril formation and function, Matrix Biol. 47 (2015) 34–43, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB, Latent TGF-beta-binding proteins, Matrix Biol. 47 (2015) 44–53, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Blaauboer ME, Boeijen FR, Emson CL, Turner SM, Zandieh-Doulabi B, Hanemaaijer R et al. , Extracellular matrix proteins: a positive feedback loop in lung fibrosis?, Matrix Biol. 34 (2014) 170–178, [DOI] [PubMed] [Google Scholar]
- [85].Hinz B, The extracellular matrix and transforming growth factor-beta1: Tale of a strained relationship, Matrix Biol. 47 (2015) 54–65, [DOI] [PubMed] [Google Scholar]
- [86].Reinboth B, Hanssen E, Cleary EG, Gibson MA, Molecular interactions of biglycan and decorin with elastic fiber components: Biglycan forms a ternary complex with tropoelastin and micrfibril-associated glycoprotein 1, J. Biol. Chem 277 (2002) 3950–3957, [DOI] [PubMed] [Google Scholar]
- [87].Schmidt G, Hausser H, Kresse H, Interaction of the small proteoglycan decorin with fibronectin. Involvement of the sequence NKISK of the core protein, Biochem. J 280 (1991) 411–414, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Winnemöller M, Schmidt G, Kresse H, Influence of decorin on fibroblast adhesion to fibronectin, Eur. J. Cell Biol 54 (1991) 10–17, [PubMed] [Google Scholar]
- [89].Winnemöller M, Schön P, Vischer P, Kresse H, Interactions between thrombospondin and the small proteoglycan decorin: interference with cell attachment, Eur. J. Cell Biol 59 (1992) 47–55, [PubMed] [Google Scholar]
- [90].de Lange Davies C, Melder RJ, Munn LL, Mouta-Carreira C, Jain RK, Boucher Y, Decorin inhibits endothelial migration and tube-like structure formation: role of thrombospondin-1, Microvasc. Res 62 (2001) 26–42, [DOI] [PubMed] [Google Scholar]
- [91].Murphy-Ullrich JE, Iozzo RV, Thrombospondins in physiology and disease: New tricks for old dogs, Matrix Biol. 31 (2012) 152–154, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Murphy-Ullrich JE, Sage EH, Revisiting the matricellular concept, Matrix Biol. 37 (2014) 1–14, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Soto-Pantoja DR, Shih HB, Maxhimer JB, Cook KL, Ghosh A, Isenberg JS et al. , Thrombospondin-1 and CD47 signaling regulate healing of thermal injury in mice, Matrix Biol. 37 (2014) 25–34, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Resovi A, Pinessi D, Chiorino G, Taraboletti G, Current understanding of the thrombospondin-1 interactome, Matrix Biol. 37 (2014) 83–91, [DOI] [PubMed] [Google Scholar]
- [95].Rogers NM, Sharifi-Sanjani M, Csanyi G, Pagano PJ, Isenberg JS, Thrombospondin-1 and CD47 regulation of cardiac, pulmonary and vascular responses in health and disease, Matrix Biol. 37 (2014) 92–101, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Duquette M, Nadler M, Okuhara D, Thompson J, Shuttleworth T, Lawler J, Members of the thrombospondin gene family bind stromal interaction molecule 1 and regulate calcium channel activity, Matrix Biol. 37 (2014) 15–24, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Stenina-Adognravi O, Invoking the power of thrombospondins: regulation of thrombospondins expression, Matrix Biol 37 (2014) 69–82, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Klee S, Lehmann M, Wagner DE, Baarsma HA, Konigshoff M, WISP1 mediates IL-6-dependent proliferation in primary human lung fibroblasts, Sci Rep. 6 (2016) 20547, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Desnoyers L, Arnott D, Pennica D, WISP-1 binds to decorin and biglycan, J. Biol. Chem 276 (2001) 47599–47607, [DOI] [PubMed] [Google Scholar]
- [100].McArthur ME, Irving-Rodgers HF, Byer S, Rodgers RJ, Identification and immunolocalization of decorin, versican, perlecan, nidogen and chondroitin sulfate proteoglycans in bovine small-antral ovarian follicles, Biol. Reprod 63 (2000) 913–924, [DOI] [PubMed] [Google Scholar]
- [101].Wight TN, Kang I, Merrilees MJ, Versican and the control of inflammation, Matrix Biol. 35 (2014) 152–161, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wight TN, Kinsella MG, Evanko SP, Potter-Perigo S, Merrilees MJ, Versican and the regulation of cell phenotype in disease, Biochim. Biophys. Acta 1840 (2014) 2441–2451, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chang MY, Tanino Y, Vidova V, Kinsella MG, Chan CK, Johnson PY et al. , Reprint of: A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease, Matrix Biol. 35 (2014) 162–173, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ning L, Xu Z, Furuya N, Nonaka R, Yamada Y, Arikawa-Hirasawa E, Perlecan inhibits autophagy to maintain muscle homeostasis in mouse soleus muscle, Matrix Biol. 48 (2015) 26–35, [DOI] [PubMed] [Google Scholar]
- [105].Grindel BJ, Martinez JR, Pennington CL, Muldoon M, Stave J, Chung LW et al. , Matrilysin/matrix metalloproteinase-7(MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior, Matrix Biol 36 (2014) 64–76, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lord MS, Chuang CY, Melrose J, Davies MJ, Iozzo RV, Whitelock JM, The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling, Matrix Biol. 35 (2014) 112–122, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Farach-Carson MC, Warren CR, Harrington DA, Carson DD, Border patrol:Insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders, Matrix Biol. 34 (2014) 64–79, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Brown EL, Wooten RM, Johnson BJB, Iozzo RV, Smith A, Dolan MC et al. , Resistance to Lyme disease in decorin-deficient mice, J. Clin. Invest. 107 (2001) 845–852, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Guo B, Norris S, Rosenberg LC, Höök M, Adherence of Borrelia burgdorferi to the proteoglycan decorin, Infect. Immun. 63 (1995) 3467–3472, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Shi Y, Xu Q, McShan K, Liang FT, Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi, Infect. Immun. 76 (2008) 1239–1246, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV, Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor, J. Clin. Invest. 101 (1998) 406–412, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zhu J-X, Goldoni S, Bix G, Owens RA, McQuillan D, Reed CC et al. , Decorin evokes protracted internalization and degradation of the EGF receptor via caveolar endocytosis, J. Biol. Chem. 280 (2005) 32468–32479, [DOI] [PubMed] [Google Scholar]
- [113].Goldoni S, Seidler DG, Heath J, Fassan M, Baffa R, Thakur ML et al. , An anti-metastatic role for decorin in breast cancer, Am. J. Pathol. 173 (2008) 844–855, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Goldoni S, Iozzo RV, Tumor microenvironment: Modulation by decorin and related molecules harboring leucine-rich tandem motifs, Int. J. Cancer 123 (2008) 2473–2479, [DOI] [PubMed] [Google Scholar]
- [115].Neill T, Torres A, Buraschi S, Owens RT, Hoek J, Baffa R et al. , Decorin induces mitophagy in breast carcinoma cells via peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and mitostatin, J. Biol. Chem. 289 (2014) 4952–4968, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Iozzo RV, Buraschi S, Genua M, Xu S-Q, Solomides CC, Peiper SC et al. , Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling, J. Biol. Chem. 286 (2011) 34712–34721, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Morcavallo A, Buraschi S, Xu S-Q, Belfiore A, Schaefer L, Iozzo RV et al. , Decorin differentially modulates the activity of insulin receptor isoform A ligands, Matrix Biol. 35 (2014) 82–90, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Morrione A, Neill T, Iozzo RV, Dichotomy of decorin activity on the insulin-like growth factor-I system, FEBS J. 280 (2013) 2138–2149, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lala N, Gannareddy VG, Cloutier-Bosworth A, Lala PK, Mechanisms in decorin regulation of vascular endothelial growth factor-induced human trophoblast migration and acquisition of endothelial phenotype, Biol. Reprod. 87 (2012) 59:1–14, [DOI] [PubMed] [Google Scholar]
- [120].Moreth K, Iozzo RV, Schaefer L, Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation, Cell Cycle 11 (2012) 2084–2091, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Schaefer L, Iozzo RV, Small leucine-rich proteoglycans, at the crossroad of cancer growth and inflammation, Curr. Opin. Genet. Dev. 22 (2012) 56–57, [DOI] [PubMed] [Google Scholar]
- [122].Santiago-Garda J, Kodama T, Pitas RE, The class A scavenger receptor binds to proteoglycans and mediates adhesion of macrophages to the extracellular matrix, J. Biol. Chem. 278 (2003) 6942–6946, [DOI] [PubMed] [Google Scholar]
- [123].de Winther MP, van Dijk KW, Havekes LM, Hofker MH, Macrophage scavenger receptor class A: A multifunctional receptor in atherosclerosis, Arterioscler. Thromb. Vasc. Biol 20 (2000) 290–297, [DOI] [PubMed] [Google Scholar]
- [124].Myren M, Kirby DJ, Noonan ML, Maeda A, Owens RT, Ricard-Blum S et al. , Biglycan potentially regulates angiogenesis during fracture repair by altering expression and function of endostatin, Matrix Biol. 52-54 (2016) 141–150, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Brandan E, Retamal C, Cabello-Verrugio C, Marzolo M-P, The low density lipoprotein receptor-related protein functions as an endocytic receptor for decorin, J. Biol. Chem. 281 (2006) 31562–31571, [DOI] [PubMed] [Google Scholar]
- [126].Cabello-Verrugio C, Brandan E, A novel modulatory mechanism of transforming growth factor-β signaling through decorin and LRP-1, J. Biol. Chem. 282 (2007) 18842–18850, [DOI] [PubMed] [Google Scholar]
- [127].Cabello-Verrugio C, Santander C, Cofre C, Acuna MJ, Melo F, Brandan E, The internal region leucine-rich repeat 6 of decorin interacts with low density lipoprotein receptor-related protein-1, modulates transforming growth factor (TGF)-beta-dependent signaling, and inhibits TGF-beta-dependent fibrotic response in skeletal muscles, J. Biol Chem. 287 (2012) 6773–6787, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Brandan E, Cabello-Verrugio C, Vial C, Novel regulatory mechanisms for the proteoglycans decorin and biglycan during muscle formation and muscular dystrophy, Matrix Biol. 27 (2008) 700–708, [DOI] [PubMed] [Google Scholar]
- [129].Jungmann O, Nikolovska K, Stock C, Schulz J-N, Eckes B, Riethmuller C et al. , The dermatan sulfate proteoglycan decorin modulates α2β1 integrin and vimentin intermediate filament system during collagen synthesis, PLoS ONE 7 (2012) e50809, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Delogu G, Brennan MJ, Functional domains present in the mycobacterial hemagglutinin, HBHA, J. Bacteriol. 181 (1999) 7464–7469, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Takeuchi Y, Kodama Y, Matsumoto T, Bone matrix decorin binds transforming growth factor-β and enhances its bioactivity, J. Biol. Chem. 269 (1994) 32634–32638, [PubMed] [Google Scholar]
- [132].Kolb M, Margetts PJ, Sime PJ, Gauldie J, Proteoblycans decorin and biglycan differentially modulcate TGF-β- mediated fibrotic responses in the lung, Am. J. Physiol. Lung Cell Mol. Physiol. 280 (2001) L1327–L1334, [DOI] [PubMed] [Google Scholar]
- [133].Abdel-Wahab N, Wicks SJ, Mason RM, Chantry A, Decorin suppresses transforming growth factor-β-induced expression of plasminogen activator inhibitor-1 in human mesangial cells through a mechanism that involves Ca2+-dependent phosphorylation of Smad2 at serine-240, Biochem. J. 362 (2002) 643–649, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Mauviel A, Santra M, Chen YQ, Uitto J, Iozzo RV, Transcriptional regulation of decorin gene expression. Induction by quiescence and repression by tumor necrosis factor-α, J. Biol. Chem. 270 (1995) 11692–11700, [DOI] [PubMed] [Google Scholar]
- [135].Lee S-J, Regulation of muscle mass by myostatin, Annu. Rev. Cell Dev. Biol. 20 (2004) 61–86, [DOI] [PubMed] [Google Scholar]
- [136].Miura T, Kishioka Y, Wakamatsu J, Hattori A, Hennebry A, Berry CJ et al. , Decorin binds myostatin and modulates its activity to muscle cells, Biochem. Biophys. Res. Commun. 340 (2006) 675–680, [DOI] [PubMed] [Google Scholar]
- [137].Lipson KE, Wong C, Teng Y, Spong S, CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis, Fibrogenesis. Tissue Repair 5 (2012) S24, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Bi X, Xia X, Fan D, Mu T, Zhang Q, Iozzo RV et al. , Oncogenic activin C interacts with decorin in colorectal cancer in vivo and in vitro, Mol. Carcinog.2015), [DOI] [PubMed] [Google Scholar]
- [139].Tufvesson E, Westergren-Thorsson G, Tumor necrosis factor-a interacts with biglycan and decorin, FEBS Lett. 530 (2002) 124–128, [DOI] [PubMed] [Google Scholar]
- [140].Horvath Z, Kovalszky I, Fullar A, Kiss K, Schaff Z, Iozzo RV et al. , Decorin deficiency promotes hepatic carcinogenesis, Matrix Biol 35 (2014) 194–205, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Morrione A, Valentinis B, Xu S-Q, Yumet G, Louvi A, Louvi A et al. , Insulin-like growth factor II stimulates cell proliferation through the insulin receptor, Proc. Natl. Acad. Sci. USA 94 (1997) 3777–3782, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Seidler DG, Dreier R, Decorin and its galactosaminoglycan chain: Extracellular regulator of cellular function?, IUBMB Life 60 (2008) 729–733, [DOI] [PubMed] [Google Scholar]
- [143].Rohani MG, Parks WC, Matrix remodeling by MMPs during wound repair, Matrix Biol. 44-46 (2015) 113–121, [DOI] [PubMed] [Google Scholar]
- [144].Itoh Y, Membrane-type matrix metalloproteinases: Their functions and regulations, Matrix Biol 44-46 (2015) 207–223, [DOI] [PubMed] [Google Scholar]
- [145].Deryugina EI, Quigley JP, Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature, Matrix Biol. 44-46 (2015) 94–112, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Gaffney J, Solomonov I, Zehorai E, Sagi I, Multilevel regulation of matrix metalloproteinases in tissue homeostasis indicates their molecular specificity in vivo, Matrix Biol 44-46 (2015) 191–199, [DOI] [PubMed] [Google Scholar]
- [147].Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y, Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release, Biochem. J. 322 ( Pt 3) (1997) 809–814, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Duarte S, Baber J, Fujii T, Coito AJ, Matrix metalloproteinases in liver injury, repair and fibrosis, Matrix Biol. 44-46 (2015) 147–156, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].von Marschall Z, Fisher LW, Decorin is processed by three isoforms of bone morphogenetic protein-1 (BMP1), Biochem. Biophys. Res. Comm. 391 (2010) 1374–1378, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Vadon-Le Goff S, Hulmes DJ, Moali C, BMP-1/tolloid-like proteinases synchronize matrix assembly with growth factor activation to promote morphogenesis and tissue remodeling, Matrix Biol. 44-46 (2015) 14–23, [DOI] [PubMed] [Google Scholar]
- [151].Sartipy P, Johansen B, Gasvik K, Hurt-Camejo E, Molecular basis for the association of group IIA phospholipase A(2) and decorin in human atherosclerotic lesions, Circ. Res 86 (2000) 707714, [DOI] [PubMed] [Google Scholar]
- [152].Burke JE, Dennis EA, Phospholipase A2 structure/function, mechanism, and signaling, J. Lipid Res 50 Suppl (2009) S237–S242, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Guidetti G, Bertoni A, Viola M, Tira E, Balduini C, Torti M, The small proteoglycan decorin supports adhesion and activation of human platelets, Blood 100 (2002) 1707–1714, [PubMed] [Google Scholar]
- [154].Reininger AJ, Function of von Willebrand factor in haemostasis and thrombosis, Haemophilia. 14 Suppl 5 (2008) 11–26, [DOI] [PubMed] [Google Scholar]
- [155].Guidetti GF, Bartolini B, Bernardi B, Tira ME, Berndt MC, Balduini C et al. , Binding of von Willebrand factor to the small proteoglycan decorin, FEBS Lett. 574 (2004) 95–100, [DOI] [PubMed] [Google Scholar]
- [156].Dugan TA, Yang VWC, McQuillan DJ, Höök M, Decorin binds fibrinogen in a Zn2+-dependent interaction, J. Biol. Chem. 278 (2003) 13655–13662, [DOI] [PubMed] [Google Scholar]
- [157].Dugan TA, Yang VWC, McQuillan DJ, Höök M, Decorin modulates fibrin assembly and structure, J. Biol. Chem. 281 (2006) 38208–38216, [DOI] [PubMed] [Google Scholar]
- [158].Lackner C, Boerwinkle E, Leffert CC, Rahmig T, Hobbs HH, Molecular basis of apolipoprotein (a) isoform size heterogeneity as revealed by pulsed-field gel electrophoresis, J. Clin. Invest 87 (1991) 2153–2161, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Klezovitch O, Edelstein C, Zhu L, Scanu AM, Apolipoprotein(a) binds via its C-terminal domain to the protein core of the proteoglycan decorin. Implications for the retention of lipoprotein(a) in atherosclerotic lesions, J. Biol. Chem. 273 (1998) 23856–23865, [DOI] [PubMed] [Google Scholar]
- [160].Kovanen PT, Pentikainen MO, Decorin links low-density lipoproteins (LDL) to collagen: a novel mechanism for retention of LDL in the atherosclerotic plaque, Trends Cardiovasc. Med. 9 (1999) 86–91, [DOI] [PubMed] [Google Scholar]
- [161].Pentikainen MO, oorni K, Lassila R, Kovanen PT, The proteoglycan decorin links low density lipoproteins with collagen type I, J. Biol. Chem. 272 (1997) 7633–7638, [DOI] [PubMed] [Google Scholar]
- [162].Nakamura F, Osborn TM, Hartemink CA, Hartwig JH, Stossel TP, Structural basis of filamin A functions, J. Cell Biol 179 (2007) 1011–1025, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Bachmann AS, Howard JP, Vogel CW, Actin-binding protein filamin A is displayed on the surface of human neuroblastoma cells, Cancer Sci 97 (2006) 1359–1365, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG, An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells, Cell Stem Cell 9 (2011) 74–86, [DOI] [PubMed] [Google Scholar]
- [165].Nadesalingam J, Bernal AL, Dodds AW, Willis AC, Mahoney DJ, Day AJ et al. , Identification and characterization of a novel interaction between pulmonary surfactant protein D and decorin, J. Biol. Chem. 278 (2003) 25678–25687, [DOI] [PubMed] [Google Scholar]
- [166].Krumdieck R, Höök M, Rosenberg LC, Volanakis JE, The proteoglycan decorin binds C1q and inhibits the activity of the C1 complex, J. Immunol. 149 (1992) 3695–3701, [PubMed] [Google Scholar]
- [167].Sugars RV, Waddington RJ, Embery G, The interaction of recombinant decorin with a2HS-glycoprotein-implications for structural and functional investigations, Protein Express. Purif. 25 (2002) 180–188, [DOI] [PubMed] [Google Scholar]
- [168].Neill T, Schaefer L, Iozzo RV, An oncosuppressive role for decorin, Mol. Cell. Oncol. 2 (2015) e975645, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Neill T, Schaefer L, Iozzo RV, Decoding the matrix: Instructive roles of proteoglycan receptors, Biochemistry 54 (2015) 4583–4598, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Neill T, Schaefer L, Iozzo RV, Decorin, a guardian from the matrix, Am. J. Pathol. 181 (2012) 380–387, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Theocharis AD, Skandalis SS, Neill T, Multhaupt HA, Hubo M, Frey H et al. , Insights into the key roles of proteoglycans in breast cancer biology and translational medicine, Biochim. Biophys. Acta 1855 (2015) 276–300, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Xu W, Neill T, Yang Y, Hu Z, Cleveland E, Wu Y et al. , The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer, Gene Therapy 22 (2015) 31–40, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Yang Y, Xu WW, Neill T, Hu Z, Wang CH, Xiao X et al. , Systemic Delivery of an Oncolytic Adenovirus Expressing Decorin for the Treatment of Breast Cancer Bone Metastases, Hum. Gene Ther. 26 (2015) 813–825, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B et al. , Cooperative action of germline mutations in decorin and p53 accelerates lymphoma tumorigenesis, Proc. Natl. Acad. Sci. USA 96 (1999) 3092–3097, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Bi X, Tong C, Dokendorff A, Banroft L, Gallagher L, Guzman-Hartman G et al. , Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation, Carcinogenesis 29 (2008) 1435–1440, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Bi X, Pohl NM, Yang GR, Gou Y, Guzman G, Kajdacsy-Balla A et al. , Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice, Carcinogenesis 33 (2012) 326–330, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Buraschi S, Neill T, Owens RT, Iniguez LA, Purkins G, Vadigepalli R et al. , Decorin protein core affects the global gene expression profile of the tumor microenvironment in a triple-negative orthotopic breast carcinoma xenograft model, PLoS ONE 7 (2012) e45559, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Kalas W, Swiderek E, Switalska M, Wietrzyk J, Rak J, Strzadala L, Thrombospondin-1 receptor mediates autophagy of RAS-expressing cancer cells and triggers tumour growth inhibition, AntiCancer Res. 33 (2013) 1429–1438, [PubMed] [Google Scholar]
- [179].Launay G, Salza R, Multedo D, Thierry-Mieg N, Ricard-Blum S, MatrixDB, the extracellular matrix interaction database: updated content, a new navigator and expanded functionalities, Nucleic. Acids Res. 43 (2015) D321–D327, [DOI] [PMC free article] [PubMed] [Google Scholar]